Abstract
Multiple neurological problems have been reported in coronavirus disease-2019 (COVID-19) patients because severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) likely spreads to the central nervous system (CNS) via olfactory nerves or through the subarachnoid space along olfactory nerves into the brain’s cerebrospinal fluid and then into the brain’s interstitial space. We hypothesize that SARS-CoV-2 enters the subfornical organ (SFO) through the above routes and the circulating blood since circumventricular organs (CVOs) such as the SFO lack the blood-brain barrier, and infection of the SFO causes dysfunction of the hypothalamic paraventricular nucleus (PVN) and supraoptic nucleus (SON), leading to hydroelectrolytic disorder. SARS-CoV-2 can readily enter SFO-PVN-SON neurons because these neurons express angiotensin-converting enzyme-2 receptors and proteolytic viral activators, which likely leads to neurodegeneration or neuroinflammation in these regions. Considering the pivotal role of SFO-PVN-SON circuitry in modulating hydroelectrolyte balance, SARS-CoV-2 infection in these regions could disrupt the neuroendocrine control of hydromineral homeostasis. This review proposes mechanisms by which SARS-CoV-2 infection of the SFO-PVN-SON pathway leads to hydroelectrolytic disorder in COVID-19 patients.
Keywords: SARS-CoV-2, COVID-19, Brain, Neuroinvasion, Hydroelectrolyte balance, Hypokalemia
1. Introduction
In late 2019, a respiratory illness with severe pneumonia cases of unknown etiology occurred in Wuhan, China, which spread rapidly across the world (Huang et al., 2020). Soon after, it was discovered that a new coronavirus, named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is the cause of this illness (Gorbalenya et al., 2020). Currently, the coronavirus disease-2019 (COVID‐19) has infected people in virtually all countries of the world, with over 100 million confirmed cases and >2.2 million confirmed deaths (worldometers.info and World Health Organization, 2020), and this pandemic has drastically affected public health and people’s lives globally (Kupferschmidt and Cohen, 2020).
COVID-19 patients can be asymptomatic; however, SARS-CoV-2 infection can also promote clinical manifestations ranging from mild to severe symptoms. The symptoms frequently reported comprise fever (∼70%), dry cough (59-76%), and myalgia (38-69%) or fatigue (14-70%), in contrast to the less common symptoms such as sputum production (28-33.7%), hemoptysis (0.9-5%), headache (6-13%), diarrhea (3-10%) and vomiting (3-5%) (Guan et al., 2020; Huang et al., 2020; Wang et al., 2020; Yang et al., 2020). Besides, some cases of COVID-19 have presented with meningitis or encephalitis (6.1%) and Guillain-Barré syndrome (1.4%) (Correia et al., 2020; Moriguchi et al., 2020). After the first week of disease onset, patients can develop dyspnea and acute respiratory distress syndrome (ARDS), leading to intensive care unit (ICU) admission. Due to severe respiratory and hematological problems resulting from ARDS, death occurs in a significant percentage of ICU admitted patients (Huang et al., 2020).
The SARS-CoV-2 infection can lead to multiple central nervous system (CNS)-related symptoms, including headache, dizziness, convulsions, febrile seizures, and encephalitis (Asadi-Pooya and Simani, 2020). Furthermore, COVID-19 patients have experienced taste and smell dysfunction (Pallanti, 2020; Spinato et al., 2020; Xydakis et al., 2020). Additionally, some studies showed that SARS-CoV-2 infection is associated with a high prevalence of hypokalemia (Barkas et al., 2020; Chen et al., 2020; Mabillard et al., 2020; Moreno-P et al., 2020). Severe (18%, <3 mmol/L) and mild (37%, 3–3.5 mmol/L) low plasma potassium (K+) levels were reported in 55% of COVID-19 patients (Chen et al., 2020). Notably, the degree of hypokalemia has been strongly associated with the severity of COVID-19 and a high mortality rate. In addition, COVID-19 patients are susceptible to pro-arrhythmic events related to electrolyte imbalance (Wu et al., 2020).
SARS-CoV-2 likely reaches the CNS via olfactory nerves into the olfactory bulb or through the subarachnoid space along olfactory nerves into the brain’s cerebrospinal fluid compartment and then into the brain’s interstitial space. We hypothesize that SARS-CoV-2 enters the subfornical organ (SFO) through the above routes as well as the circulating blood since circumventricular organs (CVOs) such as the SFO lack the blood-brain barrier, and infection of the SFO causes dysfunction of the hypothalamic paraventricular nucleus (PVN) and supraoptic nucleus (SON), leading to hydroelectrolytic disorder, such as hypokalemia. Given the critical role of SFO, PVN, and SON circuitry in modulating hydroelectrolyte balance, we propose that SARS-CoV-2 infection of SFO-PVN-SON disrupts the neuroendocrine control of hydromineral homeostasis in COVID-19 patients. This review confers the neuroendocrine control of hydroelectrolytic balance, the current evidence for different routes by which SARS-CoV-2 invades the CNS, and suggests mechanisms by which the SFO-PVN-SON pathway dysfunction after SARS-CoV-2 infection results in hydroelectrolytic disorder in COVID-19 patients.
2. Neuroendocrine control of hydroelectrolytic balance
A well-vascularized SFO is a sensory CVO, responsible for detecting peripheral circulating signals involved in the regulation of cardiovascular processes and fluid and blood pressure balance by influencing the release of specific hormones such as angiotensin and vasopressin (Black et al., 2018; Coble et al., 2015, 2014; Ferguson and Bains, 1996; Ishibashi and Nicolaidis, 1981). In addition, SFO sends efferent projections to regions protected by the blood-brain barrier (BBB) in the hypothalamic autonomic and neuroendocrine control centers, such as the PVN having a role in maintaining the hydroelectrolytic balance (Anderson et al., 2001; Gutman et al., 1986; Tanaka et al., 1985; Wright et al., 1993). Therefore, SARS-CoV-2 infection-related dysfunction of the SFO-PVN pathway could result in hypokalemia in COVID-19 patients.
Classically, the intracellular concentration of K+ is higher than extracellular [K+] in eukaryotic cells. Such regulation is imperative for protein synthesis and maintenance of cell volume maintained by Na-K-ATPase activity (Stone et al., 2016). The Na-K-ATPase pump is responsible for creating an electrochemical K+ gradient that determines the membrane potential and provides energy for the action potential, muscle contractility, and ion channel activity (McDonough and Youn, 2017; Youn, 2013). In addition, [K+] homeostasis is also interrelated with dietary K+ intake and K+ excretion (Giebisch, 1998; Xu et al., 2017). In order to keep K+ concentrations within the physiological pattern (3.8 to 5 mM), K+ excretion is physiologically tightly regulated (Giebisch et al., 2007). Decreased K+ levels in the extracellular fluid alter the cell membrane polarization, which results in dysfunction of the electrical excitability of the cell (McDonough and Youn, 2017). Therefore, the regulation of [K+] homeostasis is crucial for several vital functions in the body based on integrative physiology properties. Renal K+ excretion is mainly sustained by secretion along the distal nephron, especially through the initial and cortical collecting duct (Giebisch et al., 2007). The kidney K+ excretion is regulated by extracellular K+ levels under physiological conditions. Frequently, dietary K+ intake increases extracellular K+ levels, which stimulates renal K+ excretion by directly activating K+ secretion in the collecting duct (Gennari and Segal, 2002; Youn, 2013). This secretion is determined by a set of factors, such as acid-base balance, K+ metabolism, sodium balance, as well as the action of some hormones, including vasopressin and aldosterone (Amorim et al., 2004). The effects of SARS-CoV-2 in these related mechanisms remain unknown.
