Abstract
Background:
Invasive cervical carcinoma is associated with an HIV prevalence of >0.1%. Opt-out HIV screening is recommended and cost-effective for cancer populations exceeding this threshold. HIV status is also prognostic for cancer-specific survival, but compliance with HIV screening is poor in the US and abroad.
Objectives:
This study aims to describe HIV screening practices in a US comprehensive cancer center, as we anticipate this is low. To guide quality improvement in HIV screening, we identify patient and tumor characteristics which predict compliance with screening.
Study Design:
Women treated for invasive cervical cancer of any stage between 2007 and 2017 at two institutions were identified by cancer registry and billing data. Women with incomplete data for age, race, ethnicity, payer, histology, stage, pregnancy, drug use, and HIV testing status, or with lack of a newly diagnosed cervical cancer or Gynecologic Oncology evaluation were excluded. Univariate logistical regression was performed to assess predictors of completed HIV screening.
Results:
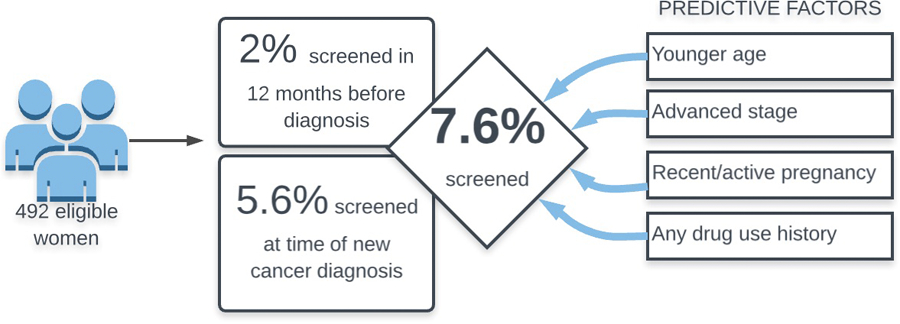
Of 1483 patients identified, 492 were eligible. No patients had a diagnosis of pre-existing HIV. HIV screening was completed within 30 days of Gynecologic Oncology evaluation in 28/492 (5.6%) women. HIV screening was documented within the preceding 12 months in 10/492 (2%) women. The cumulative screening rate was 7.6%. On univariate analysis, race, ethnicity, histology, and payer status were not associated with screening. Every 5-year increase in age was associated with a lower chance of screening (OR 0.86, 95%CI 0.75 – 0.97, p=0.015), as was earlier stage at diagnosis (OR 0.43, 95% CI 0.22–0.86, p=0.017). Only 9/492 (1.8%) of women in the cohort had a pregnancy during, or antecedent to, her cervical cancer diagnosis. Within this group, HIV screening was significantly more predictive of screening compliance (OR 10.57, 95%CI 2.71–41.14, p=0.0007). Only 8/491 (1.6%) of women in the cohort had active or former drug use, but within this group HIV screening was performed more frequently compared to no prior use (OR 22.7, 95% CI 5.2–99.2, p=<0.0001).
Conclusion:
Despite CDC recommendations for HIV screening in AIDS-defining cancers, compliance remains low. In our centers, factors including earlier age, advanced stage, active pregnancy at diagnosis, and any drug use history were predictive of greater compliance with screening. These data will inform a tailored intervention to improve compliance with HIV screening in our population.
Keywords: HIV screening, cervical cancer, quality improvement
Condensation:
Screening for HIV in women with newly diagnosed invasive cervical cancer occurs rarely and offers an opportunity for quality improvement in US Gynecologic Oncology practices.
