Abstract
Atherosclerosis is a chronic inflammatory disease of the arterial wall and the primary underlying cause of cardiovascular diseases. Approaches including in vivo imaging, cell-lineage tracing and knockout studies in mice, as well as clinical interventional studies and advanced mRNA sequencing techniques have drawn attention to the role of T cells as critical drivers and modifiers of the pathogenesis of atherosclerosis. CD4+ T cells are commonly found in atherosclerotic plaques. A large body of evidence indicates that T helper 1 (TH1) cells have pro-atherogenic roles and regulatory T (Treg) cells anti-atherogenic roles. However, Treg cells can become pro-atherogenic. The roles in atherosclerosis of other TH cell subsets such as TH2, TH9, TH17, TH22, follicular helper T cells and CD28– T cells, as well as other T cell subsets including CD8+ T cells and γδT cells, are less well understood. Moreover, some T cells seem to have both pro-atherogenic and anti-atherogenic functions. In this Review, we summarize the knowledge on T cell subsets, their functions in atherosclerosis and the process of T cell homing to atherosclerotic plaques. Much of our understanding of T cell roles in atherosclerosis is based on findings from experimental models. Translating these findings into human disease is challenging, but much needed. Targeting T cells and their specific cytokines are attractive pathways for developing new preventative and therapeutic approaches including potential T cell-related therapies for atherosclerosis.
Introduction
Atherosclerosis is a chronic inflammatory disease with an autoimmune component, and is the primary underlying cause of cardiovascular diseases1. Studies in the past 2 decades have drawn much attention to the potential role of T cells as critical drivers and modifiers in the pathogenesis of atherosclerosis2,3. CD8+ T cells and CD4+ T cells drive immune responses to peptides presented by major histocompatibility complex (MHC) class I on all nucleated cells and MHC class II on antigen-presenting cells (APCs), respectively. These responses can occur in individuals expressing a specific MHC allele with the capacity to bind the relevant peptide epitope4. Binding of a specific T cell receptor (TCR) concomitant with co-stimulatory molecules provided by APCs activates T cells and causes their clonal proliferation5. In experimental models of atherosclerosis, interactions between APCs and CD4+ T cells in the plaque result in the secretion of cytokines, many of which are pro-inflammatory3,6,7. Studies have shown that inducing the generation of regulatory T (Treg) cells by targeted vaccination reduces atherosclerosis in mice3,4,8–11.
In experimental models of atherosclerosis, many CD4+ T cells in lymph nodes and atherosclerotic plaques show an antigen-experienced phenotype. Surface markers like CD44 indicate that these T cells have already been exposed to their cognate antigen and thus are poised to respond rapidly and strongly.6. TCR sequencing of lesional T cells revealed an oligoclonal origin, suggesting that antigen-specific T cell clones might actively expand in the atherosclerotic leasion12,13. These observations, which need to be confirmed by larger studies, support the notion that atherosclerosis-specific antigens drive an immune response, which is probably initiated in the lymph nodes (Fig.1). Among the candidates that might serve as T cell-activating antigens, LDL and its core protein apolipoprotein B (ApoB) show the strongest clinical correlation with atherosclerosis in humans14. In vitro studies in CD4+ T cells from human atherosclerotic plaques show that many of these cells recognize oxidized LDL when processed and presented by APCs7. In particular, the TCRs in CD4+ T cells bind to peptide epitopes derived from ApoB presented by MHC class II molecules on APCs4,7,11. Interestingly, use of an MHC tetramer of recombinant MHC-molecules loaded with an ApoB-derived peptide enabled the detection of a naturally occurring population of CD4+ T cells in the blood in humans that recognizes the human peptide ApoB3036–30504. These findings strongly suggest that LDL is a relevant self-antigen that drives an autoimmune response in atherosclerotic plaques. In addition to LDL, heat shock proteins (HSPs) and peptides from pathogens such as human immunodeficiency virus (HIV) and cytomegalovirus (CMV) have been considered candidate antigens relevant in atherosclerosis3,8,15. These observations indicate that T cell subsets are involved in the initiation, progression, regression and ultimately rupture or erosion of atherosclerotic plaques. Much of the available evidence on the role of T cells in atherosclerosis is based on findings from experimental models. In this Review, we describe these experimental studies as well as the most relevant studies in human disease. We also discuss current knowledge on T cell homing to atherosclerotic plaques and highlight potential T cell-related therapies for atherosclerosis.
Fig. 1 |. Role of T helper cells and regulatory T cells in the pathogenesis of atherosclerosis.

a | Naive CD4+ T helper (TH) cells are primed in secondary lymphoid organs. TH cells acquire the complete phenotype of effector T (Teff) cells or regulatory T (Treg) cells after encountering antigenic peptides from apolipoprotein B (ApoB) presented by antigen-presenting cells (APCs). APCs take up and process oxidized LDL (oxLDL), migrate to the draining lymph node and present peptides from ApoB on major histocompatibility complex (MHC) class II molecules. Naive T cells recognize this complex through their specific T cell receptors (TCRs). Co-stimulatory molecules induce T cells to express transcription factors that favour the differentiation into distinct TH phenotypes. Homing receptors promote T cell migration to atherosclerotic lesions, where they secrete effector cytokines. b-c | CD4+ T cells can act in a pro-atherogenic or atheroprotective manner. Atherosclerotic lesions contain TH1, TH2, TH9, TH17, TH22, Treg, type 1 regulatory T (Tr1) cells and follicular helper T (TFH) cells. Treg cells can convert to ‘exTreg cells (loss of CD25 and FoxP3)’ (dashed arrows), acquiring properties of other TH phenotypes such as TH1, TH17 and TFH. Instability of forkhead box protein P3 (FOXP3) expression triggers the formation of antigen-specific, but dysfunctional, partially non-protective exTreg cells. AHR, aryl hydrocarbon receptor; BCL6, B-cell lymphoma 6; CCR5, C-C chemokine receptor type 5; CXCR3, C-X-C chemokine receptor type 3; EC, endothelial cell; G-CSF, granulocyte colony-stimulating factor; GATA3, GATA-binding factor 3; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFNγ, interferon-γ; LAG3, lymphocyte activation gene 3 protein; OxPL, oxidized phospholipid; PL, phospholipid; RORγt, nuclear receptor RORγt; T-bet, T-box transcription factor TBX21; TGFβ, transforming growth factor-β; TNF, tumour necrosis factor; VSMC, vascular smooth muscle cell, VCAM1; vascular cell adhesion molecule 1.
T cell research in atherosclerosis
In almost all published studies on T cells in atherosclerosis, the antigen specificity of the T cells is unknown. This is surprising, given that most T cell functions are strictly antigen dependent. However, determining the antigen specificity of T cells in atherosclerosis, or in any disease, is technically very difficult. Two methods can be used to find, isolate and study antigen-specific T cells: tetramers and restimulation assays.
MHC class II tetramers loaded with antigenic peptide can bind to CD4+ T cells and MHC class I tetramers loaded with antigenic peptide can bind to CD8+ T cells. Tetramers are recombinant proteins that must be specifically designed for the antigenic peptide in question and for the specific MHC allele(s) expressed by the study subject. Each tetramer must be validated against non-specific binding to other leukocytes. To establish specificity, the tetramer must not bind to T cells from MHC-mismatched subjects. Tetramers must co-cluster with TCR on individual cells. The number of cells identified by tetramers is usually low, in the order of 10 cells per million. In atherosclerosis, very few studies on tetramer-isolated T cells exist4.
Restimulation assays can be conducted with mixtures of antigenic peptides loaded onto APCs. Usually, the APCs are obtained from the same subject whose T cells are to be interrogated. Thus, their MHC alleles are naturally matched to the endogenous TCR repertoire. In restimulation assays, similarly to tetramers, whether the synthetic peptides that elicit a T cell response are the naturally presented peptides is not known.
T cell hybridomas have been generated with T cells from atherosclerotic Ldlr–/– mice16. A limitation of T cell hybridomas is that they grow independent of outside stimuli, which skews the T cell phenotype. Thus, almost all CD4+ T cell hybridomas reported for atherosclerosis are of T helper 1 (TH1) cells, although many other TH cells are found in atherosclerotic lesions in situ17.
Because of these technical limitations, the antigen specificity of the various T cell subsets is unknown in almost all studies in atherosclerosis. This lack of definition of the T cell antigen specificity might be one reason for the vastly different effects of T cell subsets in atherosclerosis reported in experimental studies (Box1): the same TH subset can be pro-atherogenic when recognizing an atherosclerosis-relevant antigen and potentially neutral or anti-atherogenic when detecting an unrelated antigen. This lack of knowledge of antigen specificity limits the conclusions that can be drawn about the roles of T cell subsets in atherosclerosis.
Box 1 |. Possible causes of discrepancies in the findings of experimental studies on atherosclerosis.
Several factors can account for the discrepancies in the effects of T cell subsets reported in experimental studies on atherosclerosis.
Mouse strains
One possible cause for conflicting findings is the vastly different susceptibility to atherosclerosis of inbred mouse strains223. The role of T cells in atherosclerosis has been mostly explored in Apoe–/– mice or Ldlr–/– mice on a C57BL/6 background. However, contamination with 129/Sv DNA near the targeted gene is possible if embryonic stem cells from the 129/Sv mouse strain were used to generate the knockout mice. 129/Sv contamination can decrease the susceptibility to atherosclerosis.
T cell depletion strategy
In many experimental studies on atherosclerosis, monoclonal antibodies (mAbs) are used to deplete T cell subsets. These mAbs are usually antibodies against mouse antigens generated in rats or hamsters. Therefore, the antibody itself is a foreign antigen that elicits a vigorous immune response in the mice, resulting in the production of neutralizing antibodies224. If a mAb is injected more than once, subsequent doses might become ineffective224. Therefore, careful monitoring of CD4+ T cell or CD8+ T cell levels over the course of the depletion experiment is required.
Power of the studies
Another common problem is that most of the available experimental studies on the role of T cells in atherosclerosis were underpowered, which can yield spurious results225. This can be avoided by appropriate power calculations during the experimental design phase.
Gene knockout strategy
Data from mice with systemic gene knockout are difficult to interpret, because most genes are expressed not only in T cells but also in other cell types. As a case in point, effects on atherosclerosis that were originally attributed to T cells in studies in mice with systemic deficiency in a specific receptor, transcription factor or cytokine were later found to be caused by innate lymphoid cells74. Conditional knockout mice are generated most commonly with the Cre–loxP system, in which Cre is expressed under the control of a cell-specific or tissue-specific gene promoter, in the case of T cells, for example, under the Cd4–Cre, Cd8–Cre and other subset-specific drivers, so that the Cre-mediated loxP recombination and subsequent gene loss occurs only in the targeted cells. However, the specificity of each Cre driver must be established for each targeted T cell type. When an inducible Cre system is used, such as the Cre recombinase fused to a mutant oestrogen ligand-binding domain, in which Cre is active only after administration of tamoxifen, the degree of gene knockout in the targeted cell should be monitored before and after tamoxifen administration.
Diet
The levels of dietary cholesterol in different types of diet (Chow, Western, high-cholesterol or cholate-containing diets) used in experimental studies on atherosclerosis can directly affect the phenotype and proliferation rate of T cells137,226,227. Hundreds of different diets are used in experimental atherosclerosis studies.
Gut microbiota
The gut microbiota of mice differs between different animal facilities and even between cages in the same facility. The gut microbiota can have large effects on T cell functions2,228,229 and on atherosclerosis development and progression2,230,231.
Immunohistochemistry studies18,19, single-cell RNA sequencing (scRNA-seq)17,20, and mass cytometry (CyTOF) approaches17,21 (Box2) have shown that 25–38 % of all leukocytes in mouse aortic atherosclerotic plaques and human atherosclerotic plaques are CD3+ T cells. A study using an innovative methodology, cellular indexing of transcriptomes and epitopes by sequencing (CITE-seq) (Box2), has shown that T cells account for the majority of immune cells in human atherosclerotic plaques obtained from endarterectomy22. However, flow cytometry, CyTOF, scRNA-seq and CITE-seq all require mechanical dissociation and enzymatic tissue digestion to prepare single-cell suspensions. The enzymes used for tissue digestion can alter cell surface molecules by proteolytic cleavage and the cellular processes during the digestion and isolation can alter single-cell transcriptomes. Moreover, the proportion of different cell types can be affected and skewed by the isolation procedures; because T cells are small, round and mechanically robust, these cells can survive the isolation procedure better than macrophages or dendritic cells, which are large, branched and fragile23. Analysis of gene expression signatures obtained with CITE-seq and and scRNA-seq indicated that human atherosclerotic plaques are enriched in T cells showing cytotoxicity, activation and exhaustion, whereas T cells in blood had gene signatures associated with cytokine inhibition, RNA synthesis and metabolic reprogramming22. Human and mouse studies indicate that T cells predominantly populate atherosclerotic lesions with an enrichment in the fibrous cap18,19, but are also found in the adventitia of older lesions17,24,25.
Box 2 |. Technological advances for analyzing leukocytes in atherosclerosis.
Assessment of the immune cell composition of tissues was traditionally performed by flow cytometry and immunohistochemistry. The development of high-dimensional, parametric single-cell analyses, including mass cytometry (CyTOF)232,233 and massively parallel single-cell RNA sequencing (scRNA-seq)234–239, have led to deeper insights into immune cell subsets.
Similar to flow cytometry, in CyTOF, single-cell suspensions are stained with antibody panels to detect cellular antigens. In contrast to flow cytometry, antibodies for CyTOF are conjugated with rare metal earth (lanthanide) isotopes, which enable the detection of up to 40 antigens. Flow cytometry requires substantial compensation to correct for the spectral overlap of fluorophores. In CyTOF, the mass spectra of different lanthanides do not overlap. CyTOF can simultaneously detect extracellular, intracellular and phosphorylated antigens240. CyTOF has been used to analyze immune cell composition in atherosclerosis17,21.
scRNA-seq allows the detection of single-cell transcriptomes. The commercialization of gel-bead-based approaches revolutionized the assessment of the immune landscape of complex tissues by allowing the analysis of thousands of single-cell transcriptomes in parallel241. This approach sharpens the focus on the heterogeneity of immune cell populations. On the basis of the transcriptome data, the developmental ontogeny of individual cell clusters can be assessed with trajectory algorithms242,243, the functionality of immune cell subsets can be predicted, and the likely cellular interaction partners within a tissue can be identified based on the expression of receptor-ligand pairs244. scRNA-seq enables a better understanding of aortic wall cells and particularly leukocytes17,20,25,245–247. Moreover, gene expression and cell surface phenotypes can be assessed in the same single cell with the use of antibodies conjugated with barcoded oligonucleotides, such as with the cellular indexing of transcriptomes and epitopes by sequencing (CITE-seq) or the Abseq methods22.
