Abstract
BACKGROUND AND PURPOSE:
Spinal CSF-venous fistulas are increasingly recognized as the cause of spontaneous intracranial hypotension. Here, we describe the challenges in the care of patients with CSF-venous fistulas who are morbidly or super obese.
MATERIALS AND METHODS:
A review was undertaken of all patients with spontaneous intracranial hypotension and a body mass index of >40 who underwent digital subtraction myelography in the lateral decubitus position to look for CSF-venous fistulas.
RESULTS:
Eight patients with spontaneous intracranial hypotension with a body mass index of >40 underwent lateral decubitus digital subtraction myelography. The mean age of these 5 women and 3 men was 53 years (range, 45 to 68 years). Six patients were morbidly obese (body mass indexes = 40.2, 40.6, 41, 41.8, 45.4, and 46.9), and 2 were super obese (body mass indexes = 53.7 and 56.3). Lumbar puncture showed an elevated opening pressure in 5 patients (26.5–47 cm H2O). The combination of an elevated opening pressure and normal conventional spine imaging findings resulted in a misdiagnosis (midbrain glioma and demyelinating disease, respectively) in 2 patients. Prior treatment included surgical nerve root ligation for suspected CSF-venous fistula in 3 patients. Digital subtraction myelography demonstrated a CSF-venous fistula in 6 patients (75%). Rebound high-pressure headache occurred in all 6 patients following surgical ligation of the fistula, and papilledema developed in 3.
CONCLUSIONS:
In our series, opening pressure was generally elevated in patients with morbid or super obesity. The yield of identifying CSF-venous fistulas with digital subtraction myelography in this patient population can approach that of the nonobese patient population. These patients may be at higher risk of developing rebound high-pressure headaches and papilledema.
Spontaneous intracranial hypotension (SIH) is an increasingly recognized cause of secondary headache and has also been associated with a wide variety of other neurologic disorders.1-4 The cause of SIH is a spinal CSF leak; at least 3 distinct types have been identified.5 A direct communication between the subarachnoid space and a spinal epidural vein, a so-called CSF-venous fistula, is the most recently discovered type of spinal CSF leak and may be detected in up to one-fourth of patients with SIH.6 Since the first description of spontaneous spinal CSF-venous fistulas in 2014,7 several groups have confirmed their importance, but much remains unknown about these fistulas.6-15 The main reason that this type of CSF leak remained undiscovered for so long is that it is not associated with an extradural CSF collection and its detection requires sophisticated imaging, eg, digital subtraction myelography (DSM) or dynamic CT myelography.6-15
Obesity has emerged as a major global and national health problem.16 Obesity is defined as a body mass index (BMI) of ≥30 and is associated with various diseases, such as diabetes, metabolic syndrome, and cardiovascular disease. The prevalence of obesity is increasing, and in the United States, it is among the highest in the world. According to the National Health and Nutrition Examination Survey 2015 and 2016 dataset, 39.8% of US adults live with obesity.17 Morbid obesity (BMI ≥ 40) and super obesity (BMI ≥ 50) have shown the most rapid rise in prevalence, and with data from the Behavioral Risk Factor Surveillance System, it was estimated that 6.6% of Americans had a BMI of >40 in 2010.18 Despite the importance of obesity, there is very limited information available on obesity in patients with spontaneous spinal CSF-venous fistulas. Actually, no information on BMI is available in any of the prior reports, including our own, on spontaneous spinal CSF-venous fistulas.6-15 We have noted that particularly morbid and super obesity can result in unique challenges in the diagnosis, detection, and treatment of patients with SIH with spontaneous spinal CSF-venous fistulas; therefore, we reviewed our experience with this patient population.
MATERIALS AND METHODS
This study was approved by the Cedars-Sinai Medical Center institutional review board.
