Abstract
Purpose
Phonological skills have been associated with developmental stuttering. The current study aimed to determine whether the neural processes underlying phonology, specifically for nonword rhyming, differentiated stuttering persistence and recovery.
Method
Twenty-six children who stutter (CWS) and 18 children who do not stutter, aged 5 years, completed an auditory nonword rhyming task. Event-related brain potentials were elicited by prime, rhyming, and nonrhyming targets. CWS were followed longitudinally to determine eventual persistence (n = 14) or recovery (n = 12). This is a retrospective analysis of data acquired when all CWS presented as stuttering.
Results
CWS who eventually recovered and children who do not stutter exhibited the expected rhyme effect, with larger event-related brain potential amplitudes elicited by nonrhyme targets compared to rhyme targets. In contrast, CWS who eventually persisted exhibited a reverse rhyme effect, with larger responses to rhyme than nonrhyme targets.
Conclusions
These findings suggest that CWS who eventually persisted are not receiving the same benefit of phonological priming as CWS who eventually recovered for complex nonword rhyming tasks. These results indicate divergent patterns of phonological processing in young CWS who eventually persisted, especially for difficult tasks with limited semantic context, and suggest that the age of 5 years may be an important developmental period for phonology in CWS.
Supplemental Material
Stuttering is a neurodevelopmental disorder characterized by disruptions in the production of fluent speech, which may present as sound or syllable repetitions, sound prolongations, and/or the blocking of speech progress, despite the speaker knowing exactly what they want to say (e.g., Bloodstein & Bernstein Ratner, 2008; Yairi & Seery, 2015). Stuttering is a multifactorial, dynamic disorder arising from complex interactions between genetic as well as internal and external child factors to contribute to the development of stuttering. These internal child factors, including motor, language, and cognitive abilities and emotional and temperamental characteristics, interact with external child factors, such as psychosocial pressures, family dynamics, and other characteristics specific to the child's environment, to result in epigenetic influences on neural development, especially in speech motor systems (Smith & Weber, 2017). Stuttering affects approximately 5% of young children and typically emerges between 30 and 48 months of age, during a period of rapid motor, emotional, linguistic, and cognitive development. Of children diagnosed with stuttering in the preschool years, approximately 75%–80% of children who stutter (CWS) go on to recover, while 20%–25% persist in stuttering (e.g., Bloodstein & Bernstein Ratner, 2008; Yairi & Seery, 2015). A child's ability to adapt to neurodevelopmental changes contributes to either recovery from or persistence in stuttering (Smith & Weber, 2017); however, to date, it is difficult to determine which young CWS will go on to persist and which will eventually recover.
Relationships between language and speech motor productions in CWS have been well documented (e.g., Bernstein Ratner & Sih, 1987; Gaines et al., 1991; Logan & Conture, 1997; MacPherson & Smith, 2013; Sasisekaran & Weber-Fox, 2012; Weiss & Zebrowski, 1992; Yaruss, 1999). Increased linguistic complexity across a variety of tasks has been associated with increased disfluencies (Bernstein Ratner & Sih, 1987; Gaines et al., 1991; Logan & Conture, 1997; Weiss & Zebrowski, 1992; Yaruss, 1999). Furthermore, young CWS were found to have greater variability in speech motor coordination for tasks with increased linguistic complexity, such as longer sentences or more complex phonological sequences, compared to children who do not stutter (CWNS; MacPherson & Smith, 2013; Sasisekaran & Weber-Fox, 2012; Usler et al., 2017).
Differences in language skills have been reported in CWS (e.g., Anderson & Conture, 2004; Anderso et al., 2005; Hall et al., 2007; Pellowski & Conture, 2005; for reviews, see Bernstein Ratner, 1997; Bloodstein & Bernstein Ratner, 2008; Smith & Weber, 2017). Furthermore, recent studies have identified differences in the neural processes that support language, including semantics, syntax, and phonology, in CWS compared to fluent peers, even in the absence of speaking demands (Mohan & Weber, 2015; Usler & Weber-Fox, 2015; Weber-Fox et al., 2013, 2008). Together, these findings suggest atypical neural processes for language that may impact speech production in at least some CWS. The current study evaluates whether phonological processing, which has been found to differ between CWS and CWNS (Kreidler et al., 2017; Mohan & Weber, 2015; Weber-Fox et al., 2008), may serve as an index of eventual persistence or recovery in young CWS.
Phonological Processing in CWS
Language has long been theorized to play a role in the development of stuttering. Phonological processing, a critical step in language processing and formulation (e.g., Hagoort & Levelt, 2009; Hickock, 2012; Indefrey & Levelt, 2000), has been posited as a potential point of breakdown in the process of planning for speech production (Perkins et al., 1991; Postma & Kolk, 1993; Wingate, 1988). To date, much of the literature assessing phonological abilities in CWS has employed behavioral tasks focused on phonological production (e.g., Anderson et al., 2006; Gerwin et al., 2019; Hakim & Bernstein Ratner, 2004; Melnick et al., 2003), making it difficult to separate phonological processing from phonological planning for production.
One method to increase our understanding of phonological processing is to evaluate the neural processes that underlie phonological skills, without an overt speaking requirement. Neural processes supporting phonology can be assessed through electroencephalography (EEG), a noninvasive neuroimaging technique that measures electrical activity from populations of neurons firing in synchrony (Nunez, 1995). Event-related brain potentials (ERPs) are EEG activity that is time-locked to specific stimuli (Luck, 2014). Importantly, ERPs can be acquired in the absence of speaking demands, providing insights into the neural processes that support language without additional demands of planning for speech production. ERPs have been found to index multiple aspects of language processing in children, including syntax (e.g., Friederici, 2002; Hampton Wray & Weber-Fox, 2013; Neville et al., 1993; Schneider et al., 2016; Schneider & Maguire, 2019; Usler & Weber-Fox, 2015; Weber-Fox et al., 2013), semantics (e.g., Hampton Wray & Weber-Fox, 2013; Holcomb et al., 1992; Kreidler et al., 2017; Kutas & Federmeier, 2011; Neville et al., 1993; Schneider & Maguire, 2019; Weber-Fox et al., 2013), and phonology (e.g., Coch et al., 2005; Grossi et al., 2001; Mohan & Weber, 2015; Weber-Fox et al., 2003, 2008).
Previous studies of language processing in children and adults who stutter have revealed atypical patterns in the neural processes underlying syntax (Usler & Weber-Fox, 2015; Weber-Fox & Hampton, 2008; Weber-Fox et al., 2013) and semantics (Kreidler et al., 2017; Weber-Fox, 2001; Weber-Fox & Hampton, 2008; Weber-Fox et al., 2013). A recent study examining semantic processing during a passive listening task in 5-year-old children who eventually persisted compared to those who eventually recovered from stuttering (Kreidler et al., 2017) revealed more mature semantic processes in CWS who eventually recovered (CWS-eRec) compared to CWS who eventually persisted (CWS-ePer) and CWNS. These findings suggest that eventual recovery from stuttering in young CWS may be associated with earlier maturation of neural processes underlying semantics. Importantly, these findings illustrate that subtle differences in neural processes for language, even in the absence of overt speaking or behavioral differences, can be observed using ERPs and can provide insight into early indicators of persistence or recovery in young CWS.
Neural processes supporting phonology can also be assessed using ERPs. Early and ongoing work in this area has aimed at determining whether phonological processes are distinct from semantic processes (Connolly & Phillips, 1994; Diaz & Swaab, 2007; Praamstra et al., 1994; Praamstra & Stegeman, 1993; Rugg, 1984a, 1984b; Rugg & Barrett, 1987; Van Petten et al., 1999). Studies of semantic processing have assessed semantic reliance through manipulations of sentence contexts and the use of word pairs. Tasks requiring an evaluation of phonological relatedness consistently elicit a negative ERP component, maximal between 300 and 700 ms after the onset of the stimulus, even for processing nonwords that violate phonotactical rules (Praamstra & Stegeman, 1993; Rugg, 1984a). Taken together, studies of phonological processing indicate that phonology, in addition to semantics, can modulate neural responses in this time period, and this phonological mismatch negativity likely reflects an early stage of word processing, selection mechanisms associated with phonological analysis, and segmentation (Connolly & Phillips, 1994; D'Arcy et al., 2004; Diaz & Swaab, 2007; Praamstra et al., 1994; Praamstra & Stegeman, 1993; Rugg, 1984a, 1984b). Although the nature of the relationship between phonological and semantic processing is beyond the scope of this study, previous findings suggest that, when contextual information is present, phonological processes are highly influenced by and temporally integrated with contextual and semantic processes (Diaz & Swaab, 2007; Van Petten et al., 1999).
One common way to evaluate phonological processes is to use phonological comparison or matching tasks, such as rhyming paradigms (Coch et al., 2002, 2005; Grossi et al., 2001; Praamstra et al., 1994; Praamstra & Stegeman, 1993; Rugg, 1984a, 1984b; Rugg & Barrett, 1987). Pairs of words, a prime and a target, are presented, and the listener determines whether the pairs rhyme or not. The listener must parse the onset from the rime in the first word, hold the rime in phonological working memory, and then compare the rime of the prime word with the rime of the target word. Larger ERPs are elicited by nonrhyme targets compared to rhyme targets, a difference known as the “rhyme effect” (RE; Grossi et al., 2001). The RE reflects the increased neural resources required to process the unprimed words (nonrhyme targets) compared to primed words (rhyme targets), indexing the comparison between the target and the prime already stored in phonological memory (e.g., Coch et al., 2005; Grossi et al., 2001; Rugg, 1984a, 1984b; Rugg & Barrett, 1987; Weber-Fox et al., 2003, 2008, 2004). The RE is typically largest over right-hemisphere centroparietal electrodes, a pattern that is relatively consistent in both the visual and auditory modalities in adults (Coch et al., 2008; Praamstra et al., 1994; Praamstra & Stegeman, 1993; Rugg, 1984a, 1984b; Rugg & Barrett, 1987) and children (Coch et al., 2002, 2005; Grossi et al., 2001; Mohan & Weber, 2015; Weber-Fox et al., 2003, 2008).
The RE appears to be relatively stable across development. A visual rhyme study using real words found the RE in children as young as 7 years old with a right-hemisphere parietal distribution, with RE amplitude and distribution comparable between 7-year-olds, older children, adolescents, and adults (Grossi et al., 2001). For an auditory real-word rhyme task, the distribution of the RE differed slightly—bilateral and largest over parieto-occipital electrode sites—but was still comparable in participants aged 7 years to adulthood (Coch et al., 2002). Different from visual paradigms, the auditory paradigm also revealed larger amplitudes elicited by rhyme targets over anterior sites, especially over the left hemisphere, again with no differences across age (Coch et al., 2002).
Extending the developmental literature to evaluate the development of phonological mismatch abilities, Coch and colleagues reduced the overlap and interactions between phonological and semantic processing for real words by employing a nonword rhyme paradigm in adults and in 6-, 7-, and 8-year-olds. Consistent with studies of real-word rhyme processing, 6-year-olds exhibited RE amplitudes comparable to those of older children and adults, largest over posterior medial sites (Coch et al., 2005). As found in an auditory real-word task (Coch et al., 2002), nonword rhyme targets elicited larger amplitude responses over frontal and temporal sites compared to nonrhyme targets (Coch et al., 2005). The onset of the RE was delayed in children compared to adults, suggesting that, without the support of semantic networks, the timing of initiating phonological processes is more difficult and not yet adultlike in children through the age of 8 years. Timing of the RE onset, though not RE amplitude, was associated with performance on a phonological awareness task (Coch et al., 2005).
A recent study of preschool-age children (3- to 5-year-olds) reported similar ERP patterns elicited by the same auditory nonword rhyme task (Andersson et al., 2018). In young children, anterior ERPs were larger for rhyme targets (reverse RE), whereas nonrhyme targets elicited larger responses over posterior sites (RE). The anterior reverse RE was unrelated to phonological awareness skills, whereas the posterior RE was only observed in children with stronger phonological awareness abilities. These findings may suggest that anterior REs develop earlier and have a distinct developmental time course compared to the more posterior RE (Andersson et al., 2018). Together, these studies reveal that, in children with typical development, the neural processes indexed by the RE are emerging, along with phonological awareness skills, during the preschool years and are established by the age of 6 years, for both visual and auditory presentations and for real-word and nonword rhyme paradigms.
Neural processes underlying phonology have been assessed in CWS using a visual real-word paradigm (Weber-Fox et al., 2008). CWS exhibited reduced behavioral accuracy compared to CWNS, but REs were comparable in timing and amplitude between groups. However, CWNS exhibited earlier ERP latencies over the left hemisphere compared to the right hemisphere for prime and target words, whereas CWS exhibited similar latencies across hemispheres for both word categories. These findings suggest “that the timing of the relative contributions of the left and right hemisphere functions may operate differently in CWS” (Weber-Fox et al., 2008, p. 333). Thus, although neural processes underlying rhyme were found to be similar between CWS and CWNS, subtle differences in both performance and word processing were observed between groups.
