Abstract
BACKGROUND AND PURPOSE:
Impairment of fiber integrity of the corticospinal tract in the subacute and chronic phases after ischemic stroke has been linked to poor motor outcome. The aim of the study was an assessment of fiber integrity in the acute poststroke phase and an evaluation of its association with the clinical course dependent on the infarction pattern (subtypes: peripheral versus basal ganglia infarction).
MATERIALS AND METHODS:
All patients who underwent mechanical recanalization of a large-vessel occlusion in the anterior circulation and postinterventional DTI were included (n = 165). The fractional anisotropy index of the patient-specific corticospinal tract within the posterior limb of the internal capsule was correlated to clinical parameters (NIHSS scores/mRS at 90 days), and the interaction of stroke subtype (peripheral infarcts versus basal ganglia infarction) was tested in a moderation analysis.
RESULTS:
The fractional anisotropy index was reduced in the acute poststroke phase with a correlation to clinical presentation, especially in case of peripheral infarcts (eg, with the NIHSS motor subscore: r = –0.4, P < .001). This correlation was absent for basal ganglia infarction (r = –0.008, P > .05). There was a significant association between the fractional anisotropy index and clinical outcome (mRS after 90 days, P < .01), which is moderated by stroke subtype with significant effects only for peripheral infarcts.
CONCLUSIONS:
Corticospinal tract abnormalities can be observed in the early stage after mechanical recanalization and have prognostic capacity. This finding increases the clinical value of early DTI imaging parameters. Because the effects observed were limited to peripheral infarcts, further and longitudinal evaluation of fiber integrities within basal ganglia infarction is required.
In most cases, ischemic stroke caused by acute occlusions of a large intracranial vessel of the anterior circulation leads to motor impairment, resulting in major disability and poor quality of life.1 Early prediction of functional motor outcome is essential for clinical stroke management, rehabilitation, and related research.2 The prediction of functional recovery is challenging due to high interindividual variability,3 influenced by a variety of biologic and environmental factors.4 Studies are notably missing for the rapidly rising entity of basal ganglia infarction (BGI), which emerges from high rates of successful reperfusion due to mechanical recanalization.
Several variables are valid predictors of motor recovery, such as the grade of initial motor deficit, site of infarction, stroke volume, age, demographics, comorbidities, and stroke subtype.2,5-11 Most patients are discharged from stroke units within several days after treatment, making early prediction of outcome difficult even for experienced clinicians.12 They would benefit from biomarkers that could easily be assessed in the acute poststroke phase. Imaging can provide parameters about tissue integrity that have a promising value for outcome prediction beyond the existing ones. The integrity of the corticospinal tract (CST) has already proved to be an essential factor for motor outcome.13,14 Macrostructural analyses are important, but they can give only limited information, eg, about specific tissue integrity. This can be better assessed by microstructural imaging, eg, patient-specific fiber analyses with DTI, providing more information about functional structures.
Currently, evaluation of fiber integrity using DTI is well-established.15 Microstructural damage of CST fibers has previously been analyzed in different phases after stroke, mostly in subacute and chronic stages, and the potential to predict motor outcome has been shown.16-19 A previous study used neuroimaging acquired during the acute stroke work-up, which has been able to predict motor recovery by macrostructural injury of the CST.20 However, this study did not use individual mapping of the corticospinal tract, which is mandatory for an accurate investigation of DTI metrics, especially in cases of concomitant ischemia and edema. Therefore, the present study aimed to analyze the microstructural integrity of the patient-specific CST within the posterior limb of the internal capsule (PLIC) in the acute poststroke phase for a large patient collective. The central question was whether these integrity abnormalities are associated with clinical outcome parameters. A subanalysis was performed depending on the extent of infarction (peripheral infarcts versus BGI only) and was used to evaluate effects on outcome prediction.
MATERIALS AND METHODS
Patient Characteristics
The whole study cohort consisted of 439 consecutive patients with ischemic stroke with acute occlusions of a large intracranial vessel of the anterior circulation (middle cerebral artery or carotid-T) who underwent mechanical recanalization at a single comprehensive stroke center (Klinikum Rechts der Isar, School of Medicine, Technical University of Munich) between April 2016 and December 2018. The prospectively collected clinical and imaging data were retrospectively analyzed. Basic demographic, clinical, and interventional data of patients were gathered. The NIHSS score as well as the motor subindex scores (mNIHSS), defined as the sum of arm and leg symptom values of the affect side (mNIHSS-AL) (0–8 points) and the sum of arm (A), leg (L) and facial (F) symptoms value of the affected side (mNIHSS-ALF) (0–11 points), were assessed by NIHSS-certified neurologists at admission and at the MR imaging acquisition. The mRS score was used to measure disability at day 90 assessed by experienced neurologists, either on a routinely scheduled clinical visit or by a structured telephone interview. Good clinical outcome was defined as an mRS score of ≤2 according to large clinical trials.21,22
The modified TICI (mTICI) score23 was determined by 2 experienced neurointerventionalists (C.M., B.F.) in consensus. Successful recanalization was defined as mTICI 2b–3. Time to groin puncture, time of the reperfusion, and corresponding procedure times were taken from the existing data base. Recanalization time was defined as the difference between the time to groin puncture and reperfusion. In cases in which recanalization was not successful (mTICI, <2b), the control series after the last maneuver was used as the end time point.
