Abstract
We report an enantioselective phase transfer α-chlorination of β-keto esters catalyzed by hybrid amide-based Cinchona derivatives. The chlorination process proceeds with proper quantitative yields (up to <99%) and high asymmetric induction (up to 97% ee). We show that the use of only 0.5 mol % hybrid catalyst based on a Cinchona core allows the chlorination reaction to be conducted in a highly enantioselective manner with various indanone and tetralone carboxylate esters.
Introduction
The challenge of stereoselectively introducing a halogen atom into organic compounds has become one of the forefront research issues in organic chemistry in recent years.1 The diverse pharmaceutical activity of optically active halogen-containing molecules, as well as their ability to undergo further stereoselective modifications, plays a key role in modern organic synthesis and, consequently, in the design of drug targets.2,3 Halogenated molecules often display distinct characteristics compared to their parent compounds, owing to the unique hydrophobic, space-filling, and electronic properties of the halogen atom.4 It is therefore not surprising that asymmetric construction of chlorine-containing molecules has become a fascinating and intensely investigated research field.
However, catalytic asymmetric installation of halogenated carbon centers (especially quaternary ones) poses a great synthetic challenge; hence, numerous approaches to this problem have been developed. Since Hintermann and Togni5 reported the first example of α-halogenation of β-keto esters with a Ti(IV)/TADDOL complexes, extensive studies have been undertaken. Most such procedures are based on metal catalysis, including a Lewis acid transition metal species accompanied by a chiral ligand.6,7 Organocatalytic approaches for asymmetric halogenation have also been developed, including enamine catalysis,8 diaminomethylenemalononitrile,9Cinchona-mediated processes,10 and indirect approaches using halogenated substrates in enantioselective C–C bond forming reactions.11 There are also reports related to chiral quaternary ammonium catalysts.12
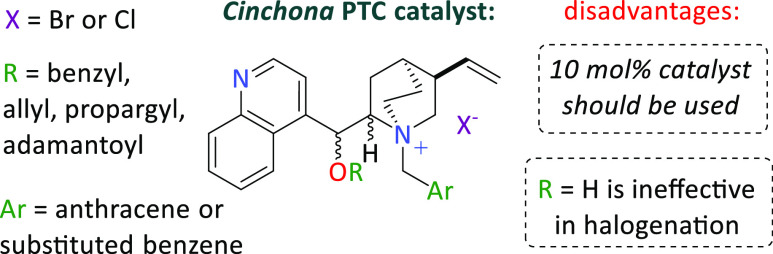
In 2013, the Luo group13 reported the first catalytic enantioselective chlorination of β-keto esters by employing this type of catalysis (Figure 1).
Figure 1.
Previously reported Cinchona PTC catalysts in asymmetric chlorination.
They found that only a bulky group at the bridgehead nitrogen of adamantoyl-derivatized Cinchona alkaloids was crucial for the expected stereoselectivity. It is worth mentioning here that these catalysts were actually inefficient when the C9 hydroxyl function was unprotected. The main decisive factor for successful enantiodiscrimination using these catalysts is their ionic interactions. However, such interactions leading to the formation of ionic pairs do not provide a defined stereochemical course of these reactions. A partial solution to such a dysfunction emerged with the introduction of a new family of hybrid catalysts14,15 having both an ionic function and a hydrogen bond donor. This approach allows for selective chlorination of β-keto esters with satisfactory values of asymmetric induction.16,17 Interestingly, the abovementioned catalysts are rather rare examples of onium, showing very good catalytic efficiency in α-halogenation reactions of carbonyl compounds. Despite extensive work in the PTC field, there are only a few cases in which the generation of a quaternary stereogenic center that contains the chlorine atom has been accomplished, and these exclusively involve the asymmetric α-chlorination reaction of dicarbonyl compounds.
Recently, our team has introduced a new class of hybrid derivatives of Cinchona alkaloids, which have been thoroughly studied in alkylation reactions of the imino glycine t-butyl ester14c and epoxidation of α,β-unsaturated ketones.15c The obtained results have demonstrated the effectiveness of the amide derivatives, showing a beneficial effect of the hydrogen bond donor in the catalyst structure. Given the satisfactory selectivities obtained so far with our library of hybrid catalysts in alkylation, we now targeted the use of these quaternary derivatives for a more challenging reaction: α-chlorination of β-keto esters.
Results and Discussion
At the beginning, a set of hybrid catalysts from the amide-based family of Cinchona derivatives were prepared according to our synthetic procedure that was described earlier,14c as presented in Scheme 1.
Scheme 1. Synthesis of Selected Amide-Based Cinchona Alkaloids.
To determine a suitable catalyst system for the enantioselective chlorination of β-keto esters, we initially applied a reaction of methyl ester indanone carboxylate 13a with N-chlorosuccinimide (NCS) as the chlorine electrophilic source. After optimization of conditions (see the Supporting Information), the reaction was carried out in toluene at room temperature for 5 min in the presence of 0.5 mol % catalyst (1–12) and potassium fluoride as a weak base. As Table 1 shows, all of the catalysts promoted the reaction with almost quantitative yields, even when a 0.5 mol % catalyst was used; however, enantioselectivity of the obtained product 14a strongly depended on the amide function attached to the bridgehead nitrogen in the catalyst.
Table 1. Catalyst Screeninga.
| entry | catalyst | yield (%)b | ee (%)c |
|---|---|---|---|
| 1 | 1 | 99 | 56 |
| 2 | 2 | 99 | 45 |
| 3 | 3 | 99 | 50 |
| 4 | 4 | 99 | 71 |
| 5 | 5 | 99 | 76 |
| 6 | 6 | 99 | –75 |
| 7 | 7 | 99 | 62 |
| 8 | 8 | 98 | 48 |
| 9 | 9 | 98 | 51 |
| 10 | 10 | 98 | 30 |
| 11 | 11 | 99 | 44 |
| 12 | 12 | 99 | 47 |
Molar ratio: 13a (0.2 mmol), NCS (0.21 mmol), catalyst 1−12 (0.5 mol %), and KF (0.4 mmol).
Isolated yields.
The ee values were determined by HPLC analysis using a chiral column Chiralcel OD-H.
Comparison of the catalysts based on cinchonidine showed that the introduction of an electron-withdrawing or -donating substituent attached to the aromatic ring reduces selectivity and enantiomeric excess in all cases is moderate (≤50% ee). The best result—71% ee—was obtained for catalyst 4 with the sterically demanding substituent 2-Ph, which can be related to the inhibition of substituent rotation. Given such a favorable result at room temperature, we decided to examine catalyst 5, based on a quinine scaffold, for which the enantiomeric induction increased to 76% ee. Thus, we also examined catalyst 6 (diastereoisomer of 5 based on quinidine), thereby obtaining product 14a with almost complete inversion of the configuration (−75% ee). Interestingly, there has been no report regarding the possibility of using two diastereoisomers of Cinchona alkaloids to generate both enantiomers of the chlorination product or especially maintaining complete inversion of configuration on the quaternary carbon atom. Finally, considering such a fast reaction (only 5 min) and almost quantitative yield, we decided to examine the impact of the temperature on enantioselectivity using the best catalyst 5. In Table 2, we presented results for different temperatures under the reaction conditions described in Table 1.
