Abstract
Hemorrhagic transformation (HT) following ischemia is one complication which worsens stroke outcome. During and after ischemia-reperfusion, persistent reduction of brain pH occurs. In a recent study, we found that GPR68 functions as a neuronal proton receptor and mediates a protective pathway in brain ischemia. Here, we asked whether GPR68 contributes HT after ischemia. At 24 hr after transient middle cerebral artery occlusion (tMCAO), 58% of the wild-type (WT) mice exhibited some degrees of mild HT. At 72 hr, 95% of the WT showed HT with 42% exhibited large “parenchymal” type hemorrhage. In the GPR68−/− mice, there was a trend of increase in both the incidence and severity of HT at both time points. Mice with severe hemorrhage exhibited significantly larger infarct than those with no to mild hemorrhage. Next, we compared % infarct of GPR68−/− vs WT based on their HT categories. GPR68 deletion increased % infarct when the HT severity is mild. In contrast, for mice exhibiting large area HT, the two genotypes had no difference in % infarct. These data showed that GPR68-dependent signaling leads to protection when HT is mild.
INTRODUCTION
Hemorrhagic transformation (HT) may occur following ischemic stroke and contribute to stroke-induced injuries [1, 2]. Previous studies have shown that multiple processes, including reperfusion-induced inflammation, disrupted metabolism, increased oxidative signaling, and disruption of basement membrane, compromise blood-brain barrier integrity and contribute to post-stroke HT [1-4]. In addition, the incidence of hemorrhagic transformation increases after thrombolytic therapy in human stroke patients[1, 3]. These studies demonstrate the importance for a better understanding of mechanisms underlying HT as well as its association with brain injury.
Following stroke, one common phenomenon is that brain becomes acidotic [5, 6]. Brain acidosis plays a key role in determining ischemia-induced brain injury [7, 8]. In previous studies, multiple ionic proton receptors, including acid-sensing ion channels and proton-activated chloride channels, contribute to acidosis-induced neuronal injury [9-11]. We recently found that GPR68, a proton receptor in brain neurons, and mediate a neuroprotective pathway in acidotic and ischemic conditions [12, 13]. This finding raised a question of whether GPR68 may also alleviate HT after ischemia.
Here, to better understand HT following tMCAO, we scored TTC stained sections for HT at both 24 and 72 hr after a 45-min tMCAO. To determine the effect of GPR68 in HT, we compared GPR68−/− with WT animals. Further, we asked whether different subgroups of HT exhibit an association with % infarct and cerebral blood flow (CBF) changes.
MATERIAL AND METHODS
Mice
Wild-type C57BL/6 and knockout mice were maintained as breeding colonies at the University of South Alabama. The GPR68−/− mice [14] were on a congenic C57BL/6 background. All knockout mice were refreshed (backcrossed to C57BL/6 wild-type) every 5–10 generations, according to the Jackson Laboratory’s recommendations. Animal care met National Institutes of Health standards. All procedures were approved by the University of South Alabama Animal Care and Use Committee.
Transient Focal ischemia
Note that the MCAO-TTC images used here for HT analysis have been previously analyzed for brain injury in our recent study [12]. The induction of transient focal ischemia in mice was performed as described previously [12, 15]. Briefly, WT and GPR68−/− male mice at 8-12 week of age and with body weight of 25 ± 3 g were used for this study. We randomly assigned the mice to experimental groups. For the 24 hr time point, the genotype of the first set of animals (7 WT 4KO) were not blinded, while that of the second cohort of the animals were blinded (5 WT 6 KO). The two sets generated comparable results and were combined and reported. For the 72 hr time point, the genotype of all animals was blinded to the operator. Following the surgery and the initial recovery in a temperature-controlled cage, all mice were returned to their home cage and have ad libitum access to water, diet gel, and food.
