Abstract
Background and aims
Few studies have reported on the use of continuous glucose monitoring (CGM) during the Covid-19 pandemic. We aimed to examine glycemic control metrics using flash glucose monitoring during insulin treatment and the clinical outcome in hospitalized patients with COVID-19.
Methods
Prospective, single-center cohort of adult patients diagnosed with type 2 diabetes or hyperglycemia and COVID-19 infection treated with basal bolus insulin regimen. Glycemic control was assessed with the use of intermittent Freestyle Libre flash glucose monitoring during the hospital stay. Outcome of interest were time in range [TIR], time above [TAR] and below [TBR] range, glycemic variability [coefficient of variation [% CV]), and differences in a composite of complications including ICU admission, acute respiratory distress syndrome (ARDS) and acute kidney injury.
Results
A total of 60 patients were included (44 known diabetes and 16 new onset hyperglycemia). In total 190,080 data points of CGM were available, of which 72.5% of values were within the target area [TIR (70–180 mg/dL)], 22% TAR (>180 mg/dL), and 3% were TBR (<70 mg/dL). During treatment, the coefficient of variation (% CV) was 30%. There were no association with TIR, but patients with TAR >180 mg/dl had higher rates of a composite of complications (22.5% vs 16%, p = 0.04).
Conclusions
Basal bolus insulin regimen was safe and effective in achieving inpatient glycemic control in most patients with COVID-19. The association between TAR and complications indicates the need for improved inpatient glycemic control in hospitalized patients with COVID-19.
Keywords: Basal bolus, Flash glucose monitoring, COVID-19, Type 2 diabetes, Inpatient
Abbreviations: A1c, glycosylated hemoglobin; AKI, acute kidney injury; AMI, acute myocardial infarction; ARDS, acute respiratory distress syndrome; BMI, body mass index; CV%, coefficient of variation; CGM, continuous glucose monitoring; GMI, glucose management indicator; HBP, high blood pressure; ICU, intensive care unit; IQR, interquartile range; LDH, lactate dehydrogenase; RCP, reactive c protein; RT-PCR, real time polymerase chain reaction; TAR, time above the range; TDDI, total daily dose of insulin; TBR, time below the range; TIR, time in range; WP, waist perimeter
1. Introduction
Diabetes is one of the most prevalent chronic conditions in the world, affecting almost 460 million people [1]. Large trials have shown that diabetes is highly prevalent in COVID-19 patients, and confers a worse prognosis [[2], [3], [4]]. A recent meta-analysis reported that hyperglycemia and diabetes are associated with higher mortality (RR 2.12 [1.44, 3.11], p < 0.001), severe COVID-19 (RR 2.45 [1.79, 3.35], p < 0.001), acute respiratory distress syndrome (ARDS) (RR 4.64 [1.86, 11.58], p = 0.001), and disease progression (RR 3.31 [1.08, 10.14], p = 0.04) [4](5) compared to patients without hyperglycemia.
During the COVID-19 pandemic, critical shortages of personal protective equipment (PPE) has resulted in increasing hospital use of CGM to minimize bedside encounters [6,7]. The result of preliminary studies have indicated that CGM readings correlates well with point of care capillary glucose testing in hospitalized patients with COVID-19, and provides an attractive option to for the management of general medicine and surgery patients with diabetes [8,9].
Several randomized controlled trials have reported that treatment with a basal-bolus regimen results in significantly lower mean daily blood glucose (BG) with a higher percentage of BG within target range in patients with type 2 diabetes [10,11]. No previous studies; however, have reported on the glycemic response to basal bolus insulin therapy in patients with COVID-19. Accordingly, we conducted a prospective study to examine the glycemic control metrics using flash glucose monitoring including time in range [TIR], time above [TAR] and below [TBR] range, coefficient of variation and glucose management indicator during basal bolus insulin treatment, as well as we report on the frequency of a composite of complications including ICU admission, acute respiratory distress syndrome (ARDS) and acute kidney injury in patients with diabetes and COVID-19 infection.
2. Methods
We conducted a pilot, prospective, single center cohort study in patients with diabetes or hyperglycemia treated with basal bolus insulin regimen at the Hospital Universitario San Ignacio (HUSI) from July 1 to September 30, 2020. We included patients older than 18 years with type 2 diabetes or non-diabetics with two point of care (POC) capillary BG greater than 180 mg/dl, and with a confirmed diagnosis of COVID-19. Patients with active neoplasms, drug dependence, poorly controlled psychiatric illness, pregnancy, or those using continuous real-time glucose monitoring prior to admission were excluded. The protocol was approved by the ethics and research committee of the Pontificia Universidad Javeriana and the Hospital Universitario San Ignacio; all patients filled out informed consent.
