Abstract
Objective
Recent studies suggest a significant association between sarcoidosis and malignancy, although the results have remained controversial. The aim of this study is to evaluate the clinical features of patients with sarcoidosis associated with malignant diseases in Japan.
Patients
We conducted a medical record review of all sarcoidosis patients in Tohoku University Hospital between January 1, 1981, and May 31, 2017.
Methods
The clinical records and pathology reports for each patient were screened, and the clinical characteristics of malignancies as well as sarcoidosis were reviewed.
Results
A total of 52 (18.8%) patients with malignancy were identified among 277 patients with sarcoidosis. Among those 52 patients, we identified 62 with malignant diseases. These patients were older and more likely to be women than the remaining 225 (81.2%) sarcoidosis patients without malignancy. The most prevalent malignant disease was breast cancer (14 cases, 22.6%), followed by stomach cancer (8 cases, 12.9%) and lung cancer (7 cases, 11.3%). Among the 14 patients with both sarcoidosis and breast cancer, 8 (57.1%) were diagnosed with breast cancer before sarcoidosis. All of these eight cases had undergone surgical resection of the cancer.
Conclusion
This study showed a higher incidence of patients with both sarcoidosis and malignancy in Japan than in some western countries. Breast cancer is the most prevalent malignant disease. The high frequency of sarcoidosis after surgical resection of breast cancer may suggest a causative association between malignancy and the development of sarcoidosis.
Keywords: sarcoidosis, malignancy, breast cancer, surgery
Introduction
Sarcoidosis is a systemic inflammatory disease characterized by non-caseating epithelioid cell granuloma and mainly affects the intrathoracic lymph nodes and the lungs. Although the etiology of sarcoidosis remains unclear, many studies have suggested that genetic, host immunologic, and environmental factors interact to cause sarcoidosis. (1,2). Environmental exposures to microbial agents, including Mycobacteria and Propionibacteria, as well as other organisms may prove causative because of their infectious and/or antigenic properties (2-4). Epidemiologic studies suggest that other environmental exposures, including not only employment as firefighters (5,6) or in the military (7) but also insecticides, agricultural employment, and microbial bioaerosols, might be associated with sarcoidosis (8).
Recent studies have suggested a significant association between sarcoidosis and malignancy (9,10). Sarcoidosis may affect the development of malignancy (10-12). It is also reported that either malignancy itself (13-15) or specific chemotherapy agents may be predisposing factors for the development of sarcoidosis (16,17). Notably, the incidence of sarcoidosis in patients with malignancy is likely to be 175 times higher after lymphoma and 38 times higher after breast cancer than in the general population (13). In addition, it has been reported that the risk of cancer, including lung cancer or lymphoma, is high within the first three months after the diagnosis of sarcoidosis, mainly due to increased surveillance, or the initial misinterpretation of cancer as sarcoidosis, and that the increased long-term (beyond 10 years after the diagnosis) risk of colon cancer or immune-related cancers was notable and unlikely to reflect surveillance bias alone (12). These reports suggest a significant association between malignancy and sarcoidosis, although the possible correlation between them has remained controversial for decades (10-12,18-20). However, little is known about what kind of cancer is typically associated with sarcoidosis in Japan and whether or not the type of treatment affects the incidence of sarcoidosis.
In this study, we investigated the clinical features of patients with sarcoidosis associated with malignant diseases in Japan to evaluate a possible causative association between the two diseases.
Materials and Methods
Patient population
All patients were diagnosed with sarcoidosis according to the American Thoracic Society (ATS)/European Respiratory Society (ERS)/ World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) statement on sarcoidosis. These criteria require histopathological evidence of non-caseating granuloma, radiographic evidence of intrathoracic sarcoidosis, compatible clinical features and the exclusion of other granulomatous diseases (21).
Study design
This was a single-center, cross-sectional survey that targeted sarcoidosis patients. This study was approved by the ethics committee of Tohoku University Graduate School of Medicine. Informed consent was waived based on the ethics guidelines of Tohoku University Graduate School of Medicine.
We conducted a medical record review of 277 consecutive sarcoidosis patients in Tohoku University Hospital between January 1, 1981, and May 31, 2017. We screened the clinical records and pathology reports for each sarcoidosis patient with malignancy. All identified patients’ charts were reviewed in detail to verify the histological, radiological and clinical features of sarcoidosis, including serum biomarkers, such as angiotensin-converting enzyme (ACE) and soluble interleukin-2 receptor (sIL-2R), the percentage of lymphocytes in bronchoalveolar lavage fluids (BALF) cell counts, the CD4/8 ratio in BALF lymphocytes and pulmonary function tests (PFTs). The procedures of the pulmonary function tests were in accordance with the ATS/ERS guidelines (22). The parameters collected were the percent predicted values of forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1) and diffusing capacity of the lung for carbon monoxide (DLco), which was measured by the single-breath method according to the ATS/ERS guidelines. The FEV1/FVC ratio was also collected. Concerning the information on malignancies, we collected data on the primary organ of malignancy and the onset timing of malignancy (before or after the diagnosis of sarcoidosis) as well as the types of treatment for breast cancer.