Introduction
Invasive cervical carcinoma (ICC) is the second most common cancer in women worldwide, with over 83% of cases occurring in the developing world1. In the US in 2016, there were 12,990 new cases and 4,120 deaths and cervical cancer remains the second leading cause of cancer death in young women age 20–39 years 2. It is well established that human papilloma virus (HPV) DNA can be detected in 95–100% of invasive cervical cancer specimens and that this sexually transmitted virus is an essential causative mechanism. Women with HIV-positivity, particularly with immunosuppression, are more likely to contract a high-risk HPV infection and this is more likely to be persistent and cause cervical dysplasia or an invasive cervical cancer3,4.
At a threshold of HIV prevalence >0.1%, it is considered an autoimmune deficiency syndrome (AIDS)-defining cancer (ADC). Invasive cervical cancer is unique in that it is the only gynecologic malignancy listed by the Center for Disease Control as an ADC5. The prevalence of HIV was 1.2% in a US study of all AIDS-defining cancers, however in the small subset of women with cervical cancer only 23/245 (9.4%) were screened for HIV6. Similarly, in a European assessment of three ADC’s, ICC had an HIV-seroprevalence of 1.7%, with screening performed in only 6/57 (10.5%) of patients.
In the National Comprehensive Cancer Network Cervical Cancer Guidelines, consideration of HIV testing is encouraged in the workup of a newly diagnosed ICC patient7. Additionally, the HIV Indicator Disease Across Europe (HIDES) trial supports that invasive cervical cancer is among a small handful of diseases that merit HIV testing upon diagnosis8. Despite these US and European recommendations, screening compliance remains low.
The primary objective of this study was therefore to establish the frequency of HIV screening in women with newly diagnosed ICC in a large comprehensive US Gynecologic Oncology practice, as well as to determine any predictive factors for screening compliance to guide efforts in quality improvement.
Materials and Methods
Data Source
For this retrospective cohort study, we identified women over age 18 using institutional tumor registries as well as billing codes (ICD-9 180.9 and ICD-10 C53.9) for invasive cervical cancer. Women were included if they had a newly diagnosed invasive cervical cancer, had been evaluated by a University of California, Irvine Gynecologic Oncologist at one of three sites of practice throughout the Orange County and southern Los Angeles areas. To reflect the implementation of the 2016 CDC HIV screening guidelines, this study spanned 1/2007 through 9/2017. Women were excluded if their billing code did not accurately reflect a newly diagnosed invasive cervical cancer diagnosis (non-cervical primary cancer, cervical dysplasia, recurrent cancer), medical records were incomplete, or they presented for initial care with someone other than a Gynecologic Oncologist at our institution.