After patients accepted to participate, an interstitial glucose sensor was implanted for intermittent glucose monitoring (Abbott Diabetes Care, Alameda, CA, USA). Education was provided to patients and the nursing staff, and were asked to take at least 3 scans a day. The CGM were cover with lead apron during diagnostic radiology images. Demographic variables and clinical outcomes during hospitalization were collected through the electronic medical record. According to the institutional protocol, the total daily dose of insulin (TDD) was calculated as 0.3–0.5 units per kilogram according to age, blood glucose at admission and creatinine [10]. Half of the total dose was given as basal insulin and the other half as prandial doses. The doses were adjusted for a glycemic goal of 100–140 mg/dl fasting and 140–180 mg/dl postprandial. In patients with a fasting glucose of 140–180 mg/dL, the daily basal insulin dose was increased by 10%, whereas in those with a fasting glucose >180 mg/dL, it was increased by 20%. CGM data was downloaded using the Libreview online platform. Critically ill patients were switched to intravenous insulin according ICU guidelines.
A confirmed case of COVID-19 infection was defined by a positive reverse transcription polymerase chain reaction (RT-PCR) for SARS-CoV-2. De novo diabetes was defined by hyperglycemia >180 mg/dl and HbA1c ≥ 6.5% in patients who had no previous history of the disease. Acute kidney injury (AKI) was defined as an increase in serum creatinine level by 0.3 mg/dL from the admission value or an increase in serum creatinine to 1.5 times baseline. Acute respiratory distress (ARDS) was defined by hypoxemia (PaO2/FiO2 <300) and bilateral opacities on chest x-ray not explained by cardiac failure or fluid overload.
The metrics provided by the CGM included time in range (TIR), which represented the percentage of time between 70 and 180 mg/dl, time below range (TBR, < 70 mg/dl) and the time above range (TAR, > 180 mg/dl) as the percentage of time outside these values. Clinically significant hypoglycemia was defined as a glucose <54 mg/dL. Values of CV >36% were defined as high variability; the coefficient of variation (%CV) results from the division between the standard deviation and the average glucose multiplied by 100.
The quantitative variables were described through means and standard deviations, or medians and interquartile ranges, as appropriate. Comparison were performed using a t-test or Mann Whitney test according with variable distribution. For the exploratory bivariate association analysis, a logistic regression model was used. ROC curves were performed to assess the discrimination capacity of each variable as a predictor of adverse outcomes. A composite outcome of acute kidney injury, admission to the ICU, and acute respiratory distress syndrome was established.
3. Results
This prospective cohort included a total of sixty patients with type 2 diabetes or hyperglycemia and Covid-19 infection. Of them, 44 had known diabetes and 16 patients had new onset hyperglycemia. A total of 190,080 data points of CGM were available. The clinical and sociodemographic characteristics are shown in Table 1 . The average age was 60.2 ± 14.1 years. Almost half of the patients had de novo diabetes (no previous history of diabetes, glucose > 180 mg/dl and HbA1c >6.5%). Most patients had an HbA1c greater than 7% on admission (58.9%). Almost half of the patients had lymphocyte counts below 1000 and PAFI (relationship between the alveolar-arterial oxygen gradient and alveolar oxygen gradient) below 200 as severity markers of COVID-19. The median length of hospitalization was 11 days (IQR 8–16).
Table 1.
Baseline clinical and sociodemographic characteristics of patients with COVID-19 diagnosis and history of diabetes or hyperglycemia.