A summary of analytic methods is shown in the Table below. Of note, all these methods require generating single-cell suspensions, which can introduce various artifacts.
| Characteristics | Microscopy | Cytometry | scRNA-seq with CITE-seq or Abseq | ||
|---|---|---|---|---|---|
| Immunofluorescence | flow cytometry | CyTOF | Antibodies | Genes | |
| Number of markers | ~3 | ~16 | ~40 | ~50 | ~2,000 |
| Number of cells | 10–1,000 | Millions | ~100,000 | ~10,000 | ~10,000 |
| Multiplexing | No | No | Yes | Yes | Yes |
| Intracellular antigens | Yes | Yes | Yes | No | N/A |
| Cell morphology | Yes | Some (forward and side scatter | No | No | No |
| Surface markers and transcriptome in the same cell | No | No | No | Yes | Yes |
| Cost per cell | High | Low | Medium | High | High |
| Spectral overlap | Yes | Yes | No | No | No |
| Enzymatic digestion | No | Yes | Yes | Yes | Yes |
| Non-proportional cell death of different cell types | No | Yes | Yes | Yes | Yes |
CD4+ T cells
CD4+ T cells are critical regulators of the adaptive immune response with the capacity to differentiate into distinct TH cell or Treg cell subtypes (Fig. 1). TH cells and Treg cells can either activate or dampen the response of other immune cells, exert direct pro-inflammatory or anti-inflammatory effects on tissue-resident cells, help B cells produce high-affinity IgG antibodies, or have cytolytic activity26. Therefore, the function of CD4+ T cells in atherosclerosis is multifaceted. Each CD4+ T cell subset has specific transcriptional programmes and patterns of cytokine secretion that can accelerate or attenuate atherosclerosis. A 2019 study showed that CD4+ effector memory T cells are enriched in plaques of patients with recent history of stroke compared with those with asymptomatic atherosclerosis22. In mice, genetic or antibody-mediated depletion of CD4+ T cells protects from atherosclerotic lesion development27,28. Depletion of CD4+ Treg cells accelerates atherosclerosis29. After antigen presentation by APCs, lesional CD4+ T cells differentiate into functionally distinct TH subtypes (TH1, TH2, TH9, TH17, TH22, follicular helper T (TFH) and CD28– T cells) and Treg subtypes (FOXP3+ Treg cells and type 1 regulatory T (Tr1) cells)3,26. Single cell data from human atherosclerotic plaques and peripheral blood mononuclear cells (PBMCs) showed that the majority of CD4+ T cells in the plaque are TH1 and TH2 cells, and that TH1 cells are enriched in atherosclerotic lesions compared with PBMCs22.
Antigen presentation can initiate and modulate CD4+ T cell responses in atherosclerosis. T cell immune responses are initiated by antigen-loaded APCs migrating to the lymph nodes30. Several types of cells function as APCs, including macrophages in the atherosclerotic plaque, B cells in the adventitia and some dendritic cell subsets such as conventional, plasmacytoid31 or IRF8-dependent32 dendritic cells, inducing antigen-experienced effector memory T cells6. Depending on co-stimulatory signals and cytokines provided by these APCs, the CD4+ T cell immune response can be polarized towards an immunogenic response or a tolerogenic response. For example, CD40 on APCs interacts with CD40 ligand on T cells, contributing to TH1 polarization33. Disrupting this signal reduces atherosclerosis in mice and cynomolgus monkeys34,35. CD80 and CD86 on APCs interact with the co-stimulatory receptor CD28 on T cells; deficiency of CD80 and CD86 in mice with atherosclerosis suppresses TH1 responses36. CD80 and CD86 on APCs also interact with cytotoxic T-lymphocyte antigen 4 (CTLA4), which is expressed on Treg cells and activated T cells. CTLA4 overexpression in Apoe–/– mice decreases atherosclerosis development compared with control mice37. Programmed cell death 1 ligand 1 (PDL1) on APCs interacts with programmed cell death 1 (PD1) and CD80 on T cells. PD1 might have a major role in limiting early T cell activation and T cell exhaustion in atherosclerosis, as has been shown in chronic inflammation and cancer38. Interestingly, T cells from human atherosclerotic plaques express high levels of PD122, and activation of the PD1–PDL1 pathway limits pro-atherogenic T cell responses in mice39. Interaction of OX40 ligand on APCs with OX40 on T cells increases TH2 cell responses; blockade of this pathway in Ldlr–/– mice with atherosclerosis led to plaque regression40. Interaction of CD137 ligand on APCs with CD137 on T cells increases T cell proliferation and survival, which can promote atherosclerosis development and progression as shown in mice with hyperlipidaemia41. Activation of transforming growth factor-β (TGFβ) receptor II signalling in dendritic cells inactivates pro-inflammatory T cell responses in Apoe–/– mice, thereby dampening inflammation in atherosclerotic plaques42. In mice with atherosclerosis, MyD88 activation in dendritic cells is required for the induction of Treg cells43, whereas C-C motif chemokine 17 (CCL17) secreted by dendritic cells suppresses Treg cell responses44. Interferon-α (IFNα), and presumably other type I interferons, secreted be dendritic cells in atherosclerotic plaques activates TH1 cell responses in mice45.
TH cells
TH1 cells.
TH1 cells express the defining transcription factor T-bet, chemokine receptors like CXCR3 and CCR5, and secrete interferon-γ. Several experimental studies have shown that TH1 cells promote atherosclerosis and are the most prominent TH cell subset in the plaque46,47. In atherosclerotic Apoe–/– mice, TH1 cells express the atherosclerotic plaque-homing receptor C-C motif chemokine receptor 5 (CCR5) in lymph nodes48. CyTOF and CITE-seq data from human atherosclerotic plaques show that CCR5 expression is upregulated in plaque-derived T cells22. In addition, TH1 cells are enriched in the plaques from patients with recent stroke compared with patients with asymptomatic atherosclerosis22. TH1 cells from mouse atherosclerotic lesions secrete IFNγ and express the T-box transcription factor TBX21 (also known as T-bet)48,49, Moreover, many CD4+ T cells in the atherosclerotic plaque express other TH1-associated pro-inflammatory cytokines in addition to IFNγ, such as IL-2, IL-3, tumour necrosis factor (TNF) and lymphotoxin, which can all activate macrophages, T cells, and other plaque cells and thereby accelerate the inflammatory response3. Deficiency of IFNγ, its receptor, or T-bet protects mice from atherosclerosis46,50,51. Consistently, IFNγ administration in Apoe–/– mice increases atherosclerosis compared with untreated mice52. IFNγ could directly reduce plaque stability by inhibiting vascular smooth muscle cell (VSMC) proliferation53, affecting macrophage polarization54 and modulating cardiovascular risk factors55. Some studies have shown that IFNγ induced VSMC proliferation56,57, which can stabilize the plaque. In one study, IFNγ-deficient bone-marrow cells transplanted into Ldlr–/– mice led to larger diet-induced atherosclerotic lesions compared with control mice58. However, this finding is confounded by the use of a cholic acid-containing diet58 (Box 1), which has known pro-inflammatory effects59. Other TH1 cytokines besides IFNγ might be important for the development of atherosclerosis, but no specific studies of other TH1 cytokines in TH1 cells are available.
TH2 cells.
Th2 cells are involved in defense against parasites, in asthma and other allergic diseases. The main TH2 cytokine is IL-4. IL-4 binds to IL-4 receptor in T cells and activates signal transducer and activator of transcription 6, which leads to the expression of the transcription factor GATA3, the master regulator of TH2 differentiation. In mouse atherosclerotic plaques, a substantial proportion of T cells express transcripts for TH2-associated cytokines such as IL-4, IL-5, IL-10 and IL-1317. However, whether TH2 cells are pro-atherogenic or atheroprotective remains unclear. Individuals with a high number of TH2 cells in the PBMC fraction have lower subclinical atherosclerosis burden, as measured by common carotid intimal media thickness, than individuals with low TH2 cell counts60. Moreover, IL-4 release from activated mononuclear leukocytes negatively correlate with clinical atherosclerosis61.
However, the role of IL-4 in atherosclerosis is unclear. IL-4 was shown to antagonize TH1 responses and diminish atherosclerotic lesion formation in Apoe–/– mice62, whereas other studies have shown that depletion of IL-4 is atheroprotective in Ldlr–/– mice fed a high-fat diet63 and administration of exogenous IL-4 did not decrease atherosclerosis in Apoe–/– mice with angiotensin II-induced atherosclerosis64. Immunization of Apoe–/– mice fed a high-fat diet with an ApoB-peptide increased IL-4 expression in T cells, which resulted in no difference in atherosclerotic lesions compared with control mice65.
Unlike IL-4, most evidence suggests that other TH2-associated cytokines, such as IL-5 and IL-13, are atheroprotective. In humans, plasma IL-5 levels inversely correlate with carotid intima media thickness66,67, but elevated IL-5 levels in plasma are associated with the presence of unstable angina and myocardial infarction68. The immunization of Ldlr–/– mice with modified LDL is atheroprotective, inducing a TH2-skewed immune response that was characterized by antigen-specific production of IL-5 and IL-13 with small amounts of IL-4 and IFNγ compared with non-immunized mice69. IL-13 administration in Ldlr–/– mice fed a high-fat diet modulates established atherosclerotic lesions by increasing lesional collagen content and reducing vascular cell adhesion molecule 1 (VCAM1) expression, resulting in decreased macrophage infiltration in plaques compared with mice treated with phosphate-buffered saline (PBS)70. IL-33 predominantly induces the production of TH2 cytokines, and IL-33 treatment reduced atherosclerosis development in high-fat diet-fed Apoe–/– mice and increased the levels of IL-4, IL-5 and IL-13 and decreased levels of IFNγ in serum and lymph nodes compared with PBS-treated mice71. By contrast, high-cholesterol diet-fed Apoe–/– mice with deficiency in IL-33 or its receptor ST2 had no differences in atherosclerosis development compared with control mice72. Of note, IL-4, IL-5 and IL-13 are also produced by subtype 2 innate lymphoid cells (ILC2)73. Genetic ablation of ILC2 in Ldlr–/– mice fed a high-fat diet accelerated atherosclerosis development, which was corrected by reconstitution with wild type ILC2 but not by reconstitution with IL-5-deficient or IL-13-deficient ILC274. The role of T cell-derived IL-5 and IL-13 has not been studied yet. Mice with specific deficiency of T cells-are needed to distinguish the effects of cytokines derived from TH2 cells and ILC2 on atherosclerosis.
TH9 cells.
TH9 cells, a subset initially associated with the TH2 phenotype, are the primary source of IL-9. The production of IL-9 in TH9 cells is stimulated by TGFβ and IL-4, and inhibited by IFNγ75. It is not known whether Th9 cells are pro- or anti-atherogenic. IL-9 plasma levels are increased in patients with coronary atherosclerosis compared with healthy individuals, and IL-9 levels were higher in human atherosclerotic plaques than in non-atherosclerotic vessel samples76. Moreover, plasma IL-9 levels are increased in patients with acute coronary syndrome compared with healthy individuals, but no differences were observed in the number of TH9 cells in blood77. In vitro, IL-9 administration increased IL-17 secretion from PBMCs of patients with unstable angina and from healthy individuals77. In Apoe–/– mice fed a Western diet, IL-9 administration exerted pro-atherogenic effects, at least partially by inducing VCAM1 expression in aortic endothelial cells, which mediates inflammatory cell infiltration into atherosclerotic lesions78.
TH17 cells.
TH17 cells are characterized by expression of the TH17-defining transcription factor nuclear receptor RORγt (RORC), are activated by IL-23 and IL-17 is their signature cytokine79. TH17 cells have distinct plasticity in different inflammatory settings80–84. In immune, endothelial and stromal cells, IL-17 induces the secretion of the pro-inflammatory cytokines IL-6, granulocyte-colony stimulating factor) and granulocyte-macrophage colony-stimulating factor, and chemokines79, all of which can be pro-atherogenic. By contrast, IL-6 and TGFβ induce a subtype of TH17 cells that produce IL-10 concomitantly with IL-1785,86, and IL-10 can be atheroprotective87. Moreover, IL-17 is also produced by γδT cells and ILC3 cells88. Because of this complexity, experimental studies in Apoe–/– mice on the role of IL-17 have yielded discrepant results: some studies suggest that IL-17A is pro-athrogenic89–91, others atheroprotective92 and other studies suggest that IL-17 has no effect on atherosclerosis93. Il17a–/–Apoe–/– mice and Il17ra–/–Apoe–/– mice fed a Western diet had smaller atherosclerotic plaques in the aortic arch and aortic roots, but similar plaque burden in the thoracoabdominal aorta, compared with Apoe–/– mice94. By contrast, in vivo administration of IL-17A reduced plaque burden in aortic roots in Ldlr–/– mice compared with control mice95. Moreover, some studies in mouse models of atherosclerosis indicate that IL-17 can promote plaque stability by increasing the production of type I collagen by VSMCs95–97.
Some clinical studies showed that plasma IL-17 levels and the number of peripheral TH17 cells are increased in patients with unstable angina or acute myocardial infarction compared with patients with stable angina and healthy individuals98,99. However, in larger clinical studies, plasma IL-17 levels were similar in individuals with or without coronary artery disease100, and low serum IL-17 levels were associated with a higher risk of cardiovascular events in patients with acute myocardial infarction101. In human atherosclerosis, IL-17 is produced concomitantly with IFNγ by T cells infiltrating into coronary artery plaques, and IL-17 and IFNγ synergistically increase pro-inflammatory responses by VSMCs100. By contrast, consistent with some experimental studies, IL-17 expression in human carotid atherosclerotic plaques was associated with a stable plaque phenotype with a lower macrophage content and a higher VSMC content95,97.
TH22 cells.
TH22 cells are unique in that they express IL-22 but not IL-17 and IFNγ, and express the master transcription factor aryl hydrocarbon receptor (AHR). The cytokines responsible for inducing TH22 cell differentiation are unknown102. It is also unknown whether Th22 cells are pro- or anti-atherogenic. Increased circulating levels of TH22 cells have been reported in patients with acute coronary syndrome compared with healthy controls77,103. IL-22 levels are higher in plasma from patients with symptomatic atherosclerotic disease compared to patients with asymptomatic atherosclerosis77,103,104. In Apoe–/– mice, IL-22 is involved in activation of vascular repair by stimulating medial VSMC differentiation into a synthetic phenotype, which might contribute to atherosclerotic plaque growth by enabling VSMC migration into the intima105. Moreover, IL-22 reduced atherosclerosis by repressing pro-atherogenic gut microbiota, shown by a study in IL-22-deficient atheroprone mice106.