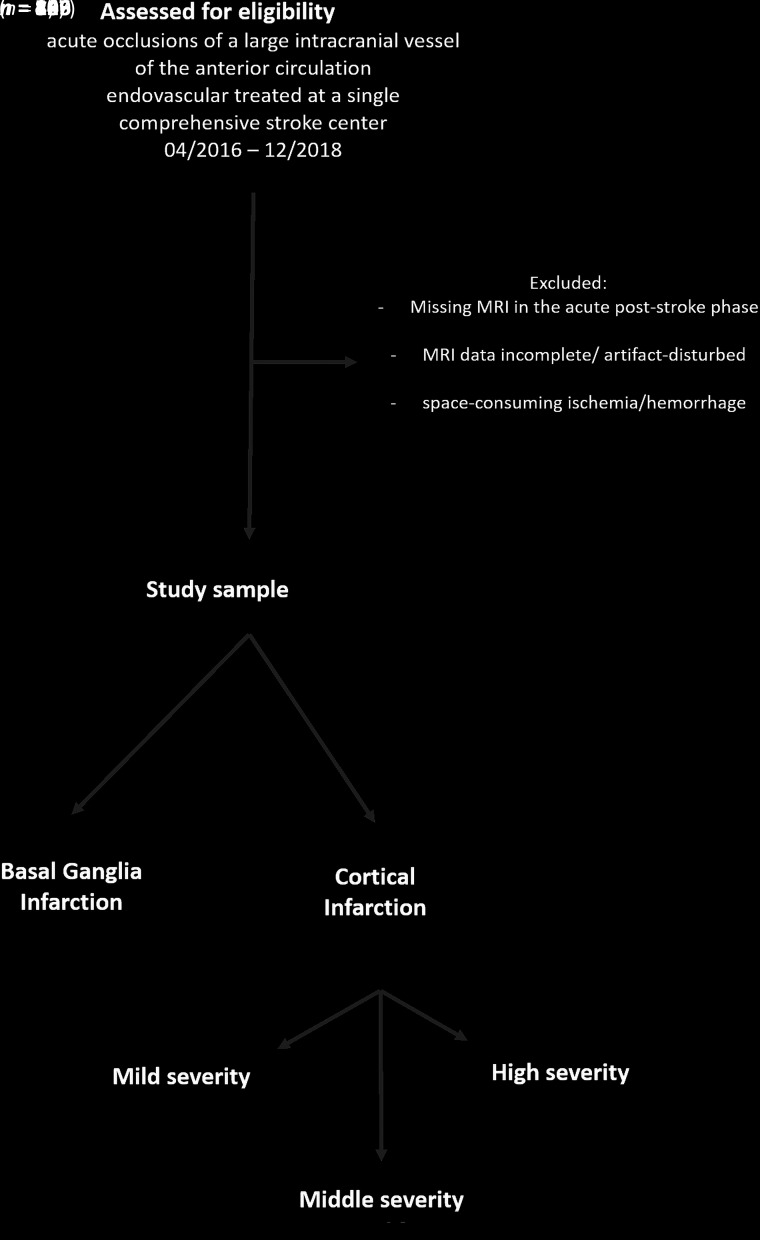
After we applied specific exclusion criteria (see flow chart, Fig 1), the final study cohort consisted of 165 patients who underwent MR imaging in the acute poststroke phase after mechanical thrombectomy (median, 3 days; interquartile range [IQR], 3–4 days; maximum, 7 days) including DTI and structural T1-weighted imaging.
FIG 1.
Flow diagram explaining the study sample.
On the basis of the infarction in the MR imaging acquisition, the stroke subtype of all patients was unanimously determined by consensus of 2 experienced neuroradiologists. It was graduated into BGI without relevant infarction of cortical structures, and peripheral infarcts, which were further divided into infarction severity. The severity assessment originates from DWI-ASPECTS (involvement of cortical regions M1–M6,24,25) and is modified by estimating the volume ratio that reflects, more importantly, the extent of peripheral infarcts (mild, below one-third; middle, between one-third and two-thirds; and high, above two-thirds of the peripheral infarct territory).
This study was approved by the local ethics committee of the Klinikum Rechts der Isar (School of Medicine, Technical University of Munich, Germany) (vote number 250/17 S), and the need for patient consent was waived.
