Abstract
A 54-year-old man was admitted to our institute with a diagnosis of infective endocarditis (IE) with vegetation on the mitral valve and severe regurgitation due to Gemella morbillorum infection together with renal dysfunction, which was eventually diagnosed as infection-related pauci-immune necrotizing crescentic glomerulonephritis. Given the refractoriness to antibiotics, the persistent activity of nephritis, and repeated cerebral hemorrhaging, we prioritized steroid therapy over early surgical mitral valve replacement. Following steroid therapy, the glomerulonephritis completely improved. Although the administration of steroid therapy in the active phase of IE remains controversial, it might be indicated if comorbid glomerulonephritis is critical.
Keywords: purpura, Gemella morbillorum, hypocomplementemia
Introduction
Infective endocarditis (IE) often accompanies glomerulonephritis via an autoimmune response, showing necrotizing glomerulonephritis or endocapillary proliferative glomerulonephritis (1). The clinical outcome of IE accompanied by renal dysfunction is poor, and renal dysfunction is an independent risk factor of mortality (2).
The therapeutic strategy for glomerulonephritis in patients with IE remains controversial. Antibiotic therapy is often insufficient, whereas surgical intervention to the infectious valve is not applicable due to poor systemic conditions and comorbidities (3). Furthermore, immunosuppressants to manage the glomerulonephritis might actually worsen the infection.
We herein report a patient with IE and crescentic necrotizing glomerulonephritis who received successful steroid therapy in addition to antibiotics, followed by surgical valve replacement.
Case Report
On admission
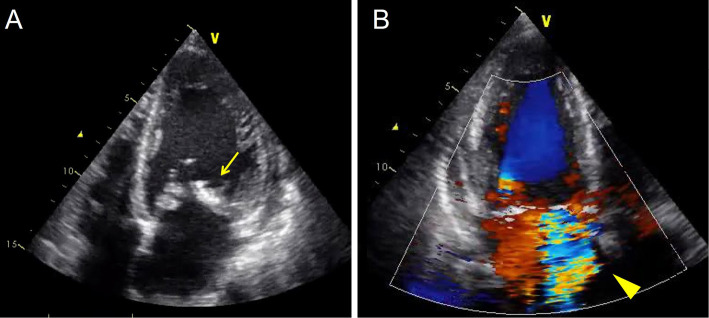
A 54-year-old man with an 18-year history of mild mitral regurgitation was admitted to our institute with a high fever, bilateral leg edema, and dyspnea on effort for the past 2 months. Given the echocardiographic findings of a 10-mm vegetation on the mitral valve with severe mitral regurgitation (Fig. 1A, B) and systemic bacteremia with Gemella morbillorum, he was diagnosed with IE.
Figure 1.
Transthoracic echocardiography showing the vegetation on the mitral valve (arrow; A) and severe mitral regurgitation (arrowhead; B)
His body temperature was 38.0 °C, blood pressure 108/56 mmHg, and pulse rate 80 beats per minute. He had Roth spots on his fundus, Osler nodules on his fingers, Janeway lesions on his palms, and purpura on his lower legs (Fig. 2).
Figure 2.
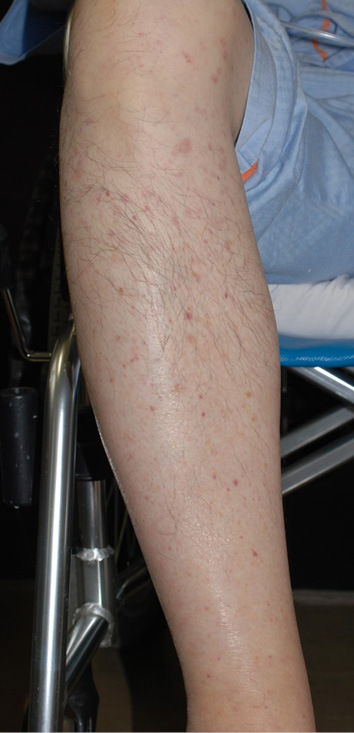
Lower leg purpura.
