Abstract
Schizophrenia remains one of the most chronic and highly disabling mental disorder. To date, the pathomechanism of schizophrenia is not fully understood and current treatments are characterized by some limitations. First- and second-generation antipsychotics have shown clinical efficacy in treating positive symptoms, while are poorly effective on both negative symptoms and cognitive deficits. Moreover, they can involve many metabolic and neurological side effects, leading to low therapeutic compliance. Many evidence suggested that serotonin may play a complex role in the neurobiology of schizophrenia. Therefore, new drugs targeting 5-HT receptors (5-HTRs) have become an important area of research in schizophrenia in the hope that treatment efficacy may be improved without inducing side effects observed with currently available antipsychotics. Research using the main database sources was conducted to obtain an overview of preclinical and clinical pharmacological 5-HTR-targeted therapies in patients with schizophrenia. We identified 17 experimental serotonergic agents, under study for their potential use in schizophrenia treatment. Particularly, AVN-211, LuAF-35700 and Brilaroxazine are currently under clinical development. Moreover, some compounds showed some pro-cognitive and antipsychotic-like properties in animal models, while other agents showed contradictory effects in improving symptoms and were removed from the development program. Although some serotonergic drugs seem promising for improving the treatment of schizophrenia, further studies regarding the pathophysiological mechanisms of schizophrenia and novel compounds as well as high-quality trials are necessary in order to improve schizophrenia outcomes.
Keywords: schizophrenia, serotonin, experimental agents, negative symptoms, cognitive deficits
Introduction
Schizophrenia (SCZ) is a chronic, highly disabling mental disorder characterized by a miscellaneous of psychopathological domains, each with different courses, patterns of treatment-response, and prognostic implications.1 Even though many patients may display a substantial reduction of positive symptoms, very few subjects reach functional recovery during the course of illness.2 Available literature highlighted that functional outcomes are most consistently predicted by social cognition and neurocognitive deficits, as well as negative symptoms.3 Nevertheless, depressive symptoms may worsen the overall psychopathology, quality of life and social functioning of patients affected by SKZ.4
Since the discovery of chlorpromazine in the treatment of SCZ, pharmacological research focused mainly on dopamine hypothesis, namely that people with SCZ have enhanced dopaminergic activity, which can be normalized by using dopamine antagonists, specifically the dopamine D2 receptor (D2R) antagonist.5 Therefore, patients were originally treated with typical or first-generation antipsychotics (FGAs) merely acting as antagonists at postsynaptic D2R with poor effect on negative, depressive and cognitive symptoms. However, accumulating evidence on the neurobiology of SCZ suggested that serotonin (5-HT) may play a complex role in the modification of dopamine neurotransmission.6 Consequently, second-generation or atypical antipsychotics (SGAs) were introduced as being equally effective or better than FGAs, particularly for negative, depressive and cognitive symptoms, as well as able to limit the risk for extrapyramidal side effects (EPS) commonly developed as a consequence of FGAs treatment.7,8 In particular, the newest antipsychotics act on 5-HT receptors (5-HTRs) in different ways including the higher blockade of 5HT2AR than for D2R, the blockade of 5-HT6 and 5-HT7Rs, the full or partial 5-HT1AR agonism, the inverse agonism of 5-HT2CR and the alpha-2 noradrenaline receptor antagonism.9,10 However, most SGAs can have non-selective interactions with receptors that are not associated with antipsychotic efficacy (histaminergic, muscarinic and alpha-adrenergic ones), leading to metabolic disturbances, akathisia and cognitive impairment. Moreover, some SGAs can be associated with sustained high levels of striatal D2R occupancy that can increase the risk of motor side effects and hyperprolactinemia.8 In addition, previous systematic reviews and meta-analyses7,11,12 estimated a modest benefit of SGAs on negative and cognitive symptoms. Some investigators also assessed the possible effectiveness of alternative compounds including Selective Serotonin Reuptake Inhibitors (SSRIs) as augmentation of both FGAs and SGAs. However, contradictory findings were reported, particularly on improvement in negative and depressive symptoms.13–15 Nevertheless, augmentation strategies were associated with a substantial risk of decreased adherence and poor tolerability as a consequence of potential pharmacokinetic interactions and worsening of psychotic symptoms.16Therefore, in the light of the mentioned unmet needs, novel treatment strategies, based on more selective receptor activity profiles, became a key area of SCZ research in order to improve the efficacy and tolerability of new compounds.17 In this regard lumateperone, an investigational drug displaying high-affinity binding to the 5-HT2AR as well to D1 and D2Rs, but with minimal binding affinity for 5-HT2CR, histaminergic and muscarinic receptors, received its first global approval in the USA for the treatment of SCZ in adults, in view of its efficacy with a favorable safety profile.18–20 So far, other 5-HTR targeted compounds are emerging as possible pharmacological treatments for SCZ. Therefore, the purpose of this review is to evaluate the main preclinical and clinical pharmacological findings concerning the therapeutic potential of 5-HTR-targeted therapies in SCZ patients.
Methods
Registries of clinical trials by the US National Institutes of Health (NIH – Clinical Trials.gov, https://www.clinicaltrials.gov) and the EU European Medicines Agency (EMA – https://www.clinicaltrialsregister.eu) were consulted in order to get information on clinical trials with selective serotonergic agents for the treatment of SCZ since 1990 to 2020. Data were accessed between June 12th and October 21st 2020. In addition, a comprehensive search of articles on MEDLINE, PsycINFO, Isi Web of Knowledge, Medscape was performed. The search was carried out using the keyword “Experimental serotonergic agents”, crossed with “schizophrenia”. At least two authors conducted the selection of appropriate papers between 1990 and 2020. A manual selection of clinical trials and papers was then performed in order to consider only those concerning the topic of the present article. No restriction criteria were established for study design. The exclusion criteria were as follows: 1) articles as reviews, meta-analyses, commentaries, letters, case reports, pooled analyses, comments, case studies; 2) off-topic clinical trials or papers; 3) studies contemplating the investigation of either non-experimental or non-serotonergic agents; 3) agents or trials evaluating patients with other psychotic disorders than SCZ, or mixed samples (eg SCZ and schizoaffective patients); 4) studies with unmentioned outcomes or results not clearly reported.
Only papers written in English were included.
Results
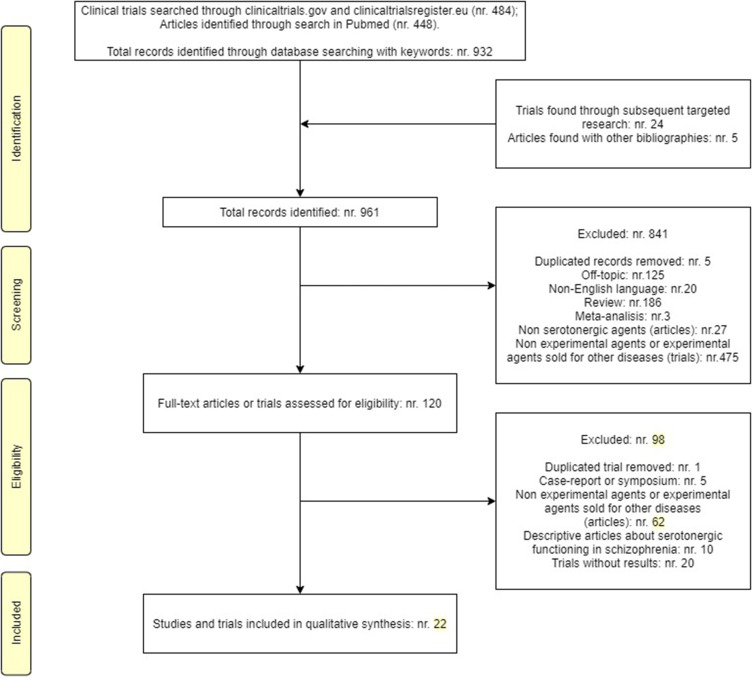
Nine hundred sixty-one records (508 clinical trials and 453 papers) were initially identified. Among these, 939 were excluded for above-mentioned criteria. Therefore, 11 clinical trials and 11 papers satisfied the inclusion criteria (Figure 1).
Figure 1.
PRISMA diagram for reviews.
Notes: PRISMA figure adapted from Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology. 2009;62(10). Creative Commons.70
We identified 13 selective 5-HTR agents including three selective 5HT1AR agonists, three 5HT2AR antagonists, one 5HT2CR agonist, four 5HT5R antagonists and two 5HT6R antagonists. Moreover, four 5-HTR compounds acting on two or more serotonin receptors were detected (Table 1).
Table 1.
