Abstract
Objective
To investigate the epidemiological dynamics, transmission patterns, and the clinical outcomes of Coronavirus disease 2019 (COVID-19) in familial cluster patients in Wuhan, China.
Methods
Between January 22, 2020, and February 4, 2020, we enrolled 214 families for this retrospective study. The COVID-19 cases were diagnosed using real-time reverse-transcriptase polymerase chain reaction (RT-PCR). The number of COVID-19 subjects in a family, their relationship with index patients, the key time-to-event, exposure history, and the clinical outcomes were obtained through telephone calls.
Results
Overall, 96 families (44.9%) met the criteria of a familial cluster, which is at least one confirmed case in addition to the index patient in the same household. The secondary attack rate was 42.9%, and nearly 95% of index patients transmitted the infection to ≤2 other family members. High transmission pattern was noted between couples (51.0%) and among multi-generations (27.1%). The median serial interval distribution in familial clusters was 5 days (95% CI, 4 to 6). The case fatality rate was 8.7% in index patients and 1.7% in non-familial clusters patients (p = 0.023).
Conclusions
There is a related higher attack rate and worse clinical outcomes in COVID-19 family clusters.
Keywords: COVID-19, Household transmission, Epidemiological dynamics, Transmission patterns
Introduction
Coronavirus disease 2019 (COVID-19), caused by a novel beta-coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is an emerging global pandemic (World Health Organization, 2021, Wang et al., 2020a). The COVID-19 outbreak began in Wuhan, China, in December 2019 but rapidly spread throughout the worldwide by early 2020 (World Health Organization, 2021, Wang et al., 2020a). Until December 15, 2020, more than 70 million COVID-19 cases had been confirmed, globally, with China recording 95167 patients and 4761 deaths (5.0%) (World Health Organization, 2021).
Emerging evidence highlights the distribution of the disease at the household level and the risk factors associated with secondary infection (Chan et al., 2020, Chen et al., 2020a, Li et al., 2020a, Li et al., 2020b, Wang et al., 2020b, Ye et al., 2020). Given the limited health facilities in Wuhan at the beginning of the COVID-19 outbreak, most patients with mild to moderate COVID-19 have had to quarantine at home (Chen et al., 2020b, Li et al., 2020c). This predisposed close contacts of the COVID-10 patients to the infection. Previous COVID-19 family infection rates have been between 16.3–30.0% (Li et al., 2020b, Wang et al., 2020b), which can cause relatively high basic reproductive rates (R0), particularly outside the Hubei province, as indicated by the World Health Organization (WHO)-China Joint Mission (The Who-China Joint Mission, 2020). However, available studies have not reported on epidemiological patterns, clinical features, and outcomes of household transmission.
Herein, we describe the integral epidemiological dynamics, transmission patterns, and risk factors of household COVID-19 transmission in Wuhan. We also investigate the clinical characteristics and outcomes of COVID-19 patients from familial clusters. These findings are vital in devising future containment measures against the spread of COVID-19.
Materials and methods
Sources of data
This was a retrospective study conducted at the fever clinics of Renmin Hospital of Wuhan University, which is one of the major designated hospitals in Wuhan, Hubei province, China. After the COVID-19 outbreak, the fever clinics were designated by the government to provide more highly effective medical care including meticulous interrogation, necessary examination, and nucleic acid tests of patients presenting with fever, defined as a temperature of 37.3 °C or higher.
We reviewed suspected COVID-19 cases from January 22, 2020, to February 4, 2020, and extracted telephone numbers, laboratory findings, and record of chest computed tomographic (CT) scans from the clinical electronic medical data. After March 6, 2020, we collected epidemiological and familial data via telephone calls. Information captured included the number of persons in a family, their relationship with the index patients, and the number of confirmed or suspected COVID-19 subjects in family. We also collected epidemiologic information such as the time from onset of illness and laboratory confirmation, history of COVID-19 exposure, protective measures after exposure employed by index and secondary patients in a family, the underlying medical history, and the clinical outcomes of all COVID-19 patients.
The data were collected in a standardized case report form by qualified health professionals. All information was captured in an Excel Database in duplicate and independently verified by two authors. Any clarifications were requested from the physicians attending to the patients. Where necessary, the inclusion and exclusions were agreed upon collectively.
Case definitions
The suspected cases were defined as illness accompanied with a fever, with or without respiratory symptoms, and/or exposure to a live animal market or close contact with confirmed or probable cases within 14 days before illness onset. According to the WHO, a confirmed case was definitively positive for SARS-COV-2 after real-time reverse-transcriptase polymerase chain reaction (RT-PCR) test or by high-throughput sequencing. A probable patient was a person only with the clinical symptoms or the radiological findings of COVID-19 (World Health Organization, 2020a). Only laboratory-confirmed COVID-19 patients with certain medical records were enrolled in the follow-up survey.
