Abstract
Background:
In patients with acute myeloid leukemia (AML), CD56 expression has been associated with adverse clinical outcome. We reported on a phenotype associated with very poor prognosis (RAM) in children enrolled in the Children’s Oncology Group trial AAML0531 (3). RAM is also characterized in part by high-intensity expression of the CD56 antigen. Herein, we investigate underlying biological and clinical differences among CD56-positive AMLs for patients in AAML0531.
Methods:
For 769 newly diagnosed pediatric patients with de novo AML enrolled in AAML0531, bone marrow specimens were submitted for flow cytometric analysis. For each patient, an immunophenotypic expression profile (IEP) was defined by mean fluorescent intensities of assayed surface antigens. Unsupervised hierarchical clustering (HCA) was completed to group patients with similar immunophenotypes. Clusters were then evaluated for CD56 expression. Principal component analysis (PCA) was subsequently applied to determine whether CD56-positive patient groups were non-overlapping.
Results:
HCA of IEPs revealed three unique phenotypic clusters of patients with CD56-positive AML, and PCA showed that these three cohorts are distinct. Cohort 1 (N=77) showed a prevalence of t(8;21) patients (72%), cohort 2 (N=52) a prevalence of 11q23 patients (69%), and cohort 3 (RAM) (N=16) a prevalence of patients with co-occurrence of the CBFA2T3–GLIS2 fusion transcript (63%). The 5-year event-free survival (EFS) for cohorts 1, 2, and 3 were 69%, 39%, and 19% respectively.
Conclusions:
When leukemia is considered by its multidimensional immunophenotype and not the expression of a single antigen, correlations are seen between genotype and there are significant differences in patient outcomes.
Keywords: AML, pediatric, CD56, multidimensional immunophenotype, flow cytometry
INTRODUCTION
Independent reports show that in patients with acute myeloid leukemia (AML), the expression of CD56, a neural adhesion molecule, is associated with adverse clinical outcomes such as decreased overall survival and low rates of complete remission (1, 2). However, such studies have not been reproducible, and it has been proposed that within CD56-positive leukemias, poor prognosis is limited to a subset of patients with unique multidimensional phenotypes (3).
We recently reported a unique phenotype within the Children’s Oncology Group (COG) trial AAML0531. The RAM phenotype is characterized in part by high-intensity expression of the CD56 antigen and is associated with extremely poor outcomes (16% 3-year event-free survival (3)). This phenotype is defined by not only remarkable CD56 expression but also multiple other antigens, suggesting that it is not merely the expression of this single antigen but a combination of antigens detected in multidimensional space. In comparison, patients that were CD56 positive that did not have RAM phenotype had a 3 yr EFS of 52%, while patients with AML that did not express CD56 had a 51% EFS (3).
This concept of using multiple antigens to define a phenotype was extended by the immunophenotypic expression profile (IEP) as a novel way to quantify surface gene product expression patterns in AML (4). This approach is predicated by the observation that antigen intensity in normal hematopoietic cell populations is invariant throughout maturation (5). Unsupervised hierarchical clustering analysis (HCA) grouped patients with similar IEPs into 11 patient cohorts that demonstrated high associations among phenotype, genotype, morphology, and outcome.
Herein, we combined the IEP with principal component analysis (PCA) to investigate the underlying biological and clinical differences between RAM and other CD56-positive AMLs for patients enrolled in AAML0531. We identified three unique phenotypic clusters of patients exhibiting increased CD56 expression and showed that these phenotypes are associated with different cytogenetic classifications and significant differences in event-free survival (EFS)
METHODS
Patient Samples:
Between August 2006 and June 2010, COG trial AAML0531 enrolled 1022 newly diagnosed pediatric patients with de novo AML. Of these, 769 satisfied the 3 study criteria: (1) submitting a bone marrow aspirate (N=626, 81%) or peripheral blood specimen (N=143, 19%) for multidimensional flow cytometry (MDF) at diagnosis, (2) providing proper consent for correlative biology studies, and (3) MDF analysis showing leukemia comprising >10% of non-erythroid cells. Patients with acute promyelocytic leukemia were not enrolled in AAML0531, and those with Down syndrome were excluded from analysis. Details of AAML0531 have been previously published (6, 7).