| Variable | n = 60 |
|---|---|
| Age, mean (SD) | 60,2 [1,14] |
| Sex, male, n (%) | 31 (51,7) |
| Diabetes, n (%) | 44 (78,57) |
| Hyperglycemia without diabetes, n (%) | 16 (21,43) |
| BMI, median (IQR) | 27,9 (24,0–30,7) |
| Waist Circumference, median (IQR) | 98 (91–108) |
| Comorbidities, n (%) | |
| HBP | 32 (53,3) |
| Coronary artery disease | 4 [6,7] |
| Treatment prior to admission, n (%) | |
| Metformin | 12 (20) |
| Sulphonylurea | 3 [5] |
| SGLT2 inhibitor | 0 (0) |
| GLP1 agonist | 1 (1,7) |
| Insulin | 15 (25) |
| Previous Macrovascular complications n (%) | |
| AMI | 3 [5] |
| Stroke | 3 [5] |
| Microvascular complications n (%) | |
| Retinopathy | 1 [1,7] |
| Nephropathy | 4 [6,7] |
| Diabetic foot | 1 [1,7] |
| Length of hospital stay, days, median (IQR) | 11 [8–16] |
| Labs at admission n (%) | |
| HbA1c > 7% | 33 (58,9) |
| Lymphocytes < 1000 | 28 (46,7) |
| RCP > 20 | 15 (27,8) |
| LDH > 400 | 22 (38,6) |
| PAFI < 200 | 24 (40) |
| Use of steroids n (%) | |
| < 10 days | 28 (50) |
AMI: Acute myocardial infarction, BMI: Body mass index (Kg/m2), HBP: High blood pressure, LDH: Lactate dehydrogenase, RCP: reactive c protein, WP: waist perimeter, PAFI: relationship between the alveolar-arterial oxygen gradient and alveolar oxygen gradient.
The mean admission blood glucose and HbA1c was 9,03%. The mean BG during the hospital stay was 146,9 mg/dL. During treatment, the median time in range by CGM was 72.5% (IQR 54–87.5). The hypoglycemic times (TBR) of the different groups of patients were low (Median 3%, IQR 1–6). The median of coefficient of variation was 30.0 (IQR 25.8–37.8). Average device readings per patient was 5,7 time per day.
Of the 60 patients included, 21.6% required ICU admission, 25% had a diagnosis of ARDS and 21.6% had AKI. The composite outcome of complications was reported in 36.6% of patients. There were no differences between the values of TIR, TAR, TBR, CV or GMI in patients with or without admission to ICU, ARDS or AKI (Table 2 ), or with the composite of complications (Table 3 ). There were not significant association between the composite outcome and TIR (OR: 0.99, CI 95% 0.97–1.02, p: 0.72), TAR (OR: 1.01, CI 95% 0.99–1.04, p:0.19), TBR (OR: 0.89, CI 95% 0.79–1.01, p: 0.07), %CV (OR: 0.96, CI 95% 0.89–1.03, p:0.24) or GMI (OR:1.38, CI 95% 0.72–2.65, p:0.32).
Table 2.
Glycemic control metrics in patients with or without complications for COVID-19.
| General |
Hyperglycemia without diabetes |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICU (n = 13) | No ICU (n:47) | p | ARDS (n = 15) | No ARDS (n = 45) | p | AKI (n = 13) | No AKI (n = 47) | p | ICU (n = 5) | No ICU (n = 7) | p | ARDS (n = 4) | No ARDS (n = 8) | p | AKI (n = 3) | No AKI (n = 9) | p | |
| %TIR, median (IQR) | 73 (62–86) | 72 (54–89) | .68 | 73 (38–90) | 72 (55–85) | .95 | 68 (54–86) | 73 (54–90) | .77 | 86 (80–90) | 75 (56–95) | .56 | 88 (77–94) | 77,5 (64,5–95) | .49 | 73 (68–86) | 90 (75–95) | .30 |
| %TAR, median (IQR) | 18 (6–29) | 20 (4–46) | .71 | 18 (6–61) | 20 (4–40) | .47 | 23 (12–42) | 14 (3–40) | .22 | 6 (0–12) | 3 (0–5) | .67 | 9 [[3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]] | 2 (0–4,5) | .26 | 18 (12–27) | 1 (0–4) | .01 |
| %TBR, median (IQR) | 4 (0–2) | 2 (0–5) | .27 | 2 [[1], [2], [3], [4]] | 3 [[1], [2], [3], [4], [5], [6], [7]] | .49 | 2 (0–3) | 4 [[1], [2], [3], [4], [5], [6], [7]] | .09 | 4 [[2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14]] | 5 (1–43) | 1 | 3 [[2], [3], [4], [5], [6], [7], [8], [9]] | 12,5 (1–31,5) | .60 | 2 (0–14) | 5 (2–20) | .26 |
| CV%, mean (SD) | 33,4 (8,41) | 31,1 (8,24) | .39 | 31,2 (8,49) | 31,8 (8,27) | .81 | 32,1 (7,68) | 31,5 (8,47) | .82 | 29,7 (5,51) | 30,15 (7,73) | .92 | 30,1 (6,27) | 29,7 (6,76) | .92 | 33,2 (2,54) | 28,9 (6,67) | .42 |
| GMI, mean (SD) | 6,81 (1,01) | 6,88 (0,98) | .84 | 7,16 (1,20) | 6,77 (0,91) | .28 | 6,88 (0,88) | 6,86 (1,02) | .95 | 5,80 | 6,02 | .55 | 5,74 (0,55) | 6,2 (0,17) | .22 | 6,30 (0) | 5,78 (0,50) | .21 |
Table 3.