Statistical analyses
Data concerning the age and pulmonary function were expressed as the mean±standard error (SE), and data concerning the pack-years of tobacco use, serum biomarkers (ACE, sIL-2R) and BALF were expressed as the median and interquartile range (IQR, Q1-Q3). The clinical characteristics were compared using the Mann-Whitney U test, Student’s t-test and Fisher's exact test. Statistical significance was accepted as p<0.05. All statistical analyses were performed using the JMP Pro 14 software program (SAS Institute, Cary, USA).
Results
Prevalence of sarcoidosis patients with malignancy
We evaluated the medical records of patients with sarcoidosis diagnosed at Tohoku University Hospital between January 1, 1981, and May 31, 2017. The characteristics of the sarcoidosis patients are summarized in Table 1. Among 277 sarcoidosis patients, 175 (63.2%) were women, and the mean age of the total sarcoidosis patients was 49.3±15.9 years old. 128 (46.2%) patients had a smoking history, but most sarcoidosis patients had normal values on PFTs (107.5±26.0 for FVC %predicted, 80.6±8.9 for FEV1/FVC and 98.0±25.3 for DLco %predicted).
Table 1.
Characteristics of Subjects with Sarcoidosis and with or without Malignancy.
| All patients with sarcoidosis |
Sarcoidosis with malignancy |
Sarcoidosis without malignancy |
p values | ||
|---|---|---|---|---|---|
| Number of subjects, n (%) | 277 (100%) | 52 (18.8%) | 225 (81.2%) | - | |
| Female,n (%) | 175 (63.2%) | 40 (76.9%) | 135 (60.0%) | 0.03* | |
| Age, years old | 49.3±15.9 | 57.8±12.2 | 47.3±16.0 | <0.01* | |
| Smoking status | Current/Ex-smokers, n (%) | 128 (46.2%) | 20 (38.5%) | 108 (48.0%) | 0.37 |
| Pack-years | 0 (0-200) | 0 (0-189) | 15 (0-200) | 0.12 | |
| Pulmonary function | FVC% predicted | 107.5±26.0 | 106.3±17.0 | 107.8±21.6 | 0.18 |
| FEV1 % predicted | 101.5±20.5 | 105.9±19.1 | 100.5±20.6 | 0.08 | |
| FEV1/FVC (%) | 80.6±8.9 | 80.3±9.3 | 80.7±8.8 | 0.77 | |
| DLco %predicted | 98.0±25.3 | 104.7±46.3 | 98.3±25.5 | 0.20 | |
| Serum marker | ACE (IU/L) | 20.5 (14.7-27.8) | 21.8 (16.2-34.6) | 20.1 (14.5-27.1) | 0.09 |
| sIL-2R (U/mL) | 697 (453-1,170) | 666 (447-1,359) | 714 (456-1,134) | 0.72 | |
| BALF | Lymphocytes (%) | 29.5 (15.0-45.0) | 43.0 (16.0-57.6) | 27.8 (14.8-41.8) | 0.06 |
| CD4/CD8 ratio | 4.5 (2.5-8.0) | 5.6 (4.3-7.9) | 4.1 (2.3-8.1) | 0.17 | |
| Histological diagnosis, n (%) | 181 (79.7%) | 47 (90.4%) | 134 (59.6%) | <0.01* | |
| Pulmonary stage, n (%) | I | 109 (39.4%) | 23 (44.2%) | 86 (38.2%) | 0.42 |
| II | 152 (54.9%) | 27 (51.9%) | 125 (55.6%) | 0.64 | |
| III/IV | 16 (5.7%) | 2 (3.9%) | 14 (6.2%) | 0.51 | |
| Organ involvement, n (%) | Eye | 161 (58.1%) | 28 (53.8%) | 133 (59.1%) | 0.23 |
| Skin | 61 (22.0%) | 18 (34.6%) | 43 (19.1%) | 0.02* | |
| Heart | 49 (17.7%) | 10 (19.2%) | 38 (16.9%) | 0.69 | |
| Others | 77 (27.8%) | 18 (34.6%) | 59 (26.2%) | 0.22 | |
| Oral corticosteroids use, n (%) | 117 (42.2%) | 19 (36.5%) | 98 (43.5%) | 0.44 | |
Data are expressed as the mean±standard error for the age and pulmonary function and as the median and interquartile range (IQR, Q1-Q3) for pack-years of tobacco use, biomarkers from serum (ACE, sIL-2R) and BALF. The clinical characteristics were compared using the Mann-Whitney U test, Student’s t test and Fisher’s exact test. Statistical significance was accepted as p<0.05 and marked by an asterisk. BALF: bronchoalveolar lavage fluids, FCV: forced vital capacity, FEV1: forced expiratory volume in 1 second, DLco: diffusing capacity of the lung for carbon monoxide, ACE: angiotensin-converting enzyme, sIL-2R: soluble interleukin-2 receptor
A total of 52 (18.8%) sarcoidosis patients had a history of malignant disease. These patients were older and more likely to be women and showed a higher rate of a histologically confirmed diagnosis of sarcoidosis than the remaining 225 (81.2%) patients without malignancy. No remarkable difference was found in the smoking status, pulmonary function, serum diagnostic biomarkers (ACE, sIL-2R, BALF findings), distribution of pulmonary stage or organ involvement aside from skin lesions or the amount of oral corticosteroids used between sarcoidosis patients with and without malignancy.
