Abstract
We developed an innovative therapy for ischemic acute kidney injury with discerning kidney-targeted delivery of a selective Toll-like receptor 9 (TLR9) antagonist in mice subjected to renal ischemia reperfusion injury. Our previous studies showed that mice deficient in renal proximal tubular TLR9 were protected against renal ischemia reperfusion injury demonstrating a critical role for renal proximal tubular TLR9 in generating ischemic acute kidney injury. Herein, we used 300–400 nm polymer-based mesoscale nanoparticles that localize to the renal tubules after intravenous injection. Mice were subjected to sham surgery or 30 minutes renal ischemia and reperfusion injury after receiving mesoscale nanoparticles encapsulated with a selective TLR9 antagonist (unmethylated CpG oligonucleotide ODN2088) or mesoscale nanoparticles encapsulating a negative control oligonucleotide. Mice treated with the encapsulated TLR9 antagonist either six hours before renal ischemia, at the time of reperfusion or 1.5 hours after reperfusion were protected against ischemic acute kidney injury. The ODN2088-encapsulated nanoparticles attenuated renal tubular necrosis, inflammation, decreased proinflammatory cytokine synthesis. neutrophil and macrophage infiltration and apoptosis, decreased DNA fragmentation and caspase 3/8 activation when compared to the negative control nanoparticle treated mice. Taken together, our studies further suggest that renal proximal tubular TLR9 activation exacerbates ischemic acute kidney injury by promoting renal tubular inflammation, apoptosis and necrosis after ischemia reperfusion. Thus, our studies suggest a potential promising therapy for ischemic acute kidney injury with selective kidney tubular targeting of TLR9 using mesoscale nanoparticle-based drug delivery.
Keywords: Apoptosis, inflammation, ischemia and reperfusion injury, mesoscale nanoparticle, necrosis, neutrophil
Graphical Abstract
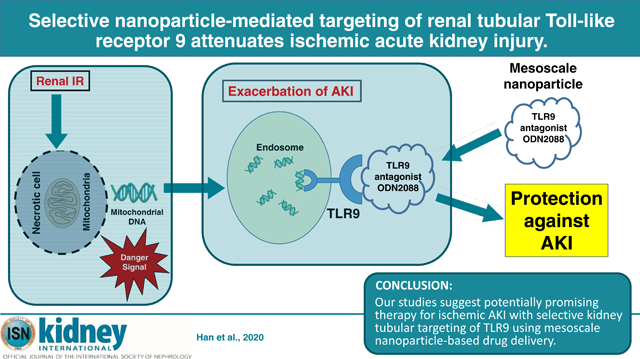
Introduction
Acute kidney injury (AKI) has been a major clinical problem with health care costs of more than $10 billion per year in the US1. Unfortunately, there is no effective preventive measure or therapy for AKI2,3. Renal ischemia reperfusion (IR) injury is a major leading cause of AKI as patients undergoing cardiac, vascular or liver transplant surgical procedures have a ~50–80% chance of developing AKI4,5. Renal IR results in rapid proximal tubular necrosis, and it is becoming increasingly clear that necrotic renal cells after IR release several damage associated molecular pattern (DAMP) ligands that orchestrate additional renal cell death6,7. Indeed, subsequent renal tubular apoptosis with rapid upregulation of pro-inflammatory cytokines and chemokines that causes influx of inflammatory leukocytes into the renal parenchyma potentiates ischemic AKI8–10.
Toll-like receptors (TLRs) are pattern recognition receptors that regulate innate as well as adaptive immunity11,12. Both cell surface and intracellular TLRs (13 identified for mice and 11 for humans) play important roles in protecting against microbial invasion11–13. Numerous endogenous DAMP ligands released after cell death activate TLRs including histones, high mobility group box 1, heat shock proteins and mitochondrial DNA.
TLR9 is a cytosolic receptor for un-methylated cytosine-phosphate-guanosine (CpG) deoxyribonucleic acid found in microbial DNA and DNA viruses14,15. In addition, TLR9 also recognizes endogenous mitochondrial DNA products released from injured cells to trigger MyD88-dependent gene transcription leading to inflammation and apoptosis14,16–18. We recently demonstrated that mice lacking renal proximal tubular TLR9 were protected against ischemic AKI with reduced renal tubular necrosis, inflammation and apoptosis when compared to wild type mice subjected to renal IR. Consistent with these findings, a selective TLR9 agonist ODN1668 exacerbated renal IR injury in wild type but not in renal proximal tubular TLR9 null mice19. TLR9-mediated renal tubular inflammation and injury after renal IR is mediated by NFκB activation as well as caspase 3 and 8 activation.
Based on our previous findings, a selective TLR9 antagonist (ODN2088) would be a potential drug therapy to treat ischemic AKI. Limitation of this approach is that systemic TLR9 antagonist administered will target every cell type in the body. As TLR9 is expressed in almost every organ system and since TLR9 activation produces diverse effects depending on cell types and organs studied15, systemic administration of TLR9 antagonist may have limited therapeutic potential. For example, TLR9 activation induces inflammation in hepatic IR and plays a role in septic AKI16,17,20,21. In contrast, TLR9 induces cytoprotective signaling in immune, cardiac and neuronal cells22–24. Although renal proximal tubular TLR9 may exacerbate renal IR injury by inducing apoptosis and inflammation, other cell types in the kidney may benefit from TLR9 signaling.
To circumnavigate these limitations, we utilized a novel approach to deliver the selective TLR9 antagonist ODN2088 to renal tubular cells to protect against ischemic AKI. We recently demonstrated that mesoscale nanoparticles (MNPs), which are polyethylene glycol (PEG)-coated poly(lactic-co-glycolic acid) (PLGA) polymer particles with diameters of ~300–400 nm, localize into renal tubule cells preferentially over other organs, with greater than 26-fold selectivity25. We packaged the selective TLR9 antagonist ODN2088 into this MNP and tested the hypothesis that selective renal tubular delivery of TLR9 antagonist would attenuate ischemic AKI in mice by reducing renal tubular necrosis, inflammation and apoptosis.
Results
Generation of mesoscale nanoparticles containing ODN2088 and confirmation of renal tubular mesoscale nanoparticle delivery
Lyophilized MNPs containing ODN2088 exhibited a mean diameter of 311.7 ± 12.1 nm and a PDI of 0.316 ± 0.048. These particles encapsulated 89 ng ODN2088 / 1 mg MNP. Lyophilized MNPs containing control ODN exhibited a mean diameter of 311.6 ± 5.0 nm and a PDI of 0.208 ± 0.032. These particles encapsulated 56.7 ng control ODN / 1 mg MNP.
To confirm renal tubular delivery of MNPs, kidney sections of mice injected with MNPs or with saline vehicle control 6 hr prior to renal IR injury were stained with anti-PEG antibody. The renal proximal tubular distribution pattern for the MNPs was confirmed by co-localization of PEG staining with PHA lectin staining in 75 mg/kg MNP injected mice (Figure 1A, 200X images shown, representative of 3 experiments). As described previously, we again show that there was almost no MNP localization to other tubular segments, endothelial cells or mesangial cells in the glomeruli25,26.
Figure 1. Confirmation of kidney selective and renal tubule cell MNP delivery.