The primary outcomes were the incidences of laboratory confirmed COVID-19 cases in a household after disease exposure from a family member. The secondary outcomes included the rate of death and hospitalization and the proportion of individuals quarantined in a centralized isolation center or at home.
Familial clusters of COVID-19 cases were defined as at least one confirmed cases in addition to the index patients in the same household within 14 days from the illness onset of index patient. The non-familial cluster was the family with only one confirmed COVID-19 patient. An index patient was defined as the first laboratory-confirmed cases in a familial cluster. Secondary patients were classified in the laboratory-confirmed cases who closely interacted with index patients for a maximum of 2 weeks. Self-protective measures by family members and strict quarantine of index patients were defined as wearing of surgical masks and separate dining at home. If that fails, we deem it to be unshielded or without intense quarantine.
Laboratory confirmation
Laboratory confirmation of the SARS-COV-2 RNA was performed by RT-PCR at Renmin Hospital of Wuhan University (Shi et al., 2020). Briefly, the nucleic acid was extracted from sputum and throat swab samples based on the Viral Nucleic Acid Kit (Health, Ningbo, China) following the manufacturer’s instructions. A COVID-19 detection kit (Bioperfectus, Taizhou, China) was used in detecting the ORF1ab and the N genes. The procedure used for RT-PCR assay was adopted from the WHO protocol (World Health Organization, 2020b). A positive test was defined as a cycle threshold value (Ct value) less than 37, while a negative test was defined by Ct value more than 40. The Ct value more than 37 were replicated twice within 48h. Laboratory confirmation of COVID-19 was based on the positive results for both ORF1ab and the N genes.
Statistical analysis
The epidemic plot of familial clusters was constructed by the day of illness onset. The serial interval distribution (the times of illness onset between index patients and secondary patients) was estimated by fitting a three-parameter lognormal distribution after Anderson-Darling test.
Continuous data were presented as mean ± standard deviation (SD) or median (interquartile range [IQR]) values, and categorical data were described as percentages. To analyze the differences between index or secondary patients from familial clusters and non-clusters patients, we used the Mann–Whitney U test or t-test depending on parametric or nonparametric data for continuous variables and the χ 2 test or Fisher’s exact test for categorical variables, as appropriate. A two-tailed p-value <0.05 was considered statistically significant.
Serial interval analysis was performed using Minitab Statistical Software V19 (Minitab LLC.). Statistical charts or boxplots were constructed using Office 2013 (Microsoft) or Prism 5 (GraphPad). Other analyses were performed using SPSS Software V19.0 (IBM Corp.).
Results
Description of the familial clusters
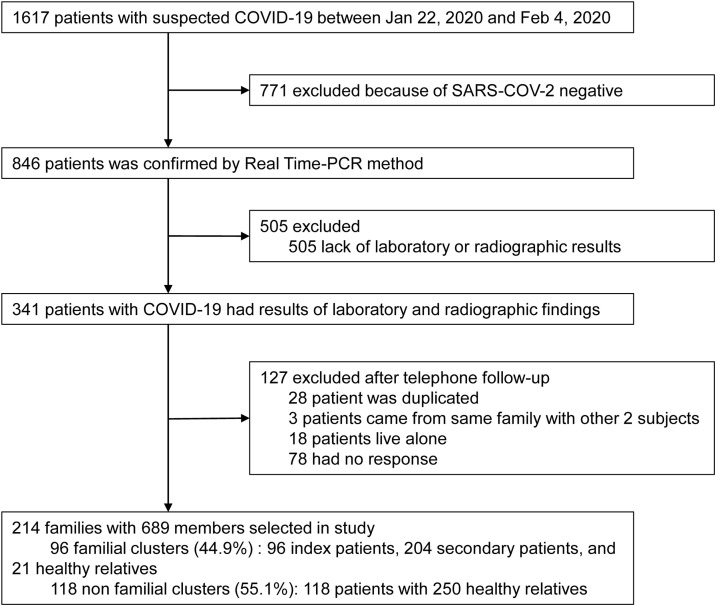
Between Jan 22, 2020, and Feb 4, 2020, 1617 suspected COVID-19 cases attended the fever clinics of Renmin Hospital of Wuhan University. Of these, 846 patients (52.3%) were confirmed as positive cases via PCR (Figure 1 ), and 341 subjects were followed-up through telephone calls for up to 1 month. Finally, 214 families comprising 689 members were included in this study. Geographically, 155 families (72.4%) reside at the Wuchang District of Wuhan City, which is more than 10 kilometers from Huanan Seafood Wholesale Market. Of the 214 families, 96 families (44.9%) met the criteria for a familial cluster.