The putative causes of hypokalemia in patients with COVID-19 include elevated gastrointestinal problems and urine loss. Gastrointestinal symptoms resulting from COVID-19 have been reported in some patients, including vomiting and diarrhea (Gu et al., 2020; Huang et al., 2020; Wang et al., 2020; Yang et al., 2020). However, the association between severe hypokalemia and diarrhea was observed in only 29% of patients, implying that hypokalemia is not the result of diarrhea alone (Chen et al., 2020). Thus, K+ gastrointestinal loss is unlikely the leading cause of hypokalemia. On the other hand, Chen and colleagues have also reported that patients with hypokalemia exhibited increased urinary K+ excretion compared to control patients with normokalemia (Chen et al., 2020), suggesting that increased urinary K+ loss is the major cause of hypokalemia in COVID-19 patients.
Aldosterone is the primary regulator of K+ levels. Most K+ excretion (>90%) is performed by the aldosterone-sensitive distal nephron of the kidney, while <10% of K+ excretion is guaranteed by the aldosterone-sensitive distal colon (Giebisch, 1998; Giebisch et al., 2007). It is noteworthy that the aldosterone-sensitive distal nephron regulates K+ excretion to match K+ intake. The increase in plasma K+ levels due to increased intake stimulates the adrenal gland to secrete aldosterone, leading to renal K+ secretion and excretion to restore normal plasma K+ levels (Todkar et al., 2015). Therefore, changes in the renin-angiotensin-aldosterone system (RAAS) may be related to hypokalemia. It has been reported that angiotensin-converting enzyme-2 (ACE2) is the main counter-regulator of the RAAS, which is responsible for modulating blood pressure, blood volume, and electrolyte balance (Santos et al., 2008). Naturally, RAAS is regulated by ACE1 and ACE2, which increase (“activator” system) and decrease (“inhibitor” system) RAAS activity, respectively (Alexandre et al., 2020; Vaduganathan et al., 2020). SARS-CoV-2 invasion of the kidney can lead to degradation of ACE2 and increased ACE1 function, which, in turn, could result in increased RAAS activity (Santos et al., 2008). Intensified RAAS activity can lead to a secondary increase in aldosterone and consequent renal K+ excretion (Chen et al., 2020; Rocha et al., 1999).
Furthermore, vasopressin (ADH) may also contribute to K+ homeostasis. ADH is produced by the PVN and supraoptic nucleus (SON) of the hypothalamus and transported to the posterior pituitary through the hypothalamic-neurohypophyseal system, and subsequently released into the systemic circulation to modulate the hydromineral homeostasis (Cocco et al., 2017). For distal nephron K+ secretion, ADH stimulates the V1 (apical membrane) and V2 (basolateral membrane) receptors, activates phospholipase C (PLC)/Ca2+/PKC and adenylate cyclase/cAMP/PKA signaling pathways, respectively, therefore acting on low and high conductance K+ channels on the apical membrane (Amorim and Malnic, 2000; Amorim et al., 2004; Barreto-Chaves and De Mello-Aires, 1997; Cassola et al., 1993; Musa-Aziz et al., 2002; Nonoguchi et al., 1995; Wheatley et al., 1998; Yoshitomi et al., 1996). Previous reports have shown that some COVID-19 patients have displayed a syndrome of inappropriate antidiuretic hormone secretion (SiADH) (Habib et al., 2020; Mabillard and Sayer, 2020; Yousaf et al., 2020). The most common consequence of SiADH is hyponatremia (Dhawan et al., 1992), which is seen in COVID-19 patients (Habib et al., 2020; Yousaf et al., 2020). However, SiADH could also increase K+ excretion (Gowrishankar et al., 1996; Musch and Decaux, 2019).
We propose that SARS-CoV-2 infection of neurons in the SFO, PVN, and SON leads to dysfunction of these regions due to either viral-mediated neurodegeneration or neuroinflammation. SARS-CoV-2 infection in SFO neurons likely disrupts the stimulus from SFO to PVN and SON to produce ADH, whereas SARS-CoV-2 infection of PVN and SON likely interferes with the release of ADH into the posterior pituitary. These changes can eventually lead to hypokalemia and hydroelectrolytic imbalance in COVID-19 patients.
Besides, the sympathetic autonomic system and the release of cortisol can influence hypokalemia. SARS-CoV-2 infection can increase sympathetic activity via changes in blood gases and immunoinflammatory factors (Porzionato et al., 2020). Autonomic activation has direct adrenergic effects on RAAS, which increases aldosterone secretion and contributes to the loss of K+ (Darbar et al., 1996; Goldstein, 2020; Gordon et al., 1967; Nayyar et al., 2017). One study also showed a relationship between the mortality of COVID-19 patients with increased plasma cortisol levels (Tan et al., 2020). Cortisol activates renal mineralocorticoid receptors and increases K+ excretion (Cushing's syndrome symptoms) (Funder, 2005; Gomez-Sanchez, 2014). Another plausible alternative is that the peripheral infection induced by COVID-19 increases the production of pro-inflammatory cytokines such as IL-6 (Han et al., 2020). The cytokine IL-6 activates the hypothalamic-pituitary-adrenocortical (HPA) axis and induces the synthesis and secretion of aldosterone, ADH, and cortisol, which can contribute to hypokalemia (González-Hernández et al., 2006; Päth et al., 1997). Therefore, hypokalemia in patients with COVID-19 is likely a result of synergy between central and peripheral factors.
3. Routes by which SARS-CoV-2 enters CNS
Several viruses have been shown to reach the CNS using different routes, including the anterograde transport through olfactory nerves into the olfactory bulb and blood circulation (Desforges et al., 2014; Durrant et al., 2016). Viruses likely also enter through the subarachnoid space along olfactory nerves passing through the cribriform plate because of the communication between the nasal lymph compartment located in the submucosa of olfactory and respiratory epithelium and ethmoidal lymphatics and the brain’s cerebrospinal fluid (CSF) compartment (Johnston et al., 2004; Zakharov et al., 2004). Once viruses are in the subarachnoid space, CSF flow can facilitate their spread into the entire brain through the interstitial space (Shetty and Zanirati, 2020), as subarachnoid CSF passes into deeper regions of the brain rapidly alongside the perivascular spaces (Iliff et al., 2012). Upon entering the interstitial space, viruses likely enter neurons or any neural cell expressing ACE2.
SARS-CoV, a beta-coronavirus with 79% of homology with SARS-CoV-2, was detected in the brain of 75% of severe acute respiratory syndrome (SARS) patients. SARS-CoV’s presence was especially seen in the hypothalamus and the cortex (Gu et al., 2005). Several studies have shown that SARS-CoV-2 can invade the human and animal brain (Conde Cardona et al., 2020; Mesci et al., 2020; Song et al., 2020b, 2020a). A study has also provided evidence of SARS-CoV-2 invasion of neurons in human brain organoids and mice overexpressing human ACE2, as well as in postmortem COVID-19 patient brain samples (Song et al., 2021). In these studies, the authors observed a unique hypermetabolic state in SARS-CoV-2-infected neurons and local vascular changes. SARS-CoV-2 preferentially infects neurons in 3D human brain organoids after 2 days of in vitro exposure, triggering dysregulation and hyperphosphorylation of the tau protein as well as neurodegeneration (Ramani et al., 2020). In addition, SARS-CoV-2 invaded neurons in iPSC-derived human brain organoids and killed cortical neurons, and promoted impaired synaptogenesis (Mesci et al., 2020). Furthermore, SARS-CoV-2 infected cerebral choroid plexus epithelium, damaging the blood-cerebrospinal fluid (CSF) barrier in human brain organoids (Pellegrini et al., 2020). Immunological changes have been identified in CSF of COVID-19 patients with neurological damage, indicating neuroinvasion of SARS-CoV-2 (Alexopoulos et al., 2020; Song et al., 2020a).