Using a prospectively maintained registry, we identified all patients with SIH and a BMI of ≥40 who underwent DSM in the lateral decubitus position to look for CSF-venous fistulas. The diagnosis of SIH was based on the criteria of the International Classification of Headache Disorders, third edition,19 with minor modifications. These criteria require objective evidence of SIH, consisting of brain MR imaging showing stigmata of SIH (ie, pachymeningeal enhancement, brain sagging, or subdural fluid collections), spinal imaging showing a CSF leak (ie, the presence of extradural CSF), or low CSF opening pressure (ie, <6.0 cm H2O). The modification consists of also including patients who do not have headaches but whose symptoms are best explained by SIH.
All patients underwent brain MR imaging and MR myelography (heavily T2-weighted MR imaging without intrathecal contrast). For DSM, the technique as described by Hoxworth et al20 was used with some minor modifications.6,8 Briefly, DSM is performed with the patient under general endotracheal anesthesia with deep paralysis and suspended respiration for maximal detail and temporal resolution. Patients are positioned in the lateral decubitus position in a biplane angiography suite, with tilt-table capability. Pillows or foam padding are placed to optimize cervicothoracic alignment. A fluoroscopically guided lumbar puncture is performed at the L2–3 level with a 22-ga needle. An opening pressure is obtained at this time using standard manometry. Then, accurate needle position is confirmed with an injection of 0.5 mL of iohexol (Omnipaque; GE Healthcare). Patients are then further positioned on the basis of the area of interest, and the table is tilted to achieve contrast flow to the cervicothoracic spine. Great care is taken, especially in these patients with obesity, to maximize contrast opacification of the lateral dural sac by adjusting the degree of tilting to patient-specific spinal curvature and anatomy. Finally, contrast is injected manually at 1 mL per second with suspended respiration for 40–100 seconds while acquiring biplane subtraction images at 2 frames per second. If the first DSM fails to identify a CSF-venous fistula, then the study is repeated on another day with the patient in the lateral decubitus position on the contralateral side.
Radiation exposure during DSM was calculated for these patients and for 2 age- and sex-matched controls, each with a BMI of <40.
RESULTS
Eight patients with SIH with a BMI of ≥40 underwent lateral decubitus DSM (Fig 1). The mean age of these 5 women and 3 men was 53 years (range, 45–68 years). Six patients were morbidly obese (BMI = 40.2, 40.6, 41, 41.8, 45.4, and 46.9), and 2 were super obese (BMI = 53.7 and 56.3). None of the patients had undergone bariatric surgery. Four patients presented with isolated orthostatic headaches; 3, with orthostatic headaches with Valsalva-induced worsening; and 1, with isolated Valsalva-induced headache. Fundoscopic examination findings were normal in all 8 patients. Brain MR imaging showed brain sagging in 7 patients and meningeal enhancement in 5 patients.
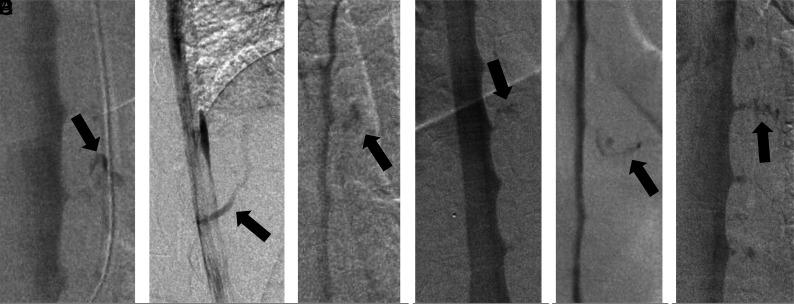
FIG 1.