Anesthesia was maintained with 1.5% isoflurane, 70% N2O, and 28.5% O2 with intubation and ventilation. Rectal and temporalis muscle temperatures were kept at 37°C ± 0.5°C with a thermostatically controlled heating pad. For cerebral blood flow monitoring, we attached a fiber optic probe to the mouse skull with cyanoacrylate adhesive. Under an operating microscope, the bifurcation of the common carotid artery (CCA) was exposed and the external carotid artery (ECA) ligated. The internal carotid artery (ICA) was isolated, the extracranial branch of the ICA was ligated, and then a 7-0 silicon coated monofilament nylon surgical suture (Doccol) was introduced through the open end of ECA into the ICA lumen and advanced ~7-8 mm past the CCA bifurcation. Once the suture reached the correct position, the tie at CCA was loosened to restore the blood flow to most of the ipsilateral side. Suture occlusion of the middle cerebral artery (MCAO) was performed for 45 min. Cerebral blood flow (CBF) for all surgery animals was monitored for the entire duration by transcranial laser Doppler (MoorVMS-LDF2). To reduce the potential concern of variabilities introduced by CBF variations, we applied the following inclusion criteria: the occluded CBF was stable at 5-20% of the original value and the reperfused CBF reaches 50%-120% of the original value. The breakdown of the animals used in the 72 hr HT analysis: a) 19 WT and 18 KO counted in the TTC and HT analysis, b) 8 WT and 5 KO with good trace but died before the 72 hr time point, and c) 9 WT and 10 KO with traces not meeting the inclusion criteria. 3 WT and 2 KO which exhibited apparent unsatisfactory occluded blood flow were euthanized before suture withdraw and thus did not have reperfusion value. For the 24 hr group: a) 12 WT and 10 KO counted in the analysis; b) 7 WT and 6 KO did not meet the CBF trace criteria; c) 6 WT and 7 KO died during the surgery. Statistically (chi square analysis) these numbers were not different between the two genotypes.
Vital dye staining and quantification of brain infarct
Mice were sacrificed at either 24 or 72 hr after tMCAO. We sectioned the brain coronally at 1 mm intervals and stained with vital dye immersion: (2%) 2,3,5-triphenyltetrazolium hydrochloride (TTC). Following staining, we fixed the sections in 4% paraformaldehyde and scanned with a photo scanner (Canon Inc). For percent infarct calculation, we used the method as described in one previous study [16]:
For quantification of edema factor, we calculated the ratio of (ipsilateral-contralateral)/contralateral brain volume [17, 18].
Quantification of cerebral blood flow
Raw blood flow before (0-2 min), during (from the 6th min till the end of occlusion), and after reperfusion (last 2 min of recording, typically at about 10 min after reperfusion) was measured by transcranial laser Doppler (MoorVMS-LDF2) and recorded by the MoorVMS software, exported and analyzed and plotted by Microsoft Excel and GraphPad software.
Quantification of hemorrhagic transformation
The categorizing of HT was modified based on a previous study [19]. Compared to the referred study, we included one additional group to better account the range of HT with small “spotty-type” hemorrhages. This is in part due to the fact that we are quantifying 6 (24 hr) or 7 (72 hr) slices per animal and thus the number of small hemorrhages can have a larger range (from 1-14). We also separated the groups with large area “parenchymal” type HT to two subgroups based on their size. Thus, there are 3 main types and 6 groups. Group (0) has no hemorrhage. Groups (I-III) show a number of small hemorrhages: (I) has a total of 1-3 small hemorrhages across all brain sections; (II) has 4-10 small hemorrhages across all brain sections; (III) has >10 small hemorrhages. Group (IV & V) exhibit large area “parenchymal” type HT: (IV) has volume of hemorrhage less than 10% when normalized to the contralateral side while (V) has volume of hemorrhage more than 10% when normalized to the contralateral side.
Statistics
Graphpad Prism and Microsoft Excel were used for statistical analysis. Two-tailed Mann-Whitney U test was used to compare two groups. When multiple comparisons were performed, we used 2-way ANOVA with Bonferroni’s post-hoc correction. Chi-square analysis was used to compare animals in included and excluded categories between the two genotypes. Differences were considered significant if p < 0.05. Mean ± SDEV was presented in figures and text.