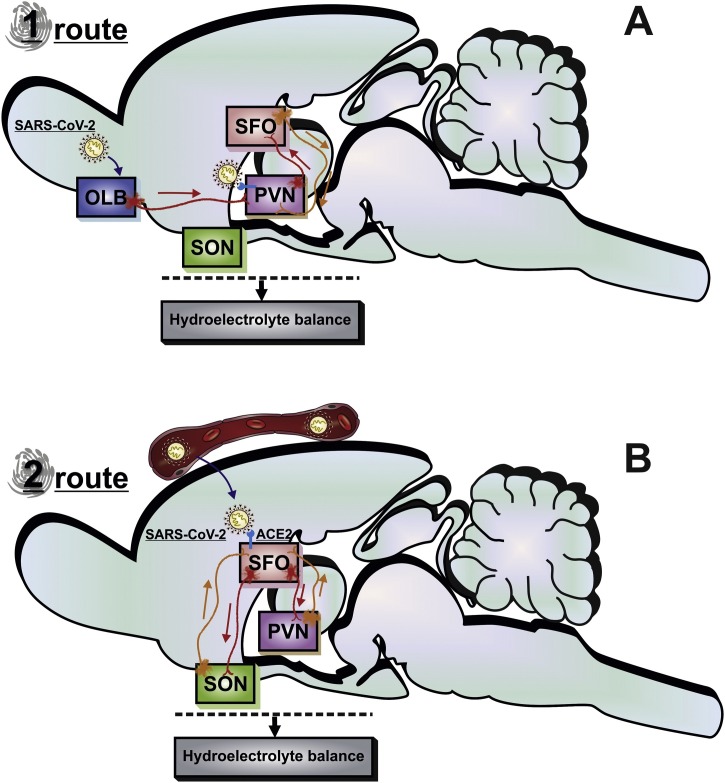
Fig. 1 illustrates the two routes by which SARS-CoV-2 can access the CNS. Anterograde transport through olfactory nerves is one of the suggested routes to reach the olfactory bulb and then the rest of the CNS (Durrant et al., 2016; Butowt and Bilinska, 2020; Conde Cardona et al., 2020). It is noteworthy that the olfactory bulb has efferent projections to the PVN (Guevara-Aguilar et al., 1988). Such trans-neuronal transmission could occur due to the neurons’ capability to transport viruses using proteins such as dynein and kinesin in retrograde and anterograde directions (Bohmwald et al., 2018). After 2 days of intranasal inoculation of SARS-CoV-2 in golden Syrian hamsters, acute inflammation was observed in the olfactory epithelium (Zhang et al., 2020). Moreover, these authors showed that SARS-CoV-2 could invade nasopharyngeal pseudo-columnar ciliated respiratory epithelial cells, mature and immature olfactory neurons, and the cells sustentacular, contributing to the olfactory dysfunction of COVID-19. Although there is enough evidence for the presence of SARS-CoV-2 in the olfactory system, the viral antigens have not yet been detected so far in the olfactory bulb (Bryche et al., 2020; Zhang et al., 2020). Therefore, it is tempting to suggest that viruses mostly enter the brain through the subarachnoid space along olfactory nerves into the brain’s cerebrospinal fluid (CSF) compartment, as described in the earlier section.
Fig. 1.
A schematic drawing of the SARS-CoV-2 invasion pathways in the brain.
SARS-CoV-2 can access the brain via the olfactory nerve or blood circulation. (A) In route 1, the virus is carried by the axons of the olfactory sensory neurons into the OLB towards the PVN. SARS-CoV-2 is transported to the cytoplasm mediated by ACE2 and proteases in PVN. Subsequently, the viral RNA is replicated, transcribed, and translated by viral proteins inside the cell. The viral protein and RNA are assembled to constitute a new virion to be released in the neuronal membrane. (B) In route 2, the SARS-CoV-2 moves from blood to extracellular fluid in circumventricular organs. This virus can enter SFO neurons through ACE2. Axonal projections from the SFO synapse on PVN and SON neurons, which regulates the hydroelectrolytic balance. SARS-CoV-2 infection can disrupt the SFO and PVN functions, leading to hydroelectrolytic imbalance. ACE2, angiotensin-converting enzyme 2; OLB, bulb olfactory; PVN, paraventricular hypothalamic nucleus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SFO, subfornical organ; SON, supraoptic hypothalamic nucleus.
The possibility of SARS-CoV-2 reaching the brain via blood circulation has also been suggested. However, the precise pathway is yet to be explored. The BBB protects the major part of the brain, and this diffusion barrier is capable of blocking the influx of the most blood-borne components to the cerebral interstitial fluid (Ballabh et al., 2004). However, the CVOs are specific brain regions allowing the entry of components from the blood without the need to cross the BBB (Ballabh et al., 2004). The typical feature of CVO is the presence of high permeability and fenestrated capillaries in a brain region that includes the neurohypophysis, the SFO, the median eminence, the vascular organ of the lamina terminalis, the subcommissural organ, the pineal gland, the choroid plexus, and the area postrema (Bodiga et al., 2013; Duvernoy and Risold, 2007; Ganong, 2000; Johnson and Gross, 1993; Simpson, 1981). A study has suggested that the expected positive result for real-time RT-PCR to SARS-CoV-2 in plasma is ∼15% (Huang et al., 2020), implying the possibility of spreading the SARS-CoV-2 via circulating blood is marginal. However, the lack of SARS-COV-2 in the plasma of most COVID-19 patients could be due to a short span of viral presence in the circulating blood. Indeed, a recent study in a ferret animal model reported that SARS-CoV-2 is present in the blood circulation and the CVO only in the acute phase of COVID-19 (Kim et al., 2020) before the seroconversion by plasma SARS-CoV-2-binding IgM and IgG antibodies from day 7 until day 20 after symptom onset (Thevarajan et al., 2020). Taken together, it appears that SARS-CoV-2 can enter the brain through several routes. Since SARS-CoV-2 could enter CVO through blood, CSF, or efferent projections from the olfactory bulb, CVO is likely one of the first brain regions to get infected by SARS-CoV-2. Examination of the CVO in post-mortem brain tissues from COVID-19 patients would likely provide additional insights.
4. Characteristics that promote SARS-CoV-2 infection
Considering the high potential of SARS-CoV-2 to reach the extracellular fluid of CVO, it is critical to draw a parallel between classical host cell infection by this virus and the cellular characteristics of the SFO area, and PVN of the hypothalamus. SARS-CoV-2 has an irregular elliptic shape discriminated by crown-like spikes on the surface and a diameter around 130 nm (Fathi, 2020). The virus particle is characterized by a positive-sense single-stranded RNA genome and proteins such as spike (S), envelope (E), membrane (M), and nucleocapsid (N). The proteins S, M, and E are anchored in the viral envelope, a phospholipid bilayer derived from the host cell membrane. Alternatively, the N protein interacts with the viral RNA into the core of the virion (Fehr and Perlman, 2015). The proteolytic activation of S glycoprotein is an elementary condition to viral entry. Generally, the S glycoprotein is cleaved by proteolytic proteins in the region between the S1 and S2 subunits, remaining non-covalently restrained in a prefusion conformation. The SARS-CoV-2 entry is functionally mediated by the viral S1 subunit of transmembrane S glycoproteins by the attachment with the ACE2 receptor in host cells. The ACE2 is known as the functional receptor of SARS-CoV-2 (Du et al., 2009; Kong et al., 1997; Lu et al., 2020; Wrapp et al., 2020). This transmembrane protein is formed by three domains: (i) the extracellular domain, which can interact with several ligands as SARS-CoV-2 in extracellular fluid, (ii) the intermediate domain connects the extracellular and intracellular milieus, and (iii) the intracellular cytoplasmatic domain (Jia et al., 2009; Peng et al., 2011). Distributed over many human tissues, ACE2 is profoundly expressed in the epithelium of the airway, vascular endothelium, lung, kidney, intestine, testis, heart, and brain (Donoghue et al., 2000; Hamming et al., 2004; Harmer et al., 2002). Indeed, it has already been reported that ACE2 is expressed in SFO, the vascular organ of the lamina terminalis, pituitary gland, median eminence, and area postrema, as well as PVN (Doobay et al., 2007). Subsequently, the S2 domain promotes the viral fusion mechanism's activation to enter into the host cells (Kong et al., 1997). Several proteases expressed in neuronal cells and extracellular liquid are capable of mediating the SARS-CoV-2 activation. The transmembrane protease/serine-type 2 (TMPRSS-2) is a trypsin-like serine protease attached to the cellular surface described as a critical protease promoting SARS-CoV-2 entry into the cell (Hoffmann et al., 2020; Qian et al., 2013). The TMPRSS-2 is also expressed in hypothalamic neurons (Ubuka et al., 2018). Thus, it is likely that PVN and SFO are territories where the SARS-CoV-2 can easily access neurons.