Glycemic control metrics in patients with or without the composite of complications.
| General |
Hyperglycemia without diabetes |
|||||
|---|---|---|---|---|---|---|
| No outcome (n = 38) | Compound outcome (n = 22) | p | No outcome (n = 7) | Compound outcome (n = 5) | p | |
| %TIR, median (IQR) | 71,5 (55–85) | 73 (54–90) | .92 | 80 (56–95) | 86 (73–90) | .80 |
| %TAR, median (IQR) | 16 (3–34) | 22,5 (6–46) | .22 | 16 (3–34) | 22,5 (6–46) | .04 |
| %TBR, median (IQR) | 5 [[1], [2], [3], [4], [5], [6], [7], [8]] | 2 (0–3) | .01 | 20 (1–43) | 2 [[2], [3], [4]] | .16 |
| %CV, mean (SD) | 32,6 (8,51) | 29,9 (7,69) | .24 | 29,72 (6,76) | 30,15 (6,27) | .92 |
| GMI, mean (SD) | 6,75 (0,94) | 7,06 (1,05) | .33 | 5,74 (0,55) | 6,20 (0,17) | .22 |
When a subgroup analysis was done for patients with hyperglycemia, a higher TAR was found in patients with AKI (18 vs 1%, p = 0.01), and in those with the composite outcome (22.5 vs 16%, p = 0,04) (Table 3).
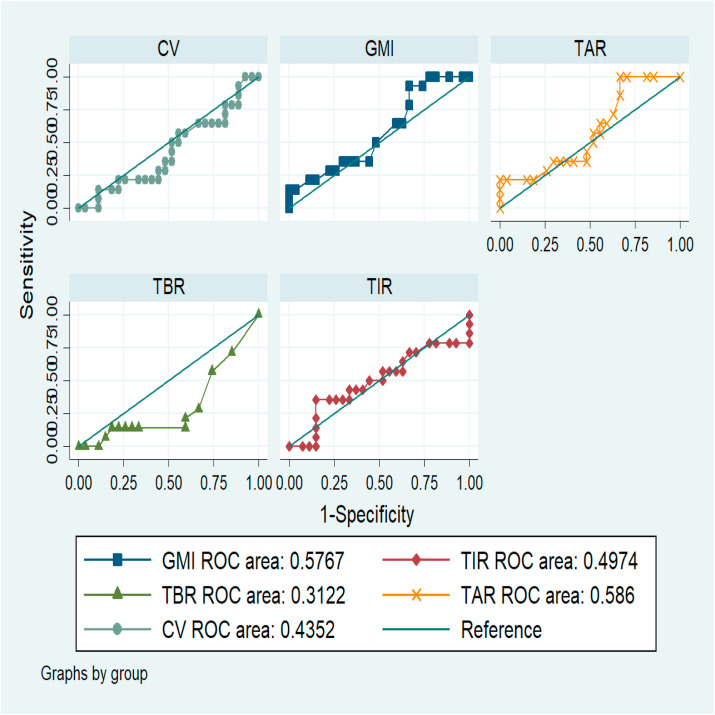
Fig. 1 presents the ROC curves describing the discrimination capacity of glycemic control variables as a predictor of adverse outcomes. For the GMI the AUC was 0.57 (95% CI 0.39, 0.76), TIR 0.49 (95% CI 0.29, 0.70), TBR 0.31 (95% CI 0.13, 0.48), TAR 0.58 (95% CI 0.40, 0.77) and % CV 0.43 (95% CI 0.24, 0.62). None of them presented an adequate discrimination capacity to predict adverse outcomes.
Fig. 1.
ROC curves describing the discrimination capacity of glycemic control variables as a predictor of adverse outcomes.