A recent retrospective analysis of auditory real-word rhyme processing based on eventual persistence or recovery status in young CWS, 4- and 5-year-olds, reported that CWS-ePer, CWS-eRec, and CWNS, who could all rhyme on a behavioral task, exhibited comparable posterior REs (Gerwin & Weber, 2020). However, larger anterior responses to rhyme targets were more consistent in CWNS than in either CWS group. These findings suggest greater variability in early phonological skills that facilitate prime–target comparisons for real-word rhyming in CWS compared to CWNS, irrespective of stuttering outcome (Gerwin & Weber, 2020).
Neural processes for rhyme were assessed using an auditory nonword rhyme task in school-age CWS and CWNS (Mohan & Weber, 2015). Children were followed for 3–5 years to determine whether they persisted in or recovered from stuttering. At the age of 7–8 years, CWS who were persisting (CWS-Per), CWS who had recovered (CWS-Rec), and CWNS completed the auditory nonword rhyme task used by Coch et al. (2005). Consistent with other studies, there were no differences between groups (CWNS, CWS-Rec, or CWS-Per) in the amplitude or timing of the posterior RE. However, CWS-Per did not exhibit the earlier anterior onset for rhyme targets compared to nonrhyme targets, which was observed in both CWS-Rec and CWNS. Additionally, this onset pattern was largest over the right hemisphere in CWS-Rec, whereas it had a bilateral distribution in CWNS (Mohan & Weber, 2015). These findings may suggest a more salient, robust neural representation of the prime word in CWNS and CWS-Rec, indexed by the earlier onset of anterior ERPs to rhyme targets. In contrast, the prime did not facilitate early phonological access in CWS-Per, where ERP onsets were comparable for rhyme and nonrhyme targets. Taken together, these patterns provide further support for differences in the earlier stages of word segmentation and rehearsal, but not in phonological integration, in CWS-Per (Mohan & Weber, 2015).
The Current Study
To date, an understanding of factors that predict persistence or recovery is relatively limited, with the strongest prediction factors including sex, family history, and age of onset (Yairi & Seery, 2015). A few recent studies have aimed to identify additional behavioral factors that may also serve as predictors for eventual outcomes. Nonword repetition skills and consonant production skills were found to be reliable predictors of stuttering outcome, with lower accuracy or more phonological errors in CWS-ePer compared to CWS-eRec and CWNS (Spencer & Weber-Fox, 2014). Additionally, earlier maturation of neural processes for semantics (indexed by the ERP component N400) was observed in CWS-eRec compared to CWS-ePer and CWNS (Kreidler et al., 2017). Although differences in ERP onset elicited by rhyme and nonrhyme target words were observed in school-age children who were persisting compared to those who had recovered from stuttering (Mohan & Weber, 2015), to date, the neural processes underlying rhyme for nonwords in younger CWS, as well as their role in eventual persistence or recovery, have not yet been investigated.
The current study aims to extend the existing literature by elucidating the differences in neural processes for rhyme with minimal semantic context in young CWS who either eventually recovered from stuttering or persisted in stuttering. Specifically, 5-year-old CWS and CWNS completed an auditory nonword rhyming task to reduce interactions between phonological and semantic processing. Children were then followed for 3–5 years. A retrospective analysis of data collected at the age of 5 years, once eventual diagnosis was known, allows for determining whether neural activity (ERPs) elicited by rhyme or nonrhyme targets differentiated CWS-ePer from CWS-eRec.
On the basis of previous findings (Gerwin & Weber, 2020; Mohan & Weber, 2015; Weber-Fox et al., 2008), we hypothesized that we would not observe significant differences in the amplitude of the RE between CWS-eRec and CWS-ePer. However, we predicted that differences would be observed in the anterior response to targets and the hemispheric distribution of the RE, as observed in older CWS during nonword processing (Mohan & Weber, 2015). Given the increased difficulty of nonword tasks compared to real-word tasks (e.g., Coch et al., 2005), an alternative hypothesis was that CWS-ePer may exhibit delayed onset of the RE compared to CWS-eRec or CWNS, as observed in younger children compared to older children (Coch et al., 2005).
Method
Participants
Participants were 5-year-old children who participated in the current study as part of a longitudinal study examining language and motor factors related to stuttering. Children were followed for 2–5 years after data acquisition. By the end of the longitudinal study, it was known which children persisted in stuttering and which children recovered from stuttering. Fifty-five CWS and 35 CWNS participated at the age of 5 years. Inclusion criteria for the current study included the following: completion of both behavioral and neurophysiological laboratory sessions, continued participation in the longitudinal study in order to determine eventual persistence in or recovery from stuttering, demonstrated ability to rhyme based on the real-word rhyme discrimination task (described below), and EEG data that did not contain excessive eye and movement artifacts. From the original 90 children, five CWS and five CWNS were excluded because they did not complete EEG testing at the age of 5 years (they completed behavioral testing only), six CWS did not continue with the longitudinal study, eight CWS and four CWNS did not meet the rhyme criteria, and seven CWS and seven CWNS did not have EEG data that were of usable quality. Four additional children, three CWS and one child who does not stutter, were excluded because their behavioral responses during the ERP task were not recorded.
Participants who met all of the above inclusion criteria included forty-four 5-year-olds. Eighteen children were judged to be perceptually fluent, with no presence or history of stuttering, whereas the remaining 26 children were determined to stutter at the time of initial testing (criteria described below). The current study is a retrospective analysis of data collected at the age of 5 years, once eventual stuttering diagnosis was known. The final groups include 14 children who eventually persisted in stuttering (10 boys, four girls), 12 children who eventually recovered from stuttering (11 boys, one girl), and 18 CWNS (10 boys, eight girls). The three groups were matched for age, F(2, 40) < 1, p = .428. All children completed either their first or second year of participation in the longitudinal project at the age of 5 years, except one child (CWNS) who completed their third year of participation (CWNS Year 2: 9/18, CWS-eRec Year 2: 9/12, CWS-ePer Year 2: 10/14). Mean ages for each group, as well as other demographic and behavioral testing data, are included in Table 1. CWS were recruited primarily from clinical settings, which may at least in part explain the higher number of CWS-ePer than CWS-eRec in the current study (Unicomb et al., 2020).
Table 1.
Group means (standard errors) and post hoc Tukey's honestly significant difference (HSD) pairwise comparisons (p values) for age, maternal education, and real-word rhyme detection at the time of testing as well as the number of stuttering-like disfluencies and scores on tests of nonverbal intelligence, receptive and expressive language, and articulatory accuracy at the initial intake.
| Post hoc Tukey's HSD pairwise comparison p values |
||||||
|---|---|---|---|---|---|---|
| Variable | CWNS | CWS-eRec | CWS-ePer | CWNS vs. CWS-eRec | CWNS vs. CWS-ePer | CWS-eRec vs. CWS-ePer |
| Age | 5.55 (0.07) | 5.45 (0.09) | 5.40 (0.09) | .672 | .428 | .939 |
| Mat Ed | 6.50 (0.19) | 5.92 (0.31) | 5.29 (0.27) | .231 | .002** | .215 |
| Rhyme word | 8.89 (0.29) | 8.17 (0.52) | 8.57 (0.44) | .425 | .832 | .782 |
| SLDs | 5.87 (1.51) | 6.99 (1.00) | .545 | |||
| CMMS | 115.89 (2.04) | 109.08 (3.49) | 107.29 (2.31) | .156 | .044* | .885 |
| TACL-3 | 125.59 (3.22) | 113.92 (3.21) | 109.14 (3.89) | .061 | .004** | .637 |
| SPELT-3 | 114.78 (2.01) | 103.08 (2.71) | 96.00 (3.60) | .013* | < .001** | .215 |
| BBTOP-CI | 103.56 (2.20) | 95.25 (3.61) | 90.57 (3.79) | .160 | .011* | .583 |
| BBTOP-PPI | 105.00 (2.24) | 94.67 (5.02) | 87.71 (4.26) | .134 | .004** | .429 |
Note. Age, Mat Ed, and real-word rhyme detection were collected when participants were at the age of 5 years, when neurophysiological data were acquired. SLDs, CMMS, TACL-3, SPELT-3, BBTOP-CI, and BBTOP-PPI were acquired at the initial intake into the longitudinal study. CWNS = children who do not stutter; CWS-eRec = children who stutter who eventually recovered; CWS-ePer = children who stutter who eventually persisted in stuttering; Mat Ed = maternal education, based on the Hollingshead scale (4 = high school, 5 = partial college, 6 = college degree, 7 = graduate degree; Hollingshead, 1975); Rhyme word = real-word rhyme discrimination task from the Phonological Awareness Test–Second Edition (Robertson & Salter, 2007); SLDs = stuttering-like disfluencies, or the number of SLDs per 100 syllables in a spoken language sample; CMMS = Columbia Mental Maturity Scale (Burgemeister et al., 1972); TACL-3 = Test for Auditory Comprehension of Language–Third Edition (Carrow-Woolfolk, 1999); SPELT-3 = Structured Photographic Expressive Language Test–Third Edition (Dawson et al., 2003); BBTOP-CI = Bankson–Bernthal Test of Phonology Consonant Inventory subtest (Bankson & Bernthal, 1990); BBTOP-PPI = Bankson–Bernthal Test of Phonology Phonological Process Inventory subtest (Bankson & Bernthal, 1990).
p < .05.
p < .01.
Consistent with the criteria proposed by Ambrose and Yairi (1999), a child was diagnosed with stuttering at their first laboratory session if (a) stuttering severity was rated as a 2 or greater on an 8-point (0–7) scale by a speech-language pathologist with expertise in stuttering (0 = no stuttering and 7 = greatest severity of stuttering), (b) stuttering severity was rated as a 2 or greater on the same 8-point (0–7) scale by the parent, and (c) the child displayed at least three stuttering-like disfluencies (SLDs) per 100 syllables during parent–child and clinician–child language samples collected in free-play in the laboratory. SLDs included part-word repetitions, sound prolongations, and/or silent blocks. Six children (two CWS-ePer and four CWS-eRec) produced less than three SLDs (range: 2.25–2.85/100 syllables), with parents reporting that their child's samples in the laboratory were not wholly representative of disfluencies produced in other contexts. This is consistent with previous findings indicating that stuttering can vary across situations (Gerwin et al., 2019; Yaruss, 1997). In these situations, in addition to parent report and rating, stuttering diagnosis was based on the speech-language pathologist's interactions with the child throughout the laboratory experience. 1 A child was determined to have recovered when they no longer satisfied these criteria for at least 2 consecutive years. The mean SLDs for the two stuttering groups at the initial intake into the project (their first laboratory visit) are included in Table 1. At initial testing, the two groups of CWS did not differ in frequency of SLDs, t(24) < 1, p = .545, d = 0.26.
All participants were native, monolingual speakers of English with no history of neurological disease or injury and no language, reading, visual, or hearing impairments other than stuttering for the experimental groups. Each participant passed a hearing screening at 20 dB SPL of 500, 1000, and 2000 Hz. Maternal education was used as a proxy for each child's socioeconomic status based on the Hollingshead scale (Hollingshead, 1975). A one-way analysis of variance (ANOVA) revealed a difference in maternal education between the three groups, F(2, 43) = 6.58, p = .003. Tukey's honestly significant difference (HSD) post hoc comparisons, a conservative method for pairwise comparisons using the MS error term from the ANOVA (Kutner et al., 2005), were calculated for pairwise group comparisons (see Table 1). Although CWNS had higher maternal education levels than CWS-ePer, maternal education levels were comparable between CWNS and CWS-eRec as well as between CWS-eRec and CWS-ePer. Mean maternal education levels for each group and Tukey's HSD statistics for each comparison are shown in Table 1.
Data were collected at Purdue University and The University of Iowa, both of which had institutional review board approval for this project. Twelve CWNS, 11 CWS-eRec, and 10 CWS-ePer participated at Purdue University. The other children (six CWNS, one child who stutters who eventually recovered, and four CWS-ePer) participated at The University of Iowa. All parents provided written consent, and all children provided verbal assent prior to participation.
Children participating in this longitudinal study completed multiple EEG and behavioral tasks. Other ERP data sets from children in the current study have been included in two other studies. Eight CWNS, two CWS-eRec, and four CWS-ePer were included in a study of nonword rhyme skills in 7- to 8-year-olds (Mohan & Weber, 2015). Four CWNS, six CWS-eRec, and five CWS-ePer were included in a retrospective analysis of the ways in which semantic processing at the age of 5 years may differentiate stuttering persistence or recovery using a different EEG task (Kreidler et al., 2017).
