Abstract
Objective:
The clinical benefit of warfarin therapy for thromboprophylaxis after incident atrial fibrillation (AF) diagnosis in late-stage chronic kidney disease (CKD) patients transitioning to dialysis is unknown. The aim of this study was to examine the efficacy and safety of warfarin initiation following the diagnosis of AF in late-stage CKD patients transitioning to dialysis.
Methods:
In this retrospective cohort analysis, the study population was a national cohort of 22,771 US veterans with incident end stage renal disease (ESRD) who developed incident AF prior to initiating renal replacement therapy. We examined the association of warfarin therapy following the diagnosis of incident AF with ischemic-cerebrovascular accidents (CVA) (ischemic stroke or transient ischemic attack), ischemic-CVA related hospitalization, major bleeding events (gastrointestinal or intracranial bleeding), bleeding event related hospitalizations and post-dialysis all-cause mortality in multivariable adjusted Cox regression analyses adjusting for demographic characteristics and comorbidities.
Results:
The mean (SD) age of the cohort was 73.5±8.8 years, 13% were African American and the mean CHA2DS2-VASc score was 5.7±2.1. Among overall cohort, 6,682 (29.3%) patients were started on warfarin during the follow up period. The hazard ratios (95% confidence intervals) for ischemic-CVA, bleeding events and death for those started on warfarin were 1.23 (1.16 to 1.30), 1.36 (1.29 to 1.44), and 0.94 (0.90 to 0.97) compared with those who received no anticoagulation. Warfarin exposure was associated with higher risk for ischemic-CVA and bleeding event related hospitalizations also.
Conclusions:
In late-stage CKD patients transitioning to dialysis, warfarin use was associated with higher risk of ischemic and bleeding events, but lower risk of mortality. Future studies such as those comparing warfarin with newer oral anticoagulant agents are needed to granularly define the net clinical benefit of anticoagulation therapy in advanced CKD patients with incident AF.
Keywords: Chronic Kidney Disease, Warfarin, Atrial fibrillation, Stroke, Bleeding
Graphical Abstract
Summary for use on social media (Twitter): The clinical benefit of warfarin therapy for thromboprophylaxis after incident atrial fibrillation (AF) diagnosis in late-stage chronic kidney disease (CKD) patients transitioning to dialysis is unknown. Our study from a national cohort of veteran population suggest that warfarin use in advanced CKD is associated with higher risk of ischemic and bleeding events, but lower risk of mortality after dialysis transition.
Condensed Abstract
The clinical benefit of warfarin therapy for thromboprophylaxis after incident atrial fibrillation (AF) diagnosis in late-stage chronic kidney disease (CKD) patients transitioning to dialysis is unknown. The present study from a national cohort of US veterans evaluates the efficacy and safety of warfarin initiation following the diagnosis of AF in late-stage CKD patients transitioning to dialysis. Our results suggest that warfarin use in advanced CKD is associated with higher risk of ischemic and bleeding events, but lower risk of mortality after end stage renal transition transition.
Introduction
Chronic kidney disease (CKD) affects 10% to 15% of the general population, and each year ~100,000 CKD patients transition to maintenance hemodialysis in the United States.(1) Warfarin has a proven benefit as a prophylactic therapy for preventing future embolic stroke events in the broader atrial fibrillation (AF) population with conflicting results from observational studies in both non-dialysis dependent (NDD) CKD and dialysis dependent population.(2–19) The management of thromboprophylaxis with anticoagulant agents in CKD patients is complex as these patients are also at higher risk of major bleeding.(8,15,17,20)
To the best of our knowledge, no literature exists describing whether warfarin treatment should be recommended in NDD-CKD patients transitioning to dialysis after incident AF. Therefore, we studied the effect of warfarin exposure after incident AF diagnosis on clinical outcomes of NDD-CKD patients transitioning to dialysis in a large nationally representative cohort of US veterans. We aimed to determine the association of warfarin exposure with measures of clinical safety (bleeding events), clinical effectiveness (ischemic-cerebrovascular accident [ischemic-CVA], death) in this population as compared to those not treated with warfarin.
Methods
Study Population and Data Source
We retrospectively examined longitudinal data from the US Renal Dialysis System (USRDS) Transition of Care in Chronic Kidney Disease study,(21) a historic cohort study examining US veterans (n=102,477) who transitioned to renal replacement therapy from October 1, 2007 through March 31, 2015.(22,23) The study population was extracted from the Veterans Affairs (VA) Inpatient and Outpatient Medical SAS Datasets and from VA/Centers for Medicare and Medicaid Services data using International Classification of Diseases, Ninth or Tenth Revisions diagnosis code (ICD-9 or ICD-10) diagnostic codes (MedPAR, Outpatient, Carrier and Inpatient files). We identified patients with an inpatient or outpatient ICD-9 code for AF or atrial flutter (n=37,390) (collectively referred to as AF in this study), as done previously.(24–28) The AF diagnosis date was used as the cohort entry date for our study. Incident non-valvular AF was defined as a new ICD 9-CM code for AF without such a diagnosis at the first encounter (excluding 9,017 patients with prevalent AF) and meeting all of the following criteria: a) no documented evidence indicating prescription of any of the following medications before the date of AF diagnosis: anticoagulant agents (warfarin, apixaban, dabigatran, rivaroxaban, edoxaban) and other rhythm conversion agents such as amiodarone, sotalol, digoxin, dofetilide, ibutilide, flecainide, propafenone, procainamide and quinidine (n= 3,608) and b) those with a diagnosis of valvular disease such as mitral stenosis and mechanical prosthetic valve (n=1,887). Any patient with a prescription for newer oral anticoagulant agents during the follow-up period was also excluded (n=107). There were 22,771 patients who met these criteria and formed the final analytic cohort. A detailed description of cohort creation is shown in Figure 1.
Figure 1.