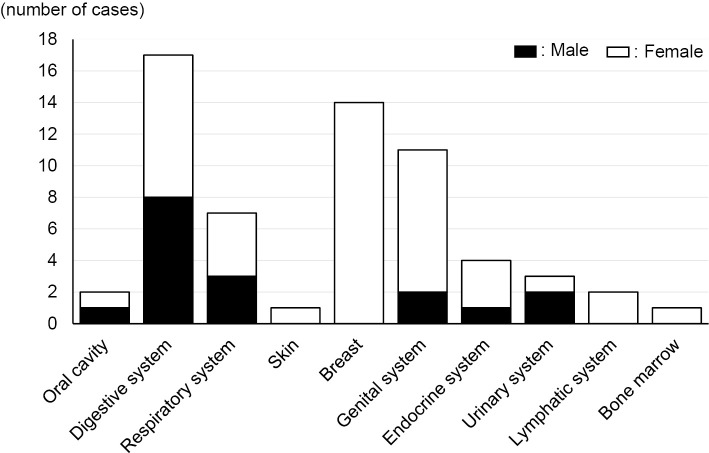
Among the 52 patients with malignancy, 62 malignant diseases (17 cases in men and 45 in women) were identified. The digestive system was the most prevalent primary lesion by organ (17 cases, 27.4%) (Fig. 1), but a more detailed analysis by tissue revealed that the most prevalent malignancy was breast cancer (14 cases, 22.6%), followed by stomach cancer (8 cases, 12.9%) and lung cancer (7 cases, 11.3%) (Table 2).
Figure 1.
Number of patients with sarcoidosis and malignancy by origins. Among 52 patients with sarcoidosis and malignancy, the malignant diseases occurred in the digestive system (17 cases, 27.4%), breast (14 cases, 22.6%) and genitourinary system (11 cases, 17.7%). The black and white parts of each bar represent the number of cases in men and women, respectively.
Table 2.
Frequencies of Cases by Types of Malignancy in Patients with Both Malignancy and Sarcoidosis.
| Primary sites of malignancy |
Number of cases (Female) | Types of malignancy | Number of cases (%) | ||
|---|---|---|---|---|---|
| Oral cavity | 2 | (1) | Tongue cancer | 1 | (1.6%) |
| Cancer of cheek mucosa | 1 | (1.6%) | |||
| Digestive system | 17 | (9) | Stomach cancer | 8 | (12.9%) |
| Esophageal cancer | 4 | (6.5%) | |||
| Colon cancer | 4 | (6.5%) | |||
| Bile duct cancer | 1 | (1.6%) | |||
| Respiratory system | 7 | (4) | Lung cancer | 7 | (11.3%) |
| Skin | 1 | (1) | Skin cancer | 1 | (1.6%) |
| Breast | 14 | (14) | Breast cancer | 14 | (22.6%) |
| Genital system | 11 | (9) | Cervix uteri cancer | 5 | (8.1%) |
| Corpus uteri cancer | 3 | (4.8%) | |||
| Vaginal cancer | 1 | (1.6%) | |||
| Prostate cancer | 1 | (1.6%) | |||
| Extragonadal germ cell tumor | 1 | (1.6%) | |||
| Endocrine system | 4 | (3) | Thyroid cancer | 3 | (4.8%) |
| Neuroendocrine tumor | 1 | (1.6%) | |||
| Urinary system | 3 | (1) | Bladder cancer | 2 | (3.2%) |
| Kidney cancer | 1 | (1.6%) | |||
| Lymphatic system | 2 | (2) | Malignant lymphoma | 2 | (3.2%) |
| Bone marrow | 1 | (1) | Multiple myeloma | 1 | (1.6%) |
| Total | 62 | (45) | Total | 62 | (100%) |
Chronological connection between malignancy and sarcoidosis
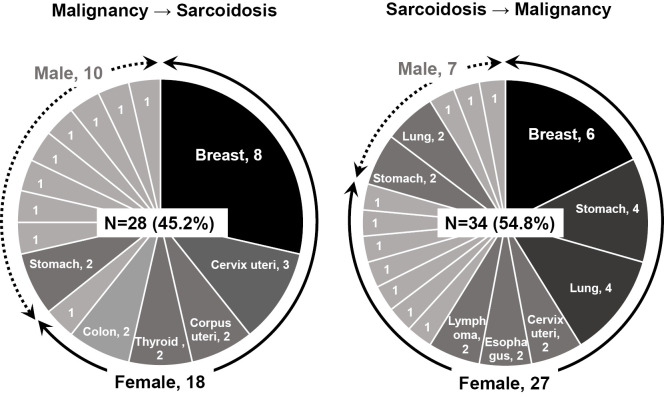
We next assessed the chronological connection between malignancy and sarcoidosis. As shown in Fig. 2, 28 cases (45.2%) were diagnosed with malignant diseases preceding sarcoidosis, and 34 cases (54.8%) were diagnosed with sarcoidosis preceding malignancy. No patient was simultaneously diagnosed with both sarcoidosis and malignancy. Breast cancer was the most prevalent malignancy in both groups (eight cases in malignancy preceding sarcoidosis, six cases in sarcoidosis preceding malignancy). No remarkable features concerning the incidence of malignancy were found among men.
