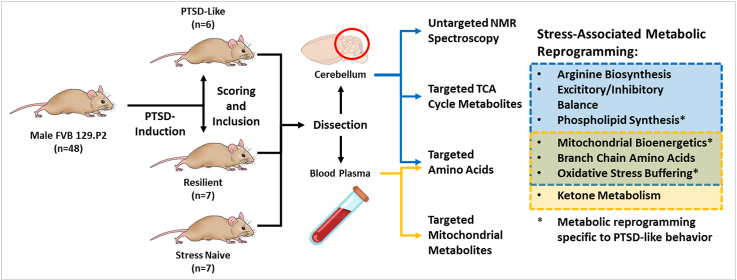
Abstract
Mitochondrial metabolism is increasingly implicated in psychopathologies and mood disorders, including post-traumatic stress disorder (PTSD). We recently reported that mice exposed to a novel paradigm for the induction of PTSD-like behavior displayed reduced mitochondrial electron transport chain (mtETC) complex activity as well as decreased multi-system fatty acid oxidation (FAO) flux. Based on these results, we hypothesized that stressed and PTSD-like animals would display evidence of metabolic reprogramming in both cerebellum and plasma consistent with increased energetic demand, mitochondrial metabolic reprogramming, and increased oxidative stress.
We performed targeted metabolomics in both cerebellar tissue and plasma, as well as untargeted nuclear magnetic resonance (NMR) spectroscopy in the cerebellum of 6 PTSD-like and 7 resilient male mice as well as 7 trauma-naïve controls. We identified numerous differences in amino acids and tricarboxylic acid (TCA) cycle metabolite concentrations in the cerebellum and plasma consistent with altered mitochondrial energy metabolism in trauma exposed and PTSD-like animals. Pathway analysis identified metabolic pathways with significant metabolic pathway shifts associated with trauma exposure, including the tricarboxylic acid cycle, pyruvate, and branched-chain amino acid metabolism in both cerebellar tissue and plasma. Altered glutamine and glutamate metabolism, and arginine biosynthesis was evident uniquely in cerebellar tissue, while ketone body levels were modified in plasma. Importantly, we also identified several cerebellar metabolites (e.g. choline, adenosine diphosphate, beta-alanine, taurine, and myo-inositol) that were sufficient to discriminate PTSD-like from resilient animals.
This multilevel analysis provides a comprehensive understanding of local and systemic metabolite fingerprints associated with PTSD-like behavior, and subsequently altered brain bioenergetics. Notably, several transformed metabolic pathways observed in the cerebellum were also reflected in plasma, connecting central and peripheral biosignatures of PTSD-like behavior. These preliminary findings could direct further mechanistic studies and offer insights into potential metabolic interventions, either pharmacological or dietary, to improve PTSD resilience.
Keywords: PTSD, Metabolomics, Mitochondria, Cerebellum, BCAA
Graphical abstract
Highlights
-
•
Trauma-exposed mice display cerebellar and multi-system metabolic reprogramming.
-
•
Stressed mice display altered multisystem mitochondrial bioenergetics.
-
•
Cerebellar metabolites are sufficient discriminate PTSD-like and resilient mice.
-
•
Plasma metabolomics identifies potential dietary promotors of trauma resilience.
-
•
Cerebellar metabolic reprogramming is reflected in plasma biosignature.
Abbreviations
- PTSD
post-traumatic stress disorder
- mtETC
mitochondrial electron transport chain
- FAO
fatty acid oxidation
- NMR
nuclear magnetic resonance
- TCA
tricarboxylic acid
- LDA
linear discriminant analysis
- PCA
principal component analysis
- B-Ala
beta-alanine
- Etn
ethanolamine
- GABA
gamma-aminobutyric acid
- Cys
L-cystine
- Cit
L-citrulline
- Val
L-valine
- Glu
L-glutamate
- PEtn
phosphoethanolamine
- Arg
L-arginine
- Orn
L-ornithine
- GPC
glycerophosphocholine
- IMP
inosine monophosphate
- AMP
adenosine monophosphate
- ADP
adenosine diphosphate
- Gly
L-glycine
- Asp
L-aspartate
- HPX
hypoxanthine
- Cho
choline
- Met
L-methionine
- AAA
alpha-aminoadipic acid
- Leu
leucine
- Ile
isoleucine
- MGA
3-methylglutaconic acid
- aKB
2-ketobutyric acid
- aKIC
2-ketoisocaproic acid
- aKIV
2-ketoisovaleric acid
- BHB
3-hydroxybutyric acid
- BMaKV
3-methyl-2-ketovaleric acid
- AcAc
acetoacetic acid
- aHB
2-hydroxybutyric acid
- PE
phosphatidylethanolamine
- PC
phosphatidylcholine
- GSH
glutathione
- BCAA
branched-chain amino acid
- NO
nitric oxide
1. Introduction
Post-traumatic stress disorder (PTSD) is a debilitating psychiatric disorder that precipitates following exposure to a traumatic event (TE), such as direct or repeated indirect exposure to death, injury, or sexual violence (Flory and Yehuda 2015). While lifetime incidence of TE exposure is high (64–70%) (Benjet et al., 2016), lifetime prevalence of PTSD remains relatively low (1.3–12.2%) (Karam et al., 2014). Given this discrepancy between incidence of trauma exposure and PTSD precipitation, understanding the biologic factors involved with vulnerability and resilience to PTSD should prove invaluable to developing improved prevention, diagnosis, and treatment of PTSD.
Mitochondrial metabolism is increasingly implicated in several psychopathologies and neurodegenerative disorder, including Alzheimer's, Parkinson's, Huntington's, bipolar disorder, anxiety, depression, and PTSD (Shao et al., 2008; Hollis et al., 2015; Pei and Wallace 2018; Morava and Kozicz 2013; Preston et al. 2018). Mitochondria play a central role in several biological functions involved in PTSD psychopathology, including synaptic plasticity, neurogenesis, apoptosis, and largescale brain network activation (Preston et al. 2018). Mitochondrial DNA variants have been associated with PTSD in humans (Flaquer et al., 2015), and reduced mtDNA copy number has been observed in peripheral blood cells of combat veterans with PTSD (Bersani et al., 2016). Altered expression of mitochondrial genes have been identified in the brains of stressed humans and rodents (Han et al. 2013; Zhang et al., 2015), and alterations in mitochondrial physiology, energy metabolism, and ATP production has been observed in stressed rodents (Xing et al., 2013; Xiao et al. 2009; Xiao et al., 2011; Li et al., 2010; Wan et al., 2016; Han et al. 2013). However, the role of mitochondria in trauma response and PTSD pathophysiology has not been fully elucidated.