RESULTS
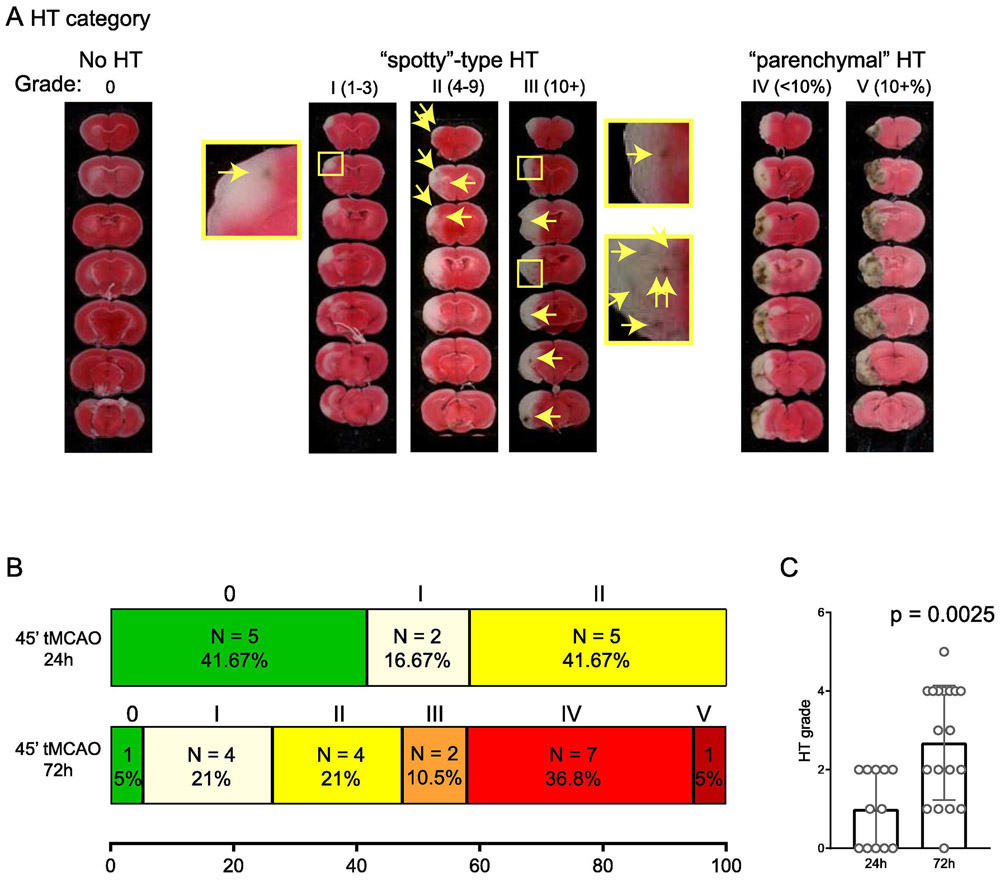
In our recent study, we examined tMCAO-induced brain infarction[12]. Our result showed that GPR68 deletion exacerbated ischemia-induced brain injury. Here, we re-analyzed the same set of TTC-stained sections for HT. To assess HT in these sections, we adopted a scoring approach similar to that described in a previous study [19]. Figure 1A illustrates the scale which we used. We classified the HT patterns to the following categories: no HT (category 0); those with “spotty” pattern of HT, which was further scored based on the number of small hemorrhages: 1-3 (category I) , 4-10 (category II), 10+ (category III); and those with large area of HT, which was further scored based on % hemorrhagic area (normalized to the contralateral side): <10% (category IV) and > 10% (category V).
FIGURE 1. Development of HT at 24 and 72 hr time points.
(A) Representative images showing the categories of HT for scoring. The categories were based on a previous report but with modifications to better fit the fact that we are scoring multiple slices from one given mouse. Arrows in middle images and enlarged insets illustrate HT spots. For categories I-III, numbers in parenthesis indicate the range of number of HT spots included in that category. For categories IV and V, numbers in parenthesis indicate the % area of HT as compared to contralateral brain. (B) Summary of HT categories at 24 and 72 hr following 45 min tMCAO. (C) Bar graph showing average HT grade at 24 and 72 hr. For quantification, categories 0-V is converted to scores of 0-5. Each dot represents one animal. P value was from 2-tailed Mann-Whitney U test.