Laboratory data showed a white blood cell count of 10,710/μL, hemoglobin 7.7 g/dL, serum albumin 2.3 g/dL, and C-reactive protein 3.0 mg/dL (Table 1). Urinary data showed proteinuria (1.16 g/g of creatinine) and >100 red blood cells/high-power field (HPF). Immunological tests showed C3 52 mg/dL, the expression of rheumatoid factor and cryoglobulin, and immunocomplex-C1q 32 μg/mL as well as the absence of hepatitis C virus (HCV) antibody, antineutrophil cytoplasmic antibodies, and anti-glomerular basement membrane antibody. The lower leg purpura was diagnosed as leukocytoclastic vasculitis given the skin biopsy findings of perivascular bleeding and infiltration of neutrophil in the superficial dermis (Fig. 3). Immunofluorescence showed no deposition of immunoglobulin or complement in the vessel walls.
Table 1.
Laboratory Data on Admission.
| Laboratory test | Result |
|---|---|
| White blood cells, mm3 | 10,710 |
| Hemoglobin, g/dL | 7.7 |
| Platelets, mm3 | 196,000 |
| Albumin, g/dL | 2.3 |
| Blood urea nitrogen, mg/dL | 14 |
| Creatinine, mg/dL | 0.8 |
| Sodium, mEq/L | 135 |
| Potassium, mEq/L | 4 |
| C-reactive protein, mg/dL | 3 |
| Immunoglobulin G, mg/dL | 1,814 |
| Immunoglobulin A, mg/dL | 194 |
| Immunoglobulin M, mg/dL | 115 |
| Complement 3, mg/dL | 52 |
| Complement 4, mg/dL | 15.1 |
| CH50, U/mL | 14 |
| Immunocomplex-C1q, μg/mL | 32 |
| Rheumatoid factor, U/mL | 25 |
| Antinuclear antibody | negative |
| MPO-ANCA | negative |
| PR3-ANCA | negative |
| Anti-GBM antibody | negative |
| Cryoglobulin | positive |
CH50: 50% hemolytic complement activity, PR3: anti-proteinase 3, MPO: anti-myeloperoxidase, ANCA: anti-neutrophil cytoplasmatic antibody, GBM: glomerular basement membrane
Figure 3.
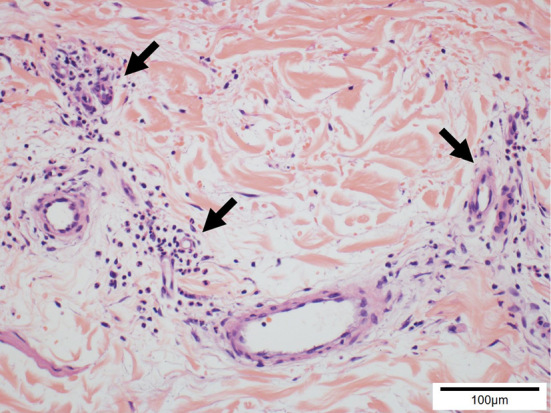
Pathological findings of his skin biopsy, showing perivascular infiltration of neutrophil and nuclear dust in the dermis (arrows) (×100).