Figure 1.
Study flow chart. COVID-19 = coronavirus disease 2019. SARS-COV-2 = severe acute respiratory syndrome coronavirus 2.
Clinical outcomes
For the primary outcome, of 475 close contacts of the first lot of COVID-19 patients, 224 persons exhibited symptoms and signs of suspected COVID-19 and 204 cases (attack rate, 42.9%) were positive for COVID-19. Most index patients (74.0%) spread the virus to 1 relative, whereas 20 cases (20.8%) transmitted the disease to 2 other family members.
At the end of the study, secondary outcomes were only evaluated in 118 non-clusters, 92 index patients, and 118 secondary patients because of the lack of some data (Table 1 ). Case fatality rate was 8.7% in index patients and 1.7% in non-familial clusters (p = 0.023). Secondary patients exhibited the highest hospitalization rate (45.8%), followed by index patients (28.3%) and non-cluster patients (12.7%).
Table 1.
Demographic and clinical outcomes of COVID-19 patients from familial clusters. Wearing of surgical masks and strict quarantine of family members with COVID 19 were the self-protective measures considered in this study. Failure to embrace these precautions was deemed as unshielded or without intense quarantine.
| Non-cluster | Familiar clusters |
||||
|---|---|---|---|---|---|
| Index patients | p Value | Secondary patients | p Value | ||
| n | 118 | 96 | – | 125 | – |
| Male(%) | 50 (42.4%) | 47 (49.0%) | 0.336 | 71 (56.8%) | 0.025 |
| Female(%) | 68 (57.6%) | 49 (51.0%) | – | 54 (43.2%) | – |
| Age, yr | 56.4 ± 13.8 | 57.3 ± 15.1 | 0.292 | 52.6 ± 17.1 | 0.203 |
| Age distribution, no./total no.(%) | |||||
| ≤30 years | 7/118 (5.9%) | 3/95 (3.2%) | 0.517 | 9/120 (7.5%) | 0.629 |
| 31–40 years | 10/118 (8.5%) | 15/95 (15.8%) | 0.099 | 26/120 (21.7%) | 0.005 |
| 41–50 years | 22/118 (18.6%) | 9/95 (9.5%) | 0.059 | 20/120 (16.7%) | 0.689 |
| 51–60 years | 36/118 (30.5%) | 25/95 (26.3%) | 0.503 | 23/120 (19.2%) | 0.043 |
| 61–70 years | 30/118 (25.4%) | 23/95 (24.2%) | 0.839 | 23/120 (19.2%) | 0.246 |
| ≥71 years | 13/118 (11.0%) | 20/95 (21.1%) | 0.044 | 19/120 (15.8%) | 0.276 |
| Exposure, no./total no.(%) | |||||
| Exposure to Huanan seafood market | 2/118 (1.7%) | 0/58 (0.0%) | 1 | – | – |
| Exposure to Other wet market | 27/118 (22.9%) | 12/58 (20.7%) | 0.742 | – | – |
| Contact to COVID-19 Patients | 8/118 (6.8%) | 12/58 (20.7%) | 0.01 | 125/125 (100.0%) | <0.001 |
| Self-protection during nursing | – | – | – | 43/101 (42.6%) | – |
| Unshielded during nursing | – | – | – | 10/101 (9.9%) | – |
| Daily contact | – | – | – | 48/101 (47.5%) | – |
| No related exposure | 69/118 (58.5%) | 33/58 (56.9%) | 0.872 | 0/125 (0.0%) | <0.001 |
| Other exposure | 12/118 (10.2%) | 1/58 (1.7%) | 0.063 | 0/125 (0.0%) | <0.001 |
| Isolation at home after illness onset, no./total no.(%) | |||||
| Stringent quarantine | 34/55 (61.8%) | 38/58 (65.5%) | 0.683 | – | – |
| Without strict isolation | 21/55 (38.2%) | 20/58 (34.5%) | – | – | – |
| Coexisting comorbidity, no./total no.(%) | |||||
| Any | 55/117 (47.0%) | 46/90 (51.1%) | 0.558 | 39/109 (35.8%) | 0.087 |
| 1 comorbidity | 34/117 (29.1%) | 28/90 (31.1%) | 0.749 | 23/109 (21.1%) | 0.169 |
| 2 comrbidities | 13/117 (11.1%) | 14/90 (15.6%) | 0.347 | 14/109 (12.8%) | 0.688 |
| ≥3 comrbidities | 8/117 (6.8%) | 4/90 (4.4%) | 0.465 | 2/109 (1.8%) | 0.104 |