We first examined wild-type mice and compared their HT scores at 24 hr and 72 hr after 45 min tMCAO (Figure 1B). At 24 hr, 41.7% (5/12) of the animal exhibited no HT while the rest exhibited HT of category I (2/12) or II (5/12). At 72 hr, there was a clear increase in both the incidence and severity of HT. 18 of 19 animals exhibited HT, with 36.8% (7/19) scored as category IV and 5% (1/19) as category V. The average HT score of the 72-hr group was 2.68 ± 1.46, significantly higher than the 0.95 ± 0.27 score of the 24-hr group (p = 0.0025, Mann-Whitney U test). Next, we asked whether GPR68 deletion alters HT. At both 24 and 72 hr time points, GPR68−/− mice exhibited a slight shift toward more severe HT (Figure 2A, B). However, the average HT scores were not different between the two genotypes.
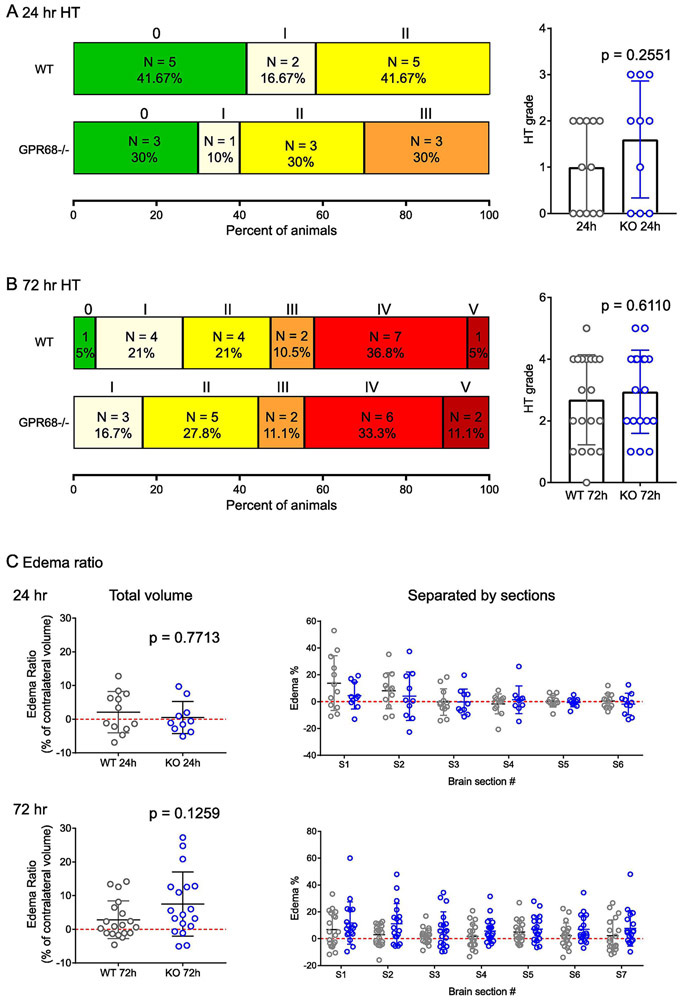
FIGURE 2. The effect of GPR68 deletion on HT.
GPR68−/− and WT mice were scored at 24 (A) and 72 (B) hr as described in Figure 1. The WT summary was re-plotted from Figure 1B to better compare with that of GPR68−/−. (C) Edema ratio at 24 and 72 hr after tMCAO. Ratio of edema was calculated based on TTC images as (ipsilateral volume – contralateral volume)/contralateral volume. P values were from 2-tailed Mann-Whitney U test.
To determine whether WT and KO exhibit any changes in edema following tMCAO, we analyzed the ipsilateral and contralateral brain volume, and calculated the ratio of (ipsilateral-contralateral)/contralateral volume [17, 18]. At 24 hr, WT and GPR68−/− showed comparable edema ratio of 2.1 ± 6.1% and 0.5 ± 4.8%, respectively (Figure 2C). At 72 hr, there was a trend toward increased edema ratio in the knockout: the average edema ratio was 2.8 ± 5.6% for WT and 7.5 ± 9.5% for GPR68−/−. We further analyzed the edema % by individual sections. None of the comparisons were statistically different. One technical note is that we fixed the TTC stained slices overnight before scanning. We speculate that this post-fixation may lead to some shrinkage of the brain sections, especially on the injury side which had higher initial water content.