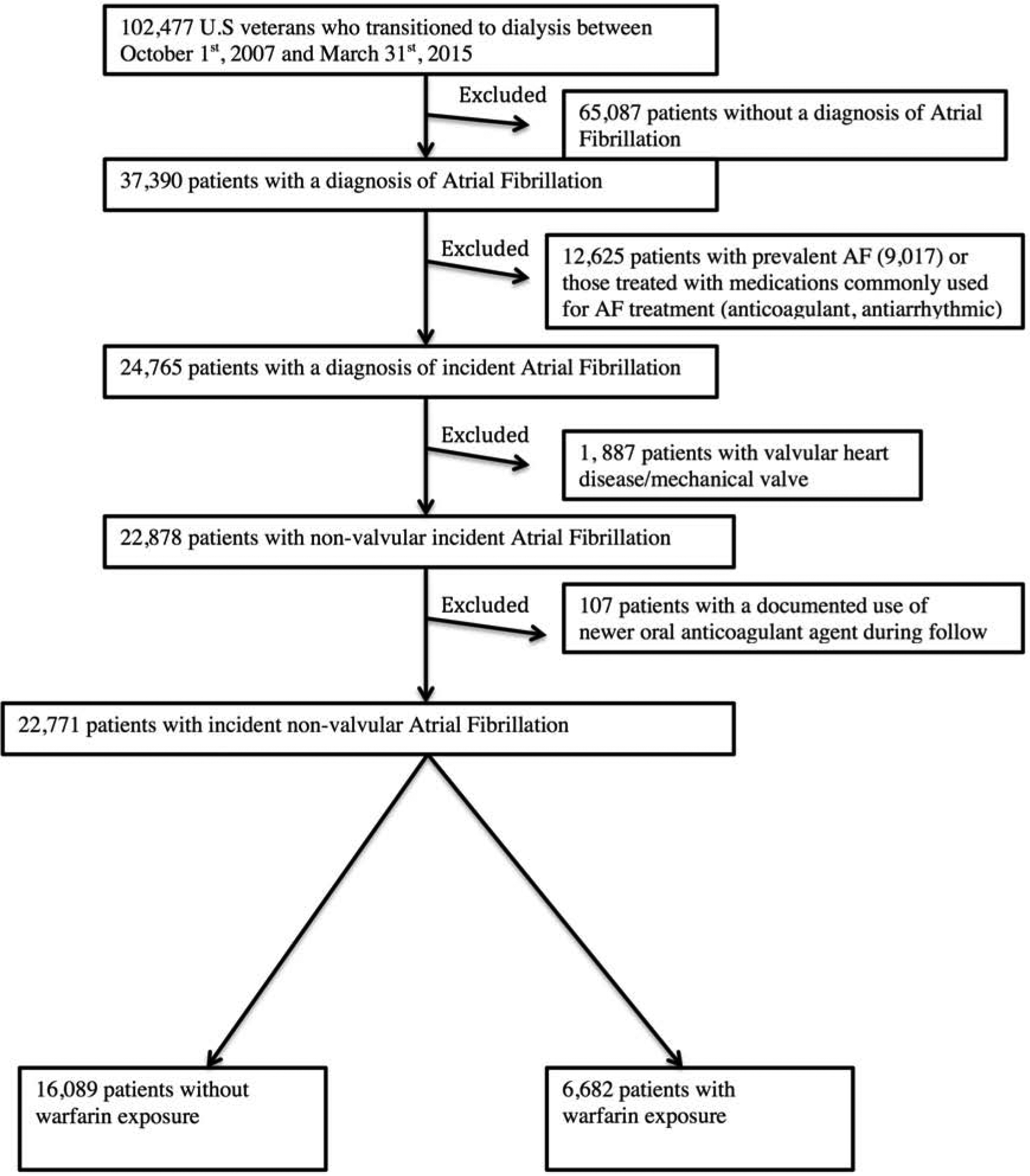
Flow chart of patient selection
Covariates
The patient characteristics at time of incident AF diagnosis, including age, sex, and self-identified race and ethnicity, were ascertained from the USRDS, VA, and CMS databases (Table 1). Preexisting comorbidities at the time of cohort entry were extracted from VA and CMS databases using ICD-9 and ICD-10 diagnostic and procedural codes and Current Procedural Terminology codes as guided by the Deyo Charlson Comorbidity Index (CCI) and CMS chronic conditions. Documentation of a diagnostic code within 3 years before AF diagnosis date during at least 1 inpatient visit or 2 outpatient visits was used to determine the presence of comorbidity, and otherwise were considered absent. The Charlson Comorbidity Index (CCI) was calculated without renal disease. We also calculated the CHA2DS2-VASc risk score using baseline comorbidity data.(8) Data related to baseline medication exposure were obtained from both CMS Medicare Part D and VA pharmacy dispensation records.(29) Patients who received at least one 30-day supply of these medications within 3 months of warfarin initiation (for the warfarin-exposed group) or date of AF diagnosis (for warfarin non-exposed group) were recorded as having been treated with these medications at baseline (Table 1).
Table 1.
