Abstract
Objective
It has been reported that anti-mitochondrial antibodies (AMAs) recognize mitochondrial antigens and are associated with some diseases involving multiple organs, such as primary biliary cholangitis, Sjögren syndrome, Hashimoto's thyroiditis, systemic sclerosis, interstitial pneumoniae, dilated cardiomyopathy, and tubulointerstitial nephritis. In the current study, we examined the prevalence of AMAs in patients with dilated cardiomyopathy (DCM) and their clinical characteristics.
Methods
We enrolled 270 patients with DCM. We measured serum AMAs and analyzed the associated factors. Out of the 270 patients, positive AMAs were detected in 3 patients (1.1%; mean age, 68 years old; 2 men). These three patients had a significantly higher prevalence of primary biliary cholangitis and myopathy and levels of alanine alkaline phosphatase than those who were negative for said antibodies. There were no significant differences in the levels of B-type natriuretic peptide, aspartate transaminase, and left ventricular ejection fraction between these groups of patients. During the follow-up period, two of the three patients died due to respiratory failure. The other patient survived but experienced type II respiratory failure.
Conclusion
The prevalence of AMAs in 270 DCM patients was only 1.1%, and these patients suffered from respiratory failure.
Keywords: anti-mitochondrial antibodies, dilated cardiomyopathy, primary biliary cholangitis
Introduction
Mitochondria are organelles that contribute to the production of respiratory adenosine triphosphate (ATP), which is found in eukaryotes (1,2). Mitochondria are also integrated into the intracellular signaling pathways and contribute to the cellular functions. Anti-mitochondrial antibodies (AMAs) recognize mitochondrial antigens and are associated with several diseases that involve multiple organs, including primary biliary cholangitis, Sjögren syndrome, Hashimoto's thyroiditis, systemic sclerosis, interstitial pneumoniae, dilated cardiomyopathy (DCM), and tubulointerstitial nephritis (3-8). Regarding cardiovascular diseases, AMAs are also related to cardiomyopathies, myocarditis, arrhythmias, and pulmonary hypertension (4,9-11).
Cardiomyopathy and arrhythmias have been reported to occur in 2.9% and 3.6% of patients with primary biliary cholangitis, respectively (12). Cardiac involvement in patients with primary biliary cholangitis has been reported to be related to a poor outcome (12). In addition, 33% to 71% of patients with AMA-associated myopathy have been reported to have cardiomyopathies and/or arrhythmias (4,9). Furthermore, a higher prevalence of supraventricular arrhythmias has been detected in AMA-positive patients than in those who were AMA-negative (13). Cardiac manifestations, such as cardiomyopathy, in patients with AMAs have been suggested; however, the prevalence and significance of AMAs in patients with DCM has not been fully investigated.
Therefore, in the current study, we examined the prevalence of AMAs in DCM patients and their clinical characteristics.
Materials and Methods
Study population
We prospectively included 270 consecutive hospitalized patients with DCM from Fukushima Medical University Hospital between January 2010 and October 2018. The diagnosis of DCM was based on the current guidelines as well as scientific statements regarding cardiomyopathy by experienced cardiologists (14-17). The baseline characteristics; co-morbidities; history of pacemaker, implantable cardioverter defibrillator (ICD), and cardiac resynchronization therapy; laboratory data; echocardiographic data; medications; and clinical courses were collected at the time of enrollment in this study. The history of interstitial pneumonia, primary biliary cholangitis, and myopathy were also investigated, as these are all AMA-associated diseases (3,4,6). Anemia was defined as hemoglobin values of <12.0 g/dL in women and <13.0 g/dL in men. Chronic kidney disease was defined as an estimated glomerular filtration rate of <60 mL/min/1.73 m2. The patients were followed up for occurrence of cardiac death, non-cardiac death, and all-cause death. Cardiac death was classified by independent experienced cardiologists as death related to the heart, such as worsened heart failure, ventricular fibrillation, or ventricular tachycardia documented by electrocardiography or implantable devices, acute coronary syndrome, or sudden cardiac death. Non-cardiac death included death due to respiratory failure, stroke infection, sepsis, cancer, digestive hemorrhaging, or other reasons.