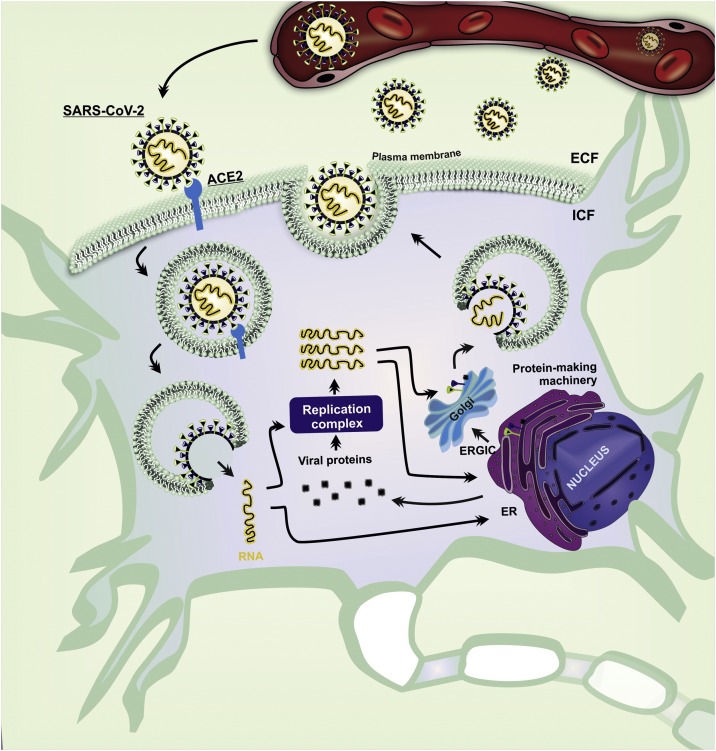
After the internalization of SARS-CoV-2, the viral genome is released in the cytoplasm (Lai and Stohlman, 1981). Some genomic RNAs are translated into nonstructural proteins (nsps) that trigger the replicase-transcriptase complex responsible for RNA synthesis, replication, and transcription of sub-genomic RNAs (Sethna et al., 1991). Subsequently, the genomic RNA of SARS-CoV-2 is transcribed into negative-strand RNA, which is used as a template for the synthesis of positive-sense genomic RNAs and subgenomic RNAs (Fehr and Perlman, 2015), to be used downstream of the replicase polyproteins (Sethna et al., 1991; Siu et al., 2008). The structural proteins S, E, and M, translated from subgenomic RNAs, are then inserted into the endoplasmic reticulum (ER) and Golgi apparatus to encapsulate viral genomes in mature virions (Kuo and Masters, 2013; Sethna et al., 1991). Then, the virions are transported to the cell surface in vesicles and released by exocytosis (Fig. 2 ). The host cells infected by SARS-CoV-2 can occasionally activate cellular lysis (Jia et al., 2005; Sethna et al., 1991; Ye and Hogue, 2007; Zhou et al., 2020), leading to overflow of virions and release of cytoplasmic ionic content that can affect adjacent neurons. In summary, we propose that, through routes described earlier, SARS-CoV-2 infects SFO-PVN-SON neurons, which alters their physiological role due to changes in neuronal function promoted by viral replication, neurodegeneration, or neuroinflammation. The SFO is a classical nucleus that modulates cardiovascular regulations and hydromineral homeostasis (Ch’ng and Lawrence, 2019). Besides, the SFO synapses on PVN and SON neurons, a well-established pathway for autonomic and neuroendocrine modulation. These neuronal connections also contribute to the negative feedback action of ADH, which maintains plasmatic water balance and plasmatic ions, like sodium and K+ ([hydromineral homeostasis] (Washburn et al., 1999). Thus, disruption of SFO-PVN-SON activity could result in hydroelectrolytic disorder.
Fig. 2.
A schematic pathway of SARS-CoV-2 infection in SFO and PVN neurons.
SARS-CoV-2 in the extracellular fluid is an essential determinant of viral infection. After the proteolytic activation of SARS-CoV-2 by proteases (TMPRSS), the S glycoprotein binds to the ACE2 in SFO and PVN neurons. The ACE2 mediates the entry of virion by fusion membrane. In the cytoplasm, SARS-CoV-2 delivers its single-stranded RNA that can be translated into viral protein or replicated. The Golgi complex assembles the viral protein and RNA to constitute a new virion in the Golgi and is released in the plasma membrane of the neuron. ACE2, angiotensin-converting enzyme 2; ECF, extracellular fluid; ER, endoplasmic reticulum; ERGIC, ER-Golgi intermediate compartment; ICF, intracellular fluid; PVN, paraventricular hypothalamic nucleus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SFO, subfornical organ; TMPRSS, transmembrane protease/serine.
5. Conclusions and future perspectives
We propose the involvement of SFO-PVN-SON network-based mechanisms for hydroelectrolytic disorder in COVID-19 patients. The SARS-CoV-2 infection could also exacerbate the HPA axis's activation, increase the secretion of ADH and aldosterone, and induce sympathetic hyperactivation. Collectively, these factors contribute directly or indirectly to the loss of K+, resulting in hypokalemia. Nonetheless, additional investigations in animal models of COVID-19 are required to fully comprehend the routes by which SARS-CoV-2 accesses the CVOs and other CNS regions at different time-points after the infection. Furthermore, careful examination of CVOs in post-mortem brain tissues from COVID-19 patients will be required to assess the extent of neurodegeneration and/or neuroinflammation in SFO, PVN, and SON vis-à-vis other brain regions.
Declaration of Competing Interest
There is no conflict of interest.
Acknowledgements
This research was supported by grants from CAPES–Prevention and Combat of Outbreaks, Endemics, Epidemics and Pandemics (#23038.014934/2020-59), CAPES/CNPq (#458143/2014), FAPEMIG (#APQ-00476-20; #APQ-02872-16; SICONV 793988/2013 and APQ-03385-18), Federal University of Uberlandia, and National Institute of Science and Technology in Theranostics and Nanobiotechnology (CNPq Process N.: 465669/2014-0). A.K.S. is supported by grants from the United States National Institutes of Health (R01NS106907-01) and the United States Department of Defense (W81XWH-17-1-0447). We also thank CAPES-Brazil for Ph.D. Research Fellowship to ISM. RS-S received a fellowship from PrInt CAPES/UFU. ACGJ received a productivity fellowship (311219/2019-5) from the CNPq (National Counsel of Technological and Scientific Development).