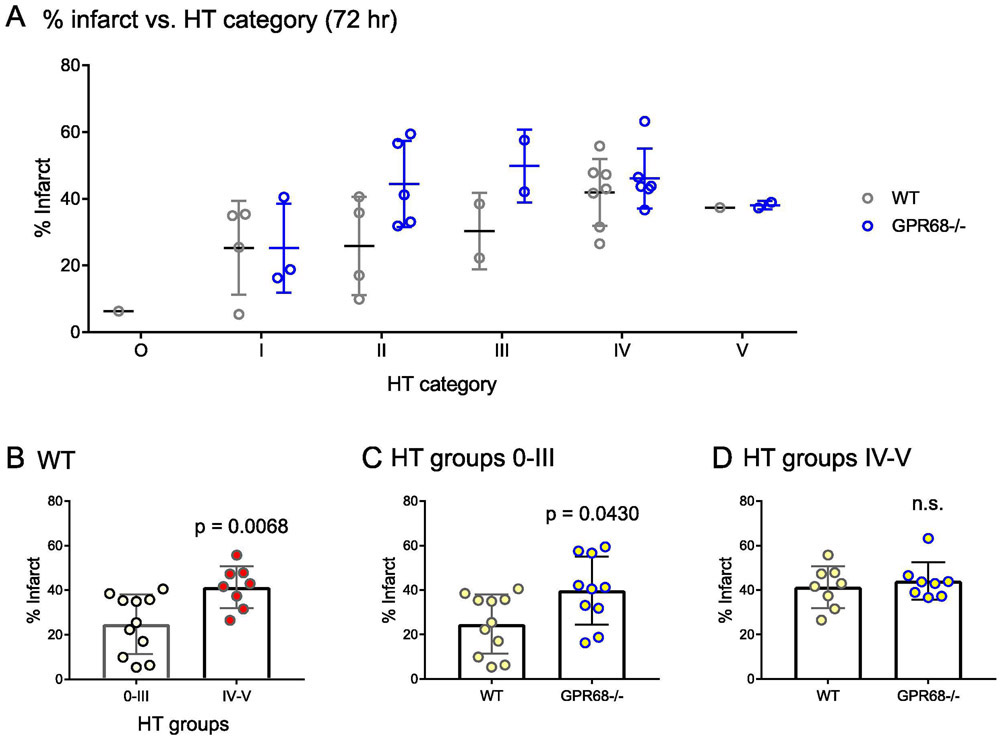
To determine whether there exists a correlation between brain injury and HT severity, we examined the % infarct in each HT categories. For WT, mice in HT categories 0-III had lower infarct as compared to those of IV and V categories (Figure 3A). Grouped comparison showed that average % infarct for IV and V group was 41.4% ± 9.4%, significantly higher than those of 0-III group 24.7 ± 13.3% (p = 0.0068, Mann-Whitney U test) (Figure 3B). In our recent study, we found that GPR68−/− mice exhibited larger tMCAO-induced infarct. To determine whether this effect correlate with HT severity, we quantified the infarct percentage of HT groups 0-III and IV-V separately and compared the numbers between the two genotypes. For HT groups 0-III, the average % infarct of GPR68−/− was 39.8 ± 15.3%, significantly higher than that (24.7 ± 13.4%) of the WT in the group (Figure 3C). However, for those exhibited large area of HT (HT groups IV and V), the two genotypes had similar levels of infarct % (Figure 3D).
FIGURE 3. Correlation between infarct size and HT severity.
(A) Scatter plot to summarize the infarct % over HT grades. (B) Quantification showing the average % infarct for WT, separated based on HT grades 0-III and IV-V. (C & D) Quantification showing the effect of GPR68 deletion on infarct percentage based on HT grades 0-III (C) or IV-V (D). P values were from 2-tailed Mann-Whitney U test.