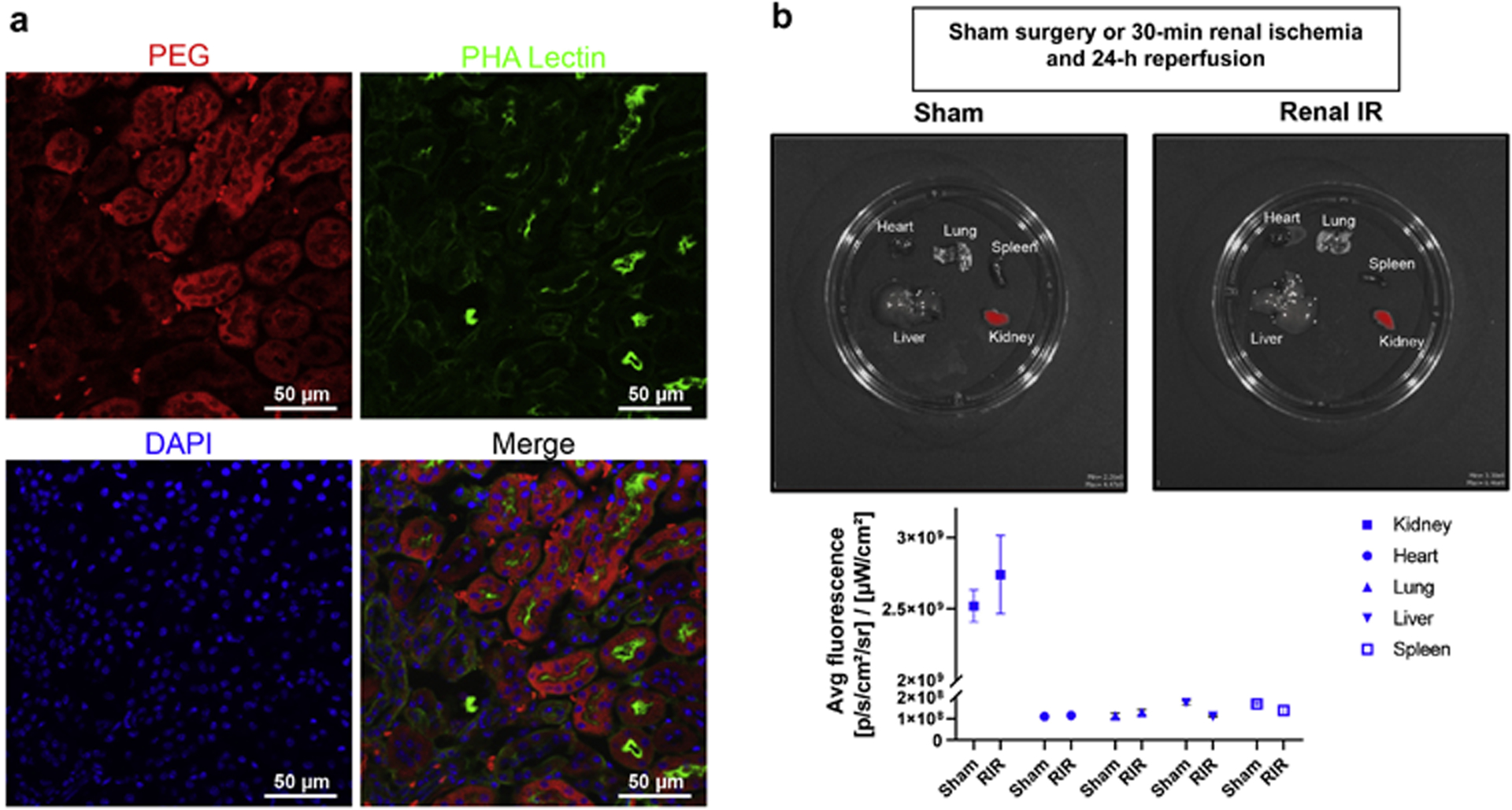
A. Mouse kidney sections stained with anti-PEG antibody to detect MNP localization and PHA-lectin to mark renal proximal tubule cells and counterstained with DAPI to visualize cell nuclei. Mice were injected with ODN2088-encapsulated MNPs or with vehicle control 6 hr before renal IR injury. Renal proximal tubular distribution pattern for PEG was confirmed by co-localization of PEG antibody staining and PHA lectin staining in MNP-injected mice (200X images shown, representative of 3 experiments). Mice injected with vehicle control showed no PEG staining (data not shown). There was almost no PEG localization in other tubular segments, endothelial cells or mesangial cells in the glomeruli. B. (top) In vivo near-infrared fluorescence images of kidney, liver, lung, heart and spleen of mice injected with 75 mg/kg i.v. Cy5 mimic 3,3′-diethylthiadicarbocyanine iodide (DEDC)-loaded MNPs 6 hr before sham-surgery or 30 min renal ischemia and 24 hr reperfusion (Renal IR). (bottom) Quantification of average fluorescence efficiency per square centimeter in each organ demonstrating ~30-fold selective delivery of Cy5 MNPs to the kidneys compared to other organs in both sham-operated mice and mice subjected to renal IR injury (N=3). Data are background subtracted and represent mean ± SEM.
We also confirmed that administration of MNPs does not result in systemic distribution. Figure 1B shows representative in vivo near-infrared fluorescence images of kidney, liver, spleen, lung and heart (top) and average fluorescence intensity quantified (bottom) after injection of 75 mg/kg Cy5-loaded MNPs given i.v. The images show ~30-fold kidney selectivity over other organs including lung, liver, spleen and heart (N=3). Furthermore, administration in mice that were subjected to 30 min renal IR also demonstrated >30-fold kidney selectivity (N=3).
Selective tubular delivery of ODN2088 encapsulated MNP protects against ischemic AKI in mice
Plasma creatinine and BUN values were similar between mice injected with MNPs encapsulated with control ODN (control MNPs) and ODN2088 encapsulated MNPs (MNP-ODN2088) subjected to sham-operation (Figure 2). Mice treated with control MNPs and subjected to renal IR had significantly higher plasma creatinine and BUN as well as kidney NGAL mRNA (N=4–5) compared to sham-operated mice (N=4). We show here that mice treated with 37.5mg/kg or 75mg/kg MNP-ODN2088 6 hr before renal ischemia were protected against ischemic AKI compared to control MNP treated mice as demonstrated by reduced plasma BUN and creatinine as well as kidney NGAL mRNA expression (N=6–7). Furthermore, mice treated with 75mg/kg MNP-ODN2088 at the time of reperfusion or 1.5 hr after reperfusion were also significantly protected against ischemic AKI compared to control MNP treated mice (N=6, Figure 2). In contrast, naked ODN2088 given i.v. 6 hr before renal ischemia failed to protect against ischemic AKI in mice (N=5–6).
Figure 2. MNP-ODN2088-mediated protection against ischemic AKI in mice.
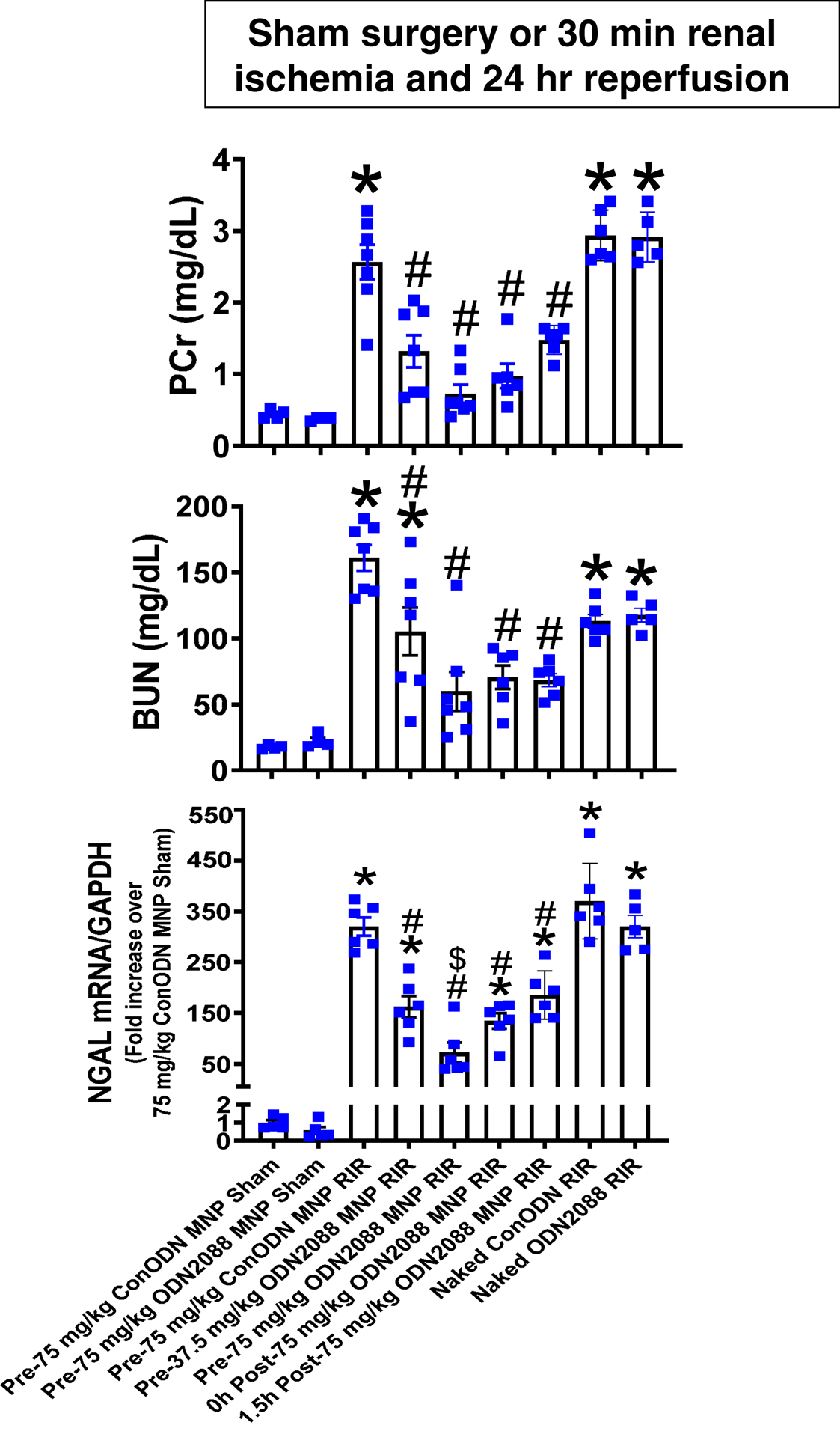
Mice were injected with control MNP-ODN or with ODN2088 encapsulated in MNPs (MNP-ODN2088) and subjected to sham-surgery (N=4) or to 30 min renal ischemia and 24 hr reperfusion (IR, N=6–7). Some mice were injected with MNPODN2088 6 hr before renal ischemia. A separate cohort of mice was injected with MNP-ODN2088 at the time of reperfusion or 1.5 hr after reperfusion. Another cohort of mice were injected with 5 mg/kg naked ODN2088 i.v. 6 hr before renal ischemia (N=5–6). Plasma BUN and creatinine as well as kidney NGAL mRNA were measured. For statistical analysis, the one-way ANOVA plus Tukey’s post hoc multiple comparison test were used to detect significant changes. *P<0.05 vs. control MNP injected mice subjected to sham surgery. #P<0.05 vs. control ODN mice subjected to renal IR. Error bars represent 1 SEM.