Experimental Serotonergic Agents in Schizophrenia
| Serotonergic Agent | Properties | Other Binding Sites | References |
|---|---|---|---|
| Bifeprunox (DU-127090) | - Partial agonist at presynaptic 5-HT1ARs (pKi=8.2) - Partial agonist at presynaptic D2Rs (pKi=8.5) |
− 5HT2A, 5HT2CRs (pKi<6) - M and H1Rs (pKi<6) |
- Bruins et al, 2005 [21] - Tadori et al, 2007 [22] - Stahl, 2002 [23] |
| 8-OH-DPAT | - Agonist at presynaptic 5-HT1ARs (pKi=9.0) | −5-HT1B (pKi=6.2), 5-HT1D (pKi=6.8), 5-HT7Rs (pKi=6.4) - D2, α1,α2Rs (pKi≤6) |
- Assié and Koek, 2000 [29] |
| LASSBio-579 | - Agonist at presynaptic 5-HT1ARs (Ki=0.22 μM) - Antagonist at postsynaptic D2-like (Ki=0.39 μM) and D4Rs (Ki=0.18 μM) |
− 5-HT2A, 5-HT2CRs (Ki around 7 μM) - MRs (Ki>30 μM), α1B and α2Rs (Ki=2.6 μM) |
- Pompeu et al, 2013 [33] - Neves et al, 2013 [34] |
| SB-773812 | -Antagonist at postsynaptic 5-HT2ARs (pKi=8.5) - Antagonist at D3Rs (pKi=9.0)* |
- D2Rs (pKi=7) | - Catafau et al, 2011 [35] |
| AM-831 | - Antagonist at postsynaptic 5-HT2ARs** - Antagonist at postsynaptic D2Rs(antagonist)** |
- M1Rs** | ACADIA Pharmaceuticals Advances, 2011 [37] |
| Zicronapine (Lu 31–130) | - Antagonist at postsynaptic 5-HT2ARs (Ki=4.2 nM) -Antagonist at postsynaptic D1 and D2Rs (Ki=19 nM) |
N/A | - Citrome, 2013 [39] |
| Vabicaserin (SCA-136) | -Agonist at postsynaptic 5-HT2CRs (Ki=3 nM) | − 5HT1A (Ki=112 nM), 5-HT2BRs (Ki=14nM) - More than 50-fold selective on Receptors such as other 5-HT, D and α1Rs (Ki≥152nM) |
- Liu et al, 2014 [41] |
| AS2030680 | - Antagonist at presynaptic 5-HT5ARs (Ki=0.58 ± 0.17 nM) | −5-HT1A (Ki=20.7 nM), 5-HT2B (Ki= 22.0 nM), 5-HT6 (Ki=39.3 nM), 5HT7Rs (Ki=10.3 nM), other 5-HTRs (Ki>300 nM) | Yamazaki et al, 2015 [43] |
| AS2674723 | - Antagonist at presynaptic 5-HT5ARs (Ki=0.75 ± 0.03 nM) | − 5-HT1A (Ki=83.8 nM), 5-HT7Rs (Ki=7.3 nM); other 5-HTRs (Ki>300 nM) | Yamazaki et al, 2015 [43] |
| ASP5736 | -Antagonist at presynaptic 5-HT5ARs (Ki=3.6 ± 0.66 nM) | − 5-HT2CR (Ki=286.8 nM); other 5-HTRs (Ki > 1000 nM) | Yamazaki et al, 2015 [43,44] |
| SB699551 | - Antagonist at presynaptic 5-HT5ARs (pKi=8.5) | − 5-HT1A and 5-HT7Rs (pKi<5.5); 5-HT1B,5-HT1D, 5-HT2A and 5-HT2C (pKi<6) | Thomas et al, 2006 [46] |
| AVN-211 | Antagonist at postsynaptic 5-HT6Rs (Ki=2.1 nM) | − 5-HT2BRs (Ki=125nM); within the other 5-HTRs group the compound showed about 100-fold less than 5-HT6R selectivity. | -Ivachtchenko et al, 2016 [47] |
| Idalopirdine (Lu-AE58054) | - Antagonist at postsynaptic 5-HT6Rs (Ki=0.83±0.12 nM) | −5-HT2A (Ki=83±17nM), 5-HT2CRs (Ki=250±23nM); other 5-HTRs: over 400-fold less affinity than for 5-HT6Rs - α1ARs (Ki=21 nM), α1BRs (Ki=22nM) |
- Arnt et al, 2010 [50] |
| RU-24969 | - Agonist at pre-synaptic 5-HT1A (pKi=9) and 5-HT1BRs (pKi=8.1) | − 5-HT1D (pKi=7.7), 5HT2B (pKi=6.9), 5HT2A (pKi=6.9), 5HT2C (pKi=6.8),5HT5A (pKi=6), 5HT6 (pKi=6.2), 5HT7Rs (pKi=6.9) | - Cassaday et al, 1993 [30] |
| Ritanserin | Antagonist at postsynaptic 5-HT2AR (pKi=9.2) and 5-HT2CRs (pKi=8.2–9.6) | − 5-HT2BRs (pKi=8.3); other 5-HTRs (pKi≦7.8) - D2 (pKi=5.8), H1 (pKi<5), α1 (pKi=6.6–7.1) and α2Rs (pKi=6.5–7) |
- Leysen et al, 1985 [53] |
| LuAF-35700 | - Antagonist at postsynaptic 5-HT2A and 5-HT6Rs** - Antagonist at postsynaptic D1>D2Rs** |
N/A | https://clinicaltrials.gov/ct2/show/NCT03230864 [54] |
| Brilaroxazine (RP5063) | - Partial agonism at presynaptic 5-HT1A (Ki=1.5nM) and postsynaptic 5-HT2A (Ki=2.5 nM); - Antagonism at presynaptic 5-HT2B (Ki=0.19 nM), postsynaptic 5-HT6**, and 5-HT7Rs (Ki=2.7 nM) - Partial agonism at D2, D3 and D4Rs** |
-SERT and nAChRα4β2** | - Cantillon et al, 2017 [56] |
Notes: *Unspecified pre or post-synaptic targets; **Unspecified degree of affinity binding.
Abbreviations: 5-HTR, 5-hydroxytryptamine receptor; 8-OH-DPAT, 8-hydroxy-2-(di-n-propylamino) tetralin; αR, adrenoreceptor; DR, dopamine receptor; H, histamine; Ki, the inhibitory constant; LASSBio-579, [1-((1-(4-chlorophenyl)-1H-pyrazol-4-yl)methyl)-4-phenylpiperazine]; MR, muscarinic receptor; μM, micromole; nAChRs, Nicotinic acetylcholine receptors; nM, nanomole; pKi, the negative log (base 10) of the Ki value eg pKi, -log10(Ki); SERT, serotonin transporter; N/A, not available.
In the following paragraphs, data about compounds targeting 5-HTR in clinical or preclinical development will be described and discussed.
Selective 5HT1AR Agonists
Bifeprunox
Bifeprunox is a partial agonist of D2R and 5-HT1AR.21 Moreover, bifeprunox has a higher intrinsic efficacy upon D2Rs than other SGAs, since it acts as a dopamine system stabilizer, increasing dopamine levels where they are too low and decreasing them where they are high.22 Bifeprunox has been investigated in two multi-centric Randomized Clinical Trials (RCTs) according to its potential efficacy in reducing both positive and negative symptoms with possible low impact on weight gain and serum lipid levels.23 In the first 6-month, Phase III, double-blind RCT (2004–002185-38, Sponsor Protocol Number: 10200) conducted on 93 patients with SCZ in an acute phase, it was found that both bifeprunox and risperidone treatment showed improvement from baseline in Positive and Negative Syndrome Scale (PANSS) total scores as well as positive, negative and general psychopathology subscale scores. However, the treatment with bifeprunox (30 or 40 mg/day) was less effective than treatment with risperidone (4 or 6 mg/day) at all time-points, albeit extrapyramidal symptoms were more common during treatment with risperidone than during treatment with bifeprunox.24 In a subsequent one-year double-blind RCT (2007–001097-90, Sponsor Protocol Number: 11915A) including 223 patients in the maintenance phase of SCZ, with the aim to compare the efficacy of fixed doses of bifeprunox (20 mg/day) versus fixed doses of quetiapine (600 mg/day), the PANSS total score and all subscale scores improved significantly after 12 weeks of treatment with bifeprunox, although no clinically relevant changes in functional outcome, quality of life, or treatment compliance were detected. The overall incidence of adverse events with bifeprunox was comparable to that with quetiapine.25 Finally, in two consecutive open-label studies (2004–000707-18, Sponsor Protocol Number: 10206; 2005–000497-50, Sponsor Protocol Number: 11051), bifeprunox was found to be safe in flexible doses (20–40 mg/day) in the long-term treatment of SCZ.26,27 However, although bifeprunox was in Phase III of clinical trials for the treatment of SCZ and bipolar depression as well as in Phase I for Parkinson’s disease, the drug development was halted in august 2007 after the Food and Drug Administration (FDA) judgment to reject its use for acute or long-term symptoms of SCZ, according to its inadequate efficacy.28
8-HYdroxy-2-(Di-n-Propylamino) Tetralin
8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT) is a compound that was developed in the 1980s in order to study the function of the 5-HT1AR. It has a higher affinity for 5-HT1AR compared with other 5-HTRs, and it exhibits a low affinity for D2R.29 The effect of this compound has been evaluated on cognitive impairment in some animal models of SCZ (Table 2). However, contradictory findings were reported. Particularly, Cassaday and co-authors30 failed to find significant improvement of attentional processes (reduction in latent inhibition) in pre-exposed rats with 8-OH-DPAT, although the limitation of 8-OH-DPAT low dosage was hypothesized. On the other hand, a subsequent pre-clinical study by Winstanley and collaborators31 reported that rats receiving intra-medial prefrontal cortex infusion of 8-OH-DPAT experienced attentional improvements and better impulse control. Finally, 8-OH-DPAT was evaluated in the definition of 5-HT and 5-HT1ARs contributions to the impaired sensorimotor gating (prepulse inhibition – PPI) in SCZ, as reported in animal models. In particular, it has been reported that low doses of 8-OH-DPAT or direct administration to the dorsal raphe nucleus might preferentially increase PPI by activating high-affinity 5-HT1A autoreceptors in the raphe nuclei of rats. On the other hand, systemic 8-OH-DPAT may act at post-synaptic 5-HT1ARs, or at least 5-HT1ARs located in regions different from the dorsal raphe nucleus, resulting in disruption of PPI. Therefore, it has been reported that 5-HT1ARs are not only pre-synaptic receptors but are also active as post-synaptic 5-HT1ARs.32
Table 2.