A, Anterior-posterior DSM demonstrates a CSF-venous fistula (BMI = 46.9 kg/m2, weight 149 kg, height = 178 cm). B, Lateral DSM demonstrates a CSF-venous fistula (BMI = 40.6 kg/m2, weight = 94.5 kg, height = 152 cm). C, Anterior-posterior DSM demonstrates a CSF-venous fistula (BMI = 40.2 kg/m2, weight = 113 kg, height = 167 cm). D, Anterior-posterior DSM demonstrates a CSF-venous fistula (BMI = 53.7 kg/m2, weight = 151 kg, height = 167 cm). E, Anterior-posterior DSM demonstrates a CSF-venous fistula (BMI = 41 kg/m2, weight = 137 kg, height = 183 cm). F, Anterior-posterior DSM demonstrates a CSF-venous fistula (BMI = 45.4 kg/m2, weight = 161 kg, height = 188 cm). In 2 other patients (BMI = 56.3 kg/m2, weight = 149 kg, height = 163 cm and BMI = 41.8 kg/m2, weight = 140 kg, height = 182 cm, respectively), DSM failed to demonstrate a CSF-venous fistula. Arrows indicates CSF-venous fistulas.
Before referral to our medical center, a lumbar puncture in the lateral decubitus position had been performed in 6 patients, showing a low opening pressure (<6 cm H2O) in 1 patient and an elevated opening pressure (26.5–36 cm H2O) in 5 patients. A lumbar puncture performed with the patient in the prone position yielded a normal opening pressure (11.5 cm H2O) in the remaining patient. All patients had undergone a spine MR imaging, 5 had undergone a conventional CT myelogram, 3 had undergone dynamic CT myelography, and 2 had undergone DSMs in the lateral decubitus position (using general or local anesthesia). None of these investigations had shown extradural CSF collections or a clear evidence of a CSF-venous fistula. The combination of an elevated opening pressure and normal spine imaging findings resulted in the diagnosis of SIH being discarded in 2 patients who were subsequently misdiagnosed with demyelinating disease (T2 signal abnormality of the spinal cord in the setting of severe brain sagging, Fig 2) and a midbrain glioma (T2 signal abnormality in the setting of severe brain sagging), respectively. Three patients had been treated with multiple epidural blood patches or fibrin glue injections as well as surgical ligation of thoracic nerve roots for the suspicion of a CSF-venous fistula. One patient had undergone an exploratory operation at the site of a disc herniation.
FIG 2.
Sagittal T2-weighted MRIs showing brain sagging and T2 signal change within the cervical spinal cord (presyrinx) (A) and resolution of the presyrinx after surgical ligation of the thoracic CSF-venous fistula due to resolution of brain sagging and cerebellar tonsillar herniation (B).
Using DSM with the patient in the lateral decubitus position, we were able to detect a CSF-venous fistula in 6 of the 8 patients (75%). All fistulas were located in the thoracic spine, 3 on the right side and 3 on the left side. All were associated with a spinal meningeal diverticulum. A normal opening pressure was found in 3 patients (13, 17, and 20 cm H2O), a borderline elevated opening pressure was found in 2 patients (23 and 24 cm H2O), and an elevated opening pressure was found in 3 patients (28, 29, and 47 cm H2O). These opening pressure readings were obtained with the patient in the lateral decubitus position under general anesthesia. Radiation exposure was higher in these patients (mean, 1753 mGy; range, 1271–2314 mGy) than in controls (mean, 488 mGy; range, 167–979 mGy).
All 6 patients with a spinal CSF-venous fistula underwent an uneventful thoracic foraminotomy, with ligation of the fistula resulting in symptom resolution and normalization of the MR imaging findings. However, rebound high-pressure headache requiring treatment with acetazolamide developed in all 6 patients, and this was complicated by visual blurring and papilledema in 3 patients. A lumbar puncture was performed with the patient in the lateral decubitus position in 2 of these patients with papilledema, and this showed an increase of opening pressure from 24 to 30 cm H2O in one patient (spontaneous respirations) and from 47 to 51 cm H2O in the other (at the time of repeat DSM with general anesthesia). Vision symptoms resolved with the acetazolamide treatment.
