Abstract
Rapidly progressive ascites is a frequent clinical manifestation of advanced abdominal malignancies or portal hypertension due to liver diseases. We report a case of 61-year-old man who presented with rapidly progressive ascites. The presence of ascites, generalised lymphadenopathy, osteosclerosis on imaging and hepatosplenomegaly initially pointed towards the diagnosis of advanced high-grade lymphoma or accelerated myeloid neoplasm. Lymph node biopsy revealed infiltration by CD45, cKIT and CD30; tryptase and toluidine blue-positive mast cells (MCs). Bone marrow examination revealed infiltration by MCs and next generation sequencing revealed the pathognomic exon 17 D 816V KIT mutation. The patient was started on weekly pegylated interferon with significant symptom relief. Systemic mastocytosis should be considered as a differential diagnosis in a clinical case of ascites of unknown aetiology even in the absence of typical skin manifestations.
Keywords: liver disease, portal hypertension
Background
Ascites is a common presenting symptom in clinical practice. Despite having distinctive clinico-pathological features, systemic mastocytosis (SM) is rarely suspected as a differential diagnosis in patients who presented with ascites especially when cutaneous manifestations are absent. Mastocytosis is characterised by pathological expansion and accumulation of mast cells (MCs) in diverse tissues such as skin, bone marrow (BM), spleen, liver, lymph nodes and gastrointestinal tract. In the revised WHO classification of myeloid neoplasms, SM has been designated as a separate entity from other myeloproliferative neoplasms due to its unique clinico-pathological features.1 Hepatic involvement is seen in at least two-third of the cases of aggressive systemic mastocytosis (ASM).2
Case presentation
A 61-year-old man presented with rapidly progressive painless ascites of 2 weeks duration. He reported 1-year history of involuntary weight loss of 22 kg, asthenia and low-grade fever. He denied history of jaundice, haematemesis, melena, altered bowel habits, transfusion, smoking, alcohol or drug consumption or high-risk sexual behaviour. He also denied history of pruritus, headaches, diarrhoea, skin lesions and anaphylaxis. Physical examination showed cachexia, pallor, firm 2×3 cm axillary and 1×2 cm cervical lymphadenopathy. Abdominal examination revealed shifting dullness, enlarged firm liver (span 14 cm) and spleen that was palpable 3 cm below the costal margin.
Investigations
Haemogram revealed normocytic normochromic anaemia (haemoglobin 91 g/L), normal leucocyte count (6.2×109/L) with normal differential counts, thrombocytopenia (45×109/L) and normal coagulogram. Liver function tests were normal except for high alkaline phosphatase 370 U/L (ref: 42–128 U/L) and hypoalbuminemia (2.8 g/dL). For his tense ascites, he required three sessions of large volume paracentesis in a span of 1 week. Ascitic fluid analysis showed high serum ascites albumin gradient (SAAG), low protein ascites (SAAG 1.3; ascitic fluid albumin 1.5 g/dL) with no evidence of spontaneous bacterial peritonitis or infiltration by atypical cells. Doppler ultrasonography of the liver revealed dilated portal vein (15 mm at porta) with hepatofugal portal flow. Upper gastrointestinal endoscopy showed multiple nodular lesions in first part of duodenum and antrum, however, there was no evidence of portal hypertension. Serological testing for hepatitis B, C, HIV and autoimmune markers were negative. Serum immunoglobulins were in the normal range with mildly decreased IgM levels and serum lactate dehydrogenase level was elevated (324 U/L).