MNP-ODN2088 reduces renal tubular necrosis after ischemic AKI
Figure 3A shows representative kidney H&E images of control MNP injected or MNP-ODN2088 injected mice subjected to sham surgery or 30 min renal IR and 24 hr reperfusion (magnification 200X, N=6–7). Control MNP injected mice subjected to renal IR showed severe tubular necrosis and proteinaceous casts as well as increased tubular dilatation and congestion (Figure 3A). MNP-ODN2088 treatment 6 hr before renal ischemia, at the time of reperfusion or 1.5 hr after reperfusion led to decreased renal tubular necrosis, congestion, and cast formation compared to control MNP injected mice subjected to renal IR. Kidneys from MNP-ODN2088 injected mice had significantly reduced renal tubular injury scores compared to control MNP injected mice after IR (Figure 3B). In contrast, naked ODN2088 given i.v. 6 hr before renal ischemia failed to reduce kidney necrosis score in mice subjected to renal IR (N=5–6).
Figure 3. MNP-ODN2088-mediated reduction of renal tubular necrosis after ischemic AKI.
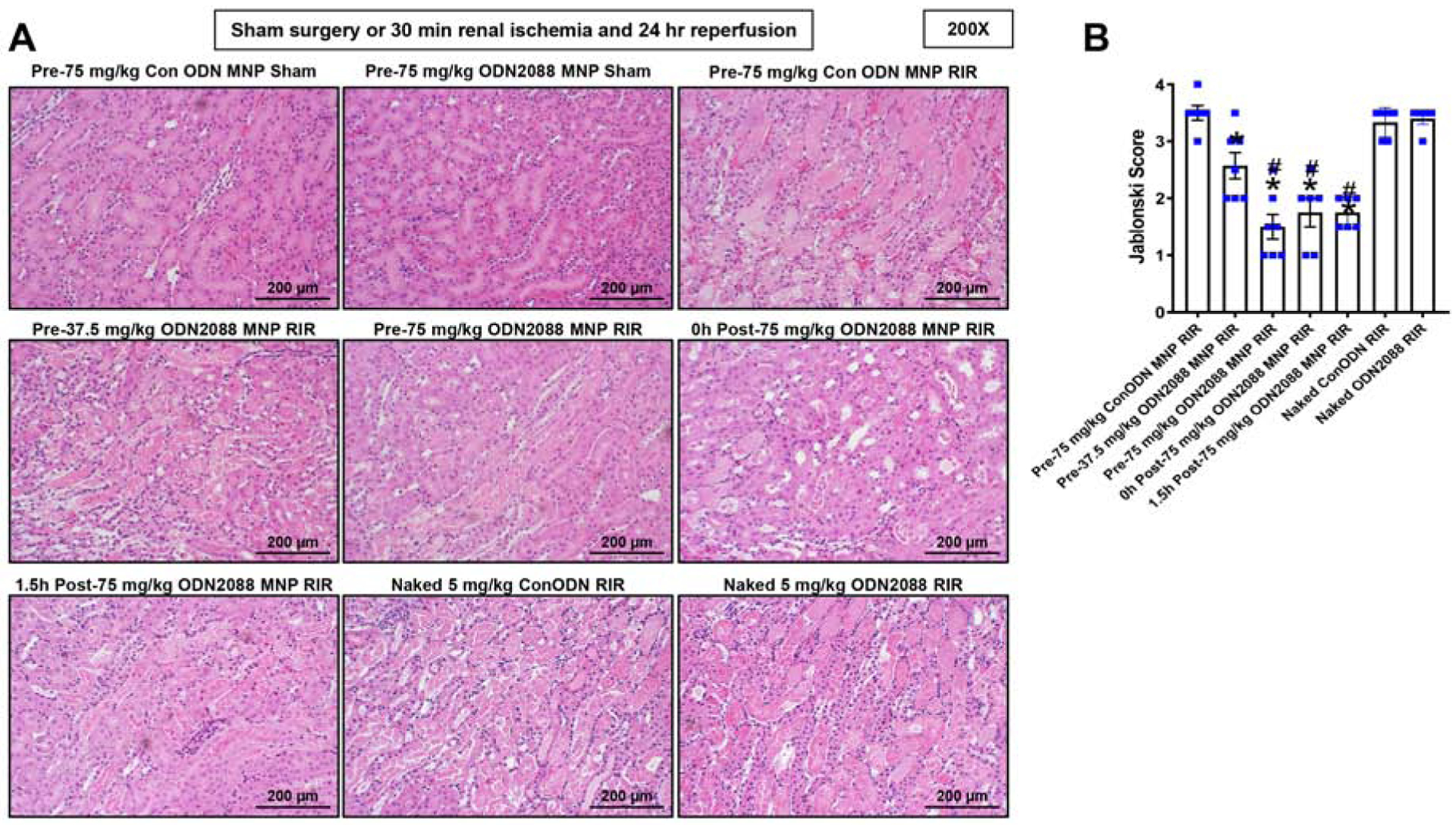
Representative H&E images (from 4–7 experiments) of kidneys from mice injected with control MNPs or with MNP-ODN2088 and subjected to sham surgery (N=4) or to 30 min renal ischemia and 24 hrs reperfusion (N=6–7, magnification 200X). Some mice were injected with MNP-ODN2088 6 hr before renal ischemia. A separate cohort of mice was injected with MNP-ODN2088 at the time of reperfusion or 1.5 hr after reperfusion. Another cohort of mice were injected with 5 mg/kg naked ODN2088 i.v. 6 hr before renal ischemia (N=5–6). Renal injury scores assessing the degree of renal tubular necrosis are also shown here (scale: 0–4) 24 hrs after renal IR. *P<0.05 vs. control MNP injected mice subjected to renal IR injury. For statistical analysis, the Mann–Whitney nonparametric test was used to detect significant changes.
MNP-ODN2088 attenuates kidney apoptosis in mice after ischemic AKI
Figure 4A shows representative TUNEL stained images indicative of renal apoptosis and Figure 4B shows counts of TUNEL positive kidney cells from control MNP injected and MNP-ODN2088 injected mice subjected to sham surgery (N=4) or to renal IR (N=6, magnification 200X). Many TUNEL (fragmented DNA) positive cells were detected, suggestive of renal tubular apoptosis in the kidneys from control MNP injected mice subjected to renal IR injury. TUNEL positive kidney cell counts were significantly reduced in MNPODN2088 injected mice given 6 hr prior to renal ischemia, at the time of reperfusion or 1.5 hr after reperfusion. In contrast, naked ODN2088 given i.v. failed to reduce kidney apoptosis in mice subjected to renal IR (N=5–6). We also detected caspase 3 and caspase 8 activation by measuring cleaved caspase 3 and caspase 8 in the kidney lysates from sham-operated mice and mice subjected to renal IR injury (Figure 4C). Caspase 3 and caspase 8 cleavages were increased in control MNP injected mice subjected to renal IR that were significantly reduced in MNP-ODN2088 injected mice 6 hr before renal ischemia. To show specific inhibition of the TLR9 signaling pathway in tubular cells treated with ODN2088-encapsulated MNPs, freshly isolated mouse proximal tubule cells were treated with a selective murine TLR9 ligand 5 μM ODN1668 for 3 days with or without pretreatment of 1 μM ODN2088 encapsulated MNPs (N=3). We show that MNP encapsulating ODN2088 completely prevented ODN1688-mediated induction of caspase 3 and caspase 8 fragmentation (Figure 4D).
Figure 4. MNP-ODN2088 attenuates kidney apoptosis after ischemic AKI.