Before the kidney biopsy
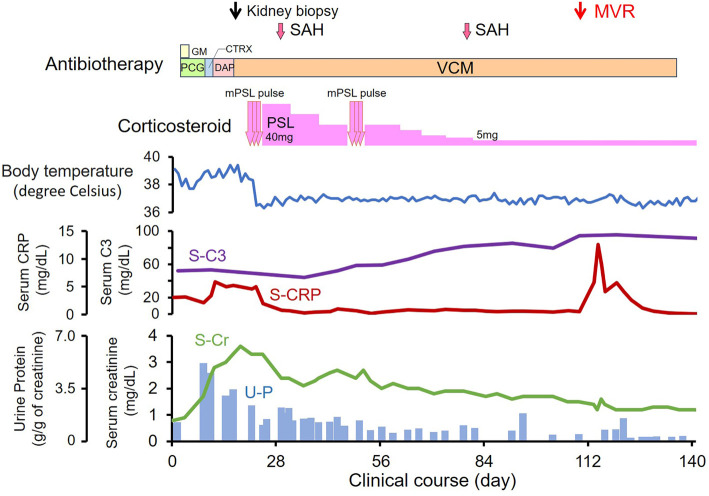
Following the intravenous administration of penicillin G and gentamicin, the bacteremia immediately improved, but the high fever and inflammatory reaction remained, and renal dysfunction again progressed from day 9 (Fig. 4). Despite the conversion of antibiotics from gentamicin to ceftriaxone, daptomycin, and vancomycin, the serum creatinine level increased to 3.6 mg/dL, lower leg purpura and proteinuria progressed gradually, and hypocomplementemia remained, which was eventually diagnosed as rapidly progressive glomerulonephritis (RPGN).
Figure 4.
Clinical course. CTRX: ceftriaxone, DAP: daptomycin, GM: gentamicin, mPSL: methylprednisolone, MVR: mitral valve replacement, PCG: penicillin G, PSL: prednisolone, SAH: subarachnoid hemorrhaging, VCM: vancomycin
The kidney biopsy
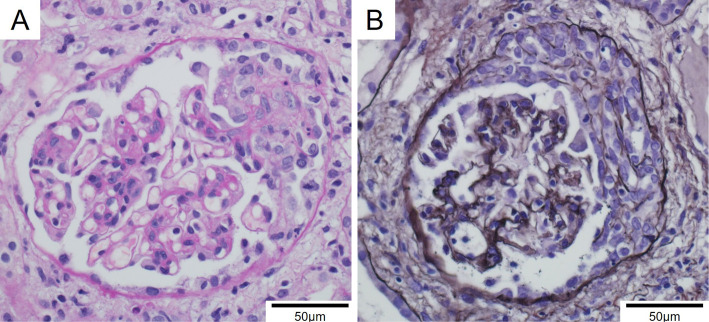
A renal biopsy was performed on day 18. Cellular crescent formation was found in 6/16 glomeruli (Fig. 5). There was no deposition of immunoglobulins or complements on immunofluorescence staining. There were also no electron-dense deposits on electron microscopy. We eventually pathologically diagnosed him with pauci-immune necrotizing crescentic glomerulonephritis. Given the lack of deposition of immune complex in the kidney against the positive immunological test of cryoglobulin, we excluded cryoglobulinemia as a cause of nephritis.
Figure 5.
Pathological findings of his kidney biopsy. A: Necrotizing and extracapillary proliferative lesion (periodic acid-Schiff stain, ×200). B: Cellular crescent formation in another glomerulus (Periodic acid-methenamine-silver stain, ×200).
Post-diagnosis course
Given the persistent inflammation despite the inversion of bacteremia, we highly suspected vasculitis. On day 22, under careful monitoring of recurrent bacteremia, we decided to perform 3-day steroid pulse therapy with the intravenous administration of methylprednisolone 500 mg/day, followed by oral prednisolone 40 mg/day, which immediately improved the high fever and lower leg purpura. Given the re-increase in the serum creatinine level as well as persistent proteinuria and mild hypocomplementemia, a second round of steroid pulse therapy was performed on day 50, which gradually improved all of these signs. The trough level of vancomycin was 15 μg/mL. The daily dose of vancomycin was between 400 and 700 mg.
As extra-renal comorbidities, on days 30 and 81, he suffered repeated subarachnoid hemorrhaging. Given the risk of perioperative cerebral hemorrhaging, we wanted to avoid early surgical mitral valve replacement, which was eventually performed on day 113 without any perioperative comorbidities and enabled us to terminate steroid therapy. After two years without steroid therapy, the serum creatinine level had normalized to 1.0 mg/dL, and all urine abnormalities, including hematuria and proteinuria, remained absent.