The investigation conformed to the principles outlined in the Declaration of Helsinki, and the study protocol was approved by the Ethical Committee of Fukushima Medical University. Written informed consent was obtained from all patients.
The analysis of AMAs and anti-mitochondrial M2 antibodies (AMA M2)
Blood samples were collected for the measurement of AMAs and AMA M2 at the time of admission. The AMAs were analyzed in all patients by an indirect immunofluorescence method. The AMA M2 antibodies were analyzed by a fluorescence-enzyme immunoassay only in AMA-positive patients. These measurements were performed by BML (Tokyo, Japan), who were blind to the patients' information.
Echocardiography
Echocardiography was performed by experienced echocardiographers using standard techniques (18). Two-dimensional echocardiographic images were obtained from the parasternal long and short axes, apical long axis, and apical four-chamber views. The following echocardiographic parameters were investigated: left ventricular end-diastolic diameter, left ventricular ejection fraction, tricuspid regurgitation pressure gradient, and right ventricular fractional area change. The left ventricular ejection fraction was calculated using Simpson's method in a four-chamber view. The right ventricular fractional area change, defined as (end diastolic area - end systolic area) / end diastolic area ×100, was a measure of the right ventricular systolic function (19).
Statistical analyses
Data were analyzed using the Statistical Package for Social Sciences version 26 software program (SPSS, Chicago, USA). Continuous data are expressed as mean ± standard deviation (SD), and skewed data are presented as the median and interquartile range. Categorical variables are expressed as numbers and percentages. The statistical significance of differences was analyzed using Student's t-test for parametric continuous variables and the Mann-Whitney U-test for nonparametric continuous variables. Categorical variables were compared using the chi-square test or Fisher's exact test. A p value of <0.05 was considered statistically significant for all comparisons.
Results
Out of the 270 patients, 3 (1.1%; mean age, 68 years old; 2 men) were positive for AMAs. These three patients underwent an AMA M2 analysis, for which they were also positive. We divided the patients into two groups according to presence or absence of AMAs for comparisons (Table 1). In the baseline characteristics, the AMA-positive patients had a significantly higher prevalence of primary biliary cholangitis and myopathy and higher levels of alanine alkaline phosphatase than the AMA-negative patients. There were no significant differences in the prevalence of atrial fibrillation or intestinal pneumonia nor in the levels of aspartate aminotransaminase, estimated glomerular filtration rate, B-type natriuretic peptide, left ventricular ejection fraction, tricuspid regurgitation pressure gradient, or right ventricular fractional area change between the groups.
Table 1.
Baseline Characteristics.
| AMA-positive (n=3) | AMA-negative (n=267) | p value | |
|---|---|---|---|
| Age, years | 68±13 | 60±15 | 0.356 |
| Female | 1 (33.3) | 76 (28.5) | 0.636 |
| Body mass index, kg/m2 | 20±2 | 24±4 | 0.101 |
| NYHA, I/II/III/IV | 1/2/0/0 | 57/136/45/29 | 0.759 |
| Co-morbidities | |||
| Atrial fibrillation | 2 (66.7) | 104 (38.9) | 0.341 |
| Diabetes mellitus | 1 (33.3) | 108 (40.4) | 0.643 |
| Hypertension | 1 (33.3) | 152 (56.9) | 0.400 |
| Intestinal pneumonia | 1 (33.3) | 6 (2.2) | 0.076 |
| Chronic obstructive pulmonary disease | 0 (0) | 53 (19.9) | 0.518 |
| Primary biliary cholangitis | 2 (66.7) | 1 (0.4) | <0.001 |
| Myopathy | 1 (33.3) | 0 (0) | 0.011 |
| Chronic kidney disease | 1 (33.3) | 144 (54) | 0.444 |
| Anemia | 2 (66.7) | 92 (34.4) | 0.278 |
| Laboratory data | |||
| Total bilirubin, mg/dL | 0.8±0.1 | 1.1±0.8 | 0.458 |