| Diabetes | 19/117 (16.2%) | 9/90 (10.0%) | 0.193 | 9/109 (8.3%) | 0.069 |
| Hypertension | 31/117 (26.5%) | 29/90 (32.2%) | 0.368 | 21/109 (19.3%) | 0.197 |
| CHD | 7/117 (6.0%) | 11/90 (12.2%) | 0.114 | 2/109 (1.8%) | 0.173 |
| Cancer | 4/117 (3.4%) | 2/90 (2.2%) | 0.699 | 3/109 (2.3%) | 1 |
| Pulmonary diseases | 4/117 (3.4%) | 1/90 (1.1%) | 0.39 | 3/109 (2.3%) | 1 |
| CVD | 2/117 (1.7%) | 2/90 (2.2%) | 1 | 2/109 (1.8%) | 1 |
| Others | 14/117 (12.0%) | 13/90 (14.4%) | 0.6 | 13/109 (12.0%) | 0.993 |
| Outcome at data cutoff, no./total no.(%) | |||||
| Quarantine | 65/118 (55.1%) | 38/92 (41.3%) | 0.047 | 37/118 (31.4%) | <0.001 |
| Stay at home | 36/118 (30.5%) | 22/92 (24.0%) | 0.289 | 23/118 (19.5%) | 0.051 |
| Hospitalization | 15/118 (12.7%) | 26/92 (28.3%) | 0.005 | 54/118 (45.8%) | <0.001 |
| Death | 2/118 (1.7%) | 8/92 (8.7%) | 0.023 | 4/118 (3.4%) | 0.683 |
CVD = cerebrovascular disease. CHD = coronary heart disease.
The transmission model of familial clusters
There were four major transmission patterns in 96 familial clusters: transmission between couples (Model 1, 49/96, 51.0%), transmission strictly from parents to offspring (Model 2, 13/96, 13.5%), spread from offspring to parents only (Model 3, 8/96, 8.3%), and multi-transmission (Model 4, 26/96, 27.1%).
Key time-to-event intervals of familial clusters
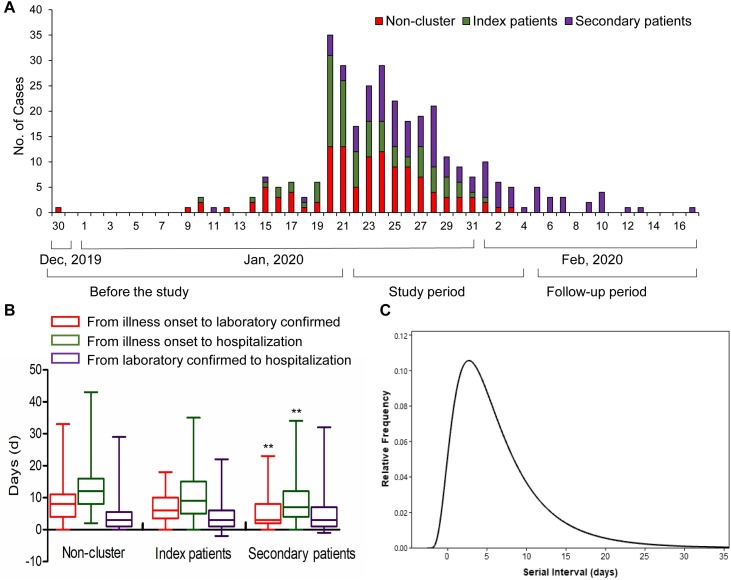
The epidemic plot showed that incidences of familial clusters occurred before January 20, 2020 (Figure 2 A). Subsequently, they gradually increased, particularly between January 20 and February 3. The median serial interval distribution in familial clusters was estimated to be 5 days (95% CI, 4 to 6) (Figure 2C). No substantial difference in the key time-to-event intervals between index and non-clusters patients was observed. For secondary patients, the median times from illness onset to laboratory confirmation were shorter than that of non-clusters cases (3 days vs. 8 days, p < 0.001) and the median days from illness onset to hospitalization (7 days vs 9 days, p < 0.001) (Figure 2B).
Figure 2.
The key time-to-event intervals of familial clusters. (A) The epidemic distribution of the COVID-19 cases based on the day of illness onset. (B) The key time-to-event intervals among COVID-19 cases in the non-familial clusters and index and secondary patients from familial clusters. (C) The serial interval distribution in familial clusters. **present p < 0.001 when compared with non-cluster group.
Profile of social and family-related factors
There were 3 family members in both non-familial clusters (range from 2 to 6) and familial clusters (range from 2 to 10) (p = 0.3661). However, the proportion of exposure to positively COVID-19 cases was higher in index patients (20.7% vs. 6.8%, p = 0.01). The median number of days of staying at home after SARS-COV-2 infection (defined as the time from onset of illness to hospitalization) was slightly higher in index patients than in non-familial clusters patients (12 days vs. 9 days) but was statistically insignificant (p = 0.198), (Figure 2B). During the home isolation period, 61.8% of subjects in non-familial clusters and 65.5% index patients observed strict quarantine measures (p = 0.683), whereas 42.6% of secondary patients only wore masks to prevent infections from index patients.