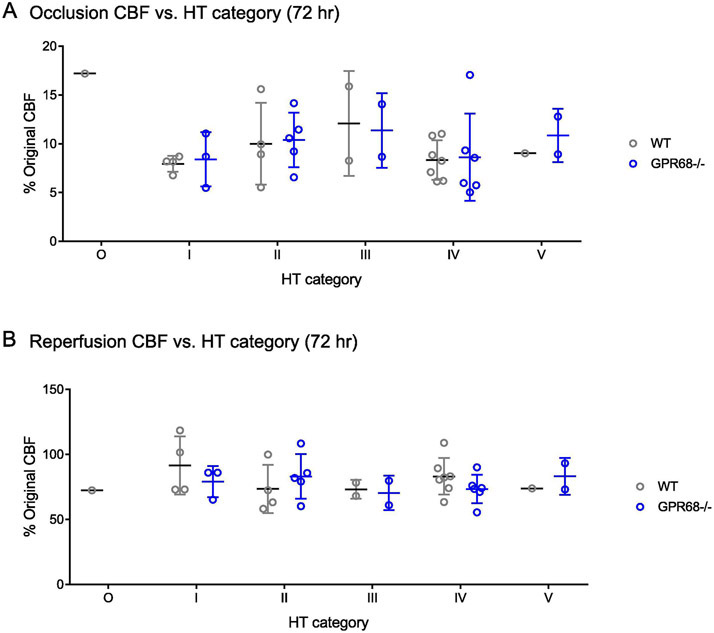
For our MCAO animals, we used the following inclusion criteria: an occluded cerebral blood flow (CBF) between 5-20% of the original value and a reperfused CBF to 50-120% of the original value. These criteria apparently pre-selected a cohort of animals based on their CBF changes during MCAO. Nevertheless, we wondered whether the observed HT groups preferentially associate with a specific range of occluded or reperfused CBF. To answer this question, we plotted CBF changes according to their HT categories. All HT groups exhibited comparable occluded (Figure 4A) or reperfused (Figure 4B) CBF.
FIGURE 4. Correlation between cerebral blood flow (CBF) changes and HT severity.
Scatter plot in (A) shows the CBF during occlusion while that in (B) shows the reperfused value over HT grades.
DISCUSSION
In a previous study, the authors used thrombin-induced clot to induce a permanent MCAO [19]. At 24 hr, all animals exhibited some degree of hemorrhages with 23% showing large “parenchymal” type hemorrhage. Here, we examined the changes following transient suture-based occlusion. Our results showed that HT at 24 hour after tMCAO is milder as compared to the previous permanent model. In our experiments, 59% of the animals exhibit hemorrhages; all in categories I & II. However, HT apparently continued to develop after day 1. At 72 hr after tMCAO, about 95% of the WT and 100% of the GPR68−/− mice exhibited some forms of HT. In addition, a significant fraction (over 40%) of the animals developed large area HT at this later time point. The contrast between these results are interesting. The permanent clot model enables the assessment of tPA effect as described in the previous study [19]. The transient suture model, on the other hand, will allow us to better understand how the time-dependent development of HT following the ischemia-reperfusion paradigm (without the confounding factor of tPA treatment).
The continued development of HT during the first three days after reperfusion is consistent with previous reported timeline of ischemia-induced disruption of blood-brain barrier (BBB) [2, 20-22]. In rodent models of transient ischemia, blood-brain barrier (BBB) exhibited biphasic opening within the first few days. The initial opening occurs within hours, followed by a reduced leakage around 24 hour and a second increase in leakage around 48-72 hour after reperfusion [20-22]. Thus, the increased HT at 72 hr (as compared to 24 hr) in our study likely reflects the second phase of BBB disruption after ischemia-reperfusion. One technical note is that analyzing TTC stained sections apparently is not the most accurate approach to quantify HT. However, this approach has its advantage in allowing a direct correlation between brain injury with HT category on the same animal. To better interpret these changes, it will be of future interest to perform additional analysis such as H&E staining and immunostaining against various neuroinflammatory marker. In addition, we speculate that our post-fixation led to, at least in part, an underestimation of the true edema percentage. Since we applied the same procedure to both WT and knockouts, this caveat would affect both genotypes in the same way, and thus unlikely to alter the main conclusion on edema comparison. Nevertheless, to gain more quantitative measurements, it will be important to perform similar analysis using freshly stained sections or measuring the water content.