Table 2. Optimization of the Temperature under the Reaction Conditions Described in Table 1a.
| entry | temperature (oC) | time (min) | yield (%)b | ee (%)c |
|---|---|---|---|---|
| 1 | 25 | 5 | 99 | 78 |
| 2 | 0 | 5 | 99 | 82 |
| 3 | –20 | 10 | 99 | 87 |
| 4 | –40 | 15 | 99 | 91 |
| 5 | –50 | 30 | 99 | 93 |
| 6 | –79 | 80 | 99 | 92 |
Molar ratio: 13a (0.2 mmol), NCS (0.21 mmol), KF (0.4 mmol), and catalyst 5 (0.5 mol %).
Isolated yields.
The ee values were determined by HPLC analysis using a chiral column Chiralcel OD-H.
As might be expected, lowering of the temperature to values below 0 °C highly increased the enantioselectivity (to 93% ee at −50 °C). Notably, the reaction proceeded very well and the yield of the isolated product was excellent despite such rigorous conditions.
Next, we examined how the type of ester group in the substrate can affect the enantiomeric excess of the obtained products. To this end, we used the set of nine β-keto esters 13a–13i in Scheme 2.
Scheme 2. Study of the Ester Group.
For example, compound 14a was obtained using 13a (1.0 mmol), NCS (1.05 mmol), KF (2 mmol), and catalyst 5 (0.5 mol%) in toluene (5 mL) for 30 min at −50 °C.
The reaction with simple methyl and ethyl esters of indanone carboxylates with NCS provided the corresponding products 14a, 14a′, and 14b in excellent yields and with very good enantioselectivities (up to 93% ee). To our knowledge, only Miura group’s presented organocatalytic synthesis of these products with moderate enentioselectivities (78% for methyl ester).9 Likewise, the use of bulky esters such as i-propyl, t-butyl, or adamanthyl in the chlorination reaction gave chlorinated products 14c–14e and 14e′ with very high enantiomeric excess (up to 97%). For 14g–14i derivatives based on tetralone, a decrease in the asymmetric induction is observed; however, it is still a satisfactory value (up to 86% ee), which is not always achievable with the use of known organocatalysts.
Based on the best results for catalyst 5, we proposed a plausible transition state model depicted in Figure 2.
Figure 2.

Proposed transition state model for catalyst 5 and substrate 13a.
As can be seen, the negatively charged enolate is stabilized by the hydrogen bonds from the amide function. A key element determining the high enantioselective manner is the phenyl ring which blocks the si-face of the enolate. In addition, the hydroxyl group of the catalyst directs the electrophile from the re-face of the enolate, further increasing the selectivity of the underlying nucleophile attack, which is agreement with the obtained results. This hypothesis can be supported by reaction with the use of an N-methylated analogue of catalyst 5 which generated nearly racemic product 14a.
With the reaction conditions optimized, we attempted to explore the substrate scope (Scheme 3).
Scheme 3. Study of β-Keto Derivative Scope.
(a)Molar ratio: NCS (1.05 equiv), catalyst 5 (0.005 equiv), and KF (2 equiv). (b) Isolated yields. (c) The ee values were determined by HPLC analysis using a chiral column Chiralcel OD-H.
As shown in Scheme 3, variously substituted cyclic β-keto esters 13(j–p), including both electron-withdrawing and electron-donating substituents in the phenyl ring, were succesfully chlorinated with excellent yields (99%) and high enantiopurity (up to 93% ee) within 30 min. Substrates with the halogen-containing substituent in the phenyl ring gave similar results.
Conclusions
In conclusion, we successfully carried out the highly enantioselective α-chlorination of a wide range of β-keto esters using cheap and readily available hybrid amide-based catalysts. Significantly, only 0.5 mol % Cinchona catalyst is enough to give excellent yields (up to 99%) and very high enantiomeric excess (ee up to 97%), even when methyl ester of indanone carboxylate was used as a substrate. In addition, we have also shown the possibility of obtaining both enantiomers of the chlorination product, while maintaining a high asymmetric induction value. Further investigation involving such substrates and a wide range of various electrophiles is currently underway at our laboratory.
Experimental Section
Materials and Methods
Chemicals and solvents were purchased from commercial suppliers and used without further purification. Column chromatography was carried out using Merck Kieselgel 60 (63–100 μm mesh size), and TLC was carried out on Merck Kieselgel F254 plates. Melting points were determined using a Boëtius M HMK hot-stage apparatus and were uncorrected. The NMR spectra were recorded on a Bruker Mercury 400 instrument. Chemical shifts are reported in ppm and are set to the solvent residue peak. The splitting pattern of multiplets is described by abbreviations (s—singlet, d—doublet, t—triplet, q—quartet, dd—doublet of doublets, m—multiplet, c—covered signal, and b—broad peak). J coupling constants values are reported in Hz. Mass spectral analyses were performed with the ESI-TOF technique on a Mariner mass spectrometer from PerSeptive Biosystem. Specific rotations were measured using a JASCO P-2000 polarimeter. [α]D values are given in units of 10–1 deg cm2 g–1. The enantiomeric excesses of products were determined by chiral HPLC analysis.
Initially, β-keto esters were synthesized according to the literature procedures.18 Amide-based Cinchona catalysts 1–12 were prepared according to our previous paper.14c Catalysts 5 and 6 have not been previously reported. Chlorination products 13(a–p) are known from the literature, and their analytical data (NMR spectra and MS) fully matched those reported previously in the literature.13,17,19−21
(1S,2S,4S)-1-[({[1,1′-Biphenyl]-2-yl}carbamoyl)methyl]-5-ethenyl-2-[(R)-hydroxy(6-methoxyquinolin-4-yl)methyl]-1-azabicyclo[2.2.2]octan-1-ium Bromide (5)
Following the literature procedure14c and using the corresponding bromoamide (1.0 g, 3.4 mmol), the product 5 (2.00 g, 3.23 mmol, 95%) was obtained as colorless powder (mp 114–115 °C). [α]D20 = −110.6 (c = 1.0, MeOH). 1H NMR (500 MHz, DMSO-d6): δ 10.63 (s, 1H), 8.78 (d, J = 4.5 Hz, 1H), 7.92 (d, J = 9.2 Hz, 1H), 7.71 (d, J = 4.5 Hz, 1H), 7.45 (dd, J = 8.4, 5.8 Hz, 6H), 7.35 (t, J = 7.6 Hz, 3H), 7.22–7.18 (m, 2H), 6.69 (d, J = 2.0 Hz, 1H), 5.78 (s, 1H), 5.64–5.54 (m, 1H), 5.00 (d, J = 17.4 Hz, 1H), 4.93 (d, J = 10.6 Hz, 1H), 4.71 (d, J = 16.6 Hz, 1H), 4.48–4.35 (m, 3H), 3.75 (d, J = 12.5 Hz, 1H), 3.64–3.51 (m, 5H), 2.76 (s, 1H), 2.07 (s, 2H), 1.91 (d, J = 5.2 Hz, 1H), 1.89–1.81 (m, 1H), 0.93 (t, J = 9.7 Hz, 1H). 13C{1H} NMR (125 MHz, DMSO-d6): δ 163.5, 157.8, 147.2, 143.6, 143.4, 138.9, 138.0, 137.9, 132.8, 131.3, 130.3, 128.4, 128.3, 128.0, 127.5, 127.2, 127.1, 125.4, 122.2, 120.2, 115.3, 100.9, 65.7, 62.8, 59.5, 58.6, 56.6, 55.7, 36.5, 25.3, 24.8, 21.3. HRMS ESI (m/z): calcd for C34H36N3O3 [M]+, 534.2757; found, 534.2760.