The cerebellum is an emerging region of interest in susceptibility to psychopathology, including PTSD (Holmes et al., 2018; Rabellino et al., 2018; Baldaçara et al., 2012), and has recently been implicated in vulnerability to psychopathology generally (Hariri 2019). We recently reported (Preston et al., 2020) reduced mtETC complex activity in the cerebellum and skeletal muscle, and evidence of reduced FAO oxidation flux in both the cerebellum and circulating plasma of mice exposed to a recently-developed paradigm of PTSD-induction (Lebow et al., 2012). This paradigm precipitated a robust and clinically-relevant PTSD-like behavioral phenotype in 12.5% of exposed animals, a comparable rate to PTSD incidence in humans exposed to a TE (Karam et al., 2014). Reduced mtETC complex activity in the cerebellar tissue of these animals directly correlated with PTSD-like behavioral symptom score. Animals exposed to the paradigm also displayed reduced mtDNA copy number in cerebellar tissue and peripheral blood cells, as well as reduced circulating carnitine. These data point to cerebellar and multi-stem alterations in mitochondrial physiology and metabolism in this animal model of PTSD.
Patients with inherited mtETC complex deficiencies display a wide range of metabolic changes consistent with mitochondrial energy metabolic remodeling, including elevated lactic acid, altered TCA cycle metabolites, elevated ketones, and biomarkers for oxidative stress (Thompson Legault et al., 2015). We hypothesized that stressed animals will display metabolic remodeling consistent with increased energetic demand and mitochondrial metabolic reprogramming in both cerebellum and plasma, including evidence of altered bioenergetics, and increased oxidative stress relative to stress-naïve animals, and that these metabolic differences will be associated with PTSD-like symptomatology. Conversely, we hypothesized that PTSD-resilient animals will display metabolic changes consistent with active metabolic adaptation, including increased energy recruitment and oxidative stress buffering. We performed targeted metabolomics to assay for amino acids and TCA cycle metabolites in both cerebellum and plasma. We also performed untargeted nuclear magnetic resonance (NMR) for metabolites in the cerebellum, and targeted metabolomics for mitochondrial-specific metabolites in plasma. Consistent with our hypothesis, stressed animals displayed changes consistent with metabolic reprogramming and increased oxidative stress, as well as altered mitochondrial phospholipid and arginine metabolism in the cerebellum, as well as evidence of increased energetic demand and decreased energy production in the plasma. Additionally, metabolites associated with reduced cerebellar energy metabolism and increased oxidative stress were identified as discriminative of PTSD-like and resilient animals.
2. Methods and materials
2.1. Animal background and maintenance
Animals were housed four-to-a-cage in ventilated microisolator cages on a 12-h light/dark cycle in a pathogen free, temperature-controlled animal housing facility, with food and water ad libitum. FVB.129P2 mice, a strain of “sighted” FVB mouse wildtype for the Pde6b retinal degeneration allele, were acquired from The Jackson Laboratory.
2.2. PTSD induction and behavioral assessment
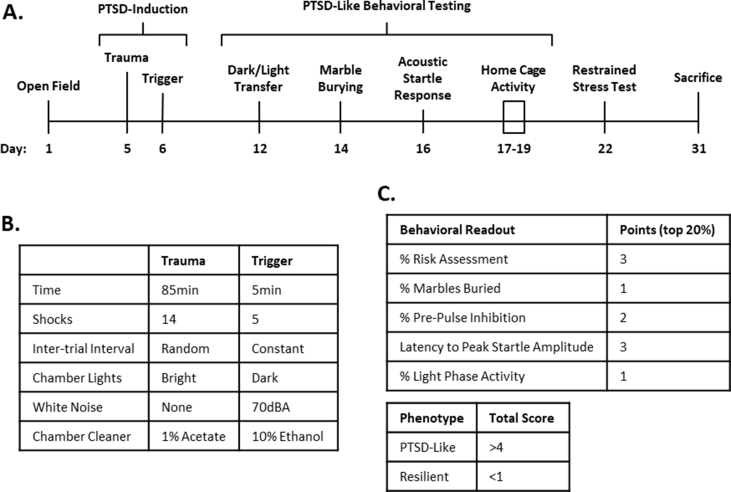
48 male FVB.129P2 mice were exposed to the PTSD induction paradigm published by Lebow et al.20. PTSD was induced through two decontextualized sessions of inescapable electric foot shock over two consecutive days (Figs. S1A and B). Animals were assessed for risk assessment, marble burying, %pre-pulse inhibition, latency to peak startle amplitude, and light phase activity (Fig. S1A). 6 PTSD-like and 7 resilient animals were identified based on performance in these behavioral measures using a scoring and inclusion criteria (Fig. S1C). All animal experiments were carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals, and the Tulane University Institutional Animal Care and Use Committee. All efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilize alternatives to in vivo techniques, if available. See Supplementary Methods for the detailed PTSD induction protocol. The results of the behavioral assessment for this cohort of animals have been previously published (Preston et al., 2020). It is well documented that the incidence of PTSD is significantly higher in female patients than in male patients (Kessler et al., 1995). However, the PTSD-induction paradigm used in this study has not yet been used to assess female mice. We therefore restricted our study to male mice. Further development of this paradigm will be required to validate its use in female animals.
2.3. Sacrifice and necropsy
All animals were sacrificed via live decapitation without the use of anesthetic. Trunk blood was collected with EDTA and centrifuged at 10,000 RCF for 10 min, plasma was collected and flash frozen. The brain was removed and dissected coronally, separating the cerebellum from the forebrain and the cerebellum was flash frozen.
2.4. Metabolomics analysis
Metabolomics analysis was performed by the Mayo Clinic Metabolomics Core, Rochester MN (Wilkins et al., 2019). Amino acids in cerebellar tissue and plasma were separated on a Waters BEH C18 column (2.1 mm × 150 mm × 1.7 μm) and analyzed using Thermo TSQ Quantum Ultra mass spectrometer (West Palm Beach, FL) coupled with a Waters ACQUITY ultra performance liquid chromatography (UPLC) system. TCA cycle metabolites in cerebellar tissue were separated on an Agilent DB-5MS column (30 m × 0.25 mm × 0.25 μm) and detected with an Agilent 5977A gas chromatography/mass spectrometry (GC/MS) under electron impact and single ion monitoring conditions in positive mode. Untargeted NMR spectrometry was performed in cerebellar tissue using a Bruker AVANCE III 600 MHz NMR spectrometer (Bruker, Billerica, USA). Mitochondria-specific metabolites in plasma were run by GC/MS with: HP-5 (Cross linked 5% phenylmethyl silicone), (25m; ID 0.20 mm; film thickness 0.33 μm) on Agilent 7890B Gas Chromatograph/Mass Spectrometer (GC/MS) Agilent 7693 Autosampler, with Agilent Masshunter Acquisition 10.0.368. All metabolite abundances were normalized to total metabolite abundance in each sample.