Discussion
IE and renal dysfunction
One-third of patients with IE have renal dysfunction due to vegetation embolism, adverse effects of antibiotics, hemodynamics-related pre-renal dysfunction, and glomerulonephritis, which often progress as RPGN. In the analyses of 49 renal biopsies, 53% showed necrotizing crescentic glomerulonephritis, and 37% showed endocapillary proliferative glomerulonephritis (4). An immune response to the infection has been proposed as a mechanism underlying glomerulonephritis, given the presence of comorbid hypocomplementemia, rheumatoid factor, and antineutrophil cytoplasmic antibodies.
In our patient, a renal histopathological assessment showed pauci-immune necrotizing crescentic glomerulonephritis without any electron-dense deposits on electron microscopy, despite hypocomplementemia and cryoglobulinemia. Our patient did not express any antineutrophil cytoplasmic antibodies, despite a renal histopathological assessment showing necrotizing crescentic glomerulonephritis. Of note, among those with IE-related glomerulonephritis, electron-dense deposits are negative in 10%, and antineutrophil cytoplasmic antibodies are negative in 72% (4).
Causative bacteria in this case
G. morbillorum, the Gram-positive streptococci with hypovirulence that we found in this case, rarely causes IE and associating glomerulonephritis (5). Kumar et al. reported a pediatric patient with IE-related crescentic glomerulonephritis due to G. morbillorum (6). Another team reported a patient with cerebral ventricle-atrial shunt-related nephritis due to G. morbillorum (Table 2) (7). The duration is associated with the severity of nephritis. A relatively long duration of infection due to the hypovirulence of G. morbillorum might actually cause an immune response and glomerulonephritis.
Table 2.
Features of Reported Cases of Gemella morbillorum-associated Glomerulonephritis.
| Case | [6] | [7] | Present report |
|---|---|---|---|
| Age (years)/sex | 17/F | 12/F | 54/M |
| Infectious disease | VA shunt infection | endocarditis | endocarditis |
| C3/C4 levels | low/low | low/normal | low/normal |
| ANCA | PR3-ANCA | negative | negative |
| Light microscopy | MPGN | crescentic GN | crescentic GN |
| IF microscopy | IgG/IgM/C1q/C4 | C3 | pauci-immune |
| Therapy | removal of shunt, antibiotics, PSL, mPSL pulse |
antibiotics, PSL, mPSL pulse, rituximab, plasmapheresis |
valve replacement surgery, antibiotics, PSL, mPSL pulse |
| Renal outcome | improved | improved | improved |
| Clinical outcome | improved | improved | improved |
IF: immunofluorescence, ANCA: antineutrophil cytoplasmic autoantibody, VA: ventricle-atrial, PR3: proteinase 3, MPGN: membranoproliferative glomerulonephritis, GN: glomerulonephritis, PSL: prednisolone, mPSL: methylprednisolone
Therapeutic strategy of IE-related glomerulonephritis
The therapeutic strategy of IE-related glomerulonephritis remains controversial. Antibiotics therapy alone might not be sufficient to improve the renal function (3). In addition to applying steroid therapy as we did in the present case (8), plasma exchange is recommended (9). However, we should pay close attention, as these therapies can actually worsen the severity of infection. One study reported that immunosuppressant therapy increased the mortality (10), although another argued the safety of steroid therapy with appropriate concomitant antibiotics (11). Careful case-by-case discussion considering the risks and benefits and continuous monitoring of recurrent bacteremia are essential for steroid therapy in such situations.
Another option is surgical intervention to remove the pathogen causing nephritis, i.e. vegetation in the present case. However, there are no recommendations concerning early surgical intervention in cases of comorbid glomerulonephritis (12,13). Surgical intervention is often challenging in patients with unstable hemodynamics or IE-related comorbidities, including renal dysfunction (14). However, successful early surgery might improve renal dysfunction (15).