Behavioral Assessments
A battery of behavioral assessments was administered to ensure typical speech and language development. Nonverbal intelligence was assessed using the Columbia Mental Maturity Scale (Burgemeister et al., 1972), in which children identify the image that does not belong with other images. Receptive language skills were assessed using the Test for Auditory Comprehension of Language–Third Edition (Carrow-Woolfolk, 1999), which evaluated vocabulary, grammatical morphology, and syntactic knowledge using elaborated phrases and sentences. Expressive language was assessed via the Structured Photographic Expressive Language Test–Third Edition (Dawson et al., 2003), in which children produce morphological and syntactic structures based on pictures of situations or activities. Sound production skills were assessed using the Bankson–Bernthal Test of Phonology Consonant Inventory subtest and Phonological Process Inventory subtest (Bankson & Bernthal, 1990), which use pictures to elicit sound production in word-initial and word-final positions. All participants performed within normal limits (see Table 1).
One-way ANOVAs were used to determine whether groups performed similarly on behavioral tasks. If the three-group interaction was significant, Tukey's HSD post hoc comparisons were calculated to determine whether differences existed between each group. Three-way group interactions were observed for performance on the standardized tests described above. Post hoc Tukey analyses (see Table 1) revealed that CWNS scored higher than CWS-ePer on tests of nonverbal intelligence, receptive language, expressive language, articulatory production, and phonological production. CWNS also exhibited better performance on the expressive language task than CWS-eRec and a tendency to perform better on the receptive language task. CWNS and CWS-eRec performed comparably on all other tasks. Importantly, the two groups of CWS did not differ on each of these skills: nonverbal intelligence, receptive language, expressive language, articulatory production, and phonological production.
Phonological awareness and rhyming abilities were assessed using the Phonological Awareness Test–Second Edition (Robertson & Salter, 2007). Children had to determine whether pairs of real words rhymed or not. Children had to complete the task with a minimum of 60% accuracy on the rhyme discrimination task on the Phonological Awareness Test–Second Edition to be included in the current study, with the majority of children in each group completing the task with 80% accuracy or greater (86% of CWS-ePer, 67% of CWS-eRec, and 94% of CWNS). As seen in Table 1, there were no differences between groups on the rhyme discrimination task, F(2, 41) < 1, p = .458.
ERP Rhyme Task Stimuli
Auditory stimuli were taken from the study of nonword auditory rhyme by Coch et al. (2005) and were used in a previous study of nonword rhyme in older CWS (Mohan & Weber, 2015). Stimuli consisted of 88 pseudowords or speakable nonwords that followed the phonological rules of English, with no semantic value and were based loosely on real-word rhyme pairs used in previous studies (Coch et al., 2002; Grossi et al., 2001). The complete list of rhyme word pairs is available in Supplemental Material S1. All words were monosyllabic except for one pair (fauer–blauer). Forty-four pairs rhymed (e.g., nef–gef), and 44 pairs did not rhyme (e.g., jate–yise). The first pseudoword of the word pair was labeled the “prime,” and the second was labeled the “target.” The target either rhymed or did not rhyme with its prime. Each nonword was recorded by a female with a native American English accent using Praat (Boersma & Weenink, 2018) at a digitization rate of 22.5 kHz. The average word duration was 582.4 ms (SD = 72.1). Two presentation orders were created, with each nonword occurring as a target only one time in each list, consistent with previous studies (Coch et al., 2005; Mohan & Weber, 2015). Presentation orders were counterbalanced within and between groups.
Procedure
The procedure is similar to that used by Mohan and Weber (2015). Participants were introduced to the laboratory setting during a prior visit. Behavioral testing and EEG testing were completed on separate days. For EEG testing, the electrode cap was placed while participants viewed a movie or played a video game. Once the cap was in place, the child stopped watching the movie/playing the game and transitioned to a sound-attenuating booth, seated next to a researcher. The children received the following instructions:
Now you will listen to pretend words. Sometimes the words will rhyme, like “zoof” and “noof.” Sometimes they won't rhyme, like “jat” and “misk.” Listen to the pairs of words and try to tell if they rhyme or if they don't rhyme. It is important that you sit very still. While you are listening to the pretend words, look at the mark on the screen in front of you. Sometimes I will ask you if the words rhyme or not, so listen carefully. Every once in a while you will see a picture pop up. This is the time when you can move! Every time you see a picture, you can play a turn of our game. When you finish the game, you will get a surprise! We will start now. Remember to listen carefully to the words that rhyme and don't rhyme. Let's practice listening to a few now. Are you ready?
All stimuli were presented via a speaker at the child's midline, located directly above a computer monitor that was 160 cm from the child. Throughout the paradigm, a small cross-hair appeared on the screen to help the children maintain visual focus. Nonword stimuli were presented using Presentation (Version 14.9, Neurobehavioral Systems) at a level of 70–75 dB SPL.
The researcher initiated each trial by pressing a button. After 1,080 ms, the prime was presented and then followed by an interstimulus interval of 1,070 ms. Then, the target was presented. Ten times throughout the paradigm, the researcher asked the child, “Did those two rhyme?” The child's response was recorded, after which the researcher pressed a button to begin the next trial. Eleven blocks of stimuli were presented, with eight word pairs per block. Between each paradigm block and at the conclusion of the experiment, the children were provided with reinforcers (e.g., stickers and a small prize).
Electrophysiological Recording
EEG recording, ERP analyses, and statistical measures were consistent with the guidelines specified by Picton et al. (2000) and Luck (2014). At Purdue University and The University of Iowa, electrical activity was recorded at the level of the scalp using an elastic cap (Quick-Cap, Compumedics Neuroscan) with 32 Ag/AgCl embedded electrodes. Electrode positions were consistent with the International 10–10 system (American Electroencephalographic Society, 1994). The electrode channels included lateral sites (F7/F8, FT7/FT8, T7/8, TP7/TP8, P7/P8), midlateral sites (F3/F4, FC3/FC4, C3/4, CP3/CP4, P3/P4), and midline sites (Fz, FCz, Cz, CPz, Pz). Scalp electrode locations are illustrated in the bottom-left corner of Figure 1. Vertical and horizontal eye movements were recorded via linked electrodes placed on the left superior and inferior orbital ridge (vertical electrooculogram) and over the left and right outer canthi (horizontal electrooculogram). EEG data were referenced online to linked electrodes placed over the left and right mastoids. All electrode impedances were adjusted to 5 kΩ or less. Electroencephalographic data were bandpass filtered between 0.1 and 100 Hz and digitized online (Neuroscan 4.2) at the rate of 500 samples per second.
Figure 1.
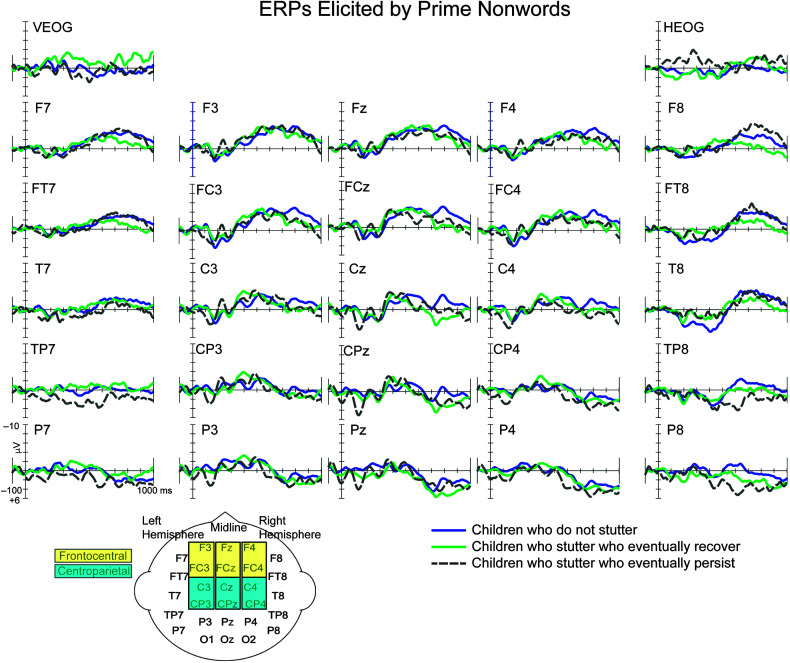
Grand-averaged event-related brain potentials (ERPs) elicited by prime words for children who do not stutter (blue lines), children who eventually recovered from stuttering (green lines), and children who eventually persisted in stuttering (gray dashed lines). For this and all subsequent figures, negative is plotted upward, and ERP figures have been low-pass filtered at 20 Hz for illustrative purposes only.
EEG Data Analyses
EEG analyses were completed using MATLAB (MathWorks) as well as EEGLAB (Delorme & Makeig, 2004) and ERPLAB (Lopez-Calderon & Luck, 2014) toolboxes. Off-line, to reduce file size, EEG data were downsampled to 250 Hz. Eye movement and blink artifacts were removed using independent component analysis (ICA), consistent with previous ERP studies in young children (Astheimer et al., 2014; Ervast et al., 2015; Gerwin & Weber, 2020; Hodel et al., 2019; Usler & Weber-Fox, 2015; Weber-Fox et al., 2013). ICA sources containing ocular movements, specifically blink and horizontal eye movements, were identified for each data set independently by two trained research assistants. ICA sources for ocular movement were identified using three criteria: (a) topographic maps of ICA source scalp distribution, (b) ICA source time–amplitude maps aligned with EEG data to ensure ICA source amplitude changes aligned in time with ocular movements in the EEG data, and (c) ensuring removal of ICA sources resulted in minimal effects on EEG data where ocular movements were not observed. If independent raters did not agree on which ICA sources contained only ocular movement, discrepancies were resolved by an expert third rater (first author). Ocular components were removed from the continuous EEG data. EEG signals were then low-pass filtered at 40 Hz with a 12-dB roll-off to reduce high-frequency noise. Continuous EEG data were epoched from 100 ms prior to 1,000 ms after stimulus onset. The 100 ms prior to nonword onset served as the baseline period. Automatic artifact rejection was performed on each epoch using a 50-ms moving-window voltage-dependent artifact detection algorithm. Artifacts exceeding ± 100 μV within a 200-ms window in eye channels and/or exceeding ± 200 μV in all other channels were marked as artifact. Manual inspection of each epoch was subsequently performed to ensure artifacts were removed from the data. Artifact-free trials for primes, rhyme targets, and nonrhyme targets were averaged together. Separate grand averages were created for each group, CWNS, CWS-eRec, and CWS-ePer. One-way ANOVAs for each condition revealed that the mean number of trials accepted per condition did not differ between groups, all Fs(2, 43) < 1, ps > .541. The number of trials accepted per condition per group is included in Table 2.
Table 2.
Mean (standard error) number of trials accepted for each condition and behavioral accuracy for the event-related brain potential nonword rhyme paradigm for each group.
| Variable | CWNS | CWS-eRec | CWS-ePer |
|---|---|---|---|
| Primes | 39.67 (3.03) | 41.92 (4.43) | 41.14 (2.84) |
| Rhyme target | 18.28 (1.78) | 20.75 (2.59) | 17.71 (1.38) |
| Nonrhyme target | 17.56 (1.74) | 19.67 (2.49) | 18.57 (1.30) |
| Rhyme task accuracy (/10) | 8.00 (0.47) | 6.33 (0.71) | 6.71 (0.62) |
Note. CWNS = children who do not stutter; CWS-eRec = children who stutter who eventually recovered; CWS-ePer = children who stutter who eventually persisted in stuttering.
Mean amplitudes of ERPs elicited by prime and targets were calculated relative to baseline (Luck, 2014). The RE was calculated by subtracting the mean amplitudes elicited by the rhyme target from the nonrhyme target (RE = nonrhyme target − rhyme target). Based on timing and distribution of the RE in previous rhyme studies that include children (Andersson et al., 2018; Coch et al., 2002; Gerwin & Weber, 2020; Mohan & Weber, 2015) and on visual inspection of the current data, a priori analyses included a mean amplitude time window of 400–700 ms after target onset. Two regions of interest (ROIs) that allowed for assessment of the previously reported anterior and posterior REs as well as potential hemispheric differences in CWS were created across midlateral frontocentral and centroparietal sites, as illustrated in Figure 1. Midlateral ROIs were calculated by taking the mean amplitude across electrodes within the region, specifically left hemisphere frontocentral (F3, FC3), right hemisphere frontocentral (F4, FC4), left hemisphere centroparietal (C3, CP3), and right hemisphere centroparietal (C4, CP4). Two midline ROIs were computed for midline sites: frontocentral (Fz, FCz) and centroparietal (Cz, CPz). Given the broad and ongoing nature of the ERP component elicited by both prime and target nonwords in the current study, mean amplitudes were also calculated across a later time window, 700–1,000 ms, similar to the later window used in another study of the RE in young children (Andersson et al., 2018). REs elicited by this paradigm were relatively broad, without a pronounced peak; therefore, peak latency measures were not calculated.