2.5. Statistical analyses
All statistical analyses were performed using GraphPad Prism or SPSS. Student's T-test was used to compare two groups, with false discovery rate set to 10%. Data are expressed as averages ± standard deviation. Linear discriminant analysis (LDA) was performed using SPSS. Principal component analysis (PCA) and pathway analysis was performed using Metaboanalyst 4.0 (Chong et al. 2019). For comparisons, the 6 identified PTSD-like animals were compared to the 7 identified resilient (trauma-exposed control) animals. To assess the impact of trauma exposure the combined group of PTSD-like and resilient (stressed) animals were compared to 7 animals not exposed to trauma (naïve).
3. Results
3.1. Cerebellar amino acids and TCA cycle metabolites
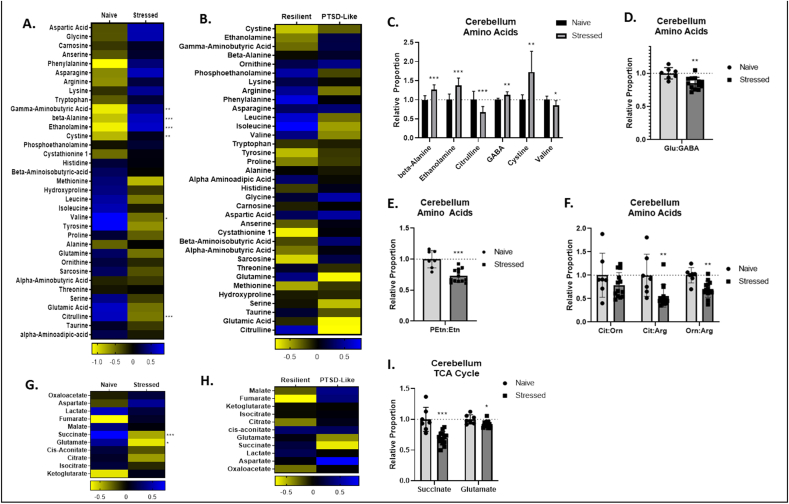
Stressed animals displayed elevated abundance of beta-alanine (B-Ala), ethanolamine (Etn), gamma-aminobutyric acid (GABA), and L-cystine (Cys), and reduced abundance of L-citrulline (Cit) and L-valine (Val) (Fig. 1A, C). Stressed animals also displayed reduced ratios of L-glutamate (Glu):GABA (Fig. 1D), phosophoethanolamine (PEtn):Etn (Fig. 1E), and reduced ratios of Cit:L-arginine (Arg) and L-ornithine (Orn):Arg (Fig. 1F). With regard to TCA cycle metabolites we found that stressed animals displayed reduced abundance of succinate and Glu (Fig. 1F, I). Notably, while the abundances of several cerebellar amino acids and TCA cycle metabolites were significantly altered in stressed animals relative to naïve animals, no metabolite abundance varied significantly between PTSD-like and resilient animals (Fig. 1B, H).
Fig. 1.
Cerebellar amino acids and tricarboxylic acid (TCA) cycle metabolites. A,B,G,H. Heat maps represent median amino acid and TCA cycle concentration Z values in stressed and naïve (A,G) and PTSD-like and resilient (B,H) mice. C,I. Relative concentrations of significant cerebellar amino acid (C) and TCA cycle (I) metabolite changes in stressed animals relative to naïve animals. D. Relative ratio of cerebellar gamma-aminobutyric acid (GABA):glutamate (Glu) in stressed and naïve mice. E. Relative ratio of cerebellar phosphoethanolamine (PEtn):ethanolamine (Etn) in stressed and naïve mice. F. Relative ratios of cerebellar urea and nitric oxide cycle metabolites in stressed and naïve mice. p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), p < 0.0001 (****).
3.2. Cerebellar untargeted NMR
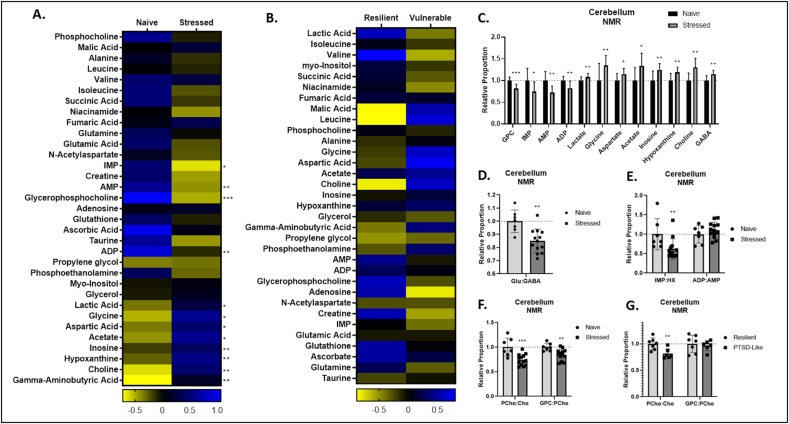
Untargeted NMR identified numerous metabolic changes in the cerebella of stressed animals. Stressed animals displayed reduced abundances of glycerophosphocholine (GPC), inosine mono-phosphate (IMP), adenosine monophosphate (AMP), and adenosine diphosphate (ADP), and elevated abundance of lactate, L-glycine (Gly), L-aspartate (Asp), acetate, inosine, hypoxanthine (HPX), choline (Cho), and GABA (Fig. 2A, C). As in the amino acid panel, stressed animals displayed reduced ratios of Glu:GABA (Fig. 2D).
Fig. 2.
Cerebellar metabolites identified through nuclear magnetic resonance (NMR) spectroscopy. A,B. Heat maps represent median cerebellar metabolite concentration Z values in stressed and naïve (A) and PTSD-like and resilient (B) mice. C. Relative concentrations of cerebellar NMR metabolites in stressed animals relative to naïve animals. D. Relative ratio of cerebellar gamma-aminobutyric acid (GABA):glutamate (Glu) in stressed and naïve mice. E. Relative ratios of cerebellar inosine monophosphate (IMP):hypoxanthine (HPX) and adenosine diphosphate (ADP):adenosine monophosphate (AMP). F,G. Relative ratios of cerebellar phosphocholine (PCho):choline (Cho) and glycerophosphocholine (GPC):phosphocholine (PCho) in stressed and naïve (F) and PTSD-like and resilient (G) mice. p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), p < 0.0001 (****).