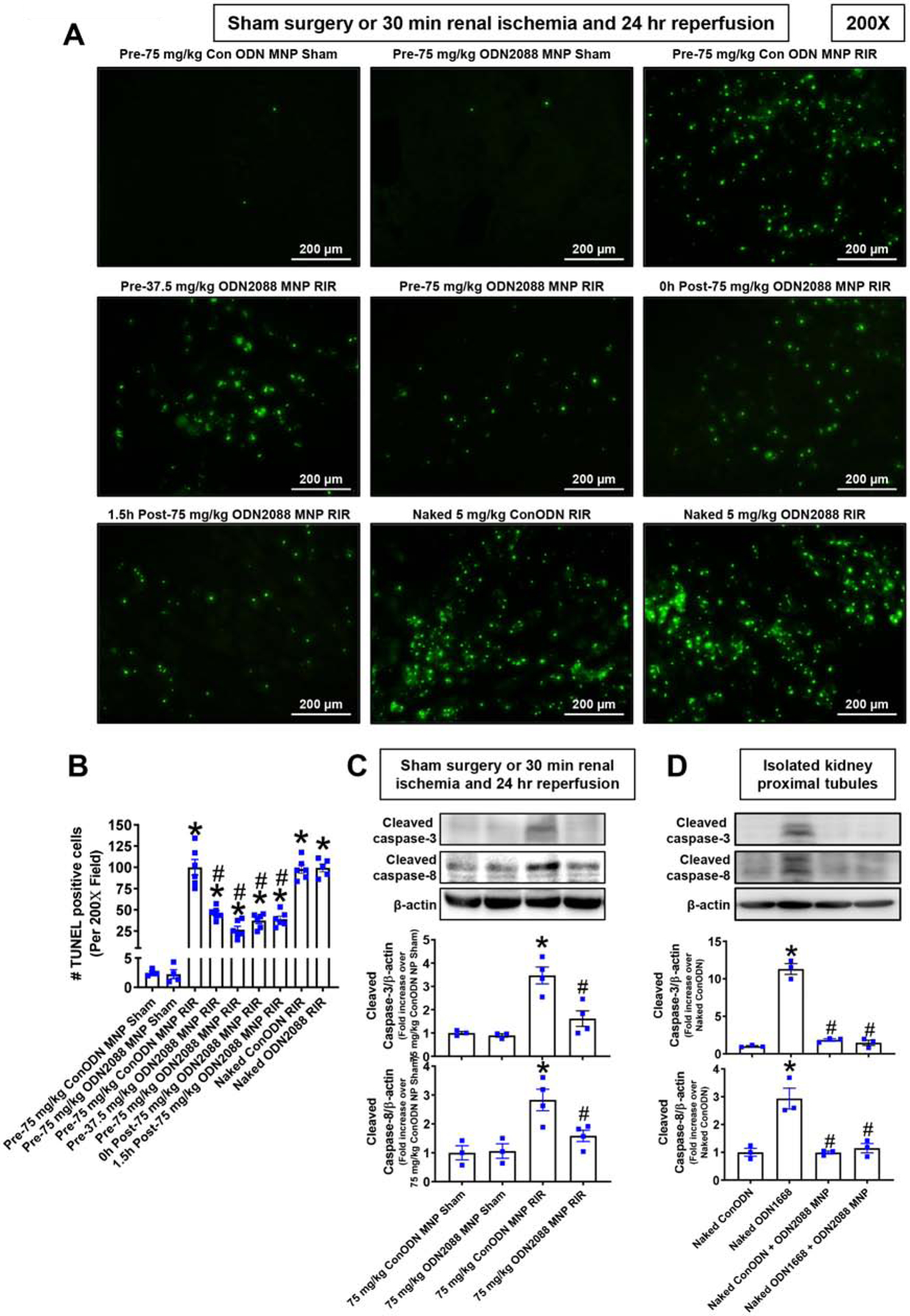
A. Representative images of terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) staining indicative of renal tubular apoptosis and counts of TUNEL positive kidney cells (B) in the kidneys of control MNP injected and MNPODN2088 injected mice subjected to sham-surgery (N=4) or to 30 min renal ischemia and 24 hr reperfusion (N=6, magnification 200X). Some mice were injected with MNP-ODN2088 6 hr before renal ischemia. A separate cohort of mice was injected with MNP-ODN2088 at the time of reperfusion or 1.5 hr after reperfusion. Another cohort of mice were injected with 5 mg/kg naked ODN2088 i.v. 6 hr before renal ischemia (N=5–6). C. We detected caspase 3 and caspase 8 activation by measuring cleaved caspase 3 and caspase 8 in the kidney lysates from sham-operated mice and mice subjected to renal IR injury after control MNP or MNP-ODN2088 treatment. *P<0.05 vs. control MNP injected mice subjected to sham surgery. #P<0.05 vs. control ODN mice subjected to renal IR. Error bars represent 1 SEM. D. We detected caspase 3 and caspase 8 activation in primary cultures of mouse renal proximal tubule cells treated with control ODN or with 5 mM ODN1668 (a selective mouse TLR9 activating ligand). Some proximal tubule cells were pretreated with MNPs encapsulating control ODN or MNPs encapsulating 1 mM ODN2088. *P<0.05 vs. control ODN treated mouse proximal tubule cells. #P<0.05 vs. ODN1668 treated mouse proximal tubule cells. Error bars represent 1 SEM.
MNP-ODN2088 reduces kidney neutrophil infiltration after ischemic AKI
Figure 5A shows representative immunohistochemistry images and Figure 5B shows counts of infiltrating kidney neutrophils in the kidneys of control MNP injected mice and MNP-ODN2088 injected mice subjected to sham surgery (N=4) or renal IR (N=6, magnification 200X). Kidney neutrophil infiltration increased significantly in control MNP injected mice subjected to renal IR. MNP-ODN2088 treatment either 6 hr before renal ischemia, at the time of reperfusion, or 1.5 hr after reperfusion significantly attenuated kidney neutrophil infiltration after renal IR compared to MNP-ODN2088 injected mice. In contrast, naked ODN2088 given i.v. 6 hr before renal ischemia failed to neutrophil infiltration after renal IR in mice (N=5–6).
Figure 5. MNP-ODN2088 decreases kidney neutrophil infiltration after ischemic AKI.
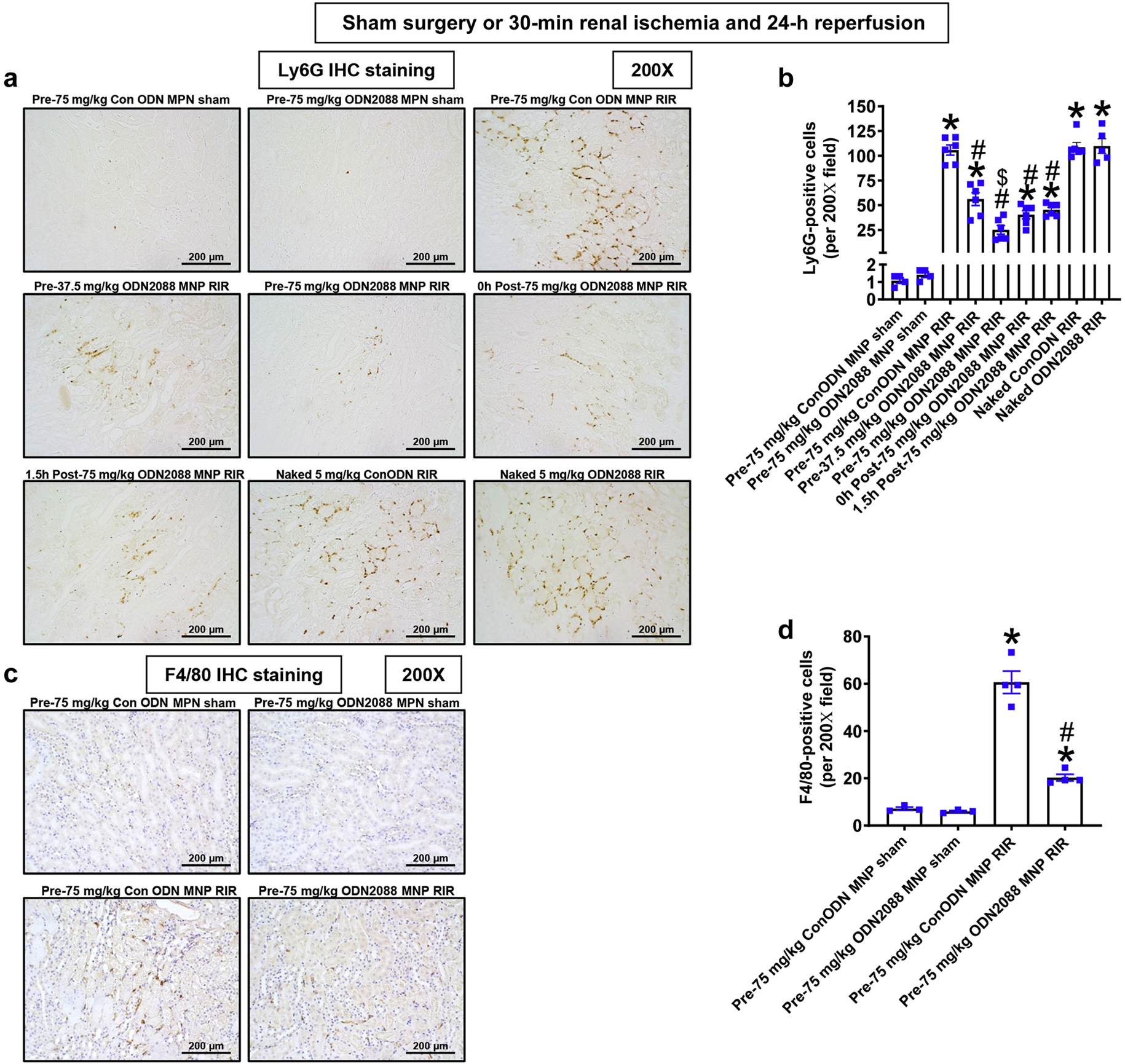
A. Representative images of immunohistochemistry for neutrophils (dark brown) and counts of infiltrating kidney neutrophils (B) in the kidneys of control MNP injected and MNP-ODN2088 injected mice subjected to sham-surgery (N=4) or to 30 min renal ischemia and 24 hr reperfusion (N=6, magnification 200X). Some mice were injected with MNP-ODN2088 6 hr before renal ischemia. A separate cohort of mice was injected with MNP-ODN2088 at the time of reperfusion or 1.5 hr after reperfusion. Another cohort of mice were injected with 5 mg/kg naked ODN2088 i.v. 6 hr before renal ischemia (N=5–6). *P<0.05 vs. control MNP injected mice subjected to sham surgery. #P<0.05 vs. control ODN mice subjected to renal IR. Error bars represent 1 SEM.
MNP-ODN2088 reduces kidney macrophage infiltration after ischemic AKI
Figure 5C shows representative immunohistochemistry images and Figure 5D shows counts of infiltrating kidney macrophages in the kidneys of control MNP injected mice and MNP-ODN2088 injected mice subjected to sham surgery (N=3) or renal IR (N=4, magnification 200X). Kidney macrophage infiltration increased significantly in control MNP injected mice subjected to renal IR. MNPODN2088 treatment either 6 hr before renal ischemia significantly attenuated kidney macrophage infiltration after renal IR compared to MNP-ODN2088 injected mice.
MNP-ODN2088 downregulates pro-inflammatory chemokine and cytokine induction after ischemic AKI
Figure 6 shows fold increases in pro-inflammatory mRNAs normalized to GAPDH for each indicated mRNA in the kidneys of control MNP injected mice and MNP-ODN2088 injected mice subjected to sham surgery (N=4–5) or renal IR (N=6). Ischemic AKI increased all pro-inflammatory genes measured in control MNP injected mice. Consistent with the renal protective role of TLR9 receptor antagonism via reduction of neutrophil and macrophage attracting chemokines, we show that MIP-2 and MCP-1 expression was significantly attenuated in mice injected with MNP-ODN2088 6 hr before renal ischemia, at the time of reperfusion or 1.5 hr after reperfusion. Moreover, IL-6, KC as well as TNF-a induction was attenuated in MNP-ODN2088 injected mice. In contrast, naked ODN2088 given i.v. 6 hr before renal ischemia failed to attenuate pro-inflammatory cytokines in the kidney of mice subjected to renal IR injury (N=5–6).