Statistical Analyses
Performance on the rhyme discrimination task during the ERP paradigm was analyzed using a one-way ANOVA between the three groups. Post hoc pairwise analyses were completed using Tukey's HSD comparisons.
Consistent with previous studies (e.g., Grossi et al., 2001; Usler & Weber-Fox, 2015; Weber-Fox et al., 2013, 2004, 2008), mixed-effects repeated-measures ANOVAs were employed for the ERP measures separately for the primes and the RE. For primes and the RE, ANOVAs included a between-subjects factor of group (CWNS, CWS-eRec, and CWS-ePer) and within-subject factors of hemisphere (left, right) and anterior–posterior distribution (frontocentral, centroparietal). 2 Significance was set at p < .05. For all interactions with greater than one degree of freedom in the numerator, Huynh–Feldt adjusted p values are reported. Effects sizes, indexed by partial eta squared (ηp 2), are reported for all significant effects. Tukey's HSD post hoc comparisons, as used for the behavioral performance described above, were used as a conservative method for pairwise comparisons without increasing alpha level (Kutner et al., 2005) and to determine whether each group differed from the others.
Results
Rhyme Judgment Accuracy
Rhyme judgments made during the ERP task were comparable between groups, F(2, 43) = 2.38, p = .105. No differences between pairs of groups were observed (all ps > .123). Mean response accuracy for each group is presented in Table 2.
ERPs Elicited by Primes
The ERP grand averages elicited by the primes for CWNS, CWS-eRec, and CWS-ePer are illustrated in Figure 1. ERP responses elicited by primes consisted of an early positivity, followed by a broad negativity for all three groups, consistent with a previous study of auditory nonword rhyme in children (Coch et al., 2005). Statistical results from both midlateral and midline repeated-measures ANOVAs are included in Table 3.
Table 3.
Analyses of between-groups effects for prime nonwords and the rhyme effect over midlateral and midline regions of interest for each time window.
| Group |
400–700 |
700–1,000 |
||||||
|---|---|---|---|---|---|---|---|---|
| Factor(s) | df | F | P | ηp 2 | df | F | p | ηp 2 |
| Midlateral regions of interest | ||||||||
| Primes | ||||||||
| 3 groups | ||||||||
| G | 2, 41 | < 1 | .924 | .004 | 2, 41 | 1.15 | .327 | .053 |
| Hemi × G | 2, 41 | < 1 | .433 | .040 | 2, 41 | < 1 | .764 | .013 |
| AP × G | 2, 41 | < 1 | .716 | .016 | 2, 41 | < 1 | .765 | .013 |
| Hemi × AP × G | 2, 41 | < 1 | .989 | .001 | 2, 41 | < 1 | .620 | .023 |
| Rhyme effect | ||||||||
| 3 groups | ||||||||
| G | 2, 41 | 4.20 | .022 * | .170 | 2, 41 | 8.21 | .001 ** | .286 |
| Hemi × G | 2, 41 | 1.05 | .359 | .049 | 2, 41 | < 1 | .762 | .013 |
| AP × G | 2, 41 | 3.31 | .047 * | .139 | 2, 41 | 2.74 | .076 | .118 |
| Hemi × AP × G |
2, 41 |
< 1 |
.666 |
.020 |
2, 41 |
1.45 |
.246 |
.066 |
|
Midline regions of interest
| ||||||||
| Primes | ||||||||
| 3 groups | ||||||||
| G | 2, 41 | < 1 | .928 | .004 | 2, 41 | 2.07 | .139 | .092 |
| AP × G | 2, 41 | 3.92 | .028 * | .160 | 2, 41 | 2.27 | .116 | .100 |
| Rhyme effect | ||||||||
| 3 groups | ||||||||
| G | 2, 41 | 1.60 | .214 | .072 | 2, 41 | 2.93 | .065 | .125 |
| AP × G | 2, 41 | < 1 | .559 | .028 | 2, 41 | < 1 | .912 | .004 |
Note. G = Group; Hemi = Hemisphere; AP = Anterior–Posterior Distribution.
p < .05.
p < .01.
400- to 700-ms Time Window
In the 400- to 700-ms time window, no significant overall group effects or interactions including group were present over midlateral ROIs. Over midline ROIs, the overall group effect was not significant. However, a significant interaction between anterior–posterior distribution and group was observed. Separate one-way ANOVAs with Tukey's HSD post hoc comparisons were conducted for frontocentral and centroparietal midline ROIs. No significant effect of group was observed across midline frontocentral sites, F(2, 43) < 1, p = .571, and pairwise group effects were not significant (Tukey's HSD: all ps > .627). Similarly, no overall or pairwise group effects were observed across midline centroparietal sites, F(2, 43) < 1, p = .552 (pairwise Tukey's HSD: all ps > .529). These step-down analyses revealed comparable scalp distribution of mean amplitudes elicited by primes between groups.
700- to 1,000-ms Time Window
In the later time window, no overall effects of group or interactions including group were observed for midlateral or midline ROIs (see Table 3).
Summary of ERPs Elicited by Primes
No differences were observed in ERPs elicited by prime nonwords between CWNS, CWS-eRec, and CWS-ePer for earlier or later time windows across both scalp regions.
ERPs Elicited by Targets—The RE
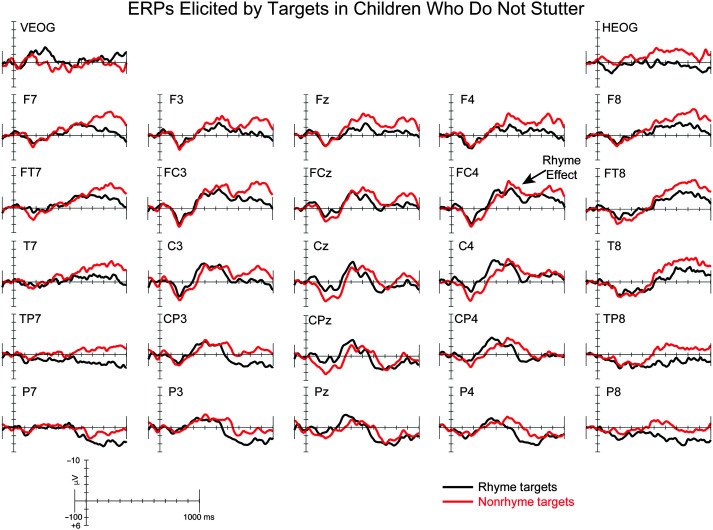
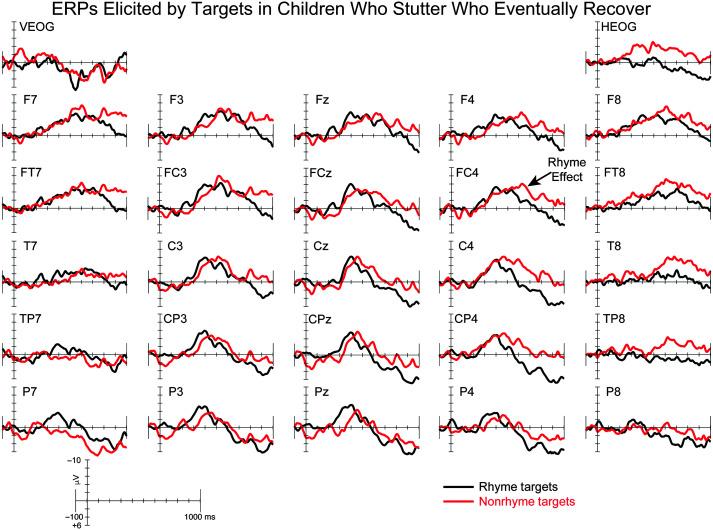
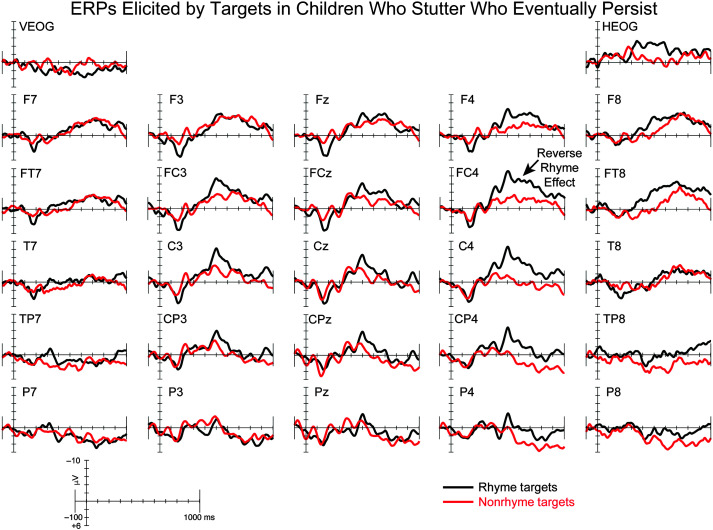
The ERP grand averages elicited by rhyme and nonrhyme targets for CWNS, CWS-eRec, and CWS-ePer are illustrated in Figures 2, 3, and 4, respectively. Rhyme and nonrhyme targets elicited an early positivity followed by a broad negativity in each group, consistent with previous studies of auditory nonword rhyme in children (Andersson et al., 2018; Coch et al., 2005). Visual inspection of the waveforms revealed the expected RE, that is, a larger negativity elicited by nonrhyme targets compared to rhyme targets, in CWNS and CWS-eRec. However, a reverse RE, with larger negativities elicited by rhyme targets compared to nonrhyme targets, was observed in CWS-ePer. These findings were confirmed by statistical analyses (see Table 3), described below.
Figure 2.
Grand-averaged event-related brain potentials (ERPs) elicited by rhyme (black) and nonrhyme (red) targets for children who do not stutter. For illustrative purposes, the rhyme effect is labeled at FC4.
Figure 3.
Grand-averaged event-related brain potentials (ERPs) elicited by rhyme (black) and nonrhyme (red) targets for children who eventually recovered from stuttering. For illustrative purposes, the rhyme effect is labeled at FC4.
Figure 4.
Grand-averaged event-related brain potentials (ERPs) elicited by rhyme (black) and nonrhyme (red) targets for children who eventually persisted in stuttering. For illustrative purposes, the reverse rhyme effect is labeled at FC4.
400- to 700-ms Time Window
Between-groups comparisons over midlateral ROIs revealed a significant main effect of group (see Table 3) across the three groups, reflecting the expected RE in CWNS and CWS-eRec as well as the reverse, or positive, RE in CWS-ePer (see Figure 5). Tukey's HSD post hoc comparisons used for pairwise group comparisons revealed a significant effect of group was observed between CWNS and CWS-ePer (p = .034), and the effect was almost significant between CWS-eRec and CWS-ePer (p = .050). The group effect was not significant between CWNS and CWS-eRec (p = .995). These findings reflect the reverse RE observed in CWS-ePer and reveal that the RE was similar between CWNS and CWS-eRec.
Figure 5.
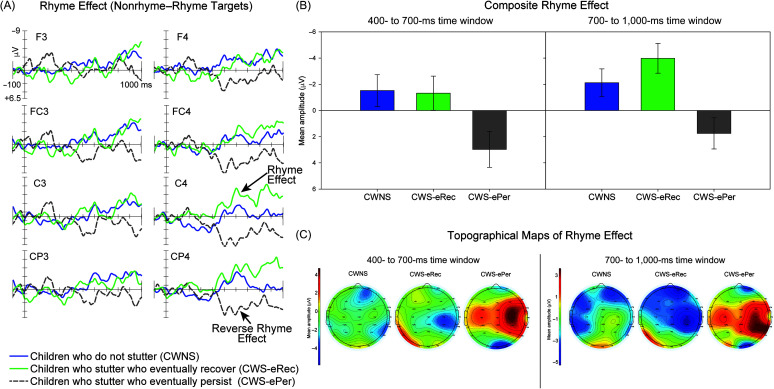
(A) Rhyme effects elicited by targets for children who do not stutter (CWNS), children who eventually recovered from stuttering (CWS-eRec), and children who eventually persisted in stuttering (CWS-ePer) are illustrated for the midlateral electrode sites included in analyses. CWNS and CWS-eRec exhibited the expected rhyme effect, with larger amplitudes elicited by nonrhyme targets compared to rhyme targets. In contrast, CWS-ePer exhibited a reverse rhyme effect, with larger amplitudes elicited by rhyme targets compared to nonrhyme targets. (B) Composite rhyme effect values, calculated as the mean across midlateral electrodes included in analyses (illustrated in Panel A), are plotted for the two analysis time windows. (C) Topographical head plots show the distribution of the rhyme effect across the scalp for each group for each time window. Cooler colors (blue) indicate more negative mean amplitudes, and warmer colors (red) indicate more positive mean amplitudes.