Figure 2.
Frequencies of cases by types of malignancy in patients with both malignancy and sarcoidosis. The left panel shows frequencies of cases with malignancy by primary sites in patients who were diagnosed with malignancy before sarcoidosis, and the right panel shows those values in patients diagnosed with sarcoidosis before malignancy. The continuous line with arrow heads outside the circle graph indicate cases in women, and the dashed line indicates cases in men. The primary sites of malignancy are indicated in the circle graph when more than two cases were included.
Causative association between breast cancer and sarcoidosis
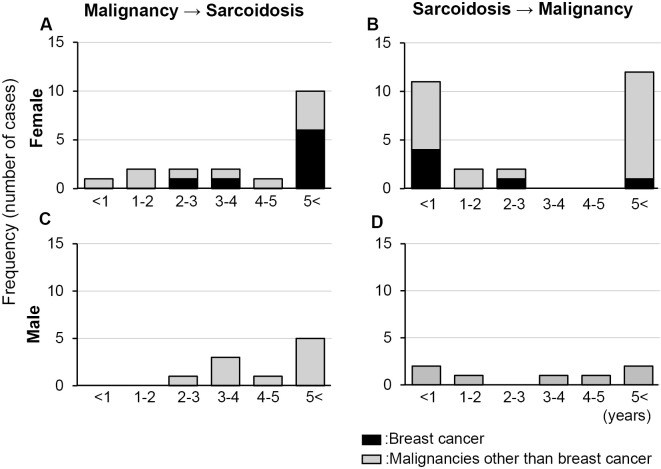
We focused on the clinical features of patients with both sarcoidosis and breast cancer. The time intervals between the diagnosis of breast cancer and sarcoidosis are summarized in Table 3. Among the 8 patients with breast cancer diagnosed before sarcoidosis, as many as 6 (75%) developed sarcoidosis more than 5 years after the diagnosis of breast cancer. In contrast, 4 (66.7%) out of 6 patients with sarcoidosis diagnosed before breast cancer developed breast cancer within 1 year after the diagnosis of sarcoidosis. The distribution of the time intervals between the diagnosis of all malignancies and sarcoidosis in women shows similar features in the case of breast cancer and sarcoidosis (Fig. 3A). Concerning the distribution of the time intervals between sarcoidosis and all malignancies in women (Fig. 3B) or those between both diseases (Fig. 3C, D) in men, no remarkable features was found.
Table 3.
Time Interval between the Diagnoses of Breast Cancer and Sarcoidosis.
| Time interval (years) | Breast cancer → Sarcoidosis, n (%) | Sarcoidosis → Breast cancer, n (%) | ||
|---|---|---|---|---|
| <1 | 0 | (0%) | 4 | (66.7%) |
| 1-2 | 0 | (0%) | 0 | (0%) |
| 2-3 | 1 | (12.5%) | 1 | (16.7%) |
| 3-4 | 1 | (12.5%) | 0 | (0%) |
| 4-5 | 0 | (0%) | 0 | (0%) |
| 5< | 6 | (75.0%) | 1 | (16.7%) |
| Total | 8 | (100%) | 6 | (100%) |
Figure 3.