| Aspartate aminotransferase, U/L | 29±7 | 59±220 | 0.811 |
| Alanine aminotransferase, U/L | 23±17 | 54±190 | 0.776 |
| Alkaline phosphatase, U/L | 559±457 | 262±121 | <0.001 |
| γ-glutamyl transpeptidase, U/L | 96±127 | 79±96 | 0.757 |
| eGFR, mL/min/1.73 cm2 | 68±57 | 58±22 | 0.423 |
| Creatine kinase, U/L | 123±31 | 127±142 | 0.967 |
| B-type natriuretic peptide*, pg/mL | 318 (195–483) | 330 (112–719) | 0.793 |
| Echocardiography | |||
| Left ventricular diastolic diameter, % | 60±5 | 61±9 | 0.732 |
| Left ventricular systolic diameter, % | 50±7 | 52±10 | 0.735 |
| Left ventricular ejection fraction, mm | 44±7 | 35±14 | 0.157 |
| TRPG, mmHg | 29±8 | 25±11 | 0.494 |
| RV-FAC, % | 42±11 | 39±14 | 0.608 |
| Medications/ Treatments | |||
| Renin-angiotensin system inhibitor | 3 (100.0) | 242 (90.3) | 0.737 |
| Beta-blocker | 3 (100.0) | 259 (97.0) | 0.913 |
| Diuretics | 3 (100.0) | 208 (77.9) | 0.476 |
| Inotropes | 1 (33.3) | 82 (30.7) | 0.669 |
| Amiodarone | 2 (66.7) | 1 (33.3) | 0.395 |
| Prednisolone | 1 (33.3) | 4 (1.5) | 0.055 |
| Pacemaker | 1 (33.3) | 24 (9.0) | 0.254 |
| Implantable cardioverter defibrillator | 1 (33.3) | 106 (39.7) | 0.654 |
| Cardiac resynchronization therapy | 1 (33.3) | 96 (35.6) | 0.711 |
| Prognosis | |||
| Cardiac death | 0 (0) | 49 (18.4) | 0.547 |
| Non-cardiac death | 2 (66.7) | 21 (7.9) | 0.020 |
| All-cause death | 2 (66.7) | 72 (27.0) | 0.183 |
Numbers are expressed as number, number (%), mean±SD, or *Median (IQR)
AMA: anti-mitochondrial antibodies, NYHA: New York Heart Association, eGFR: estimated glomerular filtration ratio, TRPG: Tricuspid regurgitation pressure gradient, RV-FAC: right ventricular fractional change, IQR: interquartile range
During the median follow-up period of 1,435 days, 49 cardiac deaths, 23 non-cardiac deaths, and 2 deaths of unknown causes occurred (Table 1). Of the cardiac deaths, 36 were from heart failure, 9 were from sudden death, and 4 were from lethal arrhythmia. Of the non-cardiac deaths, 12 were from cancer, 7 were from respiratory failure, 2 were from kidney failure, and 1 each was from brain infarction and sepsis. Two of the three AMA-positive patients died due to non-cardiac death related to respiratory failure.
The detailed clinical courses of the three patients with AMAs are shown below.
• Case 1
A woman in her 60s suffered from complete atrioventricular block and had a pacemaker implanted. Ten years later, she was admitted to the hospital with heart failure and diagnosed with DCM. Coronary angiography revealed no significant stenosis (Fig. 1a, b). The patient underwent cardiac resynchronization therapy pacemaker (CRTP) implantation, which was an upgrade from the patient's pacemaker. Six years later, she was referred to our hospital for exchange of the CRTP.
Figure 1.
a, b: Coronary angiography revealed no significant stenosis. c: Electrocardiography showed a pacing rhythm and a heart rate of 46 bpm. d: Chest X-ray showed no sign of congestion and a cardiothoracic ratio of 66.5% with a cardiac resynchronization therapy pacemaker. e: Echocardiography detected a reduced ejection fraction and a large left ventricular diastolic diameter.
On admission, laboratory findings revealed an elevated B-type natriuretic peptide level of 648.1 pg/mL and an alkaline phosphatase level of 256 U/L. Laboratory data and treatments are shown in Table 2. Electrocardiography showed a pacing rhythm and heart rate of 46 bpm, and chest X-ray showed no sign of congestion and a cardiothoracic ratio of 66.5% with a CRTP (Fig. 1c, d). Echocardiography detected a reduced ejection fraction of 39.0% and a large left ventricular diastolic diameter of 59.6 mm (Fig. 1e, Table 2). Exchange surgery of the CRTP was successfully performed, and the patient was discharged. At 586 days after this hospitalization, the patient died of respiratory failure due to pneumonia.