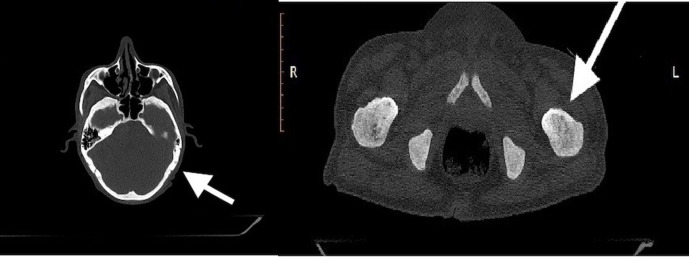
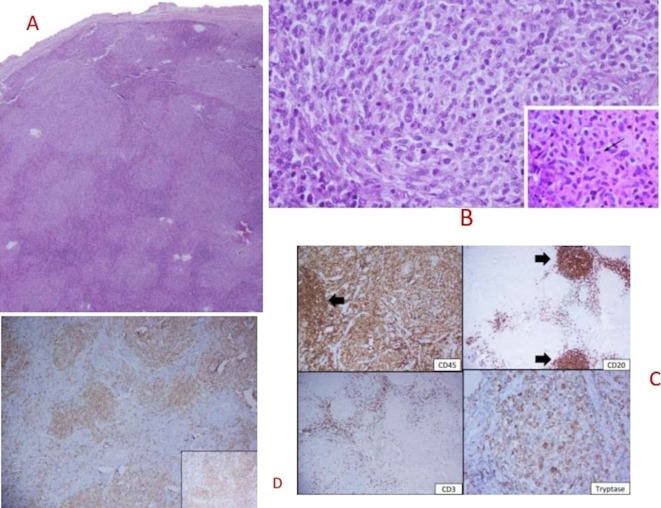
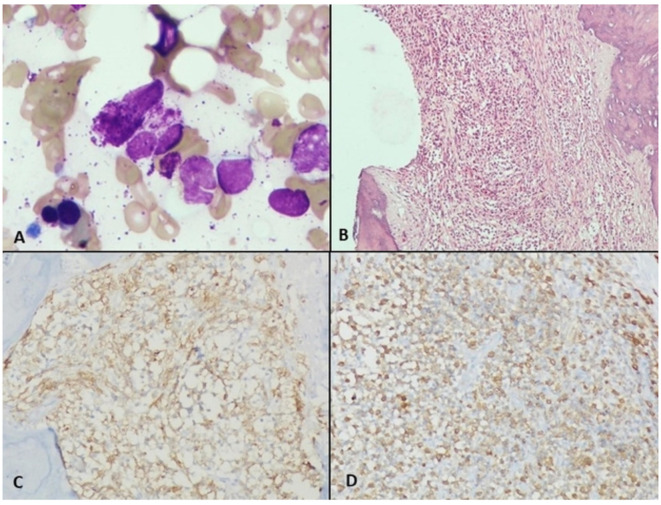
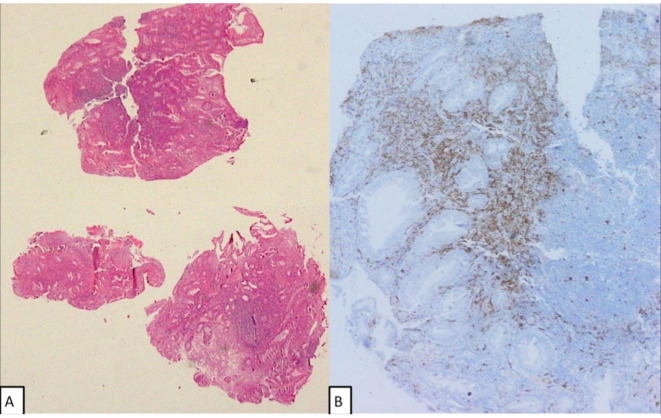
CT scan confirmed hepatosplenomegaly (liver and spleen size: 18.1 cm and 17.6 cm respectively), ascites and non-necrotic axillary (38×18 mm); mediastinal (16×8 mm) and retroperitoneal lymphadenopathy (12×18 mm). There was no peritoneal enhancement or deposit. Besides, there was patchy sclerosis in calvarial bones, clavicle, pelvis, ribs, femori and humeri (figure 1). Considering the constellation of ascites, generalised lymphadenopathy, hepatosplenomegaly and sclerotic bone disease; advanced high-grade lymphoma or accelerated myeloid neoplasm were considered in the differential diagnosis. Axillary lymph node biopsy showed distorted architecture with obliteration of sinuses and infiltration of paracortex with spindle-shaped neoplastic cells that were arranged in whorls and nodules. They had oval to elongated nuclei, pale cytoplasm and scarce mitosis. On immunohistochemistry (IHC), these cells were positive for CD45, c-KIT, CD30, tryptase, toluidine blue and negative for CD 3/20/35/240/23/7/2/5, S100 and ALK1 (figure 2A–D). BM examination revealed multifocal infiltration of atypical MCs in nodular aggregates (>15 aggregates) in the background of fibrosis. On IHC, these cells were diffusely positive for mast cell tryptase and CD 117 (figure 3A–D). Biopsy from duodenal nodules also revealed MC infiltration (figure 4A–B). Next generation sequencing (NGS) from peripheral blood and lymph node biopsy block revealed the pathognomic- exon 17 D 816V KIT mutation. A final diagnosis of ASM with BM failure, ascites, portal hypertension and splenomegaly was made.