The study was approved by the central institutional review board (IRB) at the National Cancer Institute and IRBs at each of the 184 enrolling centers. Patients and their families provided informed consent or assent as appropriate. The trial was conducted in accordance with the Declaration of Helsinki and registered at www.clinicaltrials.gov as NCT00372593.
Flow Cytometric Analysis:
Bone marrow aspirates or peripheral blood samples were drawn in heparin (the preferred anticoagulant) or EDTA for phenotypic characterization. MDF analysis was performed centrally at Hematologics, Inc., with a standardized panel of monoclonal antibodies designed to detect measurable residual disease with a difference-from-normal approach (Supplementary Figure 1). CD56 PE clone NCAM16.2 was used. A comprehensive flow cytometric work up was performed at the contributing institution but was not centrally reviewed. Specimens were processed as previously described (8).
Immunophenotypic Expression Profile and Hierarchical Clustering Analysis:
The diagnostic AML population for each patient was identified by CD45 versus log–side scatter (SSC) gating using WinList (Verity Software House, Topsham, ME). Log mean fluorescence intensities of 12 cell surface antigens were calculated and incorporated into each patient’s IEP along with the average forward scatter and SSC of leukemic cells. Further, the coefficient of variation (CV) of CD34 was included in the IEP to assess the heterogeneity of leukemic cells for this antigen. For patients that are CD34-negative, the CV approaches zero and instead of maturation, reflects aberrant loss of CD34. It is noted that CD34-negative AML will often demonstrate a small aberrant population of CD34-positive cells. Together, these independently quantified characteristics defined the IEP for each patient as a location in a 15-dimensional data space.
Mutation screening and cytogentic karyotyping:
Genomic DNA was extracted from diagnostic bone marrow and CEBPA, FLT3-ITD, WT1, and NPM1 testing was completed. Cytogenetic karyotyping was completed with standard G-banding techniques. Further methods have been previously described (4).
Analysis of CD56 Expression:
To investigate the relationship between CD56 expression and prognosis, the distribution of mean CD56 intensities for the 769 patients eligible for this study was analyzed. The distribution revealed a prominent local minimum intensity at 1.55 log units (Supplementary Figure 1). Unsupervised HCA of the IEP for each of the 769 patients (previously published, (4)) showed three clusters in which a minimum of 80% of patients exhibited CD56 expression above 1.55 log units (new data). A total of 145 patients fell into one of these three clusters, while 623 patients did not undergo further analysis.
Principal Component Analysis:
The IEPs of patients within the three CD56-positive clusters were compared and visualized with PCA. Principal components were computed without scaling of IEPs, using the prcomp function within R. The first three principal components were used to reduce dimensionality of the IEP and display multidimensional variance among CD56-positive IEPs.
Statistical Methods:
OS was defined as time from study entry until death. EFS was defined as time from study entry until induction failure, relapse, or death. Children lost to follow-up were censored at their date of last known contact. The Kaplan–Meier method was used to estimate OS and EFS. Differences between groups of patients were tested by the log-rank test.
RESULTS
As previously reported, our IEP approach divided the AAML0531 diagnostic dataset into 11 clusters of patients with unique multidimensional phenotypes (4). Of these 11 defined clusters, clusters B, E, and K (referred to as cohorts 1, 2, and 3, respectively, in this study; Table 1) each had more than 80% of patients with a CD56 expression greater than 1.55 log units (Supplementary Figure 1A). In addition, each of these cohorts had an mean linear mean fluorescent intensity (MFI) that was increased at least 7-fold compared the mean MFI of all 11 clusters combined (the mean linear MFI for the entire cohort was 16.6, Supplementary figure 1B).
Table 1.