Of 1483 women identified by cancer registry or billing data, 991 of these were excluded. Of these 450 had no new ICC diagnosis, 263 were seen by an outside provider at diagnosis, 160 were prior to the 1/2007 initiation date, and 118 had incomplete medical records. This resulted in 492 women included in the analysis (Figure 1).
Figure 1.
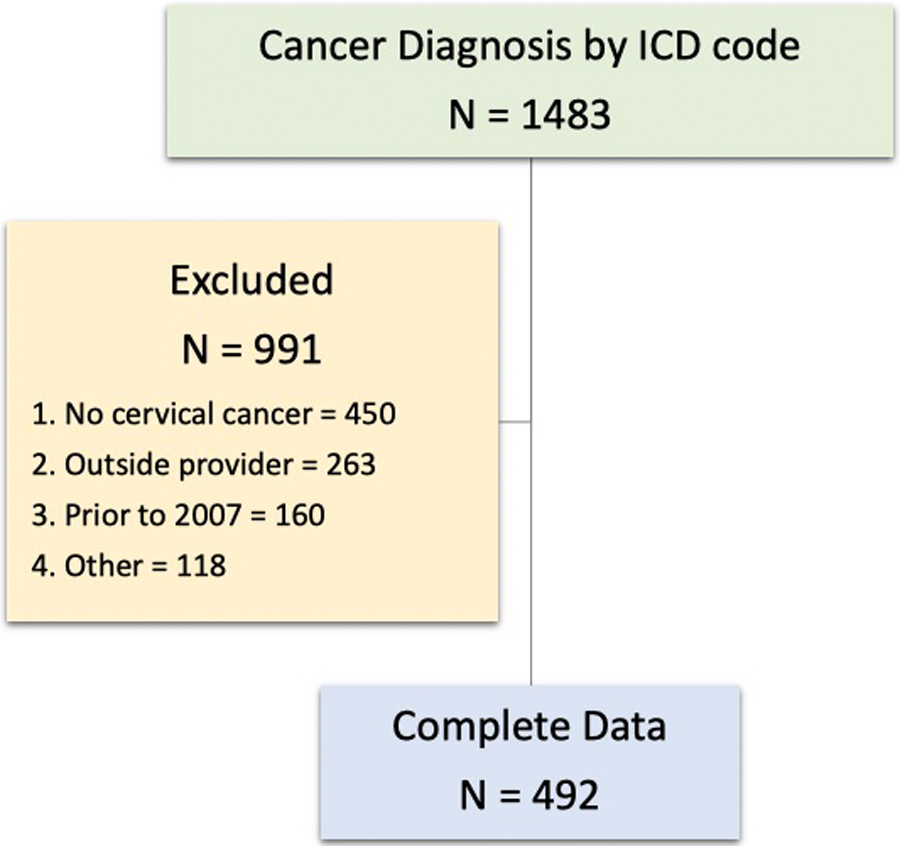
Study population flow diagram
This study was approved by the institutional review boards at University of California, Irvine, the Cancer Prevention and Treatment Center of St Joseph’s Hospital, and the MemorialCare Health System, all of whom waived the requirement for informed consent.
Study Objectives
The aim of this study was to establish the baseline frequency with which HIV screening is being performed. HIV screening was considered complete if it was documented in the clinical notes, an HIV-1/2 antibody test by enzyme-linked immunosorbent assay was resulted within 12 months prior to diagnosis, or if an HIV-1/2 antibody test by enzyme-linked immunosorbent assay was ordered within 30 days of the encounter for newly diagnosed ICC. Possible confounding variables were assessed including age, age, race, ethnicity, payer status, pregnancy in the preceding 12 months or at ICC diagnosis, former or active drug use, and tumor factors including histology and stage. Pregnancy was established using ICD9 and ICD10 codes or review of medical records, with the timing of the pregnancy, HIV testing, and cervical cancer diagnosis noted temporally. Drug use was established using ICD9 or ICD10 codes for drug or substance dependency or reviewed of medical records. Identification of variables and HIV testing was limited to the available paper or electronic medical records associated with the initial encounter with a UCI Gynecologic Oncologist and patients were only included once if they had visits with multiple sites or providers within our group.
Statistical Analyses
Frequency of HIV testing was the primary endpoint of this study. We employed descriptive statistics to define the screening frequency in newly diagnosed women and in the 12 months preceding diagnosis. Univariate modeling was performed comparing demographic, tumor, drug and pregnancy variables between those patients who were screened and were not screened for HIV to determine the predictors of HIV testing. Association(s) between categorical variables and screening performance were reported as an odds ratio with a 95% confidence interval. Wald-Chi square statistics were used to analyze age effect on screening performance. For statistical analysis, we used SAS v9.4 Software9.
Results:
Screening Frequencies
Of the 1483 women identified by registry and billing data, 492/1483 met inclusion criteria, as described in figure 1. A total of 38/492 (7.6%) patients had some form of HIV screening completed in association with their new ICC diagnosis. No women had known pre-existing HIV at diagnosis. Of these, 10/492 (2%) had documented HIV screening performed within 12 months prior to diagnosis, and 28/492 (5.6%) had a new screening test ordered within 30 days of diagnosis (Figure 2).
Figure 2.