Demographic and clinical characteristics
The demographic features of patients included in this study were summarized in Table 1. The median age of the 118 non-clusters patients was 56.4 ± 13.8 years, whereas the median age of index and secondary patients in the familial clusters were 57.3 ± 15.1 years and 52.6 ± 17.1 years, respectively. Compared with the patients from non-familial clusters, index patients in familial cluster was older, generally above 70 years of age (21.1% vs 11.0%, p = 0.044). Conversely, the secondary patients were generally younger, with the majority being males. There were 51.1% index patients with at least one underlying comorbidity, such as hypertension (32.2%), coronary heart disease (12.2%), diabetes (10.0%), and pulmonary diseases (1.1%).
Table 2 presents clinical characteristics of patients in various group clusters. In the full cohort, the most common initial symptoms were fever (75.5–84.9%), dry cough (33.3–37.7%), and fatigue (26.7–35.8%). There were only 2 asymptomatic cases in secondary patients. Compared with patients in non-familial clusters, those in the familial clusters were more likely to have dyspnea or pant (index patients, 24.5% vs 11.0%, p = 0.023; secondary patients, 24.4% vs 11.0%, p = 0.031) but less likely to present with digestive-system-related symptoms (index patients, 7.5% vs 22.9%, p = 0.016), such as vomiting, diarrhea, and/or nausea. No significant difference in other clinical symptoms was found between familial and non-clusters.
Table 2.
Clinical features of COVID-19 patients in the familial clusters.
| Non-cluster | Familiar clusters | ||||
|---|---|---|---|---|---|
| Index patients | p Value | Secondary patients | p Value | ||
| n | 118 | 53 | – | 45 | – |
| Symptoms, n (%) | |||||
| Asymptomatic | 0 (0.0%) | 0 (0.0%) | – | 2 (4.4%) | 0.08 |
| Fever | 97 (82.2%) | 45 (84.9%) | 0.663 | 34 (75.6%) | 0.339 |
| 37.3–38.0 °C | 29 (24.6%) | 16 (30.2%) | 0.441 | 9 (20.0%) | 0.537 |
| 38.1–39.0 °C | 53 (44.9%) | 22 (41.5%) | 0.678 | 21 (46.7%) | 0.841 |
| >39.0 °C | 15 (12.7%) | 7 (13.2%) | 0.929 | 4 (8.9%) | 0.497 |
| Dy cough | 43 (36.4%) | 20 (37.7%) | 0.871 | 15 (33.3%) | 0.711 |
| Sputum production | 8 (6.8%) | 2 (3.8%) | 0.726 | 4 (8.9%) | 0.738 |
| Fatigue | 40 (33.9%) | 19 (35.8%) | 0.804 | 12 (26.7) | 0.376 |
| Myalgia | 8 (6.8%) | 6 (11.3%) | 0.369 | 2 (4.4%) | 0.728 |
| Dyspnoea/pant | 13 (11.0%) | 13 (24.5%) | 0.023 | 11 (24.4%) | 0.031 |
| Vomiting/diarrhea/nausea | 27 (22.9%) | 4 (7.5%) | 0.016 | 10 (22.2%) | 0.928 |
| Sore throat/throat discomfort | 7 (5.9%) | 3 (5.7%) | 1 | 2 (4.4%) | 1 |
| Headache/dizziness | 4 (3.4%) | 2 (3.8%) | 1 | 2 (4.4%) | 0.668 |
| Chest distress | 10 (8.5%) | 3 (5.7%) | 0.757 | 2 (4.4%) | 0.519 |
| Laboratory findings | |||||
| White blood cell count (×109/L) (n, (%)) | 5.6 (4.0–7.0) | 4.8 (3.9–5.9) | 0.3523 | 5.8 (4.5–7.2) | 0.3345 |
| <3.5 | 19 (16.1%) | 7 (13.2%) | 0.626 | 5 (11.1%) | 0.421 |
| 3.5–9.5 | 94 (79.7%) | 40 (75.5%) | 0.538 | 37 (82.2%) | 0.713 |
| >9.5 | 5 (4.2%) | 6 (11.3%) | 0.097 | 3 (6.7%) | 0.686 |
| Neutrophil count (×109/L) (n,(%)) | 3.7 (2.4–4.9) | 3 (2.5–5.7) | 0.5224 | 3.9 (2.7–4.7) | 0.5135 |
| <1.8 | 8 (6.8%) | 9 (17.0%) | 0.039 | 0 (0.0%) | 0.108 |