A significant interaction between anterior–posterior distribution and group was also observed for midlateral ROIs. To better understand this interaction, separate one-way ANOVAs with Tukey's HSD post hoc comparisons were conducted across the midlateral frontocentral and midlateral centroparietal ROIs. No significant effect of group was observed across midlateral frontocentral sites, F(2, 43) = 2.59, p = .087, and pairwise group effects were not significant (Tukey's HSD: CWNS and CWS-ePer, p = .084; CWS-eRec and CWS-ePer, p = .240; CWNS and CWS-eRec, p = .929). A significant group effect was observed over midlateral centroparietal sites, F(2, 43) = 4.84, p = .013. Tukey's HSD post hoc comparisons revealed a larger RE over centroparietal sites for CWNS compared to CWS-ePer (p = .019) and for CWS-eRec compared to CWS-ePer (p = .038). RE amplitudes did not differ between CWNS and CWS-eRec (p = .847). No effects of group or interactions with group were observed across midline ROIs (see Table 3).
700- to 1,000-ms Time Window
Similar to the time window of 400–700 ms, between-groups ANOVAs revealed a significant group effect across the three groups for the midlateral ROIs (see Table 3). This reflected the RE in CWNS and CWS-eRec and the reverse RE in CWS-ePer. Tukey's HSD post hoc comparisons revealed significant group effects between CWNS and CWS-ePer (p = .014) and between CWS-eRec and CWS-ePer (p = .001). Group effects were not significant between CWNS and CWS-eRec (p = .403). No effects of group or interactions with group were observed across midline ROIs (see Table 3).
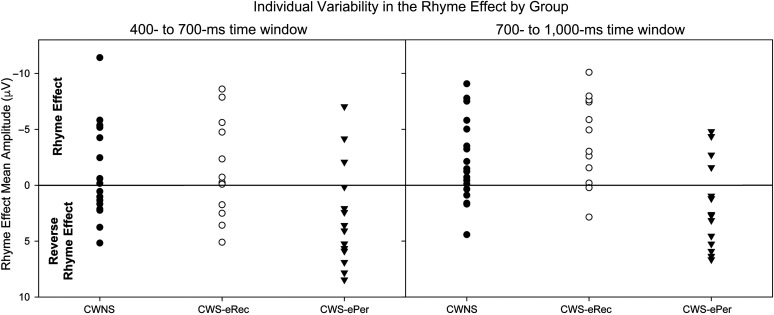
Individual Variability in the RE
To visualize individual variability in the RE, the composite midlateral RE, averaged across frontocentral and centroparietal sites, was plotted for individual participants in each group for each time window, as illustrated in Figure 6. The presence of an RE was defined as mean amplitude ≤ −0.5 μV. In the 400- to 700-ms time window, the expected RE was observed in nine out of 18 (50.0%) CWNS and six out of 12 (50.0%) CWS-eRec, but only three out of 14 (21.4%) CWS-ePer. In contrast, the reverse RE (≥ 0.5 μV) was observed in 10 out of 14 (71.4%) CWS-ePer, but only eight out of 18 (44.4%) CWNS and four out of 12 (33.3%) CWS-eRec. The remaining children in each group did not exhibit differences in mean amplitudes between rhyme and nonrhyme conditions. Fisher's exact test of independence (e.g., Heumann & Schomaker, 2016) revealed that the number of children presenting with REs or reverse REs did not differ between groups (p = .262).
Figure 6.
Composite rhyme effect values, calculated as the mean across midlateral electrodes included in analyses (F3/4, FC3/4, C3/4, CP3/4), for each participant in each group: children who do not stutter (CWNS; closed circles), children who stutter who eventually recovered (CWS-eRec; open circles), and children who stutter who eventually persisted in stuttering (CWS-ePer; triangles). Rhyme effects are plotted for individual participants for the time windows of 400–700 ms and 700–1,000 ms. While the majority of CWNS and CWS-eRec exhibited the expected rhyme effect, only a small number of CWS-ePer exhibited the rhyme effect. For consistency with event-related brain potential plots, negative is plotted upward.
In the later, 700–1000 ms time window, 11 out of 18 (61.1%) CWNS, nine out of 12 (75.0%) CWS-eRec, and four out of 14 (28.6%) CWS-ePer exhibited the RE, whereas the reverse RE was observed in 10 out of 14 (71.4%) CWS-ePer, but only four out of 18 (22.2%) CWNS and one out of 12 (8.3%) CWS-eRec. No differences in mean amplitudes between rhyme and nonrhyme conditions were observed for the other children in each group. Applying Fisher's exact test, significant differences were observed in the number of children presenting with REs versus reverse REs (p = .006). Pairwise group comparisons using Fisher's exact test, corrected for multiple comparisons using Bonferroni correction, revealed significant differences in the number of children presenting with REs and reverse REs between CWNS and CWS-ePer (p = .015) as well as between CWS-eRec and CWS-ePer (p = .002), but no differences between CWNS and CWS-eRec (p = .854).
Summary of ERPs Elicited by Targets
In summary, CWNS and CWS-eRec exhibited an RE that was comparable between the two groups. In contrast, CWS-ePer exhibited an extended reverse RE across midlateral ROIs that differed significantly from the RE observed in CWNS and CWS-eRec for both time windows.
Discussion
The current study aimed to determine whether neural processes underlying rhyme differed between children who eventually persisted in stuttering from children who eventually recovered. Five-year-olds completed a nonword rhyme task during which ERP data were acquired. Children were then followed for approximately 3 years to determine whether they persisted in or recovered from stuttering; this study is a retrospective analysis of the data collected when they were all presenting as CWS. The current findings reveal that the broad negative ERP components elicited by primes were comparable between CWNS, CWS-eRec, and CWS-ePer and suggest that single nonword processing is generally similar in young CWS, regardless of eventual stuttering outcome. For the RE elicited by target words, neural processes distinguished CWS-ePer from CWNS and CWS-eRec. CWNS and CWS-eRec exhibited the expected RE—larger responses elicited by nonrhyme targets compared to rhyme targets—that was comparable between groups. However, CWS-ePer exhibited a reverse RE, with larger amplitudes elicited by rhyme targets compared to nonrhyme targets. These findings indicate that, at the age of 5 years, CWS-ePer are not receiving the benefits of priming in the rhyme condition and may suggest that, when semantic context is minimal, young CWS who go on to persist in stuttering exhibit divergent patterns of neural processes underlying phonology.
Comparable Neural Activity for Primes Across Groups
The current findings of overall comparable mean amplitudes for the later negativity elicited by primes between CWNS, CWS-eRec, and CWS-ePer are consistent with previous findings using the same paradigm in 7- to 8-year-old children (Mohan & Weber, 2015). These findings also align with comparable mean amplitudes elicited by prime words in 9- to 13-year-old CWNS and CWS using a visual real-word rhyme paradigm (Weber-Fox et al., 2008). Together, these findings suggest that, by the age of 5 years, children who eventually persisted in stuttering and children who eventually recovered from stuttering are exhibiting comparable later neural processes for single nonwords. Furthermore, these skills appear to be maintained with age, regardless of eventual stuttering persistence or recovery status and across modalities.
RE Distinguishes Children Who Eventually Persisted From Those Who Eventually Recovered From Stuttering
We hypothesized that both CWS-eRec and CWS-ePer would exhibit the expected RE, with differences in scalp distribution, similar to existing findings (Gerwin & Weber, 2020; Mohan & Weber, 2015). Consistent with our hypothesis, CWNS and CWS-eRec exhibited the expected RE, with larger ERP amplitudes elicited by nonrhyme targets compared to rhyme targets, which did not differ between these two groups. Together with previous findings, the current findings suggest that 5-year-olds who eventually recovered from stuttering exhibit neural processes for rhyme that are comparable to those observed in CWNS.
Although previous studies (Andersson et al., 2018; Gerwin & Weber, 2020; Mohan & Weber, 2015) reported a larger anterior ERP effect for rhyme targets compared to nonrhyme targets, with a posteriorly distributed RE, this pattern did not reach statistical significance in the current study. The difference between current and previous findings may reflect the additional difficulty of nonword rhyme for young children. Without the support of semantic networks, neural responses may be more variable, making anterior versus posterior differences harder to detect, or may require broader engagement of phonological networks to complete the task, resulting in broadly distributed responses to targets. Future studies across early age ranges are needed to better understand the developmental trajectory of anterior versus posterior REs and factors that may impact the magnitude of these responses, as well as the potential impact of those differences on behavior.
Surprisingly, findings in CWS-ePer differed from our hypothesis and from previous findings (Gerwin & Weber, 2020; Mohan & Weber, 2015; Weber-Fox et al., 2008). CWS-ePer exhibited a broad reverse RE, with larger amplitudes elicited by rhyme targets compared to nonrhyme targets. These differences were largest over centroparietal sites in the 400- to 700-ms time window, where REs are typically most pronounced (Andersson et al., 2018; Coch et al., 2002, 2005; Grossi et al., 2001; Praamstra et al., 1994; Praamstra & Stegeman, 1993; Rugg, 1984a, 1984b; Rugg & Barrett, 1987; Weber-Fox et al., 2003) and were more broadly distributed across the scalp in the later time window of 700–1,000 ms. Previous studies reported the expected RE in CWS with persistent stuttering with comparable mean amplitudes to CWNS and/or CWS-Rec (Gerwin & Weber, 2020; Mohan & Weber, 2015; Weber-Fox et al., 2008). Completion of the rhyming task requires parsing the prime into its onset and rime, holding the rime in working memory, and then comparing the rime of the prime to the rime of the target, a skill exhibited by children as young as 5 years old (Goswami & East, 2000; Ziegler & Goswami, 2005). The phonological neighborhood of the rime is activated by the prime, which facilitates the processing of the rhyme target word, both behaviorally, often indexed by better accuracy and faster reaction times, as well as neurophysiologically, indexed by smaller mean amplitudes (e.g., Coch et al., 2002; Praamstra & Stegeman, 1993; Rugg, 1984a). CWS-ePer did not appear to receive the benefit of phonological nonword priming, indexed by larger amplitudes for the rhyme targets compared to nonrhyme targets.
The distinct RE patterns for real-word and nonword rhyme processing in CWS-ePer of similar age range (4- and 5-year-olds) may suggest a different strategy for phonological processes in CWS-ePer compared to CWNS and CWS-eRec. In real-word rhyme tasks, semantic processes, which overlap in time with phonological processes (Diaz & Swaab, 2007), may help facilitate prime–target comparisons, resulting in smaller amplitudes for rhyme targets compared to nonrhyme targets in young CWS-ePer (Gerwin & Weber, 2020). In the current study, with minimal semantic context, rhyme processing is more reliant on phonological networks. The current findings suggest that, instead of facilitating or easing target processing when the prime and the target rhyme, target processing for rhyme pairs recruits additional neural resources, reflected by larger ERP responses to rhyme targets. This pattern suggests less efficient phonological processes for targets in CWS-ePer, most pronounced when semantic context is limited.
The current findings align with and extend previous findings indicating that performance on a nonword repetition task (Dollaghan & Campbell, 1998) predicted eventual persistence in or recovery from stuttering in preschool-age CWS (Spencer & Weber-Fox, 2014). Together, these results suggest that young CWS-ePer exhibit less efficient neural processes underlying phonology, which may be observed, at least in some CWS-ePer, as reduced performance on a nonword repetition task compared to peers who eventually recovered from stuttering. The phonological complexities of a nonword repetition task and the current nonword rhyming task may increase cognitive demands on a vulnerable phonological system in CWS-ePer, resulting in the differences between groups that were not observed in real-word studies. Future studies directly assessing neural processes during multiple types of nonword tasks could help further refine our understanding of the role of phonological processing in persistent stuttering and provide greater specificity as to the nature of differences in phonological skills in children who eventually persisted in stuttering.