Distributions of frequencies of cases by each time interval between the diagnoses of malignancy and sarcoidosis. The left panels (A and C) show the frequencies of cases with both malignancy and sarcoidosis by years between the two diagnoses in patients who were diagnosed with malignancy before sarcoidosis, and the right panels (B and D) show those values by years between the diagnoses of sarcoidosis and malignancy in patients who were diagnosed with sarcoidosis before malignancy. The upper panels (A and B) show those values in women, and the lower panels (C and D) show those values in men. The black and gray parts of each bar represent the number of cases with breast cancer and malignancies other than breast cancer, respectively.
The treatment for breast cancer in the eight patients with breast cancer diagnosed before sarcoidosis is summarized in Table 4. The mean age at the diagnosis of breast cancer and sarcoidosis was 46.8±4.4 and 55.6±3.3 years old, respectively. The mean time interval between the diagnoses was 9.0±5.0 years. Notably, all eight cases underwent surgical resection of the breast cancer. Concerning adjuvant treatment after surgery for breast cancer, the number of cases that received radiation therapy, hormone therapy or chemotherapy was 5, 4 and 2, respectively. Chemotherapy treatments for breast cancer include epirubicin hydrochloride and cyclophosphamide but not immune checkpoint inhibitors.
Table 4.
Therapies for the Eight Patients Diagnosed with Breast Cancer before Sarcoidosis.
| Cases | Age at the diagnosis of breast cancer (years old) |
Therapies for breast cancer | Age at the diagnosis of sarcoidosis (years old) |
Time interval (years) | |||
|---|---|---|---|---|---|---|---|
| 1 | 29 | Sur | +Chem | 42 | 13 | ||
| 2 | 34 | Sur | +Rad | +Hor | 45 | 12 | |
| 3 | 40 | Sur | +Rad | 54 | 14 | ||
| 4 | 42 | Sur | +Rad | 51 | 9 | ||
| 5 | 49 | Sur | +Hor | +Chem | 54 | 5 | |
| 6 | 50 | Sur | +Rad | 64 | 14 | ||
| 7 | 62 | Sur | +Hor | 64 | 2 | ||
| 8 | 68 | Sur | +Rad | +Hor | 71 | 3 | |
| Average | 46.8±4.4 | 55.6±3.3 | 9.0±5.0 | ||||
Data are expressed as the mean±standard error.
Sur: surgery, Rad: radiation therapy, Hor: hormone therapy, Chem: chemotherapy
Discussion
In the present study, among 277 patients with sarcoidosis, we found that 52 (18.8%) had a history or coexistence of malignancy, and these patients were older and more likely to be women than the remaining 225 (81.2%) patients without malignancy. This incidence was higher than that described in previous reports [1.2% by Brincker et al. (11), 4.3% by Ungprasert et al. (19) and 14% by Blank et al. (18)]. A literature overview is provided in Table 5 (11,18,19,23,24).
Table 5.
Literature.
| References | Country | Year of Sarcoidosis | No. of patients | No. of patients with malignancy | Breast cancer | Lymphoma | Other malignancies | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| [11] | Denmark | 1962–1971 | 2,544 | 48 | (1.2%) | 4 | (8.3%) | 6 | (12.5%) | 38 | (79.2%) |
| [23] | Sweden | 1964-1994 | 8,541 | 653 | (7.6%) | 87 | (13.3%) | 35 | (5.4%) | 531 | (81.3%) |
| [24] | Sweden | 1964-2004 | 10,037 | 1,045 | (10.4%) | 125 | (12.0%) | 99 | (9.5%) | 821 | (78.6%) |
| [18] | Germany | 1960–2007 | 425 | 61 | (14.0%) | 13 | (21.3%) | 18 | (29.5%) | 30 | (49.2%) |
| [19] | USA | 1976-2013 | 345 | 36 | (4.3%) | 5 | (13.9%) | 6 | (16.7%) | 25 | (69.4%) |
| This report | Japan | 1981-2017 | 277 | 52 | (18.8%) | 14 | (22.6%) | 2 | (3.8%) | 36 | (69.2%) |
These differences among patients with sarcoidosis do not seem surprising, since the clinical features and severity of sarcoidosis vary among ethnic groups (1). In addition, given the unique character of the Japanese national health insurance system and the long follow-up periods, both sarcoidosis and malignancy can be frequently evaluated by close examinations, including chest computed tomography, in every general hospital in Japan. In this study, no patients with sarcoidosis were incidentally diagnosed with malignancy by fluorodeoxyglucose-positron emission tomography. Concerning the smoking habits, we found no significant differences in the smoking status between sarcoidosis patients with and without malignancy (Table 1). Smoking habits are strongly related to the risk of malignancy in the general population, although smoking was reported to likely be associated with a reduced risk of developing sarcoidosis in the ACCESS study (8). A smoking habit seems to have conflicting effects on these two diseases.