Table 2.
Characteristics in Each Patient with Anti-mitochondrial Antibodies on Admission.
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Laboratory data | |||
| Total bilirubin, mg/dL | 0.8 | 0.9 | 0.7 |
| Aspartate aminotransferase, U/L | 28 | 23 | 36 |
| Alanine aminotransferase, U/L | 11 | 15 | 43 |
| Alkaline phosphatase, U/L | 256 | 336 | 1,084 |
| γ-glutamyl transpeptidase, U/L | 28 | 18 | 243 |
| eGFR, mL/min/1.73 cm2 | 13 | 66 | 126 |
| Creatine kinase, U/L | 147 | 88 | 135 |
| B-type natriuretic peptide, pg/mL | 648.1 | 318.3 | 71.7 |
| IgM, mg/dL | - | 148 | - |
| Echocardiography | |||
| Left ventricular diastolic diameter, % | 59.6 | 65.3 | 55.2 |
| Left ventricular systolic diameter, % | 51.3 | 56.4 | 43.4 |
| Left ventricular ejection fraction, mm | 39.0 | 41.2 | 52.0 |
| TRPG, mmHg | 36.0 | 29.0 | 20.6 |
| RV-FAC, % | 42.1 | 54.2 | 31.6 |
| Medications/ Treatments | |||
| Renin-angiotensin system inhibitor | + | + | + |
| Beta-blocker | + | + | + |
| Diuretics | + | + | + |
| Inotropes | - | + | - |
| Amiodarone | + | + | - |
| Prednisolone | - | + | - |
| Pacemaker | + | - | - |
| Implantable cardioverter defibrillator | - | - | + |
| Cardiac resynchronization therapy | + | - | - |
eGFR: estimated glomerular filtration ratio, TRPG: Tricuspid regurgitation pressure gradient, RV-FAC: right ventricular fractional change,+: use, -: no use.
• Case 2
A man in his sixties was diagnosed with DCM. His blood test revealed an elevated creatinine kinase level and positive results of anti-nuclear antibody, anti-centromere antibody, and AMA M2. He was diagnosed with systemic scleroderma, dermatomyositis, and primary biliary cholangitis, and medical treatment with prednisolone and ciclosporin was initiated.
Three years later, a Holter echocardiogram detected frequent multifocal premature ventricular contractions of 27,072 beats per day (percentage of premature ventricular contractions per total beats: 30%). The patient was admitted to our hospital for radiofrequency catheter ablation of premature ventricular contractions. Radiofrequency catheter ablation for the premature ventricular contractions was performed, but the patient refused implantation of a cardiac device.
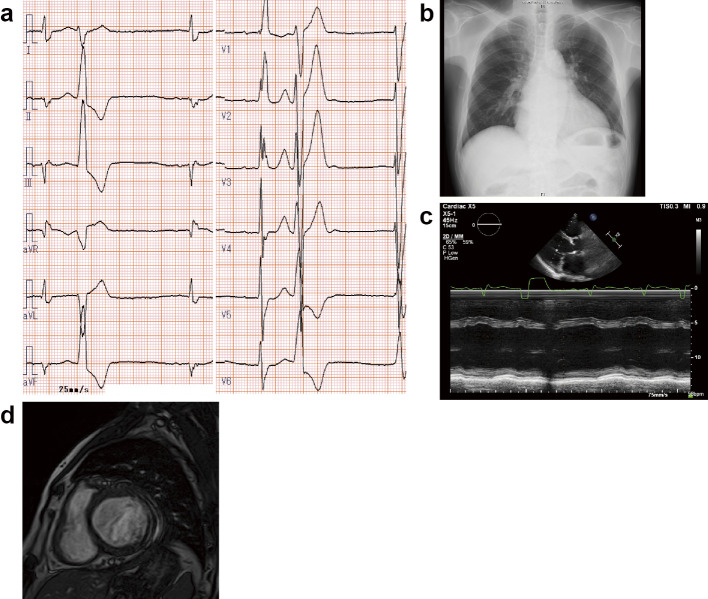
On admission, laboratory findings revealed an elevated B-type natriuretic peptide level of 318.3 pg/mL and an elevated alkaline phosphatase level of 336 U/L. Laboratory data and treatment are shown in Table 2. Electrocardiography showed a sinus rhythm, premature ventricular contraction, and a heart rate of 64 bpm, and chest X-ray showed slight congestion and a cardiothoracic ratio of 59.7% (Fig. 2a, b). Echocardiography detected a reduced left ventricular ejection fraction of 41.2% and a large left ventricular diastolic diameter of 65.3 mm (Fig. 2c, Table 2). Cardiac magnetic resonance imaging revealed late gadolinium enhancement in the left ventricular lateral wall (Fig. 2d). Spirometry showed a reduced percentage of predicted vital capacity of 61.3% and a normal percentage of predicted forced expiratory volume in 1 second of 90.0%. In addition, he was diagnosed with type II respiratory failure and received noninvasive positive pressure ventilation therapy at home. During the follow-up period of 1,391 days, he experienced worsening heart failure once, but he survived.