MR Imaging Data Acquisition
MR imaging data were acquired on a 3T scanner (Achieva; Philips Healthcare) with a standard 8-channel head coil using consistent sequences and parameter settings.
Diffusion tensor images were acquired using a single-shot spin-echo echo-planar imaging sequence, resulting in 1 non-diffusion-weighted image (b = 0 s/mm2) and 15 diffusion-weighted images (b = 800 s/mm2 in 15 noncolinear gradient directions) covering the whole brain with the following parameters: TE = 55 ms, flip angle = 90°, FOV = 224 × 224 × 146 mm, 73 transverse slices, section thickness = 2 mm, 0-mm interslice gap, voxel size = 2 × 2 × 2 mm.
A whole-head, high-resolution 3D gradient-echo T1-weighted image was acquired using the following parameters: TE = 4 ms, TR = 9 ms, flip angle = 8°, FOV = 240 × 252 × 200.25 mm, 267 sagittal slices, section thickness = 1 mm, 0-mm interslice gap, voxel size = 1 × 1 × 1 mm.
DTI Data Processing
DTI data were processed using FSL (written mainly by members of the Analysis Group, FMRIB, Oxford, UK26) applying the following steps: DTI preprocessing, DTI data quality check, and probabilistic tractography of the bilateral CST (seed, cerebral peduncle [PED], and target [precentral gyrus], ROI-based). In a next step, the individual CST was characterized within the PLIC and PED (atlas-based ROIs, transformed from standard to individual diffusion space) through an analysis of fractional anisotropy (FA) values. FA is commonly used to measure the integrity of white matter tracts by assessing the anisotropy.
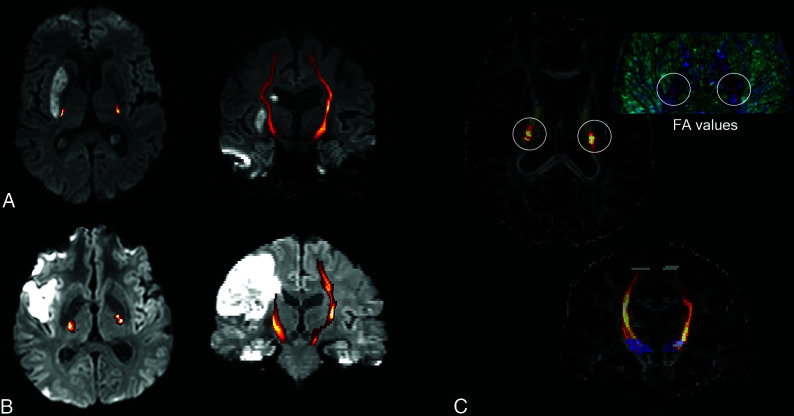
The individual FA indices for PLIC and PED were calculated to get quantitative values of FA alterations of the infarcted CST (I) in comparison with the healthy, nonaffected CST (H) (Fig 2). FA index = (FAI – FAH)/(FAI + FAH). The FA index was used to characterize the microstructural integrity of the CST within the PLIC and PED. More detailed descriptions can be found in the Online Appendix.
FIG 2.
Examples of 2 patients 3 days after mechanical recanalization of a right middle cerebral artery occlusion and consecutive basal ganglia infarction (A, patient 1)/peripheral infarct (B, patient 2). The CST, reconstructed by probabilistic fiber tracking (C, demonstrated on FA maps, blue seed ROI, gray target ROI, yellow posterior limb of the internal capsule) is overlaid on the reconstructed DWI trace picture in A and B (CST connectivity values, increasing probability from red to yellow before CST thresholding). For both patients, lower FA values of the CST within the posterior limb of the internal capsule are found for the infarcted right side in comparison with the healthy, nonaffected left side (C, illustrated using 3D Slicer; http://www.slicer.org).
Statistical Analysis
The mean value comparison of the FA index among the different infarction groups was performed by means of a 2-sample t test for independent samples. Wilcoxon rank sum tests were used for comparison of NIHSS and mRS values among the different infarction groups.
To explore the relevance of the FA index for clinical presentation, we tested the relationships between the FA index and NIHSS score as well as the mNIHSS (at MR imaging date and the percentage improvement compared with admission) using the following procedure: Partial correlation analyses were performed between the FA index and NIHSS-/mNIHSS at the time of MR imaging as well as with their percentage improvement. The analyses were corrected for age, sex, time between recanalization and MR imaging, and recanalization time.
In a multivariate logistic regression model, the association of the FA index (CST-PLIC) and further variables such as age, sex, time between recanalization and MR imaging, and recanalization time with good clinical outcome (mRS after 90 days, ≤2) was tested by means of a stepwise forward variable selection method. To check the interaction of stroke subtype (BGI versus peripheral infarcts) on the association of the FA index and the mRS after 90 days, we performed moderation analysis under consideration of the same covariates, age, sex, time between recanalization and MR imaging, and recanalization time.