DISCUSSION
In this study of morbidly and super obese patients with spontaneous spinal CSF-venous fistulas, we found specific challenges in the diagnosis, imaging, and treatment.
Although it has been well-documented since the 1990s that patients with SIH often do not have abnormally low CSF pressure,21-23 the current study showed that opening pressure can be markedly elevated in these patients. This finding shows that just like the presence of a normal opening pressure, an abnormally high opening pressure does not preclude the diagnosis of SIH. Prior studies had already concluded that some patients with SIH may have borderline elevated CSF pressure,22,23 and a patient with SIH and an opening pressure of 31 cm H2O was reported by Kranz et al.23 The combination of an elevated opening pressure in addition to normal results of conventional spinal imaging, ie, the absence of any extradural CSF collection, caused the diagnosis of SIH to be discarded in some of the presently reported patients, despite the presence of the typical brain MR imaging findings. This step resulted in an erroneous diagnosis of demyelinating disease in one patient and of a midbrain glioma in another. The presence of elevated CSF pressure also has possible pathophysiologic implications for the development of spinal CSF-venous fistulas. Similar to spontaneous skull base CSF leaks in patients with idiopathic intracranial hypertension,24–27 chronically elevated CSF pressure could also predispose to spontaneous spinal CSF leaks. This possibility also had been reported in patients with pre-existing idiopathic intracranial hypertension who developed SIH.2 The presently reported patients had normal fundoscopic examination findings on presentation and did not have idiopathic intracranial hypertension. Of note, none of the patients in the present study had undergone bariatric surgery, a risk factor for developing SIH.28
The detection of CSF-venous fistulas requires sophisticated imaging, and we have been using lateral decubitus DSM with the patient under general anesthesia for this purpose with excellent results.6 Although the body habitus of morbidly and super obese patients may be intimidating, it should not result in a defeatist attitude. In the present study, we detected CSF-venous fistulas in three-fourths of morbidly and super obese patients with SIH and no extradural CSF collections on spinal imaging, thus demonstrating that the yield of identifying such fistulas in this patient population can approach that of the nonobese patient population.6 In a prior study of 23 consecutive patients with SIH and the stigmata of SIH on brain MR imaging but no extradural CSF collections on spinal imaging who underwent lateral decubitus DSM, we found CSF-venous fistulas in 17 patients (74%).6
Postoperatively, rebound high-pressure headaches occurred in all 6 patients who underwent surgical ligation of the CSF-venous fistula, and this was complicated by papilledema and visual symptoms in 3 patients. The development of papilledema was associated with only a modest increase in opening pressure. This compares with a previous study in which about one-fourth of 113 patients developed rebound high-pressure headache requiring administration of acetazolamide following treatment of SIH, and papilledema was noted in only 2 patients.29 Similarly, Wang et al30 reported rebound high-pressure headaches requiring treatment with acetazolamide in 5 (25%) of 20 patients with SIH following surgical ligation of a spinal CSF-venous fistula. Although the number of patients in the current study is small, this suggests that morbidly and super obese patients with CSF-venous fistulas are at increased risk of developing rebound high-pressure headaches and papilledema postoperatively and that consideration should be given to pharmacologic prophylaxis with, for example acetazolamide, before treating the CSF-venous fistula and that careful ophthalmologic evaluation postoperatively is indicated.
CONCLUSIONS
In patients with SIH due to a spinal CSF-venous fistula who are morbidly or super obese, CSF pressure often is elevated and the risk of posttreatment rebound high-pressure headache and papilledema is increased. The yield of finding a CSF-venous fistula in this patient population using DSM is similar to that in patients who are not obese.
ABBREVIATIONS:
- BMI
body mass index
- DSM
digital subtraction myelography
- SIH
spontaneous intracranial hypotension
Footnotes
Disclosures: Wouter I. Schievink—UNRELATED: Board Membership: I am on the medical advisory board of the Spinal CSF Leak Foundation; this is a nonpaying position, and I have not received any funds toward this project. Marcel Maya—UNRELATED: Board Membership: I am on the medical advisory board of the Spinal CSF Leak Foundation; this is a nonpaying position, and I have not received any funds toward this project.