Stressed animals displayed reduced ratios of IMP:HPX (Fig. 2E), phosphocholine (PCho):Cho, and GPC:PCho (Fig. 2F). Again, while no single metabolite abundance varied significantly between PTSD-like and resilient animals (Fig. 2B), PTSD-like animals displayed reduced PCho:Cho relative to PTSD-resilient animals (Fig. 2G).
3.3. Plasma amino acids and mitochondrial metabolites
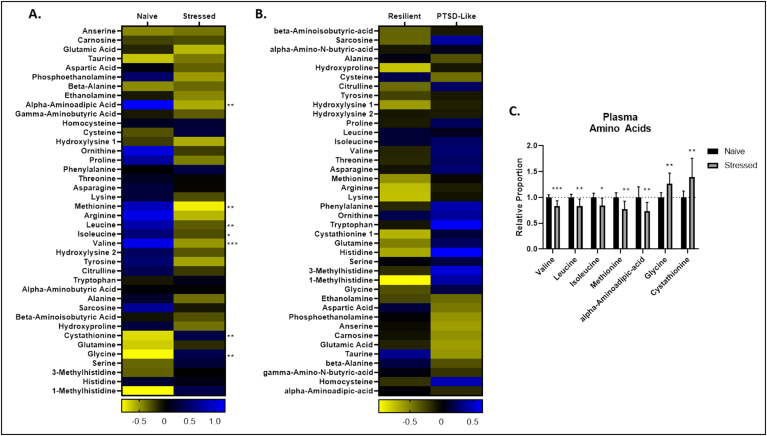
Stressed animals displayed numerous changes in circulating amino acids, including elevated abundance of Gly, and L-cystathionine, and reduced abundance of Val, L-methionine (Met), alpha-aminoadipic acid (AAA), L-leucine (Leu), and L-isoleucine (Ile) (Fig. 3A, C).
Fig. 3.
Plasma amino acids. A,B. Heat maps represent median plasma metabolite concentration Z values in stressed and naïve (A) and PTSD-like and resilient (B) mice. C. Relative concentrations of cerebellar amino acid changes in stressed animals relative to naïve animals. p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), p < 0.0001 (****).
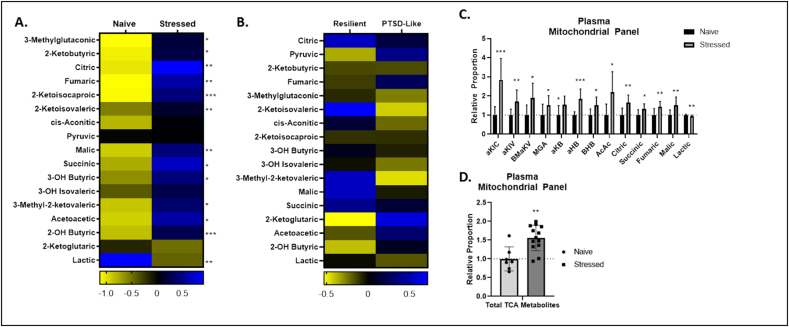
Stressed animals displayed elevated abundance of 3-methylglutaconic acid (MGA), 2-ketobutyric acid (aKB), citric acid, fumaric acid, 2-ketoisocaproic acid (aKIC), 2-ketoisovaleric acid (aKIV), malic acid, succinic acid, 3-hydroxybutyric Acid (BHB), 3-methyl-2-ketovaleric acid (BMaKV), acetoacetic acid (AcAc), and 2-hydroxybutyric acid (aHB), and slightly reduced abundance of lactic acid (Fig. 4A, C). Stressed animals also displayed elevated total abundance of plasma TCA metabolites, which has been observed in congenital mtETC dysfunction (Fig. 4D). It is notable that stressed animals displayed significantly altered abundances of almost all metabolites measured in the targeted mitochondrial metabolite panel, suggesting profound, mitochondrial metabolic reprogramming with exposure to trauma. Again, however, PTSD-like animals displayed no significant changes in any single plasma metabolite relative to resilient animals (Figs. 3B and 4B).
Fig. 4.
Plasma mitochondrial metabolite panel. A,B. Heat maps represent median plasma metabolite concentration Z values in stressed and naïve (A) and PTSD-like and resilient (B) mice. C. Relative concentrations of cerebellar mitochondrial metabolites in stressed animals relative to naïve animals. D. Total plasma tricarboxylic acid (TCA) cycle metabolite concentrations in naïve and stressed animals. p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), p < 0.0001 (****).