| 1.8–6.3 | 95 (80.5%) | 37 (69.8%) | 0.123 | 41 (91.1%) | 0.104 |
| >6.3 | 15 (12.7%) | 7 (13.2%) | 0.929 | 4 (8.9%) | 0.497 |
| Lymphocyte count (×109/L) (n, (%)) | 1.1 (0.9–1.5) | 1 (0.7–1.2) | 0.2596 | 1.3 (0.8–1.7) | 0.3769 |
| <1.1 | 55 (46.6%) | 28 (52.8%) | 0.452 | 16 (35.6%) | 0.203 |
| 1.1–3.2 | 62 (52.6%) | 25 (47.2%) | 0.516 | 29 (64.4%) | 0.171 |
| >3.2 | 1 (0.8%) | 0 (0.0%) | 1 | 0 (0.0%) | 1 |
| Monocyte count (×109/L) | 0.5 (0.3–0.7) | 0.4 (0.3–0.6) | 0.7996 | 0.5 (0.4–0.6) | 0.4933 |
| Haemoglobin (g/L) | 140.0 (130.3–153.0) | 137.5 (129.0–147.0) | 0.0063 | 139.5 (127.5–151.0) | 0.9826 |
| Platelet count (×109/L) | 175.0 (143.0–214.3) | 150.5 (109.0–175.8) | 0.1491 | 190 (155.0–221.0) | 0.2559 |
| CRP (mg/L) | 16.9(3.3–37.5) | 22.9 (5.1–94.5) | 0.9568 | 6.3 (1.1–32.5) | 0.2499 |
| SAA (mg/L) | 81.0 (31.5–300.0) | 279.3 (58.3–300.0) | 0.2239 | 56.2 (5.8–245.7) | 0.1146 |
| Findings on chest CT | |||||
| Normal | 2 (1.7%) | 1 (1.9%) | 1 | 0 (0.0%) | 1 |
| Ground-glass opacity | 95 (80.5%) | 37 (69.8%) | 0.123 | 35 (77.8%) | 0.698 |
| Bilateral distribution | 89 (75.4%) | 33 (62.3%) | 0.078 | 28 (62.2%) | 0.094 |
| Left lung | 3 (2.5%) | 1 (1.9%) | 1 | 1 (2.2%) | 1 |
| Right lung | 3 (2.5%) | 3 (5.7%) | 0.375 | 6 (13.3%) | 0.014 |
| Peripheral distribution | 11 (9.3%) | 7 (13.2%) | 0.466 | 6 (13.3%) | 0.568 |
| Consolidation | 4 (3.4%) | 2 (3.8%) | 1 | 6 (13.3%) | 0.028 |
| Increased bronchovascular shadows | 37 (31.4%) | 18 (34.0%) | 0.736 | 13 (28.9%) | 0.76 |
| Patch shadows/linear opacities | 18 (15.3%) | 15 (28.3%) | 0.046 | 9 (20.0%) | 0.466 |
CRP = C-reaction protein. SAA = serum amyloid A. CT = computed tomographic.
Laboratory assessment revealed the median levels of mostly clinical parameters such as leukocyte, neutrophil, lymphocyte, monocyte, and platelet counts were within the normal range in the three groups. Compared with non-cluster patients, index patients had a relatively high proportion of neutropenia (17.0% vs 6.86.8%, p = 0.039). The median SAA values for the three groups were increased (81.0 mg/L in non-cluster, 279.3 mg/L in index patients, and 56.2 mg/L in secondary patients). Relatively higher CRP levels were observed in non-cluster and index patients (16.9 mg/L and 22.9 mg/L, respectively), but not in secondary patients (6.3 mg/L).
Chest CT revealed that only 2 (1.7%) cases in non-clusters and 1 index patient (1.9%) had normal chest anatomy. The most common abnormal manifestation of COVID-19 was ground-glass opacity (69.8–80.5%), particularly bilateral lung involvement and higher bronchovascular shadows (28.9–34.0%). The inchoate presentations of CT imaging (patch shadows and/or linear opacities) were found in 15 index patients (28.3%), which is significantly higher than in non-clusters patients (15.3%, p = 0.046).
Discussion
In this study, among 214 families with 689 members, 44.9% of the families in Wuhan, Hubei Province, China, experienced household transmission. The overall households transmission rate was 42.9%, comparable with findings in other cities outside Wuhan (16.3% for Hubei province and 3-34% for outside Hubei province) (Li et al., 2020b, Wang et al., 2020b, The Who-China Joint Mission, 2020).