(1S,2R,4S)-1-[({[1,1′-Biphenyl]-2-yl}carbamoyl)methyl]-5-ethenyl-2-[(S)-hydroxy(6-methoxyquinolin-4-yl)methyl]-1-azabicyclo[2.2.2]octan-1-ium Bromide (6)
Following the literature procedure14c and using the corresponding bromoamide (1.00 g, 3.4 mmol), the product 6 (1.99 g, 3.26 mmol, 95%) was obtained as colorless powder (mp 137–138 °C). [α]D20 = 117.5 (c = 1.0, MeOH). 1H NMR (500 MHz, DMSO-d6): δ 10.54 (s, 1H), 8.78 (d, J = 4.5 Hz, 1H), 7.93 (d, J = 9.8 Hz, 1H), 7.72 (d, J = 4.5 Hz, 1H), 7.54–7.48 (m, 1H), 7.47–7.33 (m, 9H), 7.28 (t, J = 7.1 Hz, 1H), 6.71 (s, 1H), 6.01–5.93 (m, 1H), 5.89 (s, 1H), 5.26 (dd, J = 13.7, 6.2 Hz, 2H), 4.67 (d, J = 16.5 Hz, 1H), 4.42–4.27 (m, 3H), 3.73 (t, J = 10.0 Hz, 1H), 3.65 (t, J = 11.0 Hz, 1H), 3.55 (s, 3H), 3.43–3.36 (m, 1H), 2.78 (dd, J = 16.2, 8.7 Hz, 1H), 2.04 (t, J = 12.0 Hz, 1H), 1.90 (s, 1H), 1.84 (d, J = 7.7 Hz, 2H), 0.87 (t, J = 7.2 Hz, 1H). 13C{1H} NMR (125 MHz, DMSO-d6): δ 163.5, 157.8, 147.2, 143.6, 143.4, 138.9, 138.0, 137.9, 132.8, 131.3, 130.3, 128.4, 128.3, 128.0, 127.5, 127.2, 127.1, 125.4, 122.2, 120.2, 115.3, 100.9, 65.7, 62.8, 59.5, 58.6, 56.6, 55.7, 36.5, 25.3, 24.8, 21.3. HRMS ESI (m/z): calcd for C34H36N3O3 [M]+, 534.2757; found, 534.2758.
General Procedure for the Asymmetric Chlorination of β-Keto Esters 13(a–p)
A mixture of substrate 13(a–p) (0.2 mmol), the catalyst (0.001 mmol), and solid KF (2 equiv) was stirred in toluene (1 mL) at room temperature for 20 min. Then, the mixture was cooled to appropriate temperature, and N-chlorosuccinimide (0.21 mmol) was added in one portion. The reaction was conducted for 5–80 min at this temperature and quenched with saturated ammonium chloride solution. The product 14(a–p) was extracted with ethyl acetate (2 × 10 mL), dried over Na2SO4, and evaporated to dryness. The purification process was carried out using flash chromatography (SiO2, ethyl acetate/hexane = 2:8) to afford the pure product in the reported yields and enantiopurities.
Methyl (2R)-2-Chloro-1-oxo-2,3-dihydro-1H-indene-2-carboxylate (14a) and Methyl (2S)-2-Chloro-1-oxo-2,3-dihydro-1H-indene-2-carboxylate (14a′)
Following the general procedure and using 13a (38 mg, 0.2 mmol), the products 14a and 14a′ (45 mg, 0.2 mmol, 99%) were obtained as colorless oils after flash chromatography (SiO2, ethyl acetate/hexane = 2:8). For the reaction using 1 mmol of 13a (190 mg), the product 14a was obtained in an amount of 225 mg (1.0 mmol, 99%). [α]D20 = −46.3 (c = 1.0, CHCl3, 93% ee) (R-enantiomer) and [α]D = +11.7 (c = 0.25, CHCl3, 90% ee) (S-enantiomer). 1H NMR (500 MHz, CDCl3): δ 7.86 (d, J = 7.5 Hz, 1H), 7.71 (t, J = 7.1 Hz, 1H), 7.51–7.45 (m, 2H), 4.11 (d, J = 17.6 Hz, 1H), 3.82 (s, 3H), 3.57 (d, J = 17.8 Hz, 1H). 13C{1H} NMR (125 MHz, CDCl3): δ 194.3, 167.6, 150.5, 136.5, 132.4, 128.6, 126.3, 126.0, 67.9, 54.1, 43.4. HPLC-separation conditions: Chiralcel OD-H, 20 °C, 254 nm, 80/20 hexane/i-PrOH, 1.0 mL/min; t1 = 6.71 min, t2 = 7.55 min. Absolute configuration was determined by comparison of the retention times and the [α]D value with reported data.20
Ethyl (2R)-2-Chloro-1-oxo-2,3-dihydro-1H-indene-2-carboxylate (14b)
Following the general procedure and using 13b (41 mg, 0.2 mmol), the product 14b (48 mg, 0.2 mmol, 99%) was obtained as colorless oil after flash chromatography (SiO2, ethyl acetate/hexane = 2:8). [α]D20 = −41.6 (c = 1.0, CHCl3, 91% ee). 1H NMR (500 MHz, CDCl3): δ 7.86 (d, J = 7.6 Hz, 1H), 7.70 (t, J = 7.4 Hz, 1H), 7.51–7.45 (m, 2H), 4.27 (q, J = 7.0 Hz, 2H), 4.09 (d, J = 17.7 Hz, 1H), 3.56 (d, J = 17.7 Hz, 1H), 1.27 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ 195.0, 167.1, 150.5, 136.3, 132.5, 128.5, 126.3, 125.9, 68.0, 63.4, 43.4, 13.9. HPLC-separation conditions: chiralcel OD-H, 20 °C, 254 nm, 80/20 hexane/i-PrOH, 1.0 mL/min; tmajor = 5.73 min, tminor = 6.33 min. Absolute configuration was determined by comparison of the retention times and the [α]D value with reported data.20b
Propan-2-yl (2R)-2-Chloro-1-oxo-2,3-dihydro-1H-indene-2-carboxylate (14c)