3.4. Linear discriminate analysis (LDA)
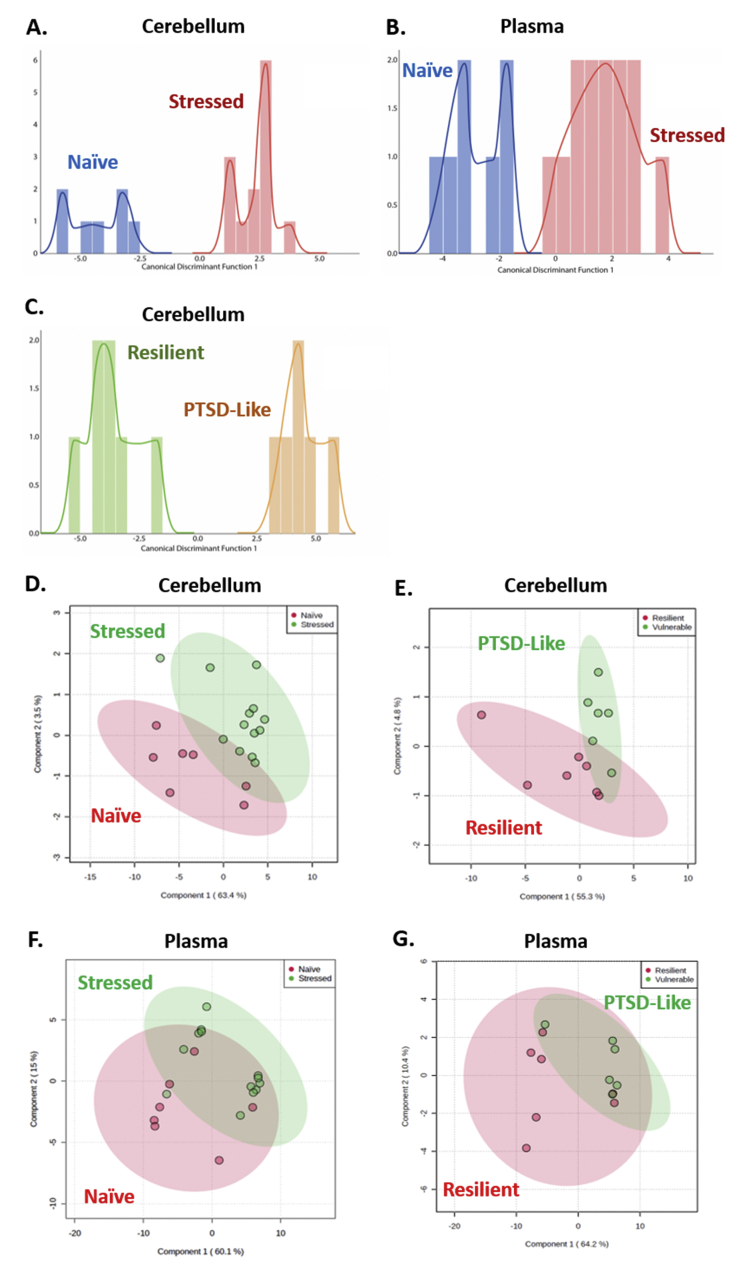
Given the large number of metabolites altered in stressed animals relative to naïve animals, and the near absence of individual metabolites varying between PTSD-like and resilient animals, linear discriminant analysis (LDA) was performed to determine which populations of metabolites, if any, would be able to discriminate stressed and naïve, and PTSD-like and resilient animals. LDA identified GABA, Val, Orn, HPX, and Ile abundance as primary discriminators of stress-naïve and stressed animals in the cerebellum (Fig. 5A, Table 1), and citric acid, Ile, B-Ala, and cis-aconitic acid abundance as primary discriminators of stress-naïve and stressed animals in plasma (Fig. 5B, Table 1).
Fig. 5.
Linear Discriminant Analysis and Principal Component Analysis of cerebellar and plasma metabolites. A,B,C. Histogram of metabolites discriminating stressed and naïve (A) and PTSD-like and resilient (C) animals in cerebellum, and metabolites discriminating stressed and naïve animals in plasma (B). Plot of metabolites identified by principal component analysis in cerebellum between stressed and naïve (D) and PTSD-like and resilient (E) animal, and in plasma between stressed and naïve (F) and PTSD-like and resilient (G) animals.
Table 1.
Factors identified by linear discriminant analysis as discriminating stressed and naïve, and PTSD-like and resilient animals in cerebellum and plasma.
| Tissue | Groups | Discriminator | Sign |
|---|---|---|---|
| Cerebellum | Stressed vs. Naïve | GABA | + |
| Valine | – | ||
| Ornithine | + | ||
| Hypoxanthine | – | ||
| Isoleucine | – | ||
| PTSD-Like vs. Resilient | Choline | + | |
| ADP | – | ||
| beta-Alanine | + | ||
| Taurine | – | ||
| Myo-inositol | + | ||
| Plasma | Stressed vs. Naïve | Citrate | + |
| Isoleucine | – | ||
| Alanine | – | ||
| Cis-aconitate | + |
LDA identified Cho, ADP, B-Ala, taurine, and myo-inositol abundance as primary discriminators of PTSD-vulnerable and –resilient animals in the cerebellum (Fig. 5C, Table 1). No factors able to discriminate PTSD-resilient and –vulnerable animals were identified in plasma.
Notably, primary discriminators of both stress-naïve and stressed, as well as PTSD-like and resilient animals identified in the cerebellum were able to correctly identify group membership in 100% of animals analyzed.
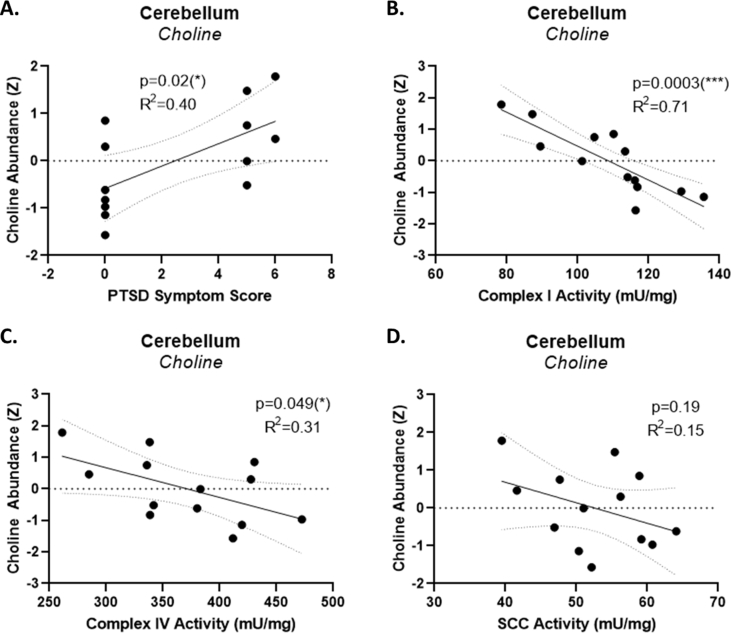
Also notably, the primary discriminator of PTSD-like and resilient animals identified in this study, cerebellar choline abundance, directly correlated with the PTSD-symptom score used to identify PTSD-like and resilient animals (Fig. S1A), and inversely correlated with two powerful predictors of PTSD-like behavior identified in a previous study of these animals (Preston et al., 2020), the activities of cerebellar mitochondrial complex I (Fig. S2B) and complex IV (Fig. S2C). Cerebellar choline abundance did not significantly correlate with the activity of succinate:cytochrome c oxidase (SCC) activity, a third predictor of PTSD-like activity identified in a previous study (Preston et al., 2020).
3.5. Pathway analysis
Metaboanalyst 4.0 identified eleven pathways showing a significant number (FDR p < 0.05) of affected cerebellar metabolites in stressed animals relative to naïve animals: aminoacyl-tRNA biosynthesis; alanine, aspartate and glutamate metabolism; glyoxylate and dicarboxylate metabolism; arginine biosynthesis; citrate cycle (TCA cycle); histidine metabolism; beta-alanine metabolism; glycine, serine, and threonine metabolism; pyruvate metabolism; glutamine and glutamate metabolism; and valine, leucine, and isoleucine biosynthesis (Table 2). Alternately, Metaboanalyst 4.0 identified eight pathways showing a significant number of affected plasma metabolites in stressed animals relative to naïve animals: glyoxylate and dicarboxylate metabolism; valine, leucine, and isoleucine biosynthesis; glycine, serine and threonine metabolism; synthesis and degradation of ketone bodies; Citrate cycle (TCA cycle); pyruvate metabolism; aminoacyl-tRNA biosynthesis; and alanine, aspartate and glutamate metabolism (Table 1). An insufficient number of metabolites varying significantly between PTSD-like and resilient animals were identified to perform pathway analysis.