Summary of Animal and Human Studies About Experimental Serotonergic Agents Under Study for SCZ Treatment
| Serotonergic Agent | Study | Phase | Duration | N | Aim | Study Design and Drug Doses | Primary Outcomes | Results | Status | Ref | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal studies | |||||||||||
| 8-OH-DPAT | Comparative experiment | Preclinical | 7 days | 26 male, Lister Hooded rats | Involvement of 5-HT1A and 5-HT2A in mPFC on 5CSRT | Training on 5CSRT. Intra-mPFC infusion through bilateral cannulae. Experimental (8-OH-DPAT or M100907) vs vehicle |
Percentage of correct responses on 5CSRT (stimulus duration = 0.5 s) |
8-OH-DPAT and M100907 enhanced attention and impulse control in every experiment (p<0.05) | Completed | https://doi.org/10.1007/s00213-003-1398-x [32] | |
| Comparative experiment | Preclinical | 3 days | 39 male Sprague –Dawley rats | Contribution of pre- or post-synaptic 5-HT1A receptors in PPI* disruption, caused by 8-OH-DPAT |
Experiment 1- Central DRN microinjections: 7 sham-operated male rats vs 10 castrated Experiment 2- DRN lesions: 12 sham-operated male rats vs 10 rats which received a selective serotonergic lesion of the DRN |
Experiment 1: Body and seminal vesicle weight, startle amplitude, PPI* Experiment 2: 5HT-content, startle amplitude, PPI* |
DRN lesion: ↓ 5-HT content in frontal cortex (↓70%), striatum (↓69%), ventral hippocampus (↓76%) Activation of post-synaptic 5-HT1A → PPI* disruption |
Completed | https://doi.org/10.1016/j.pbb.2005.05.007 [31] | ||
| Comparative experiment | Preclinical | 3 days | 72 male Sprague-Dawley rats 2 groups of 6 for each treatment |
Effect of 8-OH-DPAT on attentional processes (LI**) | Treatment: pre-exposed vs not pre-exposed. Experiment 1 Ritanserin 0.67 and 2.0 mg/kg in saline. Experiment 2 RU 24969 0.5 and 10.0 mg/kg in saline Experiment 3 8-OH-DPAT 0.19 and 0.38 mg/kg in saline |
Time to complete a preset number of licks (Test: suppression of drinking procedure) | ↓ LI**for all the three active compounds with a statistical significance for ritanserin and RU 24969 | Completed | https://doi.org/10.1177/026988119300700110 [30] | ||
| RU-24969 | ~ | ~ | ~ | ~ | Effect of RU-24969 on LI** | ~ | ~ | ↓ LI** at both doses: PE > NPE (p<0.001) |
Completed | https://doi.org/10.1177/026988119300700110 [30] | |
| Ritanserin | ~ | ~ | ~ | ~ | Effect of Ritanserin on LI** | ~ | ~ | ↓ LI**: - at low dose (p<0.001) - at high dose (p>0.05) |
Completed | https://doi.org/10.1177/026988119300700110 [30] | |
| LASSBio-579 | Comparative experiment | Preclinical | N/A | Adult male CF1 mice | To increase the affinity of LASSBio-579 for 5-HT2A receptor synthesizing 5 new N-phenylpiperazine derivatives | In-vivo pharmacological evaluation: intraperitoneally and orally (10 mL/kg) or subcutaneously (5 mL/kg) administration of compounds |
Classical competition assays; GTP-shift assay; climbing behavior in mice |
↑ 3–10-fold affinity for 5-HT2A (p<0.001) = affinity for the D2-like and 5-HT1A |
Completed | https://doi.org/10.1016/j.ejmech.2013.05.027 [33] | |
| Comparative experiment | Preclinical | N/A | Adult male CF1 mice | Pharmacological evaluation LASSBio-579, −580 and −581 to investigate potential APS activity |
Mice received a combination of sub-effective dose of LASSBio- 579 + haloperidol, LASSBio-579 + clozapine or haloperidol + clozapine |
Climbing behavior in mice; apomorphine-induced climbing and apomorphine-induced hypothermia in mice; motor parameter; PPI* of startle reflex |
- ↓ locomotor activity (p<0.022) - effect on apomorphine-induced climbing test at 5 mg/kg (p=0.758) - effective on preventing PPI* (80 dB) at 0.5 mg/kg (p<0.02) - effective on preventing PPI* (8085 dB) at 1 and 5 mg/kg (p<0.022) |
Completed | https://doi.org/10.1016/j.bbr.2012.09.016 [34] | ||
| AM-831 | N/A | Preclinical | N/A | N/A | N/A | N/A | N/A | AM-831 did not meet predetermined criteria for further development in Phase I testing | Interruption of the development of the compound | https://adisinsight.springer.com/drugs/800016551 [38] | |
| ASP5736, AS2030680 and AS2674723 | In vitro and in vivo (comparative experiments) | Preclinical | N/A | Groups of 10 (mice), 5 (young rats) or 3 (aged rats) | To investigate the relationship between the 5-HT5A receptor and cognitive function | In-vitro analysis: - 5-HT5A receptors expression in cell membranes - affinity of ASP5736, AS2030680 and AS2674723 for 5-HT5A receptors In-vivo analysis: - drug concentrations in biological samples - 5-HT5A influence on cognitive function |
Spatial working memory: - Y-maze test after scopolamine administration - Morris water maze test Ex-vivo receptor occupancy for 5-HT5A rat receptor |
- high affinity for 5-HT5A of each species - low affinity for 5-HT2B and 5-HT7 - Each of the three compounds ameliorated scopolamine-induced working memory deficit (p<0.05) |
Completed | https://doi.org/10.1016/j.jphs.2015.02.006 [43] | |
| ASP5736 | In vitro and in vivo (comparative experiments) | Preclinical | N/A | Groups of 10 mice | Evaluation of: - in-vitro inhibitory effects of ASP5736 on 5-HT5A - ASP5736 affinity for various receptors - ASP5736 pharmacokinetics - ASP5736 efficacy on positive -like and cognitive symptoms - efficacy and safety of ASP5736 + OLZ |
In-vitro analysis: - 5-HT5A expression in cell membranes - affinity of ASP5736 for 5-HT5A In-vivo analysis: - working memory deficit in MK-801-treated mice and visual learning deficit in neonatally PCP-treated mice - MK-801- and MAP-induced hyperactivity |
Y-maze test; NORT | ↓ MK-801- and PCP-induced cognitive deficit (p<0.001) Addition of OLZ was safe and did not compromise ASP5736 efficacy on cognitive deficits |
Completed | https://doi.org/10.1016/j.euroneuro.2014.07.009 [44] | |
| ASP5736 and SB699551 | Dose–response determination experiments | Preclinical | N/A | 13 male Sprague Dawley rats | To test the involvement of 5-HT5A in LSD behavioural effects | - Intraperitoneal administration of LSD (0.08 mg/kg) or sterile water - 10 training sessions - Drug discrimination tests |
Response rate and numbers of rats completing the test | ↓ subjective effects of LSD in rats (p<0.012) | Completed | https://doi.org/10.1177/0269881119867603 [45] | |
| SB699551 | In vitro and in vivo | Preclinical | N/A | Male Dunkin Hartley guinea pigs | Receptorial affinity, pharmacokinetics and action of SB699551 | - In vitro: fast cyclic voltammetry - In-vivo pharmacokinetic analysis; microdialysis (animals were treated with either vehicle (NaCl) or SB699551/WAY-100365***) |
Mean discharge frequency during the final 2 min of exposure to each concentration of drug | - high affinity for guinea pig 5-HT5A receptor - antagonist profile on 5-HT5A receptors - in association with WAY-100635***: ↑ extracellular levels of cortical 5-HT |
Completed | https://doi.org/10.1016/j.neuropharm.2006.04.019 [46] | |
| Human studies | |||||||||||
| Brilaroxazine (RP5063) | RCT | I | 10 days | 19 male pts with stable disease in 4 cohorts |
- Safety in healthy volunteers - safety and initial clinical activity in stable SCZ pts |
Ascending-dose, double-blind - single‐dose in healthy volunteers: 10, 15 mg -multiple‐dose in SCZ pts: 10, 20, 50, 100 mg |
PANSS, CG-I, CGI-S, CDSS | ↓ PANSS positive subscale in pts with PANSS ≥ 50 at BL: RP5063>PBO (p<0.05) | Completed | https://doi.org/10.1111/cts.12545 [57] | |
| RCT | II | 57 days | 234 SCZ pts | Safety and efficacy in acute SCZ | Double-blind, multi-centric Brilaroxazine (15, 30 or 50 mg) vs ARI (15 mg) or PBO once daily |
PANSS | ↓ PANSS: Brilaroxazine 15 or 50 mg > PBO (p<0.05) |
Completed |
https://clinicaltrials.gov/ct2/show/NCT01490086 [58] |
||
| SB-773812 | RCT | II | 12-week treatment 2 years extension |
338 SCZ pts | Efficacy, safety and tolerability of SB-77381 | Double-blind, multi-centric 3 arms: - SB-773812 (60 or 120 mg) - OLZ (15 mg) - PBO |
PANSS, BPRS, CGI-S. CGI-I, CDSS | ↓ PANSS: - SB-773812 60 mg=PBO (p>0.05) - SB-773812 60 and 120 mg = OLZ > PBO (p<0.05) |
Completed | https://www.clinicaltrialsregister.eu/ctr-search/trial/2005-002883-27/results [36] | |
| Vabicaserin (SCA-136) | RCT | II | 4 weeks | 202 SCZ pts 37 PBO 43 RIS 122 Vabicaserin |
Efficacy, safety, and tolerability in acute SCZ | Double-blind, multi-centric RIS: 4 mg/day Vabicaserin: 50, 100, 150, 200, 300, 400, 600 mg/day) |