In addition, within the restricted isolation centers, the estimate of R0 in intra-familial transmission was limited to the fixed numbers per family. Intensified awareness and subsequent control measures rapidly reduced transmissibility. Accordingly, we only analyzed the number of infections arising from contact with first COVID-19 patients within a family, rather than calculating the R0. Notably, in 96 COVID-19 familial clusters (44.9%), 74.0% of the index patients spread the SARS-COV-2 virus to 1 contact person, whereas 20.8% of the individuals transmitted the disease to 2 people. This finding indicates that home isolation ensured that the limited health facilities in Wuhan at the time were not overwhelmed by the epidemic; however, it increased infection risk of COVID-19 among familial members. Thus, home quarantine is an important but not ideal measure of controlling the spread of COVID-19.
Previous reports indicate that the spouses were at risk of contracting the virus from their partners (Li et al., 2020b). Notably, we also found that Model 1 was the most common means of transmission between husband and wife (51.0%). Conversely, 13.5% families were more likely to transmit SARS-COV-2 virus from single parents to siblings (Model 2); for example, a 67-year-old mother transmitted the disease to her adult daughter. These phenomena can be explained by the structure of the home-based care system. In China, the spouse or an adult child primarily provide cares for the elderly in case of an illness (Shi, 2017). In stem families, the grandparent assume the homecare responsibility for their grandchildren as their adult children fend for the family (Shi, 2017), which also potentially increases the risk of COVID-19 transmission (Model 4, 27.1%).
It has been reported that the duration of home care, poor hand hygiene practices, and poor use of masks rather than the family size are associated with higher secondary household risk of SARS (Wilson-Clark et al., 2006). However, in this study, there was no substantial difference in the number of family members, days of home isolation after the onset of illness, the proportion of individuals wearing a surgical mask, and isolated eating between index and non-familial clusters patients (Figure 2 and Table 1). Similarly, the time between the onset of illness and hospitalization of index patients was not associated with the household transmission of COVID-19 (OR, 0.9; 95% CI, 0.61–1.33) (Li et al., 2020b). These findings suggested that the above factors did not impact the transmission patterns of COVID-19 among familial members. These unique results may be attributed to a difference in viral tropism between COVID-19 and SARS (Wang et al., 2020b, Guan et al., 2020) and the nonstandard home care practices. Thus, there is an urgent need to accurately uncover risk factors for household COVID-19 transmission to design standard infection prevention and control measures for the public at home.
Familial clustering demonstrated a clinical spectrum of patients typical with COVID-19 symptoms testing negative for SARS-COV-2, asymptomatic carriers, atypical symptomatic cases, and mild to moderate illness and deaths. Most household contacts (57.1%) remained healthy at the end of study, in which 20 cases with pneumonia were confirmed as SARS-COV-2 negative via RT-PCR laboratory tests. Furthermore, there were 2 asymptomatic carriers from 45 secondary patients (4.4%) (Table 2), which is lower than other proportions reported outside Wuhan (14.1%) (Li et al., 2020b) and outside Hubei (20.0%) (Bi et al., 2020). The most common symptoms COVID-19 symptoms at the onset of the disease were fever (temperature of more than 37.3 °C), dry cough, and fatigue, which is consistent with earlier studies (Guan et al., 2020, Xu et al., 2020). However, more than 20% of patients from familial clusters exhibited dyspnea and/or panting, whereas 22.9% of non-familial clusters cases present with gastrointestinal symptoms, such as vomiting, diarrhea, and nausea. Additionally, less than half of COVID-19 patients exhibited hematologic abnormalities. Further, index patients were more likely to suffer from neutropenia and lower hemoglobin level. Although the characteristic appearances of chest CT are ground-glass opacity (Guan et al., 2020, Xu et al., 2020), 1.9% of index patients presented with normal chests, with only 8.3% of the cases exhibited patch shadows and/or linear opacities. Therefore, this study underscores the significant role of the family setting in studying the natural history of COVID-19.
To our knowledge, this is the first study reporting worse clinical outcomes (the rate of death and hospitalization at the time of data cutoff) for COVID-19 patients in familial clusters. We found that the case fatality rate in index patients was 5 times that of non-cluster subjects (8.7% vs 1.7%, p = 0.023) and higher than the overall fatality ratio in China (5.0%) (World Health Organization, 2021). In particular, old age and elevated respiratory rate (dyspnea) are risk factors for COVID-19 associated mortalities (Fei Zhou et al., 2020), as demonstrated in familial clusters (Table 1, Table 2). There were 28.3% index and 45.8% secondary patients still hospitalized at the end of the study, which implies that additional medical care is needed.