Table 2.
Metabolic pathways in cerebellum and plasma significantly affected by exposure to the PTSD-induction paradigm, including 10% false discovery rate (FDR) p values. Pathways in bold typeface were significantly altered in both cerebellum and plasma.
| Tissue | Groups | Pathway | FDR p |
|---|---|---|---|
| Cerebellum | Naïve vs. Stressed | Aminoacyl-tRNA biosynthesis | 2.1E-12 |
| Alanine, aspartate and glutamate metabolism | 7.1E-8 | ||
| Glyoxylate and dicarboxylate metabolism | 3.8E-6 | ||
| Arginine biosynthesis | 2.6E-5 | ||
| Citrate cycle (TCA) | 2.3E-4 | ||
| Histidine Metabolism | 8.9E-4 | ||
| Beta-Alanine metabolism | 3.1E-3 | ||
| Glycine, serine and threonine metabolism | 3.1E-3 | ||
| Pyruvate metabolism | 3.1E-3 | ||
| D-Glutamine and D-glutamate metabolism | 3.9E-3 | ||
| Valine, leucine, and isoleucine biosynthesis | 9.4E-3 | ||
| Plasma | Naïve vs. Stressed | Glyoxylate and dicarboxylate metabolism | 1.6E-3 |
| Valine, leucine and isoleucine biosynthesis | 3.0E-3 | ||
| Glycine, serine and threonine metabolism | 1.2E-2 | ||
| Synthesis and degradation of ketone bodies | 2.16E-2 | ||
| Citrate cycle (TCA cycle) | 2.16E-2 | ||
| Pyruvate metabolism | 2.16E-2 | ||
| Aminoacyl-tRNA biosynthesis | 2.16E-2 | ||
| Alanine, aspartate and glutamate metabolism | 3.78E-2 |
Principle component analysis (PCA) identified cystine, butyrylcarnitine (C4) and glycine as the principle metabolites associated with trauma exposure in the cerebellum (Fig. 5D), while ascorbate and choline were identified as the principle metabolites associated with PTSD-like behavior in the cerebellum (Fig. 5E). In plasma, PCA identified 1-methylhistidine as the principle metabolite associated with both trauma exposure, as well as PTSD-like behavior (Fig. 5F and G).
4. Discussion
The field of metabolomics continues to improve our understanding of the pathology of numerous disease states, including inborn errors of metabolism, acquired metabolic dysfunction, and even psychiatric disorders (Tebani et al., 2016; Klein and Shearer, 2016; Kawamura et al., 2018).Understanding the diverse metabolic changes associated with a disease state can provide valuable insights into avenues of diagnosis, prevention, and treatment, and is indispensable to the emerging field of personalized medicine (Chan and Ginsburg 2011). Recent metabolomic studies have elucidated the metabolic pathologies of both primary mitochondrial disease (Thompson Legault et al., 2015) as well as PTSD (Mellon et al., 2019). We recently reported stress-induced mitochondrial electron transport chain and fatty acid oxidation alterations in the cerebellar tissue of mice displaying PTSD-like behavior, as well as evidence of multisystem fatty acid oxidation changes. Here, we report evidence of profound metabolic rewiring in animals exposed to trauma capable of precipitating PTSD-like behavior, and metabolic biosignatures discriminating PTSD-like and resilient animals.
We hypothesized that stressed animals would display metabolic changes consistent with altered mitochondrial energy metabolism in the cerebellum. Consistent with our hypothesis, stressed animals displayed reduced abundances of cerebellar ADP and AMP, and elevated abundances of IMP and HPX, measured by NMR spectroscopy. ATP rapidly degrades to ADP and is rarely detected by NMR. Hence we were unable to measure ATP in this study. Elevated HPX, which significantly correlated with reduced AMP, was identified by LDA as a discriminator of stressed from naïve animals in the cerebellum. Stressed animals also displayed significantly decreased abundances of the phosphorylated metabolites IMP, PCho, and PEtn relative to their precursors HPX, Cho, and Etn, respectively. These metabolic alterations are consistent with reduced cerebellar ATP and, along with the elevated lactate, corroborate the reduced mtETC complex activity previously reported in these tissues (Preston et al., 2020). In addition, ADP was identified by LDA as a primary discriminator of PTSD-like from resilient animals. PTSD-like animals also displayed reduced cerebellar PCho:Cho relative to resilient animals.
Stressed animals also displayed elevated cerebellar GABA in both amino acids and NMR, and LDA identified GABA as the primary discriminator of stress-naïve and stressed animals. GABA is the primary inhibitory neurotransmitter in the brain, and has been shown to be elevated in the rat cerebellum in response chronic stress (Kazi and Oommen 2014). Stressed animals also displayed an elevated ratio of GABA:Glu, the primary excitatory neurotransmitter in the brain, indicating a reprogramming of cerebellar excitation and inhibition associated with trauma exposure. Pathway analysis identified a significant number of metabolites altered in the glutamate and glutamine pathways between stressed and naïve animals. Reduced GABA has been identified in cortical regions of both chronically stressed rats (Kazi and Oommen 2014), as well as patients both exposed to trauma (Sheth et al., 2019) and those suffering from PTSD (Meyerhoff et al., 2014). Altered GABA:Glu has also been implicated in the pathophysiology of psychopathologies including major depressive disorder and schizophrenia (Liriano et al. 2019; Chen et al., 2017). This study suggests that reduced GABA:Glu balance in the cerebellum may contribute to PTSD-like behavior in mice.