Figure 6. MNP-ODN2088 attenuates kidney pro-inflammatory chemokine/cytokine induction and neutrophil infiltration after ischemic AKI.
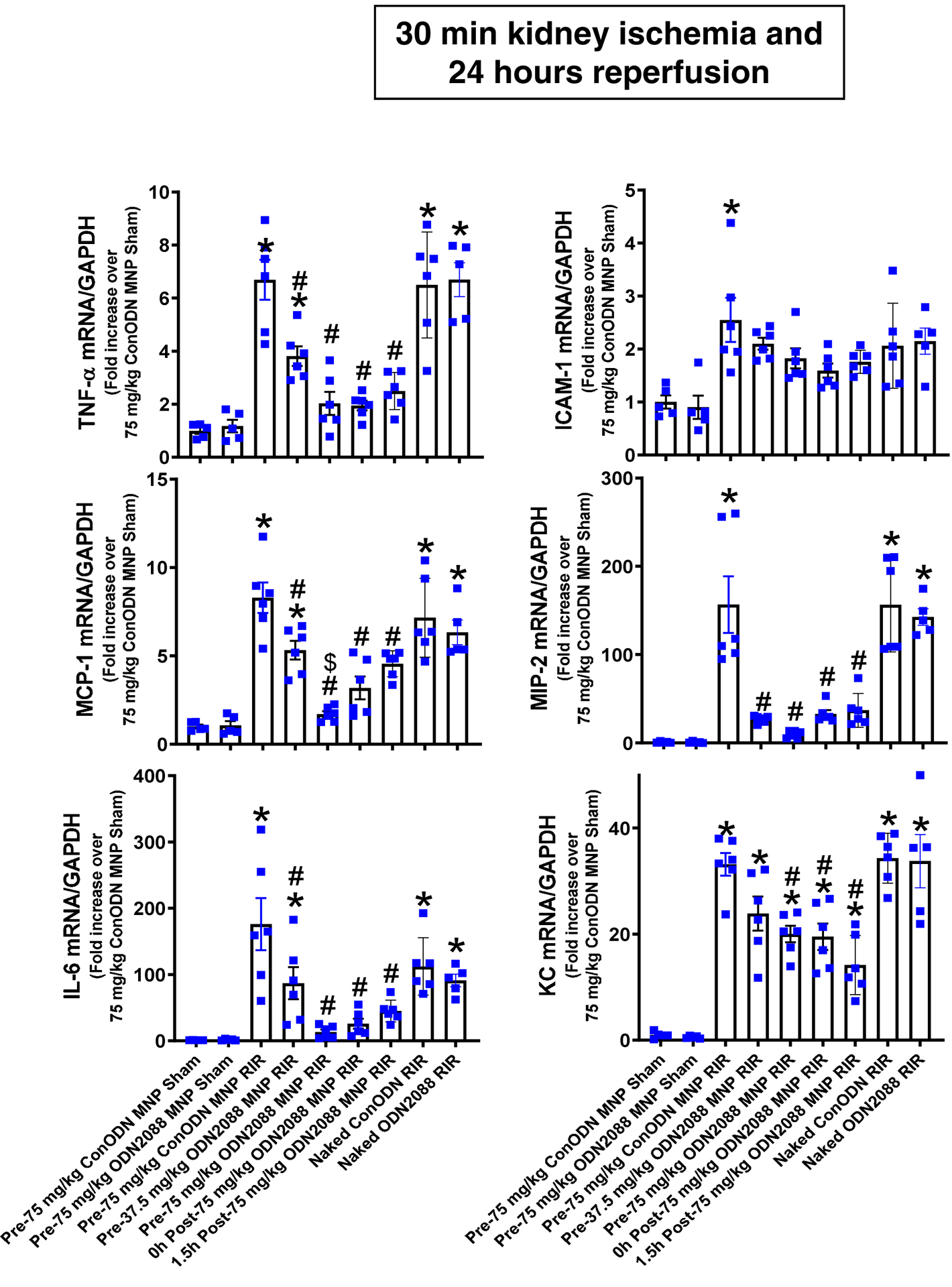
With quantitative RT-PCR, we measured the expression of pro-inflammatory cytokine and chemokine mRNAs in the kidney [keratinocyte-derived cytokine (KC), monocyte chemoattractive protein-1 (MCP-1), macrophage inflammatory protein-2 (MIP-2), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and intercellular adhesion molecule-1 (ICAM-1)] 24 hr after sham-surgery or 30 min renal ischemia. Some mice were injected with MNP-ODN2088 6 hr before renal ischemia. A separate cohort of mice was injected with MNP-ODN2088 at the time of reperfusion or 1.5 hr after reperfusion. Another cohort of mice were injected with 5 mg/kg naked ODN2088 i.v. 6 hr before renal ischemia. Fold increases in pro-inflammatory mRNAs normalized to GAPDH from quantitative RT-PCR reactions for each indicated mRNA (N=4–6) are shown. *P < 0.05 vs. control MNP injected sham-operated mice. #P < 0.05 vs. control ODN injected mice subjected to renal IR injury. Error bars represent 1 SEM.
Discussion
In this study, we found that mesoscale nanoparticle technology allows selective renal tubular delivery of a potent TLR9 antagonist ligand oligonucleotide ODN2088 to protect against ischemic AKI. The MNP-loaded ODN2088 reduced renal tubular necrosis, noted by decreased plasma BUN and creatinine as well as kidney NGAL mRNA induction compared to control MNP injected mice. Increasing clinical significance further, MNP-ODN2088 given at the time of reperfusion or 1.5 hr after reperfusion also protected against renal IR injury.
After IR, necrotic renal cells release mitochondrial DNA that can target intracellular renal tubular TLR920. We previously demonstrated that renal proximal tubular TLR9 activation exacerbates ischemic AKI by promoting renal tubular epithelial apoptosis and inflammation. Mice deficient in renal proximal tubular TLR9 were protected against ischemic AKI with reduced plasma creatinine and kidney NGAL expression as well as renal tubular apoptosis and necrosis19. Furthermore, a selective TLR9 agonist exacerbated renal tubular injury in wild type mice but not in mice deficient in renal tubular TLR9 subjected to renal IR injury. These findings suggest a potential therapeutic role for a specific TLR9 antagonist in protecting against ischemic AKI. However, systemic administration of a selective TLR9 antagonist may not be effective as a treatment for ischemic AKI. This is because TLR9 activation produces complex and perhaps divergent effects depending on cell types and organ systemic affected22,23,27 and renal IR injury results in systemic inflammatory response and extra-renal organ dysfunction28,29. Renal tubular epithelial TLR9 promotes cell injury and death, whereas TLR9 signaling in other cell types may promote cytoprotective effects. Indeed, TLR9 activation protects against cerebral as well as cardiac IR injury via activation of PI3K/AKT signaling22,23. In addition, TLR9 activation may mediate cardiac and neuronal protection by modulating energy metabolism27. Furthermore, in cisplatin-induced AKI, TLR9 may promote tissue protection by promoting accumulation of beneficial regulatory T-cells 30. Consistent with these findings, global TLR9 deficient mice were not protected against ischemic AKI again demonstrating the complex and differential effects of TLR9 activation in different organs and cell types19,31. In agreement with the global TLR9 KO mice studies, we demonstrated here that systemic TLR9 blockade with 5 mg/kg naked ODN2088 (15 nucleotides in length) failed to protect against ischemic AKI in mice. It is possible that global TLR9KO mice or mice injected with naked ODN2088 systemically i.v. were not protected against ischemic AKI, as they may lack the tissue protective regulatory T-cells modulated by TLR9 signaling.
Previous studies demonstrated that i.v. injection of oligonucleotides (~16–21 nucleotides) display broad distribution into most organ systems (liver, kidney, bone marrow, adipocytes and lymph nodes) except CNS, due to the blood brain barrier32. Therefore, broad biodistribution of naked TLR9 antagonist oligonucleotide again demonstrates the need for kidney selective delivery to effect renal protection. It is exciting and clinically relevant that MNPs encapsulating 3.26 or 6.5 mg/kg ODN2088 provided protection against ischemic AKI and attenuated renal tubular necrosis, inflammation, and apoptosis.
The highest dose of MNPs encapsulating ODN2088 (75 mg/kg) used here is higher than doses administered (25–50 mg/kg) to characterize the kidney selective delivery of MNP in previous studies25,26. However, the imaging studies still resulted in similar ~30-fold kidney specific delivery of MNPs compared to other organs studies (Figure 1B). Furthermore, it was unknown until this study whether kidneys subjected to IR would still allow similar kidney selectively delivery of MNPs given i.v., since renal tubular necrosis and inflammation have severe effects on kidney function. Our studies found that MNPs delivered i.v. continued to demonstrate kidney selective delivery, even in mice that were subjected to ischemic AKI, further adding clinical relevance to these studies.