The distribution of the patient population of this study does not show a remarkable deviation from that in a previous report (25). Patients with both sarcoidosis and malignancy were predominantly women in our study (Table 1), which is also consistent with other reports (15,26). We do not have a clear explanation, but it seems highly likely that an increase in the number of patients with female-specific cancers, such as breast or uterus cancer, impacts the female-dominated incidence of both sarcoidosis and malignancy (Table 2, Fig. 2). In line with several previous reports (13,26), our study showed that breast cancer is the most prevalent malignant disease in patients with sarcoidosis (Fig. 2). In Japan, breast cancer is the most frequent cancer in women and the number of patients is growing, especially among women in their 40s and 50s (27,28). The incidence of breast cancer by age-standardized rate (cases per 100,000 population) is 61.8 and 79.7 in the world and Japan, respectively (27). In addition, the age-specific incidence in women shows a biphasic pattern, with the first peak at 25-39 years old and the second peak in the 50s and 60s, ages that are known to be susceptible to developing breast cancer (25,27). Indeed, these epidemiological findings are likely to affect the female-dominated incidence of both sarcoidosis and malignancy. Nevertheless, we are curious about the potential causative association between breast cancer and the subsequent development of sarcoidosis.
Brincker and Wilbek reported an increased cancer incidence in patients with respiratory sarcoidosis (11). Bonifazi et al. conducted a systematic review and meta-analysis of observational studies on cancer and sarcoidosis. Their analysis suggested a significant association between sarcoidosis and malignancy (10). In contrast, Ungprasert et al. reported no difference in the prevalence or cumulative incidence of malignancy among patients with sarcoidosis compared to non-sarcoidosis subjects (19). Søgaard et al. reported that, in the case of lung cancer, tonsil cancer and lymphoma, the cancer risk in patients with sarcoidosis was particularly high within the first three months after the diagnosis of sarcoidosis compared to that beyond three months, whereas breast, skin and reproductive system cancers occurred as frequently as expected throughout the observation period for ≥10 years (12). They concluded that the short-term increased risk of cancer in patients with sarcoidosis may be due to increased surveillance or an initial misinterpretation of cancer as sarcoidosis and that the increased long-term risk of some cancers is notable and unlikely to reflect surveillance bias alone. In the present study, as many as 6 (75%) out of 8 patients with breast cancer diagnosed before sarcoidosis eventually developed sarcoidosis more than 5 years after the diagnosis of breast cancer. These findings suggest that the increased frequency of breast cancer before the diagnosis of sarcoidosis was not due to surveillance bias.
Propionibacterium acnes has been reported to be a candidate for the etiology as an environment factor (4,29). P. acnes is an anaerobic bacterium indigenous to the skin and mucosa surfaces and the only microorganism isolated from sarcoid lesions by bacterial culture (29). Genomes of P. acnes were also found in the lymph nodes of sarcoidosis patients (4). In addition, intravenous injection of P. acnes into sensitized rabbits induced massive pulmonary granulomas (30). These findings strongly suggest that P. acnes plays an important role in the pathophysiology of sarcoidosis. In the present study, we showed that breast cancer was the most prevalent malignant disease in patients with sarcoidosis (Table 3), and all patients with breast cancer diagnosed before sarcoidosis underwent surgery (Table 4). We speculate that surgical resection of breast cancer is likely to enable P. acnes to enter the blood vessels or lymph vessels, resulting in the formation of granulomas in the regional draining lymph nodes (31) or even the lung fields. In addition, it is possible that sarcoidosis represents an immune reaction to malignancy (32) and that chemotherapy agents may predispose patients to develop sarcoidosis (17,33). In our study, however, chemotherapy agents appeared unlikely to be the cause of sarcoidosis, as only two of the eight breast cancer patients received chemotherapies (Table 4). In line with our results, Schweitzer et al. also reported that, among 10 patients with a sarcoidosis onset after breast cancer, 6 (60%) were treated with chemotherapy, while 9 (90%) underwent surgery (34). Further studies are necessary to conclude the presence of a causal association between P. acnes through surgical resection of breast cancer and sarcoidosis.
Sarcoidosis treatment with immunosuppressive agents (glucocorticoid, methotrexate, etc.) may induce the development of cancer (35). However, there was no remarkably high incidence of malignancy among the patients with sarcoidosis diagnosed before malignancy, although breast cancer cases were also the most prevalent (18.5%) (Table 3). Sarcoidosis is a systemic inflammatory disease (1), and inflammatory conditions are known to promote or cause malignant disease. Asbestos-related mesothelioma, Helicobacter pylori gastritis-related gastric cancer and chronic hepatitis-related hepatocellular carcinoma are good examples of the interaction between chronic inflammatory conditions and malignancy (36). Tumor necrosis factor (TNF)-α, which plays an important role in the recruitment and maintenance of granuloma (37), is released from the alveolar macrophages of patients with sarcoidosis (38). TNF-α induces inducible nitric oxide synthase and an inducible isoform of cyclooxygenase that generates free radicals, thus causing DNA damage (39). Therefore, it is possible that sarcoidosis is associated with an increased risk for malignant disease in the affected tissue.