TFH cells.
TFH cells, which express the defining transcription factor B-cell lymphoma 6 (BCL6), are found in B cell follicles and maintain and form germinal centres together with B cells. TFH cells are required for antibody isotype switching in germinal centre B cells107. TFH cells are most likely pro-atherogenic. The atherogenic environment increases the autoimmune responses of CXCR3+ TFH cells in atherosclerosis-prone mice108. However, in Apoe−/− mice fed a high-cholesterol diet, marginal zone B cells inhibit the response of TFH cells, thereby limiting atherosclerosis development and progression109. Moreover, the pro-atherogenic TFH cell activity in germinal centres can be regulated by a subset of CD8+ T cells with regulatory function, which might be defective in Apoe–/– mice110. The pathway involving the co-stimulatory molecule inducible T-cell co-stimulator (ICOS) and its ligand ICOSL is crucial for the differentiation and maintenance of TFH cells: blocking ICOS–ICOSL signalling in Apoe–/– mice reduces atherosclerosis burden, with reduced numbers of TFH cells in secondary lymphoid organs111. Ageing increases the proportion of TFH cells in Apoe–/– mice, but not in wild-type mice, whereas ageing did not modify the overall percentage of CD4+ T cells in Apoe–/– mice110. Interestingly, TFH cells can derive from Treg cells. These ‘switched’ TFH cells are pro-atherogenic and their depletion reduces atherosclerosis in Apoe–/– mice111. In patients with coronary artery disease, plasma levels of IL-21, a major TFH cytokine, negatively correlated with FOXP3 expression in Treg cells111.
CD28null T cells.
CD4+CD28–T cells are characterized by the lack of CD28 expression, which is the main co-stimulatory receptor of naive CD4+ T cells. CD28– T cells are pro-inflammatory and cytotoxic. Interestingly, CD28– T cells are only found in humans and non-human primates, not in mice112. Patients with acute coronary syndrome have a higher number of CD28– T cells in blood than healthy individuals113–115, and CD28– T cells from these patients are resistant to apoptosis116. Moreover, human unstable atherosclerotic plaques can be invaded by clonally expanded T cells, including a large monoclonal population of CD28– T cells115. In addition, some CD28– T cell subsets are reactive to HSP60 and HSP70117, which is a possible atherosclerosis antigen118. Among patients with end-stage renal disease, those with atherosclerosis had higher expansion of the CD28– T cell subset than those without atherosclerosis119.
Treg cells
FOXP3+ Treg cells.
In humans, the classical Treg cell subset is characterized by expression of the transcription factor forkhead box protein P3 (FOXP3), the IL-2 receptor subunit-α (IL-2RA; also known as CD25, which is part of the trimeric high-affinity IL-2 receptor) and CTLA4, and by lack of CD127 expression. Of note, FOXP3 is a reliable Treg cell marker in mice, but is not a reliable marker for human Treg cells because FOXP3 is transiently expressed by other CD4+ T cells during activation120. In mice, Treg cells protect against atherosclerosis29,121. Similarly, clinical data suggest a strong inverse relationship between Treg cells and atherosclerosis: Treg cell numbers and IL-10, a cytokine secreted by Tregs are lower in patients with myocardial infarction than in patients with stable angina or individuals without coronary artery disease122,123, and a low Treg cell to CD4+ T cell ratio in blood predicted a higher rate of cardiovascular events in a large cohort study124. However, in a large, prospective, case-control study excluding patients with diabetes mellitus, individuals with a low Treg cell to total T cell ratio in blood had a lower risk of myocardial infarction, but the association was not significant when additional cardiovascular disease risk factors were included in the model125. Another study showed no correlation between Treg cell numbers in blood and carotid and coronary atherosclerosis126. This discrepancy might be due to differences in the study designs127.
Like in other T cells, the activity of Treg cells increases when their TCR binds its cognate antigenic peptides presented by MHC class II molecules. However, like for other T cell subsets, in studies on atherosclerosis the antigen specificity of Treg cells is usually unknown. Upon stimulation, Treg cells can produce high levels of IL-10 and TGFβ. IL-10 is an anti-inflammatory cytokine, whose deficiency increases atherosclerosis in atheroprone mice87; of note, in this early study the source of IL-10 was not identified. TGFβ has plaque-stabilizing effects in Apoe–/– mice128. Treg cells exert their atheroprotective properties by secreting IL-10 and TGFβ, and by suppressing the proliferation of pro-inflammatory effector T cells129. The atheroprotective effects of treatment with IL-2 complexes130 and anti-CD3 treatment131,132 in atheroprone mice have been attributed to an increase of Treg cell numbers relative to other T cells.
A splice variant of FOXP3 controls some Treg cell effector functions and is associated with human atherosclerotic plaque stability133. A study in Apoe–/– mice with MHC class II deficiency showed that lack of antigen presentation on MHC class II molecules aggravates atherosclerosis via decreased Treg cell numbers134. In patients with subclinical atherosclerosis, Treg cell numbers in blood correlate positively with plasma LDL levels135. Likewise, in mice, hypercholesterolaemia initially favours the differentiation of Treg cells followed by increased TCR downstream signalling events136, an effect that might be a response to elevated inflammation137, intracellular lipid accumulation in Treg cells138 or an antigen-specific response. These findings suggest that a subpopulation of Treg cells responds to antigens associated with increased plasma LDL levels or to components of LDL particles. Such LDL-reactive or ApoB-reactive Treg cells have TCRs specifically responding to atherosclerosis antigens. One class of atherosclerosis-related antigens are ApoB peptides. The existence of ApoB peptide-reactive T cells was shown in humans and mice with the use of human and mouse MHC class II tetramers loaded with a sequence-identical human and mouse ApoB peptide4.
Pathological conversion of Treg cells.
The plasticity of FOXP3+ Treg cells has been investigated in experimental atherosclerosis139. Circulating and atherosclerotic plaque Treg cell numbers in established mouse atherosclerosis decline in later stages of the disease, whereas total CD4+ effector T cells and splenic Treg cells increase with increased atherosclerotic lesion size137. Treg cells in late atherosclerosis in mice simultaneously express FOXP3 and T-bet, lose their capacity to regulate and to protect from atherosclerosis, while retaining some phenotypic similarity with Treg cells48,49,137. These findings suggest that the immunosuppressive phenotype of Treg cells disappears as atherosclerosis progresses.
In autoimmune conditions, such as experimental autoimmune encephalitis or arthritis, instability of FOXP3 expression triggers the formation of antigen-specific, but dysfunctional, partially non-protective former Treg cells (known as exTreg cells)140–142. A study in Apoe–/– mice fed a Western diet showed that Treg cells lose FOXP3 expression and their immunosuppressive function during atherosclerosis progression, leading to the conversion of a fraction of these cells into pro-atherogenic TFH cells expressing BCL6111. Transgenic CD4+ T cells expressing a TCR reacting to human LDL differentiated into TFH cells after transfer into transgenic Ldlr–/– mice expressing human ApoB100143. In humans, 67% of ApoB3036–3050-reactive CD4+ T cells from individuals without cardiovascular disease expressed FOXP3, indicative of ApoB-reactive Treg cells4, but not the TH17-defining transcription factor RORγt or the TH1-associated transcription factor T-bet, commonly found in ex-Tregs. By contrast, in patients with subclinical atherosclerosis, the percentage of T cells expressing only FOXP3+ declined to about 30%, and a substantial proportion of the remaining FOXP3+ T cells acquired simultaneous expression of the Th17-defining transcription factor RORγt or the TH1-asscoiated transcription factor T-bet4.
The instability of FOXP3 expression might be caused by increased methylation of the FOXP3 locus, which is observed in patients with severe coronary artery disease144. Treg cell instability might also be induced by increased intracellular cholesterol levels or by decreased IL-2 signalling111,140. In addition, the function of FOXP3 might be regulated by alternative splicing favouring a FOXP3 isoform that induces pathogenic transcriptional programmes133. These data suggest that the initial protective immune response by Treg cells can shift to a pathogenic response as atherosclerosis progresses139. Although some possibilities have been suggested, the mechanisms by which this switching occurs remain largely unknown. Inhibiting the enzyme MALT1, which is part of the CARMA1–BCL10–MALT1 (CBM) signalosome complex, promotes Treg cell conversion towards IFNγ expression and TH1 cell polarization145,146. This combination is reminiscent of the FOXP3+T-bet+ T cell type observed in atherosclerosis in mice48,49. Conversely, blocking or knocking out cyclin-dependent kinase 8 (CDK8) or CDK19 stabilizes Treg cells even under conditions of inflammation147.
Tr1 cells.
Tr1 cells lack FOXP3 expression and are characterized by the expression of lymphocyte activation gene 3 protein and CD49b, which is the α2 subunit of the α2β1 collagen receptor. Tr1 cells prominently secrete IL-10148. An experimental study in Apoe–/– mice showed that Tr1 cells are atheroprotective via IL-10 secretion149. A study in humans has shown that the frequency of Tr1 cells secreting IL-10 is significantly lower in the blood of patients with coronary artery disease than in healthy individuals150.
Other T cell subsets
CD8+ T cells
CD8 T cells recognize antigenic peptides presented by MHC-I. They can mature to cytotoxic T cells that can kill virus-infected and other abnormal (like cancer) cells using various cytotoxic pathways. Patients with coronary artery disease have increased levels of cytotoxin-producing CD8+ T cells in blood compared with healthy individuals151,152, and CD8+ T cells are abundant in atherosclerotic plaques in humans and mice153–155. In advanced human atherosclerotic lesions, CD8+ T cells outnumber CD4+ T cells22,153,156, and are predominantly found in fibrous cap areas157. TCR sequencing of T cells from human atherosclerotic plaques identified a subpopulation of CD8+ T cells whose frequencies correlated with TCR clonality, suggesting clonal expansion in the plaque22. However, similar to CD4+ T cells, in most studies on atherosclerosis the antigen specificity of plaque CD8+ T cells is unknown.
In experimental studies, both pro-atherogenic and atheroprotective roles of CD8+ T cells have been reported. The cytotoxic activity of CD8+ T cells towards lesion-stabilizing cells such as VSMCs and the inflammatory cytokines produced by of CD8+ T cells might exacerbate the inflammatory responses in atherosclerotic plaques and drive the progression and instability of the lesions (Fig. 2a). Conversely, CD8+ T cell cytotoxic activity towards APCs might limit atherosclerosis (Fig. 2a). MHC class I-dependent cytotoxic CD8+ T cells have been suggested to contribute to plaque inflammation and the build-up of the necrotic core. One study identified ApoB-reactive CD8+ T cells in Apoe–/– mice158. CD8+ T cell depletion with antibodies reduced atherosclerosis in atheroprone mice159–161, suggesting that CD8+ T cells are pro-atherogenic. These pathogenic CD8+ T cells have higher IFNγ and granzyme B production than CD8 T cells from non-atherosclerotic mice161. A study in Ldlr–/– mice fed a high-fat diet showed that CD8+ T cells promote atherosclerosis by regulating monopoiesis and the levels of peripheral monocytes via IFNγ production159. However, another study showed that IFNγ produced by CD8+ T cells does not have a role in atherosclerosis in Apoe–/– mice fed a high-fat diet160. In this mouse model, studies of adoptive transfer of CD8+ T cells deficient in either perforin, granzyme B, TNF or IFNγ into lymphopaenic Apoe–/– mice suggested that CD8+ T cells promote atherosclerotic plaque development by perforin-mediated and granzyme B-mediated apoptosis of macrophages, VSMCs and endothelial cells, followed by increased inflammation promoted by TNF secretion160. Of note, in these adoptive transfer studies, most CD8+ T cells are probably not specific for atherosclerosis antigens.
Fig. 2 |. Role of CD8+ T cells, iNKT cells and γδT cells in atherosclerosis.