References
- Alexandre J., Cracowski J.L., Richard V., Bouhanick B. Renin-angiotensin-aldosterone system and COVID-19 infection. Ann. Endocrinol. (Paris). 2020;81:63–67. doi: 10.1016/j.ando.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos H., Magira E., Bitzogli K., Kafasi N., Vlachoyiannopoulos P., Tzioufas A., Kotanidou A., Dalakas M.C. Anti-SARS-CoV-2 antibodies in the CSF, blood-brain barrier dysfunction, and neurological outcome: Studies in 8 stuporous and comatose patients. Neurol. Neuroimmunol. neuroinflammation. 2020;7 doi: 10.1212/NXI.0000000000000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorim J.B., Malnic G. V(1) receptors in luminal action of vasopressin on distal K(+) secretion. Am. J. Physiol. Renal Physiol. 2000;278:F809–16. doi: 10.1152/ajprenal.2000.278.5.F809. [DOI] [PubMed] [Google Scholar]
- Amorim J.B.O., Musa-Aziz R., Mello-Aires M., Malnic G. Signaling path of the action of AVP on distal K+ secretion. Kidney Int. 2004;66:696–704. doi: 10.1111/j.1523-1755.2004.00800.x. [DOI] [PubMed] [Google Scholar]
- Anderson J.W., Smith P.M., Ferguson A.V. Subfornical organ neurons projecting to paraventricular nucleus: whole-cell properties. Brain Res. 2001;921:78–85. doi: 10.1016/s0006-8993(01)03093-1. [DOI] [PubMed] [Google Scholar]
- Asadi-Pooya A.A., Simani L. Central nervous system manifestations of COVID-19: A systematic review. J. Neurol. Sci. 2020;413 doi: 10.1016/j.jns.2020.116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabh P., Braun A., Nedergaard M. The blood-brain barrier: An overview: Structure, regulation, and clinical implications. Neurobiol. Dis. 2004 doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Barkas F., Filippas-Ntekouan S., Liontos A., Kosmidou M., Kalambokis G., Milionis H. Multifaceted persistent hypokalaemia in a patient with coronavirus disease 2019. Intern. Med. J. 2020 doi: 10.1111/imj.14969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto-Chaves M.L.M., De Mello-Aires M. Luminal arginine vasopressin stimulates Na+-H+ exchange and H+- ATPpase in cortical distal tubule via V1 receptor. Kidney Int. 1997;52:1035–1041. doi: 10.1038/ki.1997.425. [DOI] [PubMed] [Google Scholar]
- Black E.A.E., Smith P.M., McIsaac W., Ferguson A.V. Brain-derived neurotrophic factor acts at neurons of the subfornical organ to influence cardiovascular function. Physiol. Rep. 2018;6 doi: 10.14814/phy2.13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodiga V.L., Bodiga S., Kuroiwa Y., Rao K.S.J., Tokuno H. Renin Angiotensin System in Cognitive Function and Dementia. Asian J. Neurosci. 2013;2013 doi: 10.1155/2013/102602. [DOI] [Google Scholar]
- Bohmwald K., Gálvez N.M.S., Ríos M., Kalergis A.M. Neurologic alterations due to respiratory virus infections. Front. Cell. Neurosci. 2018 doi: 10.3389/fncel.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryche B., St Albin A., Murri S., Lacôte S., Pulido C., Ar Gouilh M., Lesellier S., Servat A., Wasniewski M., Picard-Meyer E., Monchatre-Leroy E., Volmer R., Rampin O., Le Goffic R., Marianneau P., Meunier N. Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain. Behav. Immun. 2020;89:579–586. doi: 10.1016/j.bbi.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butowt R., Bilinska K. SARS-CoV-2: Olfaction, Brain Infection, and the Urgent Need for Clinical Samples Allowing Earlier Virus Detection. ACS Chem. Neurosci. 2020 doi: 10.1021/acschemneuro.0c00172. [DOI] [PubMed] [Google Scholar]
- Cassola A.C., Giebisch G., Wang W. Vasopressin increases density of apical low-conductance K+ channels in rat CCD. Am. J. Physiol. 1993;264:F502–9. doi: 10.1152/ajprenal.1993.264.3.F502. [DOI] [PubMed] [Google Scholar]
- Ch’ng S.S., Lawrence A.J. The subfornical organ in sodium appetite: Recent insights. Neuropharmacology. 2019 doi: 10.1016/j.neuropharm.2018.08.012. [DOI] [PubMed] [Google Scholar]
- Chen D., Li X., Song Q., Hu C., Su F., Dai J., Ye Y., Huang J., Zhang X. Assessment of Hypokalemia and Clinical Characteristics in Patients With Coronavirus Disease 2019 in Wenzhou, China . JAMA Netw. open. 2020;3 doi: 10.1001/jamanetworkopen.2020.11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coble J.P., Cassell M.D., Davis D.R., Grobe J.L., Sigmund C.D. Activation of the renin-angiotensin system, specifically in the subfornical organ is sufficient to induce fluid intake. Am. J. Physiol. - Regul. Integr. Comp. Physiol. 2014;307:R376. doi: 10.1152/ajpregu.00216.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coble J.P., Grobe J.L., Johnson A.K., Sigmund C.D. Mechanisms of brain renin angiotensin system-induced drinking and blood pressure: Importance of the subfornical organ. Am. J. Physiol. - Regul. Integr. Comp. Physiol. 2015 doi: 10.1152/ajpregu.00486.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocco C., Brancia C., Corda G., Ferri G.L. The hypothalamic-pituitary axis and autoantibody related disorders. Int. J. Mol. Sci. 2017 doi: 10.3390/ijms18112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde Cardona G., Quintana Pájaro L.D., Quintero Marzola I.D., Ramos Villegas Y., Moscote Salazar L.R. Neurotropism of SARS-CoV 2: Mechanisms and manifestations. J. Neurol. Sci. 2020 doi: 10.1016/j.jns.2020.116824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia A.O., Feitosa P.W.G., Moreira J.L., de S., Nogueira S.Á.R., Fonseca R.B., Nobre M.E.P. Neurological manifestations of COVID-19 and other coronaviruses: A systematic review. Neurol. Psychiatry Brain Res. 2020 doi: 10.1016/j.npbr.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbar D., Smith M., Mörike K., Roden D.M. Epinephrine-induced changes in serum potassium and cardiac repolarization and effects of pretreatment with propranolol and diltiazem. Am. J. Cardiol. 1996;77:1351–1355. doi: 10.1016/S0002-9149(96)00204-4. [DOI] [PubMed] [Google Scholar]
- Desforges M., Le Coupanec A., Stodola J.K., Meessen-Pinard M., Talbot P.J. Human coronaviruses: viral and cellular factors involved in neuroinvasiveness and neuropathogenesis. Virus Res. 2014;194:145–158. doi: 10.1016/j.virusres.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan A., Narang A., Singhi S. Hyponatraemia and the inappropriate ADH syndrome in pneumonia. Ann. Trop. Paediatr. 1992;12:455–462. doi: 10.1080/02724936.1992.11747614. [DOI] [PubMed] [Google Scholar]
- Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R., Breitbart R.E., Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 2000;87 doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- Doobay M.F., Talman L.S., Obr T.D., Tian X., Davisson R.L., Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am. J. Physiol. - Regul. Integr. Comp. Physiol. 2007;292:R373. doi: 10.1152/ajpregu.00292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., He Y., Zhou Y., Liu S., Zheng B.J., Jiang S. The spike protein of SARS-CoV - A target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009 doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant D.M., Ghosh S., Klein R.S. The Olfactory Bulb: An Immunosensory Effector Organ during Neurotropic Viral Infections. ACS Chem. Neurosci. 2016 doi: 10.1021/acschemneuro.6b00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy H.M., Risold P.Y. The circumventricular organs: An atlas of comparative anatomy and vascularization. Brain Res. Rev. 2007 doi: 10.1016/j.brainresrev.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Fathi M.B. The Crystallography of SARS-CoV-2 Suggests Its Deactivation. Iran. Red Crescent Med. J. 2020;22 doi: 10.5812/ircmj.102812. [DOI] [Google Scholar]