For details of the mediation analysis see the Online Appendix.
All statistical analyses were performed using SPSS Statistics (Version 25; IBM).
RESULTS
Patient Characteristics
In total, 165 patients met the required inclusion criteria. Online Table 1 provides an overview of demographic, clinical, and interventional data of patients as well as their stroke subtypes assessed on the MR imaging examination.
Microstructural Integrity Changes of the CST within the PLIC
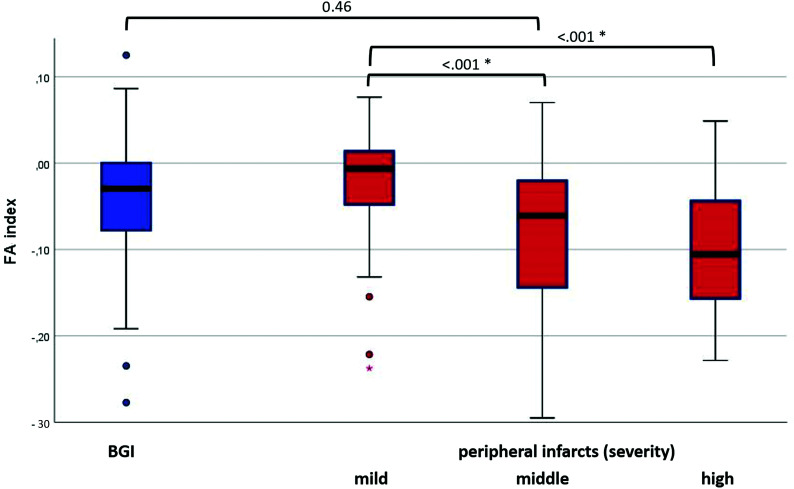
The distributions of the FA index of the CST within the PLIC are plotted in Fig 3 for the different stroke subtypes. The FA indices presented mostly negative values, meaning a loss of CST-FA values within the PLIC for the affected side. The subgroup of BGI showed FA indices (mean, −0.050 [SD, 0.098]) similar to those of peripheral infarcts in general (mean, −0.039 [SD, 0.076], P = .46). Within the group of peripheral infarcts, those of mild infarction severity (mean, −0.020, [SD, 0.058]) showed significantly higher FA indices than those of middle infarction severity (mean, −0.091 [SD, 0.108], P < .001) or of high infarction severity (mean −0.101 [SD, 0.079], P < .001).
FIG 3.
Distribution of the FA index of the corticospinal tract within the posterior limb of the internal capsule. Boxplots for the different stroke subtypes (BGI versus peripheral infarcts, which were further divided according to infarction severity). The asterisk indicates a statistically significant difference between the subgroups.
To estimate how specific the CST alterations are for the PLIC in the acute poststroke phase, we added additional analyses of the FA index within the PED. For both stroke subgroups, a weak tendency toward negative FA indices (for BGI: mean, −0.013 [SD, 0.047]; for peripheral infarcts: mean −0.003 [SD, 0.062]) was found, possibly suggesting real white matter alterations of the CST on the affected side in general, not only locally in the PLIC in the acute poststroke phase.
Correlations of Microstructural CST Integrity Changes with Clinical Presentation
For the whole study cohort, the FA index of PLIC showed weak negative correlations to the NIHSS at the time of MR imaging (r = −0.246, P = .002), mNIHSS-AL (r = −0.278, P = .001), and mNIHSS-ALF (r = −0.284, P < .001). The analyses were corrected for age, sex, time between recanalization and MR imaging, and recanalization time.
The FA indices of the additional PED testing showed no significant association with NIHSS/mNIHSS-AL/mNIHSS-ALF (r = −0.110/−0.028/−0.047; P = .183, .733, .566).
Subgroup of BGI.
The analyses to test an association of the FA index of the PLIC with the clinical presentation were repeated within the subgroup of BGI (n = 59). None of the correlation analyses with NIHSS/mNIHSS-AL/mNIHSS-ALF at the time of MR imaging as well as with the percentage improvement of these parameters from pretreatment to time of MR imaging showed significant results (P > .05, respectively) (Online Table 2).
Subgroup of Peripheral Infarcts with Impact of Infarction Severity.
The analyses to test an association of the FA index of the PLIC with the clinical presentation were repeated within the subgroup of peripheral infarcts with mild-to-middle infarction severity (n = 94, 12 patients with high severity were excluded to get a more homogeneous sample). All analyses showed statistically significant associations with higher correlation coefficients than in the analyses within the whole study cohort (Online Table 2). The mNIHSS-AL is moderately correlated to the FA index (r = –0.40, P < .001) under consideration of all above-mentioned covariates. It shows that a greater motor impairment is associated with a microstructural integrity loss of the corresponding CST.