Following the general procedure and using 13c (44 mg, 0.2 mmol), the product 14c (50 mg, 0.2 mmol, 99%) was obtained as a colorless solid (mp 40–41 °C) after flash chromatography (SiO2, ethyl acetate/hexane = 2:8).[α]D20 = −36.6 (c = 1.0, CHCl3, 92% ee). 1H NMR (500 MHz, CDCl3): δ 7.79 (d, J = 7.7 Hz, 1H), 7.63 (t, J = 7.5 Hz, 1H), 7.43–7.36 (m, 2H), 5.07–4.98 (m, 1H), 3.99 (d, J = 17.7 Hz, 1H), 3.48 (d, J = 17.7 Hz, 1H), 1.17 (dd, J = 6.3, 1.8 Hz, 6H). 13C{1H} NMR (125 MHz, CDCl3): δ 195.2, 166.6, 150.6, 136.3, 132.6, 128.5, 126.3, 125.9, 71.5, 68.1, 43.4, 21.5, 21.4. HPLC-separation conditions: Chiralcel OD-H, 20 °C, 254 nm, 99/1 hexane/i-PrOH, 1.0 mL/min; tmajor = 9.50 min, tminor = 10.97 min. Absolute configuration was determined by comparison of the retention times and the [α]D value with reported data.20b
tert-Butyl (2R)-2-Chloro-1-oxo-2,3-dihydro-1H-indene-2-carboxylate (14d)
Following the general procedure and using 13d (46 mg, 0.2 mmol), the product 14d (53 mg, 0.2 mmol, 99%) was obtained as a colorless solid (mp 52–53 °C) after flash chromatography (SiO2, ethyl acetate/hexane = 2:8). [α]D20 = −32.1 (c = 1.0, CHCl3, 96% ee). 1H NMR (600 MHz, CDCl3): δ 7.84 (d, J = 7.7 Hz, 1H), 7.67 (t, J = 8.0 Hz, 1H), 7.48–7.42 (m, 2H), 4.00 (d, J = 17.6 Hz, 1H), 3.52 (d, J = 17.6 Hz, 1H), 1.41 (s, 9H). 13C{1H} NMR (150 MHz, CDCl3): δ 195.1, 166.6, 150.5, 136.3, 132.6, 128.5, 126.3, 125.9, 71.4, 68.1, 43.4, 21.4, 21.3. HPLC-separation conditions: Chiralcel OJ, 20 °C, 254 nm, 90/10 hexane/i-PrOH, 1.0 mL/min; tminor = 6.83 min, tmajor = 9.74 min. Absolute configuration was determined by comparison of the retention times and the [α]D value with reported data.20
Adamantan-1-yl (2R)-2-Chloro-1-oxo-2,3-dihydro-1H-indene-2-carboxylate (14e) and Adamantan-1-yl (2S)-2-Chloro-1-oxo-2,3-dihydro-1H-indene-2-carboxylate (14e′)
Following the general procedure and using 13e (63 mg, 0.2 mmol), the products 14e and 14e′(69 mg, 0.2 mmol, 99%) were obtained as colorless oils after flash chromatography (SiO2, ethyl acetate/hexane = 2:8). [α]D20 = −24.7 (c = 1.0, CHCl3, 97% ee) (R-enantiomer) and [α]D = +16.5 (c = 1.0, CHCl3, 92% ee) (S-enantiomer). 1H NMR (500 MHz, CDCl3): δ 7.78 (d, J = 7.7 Hz, 1H), 7.61 (td, J = 7.7, 1.1 Hz, 1H), 7.42–7.35 (m, 2H), 3.94 (d, J = 17.7 Hz, 1H), 3.46 (d, J = 17.6 Hz, 1H), 2.07 (s, 3H), 1.97 (d, J = 3.0 Hz, 6H), 1.55 (s, 6H). 13C{1H} NMR (125 MHz, CDCl3): δ 187.51, 167.98, 142.5, 134.4, 129.5, 129.0, 128.8, 127.3, 70.7, 53.8, 35.0, 25.5. HPLC-separation conditions: Chiralcel OD-H, 20 °C, 254 nm, 90/10 hexane/i-PrOH, 0.8 mL/min; t1 = 7.13 min, t2 = 8.16 min. Absolute configuration was determined by comparison of the retention times and the [α]D value with reported data.17b,19b
Benzyl (2R)-2-Chloro-1-oxo-2,3-dihydro-1H-indene-2-carboxylate (14f)
Following the general procedure and using 13f (53 mg, 0.2 mmol), the product 14f (59 mg, 0.2 mmol, 98%) was obtained as colorless oil after flash chromatography (SiO2, ethyl acetate/hexane = 2:8). [α]D20 = −32.5 (c = 1.5, CHCl3, 79% ee). 1H NMR (500 MHz, CDCl3): δ 7.78 (d, J = 7.7 Hz, 1H), 7.61 (t, J = 7.2 Hz, 1H), 7.39 (t, J = 6.9 Hz, 2H), 7.23 (qd, J = 13.4, 6.7 Hz, 5H), 5.16 (q, J = 12.4 Hz, 2H), 3.99 (d, J = 17.7 Hz, 1H), 3.48 (d, J = 17.7 Hz, 1H). 13C{1H} NMR (125 MHz, CDCl3): δ 194.8, 167.0, 150.4, 136.4, 134.7, 132.5, 128.6, 128.5, 127.9, 126.3, 126.0, 68.61, 68.0, 43.3. HPLC-separation conditions: chiralcel OD-H, 20 °C, 254 nm, 80/20 hexane/i-PrOH, 1.0 mL/min; tmajor = 7.39 min, tminor = 8.35 min. Absolute configuration was determined by comparison of the retention times and the [α]D value with reported data.20b
Methyl (2R)-2-Chloro-1-oxo-1,2,3,4-tetrahydronaphthalene-2-carboxylate (14g)
Following the general procedure and using 13g (41 mg, 0.2 mmol), the product 14g (47 mg, 0.2 mmol, 99%) was obtained as colorless oil after flash chromatography (SiO2, ethyl acetate/hexane = 2:8). [α]D20 = −24.6 (c = 1.0, CHCl3, 79% ee). 1H NMR (500 MHz, CDCl3): δ 8.01 (dd, J = 7.9, 0.9 Hz, 1H), 7.47 (td, J = 7.6, 1.3 Hz, 1H), 7.29 (t, J = 7.6 Hz, 1H), 7.22–7.18 (m, 1H), 3.77 (s, 3H), 3.25–3.17 (m, 1H), 2.98–2.88 (m, 2H), 2.49–2.43 (m, 1H). 13C{1H} NMR (125 MHz, CDCl3): δ 187.5, 168.0, 142.5, 134.4, 129.5, 129.0, 128.8, 127.3, 70.7, 53.8, 35.0, 25.5. HPLC-separation conditions: chiralcel OD-H, 20 °C, 254 nm, 90/10 hexane/i-PrOH, 0.8 mL/min; tmajor = 9.77 min, tminor = 12.00 min. Absolute configuration was determined by comparison of the retention times and the [α]D value with reported data.19a
tert-Butyl (2R)-2-Chloro-1-oxo-1,2,3,4-tetrahydronaphthalene-2-carboxylate (14h)