While multiple mechanisms contribute to HT, the contribution to this process from brain acid receptors remain unclear. Following brain ischemia, pH in the brain can reduce to 6.5-6.0 or even lower [8, 23-25]. Through activating acid-sensing ion channels and proton-activated chloride channel, acidosis contributes to ischemia-induced brain injury [9, 10]. In our recent study, we showed that GPR68 functions as a metabotropic proton receptor in brain neurons. GPR68 contributes to hippocampal LTP and fear memory and mediates a protective pathway in brain ischemia [12, 13]. Deleting GPR68 led to increased ischemia-induced brain injury while GPR68 overexpression resulted in protection. At least part of the effect of GPR68 depends on PKC-dependent signaling [12].
Here, we showed that GPR68 deletion did not alter the average score of HT, although there appeared to have a shift, especially at 24 hr, toward more severe categories. This result suggests that the primary site of GPR68 function is not at the BBB. This speculation is consistent with the predominantly neuronal expression of GPR68 and sporadic or low expression of this receptor in brain endothelial cells [12, 26]. How GPR68, which primarily presents in neurons, alters HT warrants further analysis. In our RNA-Seq analysis, we found that GPR68 deletion altered the pathways involved in ER stress and oxidation/reduction responses (unpublished result). We speculate that an altered stress/oxidative response contributes to worsened HT in the knockout. Nevertheless, to better understand the mechanism associated with these changes, it will be important to assess whether GPR68 deletion alters BBB integrity in a future study.
CONCLUSIONS
Our results showed a continued development of HT from 24 to 72 hr after tMCAO. There was no apparent correlation between HT severity at 72 hr with CBF changes, either during occlusion or after reperfusion. GPR68−/− mice exhibited larger brain infarct when HT is mild.
Acknowledgements
We thank Dr. Yan Xu (Indiana University) for providing the GPR68−/− mouse.
Funding
The study was supported in part by NIH/NINDS R01NS102495 (XMZ) and an intramural grant #1341 from University of South Alabama College of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest
The authors declare no competing interests.
Submission declaration
We re-analyzed the same set of MCAO-TTC images which have been previously analyzed in another study for brain injury. The % infarct data was the same as in that study. However, the HT result is new and its correlation analysis with injury and blood flow is also new. These results have not been published previously or under consideration at another place.
REFERENCES
- 1.Khatri R, McKinney AM, Swenson B, Janardhan V: Blood-brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology 2012, 79(13 Suppl 1):S52–57. [DOI] [PubMed] [Google Scholar]
- 2.Okada T, Suzuki H, Travis ZD, Zhang JH: The Stroke-Induced Blood-Brain Barrier Disruption: Current Progress of Inspection Technique, Mechanism, and Therapeutic Target. Curr Neuropharmacol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang C, Hawkins KE, Dore S, Candelario-Jalil E: Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am J Physiol Cell Physiol 2019, 316(2):C135–C153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao Y: Basement membrane and stroke. J Cereb Blood Flow Metab 2019, 39(1):3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinstein PR, Hong S, Sharp FR: Molecular identification of the ischemic penumbra. Stroke 2004, 35(11 Suppl 1):2666–2670. [DOI] [PubMed] [Google Scholar]
- 6.Siesjo BK: Pathophysiology and treatment of focal cerebral ischemia. Part II: Mechanisms of damage and treatment. J Neurosurg 1992, 77(3):337–354. [DOI] [PubMed] [Google Scholar]