Correlation coefficients differed significantly between the 2 subgroups (peripheral infarcts versus BGI) in most cases, as shown by the use of the Fisher-Z test (Online Table 2).
Association of Microstructural CST Integrity Changes with Clinical Outcome
In a logistic regression analysis, the FA index (CST-PLIC) was associated with good clinical outcome (mRS after 90 days, ≤2; regression coefficient, 10.3, standard error, 3.8, P < .01). Age was inversely associated with good clinical outcome (regression coefficient, −0.12; standard error, 0.04; P < .01); the other covariates showed no significant effect (excluded with stepwise forward variable selection method).
In a second step, the impact of the FA index on the mRS (after 90 days) was tested depending on the moderator variable stroke subtype under consideration of the same covariates. Due to the interaction of this moderator variable, a significant change in R2 (.05) was shown (P < .001). The conditional effect of the FA index on clinical outcome at the 2 different moderator values was as follows: No significant effect could be seen for the stroke subgroup BGI (effect, −0.9; 95% CI containing 0 [−4.4−2.6], P = .61), but for peripheral infarcts, a strong effect of the FA index on clinical outcome existed (effect, −11.0; 95% CI, 5.2 to −6.8, P < .0001).
Impact of Microstructural CST Integrity on the Effect between Infarction Severity and Clinical Outcome
The 2 groups of mild and middle infarction severity differed not only in their FA indices (see above), with lower values for higher infarction severity, but also in their clinical parameters showing higher affection (NIHSS/mRS) for the higher infarction severity subgroup (P < .001, .002).
In a mediation analysis (Online Figure), the effect of group (mild or middle infarction severity) on clinical outcome (mRS after 90 days) (total effect c = 1.10 ± 0.35, P = .003) was still present but reduced when controlling for the FA index (direct effect c´ = 0.89 ± 0.36, P = .014); critically, the bootstrapped 95% confidence interval for the indirect effect (ie, mediation: total-direct effect) was different from zero (95% CI, 0.02−0.58), indicating that the FA index significantly mediated the relationship between infarction severity and clinical outcome.
DISCUSSION
When we integrated the whole cohort of patients with large intracranial vessel occlusions of the anterior circulation, structural patient-specific CST abnormalities were found in the acute poststroke phase after mechanical recanalization. Reduced FA indices indicate a loss of CST integrity within the PLIC. These structural alterations were associated with the clinical presentation, especially for peripheral infarcts. The level of integrity was negatively correlated to symptom severity (NIHSS/mNIHSS values) and positively correlated to the percentage improvement of symptom severity from the onset to the date of MR imaging. CST integrity within the PLIC was also associated with good clinical outcome after 90 days, as measured by the mRS, suggesting that impairment of the CST fiber integrity within the PLIC has a negative impact on clinical presentation in general.
Beside the previously shown prediction capacity of macrostructural CST injury in the acute poststroke phase,20 the present study is powered with an adequate patient number, enabling us to detect individual microstructural CST integrity changes within the PLIC, also suggesting an impact on the clinical presentation and outcome. However, occlusions of the anterior circulation result in different infarction patterns, especially since the establishment of mechanical recanalization of intracranial large-vessel occlusions with high rates of successful reperfusion and, consequently, an increasing occurrence of isolated basal ganglia infarction. To adequately address these different subgroups of infarction patterns and severity, we performed subgroup analyses.
The association of CST integrity with clinical outcome was predominantly based on the subgroup of peripheral infarcts. For BGIs, which are increasingly common in the era of mechanical recanalization, this relation could not be observed. Within the subgroup of peripheral infarcts, a further subdivision based on infarction severity was made, showing a nearly linear impairment of CST integrity and clinical presentation in relation to infarction severity as expected. Furthermore, the impact of infarction severity on the clinical outcome at day 90 was mediated by the FA index, shown by a statistically significant reduction of the association between infarction severity and outcome. It implies that CST integrity changes within the PLIC can partially explain this relationship, but the still visible effect of infarction severity on mRS suggests that the FA index is not the only mediator. Possibly other structural integrity changes, eg, within the extrapyramidal system,19 could also have an influence, requiring further research in this field.
Several studies exist that aim to examine the role of DTI in predicting motor recovery after stroke, inspired by the limitations of using clinical scores alone.16 Conventional imaging does not have the potential to detect CST damage accurately, especially when the CST is not apparently infarcted. Thus, most studies used DTI with FA calculations to characterize microstructural fiber integrity.17,18,27-33 Identifying the voxels of the individual CST fibers by probabilistic fiber-tracking is accurate. This technique was used in the present study in combination with FA assessment within a specific tract section (PLIC), which was atlas-based. The FA index that is equivalent to the FA asymmetry as part of the predicting recovery potential was used as a most popular predictor variable in previous DTI studies and showed a good association to clinical parameters,16,29,34 providing the rationale for applying it in the present study.