- Fehr A.R., Perlman S. Coronaviruses: Methods and Protocols. Springer; New York: 2015. Coronaviruses: An overview of their replication and pathogenesis; pp. 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A.V., Bains J.S. Electrophysiology of the circumventricular organs. Front. Neuroendocrinol. 1996;17:440–475. doi: 10.1006/frne.1996.0012. [DOI] [PubMed] [Google Scholar]
- Funder J.W. Mineralocorticoid receptors: Distribution and activation. Heart Fail. Rev. 2005;10:15–22. doi: 10.1007/s10741-005-2344-2. [DOI] [PubMed] [Google Scholar]
- Ganong W.F. Circumventricular organs: Definition and role in the regulation of endocrine and autonomic function, in: Clinical and Experimental Pharmacology and Physiology. pp. 422–427. 2000 doi: 10.1046/j.1440-1681.2000.03259.x. [DOI] [PubMed] [Google Scholar]
- Gennari F.J., Segal A.S. Hyperkalemia: An adaptive response in chronic renal insufficiency. Kidney Int. 2002;62:1–9. doi: 10.1046/j.1523-1755.2002.00350.x. [DOI] [PubMed] [Google Scholar]
- Giebisch G. Renal potassium transport: Mechanisms and regulation. Am. J. Physiol. - Ren. Physiol. 1998 doi: 10.1152/ajprenal.1998.274.5.f817. [DOI] [PubMed] [Google Scholar]
- Giebisch G., Krapf R., Wagner C. Renal and extrarenal regulation of potassium, in: Kidney International. Nature Publishing Group. 2007:397–410. doi: 10.1038/sj.ki.5002288. [DOI] [PubMed] [Google Scholar]
- Goldstein D.S. The extended autonomic system, dyshomeostasis, and COVID-19. Clin. Auton. Res. 2020 doi: 10.1007/s10286-020-00714-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Sanchez E.P. Brain mineralocorticoid receptors in cognition and cardiovascular homeostasis. Steroids. 2014 doi: 10.1016/j.steroids.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Hernández T., Afonso-Oramas D., Cruz-Muros I., Barroso-Chinea P., Abreu P., Pérez-Delgado M.D.M., Rancel-Torres N., Gonzalez M.D.C. Interleukin-6 and nitric oxide synthase expression in the vasopressin and corticotrophin-releasing factor systems of the rat hypothalamus. J. Histochem. Cytochem. 2006;54:427–441. doi: 10.1369/jhc.5A6845.2005. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L.L.M., Samborskiy D.V., Sidorov I.A., Sola I., Ziebuhr J. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon R.D., Küchel O., Liddle G.W., Island D.P. Role of the sympathetic nervous system in regulating renin and aldosterone production in man. J. Clin. Invest. 1967;46:599–605. doi: 10.1172/JCI105561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowrishankar M., Chen C.B., Mallie J.P., Halperin M.L. What is the impact of potassium excretion on the intracellular fluid volume: Importance of urine anions. Kidney Int. 1996;50:1490–1495. doi: 10.1038/ki.1996.463. [DOI] [PubMed] [Google Scholar]
- Gu J., Gong E., Zhang B., Zheng J., Gao Z., Zhong Y., Zou W., Zhan J., Wang S., Xie Z., Zhuang H., Wu B., Zhong H., Shao H., Fang W., Gao D., Pei F., Li X., He Z., Xu D., Shi X., Anderson V.M., Leong A.S.Y. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Han B., Wang J. COVID-19: Gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W., Ni Z., Hu Yu, Liang W., Ou C., He J., Liu L., Shan H., Lei C., Hui D.S.C., Du B., Li L., Zeng G., Yuen K.-Y., Chen R., Tang C., Wang T., Chen P., Xiang J., Li S., Wang Jin-lin, Liang Z., Peng Y., Wei L., Liu Y., Hu Ya-hua, Peng P., Wang Jian-ming, Liu J., Chen Z., Li G., Zheng Z., Qiu S., Luo J., Ye C., Zhu S., Zhong N. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara-Aguilar R., Jimenez-Montufar L.L., Garcia-Diaz D.E., Wayner M.J., Armstrong D.L. Olfactory and visceral projections to the paraventricular nucleus. Brain Res. Bull. 1988;20:799–801. doi: 10.1016/0361-9230(88)90094-9. [DOI] [PubMed] [Google Scholar]
- Gutman M.B., Ciriello J., Mogenson G.J. Electrophysiological identification of forebrain connections of the subfornical organ. Brain Res. 1986;382:119–128. doi: 10.1016/0006-8993(86)90118-6. [DOI] [PubMed] [Google Scholar]
- Habib M.B., Sardar S., Sajid J. Acute symptomatic hyponatremia in setting of SIADH as an isolated presentation of COVID-19. IDCases. 2020;21 doi: 10.1016/j.idcr.2020.e00859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H., Ma Q., Li C., Liu R., Zhao L., Wang W., Zhang P., Liu X., Gao G., Liu F., Jiang Y., Cheng X., Zhu C., Xia Y. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes Infect. 2020;9:1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer D., Gilbert M., Borman R., Clark K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532:107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. www.thelancet.com. 2020;395:497. doi: 10.1016/S0140-6736(20)30183-30185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff J.J., Wang M., Liao Y., Plogg B.A., Peng W., Gundersen G.A., Benveniste H., Vates G.E., Deane R., Goldman S.A., Nagelhus E.A., Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012;4 doi: 10.1126/scitranslmed.3003748. 147ra111-147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi S., Nicolaidis S. Hypertension induced by electrical stimulation of the subfornical organ (SFO) Brain Res. Bull. 1981;6:135–139. doi: 10.1016/s0361-9230(81)80038-x. [DOI] [PubMed] [Google Scholar]
- Jia H.P., Look D.C., Shi L., Hickey M., Pewe L., Netland J., Farzan M., Wohlford-Lenane C., Perlman S., McCray P.B. ACE2 Receptor Expression and Severe Acute Respiratory Syndrome Coronavirus Infection Depend on Differentiation of Human Airway Epithelia. J. Virol. 2005;79:14614–14621. doi: 10.1128/jvi.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia P.H., Look D.C., Tan P., Shi L., Hickey M., Gakhar L., Chappell M.C., Wohlford-Lenane C., McCray P.B. Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am. J. Physiol. - Lung Cell. Mol. Physiol. 2009:297. doi: 10.1152/ajplung.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A.K., Gross P.M. Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 1993;7:678–686. doi: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- Johnston M., Zakharov A., Papaiconomou C., Salmasi G., Armstrong D. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res. 2004;1:2. doi: 10.1186/1743-8454-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.-I., Kim S.-G., Kim S.-M., Kim E.-H., Park S.-J., Yu K.-M., Chang J.-H., Kim E.J., Lee S., Casel M.A.B., Um J., Song M.-S., Jeong H.W., Lai V.D., Kim Y., Chin B.S., Park J.-S., Chung K.-H., Foo S.-S., Poo H., Mo I.-P., Lee O.-J., Webby R.J., Jung J.U., Choi Y.K. Infection and Rapid Transmission of SARS-CoV-2 in Ferrets. Cell Host Microbe. 2020 doi: 10.1016/j.chom.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W., Mcconalogue K., Khitin L.M., Hollenberg M.D., Payan D.G., Böhm S.K., Bunnett N.W. Luminal trypsin may regulate enterocytes through proteinase-activated receptor 2. Proc. Natl. Acad. Sci. U. S. A. 1997;94:8884–8889. doi: 10.1073/pnas.94.16.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo L., Masters P.S. Functional Analysis of the Murine Coronavirus Genomic RNA Packaging Signal. J. Virol. 2013;87:5182–5192. doi: 10.1128/jvi.00100-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferschmidt K., Cohen J. Will novel virus go pandemic or be contained? Science (80-.) 2020 doi: 10.1126/science.367.6478.610. [DOI] [PubMed] [Google Scholar]