4. Discussion
This is the first study that describes the glycemic response to basal bolus insulin using flash glucose monitoring in patients with diabetes or hyperglycemia hospitalized with COVID-19. The results show that most patients treated with our standard hospital hyperglycemia management protocol improved their metabolic control, reaching times in range, low glycemic variability with low rates of hypoglycemia similar to previous studies in general non-COVID populations [12]. We found no association between adverse outcomes and poor glycemic control in patients with known diabetes, however, patients without a history of diabetes, a statistically significant difference was found in the composite of complications and time above range.
The time in range between 70 and 180 mg/dl was achieved in 72.5% of values and 22% of values were above range >180 mg/dL. The frequency of hypoglycemia was 3% (<70 mg/dL). The percentages of the coefficient of variation indicate low variability, which is positive given its relationship with hypoglycemia. Similar to our findings, a study conducted by Gómez et al. [12] in non-COVID patients hospitalized using a similar treatment protocol in the general ward reported a TIR in 89% of readings, with very low incidences of clinically significant hypoglycemia are reachable. In addition, we observed that in patients admitted to the ICU due to COVID-19 complications experienced good glycemic control, with a level of hypoglycemia similar to that reported in a non-COVID ICU study from the Hospital Universitario San Ignacio, that showed 7.5% of patients with hypoglycemia events <70 mg/dL after cardiovascular surgery [13].
Among patients with a known history of diabetes, we found no association between glycemic metrics by CGM (TIR, TAR, TBR, glycemic variability) and the rate of complications. In contrast, a clear relationship was found between percentages of TAR (hyperglycemia >180 mg/dL) and the development of complications in patients without a history of diabetes, in particular acute kidney injury. Previous report by Zhang et al. also found significant higher rates of mechanical ventilation, admission in ICU and death in patients with new-onset hyperglycemia without diabetes compared to normoglycemia patients [14]. Likewise, Copelli et al. found higher mortality in hyperglycemic patients without diabetes compared with normoglycemia, with hyperglycemia on admission been an independent predictor of mortality after the multiple adjustments [15]. Hyperglycemia is common in patients with COVID-19 infection [16] and reported in 10–40% of patients without a previous diagnosis of diabetes [17]. Several factors may contribute to hyperglycemia in our patient population including an average age of 60 years, the presence of overweight and high waist circumferences, and the use of steroid therapy. In addition, the metabolic stress of acute illness and the tropism of SARS-Cov-2 to the beta cells of the pancreas through ACE2 receptors, which could generate apoptosis of beta cells and insulinopenia [18].
The main strength of the study is the evaluation of glycemic control of hospitalized patients using the flash glucose monitoring. There are no previous trials in the literature showing the association between metrics of glycemic control including TIR, TBR and %CV and adverse outcome in patients with diabetes and hyperglycemia and COVID-19. Most of the patients had an adequate use of the device, which allowed obtaining enough data for the calculation of the metrics. Similarly, the hospital stay of the patients was generally long, which made it possible to have a good amount of data for a reliable analysis. The main weakness of the study is the small sample size of this pilot study. The sample size limits the power of the present study to find associations between COVID-19 metrics and complications, however the confidence intervals were narrow enough to state that if there is a relationship between glycemic control and complications, this association is not of great magnitude.
5. Conclusion
Our results indicate that most patients hospitalized for COVID-19 with diabetes and hyperglycemia had a good response to a standard basal bolus insulin regimen. Glycemia metrics using CGM in our study was similar to TIR, TAR, TBR and glycemic variability reported in previous non-COVID patients with diabetes. In addition, our results suggest an association between TAR the development of complications in patients with hyperglycemia without a history of diabetes. The association between TAR and complications indicates the need for close glucose monitoring and improved glycemic control in hospitalized patients with diabetes and COVID-19.
Declaration of competing interest
AMG reports speaker fees from Novo Nordisk, Elli Lilly, Boehringer Ingelheim, Abbott and Medtronic. DCH reports speaker fees from Novo Nordisk, Medtronic and Abbott.