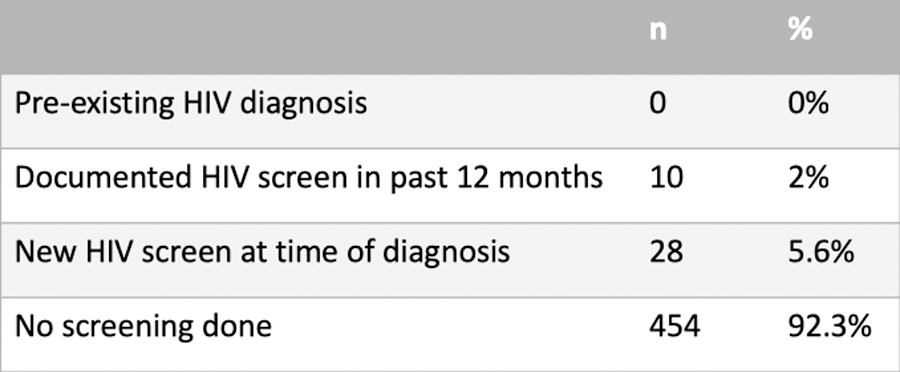
HIV screening practices
Of those with existing screening preceding their ICC diagnosis, 3/38 (37.8%) were documented only in the clinical notes while 35/38 (92%) were found only in laboratory reports within the paper or electronic medical records. Demographic factors, tumor characteristics, drug use and pregnancy are described in Figure 2.
The mean age for the cohort was 50.4 (+/− 14.9). The majority of the population was white (62%) and overall 60% of women identified as non-Hispanic. Detailed information regarding payer source at the time of diagnosis were obtained, with grouped frequencies of 18% receiving federal funding, 39% state funding, 36% private funding and 5% self-pay. Tumor characteristics included 66% were squamous cell carcinomas and 25% adenocarcinoma, with 9% of more rare histologies, and 56% were stage 1, 23% stage 2, 14% stage 3 and 9% stage IV. Only 7/492 (1.4%) women were pregnant at the time of ICC diagnosis. Of these, no new testing was ordered and only 2/7 (28.5%) had existing HIV screening documented within the prior 12 months. An additional 2 women had a pregnancy within the 12 months preceding diagnosis but not at diagnosis – of these, 0/2 had HIV documented with their pregnancy however 2/2 (100%) had new HIV screening performed within 30 days of cancer diagnosis. If pregnancy at any time during the preceding 12 months is assessed cumulatively, this would encompass 9/492 (1.8%) women with a screening performed in 4/9 (44.4%) in this population.
Predictive Factors for Screening
No demographic factors except age significantly predicted performance of HIV screening (Figure 3). The age effect on screening performance was evaluated with an OR = 0.73 (95% CI, 0.57–0.94, p = 0.015) for every 10-year increase in age. Tumor histology was not a significant predictor. Tumor stage, defined as early (FIGO stage I-II) and advanced (stage IIIIV) had an OR = 0.43 (95% CI 0.22–0.86, p = 0.017), suggesting that more advanced cancers were predictive for screening. A total of 9/492 (1.8%) women in the cohort had a pregnancy during, or antecedent to, her cervical cancer diagnosis. Within this pregnancy cohort, HIV screening was significantly correlated with performance of HIV screening, OR = 10.57 (95% CI 2.71 – 41.17, p = 0.0007). The use of drugs, either current or former, was predictive for performance of HIV screening with OR=22.7 (95% CI 5.2–99.2, p<0.0001).
Figure 3.