An alternative interpretation is that CWS-ePer may be more sensitive to and/or more attentive to phonological matching than phonological mismatch. The larger response to rhyme targets reflects increased neural resources for processing this condition. The task for this paradigm is to determine whether the two words rhyme or not. It is possible that CWS-ePer are highly sensitive to the rhyme aspect of the task, such that neural resources are increased when rhyme targets are presented. Increases in attention directed toward a task, including language and rhyme tasks, increase the amplitude of neural responses for that task (e.g., Davids et al., 2011; Hillyard et al., 1973; Kemp et al., 2019; Sanders et al., 2006). Additionally, CWS have been found to exhibit hypervigilance, or reduced distractibility (Anderson et al., 2003), and reduced skills in attentional shifting compared to CWNS (Eggers et al., 2010; Eggers & Jansson-Verkasalo, 2017). The increased amplitude for processing rhyme targets may reflect greater attentional resources directed toward rhyme targets compared to nonrhyme targets in CWS-ePer. Children were asked to determine whether the nonword pairs rhymed or not. With the difficulty of the nonword rhyme task, CWS-ePer may have more strongly attended to the rhyme aspect of the task, reflected by the increased ERP amplitudes for rhyme targets compared to nonrhyme targets. This strategy for processing prime–target pairs differs from the strategy by CWS-eRec and CWNS and may not reflect phonological skills themselves but, instead, sensitivity to or attention directed toward phonological matching versus mismatching.
While previous ERP studies have not found this pattern in CWS, a behavioral phonological priming study reported that CWS did not exhibit the same benefits of priming as CWNS. Children received two types of priming, either incremental, where they were provided the onset (initial sound) of a word, or holistic, where they were provided the entire word except the initial sound (Byrd et al., 2007). CWNS exhibited maturation in phonological encoding processes, indexed by a change from the age of 3 to 5 years in priming benefit; younger children exhibited greater benefit from holistic priming, whereas older children exhibited greater benefit from incremental priming. In contrast, CWS at the ages of 3 and 5 years showed the greatest benefit from holistic priming. These findings indicate different strategies for accomplishing rhyme tasks between CWS and CWNS. With development, the shift from holistic processing to incremental processing facilitates fluent speech production as linguistic complexity increases (Brooks & MacWhinney, 2000). Although the tasks are different (speech production/naming vs. rhyme processing), the current ERP findings may also reflect continued reliance on holistic word processing in CWS-ePer compared to CWS-eRec and CWNS. Incremental processing could facilitate the comparison of rimes between primes and targets, resulting in more efficient rhyme processes, indexed by smaller neural responses to rhyme targets. Reliance on holistic processing, which reflects a need for a more global activation of a word for processing and production (Brooks & MacWhinney, 2000), could be reflected in larger responses for matching or rhyme targets, with smaller neural responses elicited by mismatch/nonrhyme targets. Future studies are needed to determine relationships between holistic and incremental word processing and neural processes for rhyme.
Byrd et al. (2007) interpreted their findings as a delayed maturation in phonological processing/encoding in CWS. A recent ERP study identified the delayed maturation of neural processes in CWS-Per for syntactic processing when nouns and verbs had been replaced by nonwords or jabberwocky sentences (Usler & Weber-Fox, 2015). Findings by Usler and Weber-Fox (2015) have similarities to the current paradigm, in that CWS-Per exhibited neural processes for syntax that were comparable to CWS-Rec and CWNS when sentences contained real words; differences were only observed in the nonword syntax condition. The neural patterns elicited by jabberwocky sentences in CWS-Per reflected syntactic processes similar to those in younger children for real-word syntax. Given that CWS-ePer develop the expected RE for nonword rhyming by the age of 7–8 years (Mohan & Weber, 2015), the current findings may support and extend this interpretation of delayed maturation of neural processes underlying phonology in young CWS-ePer. However, the developmental trajectory of phonological processes in young children is not yet known; therefore, it is unclear whether the reverse RE in CWS-ePer is similar to patterns in younger CWNS or if it represents a divergent pattern of phonological processing in CWS-ePer. Future studies evaluating rhyme processing in young children, as well as younger CWS, are needed.
Reverse REs were observed in the majority, but not all, of CWS-ePer (see Figure 6), whereas most CWNS and CWS-eRec exhibited an RE, though some exhibited a reverse RE. Analyses of the presence of an RE or a reverse RE across individuals further supported ERP findings, with significant differences between CWS-ePer and both CWNS and CWS-eRec in the later time window. Individual variability in ERP findings has been consistently reported in previous studies of ERPs in CWS (Kaganovich et al., 2010; Kreidler et al., 2017; Smith & Weber, 2017; Usler & Weber-Fox, 2015; Weber-Fox et al., 2013) and likely reflects the heterogeneity of stuttering, reinforcing the importance of large-scale studies of developmental stuttering. Individual variability in the current study suggests that phonological processing may play a role in stuttering persistence in many, but not all, CWS.
Despite differences in neural processes elicited by rhyme and nonrhyme targets, CWS-ePer exhibited performance on the task that was comparable to CWS-eRec and CWNS. One potential explanation is that the differences in neural processes did not affect behavioral performance. This would suggest that CWS-ePer are using different neural processing strategies to achieve the same behavioral outcome. Future research needs to be conducted to determine if this interpretation is accurate, but if it holds, it could suggest that CWS-ePer are able to adequately compensate for differences in rhyme processing in order to achieve comparable task performance.
However, an alternative and more likely explanation is that the behavioral measure in the current task did not capture differences in performance between groups. Children only responded to 10 out of 88 word pairs, and responses were recorded only for accuracy, not response time. When older children responded on every trial using this paradigm (Mohan & Weber, 2015), CWNS were more accurate than both CWS-Rec and CWS-Per. When older children responded on every trial in a visual rhyme task, CWS were both less accurate and slower to respond than CWNS, especially for more difficult conditions (Weber-Fox et al., 2008). It is possible that differences between groups, especially for CWS-ePer, might have been observed if accuracy and response times were obtained for every trial in the current study. Future studies will need to collect more refined behavioral data to compare behavioral performance with neural processes.
Importantly, this study provides a snapshot of phonological processing skills at one single period of time in development, that is, at 5 years of age. Clearly, rhyme abilities and the phonological processes that support them are emerging at this age (Gerwin & Weber, 2020), and CWS exhibit different patterns of phonological processes in the absence of semantic context compared to CWNS. By the age of 7–8 years, using the same paradigm, CWS-Per exhibit phonological processes for rhyme that are largely comparable to their peers who have recovered and to children with no history of stuttering (Mohan & Weber, 2015). Thus, the current findings suggest divergent or delayed maturation of, but not disordered, phonological processing and that these patterns change relatively quickly, within 2–3 years.
These divergent patterns of phonological processes in young CWS-ePer may play a role in the development of or persistence in stuttering in some children. These findings suggest that this age may be a critical developmental period for phonological skills in CWS. Future research is needed in order to evaluate similar skills in CWS at younger ages, as well as the developmental trajectories of these skills, in order to better understand the impact of the current findings on stuttering. Additionally, if this is a critical time period in the development of phonological skills in CWS, it might serve as a target for intervention programs focusing on phonological skills. Future studies are needed to determine the impact of these divergent phonological processing patterns on behavioral performance and the potential malleability of these skills in young CWS.
Theoretical Implications
These findings support theories of developmental stuttering as a heterogeneous, dynamic, and multifactorial disorder (Smith & Weber, 2017) and suggest that atypical phonological processing may be one factor, likely among many, that contributes to persistence in or recovery from stuttering. Often, studies of neural processes in stuttering reveal not only group differences but also individual variability with overlap between groups (Hampton & Weber-Fox, 2008; Kaganovich et al., 2010; Kreidler et al., 2017; Weber-Fox et al., 2013). Although the group sizes are relatively small, the current findings suggest that the reverse RE observed in this study is a robust and reliable pattern across most 5-year-olds who eventually persisted in stuttering (see Figure 6). These findings are also consistent with previous studies suggesting a difference in maturation across multiple speech and language skills (Chow & Chang, 2017; Kreidler et al., 2017; Smith et al., 2010; Usler & Weber-Fox, 2015; Weber-Fox et al., 2008). Divergent patterns of phonological processing may interact with a vulnerable speech motor system to contribute to the development of and persistence in stuttering. Previous findings have revealed increased speech motor variability for novel and complex phonological productions in CWS compared to CWNS (MacPherson & Smith, 2013; Smith et al., 2012; Usler et al., 2017; Walsh et al., 2015) and reduced accuracy on phonology-based tasks (Anderson et al., 2006; Hakim & Bernstein Ratner, 2004; Spencer & Weber-Fox, 2014). The current findings provide a potential bridge between some of these studies, revealing differences in neural processes underlying phonology, especially for complex, unfamiliar phonological patterns with minimal semantic context, in children who eventually persisted in stuttering. These divergent neural processes may contribute to less efficient or less effective processing of incoming phonological information, resulting in less accurate and/or slower behavioral performance. They may also contribute to less efficient planning at the phonological selection and encoding stages of speech production (Hagoort & Levelt, 2009; Indefrey & Levelt, 2000), which could impact the timing and fluency of speech production.
Importantly, these findings, in conjunction with previous work, highlight that neural indices of phonology in stuttering are dynamic. More research is needed to better delineate typical developmental trajectories for phonological skills, beginning early in development into adolescence. To date, only two cross-sectional ERP studies have evaluated the development of neural processes supporting nonword rhyming (Andersson et al., 2018; Coch et al., 2005). Although 5-year-old CWNS exhibit adultlike REs, the developmental trajectory from emerging to adultlike patterns is still unknown. Future studies are necessary in order to determine whether the patterns observed in the current study in CWS-ePer are similar to patterns observed in younger CWNS, before the emergence of the RE.
Future studies also need to follow the longitudinal development of phonological skills and processes in stuttering. This will help determine the ways in which phonological processes may contribute to the development of, as well as persistence in, stuttering and how these skills may interact with other factors to contribute to persistence or recovery. While data collected from multiple locations (e.g., Purdue University and The University of Iowa) may be a limitation in the current study, large-scale studies are needed to understand the nature of developmental stuttering. This will be best completed by future studies acquiring data from large populations of children over time, either at a single location/laboratory or with carefully controlled and monitored experimental conditions across multiple laboratories. Understanding when and how these dynamic systems change over time will refine our understanding of the nature of stuttering and help refine clinical assessments and treatment programs in order to better serve CWS.
Conclusions
The findings from the current study indicate that 5-year-old children who stutter who eventually persisted exhibit a divergent pattern of phonological processes for a complex nonword rhyming task compared to children who stutter who eventually recovered. Children who eventually persisted in stuttering do not appear to benefit from phonological priming in the same way as their peers who eventually recovered when semantic context is limited and task demands are high. These findings are the first to show substantial differences in phonological processes in young children who can rhyme as a function of eventual stuttering outcome. While these children eventually develop typical phonological processes, the current study suggests that the fifth year of life may be a critical developmental period for phonology in young children who stutter.
Supplementary Material
Acknowledgments
This work was funded by National Institute on Deafness and Other Communication Disorders Grant R01 DC00559, awarded to Anne Smith and Christine Weber. We would like to thank Patricia Zebrowski and the research team at The University of Iowa for their assistance with data collection; Barb Brown for her work with participant recruitment and behavioral and electroencephalography data collection; Janna Berlin for her help with data collection; and Christine Weber, Katelyn Gerwin, Elif Isbell, and three reviewers for their feedback on earlier drafts of this article.
Funding Statement
This work was funded by National Institute on Deafness and Other Communication Disorders Grant R01 DC00559, awarded to Anne Smith and Christine Weber.
Footnotes
Statistical analyses were conducted excluding the children with less than three SLDs per 100 syllables, and the pattern of results remained unchanged. Therefore, to maximize the inclusion of children who were diagnosed at the time of testing as CWS, the children with less than three SLDs per 100 syllables were included in the study.
It is possible that differences in receptive language performance (Test for Auditory Comprehension of Language–Third Edition) and maternal education between CWNS and CWS-ePer may have had an effect on ERPs elicited by this receptive processing task. Analyses were conducted with these factors as covariates for prime and RE repeated-measures ANOVAs. Findings were the same as those reported. Given the potential confounds in the use of covariates in the analyses in the current study (e.g., Miller & Chapman, 2001), we report analyses conducted without covariates.