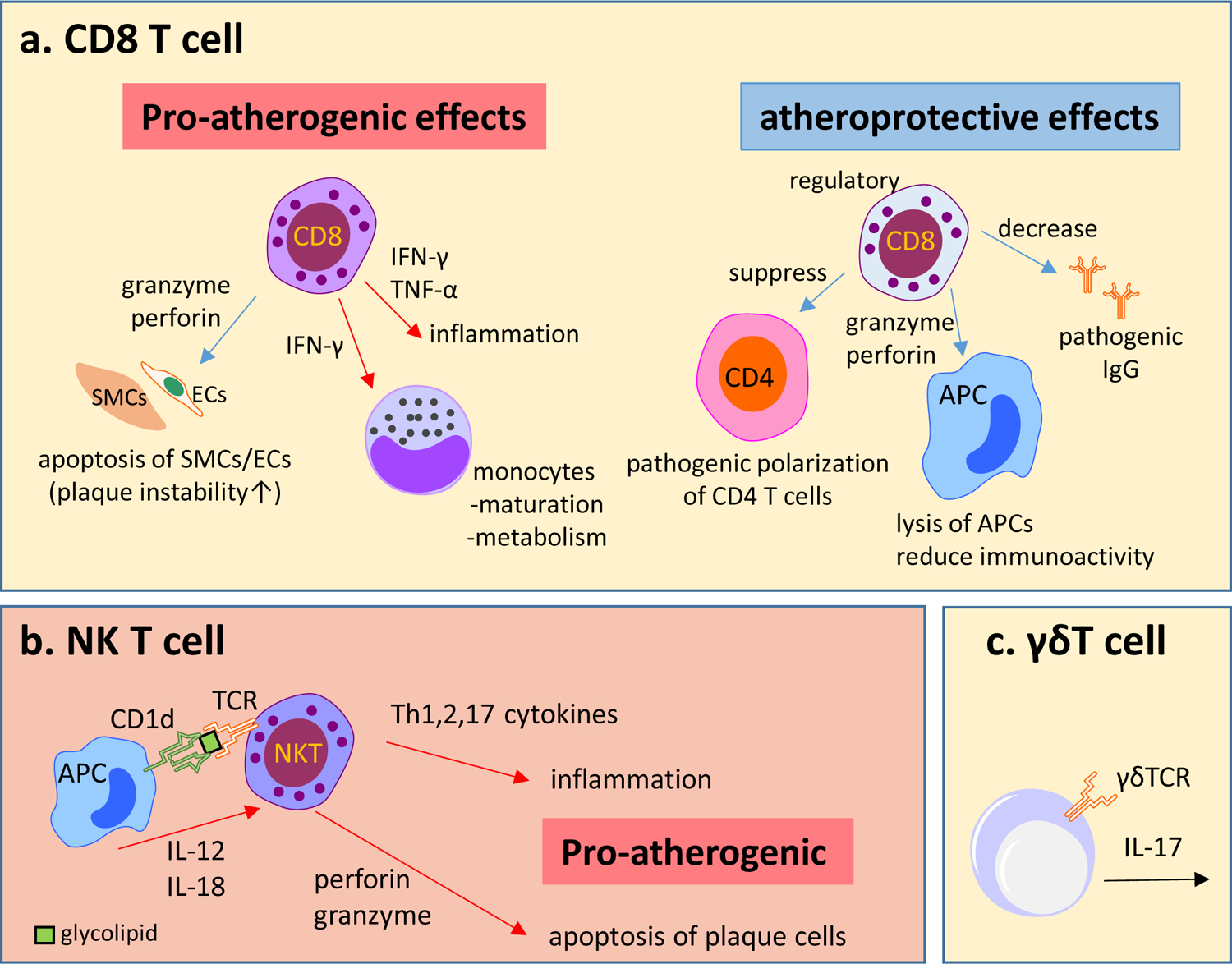
a | Overview of atheroprotective and pro-atherogenic functions of CD8+ T cells. The cytotoxic activity of CD8+ T cells towards atherosclerotic lesion-stabilizing cells, such as vascular smooth muscle cells (VSMCs) and endothelial cells (ECs), and the secretion of interferon-γ (IFNγ), tumour necrosis factor (TNF) and other pro-inflammatory cytokines exacerbate the inflammatory responses and drive the progression and destabilization of atherosclerotic lesions. Regulatory CD8+ T cell subsets can have atheroprotective effects, with high cytotoxic activity towards antigen-presenting cells (APCs) and inhibition of CD4+ T cell polarization into pro-atherogenic phenotypes. b | Invariant natural killer T (iNKT) cells can be activated by the interaction of the T cell receptor (TCR) with CD1d molecules containing antigenic glycolipids present on APCs. iNKT cells can also be activated in a CD1d-independent manner by Toll-like receptor stimulation and by the activation of APCs, which in turn secrete cytokines that activate iNKT cells, such as IL-12 and IL-18. Activation of iNKT cells results in the rapid release of TH1, TH2 and TH17 associated cytokines, which activate other immune cells in the atherosclerotic lesion. iNKT cells can also promote atherosclerosis by the induction of apoptosis of plaque cells through the release of cytotoxic proteins such as perforin and granzyme B. c | γδT cells are among the T cell subsets described in mouse atherosclerotic lesions. The intracellular cholesterol content in γδT cells regulates their activation, proliferation and effector functions. In addition, γδT cells are an abundant source of IL-17; therefore, these cells could modulate atherosclerosis via IL-17 production. However, the exact role of γδT cells in atherosclerosis is unclear.
Other experimental studies suggest that CD8+ T cells can also have an atheroprotective role. CD8+ T cells with regulatory functions in atherosclerosis have been assessed in immunization studies. CD8+ T cells mediate the atheroprotective effects of immunization with an ApoB-related peptide (p210) in Apoe–/– mice162. Adoptive transfer of CD8+ T cells from p210-immunized mice reduced atherosclerosis in Apoe–/– mice compared with transfer of CD8+ T cells from control mice162. Immunization with p210 decreased number of dendritic cells at the site of immunization and in the atherosclerotic plaque and reduced immunoreactivity of plaque macrophages compared with mice injected with PBS162. Another study of vaccination with p210 in Apoe–/– mice showed that CD8+ T cells from immunized mice had higher lytic activity against macrophages and inhibited the polarization of CD4+ T cells into TH17 cells compared with CD8+ T cells from control mice163. Depletion of CD8+ T cells in advanced atherosclerosis in Ldlr–/– mice fed a Western diet for 10 weeks resulted in less stable lesions with significantly reduced collagen content in the aortic valve area, increased macrophage content and increased necrotic core area compared with controls164. Furthermore, a specific subset of CD8+ regulatory T cells reduces atherosclerosis in Apoe–/– mice by blocking ICOS–ICOSL signalling between TFH cells and germinal centre B cells, leading to reduced levels of potentially atherogenic IgGs110.
Natural killer T cells
Natural killer T (NKT) cells are divided into two subgroups: invariant NKT (iNKT) cells (or type I), which have few variant TCRs, and type II NKT cells, which have more variable TCRs. So far, only iNKT cells have been studied in atherosclerosis. iNKT cells can be activated by the interaction of the TCR with antigen-presenting CD1d molecules containing antigenic glycolipids present on APCs165 (Fig. 2b). Some glycolipids are of microbial origin and some are self-glycolipids166. Activation of iNKT cells results in the rapid release of TH1, TH2 and TH17 cytokines, depending on the expression of the same transcription factors that define the corresponding TH cell subtypes: T-bet, GATA3 and RORγt, respectively167. Like CD8+ T cells, iNKT cells can also express the cytotoxic proteins perforin and granzyme B167. Most studies using Apoe–/– mice168–172 and Ldlr–/– mice168,169,171,173–175 fed a Western or Chow diet suggest that iNKT cells are pro-atherogenic. iNKT cells are thought to promote atherosclerosis by secreting cytokines, which can activate other immune cells present in the atherosclerotic lesion.
γδT cells
Unlike T cells expressing αβ T cells, γδT cells do not recognize specific antigens176. Only a few mouse studies on γδT cells in atherosclerosis have been published177–179 since the presence of γδT cells in human atherosclerotic lesions was reported in 1993180. γδT cells are among the T cell subsets described in mouse atherosclerotic lesions179 (Fig. 2c). Interestingly, the intracellular cholesterol content in γδT cells regulates their activation, proliferation and effector functions177. In addition, γδT cells are an abundant source of IL-17 in mice88, and could modulate atherosclerosis via IL-17 production. However, Apoe–/– mice with genetic deficiency of γδT cells had similar early atherosclerosis development as Apoe–/– mice with γδT cells178. To date, the exact role of this T cell subset in atherosclerosis is unclear.
T cell homing to atherosclerotic lesions
Upon activation, inflammatory cells including T cells increase the expression of various chemokine receptors. Many of their corresponding chemokines are overexpressed in atherosclerotic lesions181, suggesting that chemokine–chemokine receptor interactions are important for the recruitment of T cells to atherosclerotic lesions. Naive CD4+ T cells are recruited to atherosclerotic lesions via L-selectin182 or via the P-selectin glycoprotein ligand 1 (PSGL1)183. T cell recruitment to the plaque can occur via C-C motif chemokine receptors (CCRs) and their ligands (CCLs), and C-X-C motif chemokine receptors (CXCRs) and their ligands (CXCLs). The main chemokine–chemokine receptor pairs that have been studied in the context of atherosclerosis are CCL5 binding to CCR1 or CCR5, CXCL10 binding to CXCR3, CXCL16 binding to CXCR6, and CCL19 and 21 binding to CCR7184. CCR2 has been reported to be involved in Treg cell recruitment185, but this role has not been shown in atherosclerosis.
CCR1/CCR5–CCL5 axis
CCR1 and CCR5 are expressed on various cells including T cells in atherosclerotic plaques48,186,187. CCR1 deficiency enlarged atherosclerotic plaque size with increased T cell content in Apoe–/– mice186, increased plaque inflammation and IFNγ production from spleen T cells187. Consistently, transplantation of Ccr1–/– bone-marrow cells into Ldlr–/– mice increased atherosclerotic plaque size with increased T cell infiltration into the lesions compared with transplantation of Ccr1+/+ bone-marrow cells187. These data suggest that CCR1 has an atheroprotective role.
By contrast, CCR5 has a pro-atherogenic role. In Apoe–/– mice, CCR5 deficiency in Apoe–/– mice reduced diet-induced atherosclerosis and induced a more stable plaque phenotype with reduced T cell infiltration186. CCL5 blocking in Ldlr–/– mice reduced the progression of established atherosclerosis, with decreased CD4+ T cell infiltration into the lesions188. Interestingly, CCR5 is involved in the homing of TH1-like exTreg cells to atherosclerotic lesions in mice48. CCR5 is involved in promoting unstable plaque formation in Ldlr–/– mice partially via increasing TNF production from T cells189. However, CCR5 deficiency does not have an effect on early atherosclerosis in Apoe–/– mice190, and the role of CCR5 might be T cell-independent in advanced atherosclerosis191.
In addition, the structural similarity of chemokines allows them to form unique heterodimers to shape the overall signalling response of their receptors. Heterodimers of CCL5 with CXCL4, CCL2 or CCL17 act synergistically to increase T cell and monocyte arrest on human aortic endothelial cells in vitro192. Peptide inhibitors against CCL5–CXCL4 reduce atherosclerosis in mice193. Dendritic cell-derived CCL17, which is present in advanced human and mouse atherosclerosis44, limits Treg cell expansion and homing to atherosclerotic lesions in Apoe–/– mice44. In these mice, CCL17 deficiency reduced atherosclerosis, and a CCL17 blocking antibody expanded Treg cell numbers and reduced atherosclerosis progression44.
CXCR3–CXCL10 axis
TH1 cells and CD8+ T cells in mouse and human atherosclerotic plaques express high levels of CXCR3194,195, which accompanies TH1 cell differentiation196. A single-cell gene expression analysis of human samples showed that some clusters of blood CD4+ T cells or CD8+ T cells express higher CXCR3 levels in patients with symptomatic atherosclerosis than in patients with asymptomatic atherosclerosis, suggesting that these circulating cells are activated and might be prone to migrating to the atherosclerotic site22. Administration of a CXCR3 antagonist significantly inhibited atherosclerotic lesion formation in Ldlr–/– mice197, in which the draining lymph nodes of the aorta were significantly smaller, contained fewer activated T cells and more Treg cells than those from vehicle-treated mice197. Moreover, administration of an antagonist of both CCR5 and CXCR3 significantly reduced atherosclerosis in Ldlr–/– mice, with significant reduction of T cell accumulation and IFNγ expression in plaques compare with control mice198. Consistently, Cxcr3–/–Apoe–/– mice had smaller atherosclerotic lesions than Cxcr3+/+Apoe–/– mice, which correlated with a decrease in the total number of T cells, an increased number of Treg cells and an upregulation of the production of anti-inflammatory molecules including IL-10 within atherosclerotic lesions194.
Plasma CXCL10 levels are elevated in patients with coronary atherosclerosis compared with individuals without coronary atherosclerosis199. In Apoe–/– mice, Cxcl10 deletion significantly decreased atherosclerotic plaque size, with an increased number and activity of Treg cells despite a decreased overall accumulation of CD4+ T cells in the lesions200. Moreover, CXCL10 neutralization in mice prevented IFNγ-mediated T cell responses, resulting in restoration of endothelial healing201.
CXCR6–CXCL16 axis
CXCR6 is expressed on several NKT cells and other T cell subsets, and regulates TH1 cell homing202. Cxcr6–/–Apoe–/– mice show reduced atherosclerotic plaque formation and decreased IFNγ expression in the aorta203. CXCR6 regulates the recruitment of atherogenic IL-17A-producing cells (TH17 cells and γδT cells) into aortic atherosclerotic lesions in Apoe–/– mice204. Endothelial CXCL16 expression is probably involved in the recruitment of CXCR6+ cells to the arterial wall. CXCL16 is upregulated in experimental atherosclerosis205 and in patients with acute coronary syndrome compared with healthy controls206. However, Cxcl16 deletion in Ldlr–/– mice increased atherosclerosis without changes in T cell recruitment but with increased macrophage recruitment to the plaques compared with Cxcl16+/+Ldlr–/– mice207. CXCL16 is also a scavenger receptor208, and this function might be more important in atherosclerosis than its chemokine function.
CCR7
CCR7 regulates T cell, B cell and mature dendritic cell homing to lymph nodes and Peyer’s patches. CCR7 is expressed on naive T cells and central memory T cells209. Both CCR7 and its ligands, CCL19 and CCL21, have been detected in mouse and human atherosclerotic lesions210. CyTOF of immune cells from human atherosclerotic plaques and blood showed CCR7-expressing central memory CD4+ T cells22. In addition, some clusters of CD8+ effector memory T cells are enriched in atherosclerotic plaques compared with blood22. These observations suggest a possible pathological role of CCR7 in atherosclerosis. However, the role of CCR7 in atherosclerosis is still controversial because the findings from different experimental studies are contradictory211–213. One limitation of these studies is that they used mice with global knockout of Ccr7, and CCR7 is also expressed on activated dendritic cells and B cells. A second limitation is that the antigen specificity of the identified CCR7-expressing T cells is unknown.
Potential T cell-related therapies
Currently no clinically applicable therapies for atherosclerosis related to T cells are available; however, some therapeutic candidates are emerging. Current data point to IL-1β, IL-17 and TNF as effective targets to reduce cardiovascular disease progression, at least in some settings214,215. Whether the autoimmune component of atherosclerosis can be addressed by anti-inflammatory therapies targeting cytokines and inflammatory pathways is currently unknown; however, vaccination and immunomodulation might provide a future antigen-specific therapy that is expected to avoid weakening the host defence8. Tolerogenic vaccines against atherosclerosis are focused on inducing the generation of atheroprotective Treg cells against various atherosclerosis antigens. Other vaccines induce neutralizing antibodies against proprotein convertase subtilisin/kexin type 9 (PCSK9) or cholesterol ester transfer protein (CETP)8. The concept of Treg cell-inducing vaccines for atherosclerosis is based on the idea that induction of antigen-specific Treg cells would suppress atherosclerosis by suppressing effector T cell expansion, especially of TH1 cells. Treg cells would also reduce inflammatory cytokine secretion. Preventative vaccination with ApoB-peptide, LDL or HSP has been shown to reduce atherosclerotic lesions in experimental models4,11,216. Currently no evidence indicates whether any of these vaccines can be therapeutic, that is, whether vaccination would work after the onset of atherosclerosis. One concern would be that the induced Treg cells could switch their phenotype towards a pathogenic phenotype, as detailed above. A different strategy to reduce atherosclerosis by Treg cell expansion is used in clinical trials on low-dose IL-2 therapy217. The CCR5 antagonist maraviroc is currently used as a treatment in patients with HIV infection, given that some HIV strains use CCR5 to enter CD4+ T cells. Treatment of atherosclerosis-prone mice with this CCR5 antagonist reduced atherosclerotic lesions218. People living with HIV infection have an increased incidence of cardiovascular disease. One study showed that in people with HIV infection, treatment with the CCR5 antagonist reduced atherosclerotic progression219.