In the present patient, given the repeated cerebral hemorrhaging, persistent vasculitis, and progressive renal dysfunction with crescentic formation, we prioritized steroid therapy over early surgery, which probably improved the systemic inflammation and hemodynamics and reduced the perioperative risk. In patients with IE and nephritis, multidisciplinary therapy including antibiotics, steroids, and surgical intervention should be considered at an appropriate timing based on the histopathological assessment of renal tissue.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Majumdar A, Chowdhary S, Ferreira MA, et al. Renal pathological findings in infective endocarditis. Nephrol Dial Transplant 15: 1782-1787, 2000. [DOI] [PubMed] [Google Scholar]
- 2. Buchhol K, Larsen CT, Hassager C, Bruun NE. In infectious endocarditis patients mortality is highly related to kidney function at time of diagnosis: a prospective observational cohort study of 231 case. Eur J Intern Med 20: 407-410, 2009. [DOI] [PubMed] [Google Scholar]
- 3. Neugarten J, Gallo GR, Baldwin DS. Glomerulonephritis in bacterial endocarditis. Am J Kidney Dis 3: 371-379, 1984. [DOI] [PubMed] [Google Scholar]
- 4. Boils CL, Nasr SH, Walker PD, Couser WG, Larsen CP. Update on endocarditis-associated glomerulonephritis. Kidney Int 87: 1241-1249, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Terada H, Miyahara K, Sonoda M, et al. Infective endocarditis caused by an indigenous bacterium (Gemella morbillorum). Intern Med 33: 628-631, 1994. [DOI] [PubMed] [Google Scholar]
- 6. Kumar G, Al Ali AS, Gulzar Bhatti N. Rare bacteria infecting the heart and affecting the kidney of a young child. Case Rep Nephrol Dial 7: 138-143, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nagashima T, Hirata D, Yamamoto H, Okazaki H, Minota S. Antineutrophil cytoplasmic autoantibody specific for proteinase 3 in a patient with shunt nephritis induced by Gemella morbillorum. Am J Kidney Dis 37: E38, 2001. [DOI] [PubMed] [Google Scholar]
- 8. Le Moing V, Lacassin F, Delahousse M, et al. Use of corticosteroid in glomerulonephritis related to infective endocarditis. Clin Infect Dis 28: 1057-1061, 1999. [DOI] [PubMed] [Google Scholar]
- 9. Halpin M, Kozyreva O, Bijol V, Jaber BL. Plasmapheresis for treatment of immune complex-mediated glomerulonephritis in infective endocarditis: a case report and literature review. Clin Nephrol Case Stud 5: 26-31, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vergis EN, Hayden MK, Chow JW, et al. Determinants of vancomycin resistance and mortality rates in enterococcal bacteremia. A prospective multicenter study. Ann Intern Med 135: 484-492, 2001. [DOI] [PubMed] [Google Scholar]
- 11. Vanwalleghem J, Maes B, Van Damme B, Vanrenterghem Y. Steroids for deep-infection-associated glomerulonephritis: a two-edged sword. Nephrol Dial Transplant 13: 773-775, 1998. [DOI] [PubMed] [Google Scholar]
- 12. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 36: 3075-3128, 2015. [DOI] [PubMed] [Google Scholar]
- 13. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 135: e1159-e1195, 2017. [DOI] [PubMed] [Google Scholar]
- 14. Cahill TJ, Baddour LM, Habib G, et al. Challenges in infective endocarditis. J Am Coll Cardiol 69: 325-344, 2017. [DOI] [PubMed] [Google Scholar]
- 15. Yang M, Wang G, Chen P, Cheng H. Infective endocarditis-induced crescentic glomerulonephritis dramatically improved after removal of vegetations and valve replacement. Chin Med J (Engl) 128: 404-406, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]