Summary of immunophenotypic and genetic features, risk classification, and 5-year event-free survival for cohorts that were primarily CD56-positive
| Cohort | Immunophenotype summary | Prevalent Cytogenetic/molecular (Percent out of total patients in cohort) | Risk classification defined by AML0531 protocol | Five-year EFS % (±2SD) |
|---|---|---|---|---|
|
1
N=77 |
• Increased CD56 • CD34 and HLA-DR positive • CD38 intermediate |
Prevalence of t(8;21) (72%) | 82% of patients assigned low risk | 69% (±11%) |
|
2
N=52 |
• Increased CD56 • CD34 and CD117 negative |
Prevalence of 11q23 rearrangement (69%) | 94% of patients assigned standard risk | 39% (±14%) |
|
3
N = 16 |
• Increased CD56 (very bright) • HLA-DR, CD45, and CD38 negative |
Prevalence of CBFA2T3–GLIS2 fusion transcript (63%) | 100% assigned standard risk | 19% (±20%) |
SD, standard deviation.
Patients in cohort 1 demonstrated a multidimensional leukemic phenotype characterized by increased CD56 expression (the mean linear MFI for the entire cohort was 121, Supplementary figure 1B), CD34 and HLA-DR positivity, and intermediate CD38 expression (Supplementary figure 1C). Correlation with molecular and cytogenetic data revealed a prevalence of t(8;21)(q22;q22)–positive patients in cohort 1 (55 of 77 [72%] patients).
Patients in cohort 2 demonstrated a leukemic immunophenotype characterized by increased CD56 expression (the mean linear MFI for the entire cohort was 228, Supplementary figure 1B). without expression of CD34 or CD117 (Supplementary Figure 1C). In addition, the CV of CD34 intensity in these patients was higher than that of normal uncommitted progenitor cells, indicating that these patients had leukemias with maturation toward mature lineages. Correlation of genetic data for cohort 2 revealed a high proportion of patients with 11q23/KMT2A(MLL) rearrangement (35 of 51 [69%])(data was unavailable for one patient).
Patients in cohort 3 were characterized by a unique immunophenotype with exceedingly bright CD56 (the mean linear MFI for the entire cohort was 2040, Supplementary figure 1B), HLA-DR negativity, and dim to negative expression of CD45 and CD38 (Supplementary Figure 1C). This phenotype has been previously termed the RAM phenotype (3). Genetic analysis of cohort 3 revealed high prevalence of patients with the CBFA2T3–GLIS2 fusion transcript: 10 of 16 patients (63%) were positive for the fusion transcript generated by the cryptic inv(16)(p13.3q24.3) (Table 1).
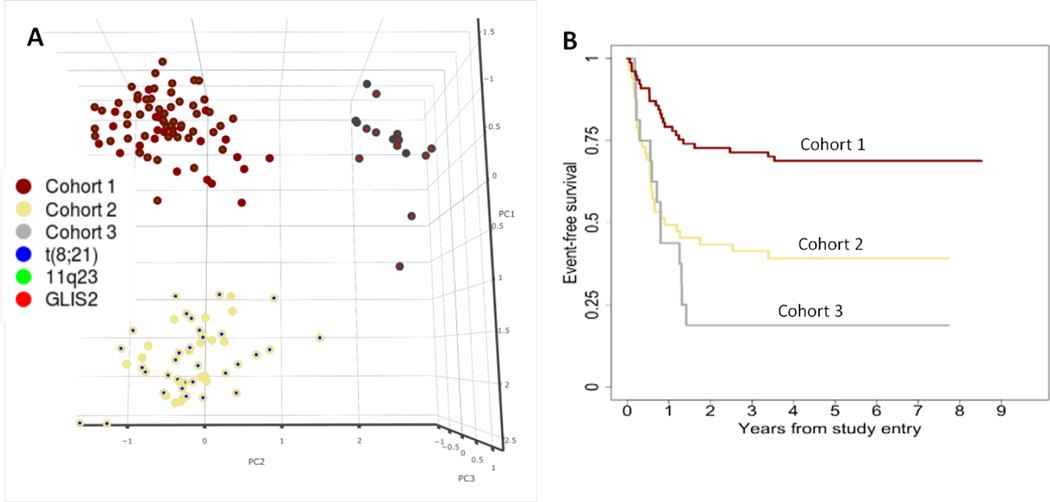
Outcome analysis was performed to assess the difference in 5-year EFS and 5-year OS between these three primarily CD56-positve cohorts. 5-year EFS and OS for patients in cohorts 1, 2, and 3 demonstrated that each multidimensional immunophenotype, despite their similarity in increased CD56 expression, corresponded with significantly different 5-year EFS (Figure 1).