Conclusion
Emerging evidence indicates that atherosclerosis is a chronic inflammatory disease with an autoimmune component. LDL and ApoB peptides seem to be the most relevant self-antigens that can both drive an autoimmune response in the atherosclerotic plaque and be atheroprotective in a vaccination setting. TH cells have been well studied in the context of atherosclerosis, and are considered to have important roles in atherosclerosis. The pro-atherosclerotic role of TH1 cells and the anti-atherogenic role of Treg cells have been clearly shown in mice. The role of the other TH subtypes and CD8+ T cells is less clear and discrepant between different studies (Box 1). Advances in single-cell analysis technologies such as scRNAseq and CyTOF (Box 2) are providing much deeper insights into T cell subsets in atherosclerotic lesions, lymph nodes and blood (Fig. 3). Techniques for sampling local molecules released from coronary plaques220,221 or debris from ruptured plaques222 are available (Fig.3a). However, all these approaches do not (yet) address the problem of identifying antigen specificity of the T cells. Newly identified MHC class II-restricted epitopes in ApoB have been used to build tetramers to detect antigen-specific T cells in atherosclerosis development in humans and mice4 (Fig.3b). Prophylactic tolerogenic vaccines that are based on ApoB and other atherosclerosis antigens are effective in animal models, but whether and how these approaches can safely be translated to the clinical setting is currently unknown.
Fig. 3 |. Tools for sampling and analysis of T cells in atherosclerosis.
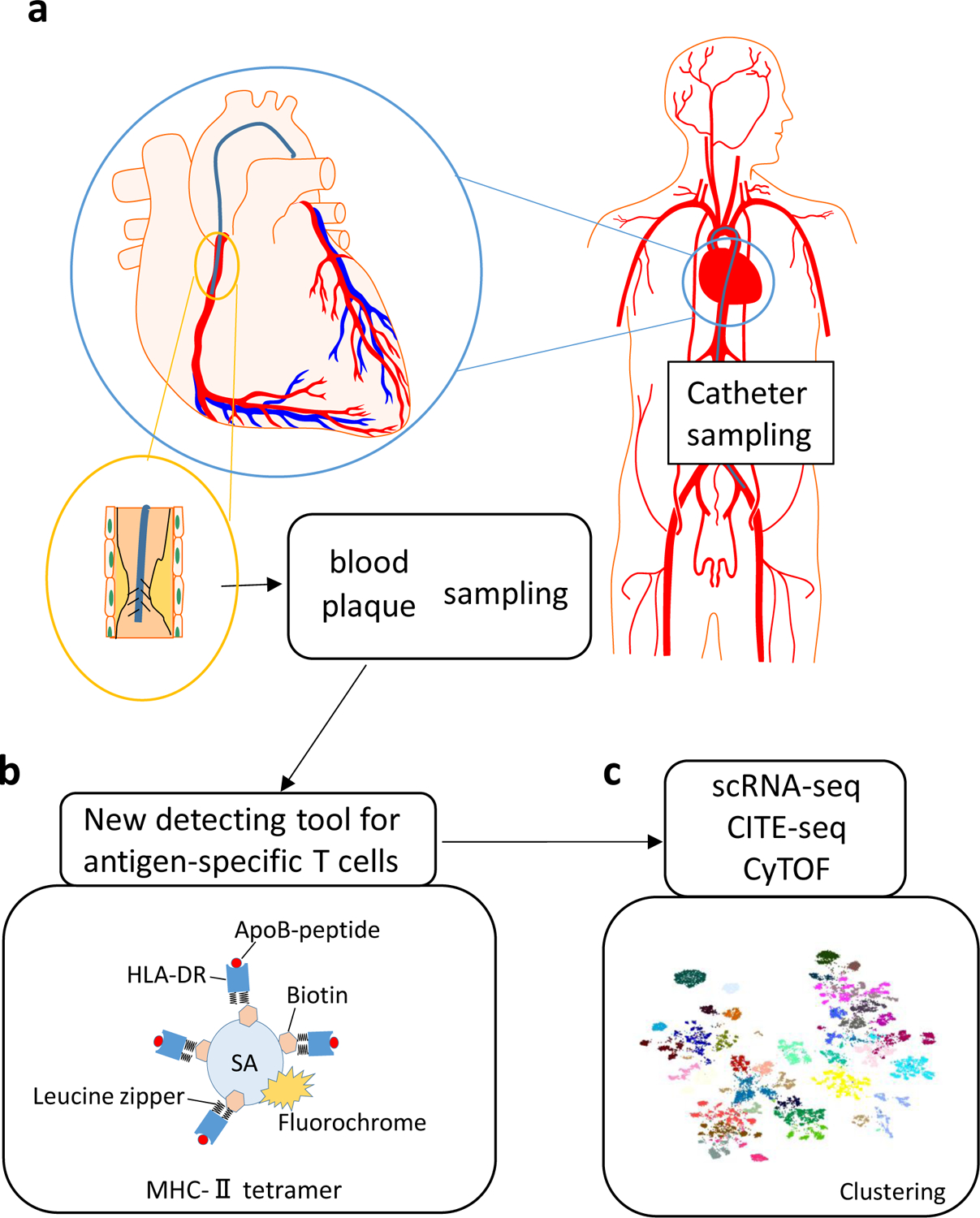
a | Advanced percutaneous catheter devices can be used to sample the boundary layer of blood close to the vessel wall upstream and downstream of intact and disrupted human coronary atherosclerotic plaques or to sample plaque debris generated by plaque disruption through stent placement. A distal protection device can be positioned in the coronary artery downstream from the atherosclerotic plaque. b | Tetramer technology to identify apolipoprotein B (ApoB)-specific T cells. The tetramer reagent consist of four specific recombinant major histocompatibility complex (MHC) class II molecules tetramerized with streptavidin (SA) and loaded with ApoB-peptide for detecting ApoB-specific T cell receptors. c | Single-cell RNA sequencing (scRNA-seq), cellular indexing of transcriptomes and epitopes by sequencing (CITE-seq) and mass cytometry (CyTOF) provide deep transcriptome data, cell surface phenotypes and T cell receptor sequencing data for ApoB-specific T cells in atherosclerosis.
Key points.
Atherosclerosis is a chronic inflammatory disease, and accumulating evidence supports the critical role of T cells as drivers and modifiers in of this condition.
T cells drive immune responses to peptide epitopes related to atherosclerosis, such as peptides derived from apolipoprotein B.
CD4+ T helper 1 (TH1) cells and natural killer T cells have pro-atherogenic roles, whereas regulatory T (Treg) cells have anti-atherogenic functions.
The role of other TH cell subsets, such as TH2, TH9, TH17, TH22 and follicular helper T (TFH) cells, and of CD8+ T cells and γδT cells remains controversial.
During atherosclerosis progression, Treg cells can convert into pro-inflammatory T cell subsets, such as TH1, TH17 or TFH cells.
T cell recruitment to the atherosclerotic plaque occurs via chemokines and chemokine receptors such as CCR5, CXCR3 and CXCR6.
Acknowledgements
This work was funded by The National Institutes of Health, HL136275, 140976, 145241, 146134, 148094 to KL, a postdoctoral fellowship from Japan Society for the Promotion of Science to RS, and a postdoctoral fellowship from the German goverment (DFG) to HW.
Glossary terms
- Major histocompatibility complex
Family of molecules that present antigen peptides to T cells. MHC class I molecules present peptides to CD8+ T cells and MHC class II molecules to CD4+ T cells. In humans, MHC molecules are very diverse (thousands of known alleles), but some alleles are more common (~10–20%) in some populations.
- T cell receptor
The TCR is a heterodimer that consists of either αβ or γδ chains, and expression of each heterodimer is mutually exclusive on the same cell. TCR signalling proceeds through transmembrane and intracellular CD3 subunits associated with the TCR. TCRs are highly polymorphic through recombination of V and J (and in some cases D) segments and through template-free nucleotide addition.
- Co-stimulatory molecules
Co-stimulatory molecules are cell surface receptors that are ligated when the TCR engages the MHC-peptide complex. The nature of the co-stimulatory molecules determined the outcome of antigen presentation248.
- TCR sequencing
Technique that uses specific primers to identify the V, D and J segments used in each α, β, γ and δ subunit. The assembly of short reads and specialized reconstruction software such as TRACER249 allow the reconstruction of the entire TCR sequence, often of paired α and β or γ and δ chains.
- MHC tetramers
Reagents used to analyse antigen-specific T cells; constructed from four recombinant truncated MHC class I or MHC class II molecules with the antigenic peptide naturally (non-covalently) or covalently bound and tetramerized by streptavidin; the streptavidin can be labelled by fluorochromes for detection with flow cytometry (Fig. 3b).
- CD4+ effector memory T cells
A type of antigen-experienced CD4 T cells that has effector functions but also forms memory (recall response after restimulation).
- T cell exhaustion
Progressive loss of T-cell functions under chronic antigen stimulation, for example in cancer or persistent virus infetions. Exhaustion can result in the deletion of the responding cells.
- Innate lymphoid cells
Cells of the innate immune system with similarity to T helper cells in the expression of key transcription factors and effector molecules, but that lack antigen-specific receptors characteristic of the adaptive immune system
- Necrotic core
The necrotic core of atherosclerotic lesions is composed of cholesterol crystals, debris of apoptotic cells such as smooth muscle cells, foam cells and macrophages, and calcium particles. Large necrotic cores are associated with vulnerable plaques.
- Central memory T cells
A type of antigen-experienced CD4 T cells that has no effector functions but forms memory and is able to recirculate to secondary lymphoid organs.
Footnotes
Competing interests
K. L. is a founder of Atherovax. The other authors declare no competing interests.
References:
- 1.Kobiyama K & Ley K Atherosclerosis. Circ. Res 123, 1118–1120 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He S et al. Gut intraepithelial T cells calibrate metabolism and accelerate cardiovascular disease. Nature 566, 115–119 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf D & Ley K Immunity and Inflammation in Atherosclerosis. Circ. Res 124, 315–327 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimura T et al. Regulatory CD4+ T Cells Recognize Major Histocompatibility Complex Class II Molecule–Restricted Peptide Epitopes of Apolipoprotein B. Circulation 138, 1130–1143 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinman RM Decisions About Dendritic Cells: Past, Present, and Future. Annu. Rev. Immunol 30, 1–22 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Koltsova EK et al. Dynamic T cell–APC interactions sustain chronic inflammation in atherosclerosis. J. Clin. Invest 122, 3114–3126 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stemme S et al. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc. Natl. Acad. Sci 92, 3893–3897 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobiyama K, Saigusa R & Ley K Vaccination against atherosclerosis. Curr. Opin. Immunol 59, 15–24 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah PK, Chyu K-Y, Dimayuga PC & Nilsson J Vaccine for Atherosclerosis. J. Am. Coll. Cardiol 64, 2779–2791 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Chyu K-Y & Shah PK In Pursuit of an Atherosclerosis Vaccine: Chasing the Holy Grail. Circ. Res 123, 1121–1123 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Tse K et al. Atheroprotective Vaccination with MHC-II Restricted Peptides from ApoB-100. Front. Immunol 4, 493 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin Z et al. Deep sequencing of the T cell receptor β repertoire reveals signature patterns and clonal drift in atherosclerotic plaques and patients. Oncotarget 8, 99312–99322 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paulsson G, Zhou X, Törnquist E & Hansson GK Oligoclonal T cell expansions in atherosclerotic lesions of apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol 20, 10–17 (2000). [DOI] [PubMed] [Google Scholar]
- 14.Colantonio LD et al. Association of Serum Lipids and Coronary Heart Disease in Contemporary Observational Studies. Circulation 133, 256–264 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pothineni NVK et al. Infections, atherosclerosis, and coronary heart disease. Eur. Heart J 38, 3195–3201 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Hermansson A et al. Inhibition of T cell response to native low-density lipoprotein reduces atherosclerosis. J. Exp. Med 207, 1081–1093 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winkels H et al. Atlas of the Immune Cell Repertoire in Mouse Atherosclerosis Defined by Single-Cell RNA-Sequencing and Mass Cytometry. Circ. Res 122, 1675–1688 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansson GK et al. Localization of T lymphocytes and macrophages in fibrous and complicated human atherosclerotic plaques. Atherosclerosis 72, 135–141 (1988). [DOI] [PubMed] [Google Scholar]
- 19.Jonasson L, Holm J, Skalli O, Bondjers G & Hansson GK Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis 6, 131–138 (1986). [DOI] [PubMed] [Google Scholar]
- 20.Cochain C et al. Single-Cell RNA-Seq Reveals the Transcriptional Landscape and Heterogeneity of Aortic Macrophages in Murine Atherosclerosis. Circ. Res 122, 1661–1674 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Cole JE et al. Immune cell census in murine atherosclerosis: cytometry by time of flight illuminates vascular myeloid cell diversity. Cardiovasc. Res 114, 1360–1371 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandez DM et al. Single-cell immune landscape of human atherosclerotic plaques. Nat. Med 25, 1576–1588 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winkels H, Ehinger E, Goshesh Y, Wolf D & Ley K Atherosclerosis in the single-cell era: Curr. Opin. Lipidol 29, 389–396 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gräbner R et al. Lymphotoxin β receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE−/− mice. J. Exp. Med 206, 233–248 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu W et al. Adventitial Cell Atlas of wt (Wild Type) and ApoE (Apolipoprotein E)-Deficient Mice Defined by Single-Cell RNA Sequencing. Arterioscler. Thromb. Vasc. Biol 39, 1055–1071 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu J & Paul WE CD4 T cells: fates, functions, and faults. Blood 112, 1557–1569 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emeson EE, Shen M-L & Bell CGH Inhibition of Atherosclerosis in CD4 T-Cell-Ablated and Nude (nu/fu) C57BL/6 Hyperlipidemic Mice. Am. J. Pathol 149, 675–685 (1996). [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou X, Robertson A-KL, Rudling M, Parini P & Hansson GK Lesion Development and Response to Immunization Reveal a Complex Role for CD4 in Atherosclerosis. Circ. Res 96, 427–434 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Ait-Oufella H et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat. Med 12, 178–180 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Koltsova EK & Ley K How dendritic cells shape atherosclerosis. Trends Immunol 32, 540–547 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zernecke A Dendritic Cells in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol 35, 763–770 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Clément M et al. Deletion of IRF8 (Interferon Regulatory Factor 8)-Dependent Dendritic Cells Abrogates Proatherogenic Adaptive Immunity. Circ. Res 122, 813–820 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Lutgens E, Lievens D, Beckers L, Donners M & Daemen M CD40 and its ligand in atherosclerosis. Trends Cardiovasc. Med 17, 118–123 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Lameijer M et al. Efficacy and safety assessment of a TRAF6-targeted nanoimmunotherapy in atherosclerotic mice and non-human primates. Nat. Biomed. Eng 2, 279–292 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolf D et al. Binding of CD40L to Mac-1’s I-Domain Involves the EQLKKSKTL Motif and Mediates Leukocyte Recruitment and Atherosclerosis—But Does Not Affect Immunity and Thrombosis in Mice. Circ. Res 109, 1269–1279 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buono C et al. B7–1/B7–2 Costimulation Regulates Plaque Antigen–Specific T-Cell Responses and Atherogenesis in Low-Density Lipoprotein Receptor–Deficient Mice. Circulation 109, 2009–2015 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Matsumoto T et al. Overexpression of Cytotoxic T-Lymphocyte–Associated Antigen-4 Prevents Atherosclerosis in Mice. Arterioscler. Thromb. Vasc. Biol 36, 1141–1151 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Wherry EJ & Kurachi M Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol 15, 486–499 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gotsman I et al. Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J. Clin. Invest 117, 2974–2982 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foks AC et al. Interruption of the OX40–OX40 Ligand Pathway in LDL Receptor–Deficient Mice Causes Regression of Atherosclerosis. J. Immunol 191, 4573–4580 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Jeon HJ et al. CD137 (4–1BB) Deficiency Reduces Atherosclerosis in Hyperlipidemic Mice. Circulation 121, 1124–1133 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Lievens D et al. Abrogated transforming growth factor beta receptor II (TGFβRII) signalling in dendritic cells promotes immune reactivity of T cells resulting in enhanced atherosclerosis. Eur. Heart J 34, 3717–3727 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Subramanian M, Thorp E, Hansson GK & Tabas I Treg-mediated suppression of atherosclerosis requires MYD88 signaling in DCs. J. Clin. Invest 123, 179–188 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weber C et al. CCL17-expressing dendritic cells drive atherosclerosis by restraining regulatory T cell homeostasis in mice. J. Clin. Invest 121, 2898–2910 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niessner A et al. Pathogen-Sensing Plasmacytoid Dendritic Cells Stimulate Cytotoxic T-Cell Function in the Atherosclerotic Plaque Through Interferon-α. Circulation 114, 2482–2489 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Buono C et al. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc. Natl. Acad. Sci 102, 1596–1601 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frostegård J et al. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis 145, 33–43 (1999). [DOI] [PubMed] [Google Scholar]