Figure 2.
a: Electrocardiography showed a sinus rhythm, premature ventricular contraction, and heart rate of 64 bpm. b: Chest X-ray showed a slight congestion and a cardiothoracic ratio of 59.7%. c: Echocardiography detected a reduced left ventricular ejection fraction and a large left ventricular diastolic diameter. d: Cardiac magnetic resonance imaging revealed late gadolinium enhancement in the left ventricular lateral wall.
• Case 3
A man in his 40s was diagnosed with primary biliary cholangitis. Two years later, he complained of palpitations and was diagnosed with DCM and ventricular tachycardia. Coronary angiography revealed no significant stenosis (Fig. 3a, b), and a myocardial biopsy showed no sign of secondary cardiomyopathies, such as sarcoidosis or amyloidosis. The patient was implanted with an ICD.
Figure 3.
a, b: Coronary angiography revealed no significant stenosis. c: Electrocardiography showed a pacing rhythm and a heart rate of 59 bpm. d: Chest X-ray showed slight congestion and a cardiothoracic ratio of 64.9% with an implantable cardioverter defibrillator. e: Echocardiography detected a preserved ejection fraction and a slight large left ventricular diastolic diameter.
Seven years later, he was admitted to our hospital for replacement of his ICD. On admission, laboratory findings revealed an elevated B-type natriuretic peptide level of 71.7 pg/mL and an elevated alkaline phosphatase level of 1,084 U/L. Laboratory data and treatments are shown in Table 2.
Electrocardiography showed a pacing rhythm and heart rate of 59 bpm, and chest X-ray showed slight congestion and a cardiothoracic ratio of 64.9% with an ICD (Fig. 3c, d). Under guideline-based treatment, echocardiography detected a preserved ejection fraction of 52.0% and a slightly large left ventricular diastolic diameter of 55.2 mm (Fig. 3e, Table 2). Replacement surgery of an ICD was performed, and he was discharged. at 1,573 days after the hospitalization, the patient died of respiratory failure due to interstitial pneumonia.
Discussion
This study demonstrated a 1.1% prevalence of AMAs among 270 DCM patients. In addition, two of the three patients with AMAs died due to respiratory failure, and the remaining patient with AMAs had type II respiratory failure.
AMAs are typically found in patients with primary biliary cholangitis (6,20). Primary biliary cholangitis is a chronic inflammatory autoimmune liver disease and can overlap with several autoimmune diseases, such as Sjögren syndrome, systemic sclerosis, systemic lupus erythematosus, autoimmune hepatitis, rheumatoid arthritis, psoriasis, chronic thyroiditis, and/or inflammatory bowel disease (7,20-26). Other AMA-related extrahepatic phenotypes have been reported to manifest in the heart, lung, kidney, and skeletal muscles (3-5,27). The following cardiac manifestations, considered to be rare AMA-associated complications, have been previously reported: atrial fibrillation, atrial flutter, sick sinus syndrome, paroxysmal supraventricular tachycardia, complete atrioventricular block, ventricular tachycardia, pulmonary hypertension, cardiomyopathy, and myocarditis (10-13,28,29). Regarding the prevalence of AMAs, AMA-M7 has been reported to be detected in 31% of congestive or hypertrophic cardiomyopathy patients; in contrast, AMA-M7 was not detected in any other subjects in the same study (30).