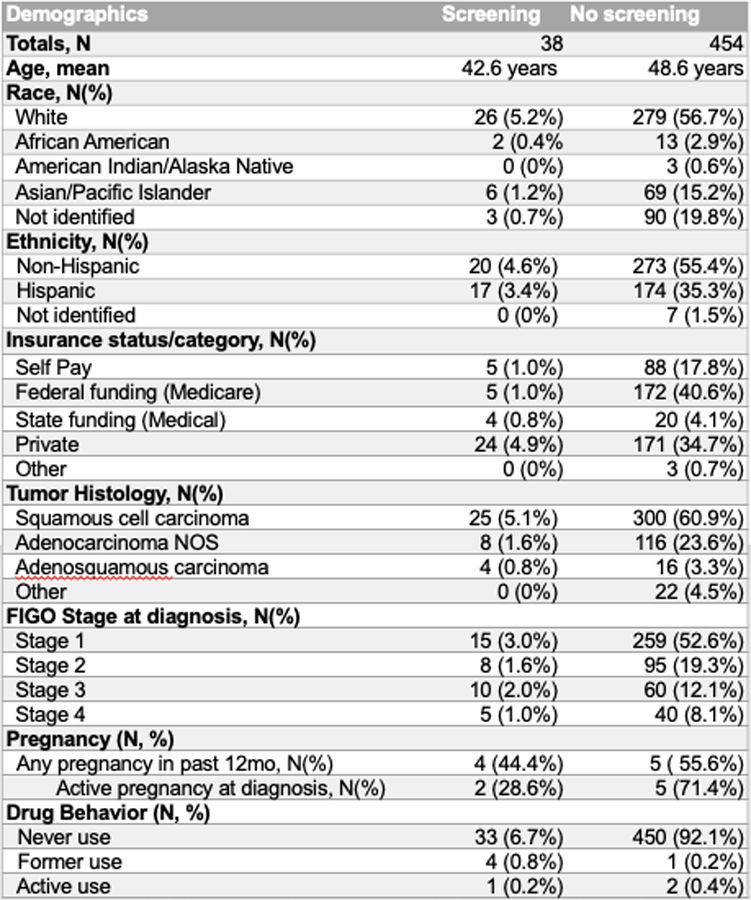
Patient and tumor characteristics
Structured Discussion/Comments:
Principal Findings
Our study allowed a broad definition of HIV screening to include the 12 months prior and the 30 days after HIV screening, with an overall rate of screening at 7.6% screening rate for HIV. In our cohort of patients under the care of academic US Gynecologic Oncologists, we found that younger age, advanced stage, active pregnancy at diagnosis, and former or active drug use were predictive of greater compliance with screening
Results Discussion
The screening rate of 7.6% our population is similar to the low incidence of screening noted in other smaller studies, including 9.4% in a US study in which ICC was one of many studied cancers, and 10.5% in a Swiss study of four ADCs 10,11.
Patient and tumor characteristic including younger age, advanced stage, active pregnancy at diagnosis, and former or active drug use were predictive of greater compliance with screening. The influence of pregnancy on HIV screening is not unexpected given the wide-spread uptake of routine opt-out screening during prenatal care. Similarly, practitioners may correctly identify drug use as a risk for HIV infection and cervical cancer risk. Despite this, however, the rate of screening even in those with pregnancy at diagnosis or drug use were relatively low. This may represent a limitation of the study in capturing all relevant screening, which may have been at outside facilities associated with the care of these specific conditions.
Clinical Implications
Previous studies stress the importance of HIV testing in newly diagnosed cancer patients, largely because earlier diagnoses of HIV allows for better management of both disease processes12. As of 2013, the most recent year when such data was available, more than 1.2 million individuals in the United States are infected with HIV, with up to 13% being unaware of their status5. Additionally, many of these individuals are diagnosed at late stages in the disease, with almost 40% of them progressing to AIDS within a year of their initial diagnosis. HIV infected patients also have worse cancer outcomes than HIV uninfected patients with the same cancers. Testing patients for HIV when they are initially diagnosed with ICC could help to better select their chemotherapy and antiretroviral regimens, thus leading to improved outcomes for these patients 10. This speaks to a pressing need to increase HIV screening programs within the US.