References
- 1.Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 2.Wu Z., Tang Y., Cheng Q. Diabetes increases the mortality of patients with COVID-19: a meta-analysis. Acta Diabetol. 2020:1–6. doi: 10.1007/s00592-020-01546-0. https://search.proquest.com/docview/2417402805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantovani A., Byrne C.D., Zheng M., Targher G. Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: a meta-analysis of observational studies. Nutr Metabol Cardiovasc Dis. 2020;30(8):1236–1248. doi: 10.1016/j.numecd.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia – a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr: Clin Res Rev. 2020;14(4):395–403. doi: 10.1016/j.dsx.2020.04.018. http://www.sciencedirect.com/science/article/pii/S1871402120300837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang S., Ma P., Zhang S. Fasting blood glucose at admission is an independent predictor for 28-day mortality in patients with COVID-19 without previous diagnosis of diabetes: a multi-centre retrospective study. Diabetologia. 2020;63(10):2102–2111. doi: 10.1007/s00125-020-05209-1. https://search.proquest.com/docview/2440541561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galindo R.J., Aleppo G., Klonoff D.C. Implementation of continuous glucose monitoring in the hospital: emergent considerations for remote glucose monitoring during the COVID-19 pandemic. J Diabetes Sci Technol. 2020;14(4):822–832. doi: 10.1177/1932296820932903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gujral U.P., Johnson L., Nielsen J. Preparedness cycle to address transitions in diabetes care during the COVID-19 pandemic and future outbreaks. BMJ Open Diabetes Res Care. 2020;8(1) doi: 10.1136/bmjdrc-2020-001520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reutrakul S., Genco M., Salinas H. Feasibility of inpatient continuous glucose monitoring during the COVID-19 pandemic: early experience. Diabetes Care. 2020;43(10):e137–e138. doi: 10.2337/dc20-1503. https://search.proquest.com/docview/2450787945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrhardt Nicole, Hirsch Irl B. The impact of COVID-19 on CGM use in the hospital. Diabetes Care. 2020;43(11):2628–2630. doi: 10.2337/dci20-0046. https://search.proquest.com/docview/2462185542 [DOI] [PubMed] [Google Scholar]
- 10.Umpierrez G.E., Smiley D., Zisman A. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial) Diabetes Care. 2007;30(9):2181–2186. doi: 10.2337/dc07-0295. https://search.datacite.org/works/10.2337/dc07-0295 [DOI] [PubMed] [Google Scholar]
- 11.Umpierrez G.E., Smiley D., Jacobs S. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery) Diabetes Care. 2011;34(2):256–261. doi: 10.2337/dc10-1407. https://search.datacite.org/works/10.2337/dc10-1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gómez A.M., Imitola Madero A., Henao Carrillo D.C. Hypoglycemia incidence and factors associated in a cohort of patients with type 2 diabetes hospitalized in general ward treated with basal bolus insulin regimen assessed by continuous glucose monitoring. J Diabetes Sci Technol. 2019;14(2) doi: 10.1177/1932296818823720. https://search.datacite.org/works/10.1177/1932296818823720 193229681882372-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gómez A.M., Pérez Cely J.A., Muñoz Velandia O.M. Factors associated with hypoglycemia in cardiovascular surgery. Diabetes Metab Syndr Clin Res Rev. 2019;13(1):420–423. doi: 10.1016/j.dsx.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y., Li H., Zhang J. The clinical characteristics and outcomes of diabetes mellitus and secondary hyperglycaemia patients with coronavirus disease 2019: a single-center, retrospective, observational study in wuhan. Diabetes Obes Metabol. 2020 doi: 10.1111/dom.14086. https://www.ncbi.nlm.nih.gov/pubmed/32406594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coppelli A., Giannarelli R., Aragona M. Hyperglycemia at hospital admission is associated with severity of the prognosis in patients hospitalized for COVID-19: the pisa COVID-19 study. Diabetes Care. 2020;43(10):2345–2348. doi: 10.2337/dc20-1380. https://search.proquest.com/docview/2450792833 [DOI] [PubMed] [Google Scholar]
- 16.Sachdeva S., Desai R., Gupta U., Prakash A., Jain A., Aggarwal A. SN comprehensive clinical medicine. 2020. Admission hyperglycemia in non-diabetics predicts mortality and disease severity in COVID-19: a pooled analysis and meta-summary of literature; pp. 1–6.https://search.proquest.com/docview/2452096352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bode B., Garrett V., Messler J. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020;14(4):813–821. doi: 10.1177/1932296820924469. https://journals.sagepub.com/doi/full/10.1177/1932296820924469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Apicella M., Campopiano M.C., Mantuano M., Mazoni L., Coppelli A., Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. The lancet. Diabetes Endocrinol. 2020;8(9):782–792. doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]