Most previous studies about CST integrity were performed in the subacute or chronic stage of stroke and showed lower FA values in the affected CST because of the beginning of Wallerian degeneration.32,35-37 These integrity alterations measured by FA asymmetry have a particular impact in the subacute and chronic stages because they are associated with the persistence of motor deficit.36,38 In the present study, the grade of CST alterations of peripheral infarcts early after mechanical recanalization was shown to correlate with the functional outcome of patients after 90 days. These results give the first evidence for using the information of fiber integrity in the acute poststroke phase for outcome predictions. This could be helpful for the patients and relatives, eg, concerning post-inpatient management. Indeed, further studies about imaging biomarkers are necessary, especially with the aim of adapting rehabilitative and follow-up strategies in the long term.
To our knowledge, a subdivision in different stroke subtypes for structural analyses, especially in the acute poststroke phase, has not been reported. Similar FA indices of the CST within the PLIC are found for the BGI and peripheral infarcts, but the BGI do not show a correlation to the clinical parameters that are collected within the clinical routine. In contrast to peripheral infarcts with secondary disintegration of dependent fibers, BGIs do not show any gray matter infarction of the cortex regions representing the fiber origins (except possibly microstructural changes that were not assessed). FA alterations within the PLIC may be based on subtle direct white matter damage or perilesional edema, which cannot be visualized without DTI, or an increase in cellularity secondary to inflammation. These tract alterations with intact primary gray matter might be partially reversible or could constitute a better precondition for secondary brain plasticity processes in the reconstitution of motor regulation. This feature could explain the lack of correlation with clinical parameters and midterm functional outcome in the present study. However, the missing correlation might also be influenced by the structure of the underlying data: The subgroup of BGI (n = 59) is smaller, implying less statistical power than in the subgroup of peripheral infarcts (n = 106). Second, due to good clinical performance of patients with BGI, their mNIHSS scores have a small range, influencing the validity of a correlation analysis.
Both the interpretation and the limitations of the BGI analysis raise the challenge to further examine BGIs, their clinical presentation, and their pathophysiologic role in future studies, because the BGI entity presents with a wide interindividual variability and heterogeneity.39
A limitation of the present study is that only common clinical parameters such as the NIHSS score, motor subindex scores, and the mRS were collected, but a large collective of almost all treated patients at a single comprehensive stroke center was included. These clinical parameters are assessed in the clinical routine for all patients independent of their condition or compliance, avoiding bias within the study cohort. However, especially the lack of motor assessment at 3 months reduces the validity concerning long-term outcome predictions. A further limitation is the lack of inclusion of patients with an incompatibility for an MR imaging examination. Additionally, these patients often present with extended ischemia or space-consuming hemorrhage, which makes a DTI analysis impossible. This subgroup of patients with distinctly worse outcome is missing in the analysis.
Further limitations of the study depend on the known technical limitations and validity of DTI, which cannot differentiate between direct white matter disintegration because of myelin sheath damage or axon collapse or processes that occur adjacent to the fibers such as inflammation or edema. These differences could impact the results of the present study that possibly differentiate these processes arising from pathophysiologic considerations of the different stroke subtypes. Additionally, DTI protocol could be improved for the future studies concerning b-values and the number of directions. The low b-value limits the accuracy of the DTI metrics and, secondarily, incurs the risk of imprecise measurements. The results should be verified in follow-up studies using the latest DTI technology and longitudinal examinations in the course of ischemia.
CONCLUSIONS
Microstructural CST alterations within the PLIC are already present in an early stage after mechanical recanalization of large intracranial vessel occlusions of the anterior circulation. For peripheral infarcts, these white matter changes can be interpreted as fiber disintegration and are associated with clinical outcome. These might be useful for the early adaption of rehabilitative and follow-up strategies. In contrast, basal ganglia infarction showed a better clinical course, and also its distinct CST alterations were not associated with patient outcome. That association requires further and longitudinal exploration of fiber integrities to examine their reversibility and potential for neuroplasticity processes.