References
- Ambrose, N. G. , & Yairi, E. (1999). Normative disfluency data for early childhood stuttering. Journal of Speech, Language, and Hearing Research, 42(4), 895–909. https://doi.org/10.1044/jslhr.4204.895 [DOI] [PubMed] [Google Scholar]
- American Electroencephalographic Society. (1994). Guideline thirteen: Guidelines for standard electrode placement nomenclature. Journal of Clinical Neurophysiology, 11, 111–113. [PubMed] [Google Scholar]
- Anderson, J. D. , & Conture, E. G. (2004). Sentence-structure priming in young children who do and do not stutter. Journal of Speech, Language, and Hearing Research, 47(3), 552–571. https://doi.org/10.1044/1092-4388(2004/043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, J. D. , Pellowski, M. W. , & Conture, E. G. (2005). Childhood stuttering and dissociations across linguistic domains. Journal of Fluency Disorders, 30(3), 219–253. https://doi.org/10.1016/j.jfludis.2005.05.006 [DOI] [PubMed] [Google Scholar]
- Anderson, J. D. , Pellowski, M. W. , Conture, E. G. , & Kelly, E. M. (2003). Temperamental characteristics of young children who stutter. Journal of Speech, Language, and Hearing Research, 46(5), 1221–1233. https://doi.org/10.1044/1092-4388(2003/095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, J. D. , Wagovich, S. A. , & Hall, N. E. (2006). Nonword repetition skills in young children who do and do not stutter. Journal of Fluency Disorders, 31(3), 177–199. https://doi.org/10.1016/j.jfludis.2006.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson, A. , Sanders, L. D. , Coch, D. , Karns, C. M. , & Neville, H. J. (2018). Anterior and posterior ERP rhyming effects in 3- to 5-year-old children. Developmental Cognitive Neuroscience, 30, 178–190. https://doi.org/10.1016/j.dcn.2018.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astheimer, L. , Janus, M. , Moreno, S. , & Bialystok, E. (2014). Electrophysiological measures of attention during speech perception predict metalinguistic skills in children. Developmental Cognitive Neuroscience, 7, 1–12. https://doi.org/10.1016/j.dcn.2013.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankson, N. W. , & Bernthal, J. E. (1990). Bankson–Bernthal Test of Phonology (BBTOP). Pro-Ed. [Google Scholar]
- Bernstein Ratner, N. (1997). Stuttering: A psycholinguistic perspective. In Curlee R. F. & Siegel G. M. (Eds.), Nature and treatment of stuttering: New directions (2nd ed., pp. 99–127). Allyn & Bacon. [Google Scholar]
- Bernstein Ratner, N. , & Sih, C. C. (1987). Effects of gradual increases in sentence length and complexity on children's dysfluency. Journal of Speech and Hearing Disorders, 52(3), 278–287. https://doi.org/10.1044/jshd.5203.278 [DOI] [PubMed] [Google Scholar]
- Bloodstein, O. , & Bernstein Ratner, N. (2008). A handbook on stuttering (6th ed.). Thomson Delmar Learning. [Google Scholar]
- Boersma, P. , & Weenink, D. (2018). Praat: Doing phonetics by computer (Version 6.0.37) [Computer software]. http://www.praat.org/
- Brooks, P. J. , & MacWhinney, B. (2000). Phonological priming in children's picture naming. Journal of Child Language, 27(2), 335–366. https://doi.org/10.1017/S0305000900004141 [DOI] [PubMed] [Google Scholar]
- Burgemeister, B. B. , Blum, L. H. , & Lorge, I. (1972). Columbia Mental Maturity Scale. Harcourt Brace Jovanovich. [Google Scholar]
- Byrd, C. T. , Conture, E. G. , & Ohde, R. N. (2007). Phonological priming in young children who stutter: Holistic versus incremental processing. American Journal of Speech-Language Pathology, 16(1), 43–53. https://doi.org/10.1044/1058-0360(2007/006) [DOI] [PubMed] [Google Scholar]
- Carrow-Woolfolk, E. (1999). TACL-3: Test for Auditory Comprehension of Language. The Psychological Corporation. [Google Scholar]
- Chow, H. M. , & Chang, S.-E. (2017). White matter developmental trajectories associated with persistence and recovery of childhood stuttering. Human Brain Mapping, 38(7), 3345–3359. https://doi.org/10.1002/hbm.23590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coch, D. , Grossi, G. , Coffey-Corina, S. , Holcomb, P. J. , & Neville, H. J. (2002). A developmental investigation of ERP auditory rhyming effects. Developmental Science, 5(4), 467–489. https://doi.org/10.1111/1467-7687.00241 [Google Scholar]
- Coch, D. , Grossi, G. , Skendzel, W. , & Neville, H. (2005). ERP nonword rhyming effects in children and adults. Journal of Cognitive Neuroscience, 17(1), 168–182. https://doi.org/10.1162/0898929052880020 [DOI] [PubMed] [Google Scholar]
- Coch, D. , Hart, T. , & Mitra, P. (2008). Three kinds of rhymes: An ERP study. Brain and Language, 104(3), 230–243. https://doi.org/10.1016/j.bandl.2007.06.003 [DOI] [PubMed] [Google Scholar]
- Connolly, J. F. , & Phillips, N. A. (1994). Event-related potential components reflect phonological and semantic processing of the terminal word of spoken sentences. Journal of Cognitive Neuroscience, 6(3), 256–266. https://doi.org/10.1162/jocn.1994.6.3.256 [DOI] [PubMed] [Google Scholar]
- D'Arcy, R. C. N. , Connolly, J. F. , Service, E. , Hawco, C. S. , & Houlihan, M. E. (2004). Separating phonological and semantic processing in auditory sentence processing: A high-resolution event-related brain potential study. Human Brain Mapping, 22(1), 40–51. https://doi.org/10.1002/hbm.20008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids, N. , van den Brink, D. , van Turennout, M. , & Verhoeven, L. (2011). Task-related influences on neurophysiological assessment of auditory rhyme: Implications for clinical studies. Clinical Neurophysiology, 122(8), 1629–1636. https://doi.org/10.1016/j.clinph.2010.12.058 [DOI] [PubMed] [Google Scholar]
- Dawson, J. I. , Stout, C. E. , & Eyer, J. A. (2003). SPELT-3: Structured Photographic Expressive Language Test–Third Edition. Janelle Publications. [Google Scholar]
- Delorme, A. , & Makeig, S. (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. https://doi.org/10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- Diaz, M. T. , & Swaab, T. Y. (2007). Electrophysiological differentiation of phonological and semantic integration in word and sentence contexts. Brain Research, 1146, 85–100. https://doi.org/10.1016/j.brainres.2006.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollaghan, C. , & Campbell, T. F. (1998). Nonword repetition and child language impairment. Journal of Speech, Language, and Hearing Research, 41(5), 1136–1146. https://doi.org/10.1044/jslhr.4105.1136 [DOI] [PubMed] [Google Scholar]
- Eggers, K. , De Nil, L. F. , & Van den Bergh, B. R. H. (2010). Temperament dimensions in stuttering and typically developing children. Journal of Fluency Disorders, 35(4), 355–372. https://doi.org/10.1016/j.jfludis.2010.10.004 [DOI] [PubMed] [Google Scholar]
- Eggers, K. , & Jansson-Verkasalo, E. (2017). Auditory attentional set-shifting and inhibition in children who stutter. Journal of Speech, Language, and Hearing Research, 60(11), 3159–3170. https://doi.org/10.1044/2017_JSLHR-S-16-0096 [DOI] [PubMed] [Google Scholar]
- Ervast, L. , Hämäläinen, J. A. , Zachau, S. , Lohvansuu, K. , Heinänen, K. , Veijola, M. , Heikkinen, E. , Suominen, K. , Luotonen, M. , Lehtihalmes, M. , & Leppänen, P. H. T. (2015). Event-related brain potentials to change in the frequency and temporal structure of sounds in typically developing 5–6-year-old children. International Journal of Psychophysiology, 98(3), 413–425. https://doi.org/10.1016/j.ijpsycho.2015.08.007 [DOI] [PubMed] [Google Scholar]
- Friederici, A. D. (2002). Towards a neural basis of auditory sentence processing. Trends in Cognitive Sciences, 6(2), 78–84. https://doi.org/10.1016/S1364-6613(00)01839-8 [DOI] [PubMed] [Google Scholar]
- Gaines, N. D. , Runyan, C. M. , & Meyers, S. C. (1991). A comparison of young stutterers' fluent versus stuttered utterances on measures of length and complexity. Journal of Speech and Hearing Research, 34(1), 37–42. https://doi.org/10.1044/jshr.3401.37 [DOI] [PubMed] [Google Scholar]
- Gerwin, K. L. , Brosseau-Lapré, F. , Brown, B. , Christ, S. , & Weber, C. (2019). Rhyme production strategies distinguish stuttering recovery and persistence. Journal of Speech, Language, and Hearing Research, 62(9), 3302–3319. https://doi.org/10.1044/2019_JSLHR-S-18-0294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwin, K. L. , & Weber, C. (2020). Neural indices mediating rhyme discrimination differ for some young children who stutter regardless of eventual recovery or persistence. Journal of Speech, Language, and Hearing Research, 63(4), 1053–1070. https://doi.org/10.1044/2020_JSLHR-19-00260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami, U. , & East, M. (2000). Rhyme and analogy in beginning reading: Conceptual and methodological issues. Applied Psycholinguistics, 21(1), 63–93. https://doi.org/10.1017/S0142716400001041 [Google Scholar]
- Grossi, G. , Coch, D. , Coffey-Corina, S. , Holcomb, P. J. , & Neville, H. J. (2001). Phonological processing in visual rhyming: A developmental ERP study. Journal of Cognitive Neuroscience, 13(5), 610–625. https://doi.org/10.1162/089892901750363190 [DOI] [PubMed] [Google Scholar]
- Hagoort, P. , & Levelt, W. J. (2009). The speaking brain. Science, 326(5951), 372–373. https://doi.org/10.1126/science.1181675 [DOI] [PubMed] [Google Scholar]
- Hakim, H. B. , & Bernstein Ratner, N. (2004). Nonword repetition abilities of children who stutter: An exploratory study. Journal of Fluency Disorders, 29(3), 179–199. https://doi.org/10.1016/j.jfludis.2004.06.001 [DOI] [PubMed] [Google Scholar]
- Hall, N. E. , Wagovich, S. A. , & Bernstein Ratner, N. (2007). Language considerations in childhood stuttering. In Conture E. G. & Curlee R. F. (Eds.), Stuttering and related disorders of fluency (pp. 153–167). Thieme. [Google Scholar]
- Hampton, A. , & Weber-Fox, C. (2008). Non-linguistic auditory processing in stuttering: Evidence from behavior and event-related brain potentials. Journal of Fluency Disorders, 33(4), 253–273. https://doi.org/10.1016/j.jfludis.2008.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton Wray, A. , & Weber-Fox, C. (2013). Specific aspects of cognitive and language proficiency account for variability in neural indices of semantic and syntactic processing in children. Developmental Cognitive Neuroscience, 5, 149–171. https://doi.org/10.1016/j.dcn.2013.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumann, C. , & Schomaker, M. (2016). Introduction to statistics and data analysis. Springer. https://doi.org/10.1007/978-3-319-46162-5 [Google Scholar]
- Hickock, G. (2012). Computational neuroanatomy of speech production. Nature Reviews Neuroscience, 13, 135–145. https://doi.org/10.1038/nrn3158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard, S. A. , Hink, R. F. , Schwent, V. L. , & Picton, T. W. (1973). Electrical signs of selective attention in the human brain. Science, 182(4108), 177–180. https://doi.org/10.1126/science.182.4108.177 [DOI] [PubMed] [Google Scholar]
- Hodel, A. S. , Brumbaugh, J. E. , Hunt, R. H. , Van Den Heuvel, S. E. , Wiltgen, A. M. , & Thomas, K. M. (2019). Individual differences in ERP measures of executive function in early childhood: Relation to low-risk preterm birth and parent-reported behavior. Child Neuropsychology, 25(7), 914–942. https://doi.org/10.1080/09297049.2018.1540690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb, P. J. , Coffey, S. A. , & Neville, H. J. (1992). Visual and auditory sentence processing: A developmental analysis using event-related brain potentials. Developmental Neuropsychology, 8(2–3), 203–241. https://doi.org/10.1080/87565649209540525 [Google Scholar]
- Hollingshead, A. B. (1975). Four factor index of social status [Unpublished manuscript]. Yale University. [Google Scholar]
- Indefrey, P. , & Levelt, W. J. (2000). The neural correlates of language production. In Gazzaniga M. S. (Ed.), The new cognitive neurosciences (2nd ed., pp. 845–865). MIT Press; http://pubman.mpdl.mpg.de/pubman/faces/viewItemOverviewPage.jsp?itemId=escidoc:148271:8 [Google Scholar]
- Kaganovich, N. , Hampton Wray, A. , & Weber-Fox, C. (2010). Non-linguistic auditory processing and working memory update in pre-school children who stutter: An electrophysiological study. Developmental Neuropsychology, 35(6), 712–736. https://doi.org/10.1080/87565641.2010.508549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp, A. , Eddins, D. , Shrivastav, R. , & Hampton Wray, A. (2019). Effects of task difficulty on neural processes underlying semantics: An event-related potentials study. Journal of Speech, Language, and Hearing Research, 62(2), 367–386. https://doi.org/10.1044/2018_JSLHR-H-17-0396 [DOI] [PubMed] [Google Scholar]