- 48.Li J et al. CCR5+T-bet+FoxP3+ Effector CD4 T Cells Drive Atherosclerosis. Circ. Res 118, 1540–1552 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Butcher MJ et al. Atherosclerosis-Driven Treg Plasticity Results in Formation of a Dysfunctional Subset of Plastic IFNγ+ Th1/Tregs. Circ. Res 119, 1190–1203 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buono C et al. Influence of Interferon-γ on the Extent and Phenotype of Diet-Induced Atherosclerosis in the LDLR-Deficient Mouse. Arterioscler. Thromb. Vasc. Biol 23, 454–460 (2003). [DOI] [PubMed] [Google Scholar]
- 51.Gupta S et al. IFN-γ Potentiates Atherosclerosis in ApoE Knock-out Mice. J. Clin. Invest 99, 2752–2761 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whitman SC, Ravisankar P, Elam H & Daugherty A Exogenous Interferon-γ Enhances Atherosclerosis in Apolipoprotein E−/− Mice. Am. J. Pathol 157, 6 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amento EP, Ehsani N, Palmer H & Libby P Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscler. Thromb 11, 1223–1230 (1991). [DOI] [PubMed] [Google Scholar]
- 54.Orecchioni M, Ghosheh Y, Pramod AB & Ley K Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS–) vs. Alternatively Activated Macrophages. Front. Immunol 10, 1084 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rocha VZ et al. Interferon-γ, a Th1 Cytokine, Regulates Fat Inflammation: A Role for Adaptive Immunity in Obesity. Circ. Res 103, 467–476 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tellides G et al. Interferon-γ elicits arteriosclerosis in the absence of leukocytes. Nature 403, 207–211 (2000). [DOI] [PubMed] [Google Scholar]
- 57.Wang Y et al. Interferon-γ Induces Human Vascular Smooth Muscle Cell Proliferation and Intimal Expansion by Phosphatidylinositol 3-Kinase–Dependent Mammalian Target of Rapamycin Raptor Complex 1 Activation. Circ. Res 101, 560–569 (2007). [DOI] [PubMed] [Google Scholar]
- 58.Niwa T et al. Interferon-γ Produced by Bone Marrow-derived Cells Attenuates Atherosclerotic Lesion Formation in LDLR-deficient Mice. J. Atheroscler. Thromb 11, 79–87 (2004). [DOI] [PubMed] [Google Scholar]
- 59.Vergnes L, Phan J, Strauss M, Tafuri S & Reue K Cholesterol and cholate components of an atherogenic diet induce distinct stages of hepatic inflammatory gene expression. J. Biol. Chem 278, 42774–42784 (2003). [DOI] [PubMed] [Google Scholar]
- 60.Tracy RP et al. T‐Helper Type 1 Bias in Healthy People Is Associated With Cytomegalovirus Serology and Atherosclerosis: The Multi‐Ethnic Study of Atherosclerosis. J. Am. Heart Assoc 2, e000117 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Engelbertsen D et al. T-Helper 2 Immunity Is Associated With Reduced Risk of Myocardial Infarction and Stroke. Arterioscler. Thromb. Vasc. Biol 33, 637–644 (2013). [DOI] [PubMed] [Google Scholar]
- 62.Davenport P & Tipping PG The Role of Interleukin-4 and Interleukin-12 in the Progression of Atherosclerosis in Apolipoprotein E-Deficient Mice. Am. J. Pathol 163, 1117–1125 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.King VL, Szilvassy SJ & Daugherty A Interleukin-4 Deficiency Decreases Atherosclerotic Lesion Formation in a Site-Specific Manner in Female LDL receptor−/− mice. Arterioscler. Thromb. Vasc. Biol 22, 456–461 (2002). [DOI] [PubMed] [Google Scholar]
- 64.King VL, Cassis LA & Daugherty A Interleukin-4 Does Not Influence Development of Hypercholesterolemia or Angiotensin II-Induced Atherosclerotic Lesions in Mice. Am. J. Pathol 171, 2040–2047 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Engelbertsen D et al. Induction of T helper 2 responses against human apolipoprotein B100 does not affect atherosclerosis in ApoE−/− mice. Cardiovasc. Res 103, 304–312 (2014). [DOI] [PubMed] [Google Scholar]
- 66.Sämpi M et al. Plasma Interleukin-5 Levels Are Related to Antibodies Binding to Oxidized Low-Density Lipoprotein and to Decreased Subclinical Atherosclerosis. J. Am. Coll. Cardiol 52, 1370–1378 (2008). [DOI] [PubMed] [Google Scholar]
- 67.Silveira A et al. Plasma IL-5 concentration and subclinical carotid atherosclerosis. Atherosclerosis 239, 125–130 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Avramakis G et al. Platelets and white blood cell subpopulations among patients with myocardial infarction and unstable angina. Platelets 18, 16–23 (2007). [DOI] [PubMed] [Google Scholar]
- 69.Binder CJ et al. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J. Clin. Invest 114, 427–437 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cardilo-Reis L et al. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol. Med 4, 1072–1086 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miller AM et al. IL-33 reduces the development of atherosclerosis. J. Exp. Med 205, 339–346 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martin P et al. Atherosclerosis severity is not affected by a deficiency in IL-33/ST2 signaling: Deficiency in IL-33 signaling does not affect atherosclerosis severity. Immun. Inflamm. Dis 3, 239–246 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walker JA, Barlow JL & McKenzie ANJ Innate lymphoid cells — how did we miss them? Nat. Rev. Immunol 13, 75–87 (2013). [DOI] [PubMed] [Google Scholar]
- 74.Newland SA et al. Type-2 innate lymphoid cells control the development of atherosclerosis in mice. Nat. Commun 8, 15781 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaplan MH Th9 cells: differentiation and disease. Immunol. Rev 252, 104–115 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gregersen I et al. Increased Systemic and Local Interleukin 9 Levels in Patients with Carotid and Coronary Atherosclerosis. PLoS ONE 8, e72769 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin Y et al. Circulating Th22 and Th9 Levels in Patients with Acute Coronary Syndrome. Mediators Inflamm 2013, 1–10 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang W et al. IL-9 aggravates the development of atherosclerosis in ApoE−/− mice. Cardiovasc. Res 106, 453–464 (2015). [DOI] [PubMed] [Google Scholar]
- 79.McGeachy MJ, Cua DJ & Gaffen SL The IL-17 Family of Cytokines in Health and Disease. Immunity 50, 892–906 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hirota K et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat. Immunol 12, 255–263 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marks BR et al. Thymic self-reactivity selects natural interleukin 17–producing T cells that can regulate peripheral inflammation. Nat. Immunol 10, 1125–1132 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou L et al. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol 8, 967–974 (2007). [DOI] [PubMed] [Google Scholar]
- 83.Zielinski CE et al. Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature 484, 514–518 (2012). [DOI] [PubMed] [Google Scholar]
- 84.Taleb S, Tedgui A & Mallat Z IL-17 and Th17 Cells in Atherosclerosis: Subtle and Contextual Roles. Arterioscler. Thromb. Vasc. Biol 35, 258–264 (2015). [DOI] [PubMed] [Google Scholar]
- 85.McGeachy MJ et al. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell–mediated pathology. Nat. Immunol 8, 1390–1397 (2007). [DOI] [PubMed] [Google Scholar]
- 86.Lee Y et al. Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol 13, 991–999 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pinderski Oslund LJ et al. Interleukin-10 Blocks Atherosclerotic Events In Vitro and In Vivo. Arterioscler. Thromb. Vasc. Biol 19, 2847–2853 (1999). [DOI] [PubMed] [Google Scholar]
- 88.Stark MA et al. Phagocytosis of Apoptotic Neutrophils Regulates Granulopoiesis via IL-23 and IL-17. Immunity 22, 285–294 (2005). [DOI] [PubMed] [Google Scholar]
- 89.Usui F et al. Interleukin-17 deficiency reduced vascular inflammation and development of atherosclerosis in Western diet-induced apoE-deficient mice. Biochem. Biophys. Res. Commun 420, 72–77 (2012). [DOI] [PubMed] [Google Scholar]
- 90.Smith E et al. Blockade of Interleukin-17A Results in Reduced Atherosclerosis in Apolipoprotein E–Deficient Mice. Circulation 121, 1746–1755 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Erbel C et al. Inhibition of IL-17A Attenuates Atherosclerotic Lesion Development in ApoE-Deficient Mice. J. Immunol 183, 8167–8175 (2009). [DOI] [PubMed] [Google Scholar]
- 92.Danzaki K et al. Interleukin-17A Deficiency Accelerates Unstable Atherosclerotic Plaque Formation in Apolipoprotein E-Deficient Mice. Arterioscler. Thromb. Vasc. Biol 32, 273–280 (2012). [DOI] [PubMed] [Google Scholar]
- 93.Madhur MS et al. Role of Interleukin 17 in Inflammation, Atherosclerosis, and Vascular Function in Apolipoprotein E–Deficient Mice. Arterioscler. Thromb. Vasc. Biol 31, 1565–1572 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Butcher MJ, Gjurich BN, Phillips T & Galkina EV The IL-17A/IL-17RA Axis Plays a Proatherogenic Role via the Regulation of Aortic Myeloid Cell Recruitment. Circ. Res 110, 675–687 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Taleb S et al. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J. Exp. Med 206, 2067–2077 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brauner S et al. Augmented Th17 differentiation in Trim21 deficiency promotes a stable phenotype of atherosclerotic plaques with high collagen content. Cardiovasc. Res 114, 158–167 (2018). [DOI] [PubMed] [Google Scholar]
- 97.Gisterå A et al. Transforming Growth Factor–β Signaling in T Cells Promotes Stabilization of Atherosclerotic Plaques Through an Interleukin-17–Dependent Pathway. Sci. Transl. Med 5, 196ra100 (2013). [DOI] [PubMed] [Google Scholar]
- 98.Hashmi S & Zeng QT Role of interleukin-17 and interleukin-17-induced cytokines interleukin-6 and interleukin-8 in unstable coronary artery disease: Coron. Artery Dis 17, 699–706 (2006). [DOI] [PubMed] [Google Scholar]
- 99.Cheng X et al. The Th17/Treg imbalance in patients with acute coronary syndrome. Clin. Immunol 127, 89–97 (2008). [DOI] [PubMed] [Google Scholar]
- 100.Eid RE et al. Interleukin-17 and Interferon-γ Are Produced Concomitantly by Human Coronary Artery–Infiltrating T Cells and Act Synergistically on Vascular Smooth Muscle Cells. Circulation 119, 1424–1432 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Simon T et al. Circulating levels of interleukin-17 and cardiovascular outcomes in patients with acute myocardial infarction. Eur. Heart J 34, 570–577 (2013). [DOI] [PubMed] [Google Scholar]
- 102.Azizi G, Yazdani R & Mirshafiey A Th22 cells in autoimmunity: a review of current knowledge. Eur. Ann. Allergy Clin. Immunol 47, 108–117 (2015). [PubMed] [Google Scholar]
- 103.Zhang L et al. Elevated Frequencies of Circulating Th22 Cell in Addition to Th17 Cell and Th17/Th1 Cell in Patients with Acute Coronary Syndrome. PLoS ONE 8, e71466 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xia Q et al. Characterisation of IL-22 and interferon-gamma-inducible chemokines in human carotid plaque. Int. J. Cardiol 154, 187–189 (2012). [DOI] [PubMed] [Google Scholar]
- 105.Rattik S et al. IL-22 affects smooth muscle cell phenotype and plaque formation in apolipoprotein E knockout mice. Atherosclerosis 242, 506–514 (2015). [DOI] [PubMed] [Google Scholar]
- 106.Fatkhullina AR et al. An Interleukin-23-Interleukin-22 Axis Regulates Intestinal Microbial Homeostasis to Protect from Diet-Induced Atherosclerosis. Immunity 49, 943–957.e9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Crotty S T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 50, 1132–1148 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ryu H et al. Atherogenic dyslipidemia promotes autoimmune follicular helper T cell responses via IL-27. Nat. Immunol 19, 583–593 (2018). [DOI] [PubMed] [Google Scholar]
- 109.Nus M et al. Marginal zone B cells control the response of follicular helper T cells to a high-cholesterol diet. Nat. Med 23, 601–610 (2017). [DOI] [PubMed] [Google Scholar]
- 110.Clement M et al. Control of the T Follicular Helper–Germinal Center B-Cell Axis by CD8+ Regulatory T Cells Limits Atherosclerosis and Tertiary Lymphoid Organ Development. Circulation 131, 560–570 (2015). [DOI] [PubMed] [Google Scholar]