ABBREVIATIONS:
- BGI
basal ganglia infarction
- CST
corticospinal tract
- FA
fractional anisotropy; IQR = interquartile range
- mNIHSS
motor subindex scores of NIHSS
- mNIHSS-AL
sum of arm (A) and leg (L) symptom values of the affected side; mNIHSS-ALF = the sum of arm (A), leg (L) and facial (F) symptoms value of the affected side; mTICI = modified TICI
- PED
cerebral peduncle
- PLIC
posterior limb of the internal capsule
Footnotes
Disclosures: Johannes Kaesmacher—UNRELATED: Grants/Grants Pending: Swiss Academy of Medical Sciences through the Young Talents in Clinical Research program/Bangerter-Rhyner Foundation, Swiss Stroke Society.* *Money paid to institution.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation 2012;125:e2–20 10.1161/CIR.0b013e31823ac046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hendricks HT, van Limbeek J, Geurts AC, et al. Motor recovery after stroke: a systematic review of the literature. Arch Phys Med Rehabil 2002;83:1629–37 10.1053/apmr.2002.35473 [DOI] [PubMed] [Google Scholar]
- 3.Prabhakaran S, Zarahn E, Riley C, et al. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil Neural Repair 2008;22:64–71 10.1177/1545968307305302 [DOI] [PubMed] [Google Scholar]
- 4.Bach-y-Rita P, Bach-y-Rita EW. Biological and psychosocial factors in recovery from brain damage in humans. Can J Psychol 1990;44:148–65 10.1037/h0084247 [DOI] [PubMed] [Google Scholar]
- 5.Puig J, Pedraza S, Blasco G, et al. Acute damage to the posterior limb of the internal capsule on diffusion tensor tractography as an early imaging predictor of motor outcome after stroke. AJNR Am J Neuroradiol 2011;32:857–63 10.3174/ajnr.A2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shelton FD, Volpe BT, Reding M. Motor impairment as a predictor of functional recovery and guide to rehabilitation treatment after stroke. Neurorehabil Neural Repair 2001;15:229–37 10.1177/154596830101500311 [DOI] [PubMed] [Google Scholar]
- 7.Feys H, Hetebrij J, Wilms G, et al. Predicting arm recovery following stroke: value of site of lesion. Acta Neurol Scand 2000;102:371–77 10.1034/j.1600-0404.2000.102006371.x [DOI] [PubMed] [Google Scholar]
- 8.Kwakkel G, Kollen BJ, van der Grond J, et al. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke 2003;34:2181–86 10.1161/01.STR.0000087172.16305.CD [DOI] [PubMed] [Google Scholar]
- 9.Chen CL, Tang FT, Chen HC, et al. Brain lesion size and location: effects on motor recovery and functional outcome in stroke patients. Arch Phys Med Rehabil 2000;81:447–52 10.1053/mr.2000.3837 [DOI] [PubMed] [Google Scholar]
- 10.Stinear CM, Byblow WD, Ackerley SJ, et al. Proportional motor recovery after stroke: implications for trial design. Stroke 2017;48:795–98 10.1161/STROKEAHA.116.016020 [DOI] [PubMed] [Google Scholar]
- 11.Smith MC, Byblow WD, Barber PA, et al. Proportional recovery from lower limb motor impairment after stroke. Stroke 2017;48:1400–03 10.1161/STROKEAHA.116.016478 [DOI] [PubMed] [Google Scholar]
- 12.Nijland RH, van Wegen EE, Harmeling-van der Wel BC, et al. ; Early Prediction of Functional Outcome After Stroke Investigators. Accuracy of physical therapists' early predictions of upper-limb function in hospital stroke units: the EPOS Study. Phys Ther 2013;93:460–69 10.2522/ptj.20120112 [DOI] [PubMed] [Google Scholar]
- 13.Zhu LL, Lindenberg R, Alexander MP, et al. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke 2010;41:910–15 10.1161/STROKEAHA.109.577023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindenberg R, Renga V, Zhu LL, et al. Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology 2010;74:280–87 10.1212/WNL.0b013e3181ccc6d9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nucifora PG, Verma R, Lee SK, et al. Diffusion-tensor MR imaging and tractography: exploring brain microstructure and connectivity. Radiology 2007;245:367–84 10.1148/radiol.2452060445 [DOI] [PubMed] [Google Scholar]
- 16.Puig J, Blasco G, Schlaug G, et al. Diffusion tensor imaging as a prognostic biomarker for motor recovery and rehabilitation after stroke. Neuroradiology 2017;59:343–51 10.1007/s00234-017-1816-0 [DOI] [PubMed] [Google Scholar]
- 17.Thomalla G, Glauche V, Koch MA, et al. Diffusion tensor imaging detects early Wallerian degeneration of the pyramidal tract after ischemic stroke. Neuroimage 2004;22:1767–74 10.1016/j.neuroimage.2004.03.041 [DOI] [PubMed] [Google Scholar]
- 18.Puig J, Pedraza S, Blasco G, et al. Wallerian degeneration in the corticospinal tract evaluated by diffusion tensor imaging correlates with motor deficit 30 days after middle cerebral artery ischemic stroke. AJNR Am J Neuroradiol 2010;31:1324–30 10.3174/ajnr.A2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leander Rimmele D, Frey BM, Cheng B, et al. Association of extrapyramidal tracts' integrity with performance in fine motor skills after stroke. Stroke 2018;49:2928–32 10.1161/STROKEAHA.118.022706 [DOI] [PubMed] [Google Scholar]