There is no effective therapy or preventive measure for clinical AKI. Many promising potential therapies based on preclinical research failed to be effective clinically33,34. Lack of effective pharmacological therapy is clearly due to the complex nature of clinical AKI in patients with multiple co-morbidities in comparison to simplified laboratory models of AKI35,36. An additional factor that may preclude effective clinical therapy for AKI is that systemic administration of drugs to treat clinical AKI is problematic for several pharmacologic reasons. Drugs given systemically (oral or intravenous) normally target every organ system and multiple cell types. Concomitantly, experimental therapeutics that produce protective effects in renal epithelial cells may produce unwanted detrimental effects in other cell types (e.g., liver, heart) as discussed above. Another reason for the failure of systemic therapies is the pharmacokinetics/biodistribution with respect to the nephron. Often, an insufficient amount of drug reaches the renal tubular cells when drugs are given in nontoxic doses. Therefore, one way to circumvent the confounding effects of multiple organ targeting of drugs and less-than-effective dosing problems is to devise a strategy to selectively deliver or concentrate drugs in the kidney by renal-specific delivery methods.
The recently developed mesoscale nanoparticle (MNP) drug delivery system may hold a promise in targeting renal cells specifically and provide potential therapy for clinical AKI. Recent studies demonstrated that the relatively large (~300–400 nm) polymer-based MNPs preferentially localized to the kidney when compared to other organs by ~26–94 fold over other organs25. MNPs injected via the i.v. route preferentially localized to renal tubular cells and remained for ~7 days. These findings are exciting, as renal tubular cells are the major site of injury during and after ischemic AKI19,37. Moreover, MNP treatment does not have any detrimental effect on renal or hepatic function, inflammation or hematological problems as MNP toxicity was tracked for up to 30 days in preclinical studies25,26,38.
Specific renal tubular localization of MNPs appears to be dependent on the large size (~300–400 nm) and hydrophilic PEG surface chemistry of the MNP25. PEGylation also appears to prevent macrophages scavenging the MNPs39,40. Clinically relevant, the major components of MNP [poly (lactic-co-glycolic acid) and PEG] are FDA approved41. Our previous studies, using both immunofluorescence and immunohistochemistry, showing renal tubular specific delivery of MNPs25, were confirmed in this study. It appears that MNP localization is greater in renal proximal tubules when compared to distal tubules and this is significant as renal proximal tubules are the most susceptible cell types for injury and death due to hypoxia after renal IR19,25,26,33,38,42. Further, i.v. injected MNPs appear to not localize in endothelial cells and renal glomeruli based on immunofluorescence and immunohistochemistry studies.
Another exciting aspect of our study is that MNP-ODN2088 delivered at the time of reperfusion as well as 1.5 hr after reperfusion were as protective as MNP-ODN2088 given 6 hr before renal ischemia. Since MNPs take ~3–6 hrs to maximally localize to the kidney26, these findings show that MNP ODN reaching renal tubular cells 3–7.5 hr after ischemic injury can be effective and equally protective as pretreatment raising clinical significance greatly as not all cases of ischemic AKI can be anticipated in advance.
Based on our findings, MNP-ODN2088 protects against ischemic AKI by reducing renal tubular necrosis and apoptosis after IR. In particular, MNPODN2088 significantly attenuated renal tubular apoptosis in mice subjected to severe ischemic AKI (reduced TUNEL staining and reduced caspase 3 and 8 activation) compared to control MNP treated mice. Moreover, MNPs encapsulating ODN2088 attenuated TLR9 agonist-mediated activation of caspase 3 and caspase 8 activation in cultured mouse proximal tubule cells. Renal IR injury causes renal proximal tubular TLR9 activation to induce kidney apoptosis via caspase activation19,43. In addition to regulating necrosis and apoptosis, MNP-ODN2088 significantly attenuated kidney pro-inflammatory cytokine expression compared to control MNP-treated mice. Consistent with reduced pro-inflammatory cytokines and chemokines, MNP-ODN2088 treated mice had reduced neutrophil infiltration after IR. Taken together, our studies suggest that selective blockade of renal tubular TLR9 effectively attenuates apoptosis and inflammatory pathways that occur during and after renal IR injury.
It is becoming increasingly clear that necrotic cell death is a highly regulated process. Necroptosis is one form of the programmed necrosis and is mediated by members of receptor-interacting protein kinases (RIPK1/3)44. Caspase 8 can protect against necroptosis by cleaving RIPK1/3 and attenuating necroptosis45. Here we show that MNPs encapsulating ODN2088 attenuated caspase 8 activation. These findings imply that in this mouse model of ischemic AKI, caspase 8 is involved in regulating apoptosis rather than necroptosis since both necrosis as well as apoptosis are significantly attenuated by MNP-ODN2088.
In prior work, we reviewed the relationship between the size of nanoparticles and their organ targeting25,26. We determined that nanoparticles less than <10 nm are rapidly cleared from the body and those between 10 and 250 nm are phagocytosed by the reticuloendothelial system and are taken up in the liver or spleen. Microparticles (>1000 nm) deposit mostly in the lungs. It is highly intriguing that mesoscale nanoparticles sizes of ~350 to 400 nm accumulated in the kidney with >30-fold selectivity25,26. It remains to be determined in future mechanistic studies exactly how MNP sizes ranging 300–400 nm injected i.v. can reach renal proximal tubules25,38. Indeed, the size of MNPs used here precludes glomerular filtration (~10 nm cutoff) and we believe they are transcytosed across the peritubular capillary endothelium25,38. Histologically, we showed that MNPs localize predominantly in the basolateral region of proximal tubule epithelial cells25,26. We hypothesize that the MNPs are transcytosed across the endothelial cells (<500 nm) of the peritubular capillaries and are subsequently released into the tubulointerstitium between the capillary and epithelial cells of the renal tubules. The MNPs would likely then be endocytosed by epithelial cells of the tubule. Due to the pressure gradient in this segment of the nephron (50–10 mmHg), we hypothesize that MNPs are endocytosed by peritubular endothelial cells rather than glomerular endothelial cells.
In summary, we demonstrate in this study a novel and innovative method to treat ischemic AKI using MNPs. Our study suggests that kidney targeted MNP-mediated selective drug delivery is an exciting method to treat AKI with improved therapeutic specificity and potentially reduced systemic toxicity by allowing lower drug dosage.
Methods
Generation of mesoscale nanoparticles incorporating selective TLR9 antagonist ODN2088
Mesoscale nanoparticles (MNPs) encapsulating the selective TLR9 antagonist ODN2088 or control ODN were formulated similarly to previously described methods with minor modifications25,26. Briefly, 38–54 kDa molecular weight poly(lactic-co-glycolic acid) (Sigma, MO) was conjugated to 5 kDa carboxylic acid-terminated polyethylene glycol (Nanocs, NY) (PLGA-PEG) prior to particle formulation. The conjugated co-polymer (100 mg) was dissolved in 2 mL acetonitrile. Then, 50 μg of oligonucleotide (ODN2088 or Control ODN; InvivoGen, CA) dissolved in water was added to the co-polymer solution and bath sonicated for 2 minutes. The resultant emulsion was added to a solution of purified water (4 mL) and Pluronic F-68 (75 mL; Fisher Scientific, NH) and centrifuged at 5400 × g for 15 minutes. The nanoparticle pellet was washed with 10 mL purified water and centrifuged under the same specifications. The resultant pellet was resuspended in a 2% sucrose solution and lyophilized for storage at −20°C.
The hydrodynamic diameter and polydispersity index (PDI) of the MNPs was characterized via dynamic light scattering (DLS; Malvern, UK) in a 10 mg/mL phosphate-buffered saline suspension. To quantify oligonucleotide loading into the particles, approximately 10 mg lyophilized particle powder was dissolved in 200 μL acetonitrile and shaken at room temperature for 30 minutes. To this solution, we added 300 μL Tris-EDTA buffer (TE; Fisher Scientific, NH) prior to centrifugation at 31,000 × g for 30 minutes. The supernatant containing liberated oligonucleotide was used for quantification via the Quant-iT RiboGreen RNA Assay Kit (Fisher Scientific, NH) according to manufacturer’s instructions.
Renal IR injury in mice
After Columbia University IACUC approval, 20–25g male C57BL/6 mice (Jackson Labs, ME) were anesthetized with pentobarbital i.p. (Sigma, MO: 50 mg/kg body weight or to effect). Some mice received i.v. MNPs encapsulating control ODN or MNPs encapsulating TLR9 antagonist ODN2088 (37.5 or 75 mg/kg MNP that delivers 3.26 or 6.5 mg/kg ODN2088) 6 hr before renal ischemia. Mice were then subjected to right nephrectomy and 30 min left renal ischemia as described46,47. Sham-operated animals underwent anesthesia followed by laparotomy, right nephrectomy, bowel manipulations and wound closure without renal ischemia. Body temperature were sustained at ~37°C during surgery as well as during recovery from anesthesia. A separate cohort of mice received i.v. 75 mg/kg MNP encapsulating control ODN or MNP encapsulating TLR9 antagonist ODN2088 at the time of reperfusion or 1.5 hr after reperfusion. Finally, some mice received naked ODN2088 6 hr before renal ischemia i.v. to determine whether systemic TLR9 blockade protects against ischemic AKI. For pain management, all mice received 0.5–1 mg/kg s.c. buprenorphine SR prior to surgery.
Detection of renal injury after IR
Twenty-four hours after renal IR or sham surgery, we measured plasma BUN and creatinine using an enzymatic creatinine reagent kit (Thermo Fisher Scientific, MA). We also performed qRT-PCR for kidney NGAL mRNA from mice subjected to sham-surgery or to renal IR injury48.