In the present study, the prevalence of AMAs was 1.1% in DCM patients (30). We showed that AMA-positive DCM patients were associated with respiratory failure. Some patients with primary biliary cholangitis experience interstitial pneumonia, which might be a respiratory complication in patients with DCM associated with AMAs (3,8,31,32). In the present study, the three AMA-positive patients underwent guideline-based medical treatments for heart failure with renin-angiotensin system inhibitors, beta blockers, amiodarone, implantable cardiac devices (CRTP, ICD), and radiofrequency catheter ablation. These intensive treatments might have improved the cardiac prognosis (e.g. arrhythmia, heart failure) in the current study's three AMA-positive patients with DCM. Non-cardiac events might thus have been involved as the cause of death in the current study's two AMA-positive patients who died.
AMAs are classified from M1 to M9. AMA M2, M4, M8, and M9 have been detected in cases of primary biliary cholangitis (33-36). AMA-M7 has been reported to be associated with cardiomyopathies and myocarditis (37). However, other studies have reported AMA M2 in patients with DCM (4,28). The present study also identified AMA M2 in all three patients with AMA-positive DCM. One anecdotal report described the infiltration of CD3-positive T cells of the myocardium in AMA M2-related cardiomyopathy (29). However, another study showed that there were no significant differences in the number of AMA M2-positive primary biliary cholangitis patients with and without cardiac involvement (12). The relationship between AMA M2 and cardiac phenotype needs to be clarified in further studies.
AMA-associated cardiac dysfunction has been reported to be improved after immunosuppressive therapy in anecdotal case reports (28,29). Complication with cardiomyopathy has been reported in patients with systemic autoimmune diseases (38). Cardiomyopathy in patients with AMAs might be caused by autoimmune inflammatory response (11). There might be a subgroup of DCM that is related to AMAs.
Several limitations associated with the present study warrant mention. First, since the prevalence of AMAs was only 1.1% in 270 DCM patients, this study may have been too weak to perform a full statistical analysis, including Kaplan-Meier and Cox proportional hazard analyses. Second, this study was unable to completely demonstrate the clinical characteristics due to the small number of AMA-positive DCM patients. Further studies with more DCM patients with AMAs are needed in order to confirm the prognosis and characteristics of these patients. Third, since this was an observational study, a causal relationship between AMAs and DCM could not be fully explained.
In conclusion, the prevalence of AMAs was only 1.1% in 270 DCM patients, and these patients suffered from respiratory failure. Thus, close attention should be paid to cases of respiratory failure in AMA-positive patients with DCM.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank Ms. Chisato Kubo, of the Office for Gender Equality Support, Fukushima Medical University, Fukushima, Japan, as well as Ms. Tomiko Miura and Ms. Shoko Sato of the Department of Cardiovascular Medicine, Fukushima Medical University, Fukushima, Japan, for their excellent technical assistance.