Following the general procedure and using 13h (47 mg, 0.2 mmol), the product 14h (55 mg, 0.2 mmol, 98%) was obtained as colorless oil after flash chromatography (SiO2, ethyl acetate/hexane = 2:8). [α]D20 = −10.2 (c = 1.0, CHCl3, 86% ee). 1H NMR (500 MHz, CDCl3): δ 1H NMR (500 MHz, CDCl3): δ 8.01 (dd, J = 7.9, 1.0 Hz, 1H), 7.45 (td, J = 7.5, 1.4 Hz, 1H), 7.28 (t, J = 7.6 Hz, 1H), 7.21–7.17 (m, 1H), 3.21–3.12 (m, 1H), 2.99–2.83 (m, 2H), 2.47–2.39 (m, 1H), 1.39 (s, 9H). 13C{1H} NMR (125 MHz, CDCl3): δ 13C NMR (126 MHz, CDCl3): δ 188.1, 166.3, 142.5, 134.3, 130.2, 128.9, 128.8, 127.2, 84.1, 71.6, 35.2, 27.7, 25.9. HPLC-separation conditions: chiralcel OJ, 20 °C, 254 nm, 99/1 hexane/i-PrOH, 0.7 mL/min; tmajor = 13.26 min, tminor = 15.13 min. Absolute configuration was determined by comparison of the retention times and the [α]D value with reported data.19a
Adamantan-1-yl (2R)-2-Chloro-1-oxo-1,2,3,4-tetrahydro-naphthalene-2-carboxylate (14i)
Following the general procedure and using 13i (58 mg, 0.2 mmol), the product 14i (64 mg, 0.2 mmol, 98%) was obtained as colorless oil after flash chromatography (SiO2, ethyl acetate/hexane = 2:8). [α]D20 = −4.7 (c = 1.0, CHCl3, 83% ee). 1H NMR (500 MHz, CDCl3): δ 8.02 (dd, J = 7.9, 0.9 Hz, 1H), 7.48–7.42 (m, 1H), 7.29 (t, J = 7.6 Hz, 1H), 7.21–7.16 (m, 1H), 3.22–3.12 (m, 1H), 3.00–2.92 (m, 1H), 2.91–2.82 (m, 1H), 2.48–2.40 (m, 1H), 2.09 (s, 3H), 2.02 (s, 6H), 1.55 (s, 6H). 13C{1H} NMR (125 MHz, CDCl3): δ 188.0, 165.8, 142.4, 134.1, 130.2, 128.8, 128.7, 127.2, 84.2, 71.7, 40.9, 36.0, 35.4, 30.9, 26.0 HPLC-separation conditions: chiralcel OD-H, 20 °C, 254 nm, 98/2 hexane/i-PrOH, 1.0 mL/min; tmajor = 16.10 min, tminor = 17.21 min. Absolute configuration was determined by comparison of the retention times and the [α]D value with reported data.19a,19b
Methyl (2R)-2-Chloro-6-methyl-1-oxo-2,3-dihydro-1H-indene-2-carboxylate (14j)
Following the general procedure and using 13j (41 mg, 0.2 mmol), the product 14j (47 mg, 0.2 mmol, 99%) was obtained as a pale yellow solid (mp 71–72 °C) after flash chromatography (SiO2, ethyl acetate/hexane = 2:8). [α]D20 = −39.6 (c = 1.0, CHCl3, 89% ee). 1H NMR (500 MHz, CDCl3): δ 7.96 (s, 1H), 7.83 (d, J = 7.8 Hz, 1H), 7.67 (d, J = 7.8 Hz, 1H), 4.36 (d, J = 17.6 Hz, 1H), 4.11 (s, 3H), 3.82 (d, J = 17.6 Hz, 1H), 2.74 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3): δ 194.9, 167.7, 147.9, 138.8, 137.8, 132.6, 125.9, 125.8, 68.3, 54.0, 43.1, 21.1. HPLC-separation conditions: chiralcel OD-H, 20 °C, 254 nm, 80/20 hexane/i-PrOH, 1.0 mL/min; tmajor = 6.01 min, tminor = 7.22 min. Absolute configuration was determined by comparison of the retention times and the [α]D value with reported data.13
Methyl (2R)-2-Chloro-6-methoxy-1-oxo-2,3-dihydro-1H-indene-2-carboxylate (14k)
Following the general procedure and using 13k (44 mg, 0.2 mmol), the product 14k (50 mg, 0.2 mmol, 99%) was obtained as a pale yellow solid (mp 57–68 °C) after flash chromatography (SiO2, ethyl acetate/hexane = 2:8). [α]D20 = −28.0 (c = 1.0, CHCl3, 85% ee). 1H NMR (600 MHz, CDCl3): 7.31–7.24 (m, 1H), 7.23–7.17 (m, 2H), 3.95 (d, J = 17.4 Hz, 1H), 3.78 (s, 3H), 3.74 (d, J = 0.8 Hz, 3H), 3.41 (d, J = 17.4 Hz, 1H)·13C{1H} NMR (150 MHz, CDCl3): δ 194.9, 167.6, 160.2, 143.4, 133.6, 127.0, 126.0, 106.7, 68.5, 55.7, 54.0, 42.8. HPLC-separation conditions: chiralcel OD-H, 20 °C, 254 nm, 80/20 hexane/i-PrOH, 1.0 mL/min; tmajor = 6.53 min, tminor = 7.55 min. Absolute configuration was determined by comparison of the retention times and the [α]D value with reported data.13
Methyl (2R)-2-Chloro-6-fluoro-1-oxo-2,3-dihydro-1H-indene-2-carboxylate (14l)
Following the general procedure and using 13l (42 mg, 0.2 mmol), the product 14l (48 mg, 0.2 mmol, 99%) was obtained as a colorless solid (mp 67–68 °C) after flash chromatography (SiO2, ethyl acetate/hexane = 2:8). [α]D20 = −48.6 (c = 1.0, CHCl3, 91% ee). 1H NMR (500 MHz, CDCl3): δ 7.44–7.38 (m, 2H), 7.38–7.33 (m, 1H), 4.00 (d, J = 17.6 Hz, 1H), 3.75 (s, 3H), 3.46 (d, J = 17.6 Hz, 1H). 13C{1H} NMR (125 MHz, CDCl3): δ 171.0, 167.3, 163.8, 145.9, 127.8, 127.7, 124.4, 124.2, 111.8, 111.6, 68.1, 54.1, 42.8. HPLC-separation conditions: chiralcel OD-H, 20 °C, 254 nm, 99/1 hexane/i-PrOH, 1.0 mL/min; tmajor = 17.19 min, tminor = 18.33 min. Absolute configuration was determined by comparison of the retention times and the [α]D value with reported data.21