Significant cerebellar metabolic changes associated with phospholipid metabolism were also observed in stressed and PTSD-like animals. Stressed animals displayed elevated abundances of Cho and GPC. In addition to the previously mentioned reduced PCho:Cho, stressed animals also displayed reduced GPC:PCho, suggesting a disruption in the PC synthesis pathway. LDA identified Cho as the primary discriminator of PTSD-like and resilient animals. As previously stated, stressed animals also displayed reduced cerebellar PEtn:Etn, and Cho and Etn were highly significantly correlated in this cohort. Etn and Cho are precursors of phosphatidylethanolamine (PE) and phosphatidylcholine (PC), respectively (Farine et al., 2015), the two most abundant phospholipid species in biologic membranes. The first step in the PE and PC synthesis pathways is the ATP-dependent phosphorylation of the precursors Etn and Cho to PEtn and PCho, respectively. Recent studies have identified altered phospholipid metabolism associated with diminished mitochondrial energy metabolism in the brains of patients with psychopathologies like depression and bipolar disorder (Modica-Napolitano and Renshaw 2004), and increased phospholipid damage and subsequent turnover has been reported following inhibition of the mtETC in vitro (Farber et al. 2000). Conversely, both altered mitochondrial phospholipid composition as well as increased abundances of phospholipid precursors have been observed to alter mitochondrial function (Modica-Napolitano and Renshaw 2004; Rubin and Rottenberg 1982), suggesting the possibility of a feed-forward interaction between mtETC and phospholipid metabolism. We have also previously reported a mtETC complex-I driven mitochondrial association between stress-exposure and PTSD-vulnerability in the cerebella of these animals, with reduced activity of both cerebellar complex I and complex IV being highly predictive of PTSD-like behavior (Preston et al., 2020). The abundance of cerebellar choline in these animals inversely correlated with activity of cerebellar complex I and complex IV (Figs. S2B and C). These data are consistent with a reduction of phospholipid synthesis pathway flux in the cerebellum associated with reduced mtETC complex activity secondary to exposure to trauma and PTSD-like symptomatology. Unfortunately, it was not possible to directly assay de novo phospholipid synthesis in the cerebellar tissues assayed in this study, and further studies will be required to directly confirm this metabolic alteration.
Elevated B-alanine was identified by LDA as a discriminator of PTSD-like and resilient animals, and is a rate limiting precursor of both carnosine and anserine (Modica-Napolitano and Renshaw 2004; Artioli et al., 2010), intracellular antioxidants and membrane stabilizers in mouse muscle and brain (Kohen et al., 1988; Boldyrev et al., 1993). LDA also identified reduced cerebellar taurine, an important buffer of mitochondrial complex I-associated oxidative stress (Jong et al., 2017), as a discriminator of PTSD-like and resilient animals. Stressed animals also displayed elevated cerebellar cysteine and glycine, the precursors for glutathione (GSH), the primary antioxidant in mammals. Taken together, these metabolic data are consistent with increased oxidative stress and inner mitochondrial phospholipid membrane alterations in the cerebella of PTSD-like animals.
Following the phospholipid and oxidative stress pathways, the most significant alterations between stress-naïve, PTSD-resilient and PTSD-like animals were associated with branched-chain amino acids (BCAA's), particularly Val. Stressed animals displayed reduced proportions of Val in the cerebellum, and reduced proportions of Val, Leu, and Ile, as well as their metabolic intermediates 2-ketoisocaproate, 2-ketoisovalerate, and 2-keto-3-methylvalerate in plasma. Reduced proportions of both valine (which was significantly correlated with isoleucine) and isoleucine (which was significantly correlated with both valine and leucine) were identified as primary discriminators of stress-naïve and stressed animals in the cerebellum, and reduced proportions of Leu (which was significantly correlated with both Val and Ile) was identified as a discriminator of stress-naïve and stressed animals in plasma. BCAA's are deaminated to keto acids, which are then able to supplement either acetyl-CoA or succinyl-CoA, and enhance flux through the TCA cycle (Kainulainen et al. 2013). Circulating valine can also be converted to BHB and then to AcAc, which are preferred substrates for the brain. However, stressed animals also displayed elevated ratios of aKIV acid and MGA, two biomarkers for altered keto acid production in the liver. Elevated citrate, the primary discriminator of stressed animals, was significantly correlated with BHB, AcAc, aKIV, and MGA. These data together suggest an increased mobilization of BCAA's and ketone bodies, and a concomitant multisystem reduction in mitochondrial oxidative phosphorylation. These data are consistent with the stress-induced multisystem fatty acid recruitment and concomitant fatty acid oxidation changes previously reported in these same animals.
BCAA's have also been shown to improve mitochondrial health by promoting mitochondrial biogenesis and reactive oxygen species buffering (D'Antona et al., 2010). Increased demand for oxidative stress buffering is a significant metabolic feature of stress and PTSD-vulnerability in these animals. Reduced plasma isoleucine, which discriminated stressed animals, correlated with plasma methionine, which was reduced in stressed animals. Reduced plasma Met is a biomarker of increased demand for the GSH precursor Cys. Homocysteine is diverted away from Met synthesis toward synthesis of cystathionine, which was elevated in stressed animals. Cleavage of cystathionine to cystine releases aKB, which is converted to aHB, both of which were elevated in stressed animals.
One proposed mechanism by which BCAA's promote mitochondrial biogenesis is by promoting the nitric oxide (NO) synthesis/urea cycle metabolic pathways (Valerio, D'Antona, and Nisoli 2011). Nitric oxide deficiencies are an important pathophysiologic feature of mitochondrial myopathy, encephalopathy, and lactic acidosis syndrome, one of the most common mitochondrial diseases in humans (El-Hattab et al. 2017), and urea cycle disorders have been linked to increased oxidative stress (Parmeggiani and Vargas 2018). Our data appear to implicate the overlapping urea cycle and NO synthesis pathways in the pathophysiology of stress in these animals. In healthy cells, Cit is metabolized to Arg, which can then be broken down to urea and Orn, or NO and Cit. Elevated cerebellar ornithine was identified as a discriminator of stress-naïve and stressed animals, and stressed animals displayed a decreased cerebellar Cit, as well as increased ratios of Arg:Cit and Arg:Orn in the cerebellum. Consistent with this, pathway analysis identified a significant number of altered metabolites in the Arginine Biosynthesis pathway in the cerebella of stressed animals. NO synthesis from arginine takes place in the mitochondria, and decreased Cit and increased Arg:Cit in the cerebella of stressed animals may confirm the mitochondrial metabolic reprogramming previously reported (Preston et al., 2020). Additionally, these data suggest that ketogenic diet or exogenous ketone body supplementation may offer therapeutic potential for patients with PTSD. The ketone bodies acetoacetate (AcAc) and beta-hydroxybutyrate (BHB) are small molecules catabolized in the liver via fatty acid oxidation during times of starvation or metabolic stress, when carbohydrates are scarce (Achanta and Rae 2017; Newman and Verdin 2014). Metabolic shifts toward ketone body catalysis have been reported in patients with schizophrenia (Yang et al., 2013), and increasing circulating ketones, through either ketogenic diets (Sussman et al. 2015; Phelps et al. 2013; Kraeuter, van den Buuse, and Sarnyai 2019; Kraft and Westman 2009; Palmer 2017; Palmer et al. 2019; Kraeuter et al., 2015; Murphy et al., 2004; Ahn et al., 2014; Evangeliou et al., 2003; Ruskin et al., 2017; Murphy and Burnham 2006) or exogenous ketone supplementation (Ari et al., 2016; Kashiwaya et al., 2013; Brownlow et al., 2017), have demonstrated therapeutic effects in psychiatric disease in both rodents and humans. AcAc and BHB freely cross the blood-brain barrier and enter the mitochondria, where they are converted to acetyl-CoA, driving ATP synthesis (Achanta and Rae 2017; Newman and Verdin 2014). Ketone bodies increase mitochondrial complex I-driven respiration, reducing reactive oxygen species production, attenuating oxidative stress, and improving brain energy metabolism (Maalouf et al., 2007; VanItallie and Nufert 2003; Sato et al., 1995). Additionally, ketones have been shown to decrease neuron hyper-excitability, and modulate GABA:Glu neurotransmitter balance (Achanta and Rae 2017; McNally and Hartman 2012), making ketone supplementation a promising potential treatment for PTSD-like symptomatology. Ketogenesis requires acetyl-CoA produced through hepatic mitochondrial fatty acid oxidation (FAO), and we have previously reported evidence of reduced flux through the hepatic FAO pathway in the animals reported on in this study (Preston et al., 2020). As such, supplementation with exogenous ketones, such as ketone salts, may ultimately prove preferable to ketogenic diet in the treatment of PTSD-like symptomatology.