References
- 1. Duchen MR. Contributions of mitochondria to animal physiology: from homeostatic sensor to calcium signalling and cell death. J Physiol 516: 1-17, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smyrnias I, Gray SP, Okonko DO, et al. Cardioprotective effect of the mitochondrial unfolded protein response during chronic pressure overload. J Am Coll Cardiol 73: 1795-1806, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shen M, Zhang F, Zhang X. Primary biliary cirrhosis complicated with interstitial lung disease: a prospective study in 178 patients. J Clin Gastroenterol 43: 676-679, 2009. [DOI] [PubMed] [Google Scholar]
- 4. Maeda MH, Tsuji S, Shimizu J. Inflammatory myopathies associated with anti-mitochondrial antibodies. Brain 135: 1767-1777, 2012. [DOI] [PubMed] [Google Scholar]
- 5. Yamaguchi S, Maruyama T, Wakino S, et al. A case of severe osteomalacia caused by Tubulointerstitial nephritis with Fanconi syndrome in asymptomotic primary biliary cirrhosis. BMC Nephrol 16: 187, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frostell A, Mendel-Hartvig I, Nelson BD, et al. Mitochondrial autoantigens in primary biliary cirrhosis. Association of disease-specific determinants with a subunit of complex I (NADH-ubiquinone reductase) of the inner mitochondrial membrane. Scand J Immunol 28: 645-652, 1988. [DOI] [PubMed] [Google Scholar]
- 7. Chalifoux SL, Konyn PG, Choi G, Saab S. Extrahepatic manifestations of primary biliary cholangitis. Gut Liver 11: 771-780, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu B, Zhang FC, Zhang ZL, Zhang W, Gao LX. Interstitial lung disease and Sjögren's syndrome in primary biliary cirrhosis: a causal or casual association? Clin Rheumatol 27: 1299-1306, 2008. [DOI] [PubMed] [Google Scholar]
- 9. Albayda J, Khan A, Casciola-Rosen L, Corse AM, Paik JJ, Christopher-Stine L. Inflammatory myopathy associated with anti-mitochondrial antibodies: a distinct phenotype with cardiac involvement. Semin Arthritis Rheum 47: 552-556, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shen M, Zhang F, Zhang X. Pulmonary hypertension in primary biliary cirrhosis: a prospective study in 178 patients. Scand J Gastroenterol 44: 219-223, 2009. [DOI] [PubMed] [Google Scholar]
- 11. Saito T, Kodani E, Katayama H, Kusama Y. Eosinophilic myocarditis associated with anti-mitochondrial M2 antibodies: a mechanism underlying the onset of myocarditis. Eur Heart J 39: 3480-3481, 2018. [DOI] [PubMed] [Google Scholar]
- 12. Bian S, Chen H, Wang L, et al. Cardiac involvement in patients with primary biliary cholangitis: a 14-year longitudinal survey-based study. PLoS One 13: e0194397, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Konishi H, Fukuzawa K, Mori S, et al. Anti-mitochondrial M2 antibodies enhance the risk of supraventricular arrhythmias in patients with elevated hepatobiliary enzyme levels. Intern Med 56: 1771-1779, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bozkurt B, Colvin M, Cook J, et al. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: a scientific statement from the American Heart Association. Circulation 134: e579-e646, 2016. [DOI] [PubMed] [Google Scholar]
- 15. Elliott P, Andersson B, Arbustini E, et al. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on myocardial and pericardial diseases. Eur Heart J 29: 270-276, 2008. [DOI] [PubMed] [Google Scholar]
- 16. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 37: 2129-2200, 2016. [DOI] [PubMed] [Google Scholar]
- 17. Yoshihisa A, Kimishima Y, Kiko T, et al. Usefulness of urinary N-terminal fragment of titin to predict mortality in dilated cardiomyopathy. Am J Cardiol 121: 1260-1265, 2018. [DOI] [PubMed] [Google Scholar]
- 18. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28: 1-39.e14, 2015. [DOI] [PubMed] [Google Scholar]
- 19. Yoshihisa A, Kimishima Y, Kiko T, et al. Liver fibrosis marker, 7S domain of collagen type IV, in patients with pre-capillary pulmonary hypertension. Int J Cardiol 258: 269-274, 2018. [DOI] [PubMed] [Google Scholar]
- 20. Rubin E, Schaffner F, Popper H. Primary biliary cirrhosis. chronic non-suppurative destructive cholangitis. Am J Pathol 46: 387-407, 1965. [PMC free article] [PubMed] [Google Scholar]