In 2006, the Center for Disease Control (CDC) and US Preventative Services Task Force (USPSTF) recommended opt-out HIV screening in areas with an HIV infection prevalence exceeds 0.1%. In the US, this translates to at least once for all individuals age 13–64 years of age, with annual screening advised for those in a high-risk group 13. This high-risk group includes those with ICC, as the incidence in AIDS-defining cancers is 1.2%, as well as in those with active injection drug use and in pregnancy.
The 2006 CDC recommendations emphasize an opt out strategy for HIV testing among the general population, an approach which has been proven to be cost effective5,14. Within this model, the consent for HIV testing should be integrated into a consent for general medical care, while still allowing a patient to specifically decline the HIV test. Many obstacles still remain for implementing wide spread opt-out practices, including physicians’ objections and state laws requiring a separate consent. However, opt-out testing may prove to be more successful when targeted at a specific population, such as women diagnosed with cervical cancer, and may play a role in reducing mortality and morbidity within this group15.
Research Implications
Identification of deficiencies in provider HIV screening practices and clarification of the patient and tumor factors that underlie these screening choices may inform quality improvement efforts. This cohort was intended to inform interventions targeted at provider and patient education as an area of future research to improve patient care.
Strengths and Limitations
Our study is unique in that it includes those with invasive cervical cancer only and includes a large sample of patients. Additionally, it deliberately assessed screening patterns after the 2006 CDC and USPSTF recommendations. This cohort represents a range of practice settings, including public, private, and community hospitals, all under the care of one group of academic Gynecologic Oncologists. While the retrospective cohort design facilitates evaluation of the relatively rare exposure of cervical cancer, the most evident weakness of the design is the limitation of both paper and electronic medical records systems in providing all possible data.
Conclusions
In light of the fact that screening is cost effective, has the potential to alter treatment courses, and improve patient outcomes, implementing HIV screening for newly diagnosed cervical cancer patients presents itself as a beneficial and feasible intervention that is grossly underutilized in the US and abroad. Our study supports that screening rates are very low despite CDC and NCCN recommendations. These data may facilitate interventions to improve compliance with HIV screening.
Figure 4.
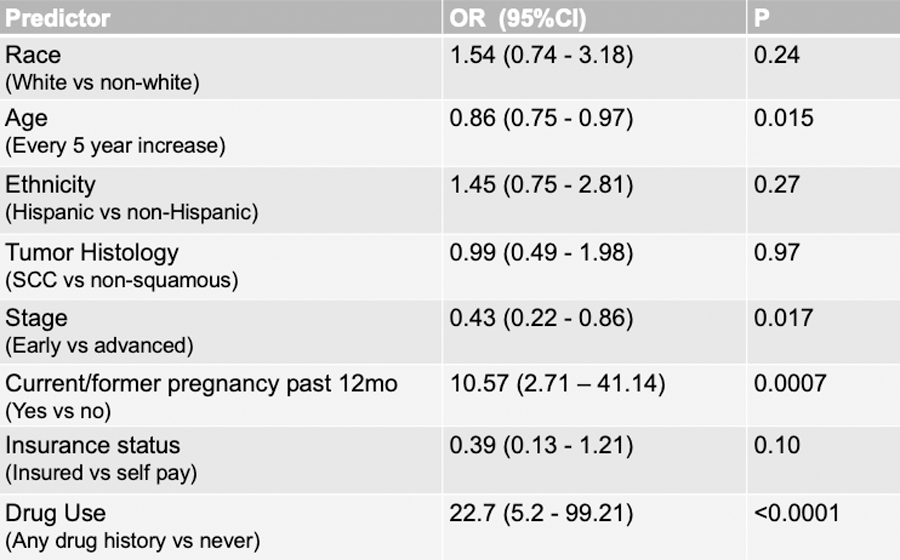
Odds Ratios for Screening Predictors
Figure 5.
Summary of HIV screening and predictive factors
AJOG at a glance:
Despite international guidelines for HIV screening in newly diagnosed cervical cancers, the compliance of US Gynecologic Oncology providers with screening has not been well studied. No study has evaluated predictive factors for screening in this cohort.