Figure 1.
CT scan showing diffuse osteosclerosis in calvaria and humeri.
Figure 2.
(A) Lymph node section showing effaced nodal architecture by pale confluent nodules (H&E, 20×). (B) These atypical nodules were composed of spindled to epithelioid cells with abundant vacuolated cytoplasm and bland nuclei (inset with arrow showing cytoplasmic granules), H&E 400×. (C) Immunohistochemistry (IHC) stains show diffuse CD45 positivity and tryptase. These nodules were negative for CD3 and CD20. (D) IHC showing diffuse staining for CD117 (cKIT).
Figure 3.
(A) Bone marrow (BM) aspirate showing normal marrow cells along with degenerated mast cell (MC) (Giemsa and MGG stain ×1000). (B) BM biopsy showing fibrosis and nodular aggregates of clear looking MCs (H&E stain ×200). (C) MCs showing positivity for MC tryptase (MCT stain ×200) (D): MCs showing positivity for CD117 (CD117 stain ×200).
Figure 4.
(A) Duodenal nodule biopsy showing infiltration by dense lymphocytic infiltrate and scattered mast cells (MCs) (H&E stain, 40×). (B) MCs showing positivity for CD117 in the duodenal mucosa (CD117 stain ×200).
Treatment
Financial constraints precluded the use of midostaurin. He was started on weekly pegylated interferon (PEG IFN) α2b + prednisolone 0.5 mg/kg/day along with low dose diuretics.
Outcome
At 3 months of follow-up, he was afebrile and had no pedal oedema or ascites. He had not required therapeutic abdominal paracentesis for past 2 months. He persisted- to have hepatosplenomegaly and is planned to continue on PEG IFN α2b until progression.
Discussion
The current classification schema divides SM into five categories among which ASM and mast cell leukaemia (MCL) are the aggressive variants with inferior outcomes.1 ASM constitutes around 12% cases of SM.2 Clinical findings that are related to organ damage from infiltrating MCs are referred to as C findings and include cytopenias, liver function abnormalities, portal hypertension, splenomegaly with hypersplenism, malabsorption with weight loss and osteolytic bone lesions.1 As per WHO 2016 criteria, the presence of one or more C findings are required for diagnosing ASM. The presence of bone marrow failure, ascites, portal hypertension and splenomegaly -qualified for the diagnosis of ASM in the current case.1 The D816V KIT mutation found in our case is present in more than 85% of patients with adult-onset mastocytosis.3
Patients of ASM commonly present with constitutional symptoms (60%), hepatosplenomegaly (50%), lymphadenopathy (30%), severe anaemia (Hgb <100 g/-L; 24%) thrombocytopenia (platelets <100×109/L; 27%) and leukocytosis (41%).1 4 Absence of cutaneous manifestations is seen in more than 50% cases of ASM, particularly in adults.1 Up to 70% patients of SM have skeletal involvement at the time of presentation.5 Osteosclerosis, osteoporosis, lytic lesions and fractures are the common patterns of bone involvement that can be seen on imaging.6 The presence of osteosclerosis that was seen in the current case is associated with an aggressive disease course and is found in ~19% cases of SM.6 Osteosclerosis is infrequently described in other hemato-lymphoid malignancies and may point towards the possibility of SM.