Baseline patient characteristics by Warfarin Exposure status
| Overall | Warfarin-Exposure | P-value | |||
|---|---|---|---|---|---|
| No | Yes | ||||
| N= 22,771 | N= 16,089 | N= 6,682 | |||
| Age | Mean (SD), y 95% CI |
73.5 (8.8) 73.4 to 73.6 |
74.3 (8.7) 74.2 to 74.5 |
71.4 (8.7) 71.2 to 71.6 |
<0.001 |
| Sex | Males | 21,729(95.4%) | 15,311(95.2%) | 6,418(96.1%) | 0.004 |
| Race | 0.076 | ||||
| Whites | 19,228(84.4%) | 13,598(84.5%) | 5,630(84.3%) | ||
| Blacks | 2,973(13.1%) | 2,068(12.9%) | 905(13.5%) | ||
| Others | 569(2.5%) | 422(2.6%) | 147(2.2%) | ||
| Ethnicity | Hispanic | 775(3.4%) | 548(3.4%) | 227(3.4%) | 0.973 |
| Marital Status | <0.001 | ||||
| Married | 14,393(68.5%) | 10,187(69.4%) | 4,206(66.5%) | ||
| Single | 878(4.2%) | 626(4.3%) | 252(4.0%) | ||
| Divorced | 2,966(14.1%) | 1,924(13.1%) | 1,042(16.5%) | ||
| Widowed | 2,782 (13.2%) | 1,952(13.3%) | 830(13.1%) | ||
| Comorbidities | |||||
| Hyperlipidemia | 14,000(61.5%) | 10,434(64.9%) | 3,566(53.4%) | <0.001 | |
| Hypertension | 17,999(79.0%) | 13,256(82.4%) | 4,743(71.0%) | <0.001 | |
| Diabetes | 13,672(60.0%) | 9,833(61.1%) | 3,839(57.5%) | <0.001 | |
| Coronary artery disease | 12,864(56.5%) | 9,651(60.0%) | 3,213(48.1%) | <0.001 | |
| Congestive Heart Failure | 11,988(52.7%) | 8,788(54.6%) | 3,200(47.9%) | <0.001 | |
| Cerebrovascular Disease | 9,050(39.7%) | 7,028(43.7%) | 2,022(30.3%) | <0.001 | |
| Peripheral vascular Disease | 9,940(43.7%) | 7,603(47.3%) | 2,337(35.0%) | <0.001 | |
| Chronic Pulmonary Disease | 9,882(43.4%) | 7,444(46.3%) | 2,438(36.5%) | <0.001 | |
| Dementia | 2,163(9.5%) | 1,830(11.4%) | 333(5.0%) | <0.001 | |
| Depression | 3,424(15.0%) | 2,723(16.9%) | 701(10.5%) | <0.001 | |
| Anemia | 9,531(41.9%) | 7,503(46.6%) | 2,028(30.4%) | <0.001 | |
| Cancer | 6,179(27.1%) | 4,860(30.2%) | 1,319(19.7%) | <0.001 | |
| Paraplegia and Hemiplegia | 993(4.4%) | 796(5.0%) | 197(3.0) | <0.001 | |
| AIDS/HIV | 137(0.6%) | 111(0.7%) | 26(0.4%) | <0.001 | |
| Liver Disease | 3,962(17.4%) | 3,189(19.8%) | 773(11.6%) | <0.001 | |
| Peptic Ulcer Disease | 1,990(8.7%) | 1,600(9.9%) | 390(5.8%) | <0.001 | |
| History of GI bleed | 5,268 (23.1%) | 4,197 (26.1%) | 1,071(16.0%) | <0.001 | |
| History of other Major bleeding | 2,512 (11.0%) | 1,985(12.3%) | 527(7.9%) | <0.001 | |
| Connective Tissue Disease | 2,868(12.6%) | 2,362(14.7%) | 506(7.6%) | <0.001 | |
| Medications | |||||
| Statin | 7,425(32.6%) | 3,643(22.6%) | 3,782(56.6%) | <0.001 | |
| Non statin lipid lowering agents | 1,392(6.1%) | 724(4.5%) | 668(10.0%) | <0.001 | |
| Non Aspirin-antiplatelet agents | 1,660(7.3%) | 901(5.6%) | 759(11.4%) | <0.001 | |
| Antiarrhythmics | 1,044(4.6%) | 91(0.6%) | 953(14.3%) | <0.001 | |
| Anti-anginals | 2,730(12.0%) | 1,245(7.7%) | 1,485(22.2%) | <0.001 | |
| Calcium channel blocker | 5,961 (26.2%) | 3,174 (19.7%) | 2,787(41.7%) | <0.001 | |
| Beta blockers | 8,081 (35.5%) | 3,659 (22.7%) | 4,422(66.2%) | <0.001 | |
| RAASi | 6,073(26.7%) | 3,040(18.9%) | 3,033(45.4%) | <0.001 | |
| Antihypertensive vasodilator agents | 1,875(8.2%) | 926(5.8%) | 949(14.2%) | <0.001 | |
| Potassium sparing diuretics | 951(4.2%) | 386(2.4%) | 565(8.5%) | <0.001 | |
| Loop diuretics | 6,478(28.5%) | 2,953(18.4%) | 3,525(52.8%) | <0.001 | |
| Thiazides | 1,896(8.3%) | 983(6.1%) | 913(13.7%) | <0.001 | |
| Antidepressants | 2,661(11.7%) | 1,315(8.2%) | 1,346(20.1%) | <0.001 | |
| NSAIDs | 17(0.1%) | 13(0.1%) | 4(0.1%) | 0.60 | |
| <0.001 | |||||
| Systolic Blood pressure, in mm Hg | 138.2(23.0) (137.8–138.6) |
138.7(22.8) (138.3–139.1) |
137.2(23.2) (136.5–137.8) |
<0.001 | |
| Diastolic Blood pressure, in mm Hg | 70.7 (13.0) (70.5–70.9) |
70.4(12.9) (70.1–70.6) |
71.3(13.1) (70.9–71.6) |
<0.001 | |
| Hemoglobin, g/dL | 12.5 (1.9) 12.5–12.5 |
12.4(1.9) 12.4–12.4 |
12.8(1.9) 12.7–12.8 |
<0.001 | |
| eGFR, mL/min/1.73 m2 | 35.8 (19.6) (35.5–36.1) |
34.8 (19.5) (34.4 – 35.2) |
37.8 (19.7) (37.3– 38.3) |
<0.001 | |
| Charlson Comorbidity | 4.5 (3.5) 4.4 to 4.5 |
4.8 (3.7) 4.8 to 4.9 |
3.5 (3.0) 3.4 to 3.6 |
<0.001 | |
| Index, Mean (SD) 95% CI | |||||
| CHA2DS2-VASc score Mean (SD) 95% CI | 5.7 (2.1) 5.7 to 5.8 |
6.0 (2.0) 6.0 to 6.0 |
5.1 (2.1) 5.19 to 5.2 |
<0.001 | |
| AF diagnosis to dialysis initiation, years Mean (SD) 95% CI | 4.0 (3.8) 4.0 to 4.1 |
3.7(3.7) 3.6 to 3.7 |
5.0 (3.8) 4.9 to 5.1 |
<0.001 | |
SD, standard deviation; CI, confidence interval; RAASi, renin angiotensin aldosterone inhibitors; NSAIDs, Non steroidal anti inflammatory drugs
Outcomes
The outcomes of interest were a) ischemic-CVA events (ischemic stroke or transient ischemic attack) b) ischemic-CVA-related hospitalization c) major bleeding events (hemorrhagic CVA, intracranial or GI bleeding events) d) major bleeding event related hospitalization e) all-cause mortality.
Exposure and Follow up period
Using both inpatient and outpatient medication data, warfarin prescription information was extracted using specific drug-class codes or names.(29) All patients were considered unexposed (i.e., without warfarin exposure) from the time of AF diagnosis until the start of warfarin treatment. We defined de-novo exposure as the initiation of warfarin following the diagnosis of incident AF. Patients who received a ≥30-day supply of warfarin with at least one refill were characterized as exposed to warfarin. Patients who were started on warfarin were considered warfarin exposed from the time of warfarin start date until date of outcome of interest (such as ischemic-CVA, major bleeding event, ischemic CVA related hospitalization, major bleeding event related hospitalization), death or date of last activity in VA system or date of administrative censoring (1st September 2015), whichever occurred first. As all patients transitioned to dialysis in our dataset, for the outcome of death the start of the follow up period was the date of first dialysis service instead of AF diagnosis date to control for immortal time bias, and then patients were followed until the date of death or last activity in VA network or date of administrative censoring (1st September 2015), whichever occurred earlier. All data on outcomes and censoring events were obtained from the USRDS, VA, and CMS datasets.