Fig. 1.
A) The first three principal components demonstrate that the three CD56-positive phenotypic cohorts are distinct when analyzed in multidimensional space. Each cohort is represented by the color of the larger circle (dark red = cohort 1, yellow = cohort 2, grey = cohort 3), and the inner circle color represents the presence of t(8;21)(blue), an MLL rearrangement (green), or CBFA2T3–GLIS2(bright red). B) Event-free survival for each cohort (dark red = cohort 1, yellow = cohort 2, grey = cohort 3)
Patients in cohort 1 were predominantly categorized as low risk by molecular and cytogenetic risk analysis (82%). Corresponding outcomes for patients in cohort 1 revealed a high 5-year EFS (69%). Patients in cohorts 2 and 3 were predominantly classified as standard risk (94% and 100% respectively). However, despite similar risk stratifications, patients in cohort 3 had poorer overall 5-year EFS than did patients in cohort 2 (19% and 39%, respectively; Table 1).
CONCLUSIONS
Conventional characterization of leukemic immunophenotypes used for lineage assignment involves calculating the proportion of cells with antigen expression above a defined threshold, but does not quantify the amount of each gene product (1, 9–11). We recently reported that antigen intensity relationships of normal hematopoietic cell populations are invariant throughout maturation from an uncommitted progenitor cell to a mature blood cell in both children and adults (5, 12). The study helped confirm that with a high degree of quality control and system stability, precise quantification of surface gene product expression can provide a robust basis to assess phenotypic deviations from normal maturation patterns that occur because of neoplastic transformation. This concept is supported by our recent report of the recurrent multidimensional immunophenotype RAM, which independently identifies high-risk pediatric AML patients at diagnosis (3).
Although there are notable differences in IEPs of patients in cohorts 1, 2, and 3, no combination of individual surface gene products clearly separates each CD56-positive group in 2 dimensions, which makes it difficult to appreciate the distinctive immunophenotypic subgroups by using standard dot plots. However, by developing the IEP, followed by unsupervised HCA, patients can be grouped by similar multidimensional immunophenotype. We previously used boosted decision tree models to quantify the importance of each surface gene product in characterizing each phenotypic cluster (8). Patients in cohorts 1, 2, and 3 had CD56 expression as either the most or second-most important identifying phenotypic feature. However, the multidimensional phenotypes of these three cohorts are distinct (Fig 1A). The PCA revealed no overlap between these cohorts, providing further evidence that these three CD56-associated phenotypes are distinct when multidimensional phenotypes are assessed. Analysis of biological and clinical features of these groups revealed that each patient cohort has unifying cytogenetic or molecular features and vastly different clinical outcomes.
Our study shows that the IEP is more informative than the historical single antigen approach. Furthermore, it suggests that multidimensional immunophenotyping at diagnosis has the potential to be implemented in future diagnostic and prognostic classifications for pediatric AML. Understanding of difference in clinical outcomes among patients that express an antigen can also help inform how patients respond to antibody-drug conjugates that currently exist or are under development.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank the patients and families for participating in AAML0531. We also thank Vani Shanker for scientific editing.
This work was supported by St. Baldrick’s Foundation and grants U10CA098543 (Chair’s grant), U10CA098413 (the Statistical Center Grant), U10CA180886 (NCTN Operations Center Grant), and U10CA180899 (NCTN Statistics & Data Center Grant).