Although hepatic involvement is seen in two-thirds of the patients of ASM, it predominantly manifests as hepatomegaly or isolated elevation of ALP.1 7 Only 4% patients with ASM develop portal hypertension while ascites has been variably reported in 7%–9% cases.1 7 However, ascites as the predominant presenting complaint is rare with only few cases reported.8–12
Infiltration by MCs, portal fibrosis, nodular regenerative hyperplasia, portal venopathy and venoocclusive disease have been described in liver biopsy specimens of patients with SM.1 4 In the current case, the presence of elevated alkaline phosphatase, hepatosplenomegaly and high SAAG ascites suggested the presence of an infiltrative hepatic disease, however ASM was not considered initially in the absence of diarrhoea, pruritus, headache, skin lesions or anaphylaxis. Therapy of mastocytosis centres on addressing symptoms related to MC activation, and using cytoreductive agents to reverse organ damage caused by neoplastic MC infiltrates in advanced SM.13 Cytoreductive therapy of ASM has evolved from the use of cladribine, IFN alpha to the use of targeted oral molecules including the imatinib and midostaurin.14 The frequency of major response is approximately 20%–30%with IFN-alpha.15 Moreover, IFN should be used with caution in patients with advanced cirrhosis (Child-Pugh score >6) as hepatic decompensation may occur.16 The use of imatinib is predominantly restricted to patients with wild-type c-KIT receptor tyrosine kinase (c-KIT) or rare non-exon 17 mutations (eg, germ line K509I; deletion of codon 419 in exon 8).14 The presence of exon 17 c-KIT mutation precluded the use of imatinib in the current case.
High response rates have been seen with small molecule inhibitors that target mutant c-KIT, including Food and Drug Administration (FDA) approved midostaurin or avapritinib as a part of clinical trial. Midostaurin was approved by the U.S. FDA for the treatment of adult patients with advanced SM in 2017 including those with hepatic dysfunction and ascites.17–19 While there is no clear consensus on sequencing of therapies, midostaurin is appropriate both as first-line treatment, particularly in MCL patients, as well as for salvage treatment in patients progressing after other cytoreductive strategies with IFN or cladribine.19 However, high costs preclude the use of midostaurin for most patients in low-to-middle income countries. Allogenic stem cell transplant (ASCT) should be considered in patients with aggressive disease (ASM and MCL) who are young and otherwise healthy, as this is the only option for a sustained response.
Prognostication in ASM initially used a scoring system based on the WHO SM classification and additional risk factors: age >70 years, platelets < 80 x 109/L and alkaline phosphatase ≥240 U/L.8 Although patients with low- and intermediate-risk disease have a survival more than 10 years, patients with advanced disease (regardless of additional risk factors) are reported to have a median survival of 3.5 years.20 The presence and number of SRSF2, ASXL1 and RUNX1 (S/A/R) mutations are associated with a poor prognosis, even in patients treated with midostaurin.21 The presence of thrombocytopenia and elevated alkaline phosphatase point towards an inferior prognosis in the current case, though none of the high-risk (S/A/R) mutations were detected on NGS. Although data are scarce, development of ascites is likely to portend a poor prognosis, and was uniformly fatal in all the five patients described by Mican et al.7
Learning points.
Rapid onset ascites can be a manifestation of many disease entities and diagnosis can be challenging.
Aggressive systemic mastocytosis (ASM) is rarely considered in the differential diagnosis of ascites though hepatic dysfunction and ascites is a common manifestation of the entity.
Diagnosis of ASM is frequently missed, especially when the physicians are unaware of the biology and aetiology of the disease, no skin lesions are present and blood counts are only mildly deranged.
Systemic mastocytosis should be considered as a differential diagnosis in patients with ascites, particularly in the presence of lymphadenopathy and osteosclerosis.
Acknowledgments
Professor Pankaj Malhotra, Dr Narender Kumar, Dr Pulkit Rastogi.