Histological detection of kidney injury
Twenty-four hours after renal IR injury or sham surgery, kidney H&E sections were assessed using a grading scale of kidney necrotic IR injury to the proximal tubules (0–4, Renal Injury Score) as outlined by Jablonski et al.49. The renal pathologist was blinded to the experimental conditions. Deidentified slides were H&E-stained coronal cross-sections of bivalved whole kidney showing full-thickness cortex and medulla. The cortical and medullary parenchyma was evaluated in its entirety in all the microscopic fields covering the entire slide to generate the Jablonski score.
Detection of kidney apoptosis
Twenty four hrs after renal IR or sham surgery, TUNEL staining detected fragmented DNA as described50. Apoptotic TUNEL positive cells were quantified in 5–7 randomly chosen 200X microscope images fields in the corticomedullary junction and results were expressed as apoptotic cells counted per 200X field. In addition, kidney caspase 3 and caspase 8 immunoblotting were performed as described previously51. Primary antibodies for mouse caspase 3 and caspase 8 were from Cell Signaling Technology (Danvers, MA).
Detection of kidney neutrophil and macrophage infiltration
We performed kidney immunohistochemistry using rat anti-mouse Ly6G monoclonal antibody or with anti-mouse F4/80 (Thermo Fisher Scientific, Inc., Pittsburgh, PA) as described52,53. Primary IgG2a antibody (MCA1212, AbD Serotec, Raleigh, NC) was utilized as a negative isotype control. Quantification of kidney infiltrating neutrophils or macrophages was performed using 5–7 randomly chosen 200X microscope image fields and results were expressed as neutrophils counted per 200x field.
Q-RTPCR for pro-inflammatory cytokine and chemokine mRNA expression
Renal inflammation after IR was also assessed by measuring pro-inflammatory mRNAs including IL-6, intercellular adhesion molecule-1 (ICAM-1), monocyte chemoattractive protein-1 (MCP-1), keratinocyte chemoattractant (KC), macrophage inflammatory protein-2 (MIP-2) and tumor necrosis factor-α (TNF-α) with qRT-PCR as described previously with primers listed in Table 152,54. To confirm equal RNA loading, GAPDH mRNA expression was also measured.
Table 1.
Primers used in quantitative reverse transcription polymerase chain reactions to amplify mouse cDNAs based on published GenBank sequences. Annealing temperatures used for each primer are also provided.
| Primers | Sequence (Sense/Antisense) | Annealing Temp (°C) |
|---|---|---|
| mouse TNF-α | 5’-TACTGAACTTCGGGGTGATTGGTCC-3’ | 65 °C |
| 5’-CAGCCTTGTCCCTTGAAGAGAACC-3’ | ||
| mouse MCP-1 | 5’-ACCTGCTGCTACTCATTCAC-3’ | 60 °C |
| 5’-TTGAGGTGGTTGTGGAAAAG-3’ | ||
| mouse MIP-2 | 5’-CCAAGGGTTGACTTCAAGAAC-3’ | 60 °C |
| 5’-AGCGAGGCACATCAGGTACG-3’ | ||
| mouse KC | 5’-CAATGAGCTGCGCTGTCAGTG-3’ | 60 °C |
| 5’-CTTGGGGACACCTTTTAGCATC-3’ | ||
| mouse IL-6 | 5’-CCGGAGAGGAGACTTCACAG-3’ | 62 °C |
| 5’-GGAAATTGGGGTAGGAAGGA-3’ | ||
| mouse ICAM-1 | 5’-TGTTTCCTGCCTCTGAAGC-3’ | 60 °C |
| 5’-CTTCGTTTGTGATCCTCCG-3’ | ||
| mouse NGAL | 5’-CACCACGGACTACAACCAGTTCGC-3’ | 66 °C |
| 5’-TCAGTTGTCAATGCATTGGTCGGTG-3’ | ||
| GAPDH | 5’-ACCACAGTCCATGCCATCAC-3’ | 65 °C |
| 5’-CACCACCCTGTTGCTGTAGCC-3’ |
Polyethylene glycol (PEG) immunohistochemistry
We performed florescent immunohistochemistry for PEG to detect renal proximal tubular localization of MNPs administered as the surface of MNPs are composed of PEGylated PLGA. Kidneys from mice treated with 75 mg/kg MNP encapsulating ODN2088 6 hr prior to renal IR injury were fixed with 4% paraformaldehyde, dehydrated with 30% sucrose, frozen in O.C.T (Tissue-Tek, Torrance, CA) and cryosectioned (5 μm). Kidney sections were incubated with anti-PEG antibody (ab94764; Abcam, MA) specific to the PEG backbone plus PHA lectin antibody (proximal tubule specific marker, Molecular probes, Eugene, OR). After washes, kidney slides were counterstained with DAPI to visualize cell nuclei, mounted with Vectashield (Vector, Burlingame, CA) mounting media, and imaged with a fluorescent microscope (Olympus IX81, Melville, NY).
In vivo imaging to test kidney selective delivery of mesoscale-nanoparticles (MNPs)
Mice were injected with fluorescent 75 mg/kg i.v. Cy5 mimic 3,3′-diethylthiadicarbocyanine iodide (DEDC) MNP 6 hr before either sham-surgery or renal IR as described above. Twenty-four hr later, heart, lungs, liver, spleen, and kidney were harvested and fluorescently imaged using an IVIS Spectrum Preclinical In Vivo Imaging System (Perkin Elmer, Waltham, MA) using 640/680 nm excitation/emission filters. Living Image Software v4.3 (Perkin Elmer) quantified average fluorescence efficiency per square centimeter in each organ of interest.
Mouse proximal tubule cell culture and TLR9 ligand treatments
Mouse kidney proximal tubules were isolated with Percoll density gradient separation as described previously55. Confluent cells were treated with control oligonucleotides (ODN) or with selective 5 mM TLR9 agonist ligand ODN1668 (InvivoGen, CA) for 3 days as described56. Some cells were pretreated with MNPs encapsulating ODN2088 (87 ng ODN2088/mg MNPs) 30 min before control ODN or ODN1668 treatment. We then performed caspase 3 and caspase 8 immunoblotting as described51.
Statistical analysis
Data were analyzed with Student’s t-test, one-way ANOVA plus Tukey’s post hoc multiple comparison test or Mann–Whitney nonparametric U test to analyze renal injury scores. All data are expressed throughout the text as means ± SEM.
Translational Statement.
Acute kidney injury (AKI) due to ischemia and reperfusion is a frequent clinical problem with high morbidity and mortality. Here, we developed an innovative therapy for ischemic AKI with kidney-targeted delivery of a selective TLR9 antagonist in mice subjected to renal ischemia and reperfusion injury. We show that kidney-targeted delivery of a TLR9 antagonist encapsulated in a mesoscale nanoparticle (MNP) that allow ~30 fold kidney selective drug delivery protects against ischemic AKI by reducing renal tubular necrosis, inflammation and apoptosis. Our studies suggest a potential promising therapy for ischemic AKI with selective kidney tubular targeting of TLR9 using MNP-based drug delivery.
Acknowledgement:
This work was supported in part by Department of Anesthesiology, Columbia University and by NIDDK (DK-109544 and DK-115694) (to H.T.L), Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education NRF-2018R1A6A3A03011633 (to S.J.H) , the National Science Foundation CAREER Award (1752506), NIDDK (DK114321), NCI (CA215719-), the Cancer Center Support Grant (P30 CA008748), the Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research, and the Experimental Therapeutics Center at Memorial Sloan Kettering Cancer Center (to D.A.H). R.M.W was supported by the American Heart Association Postdoctoral Fellowship (17POST33650043) and the City College of New York Grove School of Engineering.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
D.A.H. is a cofounder and officer with equity interest in LipidSense, Inc. and Goldilocks Therapeutics, Inc. D.A.H. is a member of the scientific advisory board of Concarlo Holdings, LLC. R.M.W. is a scientific advisor with equity interest in Goldilocks Therapeutics, Inc. E.A.J is a cofounder and chief medical officer with equity interest in Goldilocks Therapeutics Inc.