Footnotes
DISCLOSURES
Laura M. Pardo, Andrew P. Voigt, Elisabeth R. Wilson, Dana J. Paine, Fangyan Dai, Andrew J. Menssen, Denise A. Wells, Lisa Eidenschink Brodersen, and Michael R. Loken are currently or formerly employed by Hematologics, Inc. Denise A. Wells and Michael R. Loken are equity owners of Hematologics, Inc.
REFERENCES
- 1.Raspadori D, Damiani D, Lenoci M, Rondelli F, Testoni N, Nardi G, et al. CD56 antigenic expression in acute myeloid leukemia identifies patients with poor clinical prognosis. Leukemia 2001; 15:8:1161–1164. [DOI] [PubMed] [Google Scholar]
- 2.Iriyama N, Hatta Y, Takeuchi J, Ogawa Y, Ohtake S, Sakura T, et al. CD56 expression is an independent prognostic factor for relapse in acute myeloid leukemia with t(8;21). Leuk Res 2013; 37:9:1021–1026. [DOI] [PubMed] [Google Scholar]
- 3.Brodersen LE, Alonzo TA, Menssen AJ, Gerbing RB, Pardo L, Voigt AP, et al. A recurrent immunophenotype at diagnosis independently identifies high-risk pediatric acute myeloid leukemia: a report from Children’s Oncology Group. Leukemia 2016; 30:10:2077–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voigt AP, Brodersen LE, Alonzo TA, Gerbing RB, Menssen AJ, Wilson ER, et al. Phenotype in combination with genotype improves outcome prediction in acute myeloid leukemia: a report from Children’s Oncology Group protocol AAML0531. Haematologica 2017; 102:12:2058–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loken MR, Voigt AP, Brodersen LE, Fritschle W, Menssen AJ, Meshinchi S, et al. Consistent quantitative gene product expression: #2. Antigen intensities on bone marrow cells are invariant between individuals. Cytom Part A 2016; 89A:11:987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loken MR, Alonzo TA, Pardo L, Gerbing RB, Raimondi SC, Hirsch BA, Ho PA, Franklin J, Cooper TM, Gamis AS, Meshinchi S. Residual disease detected by multidimensional flow cytometry signifies high relapse risk in patients with de novo acute myeloid leukemia: a report from Children’s Oncology Group. Blood. 2012. August 23;120(8):1581–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gamis AS, Alonzo TA, Meshinchi S, Sung L, Gerbing RB, Raimondi SC, Hirsch BA, Kahwash SB, Heerema-McKenney A, Winter L, Glick K. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children’s Oncology Group trial AAML0531. Journal of Clinical Oncology. 2014. September 20;32(27):3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loken MR, Voigt AP, Gerbing RB, Brodersen LE, Menssen AJ, Pardo L, et al. Heirarchical clustering of immunophenotypic cell surface antigen expression identifies clinically meaningful cohorts in childhood AML: A report from the Children’s Oncology Group protocol AAML0531. Blood 2015; 126:23:3. [Google Scholar]
- 9.Paietta E, Goloubeva O, Neuberg D, Bennett JM, Gallagher R, Racevskis J, et al. A surrogate marker profile for PML/RAR alpha expressing acute promyelocytic leukemia and the association of immunophenotypic markers with morphologic and molecular subtypes. Cytom Part B-Clin Cytom 2004; 59B:1:1–9. [DOI] [PubMed] [Google Scholar]
- 10.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009; 114:5:937–951. [DOI] [PubMed] [Google Scholar]
- 11.Yang DH, Lee JJ, Mun YC, Shin HJ, Kim YK, Cho SH, et al. Predictable prognostic factor of CD56 expression in patients with acute myeloid leukemia with t(8 : 21) after high dose cytarabine or allogeneic hematopoietic stem cell transplantation. Am J Hematol 2007; 82:1:1–5. [DOI] [PubMed] [Google Scholar]
- 12.Loken MR, Voigt AP, Brodersen LE, Fritschle W, Menssen AJ, Wells DA. Consistent quantitative gene product expression: #3. Invariance with age. Cytom Part A 2016; 89A:11:997–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.