- Kreidler, K. , Hampton Wray, A. , Usler, E. , & Weber, C. (2017). Neural indices of semantic processing in early childhood distinguish eventual stuttering persistence and recovery. Journal of Speech, Language, and Hearing Research, 60(11), 3118–3134. https://doi.org/10.1044/2017_JSLHR-S-17-0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas, M. , & Federmeier, K. D. (2011). Thirty years and counting: Finding meaning in the N400 component of the event-related brain potential (ERP). Annual Review of Psychology, 62(1), 621–647. https://doi.org/10.1146/annurev.psych.093008.131123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutner, M. H. , Nachtsheim, C. J. , Neter, J. , & Li, W. (2005). Applied linear statistical models (5th ed.). McGraw-Hill. [Google Scholar]
- Logan, K. J. , & Conture, E. G. (1997). Selected temporal, grammatical, and phonological characteristics of conversational utterances produced by children who stutter. Journal of Speech, Language, and Hearing Research, 40(1), 107–120. https://doi.org/10.1044/jslhr.4001.107 [DOI] [PubMed] [Google Scholar]
- Lopez-Calderon, J. , & Luck, S. J. (2014). ERPLAB: An open-source toolbox for the analysis of event-related potentials. Frontiers in Human Neuroscience, 8, 213 https://doi.org/10.3389/fnhum.2014.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck, S. J. (2014). An introduction to the event-related potential technique (2nd ed.). MIT Press. [Google Scholar]
- MacPherson, M. K. , & Smith, A. (2013). Influences of sentence length and syntactic complexity on the speech motor control of children who stutter. Journal of Speech, Language, and Hearing Research, 56(1), 89–102. https://doi.org/10.1044/1092-4388(2012/11-0184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick, K. S. , Conture, E. G. , & Ohde, R. N. (2003). Phonological priming in picture naming of young children who stutter. Journal of Speech, Language, and Hearing Research, 46(6), 1428–1443. https://doi.org/10.1044/1092-4388(2003/111) [DOI] [PubMed] [Google Scholar]
- Miller, G. A. , & Chapman, J. P. (2001). Misunderstanding analysis of covariance. Journal of Abnormal Psychology, 110(1), 40–48. https://doi.org/10.1037/0021-843X.110.1.40 [DOI] [PubMed] [Google Scholar]
- Mohan, R. , & Weber, C. (2015). Neural systems mediating processing of sound units of language distinguish recovery versus persistence in stuttering. Journal of Neurodevelopmental Disorders, 7(1), Article 28 https://doi.org/10.1186/s11689-015-9124-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville, H. J. , Coffey, S. A. , Holcomb, P. J. , & Tallal, P. (1993). The neurobiology of sensory and language processing in language-impaired children. Journal of Cognitive Neuroscience, 5(2), 235–253. https://doi.org/10.1162/jocn.1993.5.2.235 [DOI] [PubMed] [Google Scholar]
- Nunez, P. L. (1995). Neocortical dynamics and human EEG rhythms (1st ed.). Oxford University Press. [Google Scholar]
- Pellowski, M. W. , & Conture, E. G. (2005). Lexical priming in picture naming of young children who do and do not stutter. Journal of Speech, Language, and Hearing Research, 48(2), 278–294. https://doi.org/10.1044/1092-4388(2005/019) [DOI] [PubMed] [Google Scholar]
- Perkins, W. H. , Kent, R. D. , & Curlee, R. F. (1991). A theory of neuropsycholinguistic function in stuttering. Journal of Speech and Hearing Research, 34(4), 734–752. https://doi.org/10.1044/jshr.3404.734 [DOI] [PubMed] [Google Scholar]
- Picton, T. W. , Bentin, S. , Berg, P. , Donchin, E. , Hillyard, S. A. , Johnson, R. , Miller, G. A. , Ritter, W. , Ruchkin, D. S. , Rugg, M. D. , & Taylor, M. J. (2000). Guidelines for using human event-related potentials to study cognition: Recording standards and publication criteria. Psychophysiology, 37(2), 127–152. https://doi.org/10.1111/1469-8986.3720127 [PubMed] [Google Scholar]
- Postma, A. , & Kolk, H. (1993). The covert repair hypothesis: Prearticulatory repair processes in normal and stuttered disfluencies. Journal of Speech and Hearing Research, 36(3), 472–487. https://doi.org/10.1044/jshr.3603.472 [PubMed] [Google Scholar]
- Praamstra, P. , Meyer, A. S. , & Levelt, W. J. M. (1994). Neurophysiological manifestations of phonological processing: Latency variation of a negative ERP component timelocked to phonological mismatch. Journal of Cognitive Neuroscience, 6(3), 204–219. https://doi.org/10.1162/jocn.1994.6.3.204 [DOI] [PubMed] [Google Scholar]
- Praamstra, P. , & Stegeman, D. F. (1993). Phonological effects on the auditory N400 event-related brain potential. Cognitive Brain Research, 1(2), 73–86. https://doi.org/10.1016/0926-6410(93)90013-U [DOI] [PubMed] [Google Scholar]
- Robertson, C. , & Salter, W. (2007). Phonological Awareness Test–Second Edition. LinguiSystems. [Google Scholar]
- Rugg, M. D. (1984a). Event-related potentials and the phonological processing of words and non-words. Neuropsychologia, 22(4), 435–443. https://doi.org/10.1016/0028-3932(84)90038-1 [DOI] [PubMed] [Google Scholar]
- Rugg, M. D. (1984b). Event-related potentials in phonological matching tasks. Brain and Language, 23(2), 225–240. https://doi.org/10.1016/0093-934X(84)90065-8 [DOI] [PubMed] [Google Scholar]
- Rugg, M. D. , & Barrett, S. E. (1987). Event-related potentials and the interaction between orthographic and phonological information in a rhyme-judgment task. Brain and Language, 32(2), 336–361. https://doi.org/10.1016/0093-934X(87)90132-5 [DOI] [PubMed] [Google Scholar]
- Sanders, L. D. , Stevens, C. , Coch, D. , & Neville, H. J. (2006). Selective auditory attention in 3- to 5-year-old children: An event-related potential study. Neuropsychologia, 44(11), 2126–2138. https://doi.org/10.1016/j.neuropsychologia.2005.10.007 [DOI] [PubMed] [Google Scholar]
- Sasisekaran, J. , & Weber-Fox, C. (2012). Cross-sectional study of phoneme and rhyme monitoring abilities in children between 7 and 13 years. Applied Psycholinguistics, 33(2), 253–279. https://doi.org/10.1017/S0142716411000348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, J. M. , Abel, A. D. , Ogiela, D. A. , Middleton, A. E. , & Maguire, M. J. (2016). Developmental differences in beta and theta power during sentence processing. Developmental Cognitive Neuroscience, 19, 19–30. https://doi.org/10.1016/j.dcn.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, J. M. , & Maguire, M. J. (2019). Developmental differences in the neural correlates supporting semantics and syntax during sentence processing. Developmental Science, 22(4), Article e12782 https://doi.org/10.1111/desc.12782 [DOI] [PubMed] [Google Scholar]
- Smith, A. , Goffman, L. , Sasisekaran, J. , & Weber-Fox, C. (2012). Language and motor abilities of preschool children who stutter: Evidence from behavioral and kinematic indices of nonword repetition performance. Journal of Fluency Disorders, 37(4), 344–358. https://doi.org/10.1016/j.jfludis.2012.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, A. , Sadagopan, N. , Walsh, B. , & Weber-Fox, C. (2010). Increasing phonological complexity reveals heightened instability in inter-articulatory coordination in adults who stutter. Journal of Fluency Disorders, 35(1), 1–18. https://doi.org/10.1016/j.jfludis.2009.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, A. , & Weber, C. (2017). How stuttering develops: The multifactorial dynamic pathways theory. Journal of Speech, Language, and Hearing Research, 60(9), 2483–2505. https://doi.org/10.1044/2017_JSLHR-S-16-0343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer, C. , & Weber-Fox, C. (2014). Preschool speech articulation and nonword repetition abilities may help predict eventual recovery or persistence of stuttering. Journal of Fluency Disorders, 41, 32–46. https://doi.org/10.1016/j.jfludis.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unicomb, R. , Kefalianos, E. , Reilly, S. , Cook, F. , & Morgan, A. (2020). Prevalence and features of comorbid stuttering and speech sound disorder at age 4 years. Journal of Communication Disorders, 84, Article 105976 https://doi.org/10.1016/j.jcomdis.2020.105976 [DOI] [PubMed] [Google Scholar]
- Usler, E. , Smith, A. , & Weber, C. (2017). A lag in speech motor coordination during sentence production is associated with stuttering persistence in young children. Journal of Speech, Language, and Hearing Research, 60(1), 51–61. https://doi.org/10.1044/2016_JSLHR-S-15-0367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usler, E. , & Weber-Fox, C. (2015). Neurodevelopment for syntactic processing distinguishes childhood stuttering recovery versus persistence. Journal of Neurodevelopmental Disorders, 7(1), 1–22. https://doi.org/10.1186/1866-1955-7-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Petten, C. , Coulson, S. , Rubin, S. , Plante, E. , & Parks, M. (1999). Time course of word identification and semantic integration in spoken language. Journal of Experimental Psychology: Learning, Memory, and Cognition, 25(2), 394–417. https://doi.org/10.1037/0278-7393.25.2.394 [DOI] [PubMed] [Google Scholar]
- Walsh, B. , Mettel, K. M. , & Smith, A. (2015). Speech motor planning and execution deficits in early childhood stuttering. Journal of Neurodevelopmental Disorders, 7(1), Article 27 https://doi.org/10.1186/s11689-015-9123-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber-Fox, C. (2001). Neural systems for sentence processing in stuttering. Journal of Speech, Language, and Hearing Research, 44(4), 814–825. https://doi.org/10.1044/1092-4388(2001/064) [DOI] [PubMed] [Google Scholar]
- Weber-Fox, C. , & Hampton, A. (2008). Stuttering and natural speech processing of semantic and syntactic constraints on verbs. Journal of Speech, Language, and Hearing Research, 51(5), 1058–1071. https://doi.org/10.1044/1092-4388(2008/07-0164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber-Fox, C. , Hampton Wray, A. , & Arnold, H. (2013). Early childhood stuttering and electrophysiological indices of language processing. Journal of Fluency Disorders, 38(2), 206–221. https://doi.org/10.1016/j.jfludis.2013.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber-Fox, C. , Spencer, R. , Cuadrado, E. , & Smith, A. (2003). Development of neural processes mediating rhyme judgments: Phonological and orthographic interactions. Developmental Psychobiology, 43(2), 128–145. https://doi.org/10.1002/dev.10128 [DOI] [PubMed] [Google Scholar]
- Weber-Fox, C. , Spencer, R. M. , Spruill, J. E. , & Smith, A. (2004). Phonologic processing in adults who stutter: Electrophysiological and behavioral evidence. Journal of Speech, Language, and Hearing Research, 47(6), 1244–1258. https://doi.org/10.1044/1092-4388(2004/094) [DOI] [PubMed] [Google Scholar]
- Weber-Fox, C. , Spruill, J. E. , Spencer, R. , & Smith, A. (2008). Atypical neural functions underlying phonological processing and silent rehearsal in children who stutter. Developmental Science, 11(2), 321–337. https://doi.org/10.1111/j.1467-7687.2008.00678.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, A. L. , & Zebrowski, P. M. (1992). Disfluencies in the conversations of young children who stutter: Some answers about questions. Journal of Speech and Hearing Research, 35(6), 1230–1238. https://doi.org/10.1044/jshr.3506.1230 [DOI] [PubMed] [Google Scholar]
- Wingate, M. E. (1988). The structure of stuttering: A psycholinguistic analysis. Springer-Verlag. [Google Scholar]
- Yairi, E. , & Seery, C. H. (2015). Stuttering: Foundations and clinical applications (2nd ed.). Pearson. [Google Scholar]
- Yaruss, J. S. (1997). Clinical implications of situational variability in preschool children who stutter. Journal of Fluency Disorders, 22(3), 187–203. https://doi.org/10.1016/S0094-730X(97)00009-0 [Google Scholar]
- Yaruss, J. S. (1999). Utterance length, syntactic complexity, and childhood stuttering. Journal of Speech, Language, and Hearing Research, 42(2), 329–344. https://doi.org/10.1044/jslhr.4202.329 [DOI] [PubMed] [Google Scholar]
- Ziegler, J. C. , & Goswami, U. (2005). Reading acquisition, developmental dyslexia, and skilled reading across languages: A psycholinguistic grain size theory. Psychological Bulletin, 131(1), 3–29. https://doi.org/10.1037/0033-2909.131.1.3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.