The limitations of this study include the small number of cases in a specialty medical center, its retrospective nature and the lack of a control population without sarcoidosis that was followed for the same amount of time. It is also difficult to determine the precise timing of the onset of both sarcoidosis and malignancy because of the naturally latent progression of these diseases. Further prospective studies are necessary to confirm the relationship between cancer and sarcoidosis.
In conclusion, this study showed a higher incidence of patients with both sarcoidosis and malignancy in Japan than in some western countries. Breast cancer is the most prevalent malignant disease among sarcoidosis patients. The high frequency of sarcoidosis after surgical resection of breast cancer may suggest a candidate mechanism for the causative association between malignancy and the development of sarcoidosis.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Muller-Quernheim J. Sarcoidosis. Lancet 383: 1155-1167, 2014. [DOI] [PubMed] [Google Scholar]
- 2. Chen ES, Moller DR. Etiologies of sarcoidosis. Clin Rev Allergy Immunol 49: 6-18, 2015. [DOI] [PubMed] [Google Scholar]
- 3. Gupta D, Agarwal R, Aggarwal AN, Jindal SK. Molecular evidence for the role of mycobacteria in sarcoidosis: a meta-analysis. Eur Respir J 30: 508-516, 2007. [DOI] [PubMed] [Google Scholar]
- 4. Ishige I, Usui Y, Takemura T, Eishi Y. Quantitative PCR of mycobacterial and propionibacterial DNA in lymph nodes of Japanese patients with sarcoidosis. Lancet 354: 120-123, 1999. [DOI] [PubMed] [Google Scholar]
- 5. Prezant DJ, Dhala A, Goldstein A, et al. The incidence, prevalence, and severity of sarcoidosis in New York City firefighters. Chest 116: 1183-1193, 1999. [DOI] [PubMed] [Google Scholar]
- 6. Hena KM, Yip J, Jaber N, et al. Clinical course of sarcoidosis in World Trade Center-exposed firefighters. Chest 153: 114-123, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McDonough C, Gray GC. Risk factors for sarcoidosis hospitalization among U.S. Navy and Marine Corps personnel, 1981 to 1995. Mil Med 165: 630-632, 2000. [PubMed] [Google Scholar]
- 8. Newman LS, Rose CS, Bresnitz EA, et al. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med 170: 1324-1330, 2004. [DOI] [PubMed] [Google Scholar]
- 9. Cohen PR, Kurzrock R. Sarcoidosis and malignancy. Clin Dermatol 25: 326-333, 2007. [DOI] [PubMed] [Google Scholar]
- 10. Bonifazi M, Bravi F, Gasparini S, et al. Sarcoidosis and cancer risk: systematic review and meta-analysis of observational studies. Chest 147: 778-791, 2015. [DOI] [PubMed] [Google Scholar]
- 11. Brincker H, Wilbek E. The incidence of malignant tumours in patients with respiratory sarcoidosis. Br J Cancer 29: 247-251, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Søgaard KK, Svaerke C, Thomsen RW, Norgaard M. Sarcoidosis and subsequent cancer risk: a Danish nationwide cohort study. Eur Respir J 45: 269-272, 2015. [DOI] [PubMed] [Google Scholar]
- 13. Arish N, Kuint R, Sapir E, et al. Characteristics of sarcoidosis in patients with previous malignancy: causality or coincidence? Respiration 93: 247-252, 2017. [DOI] [PubMed] [Google Scholar]
- 14. Paparel P, Devonec M, Perrin P, et al. Association between sarcoidosis and testicular carcinoma: a diagnostic pitfall. Sarcoidosis Vasc Diffuse Lung Dis 24: 95-101, 2007. [PubMed] [Google Scholar]
- 15. Suen JS, Forse MS, Hyland RH, Chan CK. The malignancy-sarcoidosis syndrome. Chest 98: 1300-1302, 1990. [DOI] [PubMed] [Google Scholar]
- 16. Massaguer S, Sanchez M, Castel T. Mediastinal sarcoidosis induced by high-dose alpha-2-interferon therapy in a patient with malignant melanoma. Eur Radiol 14: 1716-1717, 2004. [DOI] [PubMed] [Google Scholar]
- 17. Murphy KP, Kennedy MP, Barry JE, O'Regan KN, Power DG. New-onset mediastinal and central nervous system sarcoidosis in a patient with metastatic melanoma undergoing CTLA4 monoclonal antibody treatment. Oncol Res Treat 37: 351-353, 2014. [DOI] [PubMed] [Google Scholar]