- Lai M.M., Stohlman S.A. Comparative analysis of RNA genomes of mouse hepatitis viruses. J. Virol. 1981;38:661–670. doi: 10.1128/jvi.38.2.661-670.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabillard H., Sayer J.A. Electrolyte Disturbances in SARS-CoV-2 Infection. F1000Research. 2020;9 doi: 10.12688/f1000research.24441.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabillard H., Sayer J.A., Tedd H., Speight A., Duncan C., Price D.A. Case Report: Renal potassium wasting in SARS-CoV-2 infection. F1000Research. 2020;9 doi: 10.12688/f1000research.24621.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough A.A., Youn J.H. Potassium homeostasis: The knowns, the unknowns, and the health benefits. Physiology. 2017 doi: 10.1152/physiol.00022.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesci P., Macia A., Saleh A., Martin-Sancho L., Yin X., Snethlage C., Avansini S., Chanda S., Muotri A. Sofosbuvir protects human brain organoids against SARS-CoV-2. bioRxiv. 2020 doi: 10.1101/2020.05.30.125856. 2020.05.30.125856. [DOI] [Google Scholar]
- Moreno-P O., Leon-Ramirez J.M., Fuertes-Kenneally L., Perdiguero M., Andres M., Garcia-Navarro M., Ruiz-Torregrosa P., Boix V., Gil J., Merino E., Asensio S., Fernandez C., Candela A., del Mar García M., Sánchez R., Reus S., Ruiz P., García-Sevila R., Martínez M.Á., García-Mullor M.M., Blanes M., Guijarro J., Pascual J.C., Gonzalez I., Sanso P., Ramos J.M., Javaloy J., Llopis C., Coronado O., García E., Rodríguez G., Melgar P., Franco M., Lluís F., Zaragoza C., Alcaraz C., Carrión A., Villodre C., de la Cuesta E.R., Alenda C., Peiró F., Planelles M., Greco L., Silvia S., Francia A., Verdú I., Sales J., Palacios A., Ballester H., García-Valentín A., Márquez M., Canelo E., Juan A., Vives E., Revert A., Fuente G., Nofuentes E., Mangas C., Vera E., Ferradas A., López H., Herrera C., López B., Morillas M., Rodríguez V., Khartabil M., Giménez M., Tovar E., Martínez E., Medina L., Baile S., Salazar C., Guerra N., Moliner S., López-González M.C., Figueres B. Hypokalemia as a sensitive biomarker of disease severity and the requirement for invasive mechanical ventilation requirement in COVID-19 pneumonia: A case series of 306 Mediterranean patients. Int. J. Infect. Dis. 2020;100:449–454. doi: 10.1016/j.ijid.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., Ueno M., Sakata H., Kondo K., Myose N., Nakao A., Takeda M., Haro H., Inoue O., Suzuki-Inoue K., Kubokawa K., Ogihara S., Sasaki T., Kinouchi H., Kojin H., Ito M., Onishi H., Shimizu T., Sasaki Y., Enomoto N., Ishihara H., Furuya S., Yamamoto T., Shimada S. A first Case of Meningitis/Encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa-Aziz R., Barreto-Chaves M.L.M., De Mello-Aires M. Peritubular AVP regulates bicarbonate reabsorption in cortical distal tubule via V1 and V2 receptors. Am. J. Physiol. - Ren. Physiol. 2002;282:F256–64. doi: 10.1152/ajprenal.00056.2001. [DOI] [PubMed] [Google Scholar]
- Musch W., Decaux G. High fractional potassium excretion in symptomatic hyponatremia. Eur. J. Intern. Med. 2019 doi: 10.1016/j.ejim.2018.09.002. [DOI] [PubMed] [Google Scholar]
- Nayyar M., Yusuf J., Khan M.U., Weber K.T. K+ and Mg2+ Dyshomeostasis in Acute Hyperadrenergic Stressor States. Am. J. Med. Sci. 2017;353:422–424. doi: 10.1016/j.amjms.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Nonoguchi H., Owada A., Kobayashi N., Takayama M., Terada Y., Koike J., Ujiie K., Marumo F., Sakai T., Tomita K. Immunohistochemical localization of V2 vasopressin receptor along the nephron and functional role of luminal V2 receptor in terminal inner medullary collecting ducts. J. Clin. Invest. 1995;96:1768–1778. doi: 10.1172/JCI118222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallanti S. Importance of SARs-Cov-2 anosmia: From phenomenology to neurobiology. Compr. Psychiatry. 2020 doi: 10.1016/j.comppsych.2020.152184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Päth G., Bornstein S.R., Ehrhart-Bornstein M., Scherbaum W.A. Interleukin-6 and the Interleukin-6 Receptor in the Human Adrenal Gland: Expression and Effects on Steroidogenesis1. J. Clin. Endocrinol. Metab. 1997;82:2343–2349. doi: 10.1210/jcem.82.7.4072. [DOI] [PubMed] [Google Scholar]
- Pellegrini L., Albecka A., Mallery D.L., Kellner M.J., Paul D., Carter A.P., James L.C., Lancaster M.A. SARS-CoV-2 Infects the Brain Choroid Plexus and Disrupts the Blood-CSF Barrier in Human Brain Organoids. Cell Stem Cell. 2020;27 doi: 10.1016/j.stem.2020.10.001. 951-961.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng G., Sun D., Rajashankar K.R., Qian Z., Holmes K.V., Li F. Crystal structure of mouse coronavirus receptor-binding domain complexed with its murine receptor. Proc. Natl. Acad. Sci. U. S. A. 2011;108:10696–10701. doi: 10.1073/pnas.1104306108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porzionato A., Emmi A., Barbon S., Boscolo-Berto R., Stecco C., Stocco E., Macchi V., De Caro R. Sympathetic activation: a potential link between comorbidities and COVID-19. FEBS J. 2020 doi: 10.1111/febs.15481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Z., Dominguez S.R., Holmes K.V. Role of the Spike Glycoprotein of Human Middle East Respiratory Syndrome Coronavirus (MERS-CoV) in Virus Entry and Syncytia Formation. PLoS One. 2013;8 doi: 10.1371/journal.pone.0076469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani A., Müller L., Ostermann P.N., Gabriel E., Abida‐Islam P., Müller‐Schiffmann A., Mariappan A., Goureau O., Gruell H., Walker A., Andrée M., Hauka S., Houwaart T., Dilthey A., Wohlgemuth K., Omran H., Klein F., Wieczorek D., Adams O., Timm J., Korth C., Schaal H., Gopalakrishnan J. SARS ‐CoV‐2 targets neurons of 3D human brain organoids. EMBO J. 2020;39 doi: 10.15252/embj.2020106230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha R., Chander P.N., Zuckerman A., Stier C.T. Role of aldosterone in renal vascular injury in stroke-prone hypertensive rats. Hypertension. 1999;33:232–237. doi: 10.1161/01.hyp.33.1.232. [DOI] [PubMed] [Google Scholar]
- Santos R.A.S., Ferreira A.J., Simões E Silva A.C. Recent advances in the angiotensin-converting enzyme 2-angiotensin(1-7)-Mas axis. Exp. Physiol. 2008 doi: 10.1113/expphysiol.2008.042002. [DOI] [PubMed] [Google Scholar]
- Sethna P.B., Hofmann M.A., Brian D.A. Minus-strand copies of replicating coronavirus mRNAs contain antileaders. J. Virol. 1991;65:320–325. doi: 10.1128/jvi.65.1.320-325.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty A.K., Zanirati G. The interstitial system of the brain in health and disease. Aging Dis. 2020 doi: 10.14336/AD.2020.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J.B. The circumventricular organs and the central actions of angiotensin. Neuroendocrinology. 1981 doi: 10.1159/000123167. [DOI] [PubMed] [Google Scholar]
- Siu Y.L., Teoh K.T., Lo J., Chan C.M., Kien F., Escriou N., Tsao S.W., Nicholls J.M., Altmeyer R., Peiris J.S.M., Bruzzone R., Nal B. The M, E, and N Structural Proteins of the Severe Acute Respiratory Syndrome Coronavirus Are Required for Efficient Assembly, Trafficking, and Release of Virus-Like Particles. J. Virol. 2008;82:11318–11330. doi: 10.1128/jvi.01052-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E., Bartley C.M., Chow R.D., Ngo T.T., Jiang R., Zamecnik C.R., Dandekar R., Loudermilk R.P., Dai Y., Liu F., Hawes I.A., Alvarenga B.D., Huynh T., Mcalpine L., Rahman N.-T., Geng B., Chiarella J., Goldman-Israelow B., Vogels C.B.F., Grubaugh N.D., Casanovas-Massana A., Phinney B.S., Salemi M., Alexander J., Gallego J.A., Lencz T., Walsh H., Lucas C., Klein J., Mao T., Oh J., Ring A., Spudich S., Ko A.I., Kleinstein S.H., Derisi J.L., Iwasaki A., Pleasure S.J., Wilson M.R., Farhadian S.F. Exploratory neuroimmune profiling identifies CNS-specific alterations in COVID-19 patients with neurological involvement. bioRxiv. 2020 doi: 10.1101/2020.09.11.293464. 2020.09.11.293464. [DOI] [Google Scholar]