Methyl (2R)-5-Bromo-2-chloro-1-oxo-2,3-dihydro-1H-indene-2-carboxylate (14m)
Following the general procedure and using 13m (54 mg, 0.2 mmol), the product 14m (60 mg, 0.2 mmol, 99%) was obtained as a pale yellow solid (mp 64–65 °C) after flash chromatography (SiO2, ethyl acetate/hexane = 2:8). [α]D20 = −44.0 (c = 1.0, CHCl3, 90% ee). 1H NMR (500 MHz, CDCl3): δ 7.71 (d, J = 8.2 Hz, 1H), 7.67 (s, 1H), 7.62 (d, J = 8.2 Hz, 1H), 4.09 (d, J = 17.9 Hz, 1H), 3.82 (s, 3H), 3.54 (d, J = 17.9 Hz, 1H). 13C{1H} NMR (125 MHz, CDCl3): δ 193.7, 167.2, 151.9, 132.4, 132.1, 131.3, 129.6, 127.0, 67.6, 54.2, 42.9. HPLC-separation conditions: chiralcel OD-H, 20 °C, 254 nm, 80/20 hexane/i-PrOH, 1.0 mL/min; tmajor = 7.25 min, tminor = 8.50 min. Absolute configuration was determined by comparison of the retention times and the [α]D value with reported data.20b,13
Methyl (2R)-2-Chloro-5-fluoro-1-oxo-2,3-dihydro-1H-indene-2-carboxylate (14n)
Following the general procedure and using 13n (42 mg, 0.2 mmol), the product 14n (48 mg, 0.2 mmol, 99%) was obtained as a colorless solid (mp 68–69 °C) after flash chromatography (SiO2, ethyl acetate/hexane = 2:8).[α]D20 = −50.8 (c = 1.0, CHCl3, 87% ee). 1H NMR (500 MHz, CDCl3): δ 7.88 (dd, J = 8.3, 5.3 Hz, 1H), 7.16 (dd, J = 8.3, 4.1 Hz, 2H), 4.11 (d, J = 18.0 Hz, 1H), 3.83 (s, 3H), 3.55 (d, J = 18.0 Hz, 1H). 13C{1H} NMR (125 MHz, CDCl3): δ 193.0, 169.1, 167.3, 167.1, 153.5, 153.4, 128.8, 128.8, 128.5, 128.4, 117.2, 117.0, 113.3, 113.1, 67.8, 54.1, 43.2, 43.1. HPLC-separation conditions: chiralcel OD-H, 20 °C, 254 nm, 80/20 hexane/i-PrOH, 1.0 mL/min; tmajor = 7.13 min, tminor = 7.98 min. Absolute configuration was determined by comparison of the retention times and the [α]D value with reported data.21
Methyl (2R)-2,5-Dichloro-1-oxo-2,3-dihydro-1H-indene-2-carboxylate (14o)
Following the general procedure and using 13o (45 mg, 0.2 mmol), the product 14o (51 mg, 0.2 mmol, 99%) was obtained as a pale yellow solid (mp 64–65 °C) after flash chromatography (SiO2, ethyl acetate/hexane = 2:8).[α]D20 = −47.8 (c = 1.0, CHCl3, 87% ee). 1H NMR (600 MHz, CDCl3): δ 7.77 (d, J = 8.2 Hz, 1H), 7.47 (s, 1H), 7.45–7.42 (m, 1H), 4.07 (d, J = 17.9 Hz, 1H), 3.80 (s, 3H), 3.52 (d, J = 17.9 Hz, 1H). 13C{1H} NMR (150 MHz, CDCl3): δ 193.5, 167.2, 151.9, 143.2, 130.8, 129.5, 127.0, 126.5, 67.7, 54.2, 43.0. HPLC-separation conditions: chiralcel OD-H, 20 °C, 254 nm, 80/20 hexane/i-PrOH, 1.0 mL/min; tmajor = 6.98 min, tminor = 8.10 min. Absolute configuration was determined by comparison of the retention times and the [α]D value with reported data.13
Methyl (2R)-4-Bromo-2-chloro-1-oxo-2,3-dihydro-1H-indene-2-carboxylate (14p)
Following the general procedure and using 13p (54 mg, 0.2 mmol), the product 14p (60 mg, 0.2 mmol, 99%) was obtained as a pale yellow solid (mp 51–52 °C) after flash chromatography (SiO2, ethyl acetate/hexane = 2:8).[α]D20 = −30.1 (c = 1.0, CHCl3, 89% ee). 1H NMR (600 MHz, CDCl3): δ 7.87 (dd, J = 7.8, 0.6 Hz, 1H), 7.82 (d, J = 7.6 Hz, 1H), 7.39 (t, J = 7.7 Hz, 1H), 4.02 (d, J = 18.3 Hz, 1H), 3.83 (s, 3H), 3.51 (d, J = 18.3 Hz, 1H). 13C{1H} NMR (150 MHz, CDCl3): δ 194.3, 167.2, 150.3, 139.1, 134.4, 130.3, 124.7, 121.6, 67.3, 54.2, 44.4. HPLC-separation conditions: chiralcel OD-H, 20 °C, 254 nm, 80/20 hexane/i-PrOH, 1.0 mL/min; tmajor = 6.16 min, tminor = 7.53 min. Absolute configuration was determined by comparison of the retention times and the [α]D value with reported data.21
Acknowledgments
We would like to acknowledge Poland’s National Science Centre (Project 2016/21/B/ST5/03352) for financial support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.0c02486.
General remarks, experimental procedures, characterization of products, copies of NMR spectra for 5, 6, and 14(a–p), and HPLC chromatograms (PDF)
Author Contributions
Conceptualization, M.M.; methodology, M.M., J.J.; validation, M.M and P.G.; formal analysis, M.M.; investigation, M.M. and P.G.; resources, J.J.; data curation, M.M.; writing (original draft preparation), M.M. and J.J.; writing (review and editing), M.M. and J.J.; visualization, M.M.; supervision, J.J.; project administration, J.J.; funding acquisition, J.J.
The authors declare no competing financial interest.