PANSS, CGI-S, CGI-I, CDSS | ↓ PANSS: - Vabicaserin at all doses = PBO (p>0.05) |
Recruitment status completed | https://clinicaltrials.gov/ct2/show/NCT00563706 [42] | |
| AVN-211 | RCT | II | 4 weeks | 42 SCZ pts 17 AVN-211 25 PBO |
Efficacy on clinical and cognitive symptoms in SCZ pts on APS medications | Double-blind AVN-211: 4 mg |
PANSS, CGI-S, CGI-I, CDSS, NSA-16, WAIS, 5 attention tests | ↓ PANSS positive subscale: AVN-211>PBO (p=0.058) WAIS: AVN-211>PBO (p=0.02) AVN-211 ↓ PANSS positive subscale: p=0.007 AVN-211 ↓ CGI-S: p=0.048 AVN-211 ↓ CDSS: p<0.05 |
Completed | https://doi.org/10.1017/S1092852913000394 [48] | |
| Idalopirdine (Lu-AE58054) | RCT | II | 12 weeks | 122 SCZ pts 58 RIS+Lu-AE58054 64 RIS+PBO |
Efficacy as augmentation therapy to RIS | 2-week, prospective run-in period → double-blind Lu-AE58054 (120 mg/day) or PBO (12 weeks) RIS: 4–8 mg/day |
PANSS, BACS, CGI-I, CGI-S, SQoL |
↓ PANSS: RIS+Lu-AE58054 = RIS+PBO (p>0.05) |
Interruption of the development of the compound | https://www.clinicaltrialsregister.eu/ctr-search/trial/2008-001441-26/IT [51] | |
| Bifeprunox (DU-127090) | RCT | III | 12 months: 6-month treatment period + 6-month extension period | 93 acute SCZ pts 35 BX 58 RIS |
Long-term safety, tolerability and efficacy of BX vs RIS flexible doses | Double-blind 2 arms: - BX: 30–40 mg/day - RIS: 4–6 mg/day |
PANSS; BPRS; CGI-S; CGI-I; CDSS | - ↓ PANSS, BPRS, CGI-S, CGI-I: RIS > BX (p<0.05) - ↓ CDSS: RIS = BX (p>0.05) |
Recruitment completed. End of trial ongoing |
https://www.clinicaltrialsregister.eu/ctr-search/trial/2004-002185-38/results [24] | |
| RCT | III | 12 months | 223 SCZ pts 79 BX 76 QUE 68 PBO |
Efficacy of fixed doses BX vs PBO Efficacy, safety, tolerability, functional outcomes, quality of life, and treatment compliance BX vs QUE |
Multi-national, multi-centric, double-blind 3 arms: -BX: 20 mg/day -QUE: 600 mg/day -PBO - lead-in period (4 weeks) -PBO-controlled (12 weeks) - QUE-controlled period (9 months) |
PANSS, CGI-S score, CGI-I score, and CDSS | - ↓ PANSS at week 12: BX=QUE > PBO (p<0.05) | Prematurely ended | https://www.clinicaltrialsregister.eu/ctr-search/trial/2007-001097-90/GR [25] | ||
| Open-label | N/A | 40 weeks | 153 SCZ pts 73 OLZ → BX 80 BX → BX |
Long-term safety, tolerability and maintenance of therapeutic effects of BX flexible doses vs OLZ | 1st week double-blind: switch OLZ to BX (up to 30 mg/day) or BX continuation at 30 mg/day From 2nd week: BX flexible dose (20, 30, or 40 mg/day) OLZ: 15 mg/day |
PANSS, CGI-S, CDSS | BX → BX: - ↓ PANSS during lead-in, ↑ during continuative OLZ → BX - ↑ PANSS at Week 40 |
Completed | https://www.cliicaltrialsregister.eu/ctr-search/trial/2004-000707-18/IT [26] | ||
| Open-label | N/A | 14–531 days | 11 SCZ pts | Efficacy, long-term safety and tolerability | BX flexible doses (20, 30, or 40 mg/day) |
CGI-S | No efficacy results (small sample of pts) | Completed | https://www.clinicaltrialsregister.eu/ctr-search/trial/2005-000497-50/IT [27] | ||
| Zicronapine (Lu 31–130) | RCT | III | 6 months | 160 SCZ pts | Efficacy, safety and tolerability of ZIC vs RIS | Double-blind 2 arms: - ZIC 7.5 mg/day - RIS 5 mg/day |
PANSS; CGI-S, GAF | ↓ PANSS and CGI-S: ZIC=RIS (p>0.05) |
Completed | https://www.clinicaltrialsregister.eu/ctr-search/trial/2010-022181-28/EE [40] | |
| LuAF-35700 | RCT | III | 16 weeks | 1098 TRS pts 401 did not complete PC 235 LuAF-35700 10 mg 232 LuAF-35700 20 mg 232 continued PC treatment |
Efficacy in TRS pts | - Screening Period (3 weeks) - PC: 6-week, patient-blinded treatment with RIS (4–6 mg/day) or OLZ (15–20 mg/day) - DBT (10 weeks): LuAF-35700 10 mg or 20 mg or continued PC treatment (1:1:1) - Safety Follow-up Period (6 weeks) |
PANSS, PSP, CGI-S | ↓ PANSS: LuAF-35700 = RIS or OLZ (p>0.05) |
Recruitment status completed | https://investor.lundbeck.com/news-releases/news-release-details/lundbeck-updates-clinical-phase-iii-study-lu-af35700-treatment (Valby, Oct. 25, 2018) [54] | |
| RCT | III | 34 weeks | 119 TRS pts 68 RIS 51 OLZ 51 Non randomized 35 LuAF-35700 10 mg 33 continued PC treatment |
Testing the efficacy on symptoms of SCZ | - PC: 6-week, patient-blinded treatment with RIS (4–6 mg/day) or OLZ (15–20 mg/day) - DBT (8 weeks): LuAF-35700 10 mg or continued PC treatment (1:1) - observation period for AEs (20 weeks) |
PANSS, CGI-S, NSA-16 | Waiting for results on patients randomized in the DBT period. | Terminated (New data; the study was terminated based on new efficacy data from another study) |
https://clinicaltrials.gov/ct2/show/NCT03230864 [55] |
||
Notes: *PPI is a measure of sensorimotor gating that is deficient in schizophrenia; **Latent inhibition is the delay in responding to a stimulus which has no consequences in the first exposures. For example, when animals were exposed to a stimulus without implications they are impaired in learning that this stimulus could predict an important event such as footshocks. The impairment in comprehending danger stimulus is called pre-exposition. Amphetamine disrupts LI while antipsychotics have an opposite effect; ***WAY-100635: 5-HT1A receptor-selective antagonist.
Abbreviations: ~, The same information as the box above; 5CSRT, five-choice serial reaction time task; AEs, adverse events; APS, antipsychotic; ARI, aripiprazole; BL, baseline; BACS, Brief Assessment of Cognition in Schizophrenia; BPRS, Brief Psychiatric Rating Scale; BX, bifeprunox; CAT, Continuous Attention Task; CF1 mouse, obtained with outbreeding by Carworth Farms; CGI-S, Clinical Global Impression – Severity of Illness; CGI-I, Clinical Global Impression – Global Improvement; CDSS, Calgary Depression Scale for Schizophrenia; CSRT choice serial reaction time task; DAI-30, Drug Attitude Inventory; DBT, Double-Blind Treatment; DRN, dorsal raphe nucleus; GAF, Global Assessment of Functioning; GTP, nucleotide guanosine triphosphate; LSD, D-lysergic acid diethylamide; MAP, methamphetamine; MK-801, dizocilpine; mPFC, medial Prefrontal Cortex; N/A, not applicable; NPE, not pre-exposed; NORT, Novel object recognition test; NSA-16, Negative Symptom Assessment; OLZ, olanzapine; PBO, placebo; PC, prospective confirmation; PCP, phencyclidine; PE, pre-exposed; pts, patients; PSP, Personal and Social Performance Scale; QUE, quetiapine; RIS, risperidone; RU 24969, (5-methoxy-3 (1,2,3,6-tetrahydropyridin-4-yl); SCZ, schizophrenia; S-QoL, Schizophrenia Quality of Life; PANSS,Positive and Negative Syndrome Scale; TRS, treatment-resistant SCZ; WAIS, Wechsler Adult Intelligence Scale; ZIC, zicronapine; PPI, Prepulse inhibition; LI, latent inhibition.
LASSBio-579
LASSBio-579 [1-((1-(4-chlorophenyl)-1H-pyrazole-4-yl)methyl)-4-phenylpiperazine]33 was orally active in different rodent models of positive and negative symptoms of SCZ (Table 2), showing a moderate affinity for 5-HT1ARs, D2 and D4Rs other than a low affinity for the 5-HT2A and 5-HT2CRs.34 As reported by some experimental studies on mice, LASSBio-579 may also diminish EPS induced by both FGA and SGA.34
Selective 5HT2AR Antagonists
SB-773812
SB-773812 was designed to enhance antagonism on those receptors possibly related to antipsychotic efficacy while reducing affinity to receptors associated with side effects of older antipsychotics. SB-773812 is a moderate antagonist of D2R and a selective high-affinity antagonist of D3R and 5HT2AR.35 In a 12-week, multi-centric, phase-II, double-blind RCT (2005–002883-27, Sponsor Protocol Number: NAA104606) (n=317), including olanzapine as active control, SB-773812 showed statistically significant efficacy in improving SCZ symptoms, measured with the PANSS rating scale, at both 120 mg and 60 mg, in patients with acute exacerbation of SCZ and requiring inpatient hospitalization. Moreover, improvement at week 6 was statistically superior to placebo at both doses on most secondary efficacy measures, such as PANSS Positive and General Psychopathology subscale scores, CGI-S (Clinical Global Impression – severity scale), total BPRS (Brief Psychiatric Rating Scale) and psychosis BPRS scores. However, tremor and akathisia were reported at a higher incidence than placebo, but similar to olanzapine. Finally, SB-773812 caused a dose-related increase in prolactin at 60 mg of dosage, although similar to that caused by olanzapine.36
No recent reports of development for SB 773812 were identified for SCZ.