- Song E., Zhang C., Israelow B., Lu-Culligan A., Prado A.V., Skriabine S., Lu P., Weizman O.-E., Liu F., Dai Y., Szigeti-Buck K., Yasumoto Y., Wang G., Castaldi C., Heltke J., Ng E., Wheeler J., Alfajaro M.M., Levavasseur E., Fontes B., Ravindra N.G., Van Dijk D., Mane S., Gunel M., Ring A., Jaffar Kazmi S.A., Zhang K., Wilen C.B., Horvath T.L., Plu I., Haik S., Thomas J.-L., Louvi A., Farhadian S.F., Huttner A., Seilhean D., Renier N., Bilguvar K., Iwasaki A. Neuroinvasion of SARS-CoV-2 in human and mouse brain. bioRxiv Prepr. Serv. Biol. 2020;6:9. doi: 10.1101/2020.06.25.169946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E., Zhang C., Israelow B., Lu-Culligan A., Prado A.V., Skriabine S., Lu P., Weizman O.-E., Liu F., Dai Y., Szigeti-Buck K., Yasumoto Y., Wang G., Castaldi C., Heltke J., Ng E., Wheeler J., Alfajaro M.M., Levavasseur E., Fontes B., Ravindra N.G., Van Dijk D., Mane S., Gunel M., Ring A., Kazmi S.A.J., Zhang K., Wilen C.B., Horvath T.L., Plu I., Haik S., Thomas J.-L., Louvi A., Farhadian S.F., Huttner A., Seilhean D., Renier N., Bilguvar K., Iwasaki A. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021:218. doi: 10.1084/jem.20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinato G., Fabbris C., Polesel J., Cazzador D., Borsetto D., Hopkins C., Boscolo-Rizzo P. Alterations in Smell or Taste in Mildly Symptomatic Outpatients with SARS-CoV-2 Infection. JAMA - J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone M.S., Martyn L., Weaver C.M. Potassium intake, bioavailability, hypertension, and glucose control. Nutrients. 2016 doi: 10.3390/nu8070444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan T., Khoo B., Mills E.G., Phylactou M., Patel B., Eng P.C., Thurston L., Muzi B., Meeran K., Prevost A.T., Comninos A.N., Abbara A., Dhillo W.S. Association between high serum total cortisol concentrations and mortality from COVID-19. Lancet Diabetes Endocrinol. 2020 doi: 10.1016/S2213-8587(20)30216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J., Kaba H., Saito H., Seto K. Electrophysiological evidence that circulating angiotensin II sensitive neurons in the subfornical organ alter the activity of hypothalamic paraventricular neurohypophyseal neurons in the rat. Brain Res. 1985;342:361–365. doi: 10.1016/0006-8993(85)91137-0. [DOI] [PubMed] [Google Scholar]
- Thevarajan I., Nguyen T.H.O., Koutsakos M., Druce J., Caly L., van de Sandt C.E., Jia X., Nicholson S., Catton M., Cowie B., Tong S.Y.C., Lewin S.R., Kedzierska K. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat. Med. 2020;26:453–455. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todkar A., Picard N., Loffing-Cueni D., Sorensen M.V., Mihailova M., Nesterov V., Makhanova N., Korbmacher C., Wagner C.A., Loffing J. Mechanisms of renal control of potassium homeostasis in complete aldosterone deficiency. J. Am. Soc. Nephrol. 2015;26:425–438. doi: 10.1681/ASN.2013111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubuka T., Moriya S., Soga T., Parhar I. Identification of transmembrane protease serine 2 and forkhead box a1 as the potential bisphenol a responsive genes in the neonatal male rat brain. Front. Endocrinol. (Lausanne) 2018;9 doi: 10.3389/fendo.2018.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin–Angiotensin–Aldosterone System Inhibitors in Patients with Covid-19. N. Engl. J. Med. 2020;382:1653–1659. doi: 10.1056/nejmsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA - J. Am. Med. Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn D.L.S., Beedle A.M., Ferguson A.V. Inhibition of subfornical organ neuronal potassium channels by vasopressin. Neuroscience. 1999;93:349–359. doi: 10.1016/S0306-4522(99)00125-6. [DOI] [PubMed] [Google Scholar]
- Wheatley M., Hawtin S.R., Yarwood N.J. Structure/function studies on receptors for vasopressin and oxytocin. Adv. Exp. Med. Biol. 1998 doi: 10.1007/978-1-4615-4871-3_46. [DOI] [PubMed] [Google Scholar]
- World Health Organization . 2020. WHO Coronavirus Disease (COVID-19) Dashboard | WHO Coronavirus Disease (COVID-19) Dashboard [WWW Document] URL https://covid19.who.int/ (accessed 12.14.20) [Google Scholar]
- Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM Structure of the 2019-nCoV Spike in the Prefusion Conformation. bioRxiv. 2020 doi: 10.1101/2020.02.11.944462. 2020.02.11.944462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J.W., Roberts K.A., Stubley L.A., Hanesworth J.M., Harding J.W. Hypothalamic angiotensin release in response to AII or glutamic acid stimulation of the SFO in rats. Brain Res. Bull. 1993;31:649–654. doi: 10.1016/0361-9230(93)90136-y. [DOI] [PubMed] [Google Scholar]
- Wu C.-I., Postema P.G., Arbelo E., Behr E.R., Bezzina C.R., Napolitano C., Robyns T., Probst V., Schulze-Bahr E., Remme C.A., Wilde A.A.M. SARS-CoV-2, COVID-19 and inherited arrhythmia syndromes. Hear. Rhythm. 2020 doi: 10.1016/j.hrthm.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Lu A., Wang H., Fang H., Zhou L., Sun P., Yang T. (Pro)renin receptor regulates potassium homeostasis through a local mechanism. Am. J. Physiol. - Ren. Physiol. 2017;313:F641–F656. doi: 10.1152/ajprenal.00043.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xydakis M.S., Dehgani-Mobaraki P., Holbrook E.H., Geisthoff U.W., Bauer C., Hautefort C., Herman P., Manley G.T., Lyon D.M., Hopkins C. Smell and taste dysfunction in patients with COVID-19. Lancet. Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30293-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Hogue B.G. Role of the Coronavirus E Viroporin Protein Transmembrane Domain in Virus Assembly. J. Virol. 2007;81:3597–3607. doi: 10.1128/jvi.01472-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshitomi K., Naruse M., Hanaoka K., Yamamura Y., Imai M., Kurokawa K. Functional characterization of vasopressin V1 and V2 receptors in the rabbit renal cortical collecting duct. Kidney Int. Suppl. 1996;55:S177–82. [PubMed] [Google Scholar]
- Youn J.H. Gut Sensing of Potassium Intake and its Role in Potassium Homeostasis. Semin. Nephrol. 2013;33:248–256. doi: 10.1016/j.semnephrol.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousaf Z., Al-Shokri S.D., Al-Soub H., Mohamed M.F.H. COVID-19-associated SIADH: A clue in the times of pandemic! Am. J. Physiol. - Endocrinol. Metab. 2020 doi: 10.1152/AJPENDO.00178.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharov A., Papaiconomou C., Johnston M. Lymphatic vessels gain access to cerebrospinal fluid through unique association with olfactory nerves. Lymphat. Res. Biol. 2004;2:139–146. doi: 10.1089/lrb.2004.2.139. [DOI] [PubMed] [Google Scholar]
- Zhang A.J., Lee A.C.-Y., Chu H., Chan J.F.-W., Fan Z., Li C., Liu F., Chen Y., Yuan S., Poon V.K.-M., Chan C.C.-S., Cai J.-P., Wu K.L.-K., Sridhar S., Chan Y.-S., Yuen K.-Y. Severe Acute Respiratory Syndrome Coronavirus 2 Infects and Damages the Mature and Immature Olfactory Sensory Neurons of Hamsters. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Hou Y., Shen J., Huang Y., Martin W., Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020;6:14. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]