Only 7.6% of women with newly diagnosed cervical cancer were screened for HIV in our population. Several factors such as younger age, later cancer stage, recent pregnancy or drug use history may predict the performance of screening however this overall rate is very low.
Women with newly diagnosed cervical cancer are high risk populations who merit HIV screening. With a significantly more robust sample size than any prior assessment in the US or abroad, screening in our population was quite low. This identifies an area of practice for focused quality improvement.
Acknowledgements
We would like to thank the University of California, Irvine Department of Obstetrics and Gynecology for their financial support of this project.
Sources of financial support:
UCI Obstetrics and Gynecology Department
Footnotes
Conflicts of Interest: The authors report no conflicts of interest
Presentation information: 35th Annual Meeting of the Western Association of Gynecologic Oncologists, Park City, UT, June 2018 – oral presentation
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA: a cancer journal for clinicians. 2005;55(2):74–108. [DOI] [PubMed] [Google Scholar]
- 2.Health NI. Surveillance, Epidemiology and End Results Program. 2016; https://seer.cancer.gov/statfacts/html/cervix.html. Accessed 2.8.2017.
- 3.Minkoff H, Feldman J, DeHovitz J, Landesman S, Burk R. A longitudinal study of human papillomavirus carriage in human immunodeficiency virus-infected and human immunodeficiency virus-uninfected women. American journal of obstetrics and gynecology. 1998;178(5):982–986. [DOI] [PubMed] [Google Scholar]
- 4.Sun XW, Kuhn L, Ellerbrock TV, Chiasson MA, Bush TJ, Wright TC Jr. Human papillomavirus infection in women infected with the human immunodeficiency virus. The New England journal of medicine. 1997;337(19):1343–1349. [DOI] [PubMed] [Google Scholar]
- 5.Prevention CfDCa. HIV in the United States: At A Glance. 2016; https://www.cdc.gov/hiv/statistics/overview/ataglance.html. Accessed 3/1/2017.
- 6.Hwang JP, Granwehr BP, Torres HA, et al. HIV Testing in Patients With Cancer at the Initiation of Therapy at a Large US Comprehensive Cancer Center. Journal of oncology practice. 2015;11(5):384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Network NCC. Cervical Cancer Guidelines. 2016.
- 8.Sullivan AK, Raben D, Reekie J, et al. Feasibility and effectiveness of indicator condition-guided testing for HIV: results from HIDES I (HIV indicator diseases across Europe study). PLoS One. 2013;8(1):e52845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.SAS Enterprise Miner 13.1. [computer program]. Cary, NC.. [Google Scholar]
- 10.J H. HIV Testing in Patients with care at the Initiation of Therapy at a large US Comprehensive Cancer Center. J Oncolo Practice. 2015;11(5):384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosimann V, Cavassini M, Hugli O, et al. Patients with AIDS-defining cancers are not universally screened for HIV: a 10-year retrospective analysis of HIV-testing practices in a Swiss university hospital. HIV Med. 2014;15(10):631–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li. Testing for Human Immunideficiency Virus Among Cancer Survivors Under 65 in the United States. Prev Chronic Dis. 2014;11(E200):140274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Branson BHH, Lampe M, et al. Revised Recommendations for HIV Testing of Adults, Adolescnets and Pregnant Women in Health-Care Settings. MMWR. September/22/2006;55(RR 14);1–17. [PubMed] [Google Scholar]
- 14.G S. Cost-Effectiveness of Screening for HIV in the Era of Highly Active Antiretroviral Therapy. NEJM. 2005;352:570–585. [DOI] [PubMed] [Google Scholar]
- 15.Chiao EY, Dezube BJ, Krown SE, et al. Time for oncologists to opt in for routine opt-out HIV testing? JAMA. 2010;304(3):334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]