AM-831
AM-831 is a potential antipsychotic compound that combines muscarinic (M)1 partial agonism with both D2R and 5-HT2AR antagonism.37 Although AM-831 demonstrated a combination of strong antipsychotic properties in traditional preclinical models of psychosis, as well as pro-cognitive activity in preclinical behavioral models, it was reported by Acadia Pharmaceuticals (Acadia, US) that the compound did not meet predetermined criteria for further development in Phase I testing. Therefore, it was decided to discontinue the development of AM-831.38
Zicronapine
Zicronapine (previously known as Lu 31–130) is a potent D1, D2 and 5-HT2A antagonist.39 In the phase-II development programme, zicronapine showed robust antipsychotic effects. A subsequent 6-month, phase-III, double-blind, risperidone-controlled, fixed-dose RCT (2010–022181-28, Sponsor Protocol Number:13639A) (n=160) evaluated the effect of zicronapine versus risperidone on metabolic parameters. This study showed that zicronapine was safe and well tolerated. Moreover, the overall incidence of adverse events was similar in the treatment groups but the pattern of adverse events differed. Particularly, the prolactin values were lowest in the zicronapine group than in the risperidone group.40 In 2014, Lundbeck (Lundbeck, DK) stopped the study of this compound according to the development of Lu AF35700, which was claimed to have a better drug profile than zicronapine.
Selective 5HT-2CR Agonist
Vabicaserin, also known as SCA-136, is a potential antipsychotic and anorectic with high agonism on the 5-HT2CR but with modest additional effect on 5HT2BR. Moreover, it is more than 50-fold selective over a number of serotonergic, dopaminergic and noradrenergic receptors.41
A phase-II, double-blind, multi-centric RCT (NCT00563706) (n=404) with placebo, risperidone (4 mg/day), and up to seven treatment arms of vabicaserin (dosages of 50, 100, 150, 200, 300, 400 or 600 mg/day) was performed in order to evaluate the efficacy, safety and tolerability of vabicaserin in patients with acute exacerbations of SCZ. However, no significant improvement versus placebo was observed after 28 days of treatment according to PANSS total scores. Moreover, as a result of the failure of all vabicaserin doses to meet the primary efficacy objective, the study was prematurely terminated.42 Research of vabicaserin for the treatment of SCZ was discontinued.
Selective 5HT-5AR Antagonists
AS2030680 and AS2674723
AS2030680 and AS2674723 are two selective 5-HT5AR antagonists with modest or low affinity for other serotonin receptors.43 Yamazaki et al reported that each of the two compounds ameliorated scopolamine-induced working memory deficit related to dementia in mice and rats,43 suggesting a possible use to treat cognitive impairment associated with SCZ.
ASP5736
ASP5736 is a 5-HT5AR antagonist with a low affinity for other 5-HTRs.44 This compound was assessed on positive symptoms and cognitive impairments in different animal models of SCZ (Table 2). Particularly, a preclinical study reported that ASP5736 might improve both working memory and visual learning deficits in dizocilpine (MK-801) and phencyclidine (PCP) treated mice. Moreover, ASP5736 was associated with improvement in MK-801 and methamphetamine-induced hyperactivity in mice without important adverse effects. Olanzapine did not impact ASP5736-induced cognitive enhancement.44 ASP5736 has been also reported to ameliorate scopolamine-induced working memory deficit and reference memory impairment in aged rats.42 Interestingly, a recent study reported that ASP5736 inhibited subjective effects of D-lysergic acid diethylamide (LSD) in rats.45
SB699551
SB699551 is a compound with at least 30-fold selectivity for 5-HT5AR versus other 5-HTRs, as demonstrated in a sample of Guinea pigs.46 Similarly to ASP5736, it was reported that, in rats trained to discriminate LSD from vehicle 15 minutes after injection, the inhibitory actions of SB699551 on the subjective effects of LSD might represent a possible way to treat positive symptoms of SCZ, as emerged in rats.45
Selective 5HT-6R Antagonists
AVN-211 (CD-008-0173)
AVN-211 is a small molecule, which acts as a highly selective 5HT6R antagonist.47 A 4-week, phase-II, double-blind RCT with 42 SCZ patients who were stabilized on the antipsychotic drug was conducted. A significant difference between the groups in PANSS positive subscale score and CGI-S in favor of the treatment group was found at the endpoint. Moreover, AVN-211 improved selectivity and continuous attention in the group treated with the active compound than placebo.48 AVN-211 is undergoing development with Avineuro Pharmaceuticals.49
Idalopirdine (Lu-AE58054)
Idalopirdine displays a high affinity for 5HT6Rs, leading to 65% binding occupancy in a dose range. Moreover, it was reported that idalopirdine reversed cognitive impairment in mice after chronic treatment with PCP.50 A 12-week, phase-II, double-blind, parallel-group, fixed-dose RCT (NCT00810667) was performed in order to explore the efficacy and safety of idalopirdine (120 mg/die) as augmentation therapy to risperidone (4–8 mg/die) in 122 patients with SCZ. However, augmentation therapy with idalopirdine did not offer any treatment advantage over placebo in improving overall SCZ symptoms, as assessed by the PANSS total score. Similarly, there was no treatment advantage with 120 mg/day idalopirdine over placebo in improving patients’ overall neurocognitive profile. The safety profiles of augmentation therapies with 120mg/day idalopirdine and placebo were comparable and no new safety issues were raised in this study.51 No recent reports of idalopirdine development were identified for SCZ. Indeed, Lundbeck announced in its 2017 annual report that the results of all phase-III trials conducted on patients with mild to moderate Alzheimer’s disease did not demonstrate efficacy to support a regulatory submission of idalopirdine. Thus, it was removed from the development program.52
Other Serotonergic Compounds
RU-24969
RU-24969 is a compound that acts as a potent agonist at both the 5HT1A and the 5HT1B terminal autoreceptor sites. It was reported that this compound might improve attentional processes and associative learning, as described in a preclinical animal study (Table 2).30
Ritanserin
Ritanserin acts as a selective 5HT-2A and 5HT-2CRs antagonist, although it binds to and antagonizes other serotonin receptors.53 It was reported that ritanserin might improve attentional processes and associative learning in a preclinical animal study (Table 2).30
Although it was never marketed for clinical use, it was used in scientific research.
LuAF-35700
LuAF-35700 is an investigational antagonist of 5HT2A and 5HT6Rs in addition to D1R. In a recent 16-week, phase-III RCT with the aim to evaluate the efficacy of 10 and 20 mg/day of LuAF-35700 in patients with treatment-resistant SCZ (DAYBREAK study; 2014–003569-12, Sponsor Protocol Number:16159A) (n=1098), LuAF-35700 did not show statistical superiority versus either risperidone (4–6 mg/day) or olanzapine (15–20 mg/day on the primary endpoint (change in total PANSS)). However, LuAF-35700 showed good antipsychotic effects, it was well tolerated and it resulted to be safe at 10 mg and 20 mg doses.54 However, no data regarding differences in the safety profile between Lu AF-35700 and Olanzapine and Risperidone were found. Currently, a new study program (2017–000788-34, Sponsor Protocol Number: 17303A) is evaluating a single dose of 10 mg/day of LuAF-35700 versus placebo and it is stratifying randomization of treatment-resistant SCZ patients according to early (within the first 5 years) or chronic (after 10 years) illness. Final results are awaited.55
Brilaroxazine (RP5063)
Brilaroxazine acts as a partial agonist of D2, D3 and D4Rs and 5-HT1A and 5-HT2ARs, as well as an antagonist of 5-HT6 and 5-HT7Rs.56
A phase-I, double-blind, ascending-dose RCT was conducted in order to assess the safety of RP5063 in four cohorts of patients (treated with 10, 20, 50, and 100 mg/day). The primary aim of the study regarded the safety of single doses (10 and 15 mg) in non-affected healthy men, using an ascending-dose study design, and of different doses (10, 20, 50 and 100 mg) over 10 days in male patients affected by stable SCZ. In the single-dose study, orthostatic hypotension, nausea, and dizziness were the most common adverse events. On the other hand, in the multiple-dose study, akathisia and somnolence were the most frequent. No significant changes were seen in lipid or prolactin levels, weight, or electrocardiographic recordings. The authors observed significant improvements in positive symptoms with RP5063 over placebo in an analysis of individuals with a baseline PANSS score higher than or equal to 50. Moreover, improvements on the Trail Making A and B tests were seen for patients included in the 50-mg-dose group. Therefore, this study reported promising preliminary findings of the efficacy of RP5063 on clinical symptoms and cognition in patients with the stable illness.57
A phase-II, 57-day, double-blind, multi-centric RCT was conducted in order to assess the safety and efficacy of RP5063 in 234 patients with an acute exacerbation of SCZ disorder (REFRESH study; NCT01490086), randomized to brilaroxazine (15, 30, or 50 mg), aripiprazole (15 mg) or placebo once daily. The primary endpoint was the change from baseline to day 28 of PANSS total score. The primary analysis showed a significant improvement of PANSS scores with the doses of 15 mg (34% of patients), 30 mg (30% of patients), and 50 mg (46% of patients). The difference between treatment and placebo reached statistical significance for the 15 and 50 mg doses. Furthermore, the patients treated with brilaroxazine demonstrated cognitive improvements as shown by an amelioration of scores in the Digital symbol substitution test as well as in Trail Making A and B tests. The most common adverse events for brilaroxazine consisted of insomnia and agitation. The compound was not associated with weight changes, electrocardiogram abnormalities or increased risk of hypotension. Discontinuation for any reason for brilaroxazine was much lower than for placebo and aripiprazole.58 RP5063 phase III studies in acute and in maintenance phase SCZ patients are in preparation.59
Summary of the Findings
Our search identified 17 experimental serotonergic agents, under study for their potential use in SCZ treatment. Three compounds, namely AVN-211, LuAF-35700 and brilaroxazine, are currently under clinical development. The selective 5-HT6R antagonist AVN-211 showed promising results on both positive and cognitive symptoms in a phase-II RCT.48 As reported in a phase-III RCT, the 5-HT2A and 5-HT6Rs antagonist LuAF-35700 may have good antipsychotic effects in patients with treatment-resistant- SCZ, but without displaying superiority over conventional antipsychotics.53,54 Brilaroxazine is a candidate for a further phase-III RCT as a result of the evidence of a statistically significant improvement of positive symptoms and some neurocognitive domains in acute SCZ patients.56–58 Four selective 5-HT5AR antagonists (AS2030680, AS2674723, ASP5736 and SB699551) showed some pro-cognitive and antipsychotic-like properties in animal models, suggesting a possible clinical application to treat SCZ.43–46 Two 5-HT1AR agonists, 8-OH-DPAT and LASSBio-579, the selective 5-HT1BR agonist RU-24969 as well as the selective 5-HT2A and 5-HT2CRs antagonist ritanserin are research chemicals which were developed some years ago in order to study the role of serotonergic mechanisms in SCZ.30–34 The selective 5HT2AR antagonist SB-773812 showed contradictory results with regard to the efficacy in improving SCZ symptoms.36 Moreover, no reports of development for this compound have been recently published. Finally, bifeprunox, AM-831, zicronapine, vabicaserin and idalopirdine were removed from the development program24–27,37,40,42,52 (Table 2).