Supplementary Material
References
- See:; a Thomas G.Medicine Chemistry: An Introduction; Wiley: New York, 2000. [Google Scholar]; b Purser S.; Moore P. R.; Swallow S.; Gouverneur V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. 10.1039/b610213c. [DOI] [PubMed] [Google Scholar]; c Liu Y.; Leng H. J.; Li Q. Z.; Li J. L. Catalytic Strategies for the Asymmetric Construction of Cyclic Frameworks with a Halogenated Tetrasubstituted Stereocenter. Adv. Synth. Catal. 2020, 362, 3926–3947. 10.1002/adsc.202000540. [DOI] [Google Scholar]
- For reviews, see:; a Muñiz K. Improving Enantioselective Fluorination Reactions: ChiralN-Fluoroammonium Salts and Transition Metal Catalysts. Angew. Chem., Int. Ed. 2001, 40, 1653–1656. . [DOI] [PubMed] [Google Scholar]; b Ma J.-A.; Cahard D. Asymmetric Fluorination, Trifluoromethylation, and Perfluoroalkylation Reactions. Chem. Rev. 2004, 104, 6119–6146. 10.1021/cr030143e. [DOI] [PubMed] [Google Scholar]; c Yang X.; Wu T.; Phipps R. J.; Toste F. D. Advances in Catalytic Enantioselective Fluorination, Mono-, Di-, and Trifluoromethylation, and Trifluoromethylthiolation Reactions. Chem. Rev. 2015, 115, 826–870. 10.1021/cr500277b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected examples, see:; a Oestreich M. Strategies for Catalytic Asymmetric Electrophilic ? Halogenation of Carbonyl Compounds. Angew. Chem., Int. Ed. 2005, 44, 2324–2327. 10.1002/anie.200500478. [DOI] [PubMed] [Google Scholar]; b Shibatomi K.; Narayama A. Catalytic Enantioselective α-Chlorination of Carbonyl Compounds. Asian J. Org. Chem. 2013, 2, 812–823. 10.1002/ajoc.201300058. [DOI] [Google Scholar]; c Gómez-Martínez M.; Alonso D. A.; Pastor I. M.; Guillena G.; Baeza A. Organocatalyzed Assembly of Chlorinated Quaternary Stereogenic Centers. Asian J. Org. Chem. 2016, 5, 1428–1437. 10.1002/ajoc.201600404. [DOI] [Google Scholar]
- For reviews, see:; a Isanbor C.; O’Hagan D. Fluorine in medicinal chemistry: A review of anti-cancer agents. J. Fluorine Chem. 2006, 127, 303–319. 10.1016/j.jfluchem.2006.01.011. [DOI] [Google Scholar]; b Muller K.; Faeh C.; Diederich F. Fluorine in Pharmaceuticals: Looking Beyond Intuition. Science 2007, 317, 1881–1886. 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]; c O’Hagan D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 2008, 37, 308–319. 10.1039/b711844a. [DOI] [PubMed] [Google Scholar]
- Hintermann L.; Togni A. Catalytic Enantioselective Chlorination and Bromination of β-Keto Esters. Helv. Chim. Acta 2000, 83, 2425–2435. . [DOI] [Google Scholar]
- For selected examples, see:; a Hamashima Y.; Yagi K.; Takano H.; Tamás L.; Sodeoka M. An Efficient Enantioselective Fluorination of Various β-Ketoesters Catalyzed by Chiral Palladium Complexes. J. Am. Chem. Soc. 2002, 124, 14530–14531. 10.1021/ja028464f. [DOI] [PubMed] [Google Scholar]; b Hamashima Y.; Suzuki T.; Takano H.; Shimura Y.; Sodeoka M. Catalytic Enantioselective Fluorination of Oxindoles. J. Am. Chem. Soc. 2005, 127, 10164–10165. 10.1021/ja0513077. [DOI] [PubMed] [Google Scholar]; c Suzuki T.; Goto T.; Hamashima Y.; Sodeoka M. Enantioselective Fluorination oftert-Butoxycarbonyl Lactones and Lactams Catalyzed by Chiral Pd(II)-Bisphosphine Complexes. J. Org. Chem. 2007, 72, 246–250. 10.1021/jo062048m. [DOI] [PubMed] [Google Scholar]
- See:; a Reddy D. S.; Shibata N.; Nagai J.; Nakamura S.; Toru T.; Kanemasa S. Desymmetrization-like Catalytic Enantioselective Fluorination of Malonates and Its Application to Pharmaceutically Attractive Molecules. Angew. Chem., Int. Ed. 2008, 47, 164–168. 10.1002/anie.200704093. [DOI] [PubMed] [Google Scholar]; b Kang S. H.; Kim D. Y. Catalytic Enantioselective Fluorination of α-Chloro-β-keto Esters in the Presence of Chiral Nickel Complexes. Adv. Synth. Catal. 2010, 352, 2783–2786. 10.1002/adsc.201000515. [DOI] [Google Scholar]; c Shibatomi K.; Kotozaki M.; Sasaki N.; Fujisawa I.; Iwasa S. Williamson Ether Synthesis with Phenols at a Tertiary Stereogenic Carbon: Formal Enantioselective Phenoxylation of β-Keto Esters. Chem.—Eur. J. 2015, 21, 14095–14098. 10.1002/chem.201502042. [DOI] [PubMed] [Google Scholar]
- For selected examples, see:; a Steiner D. D.; Mase N.; Barbas C. F. Direct Asymmetric α-Fluorination of Aldehydes. Angew. Chem., Int. Ed. 2005, 44, 3706–3710. 10.1002/anie.200500571. [DOI] [PubMed] [Google Scholar]; b Marigo M.; Fielenbach D.; Braunton A.; Kjærsgaard A.; Jørgensen K. A. Enantioselective Formation of Stereogenic Carbon-Fluorine Centers by a Simple Catalytic Method. Angew. Chem., Int. Ed. 2005, 44, 3703–3706. 10.1002/anie.200500395. [DOI] [PubMed] [Google Scholar]; c Beeson T. D.; MacMillan D. W. C. Enantioselective Organocatalytic α-Fluorination of Aldehydes. J. Am. Chem. Soc. 2005, 127, 8826–8828. 10.1021/ja051805f. [DOI] [PubMed] [Google Scholar]
- Sakai T.; Hirashima S.-i.; Nakashima K.; Maeda C.; Yoshida A.; Koseki Y.; Miura T. Asymmetric Chlorination of β-Keto Esters Using Diaminomethylenemalononitrile Organocatalyst. Chem. Pharm. Bull. 2016, 64, 1781–1784. 10.1248/cpb.c16-00722. [DOI] [PubMed] [Google Scholar]
- See:; a Shibata N.; Suzuki E.; Takeuchi Y. A Fundamentally New Approach to Enantioselective Fluorination Based on Cinchona Alkaloid Derivatives/Selectfluor Combination. J. Am. Chem. Soc. 2000, 122, 10728–10729. 10.1021/ja002732x. [DOI] [Google Scholar]; b Ishimaru T.; Shibata N.; Horikawa T.; Yasuda N.; Nakamura S.; Toru T.; Shiro M. Cinchona Alkaloid Catalyzed Enantioselective Fluorination of Allyl Silanes, Silyl Enol Ethers, and Oxindoles. Angew. Chem., Int. Ed. 2008, 47, 4157–4161. 10.1002/anie.200800717. [DOI] [PubMed] [Google Scholar]; c Stockhammer L.; Schörgenhumer J.; Mairhofer Ch.; Waser M. Asymmetric α-Chlorination of β-Keto Esters Using Hypervalent Iodine-Based Cl-Transfer Reagents in Combination with Cinchona Alkaloid Catalysts. Eur. J. Org. Chem. 2020, 10.1002/ejoc.202001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected examples, see:; a Han X.; Luo J.; Liu C.; Lu Y. Asymmetric generation of fluorine-containing quaternary carbons adjacent to tertiary stereocenters: uses of fluorinated methines as nucleophiles. Chem. Commun. 2009, 2044–2046. 10.1039/b823184b. [DOI] [PubMed] [Google Scholar]; b Shibatomi K. Alternative Synthetic Strategies for Enantioselective Construction of Halogenated Chiral Carbon Centers. Synthesis 2010, 2679–2702. 10.1055/s-0030-1258164. [DOI] [Google Scholar]; c Han X.; Zhong F.; Lu Y. Highly Enantioselective Amination Reactions of Fluorinated Keto Esters Catalyzed by Novel Chiral Guanidines Derived from Cinchona Alkaloids. Adv. Synth. Catal. 2010, 352, 2778–2782. 10.1002/adsc.201000562. [DOI] [Google Scholar]