- 111.Gaddis DE et al. Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis. Nat. Commun 9, 1095 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Weng N, Akbar AN & Goronzy J CD28- T cells: their role in the age-associated decline of immune function. Trends Immunol 30, 306–312 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dumitriu IE et al. High Levels of Costimulatory Receptors OX40 and 4–1BB Characterize CD4+CD28null T Cells in Patients With Acute Coronary Syndrome. Circ. Res 110, 857–869 (2012). [DOI] [PubMed] [Google Scholar]
- 114.Téo FH et al. Characterization of CD4+CD28null T cells in patients with coronary artery disease and individuals with risk factors for atherosclerosis. Cell. Immunol 281, 11–19 (2013). [DOI] [PubMed] [Google Scholar]
- 115.Liuzzo G et al. Monoclonal T-Cell Proliferation and Plaque Instability in Acute Coronary Syndromes. Circulation 101, 2883–2888 (2000). [DOI] [PubMed] [Google Scholar]
- 116.Kovalcsik E, Antunes RF, Baruah P, Kaski JC & Dumitriu IE Proteasome-Mediated Reduction in Proapoptotic Molecule Bim Renders CD4+CD28null T Cells Resistant to Apoptosis in Acute Coronary Syndrome. Circulation 131, 709–720 (2015). [DOI] [PubMed] [Google Scholar]
- 117.Yadav AK, Kumar V & Jha V Heat Shock Proteins 60 and 70 Specific Proinflammatory and Cytotoxic Response of CD4 + CD28 null Cells in Chronic Kidney Disease. Mediators Inflamm 2013, 1–9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Roy P, Ali AJ, Kobiyama K, Ghosheh Y & Ley K Opportunities for an atherosclerosis vaccine: From mice to humans. Vaccine (2020) doi: 10.1016/j.vaccine.2019.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Okba MA et al. Expanded Peripheral CD4+CD28null T Cells and its Association with Atherosclerotic Changes in Patients with End Stage Renal Disease on Hemodialysis. Hum. Immunol 80, 748–754 (2019). [DOI] [PubMed] [Google Scholar]
- 120.Roncarolo M-G & Gregori S Is FOXP3 a bona fide marker for human regulatory T cells? Eur. J. Immunol 38, 925–927 (2008). [DOI] [PubMed] [Google Scholar]
- 121.Klingenberg R et al. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J. Clin. Invest 123, 1323–1334 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mor A, Luboshits G, Planer D, Keren G & George J Altered status of CD4+CD25+ regulatory T cells in patients with acute coronary syndromes. Eur. Heart J 27, 2530–2537 (2006). [DOI] [PubMed] [Google Scholar]
- 123.George J et al. Regulatory T cells and IL-10 levels are reduced in patients with vulnerable coronary plaques. Atherosclerosis 222, 519–523 (2012). [DOI] [PubMed] [Google Scholar]
- 124.Wigren M et al. Low Levels of Circulating CD4+FoxP3+ T Cells Are Associated With an Increased Risk for Development of Myocardial Infarction But Not for Stroke. Arterioscler. Thromb. Vasc. Biol 32, 2000–2004 (2012). [DOI] [PubMed] [Google Scholar]
- 125.Barth SD et al. The Ratio of Regulatory (FOXP3+) to Total (CD3+) T Cells Determined by Epigenetic Cell Counting and Cardiovascular Disease Risk: A Prospective Case-cohort Study in Non-diabetics. EBioMedicine 11, 151–156 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ammirati E et al. Circulating CD4+CD25hiCD127lo Regulatory T-Cell Levels Do Not Reflect the Extent or Severity of Carotid and Coronary Atherosclerosis. Arterioscler. Thromb. Vasc. Biol 30, 1832–1841 (2010). [DOI] [PubMed] [Google Scholar]
- 127.Björkbacka H Can Circulating Regulatory T Cells Predict Cardiovascular Disease? EBioMedicine 11, 15–16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Robertson A-KL et al. Disruption of TGF-β signaling in T cells accelerates atherosclerosis. J. Clin. Invest 112, 1342–1350 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Foks AC, Lichtman AH & Kuiper J Treating Atherosclerosis With Regulatory T Cells. Arterioscler. Thromb. Vasc. Biol 35, 280–287 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dinh TN et al. Cytokine Therapy With Interleukin-2/Anti–Interleukin-2 Monoclonal Antibody Complexes Expands CD4+CD25+Foxp3+ Regulatory T Cells and Attenuates Development and Progression of Atherosclerosis. Circulation 126, 1256–1266 (2012). [DOI] [PubMed] [Google Scholar]
- 131.Kita T et al. Regression of atherosclerosis with anti-CD3 antibody via augmenting a regulatory T-cell response in mice. Cardiovasc. Res 102, 107–117 (2014). [DOI] [PubMed] [Google Scholar]
- 132.Caligiuri G et al. Interleukin-10 Deficiency Increases Atherosclerosis, Thrombosis, and Low-density Lipoproteins in Apolipoprotein E Knockout Mice. Mol. Med 9, 10–17 (2003). [PMC free article] [PubMed] [Google Scholar]
- 133.Joly A-L et al. Alternative Splicing of FOXP3 Controls Regulatory T Cell Effector Functions and Is Associated With Human Atherosclerotic Plaque Stability. Circ. Res 122, 1385–1394 (2018). [DOI] [PubMed] [Google Scholar]
- 134.Wigren M et al. Lack of Ability to Present Antigens on Major Histocompatibility Complex Class II Molecules Aggravates Atherosclerosis in ApoE−/− Mice. Circulation 139, 2554–2566 (2019). [DOI] [PubMed] [Google Scholar]
- 135.Guasti L et al. Relationship between regulatory T cells subsets and lipid profile in dyslipidemic patients: a longitudinal study during atorvastatin treatment. BMC Cardiovasc. Disord 16, 26 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mailer RKW, Gisterå A, Polyzos KA, Ketelhuth DFJ & Hansson GK Hypercholesterolemia Induces Differentiation of Regulatory T Cells in the Liver. Circ. Res 120, 1740–1753 (2017). [DOI] [PubMed] [Google Scholar]
- 137.Maganto-García E, Tarrio ML, Grabie N, Bu D & Lichtman AH Dynamic Changes in Regulatory T Cells Are Linked to Levels of Diet-Induced Hypercholesterolemia. Circulation 124, 185–195 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cheng H-Y et al. Loss of ABCG1 influences regulatory T cell differentiation and atherosclerosis. J. Clin. Invest 126, 3236–3246 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ley K 2015 Russell Ross Memorial Lecture in Vascular Biology: Protective Autoimmunity in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol 36, 429–438 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bailey-Bucktrout SL et al. Self-antigen-Driven Activation Induces Instability of Regulatory T Cells during an Inflammatory Autoimmune Response. Immunity 39, 949–962 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Komatsu N et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat. Med 20, 9 (2014). [DOI] [PubMed] [Google Scholar]
- 142.Korn T et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat. Med 13, 423–431 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gisterå A et al. Low-Density Lipoprotein-Reactive T Cells Regulate Plasma Cholesterol Levels and Development of Atherosclerosis in Humanized Hypercholesterolemic Mice. Circulation 138, 2513–2526 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jia L et al. Methylation of FOXP3 in regulatory T cells is related to the severity of coronary artery disease. Atherosclerosis 228, 346–352 (2013). [DOI] [PubMed] [Google Scholar]
- 145.Pilato MD et al. Targeting the CBM complex causes Treg cells to prime tumors for immune checkpoint therapy. Nature 570, 112–116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rosenbaum M et al. Bcl10-controlled Malt1 paracaspase activity is key for the immune suppressive function of regulatory T cells. Nat. Commun 10, 2352 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Akamatsu M et al. Conversion of antigen-specific effector/memory T cells into Foxp3-expressing Treg cells by inhibition of CDK8/19. Sci. Immunol 4, eaaw2707 (2019). [DOI] [PubMed] [Google Scholar]
- 148.Gagliani N et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat. Med 19, 739–746 (2013). [DOI] [PubMed] [Google Scholar]
- 149.Mallat Z et al. Induction of a Regulatory T Cell Type 1 Response Reduces the Development of Atherosclerosis in Apolipoprotein E–Knockout Mice. Circulation 108, 1232–1237 (2003). [DOI] [PubMed] [Google Scholar]
- 150.Zhu Z et al. Function of T regulatory type 1 cells is down-regulated and is associated with the clinical presentation of coronary artery disease. Hum. Immunol 79, 564–570 (2018). [DOI] [PubMed] [Google Scholar]
- 151.Bergström I, Backteman K, Lundberg A, Ernerudh J & Jonasson L Persistent accumulation of interferon-γ-producing CD8+CD56+ T cells in blood from patients with coronary artery disease. Atherosclerosis 224, 515–520 (2012). [DOI] [PubMed] [Google Scholar]
- 152.Hwang Y et al. Expansion of CD8+ T cells lacking the IL-6 receptor α chain in patients with coronary artery diseases (CAD). Atherosclerosis 249, 44–51 (2016). [DOI] [PubMed] [Google Scholar]
- 153.Gewaltig J, Kummer M, Koella C, Cathomas G & Biedermann BC Requirements for CD8 T-cell migration into the human arterial wall. Hum. Pathol 39, 1756–1762 (2008). [DOI] [PubMed] [Google Scholar]
- 154.Kolbus D et al. CD8+ T cell activation predominate early immune responses to hypercholesterolemia in Apoe−/− mice. BMC Immunol 11, 58 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.van der Wal AC, Das PK, Bentz van de Berg D, van der Loos CM & Becker AE Atherosclerotic lesions in humans. In situ immunophenotypic analysis suggesting an immune mediated response. Lab. Investig. J. Tech. Methods Pathol 61, 166–170 (1989). [PubMed] [Google Scholar]
- 156.Rossmann A et al. T-cells from advanced atherosclerotic lesions recognize hHSP60 and have a restricted T-cell receptor repertoire. Exp. Gerontol 43, 229–237 (2008). [DOI] [PubMed] [Google Scholar]
- 157.Paul VSV Quantification of Various Inflammatory Cells in Advanced Atherosclerotic Plaques. J. Clin. Diagn. Res 10, EC35–8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dimayuga PC et al. Identification of apoB‐100 Peptide‐Specific CD8+ T Cells in Atherosclerosis. J. Am. Heart Assoc 6, e005318 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cochain C et al. CD8+ T Cells Regulate Monopoiesis and Circulating Ly6Chigh Monocyte Levels in Atherosclerosis in Mice. Circ. Res 117, 244–253 (2015). [DOI] [PubMed] [Google Scholar]
- 160.Kyaw T et al. Cytotoxic and Proinflammatory CD8+ T Lymphocytes Promote Development of Vulnerable Atherosclerotic Plaques in ApoE-Deficient Mice. Circulation 127, 1028–1039 (2013). [DOI] [PubMed] [Google Scholar]
- 161.Seijkens TTP et al. Deficiency of the T cell regulator Casitas B-cell lymphoma-B aggravates atherosclerosis by inducing CD8+ T cell-mediated macrophage death. Eur. Heart J 40, 372–382 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chyu K-Y et al. CD8+ T Cells Mediate the Athero-Protective Effect of Immunization with an ApoB-100 Peptide. PLoS ONE 7, e30780 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Honjo T et al. ApoB-100–Related Peptide Vaccine Protects Against Angiotensin II–Induced Aortic Aneurysm Formation and Rupture. J. Am. Coll. Cardiol 65, 546–556 (2015). [DOI] [PubMed] [Google Scholar]
- 164.van Duijn J et al. CD8+ T-cells contribute to lesion stabilization in advanced atherosclerosis by limiting macrophage content and CD4+ T-cell responses. Cardiovasc. Res 115, 729–738 (2019). [DOI] [PubMed] [Google Scholar]
- 165.Getz GS & Reardon CA Natural killer T cells in atherosclerosis. Nat. Rev. Cardiol 14, 304–314 (2017). [DOI] [PubMed] [Google Scholar]
- 166.Bennstein SB Unraveling Natural Killer T-Cells Development. Front. Immunol 8, 1950 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bondarenko S, Catapano AL & Norata GD The CD1d-natural killer T cell axis in atherosclerosis. J. Innate Immun 6, 3–12 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nakai Y et al. Natural killer T cells accelerate atherogenesis in mice. Blood 104, 2051–2059 (2004). [DOI] [PubMed] [Google Scholar]
- 169.Tupin E et al. CD1d-dependent Activation of NKT Cells Aggravates Atherosclerosis. J. Exp. Med 199, 417–422 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Major AS et al. Quantitative and Qualitative Differences in Proatherogenic NKT Cells in Apolipoprotein E–Deficient Mice. Arterioscler. Thromb. Vasc. Biol 24, 2351–2357 (2004). [DOI] [PubMed] [Google Scholar]
- 171.van Puijvelde G et al. Effect of natural killer T cell activation on initiation of atherosclerosis. Thromb. Haemost 102, 223–230 (2009). [DOI] [PubMed] [Google Scholar]
- 172.Li Y et al. CD4+ Natural Killer T Cells Potently Augment Aortic Root Atherosclerosis by Perforin- and Granzyme B-Dependent Cytotoxicity. Circ. Res 116, 245–254 (2015). [DOI] [PubMed] [Google Scholar]
- 173.Aslanian AM, Chapman HA & Charo IF Transient Role for CD1d-Restricted Natural Killer T Cells in the Formation of Atherosclerotic Lesions. Arterioscler. Thromb. Vasc. Biol 25, 628–632 (2005). [DOI] [PubMed] [Google Scholar]
- 174.VanderLaan PA et al. Characterization of the Natural Killer T-Cell Response in an Adoptive Transfer Model of Atherosclerosis. Am. J. Pathol 170, 1100–1107 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rogers L et al. Deficiency of invariant Va14 natural killer T cells decreases atherosclerosis in LDL receptor null mice. Cardiovasc. Res 78, 167–174 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nielsen MM, Witherden DA & Havran WL γδ T cells in homeostasis and host defence of epithelial barrier tissues. Nat. Rev. Immunol 17, 733–745 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cheng H-Y et al. Increased Cholesterol Content in Gammadelta (γδ) T Lymphocytes Differentially Regulates Their Activation. PLoS ONE 8, e63746 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cheng H-Y, Wu R & Hedrick CC Gammadelta (γδ) T lymphocytes do not impact the development of early atherosclerosis. Atherosclerosis 234, 265–269 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vu DM et al. γδT Cells Are Prevalent in the Proximal Aorta and Drive Nascent Atherosclerotic Lesion Progression and Neutrophilia in Hypercholesterolemic Mice. PLOS ONE 9, 12 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kleindienst R, Xu Q, Willeit J, Weimann S & Wick G Immunology of Atherosclerosis. Am. J. Pathol 142, 11 (1993). [PMC free article] [PubMed] [Google Scholar]