References
- 1.Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 2005; 16: 3365–3370. [DOI] [PubMed] [Google Scholar]
- 2.Kork F, Balzer F, Spies CD, et al. Minor Postoperative Increases of Creatinine Are Associated with Higher Mortality and Longer Hospital Length of Stay in Surgical Patients. Anesthesiology 2015; 123: 1301–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hilmi IA, Damian D, Al-Khafaji A, et al. Acute kidney injury following orthotopic liver transplantation: incidence, risk factors, and effects on patient and graft outcomes. Br J Anaesth 2015; 114: 919–926. [DOI] [PubMed] [Google Scholar]
- 4.Jones DR, Lee HT. Perioperative renal protection. Best Pract Res Clin Anaesthesiol 2008; 22: 193–208. [DOI] [PubMed] [Google Scholar]
- 5.Aronson S, Blumenthal R. Perioperative renal dysfunction and cardiovascular anesthesia: concerns and controversies. J Cardiothorac Vasc Anesth 1998; 17: 117–130. [DOI] [PubMed] [Google Scholar]
- 6.Rabadi MM, Kim M, Li H, et al. ATP induces PAD4 in renal proximal tubule cells via P2X7 receptor activation to exacerbate ischemic AKI. Am J Physiol Renal Physiol 2017: ajprenal 00364 02017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosin DL, Okusa MD. Dangers Within: DAMP Responses to Damage and Cell Death in Kidney Disease. J Am Soc Nephrol 2011; 22: 416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jang HR, Rabb H. Immune cells in experimental acute kidney injury. Nat Rev Nephrol 2015; 11: 88–101. [DOI] [PubMed] [Google Scholar]
- 9.Kinsey GR, Okusa MD. Expanding role of T cells in acute kidney injury. Curr Opin Nephrol Hypertens 2014; 23: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kusch A, Hoff U, Bubalo G, et al. Novel signalling mechanisms and targets in renal ischaemia and reperfusion injury. Acta Physiol (Oxf) 2013; 208: 25–40. [DOI] [PubMed] [Google Scholar]
- 11.Leventhal JS, Schroppel B. Toll-like receptors in transplantation: sensing and reacting to injury. Kidney Int 2012; 81: 826–832. [DOI] [PubMed] [Google Scholar]
- 12.Gluba A, Banach M, Hannam S, et al. The role of Toll-like receptors in renal diseases. Nat Rev Nephrol 2010; 6: 224–235. [DOI] [PubMed] [Google Scholar]
- 13.Arumugam TV, Okun E, Tang SC, et al. Toll-like receptors in ischemia-reperfusion injury. Shock 2009; 32: 4–16. [DOI] [PubMed] [Google Scholar]
- 14.Bamboat ZM, Balachandran VP, Ocuin LM, et al. Toll-like receptor 9 inhibition confers protection from liver ischemia-reperfusion Hepatology 2010; 51: 621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robson MG. Toll-like receptors and renal disease. Nephron Exp Nephrol 2009; 113: e1–e7. [DOI] [PubMed] [Google Scholar]
- 16.Yasuda H, Leelahavanichkul A, Tsunoda S, et al. Chloroquine and inhibition of Toll-like receptor 9 protect from sepsis-induced acute kidney injury. Am J Physiol Renal Physiol 2008; 294: F1050–F1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang H, Evankovich J, Yan W, et al. Endogenous histones function as alarmins in sterile inflammatory liver injury through Toll-like receptor 9 in mice. Hepatology 2011; 54: 999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen CJ, Kono H, Golenbock D, et al. Identification of a key parthway required for the sterile inflammatory response triggered by dying cells. Nat Med 2007; 13: 851–856. [DOI] [PubMed] [Google Scholar]
- 19.Han SJ, Li H, Kim M, et al. Kidney Proximal Tubular TLR9 Exacerbates Ischemic Acute Kidney Injury. J Immunol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsuji N, Tsuji T, Ohashi N, et al. Role of Mitochondrial DNA in Septic AKI via Toll-Like Receptor 9. J Am Soc Nephrol 2016; 27: 2009–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu L, Li Y, Hu Z, et al. Small interfering RNA targeting Toll-like receptor 9 protects mice against polymicrobial septic acute kidney injury. Nephron Exp Nephrol 2012; 122: 51–61. [DOI] [PubMed] [Google Scholar]
- 22.Lu C, Ha T, Wang X, et al. The TLR9 ligand, CpG-ODN, induces protection against cerebral ischemia/reperfusion injury via activation of PI3K/Akt signaling. J Am Heart Assoc 2014; 3: e000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao Z, Ren D, Ha T, et al. CpG-ODN, the TLR9 agonist, attenuates myocardial ischemia/reperfusion injury: involving activation of PI3K/Akt signaling. Biochim Biophys Acta 2013; 1832: 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens SL, Ciesielski TM, Marsh BJ, et al. Toll-like receptor 9: a new target of ischemic preconditioning in the brain. J Cereb Blood Flow Metab 2008; 28: 1040–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams RM, Shah J, Tian HS, et al. Selective Nanoparticle Targeting of the Renal Tubules. Hypertension 2018; 71: 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams RM, Shah J, Ng BD, et al. Mesoscale nanoparticles selectively target the renal proximal tubule epithelium. Nano Lett 2015; 15: 2358–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shintani Y, Kapoor A, Kaneko M, et al. TLR9 mediates cellular protection by modulating energy metabolism in cardiomyocytes and neurons. Proc Natl Acad Sci U S A 2013; 110: 5109–5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park SW, Kim M, Kim JY, et al. Paneth cell-mediated multiorgan dysfunction after acute kidney injury. J Immunol 2012; 189: 5421–5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park SW, Chen SW, Kim M, et al. Cytokines induce small intestine and liver injury after renal ischemia or nephrectomy. Lab Invest 2011; 91: 63–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alikhan MA, Summers SA, Gan PY, et al. Endogenous Toll-Like Receptor 9 Regulates AKI by Promoting Regulatory T Cell Recruitment. J Am Soc Nephrol 2016; 27: 706–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Yun Z, Tan Z, et al. The role of Toll-like receptor (TLR) 2 and 9 in renal ischemia and reperfusion injury. Urology 2013; 81: 1379 e1315–1320. [DOI] [PubMed] [Google Scholar]
- 32.Geary RS, Norris D, Yu R, et al. Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv Drug Deliv Rev 2015; 87: 46–51. [DOI] [PubMed] [Google Scholar]
- 33.Molitoris BA. Therapeutic translation in acute kidney injury: the epithelial/endothelial axis. J Clin Invest 2014; 124: 2355–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Caestecker M, Humphreys BD, Liu KD, et al. Bridging Translation by Improving Preclinical Study Design in AKI. J Am Soc Nephrol 2015; 26: 2905–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jo SK, Rosner MH, Okusa MD. Pharmacologic treatment of acute kidney injury: why drugs haven’t worked and what is on the horizon. Clin J Am Soc Nephrol 2007; 2: 356–365. [DOI] [PubMed] [Google Scholar]
- 36.Elapavaluru S, Kellum JA. Why do patients die of acute kidney injury? Acta Clin Belg Suppl 2007: 326–331. [DOI] [PubMed] [Google Scholar]
- 37.Yuan X, Lee JW, Bowser JL, et al. Targeting Hypoxia Signaling for Perioperative Organ Injury. Anesth Analg 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams RM, Jaimes EA, Heller DA. Nanomedicines for kidney diseases. Kidney Int 2016; 90: 740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yap ML, Wang X, Pietersz GA, et al. Mesoscale Nanoparticles: An Unexpected Means for Selective Therapeutic Targeting of Kidney Diseases! Hypertension 2018; 71: 61–63. [DOI] [PubMed] [Google Scholar]
- 40.Pietersz GA, Wang X, Yap ML, et al. Therapeutic targeting in nanomedicine: the future lies in recombinant antibodies. Nanomedicine (Lond) 2017; 12: 1873–1889. [DOI] [PubMed] [Google Scholar]
- 41.Tong R, Gabrielson NP, Fan TM, et al. Polymeric Nanomedicines Based on Poly(lactide) and Poly(lactide-co-glycolide). Curr Opin Solid State Mater Sci 2012; 16: 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Endre ZH, Ratcliffe PJ, Tange JD, et al. Erythrocytes alter the pattern of renal hypoxic injury: predominance of proximal tubular injury with moderate hypoxia. Clin Sci (Lond) 1989; 76: 19–29. [DOI] [PubMed] [Google Scholar]
- 43.Bao W, Xia H, Liang Y, et al. Toll-like Receptor 9 Can be Activated by Endogenous Mitochondrial DNA to Induce Podocyte Apoptosis. Sci Rep 2016; 6: 22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pefanis A, Ierino FL, Murphy JM, et al. Regulated necrosis in kidney ischemia-reperfusion injury. Kidney Int 2019; 96: 291–301. [DOI] [PubMed] [Google Scholar]
- 45.Tummers B, Green DR. Caspase-8: regulating life and death. Immunol Rev 2017; 277: 76–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim M, Park SW, Kim M, et al. Selective Renal Over-Expression of Human Heat Shock Protein 27 Reduces Renal Ischemia-Reperfusion Injury in Mice. Am J Physiol Renal Physiol 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee HT, Park SW, Kim M, et al. Interleukin-11 protects against renal ischemia and reperfusion injury. Am J Physiol Renal Physiol 2012; 303: F1216–F1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 2003; 14: 2534–2543. [DOI] [PubMed] [Google Scholar]
- 49.Jablonski P, Howden BO, Rae DA, et al. An experimental model for assessment of renal recovery from warm ischemia. Transplantation 1983; 35: 198–204. [DOI] [PubMed] [Google Scholar]
- 50.Park SW, Chen SW, Kim M, et al. Human heat shock protein 27 overexpressing mice are protected against acute kidney injury after hepatic ischemia and reperfusion. Am J Physiol Renal Physiol 2009; 297: F885–F894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee HT, Xu H, Siegel CD, et al. Local Anesthetics Induce Human Renal Cell Apoptosis. Am J Nephrol 2003; 23: 129–139. [DOI] [PubMed] [Google Scholar]
- 52.Park SW, Kim M, Brown KM, et al. Inhibition of sphingosine 1-phosphate receptor 2 protects against renal ischemia-reperfusion injury. J Am Soc Nephrol 2012; 23: 266–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park SW, Kim M, Kim M, et al. Sphingosine kinase 1 protects against renal ischemia-reperfusion injury in mice by sphingosine-1-phosphate1 receptor activation. Kidney Int 2011; 80: 1315–1327. [DOI] [PubMed] [Google Scholar]
- 54.Park SW, Kim JY, Ham A, et al. A1 adenosine receptor allosteric enhancer PD-81723 protects against renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 2012; 303: F721–F732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vinay P, Gougoux A, Lemieux G. Isolation of a pure suspension of rat proximal tubules. Am J Physiol 1981; 241: F403–F411. [DOI] [PubMed] [Google Scholar]
- 56.Tsai F, Homan PJ, Agrawal H, et al. Bim suppresses the development of SLE by limiting myeloid inflammatory responses. J Exp Med 2017; 214: 3753–3773. [DOI] [PMC free article] [PubMed] [Google Scholar]