- 18. Blank N, Lorenz HM, Ho AD, Witzens-Harig M. Sarcoidosis and the occurrence of malignant diseases. Rheumatol Int 34: 1433-1439, 2014. [DOI] [PubMed] [Google Scholar]
- 19. Ungprasert P, Crowson CS, Matteson EL. Risk of malignancy among patients with sarcoidosis: a population-based cohort study. Arthritis Care Res 69: 46-50, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ungprasert P, Srivali N, Wijarnpreecha K, Thongprayoon C, Cheungpasitporn W, Knight EL. Is the incidence of malignancy increased in patients with sarcoidosis? a systematic review and meta-analysis. Respirology 19: 993-998, 2014. [DOI] [PubMed] [Google Scholar]
- 21. Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis 16: 149-173, 1999. [PubMed] [Google Scholar]
- 22. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 26: 319-338, 2005. [DOI] [PubMed] [Google Scholar]
- 23. Askling J, Grunewald J, Eklund A, Hillerdal G, Ekbom A. Increased risk for cancer following sarcoidosis. Am J Respir Crit Care Med 160: 1668-1672, 1999. [DOI] [PubMed] [Google Scholar]
- 24. Ji J, Shu X, Li X, Sundquist K, Sundquist J, Hemminki K. Cancer risk in hospitalized sarcoidosis patients: a follow-up study in Sweden. Ann Oncol 20: 1121-1126, 2009. [DOI] [PubMed] [Google Scholar]
- 25. Morimoto T, Azuma A, Abe S, et al. Epidemiology of sarcoidosis in Japan. Eur Respir J 31: 372-379, 2008. [DOI] [PubMed] [Google Scholar]
- 26. Hunt BM, Vallieres E, Buduhan G, Aye R, Louie B. Sarcoidosis as a benign cause of lymphadenopathy in cancer patients. Am J Surg 197: 629-632, 2009. [DOI] [PubMed] [Google Scholar]
- 27. Hori M, Matsuda T, Shibata A, Katanoda K, Sobue T, Nishimoto H. Cancer incidence and incidence rates in Japan in 2009: a study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol 45: 884-891, 2015. [DOI] [PubMed] [Google Scholar]
- 28. Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 391: 1023-1075, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eishi Y. Etiologic link between sarcoidosis and Propionibacterium acnes. Respir Investig 51: 56-68, 2013. [DOI] [PubMed] [Google Scholar]
- 30. Ichiyasu H, Suga M, Iyonaga K, Ando M. Role of monocyte chemoattractant protein-1 in Propionibacterium acnes-induced pulmonary granulomatosis. Microsc Res Tech 53: 288-297, 2001. [DOI] [PubMed] [Google Scholar]
- 31. Butt S, Alzebdeh R, Kable TD, Soubani AO. Non-caseating granulomas in patients after the diagnosis of cancer: clinical characteristics and outcome. Sarcoidosis Vasc Diffuse Lung Dis 28: 44-9, 2011. [PubMed] [Google Scholar]
- 32. Reich JM. Neoplasia in the etiology of sarcoidosis. Eur J Intern Med 17: 81-87, 2006. [DOI] [PubMed] [Google Scholar]
- 33. Buss G, Cattin V, Spring P, Malinverni R, Gilliet M. Two cases of interferon-alpha-induced sarcoidosis Koebnerized along venous drainage lines: new pathogenic insights and review of the literature of interferon-induced sarcoidosis. Dermatology 226: 289-297, 2013. [DOI] [PubMed] [Google Scholar]
- 34. Schweitzer MD, Salamo O, Holt G, Donna E, Mirsaeidi M. Sarcoidosis onset after breast cancer; a potential association. Eur J Intern Med 44: e11-e12, 2017. [DOI] [PubMed] [Google Scholar]
- 35. Sorensen HT, Mellemkjaer L, Nielsen GL, Baron JA, Olsen JH, Karagas MR. Skin cancers and non-hodgkin lymphoma among users of systemic glucocorticoids: a population-based cohort study. J Natl Cancer Inst 96: 709-711, 2004. [DOI] [PubMed] [Google Scholar]
- 36. Candido J, Hagemann T. Cancer-related inflammation. J Clin Immunol 33: S79-S84, 2013. [DOI] [PubMed] [Google Scholar]
- 37. Kunkel SL, Chensue SW, Strieter RM, Lynch JP, Remick DG. Cellular and molecular aspects of granulomatous inflammation. Am J Respir Cell Mol Biol 1: 439-447, 1989. [DOI] [PubMed] [Google Scholar]
- 38. Fehrenbach H, Zissel G, Goldmann T, et al. Alveolar macrophages are the main source for tumour necrosis factor-alpha in patients with sarcoidosis. Eur Respir J 21: 421-428, 2003. [DOI] [PubMed] [Google Scholar]
- 39. Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer 3: 276-285, 2003. [DOI] [PubMed] [Google Scholar]