- 181.Zernecke A, Shagdarsuren E & Weber C Chemokines in Atherosclerosis: An Update. Arterioscler. Thromb. Vasc. Biol 28, 1897–1908 (2008). [DOI] [PubMed] [Google Scholar]
- 182.Galkina E et al. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J. Exp. Med 203, 1273–1282 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.MacRitchie N et al. The aorta can act as a site of naïve CD4+ T cell priming. Cardiovasc. Res 116, 306–316 (2020). [DOI] [PubMed] [Google Scholar]
- 184.Li J & Ley K Lymphocyte Migration Into Atherosclerotic Plaque. Arterioscler. Thromb. Vasc. Biol 35, 40–49 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mondini M et al. CCR2-Dependent Recruitment of Tregs and Monocytes Following Radiotherapy Is Associated with TNFα-Mediated Resistance. Cancer Immunol. Res 7, 376–387 (2019). [DOI] [PubMed] [Google Scholar]
- 186.Braunersreuther V et al. Ccr5 But Not Ccr1 Deficiency Reduces Development of Diet-Induced Atherosclerosis in Mice. Arterioscler. Thromb. Vasc. Biol 27, 373–379 (2007). [DOI] [PubMed] [Google Scholar]
- 187.Potteaux S et al. Chemokine Receptor CCR1 Disruption in Bone Marrow Cells Enhances Atherosclerotic Lesion Development and Inflammation in Mice. Mol. Med 11, 16–20 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Braunersreuther V et al. A Novel RANTES Antagonist Prevents Progression of Established Atherosclerotic Lesions in Mice. Arterioscler. Thromb. Vasc. Biol 28, 1090–1096 (2008). [DOI] [PubMed] [Google Scholar]
- 189.Potteaux S et al. Role of Bone Marrow–Derived CC-Chemokine Receptor 5 in the Development of Atherosclerosis of Low-Density Lipoprotein Receptor Knockout Mice. Arterioscler. Thromb. Vasc. Biol 26, 1858–1863 (2006). [DOI] [PubMed] [Google Scholar]
- 190.Kuziel WA et al. CCR5 deficiency is not protective in the early stages of atherogenesis in apoE knockout mice. Atherosclerosis 167, 25–32 (2003). [DOI] [PubMed] [Google Scholar]
- 191.Quinones MP et al. CC chemokine receptor 5 influences late-stage atherosclerosis. Atherosclerosis 195, e92–e103 (2007). [DOI] [PubMed] [Google Scholar]
- 192.von Hundelshausen P et al. Chemokine interactome mapping enables tailored intervention in acute and chronic inflammation. Sci. Transl. Med 9, eaah6650 (2017). [DOI] [PubMed] [Google Scholar]
- 193.Koenen RR et al. Disrupting functional interactions between platelet chemokines inhibits atherosclerosis in hyperlipidemic mice. Nat. Med 15, 97–103 (2009). [DOI] [PubMed] [Google Scholar]
- 194.Veillard NR et al. Differential Influence of Chemokine Receptors CCR2 and CXCR3 in Development of Atherosclerosis In Vivo. Circulation 112, 870–878 (2005). [DOI] [PubMed] [Google Scholar]
- 195.Mach F et al. Differential expression of three T lymphocyte-activating CXC chemokines by human atheroma-associated cells. J. Clin. Invest 104, 1041–1050 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Groom JR et al. CXCR3 Chemokine Receptor-Ligand Interactions in the Lymph Node Optimize CD4+ T Helper 1 Cell Differentiation. Immunity 37, 1091–1103 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.van Wanrooij EJA et al. CXCR3 Antagonist NBI-74330 Attenuates Atherosclerotic Plaque Formation in LDL Receptor–Deficient Mice. Arterioscler. Thromb. Vasc. Biol 28, 251–257 (2008). [DOI] [PubMed] [Google Scholar]
- 198.van Wanrooij EJA et al. HIV Entry Inhibitor TAK-779 Attenuates Atherogenesis in Low-Density Lipoprotein Receptor–Deficient Mice. Arterioscler. Thromb. Vasc. Biol 25, 2642–2647 (2005). [DOI] [PubMed] [Google Scholar]
- 199.Niki T et al. Elevated Concentration of Interferon-Inducible Protein of 10 kD (IP-10) Is Associated With Coronary Atherosclerosis. Int. Heart. J 56, 269–272 (2015). [DOI] [PubMed] [Google Scholar]
- 200.Heller EA et al. Chemokine CXCL10 Promotes Atherogenesis by Modulating the Local Balance of Effector and Regulatory T Cells. Circulation 113, 2301–2312 (2006). [DOI] [PubMed] [Google Scholar]
- 201.Lupieri A et al. Smooth muscle cells-derived CXCL10 prevents endothelial healing through PI3Kγ-dependent T cells response. Cardiovasc. Res 116, 438–449 (2020). [DOI] [PubMed] [Google Scholar]
- 202.Kim CH et al. Bonzo/CXCR6 expression defines type 1–polarized T-cell subsets with extralymphoid tissue homing potential. J. Clin. Invest 107, 595–601 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Galkina E et al. CXCR6 Promotes Atherosclerosis by Supporting T-Cell Homing, Interferon-γ Production, and Macrophage Accumulation in the Aortic Wall. Circulation 116, 1801–1811 (2007). [DOI] [PubMed] [Google Scholar]
- 204.Butcher MJ, Wu C-I, Waseem T & Galkina EV CXCR6 regulates the recruitment of pro-inflammatory IL-17A-producing T cells into atherosclerotic aortas. Int. Immunol 28, 255–261 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wuttge DM et al. CXCL16/SR-PSOX Is an Interferon-γ–Regulated Chemokine and Scavenger Receptor Expressed in Atherosclerotic Lesions. Arterioscler. Thromb. Vasc. Biol 24, 750–755 (2004). [DOI] [PubMed] [Google Scholar]
- 206.Andersen T et al. C-X-C Ligand 16 Is an Independent Predictor of Cardiovascular Death and Morbidity in Acute Coronary Syndromes. Arterioscler. Thromb. Vasc. Biol 39, 2402–2410 (2019). [DOI] [PubMed] [Google Scholar]
- 207.Aslanian AM & Charo IF Targeted Disruption of the Scavenger Receptor and Chemokine CXCL16 Accelerates Atherosclerosis. Circulation 114, 583–590 (2006). [DOI] [PubMed] [Google Scholar]
- 208.Minami M et al. Expression of SR-PSOX, a Novel Cell-Surface Scavenger Receptor for Phosphatidylserine and Oxidized LDL in Human Atherosclerotic Lesions. Arterioscler. Thromb. Vasc. Biol 21, 1796–1800 (2001). [DOI] [PubMed] [Google Scholar]
- 209.Sallusto F, Lenig D, Förster R, Lipp M & Lanzavecchia A Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999). [DOI] [PubMed] [Google Scholar]
- 210.Damås JK et al. Enhanced Expression of the Homeostatic Chemokines CCL19 and CCL21 in Clinical and Experimental Atherosclerosis: Possible Pathogenic Role in Plaque Destabilization. Arterioscler. Thromb. Vasc. Biol 27, 614–620 (2007). [DOI] [PubMed] [Google Scholar]
- 211.Trogan E et al. Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. Proc. Natl. Acad. Sci 103, 3781–3786 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wan W, Lionakis MS, Liu Q, Roffê E & Murphy PM Genetic deletion of chemokine receptor Ccr7 exacerbates atherogenesis in ApoE-deficient mice. Cardiovasc. Res 97, 580–588 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Luchtefeld M et al. Chemokine Receptor 7 Knockout Attenuates Atherosclerotic Plaque Development. Circulation 122, 1621–1628 (2010). [DOI] [PubMed] [Google Scholar]
- 214.Williams JW, Huang L & Randolph GJ Cytokine Circuits in Cardiovascular Disease. Immunity 50, 941–954 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ridker PM et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med 377, 1119–1131 (2017). [DOI] [PubMed] [Google Scholar]
- 216.Kimura T et al. Atheroprotective vaccination with MHC-II-restricted ApoB peptides induces peritoneal IL-10-producing CD4 T cells. Am. J. Physiol.-Heart Circ. Physiol 312, H781–H790 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhao TX et al. Low-dose interleukin-2 in patients with stable ischaemic heart disease and acute coronary syndromes (LILACS): protocol and study rationale for a randomised, double-blind, placebo-controlled, phase I/II clinical trial. BMJ Open 8, e022452 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Cipriani S et al. Efficacy of the CCR5 Antagonist Maraviroc in Reducing Early, Ritonavir-Induced Atherogenesis and Advanced Plaque Progression in Mice. Circulation 127, 2114–2124 (2013). [DOI] [PubMed] [Google Scholar]
- 219.Francisci D et al. Maraviroc Intensification Modulates Atherosclerotic Progression in HIV-Suppressed Patients at High Cardiovascular Risk. A Randomized, Crossover Pilot Study. Open Forum Infect. Dis 6, ofz112 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Libby P Seeing and Sampling the Surface of the Atherosclerotic Plaque: Red or White Can Make Blue. Arterioscler. Thromb. Vasc. Biol 36, 2275–2277 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.West NEJ et al. Percutaneous Sampling of Local Biomolecule Gradients Across Coronary Artery Atherosclerotic Plaques. JACC Basic Transl. Sci 2, 646–654 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lee R et al. A novel workflow combining plaque imaging, plaque and plasma proteomics identifies biomarkers of human coronary atherosclerotic plaque disruption. Clin. Proteomics 14, 22 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Bennett BJ et al. Genetic Architecture of Atherosclerosis in Mice: A Systems Genetics Analysis of Common Inbred Strains. PLOS Genet 11, e1005711 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Strand V et al. Immunogenicity of Biologics in Chronic Inflammatory Diseases: A Systematic Review. BioDrugs Clin. Immunother. Biopharm. Gene Ther 31, 299–316 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Krzywinski M & Altman N Power and sample size. Nat. Methods 10, 1139–1140 (2013). [Google Scholar]
- 226.Mailer RKW, Gisterå A, Polyzos KA, Ketelhuth DFJ & Hansson GK Hypercholesterolemia Enhances T Cell Receptor Signaling and Increases the Regulatory T Cell Population. Sci. Rep 7, 15655 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Pollock AH et al. Prolonged Intake of Dietary Lipids Alters Membrane Structure and T Cell Responses in LDLr−/− Mice. J. Immunol 196, 3993–4002 (2016). [DOI] [PubMed] [Google Scholar]
- 228.Brown EM, Kenny DJ & Xavier RJ Gut Microbiota Regulation of T Cells During Inflammation and Autoimmunity. Annu. Rev. Immunol 37, 599–624 (2019). [DOI] [PubMed] [Google Scholar]
- 229.Lee N & Kim W-U Microbiota in T-cell homeostasis and inflammatory diseases. Exp. Mol. Med 49, e340–e340 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Komaroff AL The Microbiome and Risk for Atherosclerosis. JAMA 319, 2381 (2018). [DOI] [PubMed] [Google Scholar]
- 231.Barrington WT & Lusis AJ Association between the gut microbiome and atherosclerosis. Nat. Rev. Cardiol 14, 699–700 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Bandura DR et al. Mass Cytometry: Technique for Real Time Single Cell Multitarget Immunoassay Based on Inductively Coupled Plasma Time-of-Flight Mass Spectrometry. Anal. Chem 81, 6813–6822 (2009). [DOI] [PubMed] [Google Scholar]
- 233.Bendall SC et al. Single-Cell Mass Cytometry of Differential Immune and Drug Responses Across a Human Hematopoietic Continuum. Science 332, 687–696 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Jaitin DA et al. Massively Parallel Single-Cell RNA-Seq for Marker-Free Decomposition of Tissues into Cell Types. Science 343, 776–779 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Klein AM et al. Droplet Barcoding for Single-Cell Transcriptomics Applied to Embryonic Stem Cells. Cell 161, 1187–1201 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Macosko EZ et al. Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell 161, 1202–1214 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Gierahn TM et al. Seq-Well: portable, low-cost RNA sequencing of single cells at high throughput. Nat. Methods 14, 395–398 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Cao J et al. Comprehensive single-cell transcriptional profiling of a multicellular organism. Science 357, 661–667 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Rosenberg AB et al. Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science 360, 176–182 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Spitzer MH & Nolan GP Mass Cytometry: Single Cells, Many Features. Cell 165, 780–791 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Zheng GXY et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun 8, 14049 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Trapnell C et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat. Biotechnol 32, 381–386 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Qiu X et al. Single-cell mRNA quantification and differential analysis with Census. Nat. Methods 14, 309–315 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Cohen M et al. Lung Single-Cell Signaling Interaction Map Reveals Basophil Role in Macrophage Imprinting. Cell 175, 1031–1044 (2018). [DOI] [PubMed] [Google Scholar]
- 245.Kim K et al. Transcriptome Analysis Reveals Nonfoamy Rather Than Foamy Plaque Macrophages Are Proinflammatory in Atherosclerotic Murine Models. Circ. Res 123, 1127–1142 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lukowski SW et al. Single-Cell Transcriptional Profiling of Aortic Endothelium Identifies a Hierarchy from Endovascular Progenitors to Differentiated Cells. Cell Rep 27, 2748–2758 (2019). [DOI] [PubMed] [Google Scholar]
- 247.Kalluri AS et al. Single-Cell Analysis of the Normal Mouse Aorta Reveals Functionally Distinct Endothelial Cell Populations. Circulation 140, 147–163 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ley K, Gerdes N & Winkels H ATVB Distinguished Scientist Award: How Costimulatory and Coinhibitory Pathways Shape Atherosclerosis. Arterioscler. Thromb. Vasc. Biol 37, 764–777 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Stubbington MJT et al. T cell fate and clonality inference from single-cell transcriptomes. Nat. Methods 13, 329–332 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]