Statistical Analysis
Baseline patient characteristics are presented as the mean ± standard deviation (SD) or median [interquartile ranges) (IQR)] for continuous variables and as frequency (percentages) for categorical variables, as appropriate. Patients were compared between warfarin-exposed and non-exposed groups using chi-square test for categorical variables and student’s t-test for continuous variables.
The exposure to warfarin was modeled in an “intention-to-treat” approach (with all de novo exposed patients kept in the treated group irrespective of subsequent discontinuation of warfarin). Given majority of incident non-valvular AF patients are not started on warfarin therapy at the time of AF diagnosis(30) and the outcomes of interest such as ischemic-CVA and bleeding events could have happened before warfarin exposure, we decided to model the exposure of warfarin initiation as a time-dependant covariate. The time between cohort entry (incident AF diagnosis date) and the start of warfarin therapy allocated to the unexposed group to avoid immortal time bias arising from the relevant follow-up period among patients treated with warfarin. (31) The comparison between warfarin exposed and untreated patients was performed with multivariable Cox regression analyses.
The influence of potential confounders was analyzed by incremental adjustments based on priori considerations: unadjusted (model 1); age, sex, race/ethnicity (whites, African Americans, Hispanics, and others), marital status (married, divorced, single, and widowed) (model 2); model 2 plus comorbid conditions (hyperlipidemia, hypertension, diabetes, history of coronary artery disease, congestive heart failure, cerebrovascular disease, peripheral vascular disease, chronic pulmonary disease, dementia, depression, anemia, cancer, paraplegia and hemiplegia, AIDS/HIV, liver disease, peptic ulcer disease, history of gastrointestinal (GI) bleeding, history of other major bleeding, connective tissue disease, CCI, CHA2DS2-VASc score and medications (statins, non-statin lipid lowering agents, non aspirin anti-platelet agents, anti-arrhythmic, anti-anginals, calcium channel blockers, beta blockers, anti-depressants, potassium sparing diuretics, loop diuretics, renin angiotensin aldosterone system inhibitors, thiazides and anti-hypertensive vasodilator agents); model 3 plus blood/serum hemoglobin, systolic blood pressure, diastolic blood pressure and estimated glomerular filtration rate (eGFR) (model 4). Model 4 had high proportions of missing data (systolic blood pressure [~39%], diastolic blood pressure [30%], hemoglobin [~39%], eGFR [~36%]); therefore, we used model 3 as the main multivariable-adjusted model. We also performed the eGFR-stratified analysis to study the association of warfarin exposure with the outcomes. (e Table1 in supplement).
We performed different sensitivity analyses to test the robustness of our findings. As we did not have information regarding the type of AF (paroxysmal vs. persistent), we studied the association estimates depending on the number of times AF was documented during the longitudinal follow up (<3 vs. ≥3 times) (e Table 2 in supplement). We studied the associations of outcomes (ischemic-CVA, major bleeding events) with the warfarin initiation by performing separate analyses for pre and post -dialysis period (e Table 3 in supplement). To address confounding by indication of treatment an inverse probability of treatment weighted (IPTW) analysis was applied (e Table 4–7 in supplement). As many patients might obtain aspirin over the counter (that will not be captured in pharmacy prescription data), we examined interactions and performed a subgroup analysis by aspirin exposure (eTable 8a–b). We examined the effect of warfarin exposure on ischemic-CVA in discrete time bins (0–7 days; 8–30 days; >30 days) after therapy initiation to differentiate the risk after warfarin initiation (early vs. late) (eTable 10)
Results are expressed as hazard ratios (HRs) for Cox regression analyses with 95% confidence intervals (CIs). P values are 2 sided and reported as significant at 0.05 for all analyses. All analyses were conducted using STATA MP, version 14 (STATA Corp LP) and SAS version 9.4 (SAS Institute Inc., Cary, NC). The institutional review board of Memphis VA Medical Center approved this study (IRB# 555872–15), and the participant written informed consent requirement was waived.
Results
The mean age of the study population was 73.50±8.8 years; the cohort was 21,729 (95.4%) male and 2,973 (13.1%) were African Americans. The mean time from AF diagnosis to dialysis initiation was 4.0± 3.8 years. 9,050 (39.7%) patients had a history of cerebrovascular disease, 5,268 (23.1%) patients had a history of GI bleeding and 2,512 (11.0%) patients had a history of other major bleeding events prior to the diagnosis of AF. The mean CCI and CHA2DS2-VASc risk score were 4.5±3.5 and 5.7±2.1 respectively. The mean eGFR was 35.8 ± 19.6 mL/min/1.73m2, systolic blood pressure was 138.2 ± 23.0 mmHg, diastolic blood pressure was 70.7 ± 13.0 mmHg and blood/serum hemoglobin value was 12.5 ± 1.9 g/dL (Table 1).
During the follow-up period, 6,682 patients (29.3%) were started on warfarin treatment. For warfarin-exposed group, the median time from AF diagnosis to warfarin initiation was 2.4 years (0.4 to 5.0 years). Compared with AF patients without warfarin initiation, those started on warfarin treatment were younger and more likely to be African American, with lower prevalence of comorbidities, and with lower mean CCI and CHA2DS2-VASc scores. They were also more likely to be on statins, beta-blockers, renin-angiotensin system inhibitors, calcium channel blockers, antiarrhythmics and antiplatelet agents (Table 1).