References
- 1.Schievink WI Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA 2006;295:2286–96 10.1001/jama.295.19.2286 [DOI] [PubMed] [Google Scholar]
- 2.Mokri B Spontaneous intracranial hypotension. Continuum (Minneap Minn) 2015;21:1086–1108 10.1212/CON.0000000000000193 [DOI] [PubMed] [Google Scholar]
- 3.Kranz PG, Malinzak MD, Amrhein TJ, et al. . Update on the diagnosis and treatment of spontaneous intracranial hypotension. Curr Pain Headache Rep 2017;21:37 10.1007/s11916-017-0639-3 [DOI] [PubMed] [Google Scholar]
- 4.Robblee J, Secora KA, Alhilali LM, et al. . Spontaneous intracranial hypotension. Pract Neurol 2020. https://practicalneurology.com/articles/2020-may/spontaneous-intracranial-hypotension-1. Accessed August 1, 2020
- 5.Schievink WI, Maya MM, Jean-Pierre S, et al. . A classification system of spontaneous spinal CSF leaks. Neurology 2016;87:673–79 10.1212/WNL.0000000000002986 [DOI] [PubMed] [Google Scholar]
- 6.Schievink WI, Maya MM, Moser FG, et al. . Lateral decubitus digital subtraction myelography to identify spinal CSF-venous fistulas in spontaneous intracranial hypotension. J Neurosurg Spine 2019. September 13. [Epub ahead of print] 10.3171/2019.6.SPINE19487 [DOI] [PubMed] [Google Scholar]
- 7.Schievink WI, Moser FG, Maya MM. CSF-venous fistula in spontaneous intracranial hypotension. Neurology 2014;83:472–73 10.1212/WNL.0000000000000639 [DOI] [PubMed] [Google Scholar]
- 8.Schievink WI, Moser FG, Maya MM, et al. . Digital subtraction myelography for the identification of spontaneous spinal CSF-venous fistulas. J Neurosurg Spine 2016;24:960–64 10.3171/2015.10.SPINE15855 [DOI] [PubMed] [Google Scholar]
- 9.Kumar N, Diehn FE, Carr CM, et al. . Spinal CSF venous fistula: a treatable etiology for CSF leaks in craniospinal hypovolemia. Neurology 2016;86:2310–12 10.1212/WNL.0000000000002776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kranz PG, Amrhein TJ, Schievink WI, et al. . The “hyperdense paraspinal vein” sign: a marker of CSF-venous fistula. AJNR Am J Neuroradiol 2016;37:1379–81 10.3174/ajnr.A4682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kranz PG, Amrhein TJ, Gray L. CSF venous fistulas in spontaneous intracranial hypotension: imaging characteristics on dynamic and CT myelography. AJR Am J Roentgenol 2017;209:1360–66 10.2214/AJR.17.18351 [DOI] [PubMed] [Google Scholar]
- 12.Farb RI, Nicholson PJ, Peng PW, et al. . Spontaneous intracranial hypotension: a systematic imaging approach for CSF leak localization and management based on MRI and digital subtraction myelography. AJNR Am J Neuroradiol 2019;40:745–53 10.3174/ajnr.A6016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schievink WI, Maya MM, Moser FG, et al. . Spontaneous spinal CSF-venous fistulas associated with venous/venolymphatic vascular malformations: report of 3 cases. J Neurosurg Spine 2019;32:305–10 10.3171/2019.8.SPINE19716 [DOI] [PubMed] [Google Scholar]
- 14.Duvall JR, Robertson CE, Cutsforth-Gregory JK, et al. . Headache due to spontaneous spinal cerebrospinal fluid leak secondary to cerebrospinal fluid-venous fistula: case series. Cephalalgia 2019;39:1847–54 10.1177/0333102419881673 [DOI] [PubMed] [Google Scholar]