- For examples, see:; a Kim D. Y.; Park E. J. Catalytic Enantioselective Fluorination of β-Keto Esters by Phase-Transfer Catalysis Using Chiral Quaternary Ammonium Salts. Org. Lett. 2002, 4, 545–547. 10.1021/ol010281v. [DOI] [PubMed] [Google Scholar]; b Wang X.; Lan Q.; Shirakawa S.; Maruoka K. Chiral bifunctional phase transfer catalysts for asymmetric fluorination of β-keto esters. Chem. Commun. 2010, 46, 321–323. 10.1039/b920099a. [DOI] [PubMed] [Google Scholar]
- Luo J.; Wu W.; Xu L.-W.; Meng Y.; Lu Y. Enantioselective direct fluorination and chlorination of cyclic β-ketoesters mediated by phase-transfer catalysts. Tetrahedron Lett. 2013, 54, 2623–2626. 10.1016/j.tetlet.2013.03.028. [DOI] [Google Scholar]
- For examples, see:; a Novacek J.; Waser M. Bifunctional Chiral Quaternary Ammonium Salt Catalysts: A Rapidly Emerging Class of Powerful Asymmetric Catalysts. Eur. J. Org. Chem. 2013, 637–648. 10.1002/ejoc.201201425. [DOI] [Google Scholar]; b Jin Q.; Zheng C.; Zhao G.; Zou G. Bifunctional Quaternary Ammonium Salts Catalyzed Stereoselective Conjugate Addition of Oxindoles to Electron-Deficient β-Haloalkenes. J. Org. Chem. 2017, 82, 4840–4850. 10.1021/acs.joc.7b00571. [DOI] [PubMed] [Google Scholar]; c Majdecki M.; Niedbala P.; Jurczak J. Amide-Based Cinchona Alkaloids as Phase-Transfer Catalysts: Synthesis and Potential Application. Org. Lett. 2019, 21, 8085–8090. 10.1021/acs.orglett.9b03065. [DOI] [PubMed] [Google Scholar]
- See:; a Wang H. Chiral Phase-Transfer Catalysts with Hydrogen Bond: A Powerful Tool in the Asymmetric Synthesis. Catalysts 2019, 9, 244–277. 10.3390/catal9030244. [DOI] [Google Scholar]; b Majdecki M.; Niedbala P.; Jurczak J. Synthesis of C2 Hybrid Amide-Based PTC Catalysts and Their Comparison with Saturated Analogues. ChemistrySelect 2020, 5, 6424–6429. 10.1002/slct.202001012. [DOI] [Google Scholar]; c Majdecki M.; Tyszka-Gumkowska A.; Jurczak J. Highly Enantioselective Epoxidation of α,β-Unsaturated Ketones Using Amide-Based Cinchona Alkaloids as Hybrid Phase-Transfer Catalysts. Org. Lett. 2020, 22, 8687–8691. 10.1021/acs.orglett.0c03272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa S.; Tokuda T.; Kasai A.; Maruoka K. Design of Chiral Bifunctional Quaternary Phosphonium Bromide Catalysts Possessing an Amide Moiety. Org. Lett. 2013, 15, 3350–3353. 10.1021/ol4013926. [DOI] [PubMed] [Google Scholar]
- See:; a Novacek J.; Waser M. Syntheses and Applications of (Thio)Urea-Containing Chiral Quaternary Ammonium Salt Catalysts. Eur. J. Org. Chem. 2014, 802–809. 10.1002/ejoc.201301594. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Novacek J.; Monkowius U.; Himmelsbach M.; Waser M. Asymmetric α-chlorination of β-ketoesters using bifunctional ammonium salt catalysis. Monatsh. Chem. 2016, 147, 533–538. 10.1007/s00706-015-1604-7. [DOI] [Google Scholar]
- See:; a Nakajima M.; Yamamoto S.; Yamaguchi Y.; Nakamura S.; Hashimoto S. Enantioselective Michael additions of β-keto esters to α,β-unsaturated carbonyl compounds catalyzed by a chiral biquinoline N,N′-dioxide-scandium trifluoromethanesulfonate complex. Tetrahedron 2003, 59, 7307–7313. 10.1016/s0040-4020(03)01139-6. [DOI] [Google Scholar]; b Wu F.; Hong R.; Khan J.; Liu X.; Deng L. Asymmetric Synthesis of Chiral Aldehydes by Conjugate Additions with Bifunctional Organocatalysis by Cinchona Alkaloids. Angew. Chem., Int. Ed. 2006, 45, 4301–4305. 10.1002/anie.200600867. [DOI] [PubMed] [Google Scholar]; c Poulsen T. B.; Bernardi L.; Alemán J.; Overgaard J.; Jørgensen K. A. Organocatalytic Asymmetric Direct α-Alkynylation of Cyclic β-Ketoesters. J. Am. Chem. Soc. 2007, 129, 441–449. 10.1021/ja067289q. [DOI] [PubMed] [Google Scholar]
- See:; a Cai Y.; Wang W.; Shen K.; Wang J.; Hu X.; Lin L.; Liu X.; Feng X. Highly enantioselective α-chlorination of cyclic β-ketoesters catalyzed by N,N′-Dioxide using NCS as the chlorine source. Chem. Commun. 2010, 46, 1250–1252. 10.1039/b922769e. [DOI] [PubMed] [Google Scholar]; b Li J.; Pan W.; Wang Z.; Zhang X.; Ding K. Access to Both Enantiomers of α-Chloro-β-keto Esters with a Single Chiral Ligand: Highly Efficient Enantioselective Chlorination of Cyclic β-Keto Esters Catalyzed by Chiral Copper(II) and Zinc(II) Complexes of a Spiro-2,2′-bischroman-Based Bisoxazoline Li. Adv. Synth. Catal. 2012, 354, 1980–1986. 10.1002/adsc.201200088. [DOI] [Google Scholar]
- See:; a Qi M.-H.; Wang F.-J.; Shi M. Synthesis of novel chiral oxazoline-Schiff base ligands for the catalytic asymmetric chlorination of β-keto esters. Tetrahedron: Asymmetry 2010, 21, 247–253. 10.1016/j.tetasy.2010.02.003. [DOI] [Google Scholar]; b Jiang J.-J.; Huang J.; Wang D.; Yuan Z.-L.; Zhao M.-X.; Wang F.-J.; Shi M. Cu(I)-catalyzed asymmetric chlorination of β-keto esters in the presence of chiral phosphine-schiff base type ligands. Chirality 2011, 23, 272–276. 10.1002/chir.20913. [DOI] [PubMed] [Google Scholar]; c Shibatomi K.; Soga Y.; Narayama A.; Fujisawa I.; Iwasa S. Highly Enantioselective Chlorination of β-Keto Esters and Subsequent SN2 Displacement of Tertiary Chlorides: A Flexible Method for the Construction of Quaternary Stereogenic Centers. J. Am. Chem. Soc. 2012, 134, 9836–9839. 10.1021/ja304806j. [DOI] [PubMed] [Google Scholar]
- Naganawa Y.; Aoyama T.; Kato K.; Nishiyama H. Cu(II)-catalyzed Enantioselective α-Hydroxylation and α-Chlorination of β-Ketoesters withN,N,O-Tridentate Chiral Phenanthroline Ligand. ChemistrySelect 2016, 1, 1938–1942. 10.1002/slct.201600449. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.