Discussion and Conclusions
To date, the available pharmacotherapy for SCZ demonstrates efficacy for the treatment of positive symptoms, but does not address the large array of negative and cognitive symptoms, as well as the management of drug-resistant SCZ. In particular, FGAs, as strong antagonists of D2Rs, are effective in reducing positive symptoms, but they may exacerbate negative and cognitive symptoms.9 Through the 5-HT2AR antagonism in combination with D2R antagonism, SGAs have reduced EPS, but they have a modest effect on negative symptoms and cognitive deficits. Moreover, the use of SGAs is associated with metabolic disturbances and akathisia.11 In order to fill these gaps, new investigational compounds targeting 5-HTRs were intensively investigated in SCZ patients. Among the 5-HTRs, the functioning of the 5-HT1ARs is the best known. In the light of the evidence of an increased 5-HT1AR density in the brains of SCZ patients, these receptors are considered as a preferable target to treat the disorder.60 Of note, the activation of 5-HT1A inhibitory autoreceptors located in the cells of raphe nucleus is associated with the inhibition of 5-HT2ARs, possibly leading to a decrease of extrapyramidal side effects and an improvement of both affective symptoms and cognitive deficits.61 Among selective 5-HT1ARs agonists, LASSBio-579 and 8-OH-DPAT showed promising results in preclinical studies, having a low propensity to induce EPS.29–34 Along with 5-HT1ARs, 5-HT2ARs are also located on neurons involved in SCZ. 5-HT2ARs can modulate the activity of dopaminergic neurons and they play an important role in the regulation of the γ-aminobutyric acid (GABA) interneurons inhibition.62 Nevertheless, the antagonism on 5-HT2ARs operated by some SGAs (ie, olanzapine, risperidone, clozapine) not only leads to reduce the excessive dopamine neurotransmission in the mesolimbic circuit but may be also related to an increase of both dopamine and glutamate release in the mesocortical pathway.10 Among investigational drugs acting on 5-HT2ARs, lumateperone could represent a novel approach to the treatment of SCZ. The combined effects of this compound (strong block of 5-HT2ARs, dopamine modulation, serotonin reuptake inhibition and indirect glutamatergic activity) resulted in reducing positive and negative symptoms, as well as in improving social functioning of SCZ patients, with low rates of EPS or metabolic side effects.19 Since many compounds binding to 5-HT2ARs also exhibit high affinity to 5-HT2CRs, it was hypothesized that 5-HT2CR antagonism may reduce the activity of dopaminergic neurons in mesolimbic and nigrostriatal pathways.63 However, the role of 5-HT2CRs in the pathophysiology of SCZ is still poorly understood and requires further studies. Similarly, the possible involvement of 5-HT5ARs in the biological mechanisms of SCZ has not been extensively defined. Some studies reported that 5-HT5ARs might be distributed in several brain areas and implicated in the control of affective states, sensory perception, as well as in learning, memory and emotional state.64 Moreover, some psychotic effects of LSD may be mediated by this receptor. So far, only some preclinical studies have shown a possible use of 5-HT5AR antagonists in treating positive symptoms and cognitive impairment associated with SCZ.45 The 5-HT6R could be a prospective pharmacological target for the treatment of cognitive dysfunction in SCZ, as well. Of note, even though the 5-HT6R had been firstly investigated as a possible therapeutic target for the treatment of Alzheimer’s disease, its distribution in cortical and limbic/striatal areas suggested its potential role in cognitive impairment associated with SCZ.65 In particular, pro-cognitive effects of 5-HT6R antagonists might be mediated by a blockade of GABAergic neurons in the hippocampus and cortex, resulting in an enhanced dopaminergic, cholinergic and glutamatergic neurotransmission.66 AVN-211 showed some promising results in improving positive symptoms and sustained attention in a phase-II RCT involving medically stabilized SCZ patients. However, new trials should be conducted in order to test the efficacy of the compound in different groups of patients.48
It is important to highlight that, next to ligands of specific serotonin receptors, molecules targeting multiple serotonin receptor subtypes should be developed as potential SCZ treatments. Of note, all SGAs have a complex pharmacological profile possibly involving high affinity to many serotonin receptors as 5-HT1AR agonism, 5-HT2A and 5-HT2B antagonism, 5-HT2CR antagonism, as well as 5-HT6R and 5-HT7R antagonism.67 In addition to RU-24969 and ritanserin, which showed pro-cognitive properties only in animal models of SCZ,30 other compounds acting on multiple 5-HTR are currently under clinical development. Even though LuAF-35700 failed to demonstrate more efficacy than controls in a sample of treatment-resistant SCZ patients, the compound seems to have less dopamine-related adverse effects than other currently available antipsychotics. However, a study in a relatively small sample of randomized patients is still ongoing to draw final data.54,55 On the other hand, brilaroxazine, also called “dopamine-serotonin system stabilizer” because of its unique action on both the dopaminergic and the serotonergic system, showed to be efficacious in reducing PANSS total scores and in ameliorating cognition of acute SCZ patients in a phase-II RCT.57 As a very promising and well-tolerated medication, it is now being developed.
Even though some promising data exist, particularly regarding AVN-211 and brilaroxazine, the directions of future research about potential serotonergic compounds for SCZ treatment could be the following:
– a better understanding of the role of some 5-HTRs in the etiology of SCZ;
– a focus on searching for novel compounds to specifically treat negative symptoms, cognitive deficits and residual/treatment-resistant positive symptoms, in particular through a multi-target approach;68
– high-quality trials in order to evaluate the efficacy and tolerability of the molecules on different domains of SCZ.69
Highlights
– Schizophrenia is a chronic and highly disabling mental disorder that is related to low rates of functional recovery.
– Current drug treatment for schizophrenia is based on antipsychotics which are poorly effective on negative and cognitive symptoms.
– New agents targeting 5-HT receptors (5-HTRs) became an important area of research in order to improve the long-term outcome of schizophrenia.
– AVN-211, LuAF-35700 and Brilaroxazine showed promising results and are currently under clinical development.
– Additional mechanisms of action and compounds require further studies to improve schizophrenia outcomes, particularly for negative and cognitive symptoms as well as treatment resistance.
Disclosure
The authors have no conflicts of interest to declare for this work.