- 15.Chazen JL, Robbins MS, Strauss SB, et al. . MR myelography for the detection of CSF-venous fistulas. AJNR Am J Neuroradiol 2020;41:938–40 10.3174/ajnr.A6521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016;387:1377–96 10.1016/S0140-6736(16)30054-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Health, United States, 2018–Data Finder. https://www.cdc.gov/nchs/hus/contents2018.htm#Table_021. Accessed June 9, 2020
- 18.Sturm R, Hattori A. Morbid obesity rates continue to rise rapidly in the United States. Int J Obes (Lond) 2013;37:889–91 10.1038/ijo.2012.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Headache Classification Committee of the International Headache Society: The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018. 38:1–211 10.1177/0333102417738202 [DOI] [PubMed] [Google Scholar]
- 20.Hoxworth JM, Patel AC, Bosch EP, et al. . Localization of a rapid CSF leak with digital subtraction myelography. AJNR Am J Neuroradiol 2009;30:516–19 10.3174/ajnr.A1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mokri B, Hunter SF, Atkinson JL, et al. . Orthostatic headaches caused by CSF leak but with normal CSF pressures. Neurology 1998;51:786–90 10.1212/wnl.51.3.786 [DOI] [PubMed] [Google Scholar]
- 22.Luetmer PH, Schwartz KM, Eckel LJ, et al. . When should I do dynamic CT myelography? Predicting fast spinal CSF leaks in patients with spontaneous intracranial hypotension. AJNR Am J Neuroradiol 2012;33:690–94 10.3174/ajnr.A2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kranz PG, Tanpitukpongse TP, Choudhury KR, et al. . How common is normal cerebrospinal fluid pressure in spontaneous intracranial hypotension? Cephalalgia 2016;36:1209–17 10.1177/0333102415623071 [DOI] [PubMed] [Google Scholar]
- 24.Brisman R, Hughes JE, Mount LA. Cerebrospinal fluid rhinorrhea. Arch Neurol 1970;22:245–52 10.1001/archneur.1970.00480210055007 [DOI] [PubMed] [Google Scholar]
- 25.Aaron G, Doyle J, Vaphiades MS, et al. . Increased intracranial pressure in spontaneous CSF leak patients not associated with papilledema. Otolaryngol Head Neck Surg 2014;151:1061–66 10.1177/0194599814551122 [DOI] [PubMed] [Google Scholar]
- 26.Bidot S, Levy JM, Saindane AM, et al. . Do most patients with a spontaneous cerebrospinal fluid leak have idiopathic intracranial hypertension? J Neuroophthalmol 2019;39:487–95 10.1097/WNO.0000000000000761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tam EK, Gilbert AL. Spontaneous cerebrospinal fluid leak and idiopathic intracranial hypertension. Curr Opin Ophthalmol 2019;30:467–71 10.1097/ICU.0000000000000603 [DOI] [PubMed] [Google Scholar]
- 28.Schievink WI, Goseland A, Cunneen S. Bariatric surgery as a possible risk factor for spontaneous intracranial hypotension. Neurology 2014;83:1819–22 10.1212/WNL.0000000000000985 [DOI] [PubMed] [Google Scholar]
- 29.Schievink WI, Maya MM, Jean-Pierre S, et al. . Rebound high-pressure headache after treatment of spontaneous intracranial hypotension: MRV study. Neurol Clin Pract 2019;9:93–100 10.1212/CPJ.0000000000000550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang TY, Karikari IO, Amrhein TJ, et al. . Clinical outcomes following surgical ligation of cerebrospinal fluid–venous fistula in patients with spontaneous intracranial hypotension: a prospective case series. Oper Neurosurg (Hagerstown) 2020;18:239–45 10.1093/ons/opz134 [DOI] [PubMed] [Google Scholar]