- 21. Yokokawa J, Saito H, Kanno Y, et al. Overlap of primary biliary cirrhosis and autoimmune hepatitis: characteristics, therapy, and long term outcomes. J Gastroenterol Hepatol 25: 376-382, 2010. [DOI] [PubMed] [Google Scholar]
- 22. Marasini B, Gagetta M, Rossi V, Ferrari P. Rheumatic disorders and primary biliary cirrhosis: an appraisal of 170 Italian patients. Ann Rheum Dis 60: 1046-1049, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koyamada R, Higuchi T, Kitada A, et al. Association of primary biliary cirrhosis-autoimmune hepatitis overlap syndrome with immune thrombocytopenia and Graves' disease. Intern Med 54: 2013-2016, 2015. [DOI] [PubMed] [Google Scholar]
- 24. Liberal R, Gaspar R, Lopes S, Macedo G. Primary biliary cholangitis in patients with inflammatory bowel disease. Clin Res Hepatol Gastroenterol 44: e5-e9, 2020. [DOI] [PubMed] [Google Scholar]
- 25. Floreani A, Cazzagon N. PBC and related extrahepatic diseases. Best Pract Res Clin Gastroenterol 34-35: 49-54, 2018. [DOI] [PubMed] [Google Scholar]
- 26. Prince MI, Ducker SJ, James OF. Case-control studies of risk factors for primary biliary cirrhosis in two United Kingdom populations. Gut 59: 508-512, 2010. [DOI] [PubMed] [Google Scholar]
- 27. Albayda J, Khan A, Casciola-Rosen L, Corse AM, Paik JJ, Christopher-Stine L. Inflammatory myopathy associated with anti-mitochondrial antibodies: a distinct phenotype with cardiac involvement. Semin Arthritis Rheum 47: 552-556, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Katahira M, Yokokawa T, Miura S, et al. A case of non-ischemic heart failure associated with anti-mitochondrial antibody-positive myositis. SHINZO 50: 1138-1144, 2018. (in Japanese). [Google Scholar]
- 29. Matsumoto K, Tanaka H, Yamana S, et al. Successful steroid therapy for heart failure due to myocarditis associated with primary biliary cirrhosis. Can J Cardiol 28: 515.e3-515.e6, 2012. [DOI] [PubMed] [Google Scholar]
- 30. Klein R, Maisch B, Kochsiek K, Berg PA. Demonstration of organ specific antibodies against heart mitochondria (anti-M7) in sera from patients with some forms of heart diseases. Clin Exp Immunol 58: 283-292, 1984. [PMC free article] [PubMed] [Google Scholar]
- 31. Hiraoka A, Kojima N, Yamauchi Y, et al. An autopsy case of primary biliary cirrhosis with severe interstitial pneumonia. Intern Med 40: 1104-1108, 2001. [DOI] [PubMed] [Google Scholar]
- 32. Weissman E, Becker NH. Interstitial lung disease in primary biliary cirrhosis. Am J Med Sci 285: 21-27, 1983. [DOI] [PubMed] [Google Scholar]
- 33. Berg PA, Klein R, Lindenborn-Fotinos J, Kloppel W. ATPase-associated antigen (M2): marker antigen for serological diagnosis of primary biliary cirrhosis. Lancet 2: 1423-1426, 1982. [DOI] [PubMed] [Google Scholar]
- 34. Klein R, Kloppel G, Garbe W, Fintelmann V, Berg PA. Antimitochondrial antibody profiles determined at early stages of primary biliary cirrhosis differentiate between a benign and a progressive course of the disease. A retrospective analysis of 76 patients over 6-18 years. J Hepatol 12: 21-27, 1991. [DOI] [PubMed] [Google Scholar]
- 35. Klein R, Berg PA. Characterization of a new mitochondrial antigen-antibody system (M9/anti-M9) in patients with anti-M2 positive and anti-M2 negative primary biliary cirrhosis. Clin Exp Immunol 74: 68-74, 1988. [PMC free article] [PubMed] [Google Scholar]
- 36. Klein R, Berg PA. Anti-M4 antibodies in primary biliary cirrhosis react with sulphite oxidase, an enzyme of the mitochondrial inter-membrane space. Clin Exp Immunol 84: 445-448, 1991. [PMC free article] [PubMed] [Google Scholar]
- 37. Otto A, Stahle I, Klein R, Berg PA, Pankuweit S, Brandsch R. Anti-mitochondrial antibodies in patients with dilated cardiomyopathy (anti-M7) are directed against flavoenzymes with covalently bound FAD. Clin Exp Immunol 111: 541-547, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Maisch B, Alter P. Treatment options in myocarditis and inflammatory cardiomyopathy: focus on i.v. immunoglobulins. Herz 43: 423-430, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]