References
- 1.Picchioni MM, Murray RM. Schizophrenia. BMJ. 2007;335(7610):91–95. doi: 10.1136/bmj.39227.616447.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine SZ, Lurie I, Kohn R, Levav I. Trajectories of the course of schizophrenia: from progressive deterioration to amelioration over three decades. Schizophr Res. 2011;126:184–191. doi: 10.1016/j.schres.2010.10.026 [DOI] [PubMed] [Google Scholar]
- 3.Silberstein J, Harvey PD. Cognition, social cognition, and Self-assessment in schizophrenia: prediction of different elements of everyday functional outcomes. CNS Spectr. 2019;24(1):88–93. doi: 10.1017/S1092852918001414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kasckow JW, Zisook S. Co-occurring depressive symptoms in the older patient with schizophrenia. Drugs Aging. 2008;25(8):631–647. doi: 10.2165/00002512-200825080-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pani L, Pira L, Marchese G. Antipsychotic efficacy: relationship to optimal D2-receptor occupancy. Eur Psychiatry. 2007;22(5):267–275. doi: 10.1016/j.eurpsy.2007.02.005 [DOI] [PubMed] [Google Scholar]
- 6.Uhl I, Kulik A, Roser P, et al. Central serotonergic function in patients with predominantly negative symptoms of schizophrenia. Schizophr Res. 2018;193:443–444. doi: 10.1016/j.schres.2017.05.041 [DOI] [PubMed] [Google Scholar]
- 7.Krause M, Zhu Y, Huhn M, et al. Antipsychotic drugs for patients with schizophrenia and predominant or prominent negative symptoms: a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci. 2018;268(7):625–639. doi: 10.1007/s00406-018-0869-3 [DOI] [PubMed] [Google Scholar]
- 8.Buoli M, Kahn RS, Serati M, Altamura AC, Cahn W. Haloperidol versus second-generation antipsychotics in the long-term treatment of schizophrenia. Hum Psychopharmacol. 2016;31(4):325–331. doi: 10.1002/hup.2542 [DOI] [PubMed] [Google Scholar]
- 9.Solmi M, Murru A, Pacchiarotti I, et al. Safety, tolerability, and risks associated with first- and second-generation antipsychotics: a state-of-the-art clinical review. Ther Clin Risk Manag. 2017;13:757–777. doi: 10.2147/TCRM.S117321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan LC, Clarke WP, Berg KA. Atypical antipsychotics and inverse agonism at 5-HT2 receptors. Curr Pharm Des. 2015;21(26):3732–3738. doi: 10.2174/1381612821666150605111236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373(9657):31–41. doi: 10.1016/S0140-6736(08)61764-X [DOI] [PubMed] [Google Scholar]
- 12.Nielsen RE, Levander S, Kjaersdam Telléus G, Jensen SO, Østergaard Christensen T, Leucht S. Second-generation antipsychotic effect on cognition in patients with schizophrenia–a meta-analysis of randomized clinical trials. Acta Psychiatr Scand. 2015;131(3):185–196. doi: 10.1111/acps.12374 [DOI] [PubMed] [Google Scholar]
- 13.Galling B, Vernon JA, Pagsberg AK, et al. Efficacy and safety of antidepressant augmentation of continued antipsychotic treatment in patients with schizophrenia. Acta Psychiatr Scand. 2018;137(3):187–205. doi: 10.1111/acps.12854 [DOI] [PubMed] [Google Scholar]
- 14.Gregory A, Mallikarjun P, Upthegrove R. Treatment of depression in schizophrenia: systematic review and meta-analysis. BJP. 2017;211(4):198–204. doi: 10.1192/bjp.bp.116.190520 [DOI] [PubMed] [Google Scholar]
- 15.Sepehry AA, Potvin S, Elie R, Stip E. Selective serotonin reuptake inhibitor (SSRI) add-on therapy for the negative symptoms of schizophrenia: a meta-analysis. J Clin Psychiatry. 2007;68(4):604–610. doi: 10.4088/jcp.v68n0417 [DOI] [PubMed] [Google Scholar]
- 16.Mao YM, Zhang MD. Augmentation with antidepressants in schizophrenia treatment: benefit or risk. Neuropsychiatr Dis Treat. 2015;11:701–713. doi: 10.2147/NDT.S62266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones CA, McCreary AC. Serotonergic approaches in the development of novel antipsychotics. Neuropharmacology. 2008;55(6):1056–1065. doi: 10.1016/j.neuropharm.2008.05.025 [DOI] [PubMed] [Google Scholar]
- 18.Davis RE, Correll CU. ITI-007 in the treatment of schizophrenia: from novel pharmacology to clinical outcomes. Expert Rev Neurother. 2016;16(6):601–614. doi: 10.1080/14737175.2016.1174577 [DOI] [PubMed] [Google Scholar]
- 19.Vanover K, Dmitrienko A, Glass S, et al. S44. Lumateperone (ITI-007) for the treatment of schizophrenia: placebo-controlled clinical trials and an open-label safety switching study. Schizophr Bull. 2018;44(Suppl1):S341. doi: 10.1093/schbul/sby018.831 [DOI] [Google Scholar]
- 20.Blair HA. Lumateperone: first Approval. Drugs. 2020;80(4):417–423. doi: 10.1007/s40265-020-01271-6 [DOI] [PubMed] [Google Scholar]
- 21.Bruins Slot LA, Kleven MS, Newman-Tancredi A. Effects of novel antipsychotics with mixed D(2) antagonist/5-HT(1A) agonist properties on PCP-induced social interaction deficits in the rat. Neuropharmacology. 2005;49(7):996–1006. doi: 10.1016/j.neuropharm.2005.05.013 [DOI] [PubMed] [Google Scholar]
- 22.Tadori Y, Kitagawa H, Forbes RA, McQuade RD, Stark A, Kikuchi T. Differences in agonist/antagonist properties at human dopamine D(2) receptors between aripiprazole, bifeprunox and SDZ 208-912. Eur J Pharmacol. 2007;574(2–3):103–111. doi: 10.1016/j.ejphar.2007.07.031 [DOI] [PubMed] [Google Scholar]
- 23.Stahl SM. Essential Psychopharmacology of Antipsychotics and Mood Stabilizers. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- 24.A 6 months double-blind, risperidone-referenced, flexible dose, parallel-group extension study of bifeprunox in patients with schizophrenia. EudraCT number: 2004-002185-38. Available from: https://www.clinicaltrialsregister.eu/ctr-search/trial/2004-002185-38/results. Accessed January19, 2020.
- 25.Lundbeck. A one-year multi-national, multi-centre, randomised, double-blind, parallel-group, fixed-dose bifeprunox study combining a 12-week placebo-controlled, quetiapine-referenced phase with a 12-month quetiapine-controlled phase in patients with schizophrenia. EudraCT number: 2007-001097-90. Available from: https://www.clinicaltrialsregister.eu/ctr-search/trial/2007-001097-90/GR. Accessed January19, 2020.
- 26.A 40-Week Open, Flexible-Dose, Expansion Study of Bifeprunox in Patients with Schizophrenia. EudraCT number: 2004-000707-18; Available from: https://www.cliicaltrialsregister.eu/ctr-search/trial/2004-000707-18/IT. Accessed January19, 2020. [Google Scholar]
- 27.Lundbeck. An Open-Label Safety Study of Bifeprunox Investigating Flexible Doses of 20, 30, or 40mg/Day in Patients with Schizophrenia Who Have Completed Studies 10206 or 10265. EudraCT Number: 2005-000497-50; Available from: https://www.clinicaltrialsregister.eu/ctr-search/trial/2005-000497-50/IT. Accessed January19, 2020. [Google Scholar]
- 28.Ye N, Song Z, Zhang A. Dual ligands targeting dopamine D2 and serotonin 5-HT1A receptors as new antipsychotical or anti-Parkinsonian agents. Curr Med Chem. 2014;21(4):437–457. doi: 10.2174/09298673113206660300 [DOI] [PubMed] [Google Scholar]
- 29.Assié MB, Koek W. [(3)H]-8-OH-DPAT binding in the rat brain raphe area: involvement of 5-HT(1A) and non-5-HT(1A) receptors. Br J Pharmacol. 2000;130(6):1348–1352. doi: 10.1038/sj.bjp.0703426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cassaday HJ, Hodges H, Gray JA. The effects of ritanserin, RU 24969 and 8-OH-DPAT on latent inhibition in the rat. J Psychopharmacol. 1993;7(1Suppl):63–71. doi: 10.1177/026988119300700110 [DOI] [PubMed] [Google Scholar]
- 31.Winstanley CA, Chudasama Y, Dalley JW, Theobald DE, Glennon JC, Robbins TW. Intra-prefrontal 8-OH-DPAT and M100907 improve visuospatial attention and decrease impulsivity on the five-choice serial reaction time task in rats. Psychopharmacology. 2003;167(3):304–314. doi: 10.1007/s00213-003-1398-x [DOI] [PubMed] [Google Scholar]
- 32.Gogos A, Kusljic S, van den Buuse M. 8-OH-DPAT-induced effects on prepulse inhibition: pre- vs. post-synaptic 5-HT1A receptor activation. Pharmacol Biochem Behav. 2005;81(3):664–672. doi: 10.1016/j.pbb.2005.05.007 [DOI] [PubMed] [Google Scholar]
- 33.Pompeu TE, Alves FR, Figueiredo CD, et al. Synthesis and pharmacological evaluation of new N-phenylpiperazine derivatives designed as homologues of the antipsychotic lead compound LASSBio-579. Eur J Med Chem. 2013;66:122–134. doi: 10.1016/j.ejmech.2013.05.027 [DOI] [PubMed] [Google Scholar]
- 34.Neves G, Antonio CB, Betti AH, et al. New insights into pharmacological profile of LASSBio-579, a multi-target N-phenylpiperazine derivative active on animal models of schizophrenia. Behav Brain Res. 2013;237:86–95. doi: 10.1016/j.bbr.2012.09.016 [DOI] [PubMed] [Google Scholar]
- 35.Catafau AM, Bullich S, Nucci G, Burgess C, Gray F, Merlo-Pich E, Barcelona Clinical Imaging in Psychiatry Group. Contribution of SPECT measurements of D2 and 5-HT2A occupancy to the clinical development of the antipsychotic SB-773812. J Nucl Med. 2011;52(4):526–534. doi: 10.2967/jnumed.110.081885 [DOI] [PubMed] [Google Scholar]
- 36.Gsk. A Multi Centre, Double-Blind, Double-Dummy, Placebo-Controlled, Randomised, Adaptive, Dose-Range Study to Evaluate the Safety and Efficacy of SB-773812 Administered Once Daily for 12 Weeks in Adults with Schizophrenia. EudraCT number: 2005-002883-27; Available from: https://www.clinicaltrialsregister.eu/ctr-search/trial/2005-002883-27/results. Accessed January19, 2020. [Google Scholar]
- 37.ACADIA Pharmaceuticals Advances AM-831 to Phase I Clinical Development in Collaboration with Meiji Seika Pharma. 2011. Available from: http://phxcorporate-irnet. Accessed January19, 2020.
- 38.ACADIA Pharmaceuticals. AM 831. Available from: https://adisinsight.springer.com/drugs/800016551. Accessed January19, 2020.