Association of Warfarin Initiation with Cerebrovascular Accidents
The median follow-up period for the outcome of ischemic-CVA was 3.2 years (1.1 to 6.3 years) and ischemic-CVA hospitalization was 4.2 years (1.7 to 7.5 years). Patients who were started on warfarin had a lower crude ischemic-CVA rate compared with patients who were untreated (105.5; 95%CI, 100.7–110.6 vs. 107.3; 95%CI, 105.0–109.6 events per 1000 person-years) but higher ischemic-CVA related hospitalizations (61.4; 95%CI, 58.1–64.9 vs. 50.8; 95% CI, 49.3–52.3 events per 1000 person-years) (Table 2). In multivariable adjusted models, warfarin exposure was associated with higher ischemic-CVA events (HR= 1.23, 95%CI [1.16–1.30]) and higher risk for ischemic-CVA related hospitalizations (HR = 1.38, 95% CI [1.28 −1.48]) (Table 3). Similar results were observed when analysis was limited to pre-dialysis period and post-dialysis period (e Table 3). In IPTW analysis, warfarin exposure was associated with higher ischemic-CVA events (HR = 1.25, 95% CI [1.18– 1.32]) (e table 4). The analysis based on eGFR stratification (e Table1) and aspirin exposure also revealed similar results (e Table 8a–8b). We found higher risk of ischemic-CVA during 0–7 days (HR = 4.03, 95% CI [0.83–19.53] and >30 days of warfarin exposure (HR = 1.24 [1.16– 1.31]) (e Table 10).
Table 2.
Outcomes depending on exposure to warfarin (based on patient-time in each risk group)
| Outcome of Interest | Overall | No-Warfarin Exposure | Warfarin Exposure |
|---|---|---|---|
| 1000 patient years | 1000 patient years | 1000 patient years | |
| [95% CI] | [95% CI] | [95% CI] | |
| Cerebrovascular Accident | 107.0 (100.7 – 110.6) | 107.3(105.0–109.6) | 105.5(100.7–110.6) |
| Cerebrovascular Accident-hospitalization | 52.7(51.4–54.0) | 50.8(49.3–52.3) | 61.4(58.1–64.9) |
| Major Bleeding Event | 131.5 (129.1 – 134.0) | 128.9(126.3–131.5) | 144.7(128.6–151.0) |
| Major Bleeding event-hospitalization | 70.3(68.7–71.9) | 66.4(64.7–68.1) | 88.6(84.4–93.0) |
| All-cause mortality | 512.1(504.8–519.5) | 536.7(527.6–545.9) | 460.9(448.9–473.3) |
CI, confidence interval
-Cerebrovascular accident defined as ischemic stroke or transient ischemic attack
-Major bleeding event defined as hemorrhagic stroke or intracranial bleeding or gastrointestinal bleeding event
Table 3.
Association of Warfarin Exposure with Outcomes in Time Varying Covariate Cox Regression model analyses.
| Outcome | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| no-warfarin | Warfarin exposed | no-warfarin | Warfarin exposed | no-warfarin | Warfarin exposed | no-warfarin | Warfarin exposed | |
| CVA, Hazard ratio (95% CI) | Ref | 1.10 (1.04–1.17) | Ref | 1.13(1.07–1.19) | Ref | 1.23(1.16–1.30) | Ref | 1.23(1.15–1.32) |
| CVA-hospitalization, Hazard ratio (95% CI) | Ref | 1.26(1.18–1.34) | Ref | 1.29(1.20–1.38) | Ref | 1.38(1.28–1.48) | Ref | 1.33(1.221.46) |
| Major Bleeding Event, Hazard ratio (95% CI) | Ref | 1.27 (1.211.33) | Ref | 1.28 (1.22 to 1.35) | Ref | 1.36(1.291.44) | Ref | 1.34(1.251.43) |
| Major Bleeding Event-Hospitalization, Hazard ratio (95% CI) | Ref | 1.47 (1.391.56) | Ref | 1.50(1.41–1.59) | Ref | 1.56(1.47–1.67) | Ref | 1.49(1.381.61) |
| All-cause post dialysis mortality, Hazard ratio (95% CI) | Ref | 0.87(0.84–0.90) | Ref | 0.92(0.90–0.96) | Ref | 0.94(0.90–0.97) | Ref | 0.93 (0.890.98) |
Ref, reference; CVA, cerebrovascular accident; CI, confidence interval
Model 1- unadjusted
Model 2- adjusted for age, sex, race, ethnicity, marital status
Model 3- adjusted for age, sex, race, ethnicity, marital status, comorbidities, chadvasc score, Charlson comorbidity index and medications
Model 4- adjusted for age, sex, race, ethnicity, marital status, comorbidities, chadvasc score, Charlson comorbidity index, medications, systolic and diastolic blood pressures, serum/blood hemoglobin values and estimated glomerular filtration rates
Association of Warfarin Initiation with Major Bleeding Events
The median follow-up period for the outcome of major bleeding events was 2.8 years (IQR= 0.9 to 5.8) and bleeding event related hospitalization was 3.8 years (IQR= 1.5 to 7.1). Patients who were started on warfarin had a higher crude major bleeding event rate (144.7; 95%CI, 128.6 – 151.0 vs. 128.9; 95%CI, 126.3– 131.5 events per 1000 person-years) and higher major bleeding related hospitalizations (88.6; 95%CI, 84.4–93.0 vs. 66.4; 95%CI, 64.7–68.1 events per 1000 person-years) compared with patients who were untreated (Table 2). In multivariable adjusted models, warfarin exposure was associated with higher major bleeding events (HR= 1.36, 95%CI [1.29–1.44]) and higher risk for bleeding event related hospitalizations (HR = 1.56, 95% CI [1.47 −1.67]) (Table 3). Similar results were observed when analysis was limited to pre-dialysis period and post-dialysis period (e Table 3). In IPTW analysis also, warfarin exposure was associated with higher major bleeding events (HR = 1.34, 95% CI [1.27– 1.41]) (e Table 4). The analysis based on eGFR stratification (e Table1) and aspirin exposure also revealed similar results (e Table 8a–8b).
Association of Warfarin Initiation with Death
The median follow-up period post dialysis initiation for the outcome of death was 1.1 years (0.4 to 2.4 years). As shown in Table 2, patients who were started on warfarin had a lower crude all-cause mortality rate compared with patients who were untreated (460.9; 95%CI, 448.9–473.3 vs. 536.7; 95%CI, 527.6 – 545.9 events per 1000 person-years) (Table 3). In multivariable adjusted Cox regression analyses, warfarin exposure was associated with significantly lower risk of death (HR = 0.94, 95%CI [0.90 – 0.97]) (Table 3). In IPTW analysis also, warfarin exposure was associated with lower risk of death (HR = 0.89, 95% CI [0.86– 0.92]) (e Table 4). The analysis based on aspirin exposure revealed similar results (e Table 8a–b).