- 20.Lin DJ, Cloutier AM, Erler KS, et al. Corticospinal tract injury estimated from acute stroke imaging predicts upper extremity motor recovery after stroke. Stroke 2019;50:3569–77 10.1161/STROKEAHA.119.025898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albers GW, Marks MP, Kemp S, et al. ; DEFUSE 3 Investigators. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018;378:708–18 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nogueira RG, Jadhav AP, Haussen DC, et al. ; DAWN Trial Investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:11–21 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 23.Zaidat OO, Yoo AJ, Khatri P, et al. ; STIR Thrombolysis in Cerebral Infarction (TICI) Task Force. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 2013;44:2650–63 10.1161/STROKEAHA.113.001972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barber PA, Hill MD, Eliasziw M, et al. ; ASPECTS Study Group. Imaging of the brain in acute ischaemic stroke: comparison of computed tomography and magnetic resonance diffusion-weighted imaging. J Neurol Neurosurg Psychiatry 2005;76:1528–33 10.1136/jnnp.2004.059261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fahed R, Lecler A, Sabben C, et al. DWI-ASPECTS (Diffusion-Weighted Imaging-Alberta Stroke Program Early Computed Tomography Scores) and DWI-FLAIR (Diffusion-Weighted Imaging-Fluid Attenuated Inversion Recovery) mismatch in thrombectomy candidates: an intrarater and interrater agreement study. Stroke 2018;49:223–27 10.1161/STROKEAHA.117.019508 [DOI] [PubMed] [Google Scholar]
- 26.Jenkinson M, Beckmann CF, Behrens TE, et al. FSL. Neuroimage 2012;62:782–90 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- 27.Byblow WD, Stinear CM, Barber PA, et al. Proportional recovery after stroke depends on corticomotor integrity. Ann Neurol 2015;78:848–59 10.1002/ana.24472 [DOI] [PubMed] [Google Scholar]
- 28.Puig J, Blasco G, Daunis IE, et al. Decreased corticospinal tract fractional anisotropy predicts long-term motor outcome after stroke. Stroke 2013;44:2016–18 10.1161/STROKEAHA.111.000382 [DOI] [PubMed] [Google Scholar]
- 29.Stinear CM, Barber PA, Petoe M, et al. The PREP algorithm predicts potential for upper limb recovery after stroke. Brain 2012;135:2527–35 10.1093/brain/aws146 [DOI] [PubMed] [Google Scholar]
- 30.Kumar P, Kathuria P, Nair P, et al. Prediction of upper limb motor recovery after subacute ischemic stroke using diffusion tensor imaging: a systematic review and meta-analysis. J Stroke 2016;18:50–59 10.5853/jos.2015.01186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindenberg R, Zhu LL, Ruber T, et al. Predicting functional motor potential in chronic stroke patients using diffusion tensor imaging. Hum Brain Mapp 2012;33:1040–51 10.1002/hbm.21266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stinear CM, Barber PA, Smale PR, et al. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain 2007;130:170–80 10.1093/brain/awl333 [DOI] [PubMed] [Google Scholar]
- 33.Schaechter JD, Fricker ZP, Perdue KL, et al. Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients. Hum Brain Mapp 2009;30:3461–74 10.1002/hbm.20770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doughty C, Wang J, Feng W, et al. Detection and predictive value of fractional anisotropy changes of the corticospinal tract in the acute phase of a stroke. Stroke 2016;47:1520–26 10.1161/STROKEAHA.115.012088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Werring DJ, Toosy AT, Clark CA, et al. Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J Neurol Neurosurg Psychiatry 2000;69:269–72 10.1136/jnnp.69.2.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moller M, Frandsen J, Andersen G, et al. Dynamic changes in corticospinal tracts after stroke detected by fibretracking. J Neurol Neurosurg Psychiatry 2007;78:587–92 10.1136/jnnp.2006.100248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radlinska B, Ghinani S, Leppert IR, et al. Diffusion tensor imaging, permanent pyramidal tract damage, and outcome in subcortical stroke. Neurology 2010;75:1048–54 10.1212/WNL.0b013e3181f39aa0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watanabe T, Honda Y, Fujii Y, et al. Three-dimensional anisotropy contrast magnetic resonance axonography to predict the prognosis for motor function in patients suffering from stroke. J Neurosurg 2001;94:955–60 10.3171/jns.2001.94.6.0955 [DOI] [PubMed] [Google Scholar]
- 39.Kaesmacher J, Huber T, Lehm M, et al. Isolated striatocapsular infarcts after endovascular treatment of acute proximal middle cerebral artery occlusions: prevalence, enabling factors, and clinical outcome. Front Neurol 2017;8:272 10.3389/fneur.2017.00272 [DOI] [PMC free article] [PubMed] [Google Scholar]