Footnotes
Contributors: AJ and VAA drafted the manuscript and were involved in management of the case. AB reviewed the biopsy. DS contributed to the patient management.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Horny HP, Akin C, Arber D. Mastocytosis : Swerdlow SH, Campo E, Harris NL, World Health Organization (WHO) classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press, 2017: 61–9. [Google Scholar]
- 2.Lim K-H, Tefferi A, Lasho TL, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood 2009;113:5727–36. 10.1182/blood-2009-02-205237 [DOI] [PubMed] [Google Scholar]
- 3.Longley BJ, Metcalfe DD, Tharp M, et al. Activating and dominant inactivating c-kit catalytic domain mutations in distinct clinical forms of human mastocytosis. Proc Natl Acad Sci U S A 1999;96:1609–14. 10.1073/pnas.96.4.1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Travis WD, Li CY, Bergstralh EJ, et al. Systemic mast cell disease. Analysis of 58 cases and literature review. Medicine 1988;67:345–68. [PubMed] [Google Scholar]
- 5.Johansson C, Roupe G, Lindstedt G, et al. Bone density, bone markers and bone radiological features in mastocytosis. Age Ageing 1996;25:1–7. 10.1093/ageing/25.1.1 [DOI] [PubMed] [Google Scholar]
- 6.Barete S, Assous N, de Gennes C, et al. Systemic mastocytosis and bone involvement in a cohort of 75 patients. Ann Rheum Dis 2010;69:1838–41. 10.1136/ard.2009.124511 [DOI] [PubMed] [Google Scholar]
- 7.Mican JM, Di Bisceglie AM, Fong TL, et al. Hepatic involvement in mastocytosis: clinicopathologic correlations in 41 cases. Hepatology 1995;22:1163–70. 10.1016/0270-9139(95)90625-8 [DOI] [PubMed] [Google Scholar]
- 8.Bloom G, Franzén S, Sirén M. Malignant systemic mast cell disease (mastocytoma) in man. Acta Med Scand 1960;168:95–102. 10.1111/j.0954-6820.1960.tb06648.x [DOI] [Google Scholar]
- 9.Ghandur-Mnaymneh L, Gould E. Systemic mastocytosis with portal hypertension. autopsy findings and ultrastructural study of the liver. Arch Pathol Lab Med 1985;109:76–8. [PubMed] [Google Scholar]
- 10.Narayanan MN, Liu Yin JA, Azzawi S, et al. Portal hypertension and ascites in systemic mastocytosis. Postgrad Med J 1989;65:394–6. 10.1136/pgmj.65.764.394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X-Y, Zhang W-H. An unusual case of aggressive systemic mastocytosis mimicking hepatic cirrhosis. Cancer Biol Med 2014;11:134. 10.7497/j.issn.2095-3941.2014.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jafari E, Hadipour A, Kalantari Khandani B, et al. Mast cell leukemia with ascites and multiple organs damage. Iran J Pathol 2019;14:265–9. 10.30699/ijp.2019.96187.1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scherber RM, Borate U. How we diagnose and treat systemic mastocytosis in adults. Br J Haematol 2018;180:11–23. 10.1111/bjh.14967 [DOI] [PubMed] [Google Scholar]
- 14.Pardanani A Systemic mastocytosis in adults: 2019 update on diagnosis, risk stratification and management. Am J Hematol 2019;94:363–77. 10.1002/ajh.25371 [DOI] [PubMed] [Google Scholar]
- 15.Hauswirth AW, Simonitsch-Klupp I, Uffmann M, et al. Response to therapy with interferon alpha-2b and prednisolone in aggressive systemic mastocytosis: report of five cases and review of the literature. Leuk Res 2004;28:249–57. 10.1016/S0145-2126(03)00259-5 [DOI] [PubMed] [Google Scholar]
- 16.Gambato M, Lens S, Navasa M, et al. Treatment options in patients with decompensated cirrhosis, pre- and post-transplantation. J Hepatol 2014;61:S120–31. 10.1016/j.jhep.2014.07.020 [DOI] [PubMed] [Google Scholar]
- 17.Gotlib J, Kluin-Nelemans HC, George TI, et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N Engl J Med 2016;374:2530–41. 10.1056/NEJMoa1513098 [DOI] [PubMed] [Google Scholar]
- 18.DeAngelo DJ, George TI, Linder A, et al. Efficacy and safety of midostaurin in patients with advanced systemic mastocytosis: 10-year median follow-up of a phase II trial. Leukemia 2018;32:470–8. 10.1038/leu.2017.234 [DOI] [PubMed] [Google Scholar]
- 19.Vette, LKasamon Y, et al. Fda approval summary: midostaurin for the treatment ofAdvanced systemic mastocytosis. The Oncologist 2018;23:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sperr, et al. Prognosticfactors and survival prediction in 1,088patients with mastocytosis collected in the registryof the European competence network onMastocytosis (ECNM registry) 2016;128:396. [Google Scholar]
- 21.Jawhar M, Schwaab J, Schnittger S, et al. Additional mutations in SRSF2, ASXL1 and/or RUNX1 identify a high-risk group of patients with KIT D816V(+) advanced systemic mastocytosis. Leukemia 2016;30:136–43. 10.1038/leu.2015.284 [DOI] [PubMed] [Google Scholar]