Discussion
This observational retrospective cohort study indicates that, in advanced CKD patients transitioning to dialysis, warfarin use is associated with higher risk of ischemic and bleeding events but lower risk of mortality. Till date, it is still unknown if thromboembolic and bleeding outcomes differ between patients with NDD-CKD who are started on warfarin therapy after incident AF while transitioning to dialysis, and those who are untreated. Our study examining a large national cohort of NDD-CKD patients provides new data highlighting the long-term outcomes of initiating warfarin therapy in incident AF patients with advanced NDD-CKD transitioning to dialysis.
The current clinical guidelines are controversial with respect to recommendations about use of warfarin in AF patients with end-stage renal disease (ESRD) or on dialysis.(10,32,33) With no randomized clinical trial studying this issue, observational studies in both ESRD and NDD-CKD patients have reported inconsistent results. (5–13,16,18,32–34) Recently Kumar et al. retrospectively examined 6,977 NDD-CKD patients (baseline GFR of <50 ml/min/1.73m2) and incident AF in a large UK database and reported warfarin use to be associated with higher bleeding (HR = 2.42, 95%CI [1.44 to 4.05]) and higher stroke events (HR= 2.60, 95%CI [2.00 to 3.38]) over a follow up period of 1.4 years.(7) In a retrospective cohort analysis of 3,587 patients in a Danish registry, investigators reported an association of anticoagulants in NDD-CKD AF patients with increased bleeding risk (HR= 1.36, 95%CI [1.17 to 1.59]) without reducing strokes (HR= 0.84, 95%CI [0.69 to 1.01]).(15) Similar observations were seen in a Canadian population with use of warfarin providing no significant reduction in stroke events (HR= 1.10, 95%CI [0.78 to 1.56]) but increasing bleeding events (HR=1.42, 95%CI [1.04 to 1.93]).(18) In contrast, others have reported warfarin treatment to be associated with a significantly lower risk of all-cause mortality (16) and CVA events.(5) In ESRD population, some studies (13,34) reported warfarin use to be associated with a nearly two-fold increase in the risk of stroke events, while others (15) have shown warfarin use to be associated with a significant reduction in thromboembolic events (HR= 0.44, 95%CI [0.26 −0.74]). Garg et al. retrospectively studied 302 AF patients on chronic hemodialysis and reported lack of clinical benefit of warfarin use in reducing risk of ischemic stroke and mortality events.(2) Nochaiwong et al. in a meta-analysis of 14 studies found no clinical benefit of warfarin therapy for the outcomes of mortality and stroke/thromboembolism and reported significantly increased risk of major bleeding among dialysis patients with AF.(12)
As an extension of these prior studies, our core objective was to focus on advanced NDD-CKD patients transitioning to dialysis, and we found warfarin exposure to be associated with increased rate of bleeding and ischemic-CVA events, but with lower risk of all-cause deaths. The differences in our findings from the previous studies could be due to disparities in study sample size, temporal discordance (short term vs. long-term follow-up), type of AF (incident vs. prevalent), patient characteristics and severity of renal disease. We examined a population with advanced CKD with progressive decline in renal function. We decided to include patients with all CHA2DS2-VASc scores in our analysis without limiting to only those with higher scores, as advanced CKD patients were not included in the pivotal studies that established the use of risk-based anticoagulation prescription.(35) The lack of benefit of warfarin for stroke prevention and perhaps increased risk as seen in our study is plausibly multifactorial driven. First, the use of Vitamin K antagonist leading to accelerated vascular calcification predisposing patients to watershed vascular territory or lacunar infarcts has been previously reported.(8,17,18) Second, advanced CKD patients on warfarin have been shown to have poorer anticoagulation control with lower proportion of patients spending time in target international normalized ratio (INR) ranges (2 to 3) when compared to those with better renal function.(20) Hence the lack of benefit in CVA prevention with warfarin might simply be a reflection of inadequate anticoagulation causing failure in prevention of cardio-embolic strokes. Third, warfarin initiation has been reported to increase risk of stroke during the early treatment phase plausibly due to a transient hypercoagulable state as also observed by us. (36) Finally, our cohort represents an older age population, which has higher vascular calcification and atherosclerotic disease burden potentially making them prone to atherothrombotic stroke events rather than embolic stroke reducing the overall benefit of warfarin for stroke prevention in AF.
CKD patients have underlying platelet and coagulation disorders such as uremic thrombocyte function disorder, and a high prevalence of risk factors for bleeding such as hypertension and old age.(8) The use of anticoagulation in this background might augment the risk of bleeding. Additionally, post transition to dialysis; these patients require systemic anticoagulation with heparin to prevent clotting that may further increase the risk of bleeding. Warfarin related nephropathy has been described in CKD population that potentially can further compromise kidney function, compounding the risk of ischemic and bleeding events. (8,37) Reduced renal function in patients receiving warfarin has been shown to be associated with a higher incidence of over anticoagulation and greater risk for major hemorrhage. (20) We also speculate that reduced mortality could also be due to the protective effects of anticoagulation against cardiovascular events such as myocardial infarction and venous thromboembolic events, which in turn might decrease the mortality of advanced CKD patients.(38) The warfarin-exposed group was more likely to be on cardioprotective medications such as statins, beta-blockers and renin-angiotensin-aldosterone system inhibitor (RAASi), and while we did adjust for these in regression analysis, we cannot exclude the possibility of residual confounding. Although the association of warfarin exposure with the reduced mortality was statistically significant in our study, ~50% patients of our cohort died within one year of dialysis initiation. Previous studies have also shown that annualized mortality rate in the first 3–6 months of dialysis initiation is ~40%.(39) Hence it is vital to consider the limited life expectancy of this population when making treatment decisions.