Our findings notwithstanding, this cohort steady has several notable limitations. First, the small sample sizes may cast doubt on the credibility of our findings. Second, the relatively higher non-response rate (22.9%) recorded in this study presents a potential non-respondent bias. Some people were apprehensive of sharing critical information having been dissatisfied with the medical care they received at the beginning of the outbreak. Third, the laboratory-confirmed cases were exclusively identified by the RT-PCR test, which may have missed few positive cases, particularly the asymptomatic carriers. Fourth, although we employed best principles and methodologies for clinical trials, the responses are subject to recall bias.
In conclusion, this study described the integral epidemiological and clinical characteristics of familial clusters with COVID-19 in Wuhan. An intriguing 42.9% of family members, particularly spouses, were infected with COVID-19 by their relatives (index patients). However, in this study, the social and family-related factors, such as the number of family members, the days of home isolation after illness onset, and separating dining, did not impact on the risk of household transmission. Nonetheless, the worse clinical outcomes of COVID-19 patients were found in family clusters. Taken together, household transmission of COVID-19 poses a serious health threat. As such, as opposed to home isolation, we recommend all confirmed or suspected cases and their close contacts should be referred to centralized quarantine.
Contributors
YB, WZY, and SX designed the study. YB, FGK, CDD, LW, QL, and HYP collected the data. YB and FGK performed analysis and interpretation of the data. YB drafted the manuscript, and FGK, WZY, and SX edited and revised the article.
Ethics approval
The study was approved by the Clinical Research Ethics Commission of Renmin Hospital of Wuhan University (WDRY2020-K051). Verbal consent was obtained from patients via telephone calls.
Funding source
This study was supported by Zhejiang Universityspecial scientific research fund for COVID-19 prevent and control (2020XGZX015), the N ational Natural Science Foundation of China (81900749), and the N ature Science Foundation of Hubei province (2018CFB150).
Conflict of interest
We declare no competing interests.
Acknowledgments
We are grateful to all the patients and their relatives who participated in the study. We express our sincere gratitude to the entire medical staff at the fever clinics for their relentless effort in managing COVID-19 patients.
References
- Bi Q., Wu Y., Mei S., Ye C., Zou X., Zhang Z. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. 2020;(April) doi: 10.1016/S1473-3099(20)30287-5. S1473-3099(20)30287-5. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Yin Q., Shi H., Du D., Chang S., Ni L. A familial cluster, including a kidney transplant recipient, of coronavirus disease 2019 (COVID-19) in Wuhan, China. Am J Transplant. 2020;20(7):1869–1874. doi: 10.1111/ajt.15903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Zhang Z., Yang J., Wang J., Zhai X., Barnighausen T. Fangcang shelter hospitals: a novel concept for responding to public health emergencies. Lancet. 2020;395(10232):1305–1314. doi: 10.1016/S0140-6736(20)30744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei Zhou T.Y., Du Ronghui, Fan Guohui, Liu Ying, Liu Zhibo, Xiang Jie. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020:1–9. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Ji F., Wang L., Wang L., Hao J., Dai M. Asymptomatic and human-to-human transmission of SARS-CoV-2 in a 2-family cluster, Xuzhou, China. Emerg Infect Dis. 2020;26(7):1626–1628. doi: 10.3201/eid2607.200718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Zhang B., Lu J., Liu S., Chang Z., Cao P. The characteristics of household transmission of COVID-19. Clin Infect Dis. 2020;71(8):1943–1946. doi: 10.1093/cid/ciaa450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J. The evolvement of family intergenerational relationship in transition: mechanism, logic, and tension. J Chin Sociol. 2017;4:20. [Google Scholar]
- Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Who-China Joint Mission . 2020. Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19) pp. 1–40. [Google Scholar]
- Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Ma W., Zheng X., Wu G., Zhang R. Household transmission of SARS-CoV-2. J Infect. 2020;81(1):179–182. doi: 10.1016/j.jinf.2020.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Clark S.D., Deeks S.L., Gournis E., Hay K., Bondy S., Kennedy E. Household transmission of SARS, 2003. CMAJ. 2006;175(10):1219–1223. doi: 10.1503/cmaj.050876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2020. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance.https://apps.who.int/iris/handle/10665/330893 January 28, 2020. [Google Scholar]
- World Health Organization . 2020. Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases: interim guidance.https://apps.who.int/iris/handle/10665/331329 2 March 2020. [Google Scholar]
- World Health Organization . 2021. Coronavirus disease 2019 (COVID-19) weekly epidemiological update and weekly operational update.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports [Google Scholar]
- Xu X.W., Wu X.X., Jiang X.G., Xu K.J., Ying L.J., Ma C.L. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F., Xu S., Rong Z., Xu R., Liu X., Deng P. Delivery of infection from asymptomatic carriers of COVID-19 in a familial cluster. Int J Infect Dis. 2020;94:133–138. doi: 10.1016/j.ijid.2020.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]