- 7.Tombaugh GC, Sapolsky RM: Evolving concepts about the role of acidosis in ischemic neuropathology. J Neurochem 1993, 61(3):793–803. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y, Jiang N, Li J, Ji YH, Xiong ZG, Zha XM: Two aspects of ASIC function: Synaptic plasticity and neuronal injury. Neuropharmacology 2015, 94:42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ et al. : Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell 2004, 118(6):687–698. [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Chen J, Del Carmen Vitery M, Osei-Owusu J, Chu J, Yu H, Sun S, Qiu Z: PAC, an evolutionarily conserved membrane protein, is a proton-activated chloride channel. Science 2019, 364(6438):395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Y, Jiang YQ, Li C, He M, Rusyniak WG, Annamdevula N, Ochoa J, Leavesley SJ, Xu J, Rich TC et al. : Human ASIC1a mediates stronger acid-induced responses as compared with mouse ASIC1a. FASEB J 2018, 32(7):3832–3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang T, Zhou G, He M, Xu Y, Rusyniak WG, Xu Y, Y. J, Simon RP, Xiong ZG, Zha XM: GPR68 is a neuroprotective proton receptor in brain ischemia. Stroke 2020. (in press; Doi: 10.1161/STROKEAHA.120.031479) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Y, Lin MT, Zha XM: GPR68 deletion impairs hippocampal long-term potentiation and passive avoidance behavior. Mol Brain 2020, 13(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Wang D, Singh LS, Berk M, Tan H, Zhao Z, Steinmetz R, Kirmani K, Wei G, Xu Y: Abnormalities in osteoclastogenesis and decreased tumorigenesis in mice deficient for ovarian cancer G protein-coupled receptor 1. PLoS One 2009, 4(5):e5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang N, Wu J, Leng T, Yang T, Zhou Y, Jiang Q, Wang B, Hu Y, Ji YH, Simon RP et al. : Region specific contribution of ASIC2 to acidosis-and ischemia-induced neuronal injury. J Cereb Blood Flow Metab 2017, 37(2):528–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR: A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab 1990, 10(2):290–293. [DOI] [PubMed] [Google Scholar]
- 17.McBride DW, Klebe D, Tang J, Zhang JH: Correcting for Brain Swelling’s Effects on Infarct Volume Calculation After Middle Cerebral Artery Occlusion in Rats. Transl Stroke Res 2015, 6(4):323–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin TN, He YY, Wu G, Khan M, Hsu CY: Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke 1993, 24(1):117–121. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Yebenes I, Sobrado M, Zarruk JG, Castellanos M, Perez de la Ossa N, Davalos A, Serena J, Lizasoain I, Moro MA: A mouse model of hemorrhagic transformation by delayed tissue plasminogen activator administration after in situ thromboembolic stroke. Stroke 2011, 42(1):196–203. [DOI] [PubMed] [Google Scholar]
- 20.Kuroiwa T, Ting P, Martinez H, Klatzo I: The biphasic opening of the blood-brain barrier to proteins following temporary middle cerebral artery occlusion. Acta Neuropathol 1985, 68(2):122–129. [DOI] [PubMed] [Google Scholar]
- 21.Huang ZG, Xue D, Preston E, Karbalai H, Buchan AM: Biphasic opening of the blood-brain barrier following transient focal ischemia: effects of hypothermia. Can J Neurol Sci 1999, 26(4):298–304. [DOI] [PubMed] [Google Scholar]
- 22.Belayev L, Busto R, Zhao W, Ginsberg MD: Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res 1996, 739(1–2):88–96. [DOI] [PubMed] [Google Scholar]
- 23.Kalimo H, Rehncrona S, Soderfeldt B, Olsson Y, Siesjo BK: Brain lactic acidosis and ischemic cell damage: 2. Histopathology. J Cereb Blood Flow Metab 1981, 1(3):313–327. [DOI] [PubMed] [Google Scholar]
- 24.Siesjo BK: Lactic acidosis in the brain: occurrence, triggering mechanisms and pathophysiological importance. Ciba Found Symp 1982, 87:77–100. [DOI] [PubMed] [Google Scholar]
- 25.Pignataro G, Simon RP, Xiong ZG: Prolonged activation of ASIC1a and the time window for neuroprotection in cerebral ischaemia. Brain 2007, 130(Pt 1):151–158. [DOI] [PubMed] [Google Scholar]
- 26.Xu J, Mathur J, Vessieres E, Hammack S, Nonomura K, Favre J, Grimaud L, Petrus M, Francisco A, Li J et al. : GPR68 Senses Flow and Is Essential for Vascular Physiology. Cell 2018, 173(3):762–775 e716. [DOI] [PMC free article] [PubMed] [Google Scholar]