Certain limitations merit consideration when interpreting our findings. We were not able to distinguish between paroxysmal and persistent AF. However, when we performed a subgroup analyses depending on the number of times AF was documented during longitudinal follow up, we observed similar results. The proportion of patients started on warfarin after incident AF diagnosis (~30%) was lower than expected and important clinical information, such as reasons of not starting warfarin treatment, is not available from the data source. However, a recent study found similar results and reported that nearly 60% of patients with AF were not receiving oral anticoagulation treatment and the median time to warfarin initiation from AF diagnosis (~2 years) was also comparable to that seen in our study.(30) This delay can possibly be from factors such as patient refusal or disinterest in warfarin therapy due to inconvenience of frequent monitoring and dose adjustment related to warfarin therapy, along with under-prescribing by providers due to concerns of patient compliance and warfarin associated bleeding complications in this high risk population. Most of our patients were male US veterans; hence, the results may not apply to women or patients from other geographic areas. As with all observational studies on drug effects, we cannot eliminate the possibility of unmeasured confounders and confounding by indication. (40) However, when we performed IPTW analyses, we found similar results. Our whole cohort transitioned to dialysis; hence we were not able to capture mortality events during the pre-dialysis period. Also since many advanced CKD patients do not transition to dialysis, the clinical applicability of our study findings may be limited. We were not able to analyze the warfarin treatment in a time dependent manner and taking into account factors such as warfarin discontinuation. We were not able to analyze the time in therapeutic INR range. Lastly, observational retrospective design of our study does not enable casual inferences.
Conclusions
This study revealed that warfarin use after incident AF diagnosis in advanced CKD is associated with higher risk of ischemic and bleeding events, but lower risk of mortality after ESRD transition. Future studies such as those comparing warfarin with newer oral anticoagulant agents are needed to granularly define the net clinical benefit of anticoagulation therapy in advanced CKD patients with incident AF.
Supplementary Material
CENTRAL ILLUSTRATION:

Warfarin Use for Atrial Fibrillation in Advanced Chronic Kidney Disease: Hazard Ratios
CVA, ischemic cerebrovascular accident (ischemic stroke or transient ischemic attack)
Funding Sources:
C.P.K., K.K.Z., E.S., and M.Z.M. are employees of the US Department of Veterans Affairs. Opinions expressed in this paper are those of the authors and do not necessarily represent the opinion of the US Department of Veterans Affairs. The results of this paper have not been published previously in whole or part. This study is supported by grant 5U01DK102163 from the National Institutes of Health (NIH) to K.K.Z. and C.P.K., and by resources from the US Department of Veterans Affairs. The data reported here have been supplied in part by the United States Renal Data System (USRDS). Support for VA/CMS data is provided by the US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004).
Abbreviations and Acronyms
- CKD
chronic kidney disease
- AF
Atrial fibrillation
- NDD
non-dialysis dependant
- CVA
cerebrovascular accident
- ICD-9 or ICD-10
International Classification of Diseases, Ninth or Tenth Revisions diagnosis code
- CCI
Charlson Comorbidity Index
- VA
Veterans Affairs
- SD
Standard Deviation
- IQR
Inter Quartile Range
- CI
Confidence Interval
- AIDS/HIV
Acquired Immunodeficiency Syndrome/ Human Immunodeficiency Virus
- GI
Gastrointestinal
- eGFR
estimated glomerular filtration rate
- IPTW
inverse probability of treatment weighted
- HR
Hazard Ratio
- ESRD
End-stage renal disease
- INR
International normalized ratio
- RAASi
renin angiotensin-aldosterone system inhibitor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETENCY IN MEDICAL KNOWLEDGE: The clinical benefit of warfarin therapy for thromboprophylaxis after incident atrial fibrillation (AF) diagnosis in late-stage chronic kidney disease (CKD) patients transitioning to dialysis is unknown. Our results from a national cohort of veteran population suggest that warfarin use in advanced CKD is associated with higher risk of ischemic and bleeding events, but lower risk of mortality after end stage renal transition.
TRANSLATIONAL OUTLOOK: Future studies such as those comparing warfarin with newer oral anticoagulant agents are needed to more granularly define the net clinical benefit of anticoagulation therapy in advanced CKD patients with incident AF.
Conflict of Interest: K.K.Z has received honoraria and/or support from Abbott, Abbvie, Akebia, Alexion, Amgen, ASN (American Society of Nephrology), Astra-Zeneca, Aveo, BBraun, Chugai, Daiichi, DaVita, Fresenius, Genentech, Haymarket Media, Hofstra Medical School, IFKF (International Federation of Kidney Foundations), ISH (International Society of Hemodialysis), International Society of Renal Nutrition & Metabolism (ISRNM), JSDT (Japanese Society of Dialysis Therapy), Hospira, Kabi, Keryx, Kissei, Novartis, OPKO, NIH (National Institutes of Health), NKF (National Kidney Foundations), Pfizer, Relypsa, Resverlogix, Dr Schaer, Sandoz, Sanofi, Shire, VA (Veterans’ Affairs), Vifor, UpToDate, ZS-Pharma. MM: served as advisor for Merck and AbbVie. Others: None
References:
- 1.US Renal Data System 2016 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2017;69:A4. [DOI] [PubMed] [Google Scholar]
- 2.Garg L, Chen C, Haines DE. Atrial fibrillation and chronic kidney disease requiring hemodialysis - Does warfarin therapy improve the risks of this lethal combination? Int J Cardiol 2016;222:47–50. [DOI] [PubMed] [Google Scholar]
- 3.Kooiman J, van Rein N, Spaans B et al. Efficacy and safety of vitamin K-antagonists (VKA) for atrial fibrillation in non-dialysis dependent chronic kidney disease. PLoS One 2014;9:e94420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai HM, Aronow WS, Kalen P et al. Incidence of thromboembolic stroke and of major bleeding in patients with atrial fibrillation and chronic kidney disease treated with and without warfarin. Int J Nephrol Renovasc Dis 2009;2:33–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonde AN, Lip GY, Kamper AL et al. Net clinical benefit of antithrombotic therapy in patients with atrial fibrillation and chronic kidney disease: a nationwide observational cohort study. J Am Coll Cardiol 2014;64:2471–82. [DOI] [PubMed] [Google Scholar]
- 6.Winkelmayer WC, Liu J, Setoguchi S, Choudhry NK. Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clin J Am Soc Nephrol 2011;6:2662–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar S, de Lusignan S, McGovern A et al. Ischaemic stroke, haemorrhage, and mortality in older patients with chronic kidney disease newly started on anticoagulation for atrial fibrillation: a population based study from UK primary care. BMJ 2018;360:k342. [DOI] [PubMed] [Google Scholar]