- 39.Citrome L. A review of the pharmacology, efficacy and tolerability of recently approved and upcoming oral antipsychotics: an evidence-based medicine approach. CNS Drugs. 2013;27(11):879–911. doi: 10.1007/s40263-013-0105-7 [DOI] [PubMed] [Google Scholar]
- 40.Lundbeck. A 6-Month, Randomised, Double-Blind, Parallel-Group, Risperidone-Controlled, Fixed-Dose Study Evaluating the Safety and Efficacy of Zicronapine in Patients with Schizophrenia. EudraCT Number: 2010-022181-28; Available from: https://www.clinicaltrialsregister.eu/ctr-search/trial/2010-022181-28/EE. Accessed January19, 2020. [Google Scholar]
- 41.Liu J, Ogden A, Comery TA, Spiros A, Roberts P, Geerts H. Prediction of Efficacy of Vabicaserin, a 5-HT2C agonist, for the treatment of schizophrenia using a quantitative systems pharmacology model. CPT Pharmacometrics Syst Pharmacol. 2014;3(4):e111. doi: 10.1038/psp.2014.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfizer. Study Evaluating Vabicaserin in Subjects with Schizophrenia. NLM identifier: NCT00563706; Available from: https://clinicaltrials.gov/ct2/show/NCT00563706. Accessed January19, 2020. [Google Scholar]
- 43.Yamazaki M, Okabe M, Yamamoto N, Yarimizu J, Harada K. Novel 5-HT5A receptor antagonists ameliorate scopolamine-induced working memory deficit in mice and reference memory impairment in aged rats. J Pharmacol Sci. 2015;127(3):362–369. doi: 10.1016/j.jphs.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 44.Yamazaki M, Harada K, Yamamoto N, et al. ASP5736, a novel 5-HT5A receptor antagonist, ameliorates positive symptoms and cognitive impairment in animal models of schizophrenia. Eur Neuropsychopharmacol. 2014;24(10):1698–1708. doi: 10.1016/j.euroneuro.2014.07.009 [DOI] [PubMed] [Google Scholar]
- 45.Popik P, Krawczyk M, Kuziak A, et al. Serotonin type 5A receptor antagonists inhibit D-lysergic acid diethylamide discriminatory cue in rats. J Psychopharmacol. 2019;33(11):1447–1455. doi: 10.1177/0269881119867603 [DOI] [PubMed] [Google Scholar]
- 46.Thomas DR, Soffin EM, Roberts C, et al. SB-699551-A (3-cyclopentyl-N-[2-(dimethylamino)ethyl]-N-[(4ʹ-{[(2-phenylethyl)amino]methyl}-4-biphenylyl)methyl]propanamide dihydrochloride), a novel 5-ht5A receptor-selective antagonist, enhances 5-HT neuronal function: evidence for an autoreceptor role for the 5-ht5A receptor in guinea pig brain. Neuropharmacology. 2006;51(3):566–577. doi: 10.1016/j.neuropharm.2006.04.019 [DOI] [PubMed] [Google Scholar]
- 47.Ivachtchenko AV, Lavrovsky Y, Ivanenkov YA. AVN-211, novel and highly selective 5-HT6 receptor small molecule antagonist, for the treatment of alzheimer’s disease. Mol Pharm. 2016;13(3):945–963. doi: 10.1021/acs.molpharmaceut.5b00830 [DOI] [PubMed] [Google Scholar]
- 48.Morozova MA, Lepilkina TA, Rupchev GE, et al. Add-on clinical effects of selective antagonist of 5HT6 receptors AVN-211 (CD-008-0173) in patients with schizophrenia stabilized on antipsychotic treatment: pilot study. CNS Spectr. 2014;19(4):316–323. doi: 10.1017/S1092852913000394 [DOI] [PubMed] [Google Scholar]
- 49.Avineuro, pharmaceuticals. Available from: www.avineuro.com. Accessed January19, 2020. [Google Scholar]
- 50.Arnt J, Bang-Andersen B, Grayson B, et al. Lu AE58054, a 5-HT6 antagonist, reverses cognitive impairment induced by subchronic phencyclidine in a novel object recognition test in rats. Int J Neuropsychopharmacol. 2010;13(8):1021–1033. doi: 10.1017/S1461145710000659 [DOI] [PubMed] [Google Scholar]
- 51.Lundbeck. A Randomised, Double-Blind, Parallel-Group, Fixed-Dose Study Exploring the Efficacy and Safety of Lu AE58054 as Augmentation Therapy to Risperidone in Patients with Schizophrenia. EudraCT Number: 2008-001441-26; Available from: https://www.clinicaltrialsregister.eu/ctr-search/trial/2008-001441-26/IT. Accessed January19, 2020. [Google Scholar]
- 52.Idalopirdine. https://www.alzforum.org/therapeutics/idalopirdine. Accessed January19, 2020.
- 53.Leysen JE, Gommeren W, Van Gompel P, Wynants J, Janssen PF, Laduron PM. Receptor-binding properties in vitro and in vivo of ritanserin: A very potent and long acting serotonin-S2 antagonist. Mol Pharm. 1985;27(6):600–611. [PubMed] [Google Scholar]
- 54.Lundbeck updates on clinical phase III study for Lu AF35700 in Treatment-Resistant Schizophrenia. Available from:https://investor.lundbeck.com/news-releases/news-release-details/lundbeck-updates-clinical-phase-iii-study-lu-af35700-treatment. (Valby, Oct. 25, 2018)
- 55.Lundbeck. Efficacy of Lu AF35700 in Patients with Early-In-Disease or Late-In-Disease Treatment-Resistant Schizophrenia (Anew). NLM identifier: NCT03230864; Available from: https://clinicaltrials.gov/ct2/show/NCT03230864. Accessed January19, 2020. [Google Scholar]
- 56.Cantillon M, Prakash A, Alexander A, Ings R, Sweitzer D, Bhat L. Dopamine serotonin stabilizer RP5063: a randomized, double-blind, placebo-controlled multicenter trial of safety and efficacy in exacerbation of schizophrenia or schizoaffective disorder. Schizophr Res. 2017;189:126–133. doi: 10.1016/j.schres.2017.01.043 [DOI] [PubMed] [Google Scholar]
- 57.Cantillon M, Ings R, Bhat L. Initial Clinical Experience of RP5063 following single doses in normal healthy volunteers and multiple doses in patients with stable schizophrenia. Clin Transl Sci. 2018;11(4):387–396. doi: 10.1111/cts.12545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reviva pharmaceuticals. RP5063 in Subjects with Schizophrenia or Schizoaffective Disorder (REFRESH). NLM identifier: NCT01490086; Available from: https://clinicaltrials.gov/ct2/show/NCT01490086. Accessed January19, 2020. [Google Scholar]
- 59.Reviva pharmaceuticals clinical trials. Available from: http://revivapharma.com/clinical-trails. Accessed January19, 2020.
- 60.Frankle WG, Lombardo I, Kegeles LS, et al. Serotonin 1A receptor availability in patients with schizophrenia and schizo-affective disorder: a positron emission tomography imaging study with [11C]WAY 100635. Psychopharmacology. 2006;189(2):155–164. doi: 10.1007/s00213-006-0543-8 [DOI] [PubMed] [Google Scholar]
- 61.Meltzer HY, Li Z, Kaneda Y, Ichikawa J. Serotonin receptors: their key role in drugs to treat schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(7):1159–1172. doi: 10.1016/j.pnpbp.2003.09.010 [DOI] [PubMed] [Google Scholar]
- 62.Burnet PW, Eastwood SL, Harrison PJ. 5-HT1A and 5-HT2A receptor mRNAs and binding site densities are differentially altered in schizophrenia. Neuropsychopharmacology. 1996;15(5):442–455. doi: 10.1016/S0893-133X(96)00053-X [DOI] [PubMed] [Google Scholar]
- 63.Pogorelov VM, Rodriguiz RM, Cheng J, et al. 5-HT2C agonists modulate schizophrenia-like behaviors in mice. Neuropsychopharmacology. 2017;42(11):2163–2177. doi: 10.1038/npp.2017.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gonzalez R, Chávez-Pascacio K, Meneses A. Role of 5-HT5A receptors in the consolidation of memory. Behav Brain Res. 2013;252:246–251. doi: 10.1016/j.bbr.2013.05.051 [DOI] [PubMed] [Google Scholar]
- 65.de Bruin NM, Kruse CG. 5-HT6 receptor antagonists: potential efficacy for the treatment of cognitive impairment in schizophrenia. Curr Pharm Des. 2015;21(26):3739–3759. doi: 10.2174/1381612821666150605112105 [DOI] [PubMed] [Google Scholar]
- 66.Woods S, Clarke NN, Layfield R, Fone KC. 5-HT(6) receptor agonists and antagonists enhance learning and memory in a conditioned emotion response paradigm by modulation of cholinergic and glutamatergic mechanisms. Br J Pharmacol. 2012;167(2):436–449. doi: 10.1111/j.1476-5381.2012.02022.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mauri MC, Paletta S, Maffini M, et al. Clinical pharmacology of atypical antipsychotics: an update. EXCLI J. 2014;13:1163–1191. [PMC free article] [PubMed] [Google Scholar]
- 68.Korcsmáros T, Szalay MS, Böde C, Kovács IA, Csermely P. How to design multi-target drugs. Expert Opin Drug Discov. 2007;2(6):799–808. doi: 10.1517/17460441.2.6.799 [DOI] [PubMed] [Google Scholar]
- 69.Correll CU, Kishimoto T, Kane JM. Randomized controlled trials in schizophrenia: opportunities, limitations, and trial design alternatives. Dialogues Clin Neurosci. 2011;13(2):155–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology. 2009;62(10). [DOI] [PubMed] [Google Scholar]