- 8.Turakhia MP, Blankestijn PJ, Carrero JJ et al. Chronic kidney disease and arrhythmias: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Eur Heart J 2018;39:2314–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai C, Marcus LQ, Patel P, Battistella M. Warfarin Use in Hemodialysis Patients With Atrial Fibrillation: A Systematic Review of Stroke and Bleeding Outcomes. Can J Kidney Health Dis 2017;4:2054358117735532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herzog CA, Asinger RW, Berger AK et al. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2011;80:572–86. [DOI] [PubMed] [Google Scholar]
- 11.Hart RG, Pearce LA, Asinger RW, Herzog CA. Warfarin in atrial fibrillation patients with moderate chronic kidney disease. Clin J Am Soc Nephrol 2011;6:2599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nochaiwong S, Ruengorn C, Awiphan R, Dandecha P, Noppakun K, Phrommintikul A. Efficacy and safety of warfarin in dialysis patients with atrial fibrillation: a systematic review and meta-analysis. Open Heart 2016;3:e000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan KE, Lazarus JM, Thadhani R, Hakim RM. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol 2009;20:2223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friberg L, Benson L, Lip GY. Balancing stroke and bleeding risks in patients with atrial fibrillation and renal failure: the Swedish Atrial Fibrillation Cohort study. Eur Heart J 2015;36:297–306. [DOI] [PubMed] [Google Scholar]
- 15.Olesen JB, Lip GY, Kamper AL et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med 2012;367:625–35. [DOI] [PubMed] [Google Scholar]
- 16.Jun M, James MT, Ma Z et al. Warfarin Initiation, Atrial Fibrillation, and Kidney Function: Comparative Effectiveness and Safety of Warfarin in Older Adults With Newly Diagnosed Atrial Fibrillation. Am J Kidney Dis 2017;69:734–743. [DOI] [PubMed] [Google Scholar]
- 17.Yang F, Hellyer JA, Than C et al. Warfarin utilisation and anticoagulation control in patients with atrial fibrillation and chronic kidney disease. Heart 2017;103:818–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keskar V, McArthur E, Wald R et al. The association of anticoagulation, ischemic stroke, and hemorrhage in elderly adults with chronic kidney disease and atrial fibrillation. Kidney Int 2017;91:928–936. [DOI] [PubMed] [Google Scholar]
- 19.Garg L, Gupta M, Sabzwari SRA et al. Atrial fibrillation in hypertrophic cardiomyopathy: prevalence, clinical impact, and management. Heart Fail Rev 2019;24:189–197. [DOI] [PubMed] [Google Scholar]
- 20.Limdi NA, Beasley TM, Baird MF et al. Kidney function influences warfarin responsiveness and hemorrhagic complications. J Am Soc Nephrol 2009;20:912–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.United States Renal Data System. Chapter 8: Transition of Care in Chronic Kidney Disease. [Google Scholar]
- 22.Streja E, Gosmanova EO, Molnar MZ et al. Association of Continuation of Statin Therapy Initiated Before Transition to Chronic Dialysis Therapy With Mortality After Dialysis Initiation. JAMA Netw Open 2018;1:e182311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molnar MZ, Gosmanova EO, Sumida K et al. Predialysis Cardiovascular Disease Medication Adherence and Mortality After Transition to Dialysis. Am J Kidney Dis 2016;68:609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bengtson LG, Kucharska-Newton A, Wruck LM et al. Comparable ascertainment of newly-diagnosed atrial fibrillation using active cohort follow-up versus surveillance of centers for medicare and medicaid services in the atherosclerosis risk in communities study. PLoS One 2014;9:e94321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agarwal MA, Garg L, Shah M et al. Relation of Obesity to Outcomes of Hospitalizations for Atrial Fibrillation. Am J Cardiol 2019;123:1448–1452. [DOI] [PubMed] [Google Scholar]
- 26.Garg L, Agrawal S, Agarwal M et al. Influence of Atrial Fibrillation on Outcomes in Patients Who Underwent Primary Percutaneous Coronary Intervention for ST-Segment Elevation Myocardial Infarction. Am J Cardiol 2018;121:684–689. [DOI] [PubMed] [Google Scholar]
- 27.Siontis KC, Zhang X, Eckard A et al. Outcomes Associated With Apixaban Use in Patients With End-Stage Kidney Disease and Atrial Fibrillation in the United States. Circulation 2018;138:1519–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bansal N, Fan D, Hsu CY, Ordonez JD, Marcus GM, Go AS. Incident atrial fibrillation and risk of end-stage renal disease in adults with chronic kidney disease. Circulation 2013;127:569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.US Department of Veterans Affairs, VA Information Resource Center (VIReC). VIReC Research User Guide: VHA Pharmacy Prescription Data. 2nd ed. Hines, IL: VIReC; 2008. http://www.virec.research.va.gov/References/RUG/RUG.htm. Accessed August 2, 2017. [Google Scholar]
- 30.Willey V, Franchino-Elder J, Fu AC et al. Treatment and persistence with oral anticoagulants among newly diagnosed patients with non-valvular atrial fibrillation: a retrospective observational study in a US commercially insured and Medicare Advantage population. BMJ Open 2018;8:e020676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molnar MZ, Kalantar-Zadeh K, Lott EH et al. Angiotensin-converting enzyme inhibitor, angiotensin receptor blocker use, and mortality in patients with chronic kidney disease. J Am Coll Cardiol 2014;63:650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.January CT, Wann LS, Calkins H et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019. [DOI] [PubMed] [Google Scholar]
- 33.Kirchhof P, Benussi S, Kotecha D et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 34.Wizemann V, Tong L, Satayathum S et al. Atrial fibrillation in hemodialysis patients: clinical features and associations with anticoagulant therapy. Kidney Int 2010;77:1098–106. [DOI] [PubMed] [Google Scholar]
- 35.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263–72. [DOI] [PubMed] [Google Scholar]
- 36.Azoulay L, Dell’Aniello S, Simon TA, Renoux C, Suissa S. Initiation of warfarin in patients with atrial fibrillation: early effects on ischaemic strokes. Eur Heart J 2014;35:1881–7. [DOI] [PubMed] [Google Scholar]
- 37.Brodsky SV, Nadasdy T, Rovin BH et al. Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int 2011;80:181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lip GY, Lane DA. Does warfarin for stroke thromboprophylaxis protect against MI in atrial fibrillation patients? Am J Med 2010;123:785–9. [DOI] [PubMed] [Google Scholar]
- 39.Saran R, Robinson B, Abbott KC et al. US Renal Data System 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2020;75:A6–A7. [DOI] [PubMed] [Google Scholar]
- 40.Walker AM. Confounding by indication. Epidemiology 1996;7:335–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