Consistent with our hypothesis, these data reveal significant metabolic shifts associated with exposure to stress, as well as PTSD-like behavior in these animals. Pathway analysis identified eleven pathways with a significant number of altered metabolite species in the cerebella of animals exposed to trauma. These data shed new light on the role of the cerebellum, and emerging area of interesting in human cognition, in the pathophysiology of stress response and trauma vulnerability/resilience. As hypothesized, the metabolic shifts observed in stressed animals were highly associated with mitochondrial function. In addition to cerebellar metabolic shifts, the evidence of multisystem mitochondrial alteration is notable. Research into traumatic event exposure and subsequent precipitation of PTSD-like symptomatology has understandably focused on the neural pathways in the brain. However, our data indicate that exposure to a traumatic event exposure results in a significant multi-system metabolic reprogramming. Nearly all plasma metabolites analyzed through the targeted mitochondrial panel were significantly altered in stressed animals relative to naïve animals. Both stressed and PTSD-like animals were characterized by metabolic patterns of reduced mitochondrial energy metabolism and increased oxidative stress, and altered phospholipid metabolism, associated with changes to neurotransmitter balance, suggesting a possible role of cerebellar excitotoxicity in the pathophysiology of PTSD.
It is notable that considerable overlap was observed between the altered metabolic pathways identified in the cerebellum, and those identified in the circulating plasma. Of the 11 metabolic pathways in the cerebellum significantly affected by exposure to trauma, 5 of those pathways were also significantly affected in the plasma. The use of biofluids such as plasma in the application of metabolomics in diagnostics is currently a major objective in the field of metabolomics research (Esterhuizen, van der Westhuizen, and Louw 2017). Our data suggest that metabolomic analysis of plasma metabolites may reveal biosignatures of impaired energetics in the brain secondary to trauma exposure, greatly increasing the utility of metabolomics in diagnosing and monitoring metabolic perturbations following trauma exposure and precipitation of PTSD-like symptomatology.
It is also notable that while the cerebellar and plasma abundances of a large number of metabolites varied significantly in stressed animals relative to naïve animals, only a few metabolites were identified by LDA as clear discriminators of PTSD-like and PTSD resilient behavior. These data therefore suggest no single putative mitochondrial biomarker for PTSD-like behavior in mice. Unlike genetic defects in a single metabolic enzyme, it is unlikely that any single metabolite or pathway would serve as a biomarker for the complex psychopathology of PTSD. These data suggest that a more complex and nuanced metabolic biosignature of PTSD may ultimately be more useful for the diagnosis and treatment of PTSD. These data also highlight the importance of paradigms of PTSD-like symptomatology that discriminate PTSD-like and resilient animals, as treating all stressed animals in this paradigm as PTSD-like would have led to a profound misunderstanding of the metabolic profile of PTSD-like individuals. Similarly, these data also highlight the challenges of utilizing paradigms that discriminate PTSD-like and resilient animals. The PTSD-induction paradigm utilized in this study precipitates PTSD-like symptomatology in ~15% of stressed animals, a rate comparable to that seen in humans. While this feature makes this paradigm particularly valuable as a pre-clinical model of PTSD, the small sample size of PTSD-like and resilient animals identified makes resolving differences between these groups challenging. Future studies therefore will aim to increase cohort size in order to increase the sample size of PTSD-like and resilient animals. Additional studies will also aim to use these data to predict the PTSD-like and resilient phenotypes, using discriminating metabolites identified in this study. It is also notable that recent investigations into metabolic shifts in the nucleus accumbens of mice exposed to chronic restraint stress identified alterations of several of the same metabolites identified in this study, though in opposing directions, including decreased abundance of Tau, Glu, Asp, Gln, PCho, and elevated GPC:Cho (Cherix et al., 2020). These data suggest that metabolic reprogramming involving pathways affecting these metabolites, while strongly implicated in stress exposure and subsequent psychopathology, may vary significantly between different brain areas in mice exposed to different stressors. These data strengthen the case for the importance of metabolomic analysis in both the diagnosis and treatment of psychopathologies, as well as the potential importance of treatments specific to individual psychiatric conditions. Ultimately, these data provide novel insights into the role of the cerebellum in the pathophysiology of both trauma exposure and PTSD-like behavior. These data also provide novel insight into the profound multi-system metabolic reprogramming associated with exposure to trauma and suggest several potential interventions both to increase trauma resilience in high risk populations, including supplementation with BCAA's and ketones. However, these findings remain preliminary, and further studies will be required to confirm that the metabolic shifts identified in this study are able to predict either PTSD-like behavior or susceptibility to PTSD-like behavior, and whether influencing these metabolic shifts through supplementation or therapeutic intervention can mediate PTSD-like symptomatology.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for profit sectors.
No funding sources were involved in the study design; collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit this article for publication.
CRediT authorship contribution statement
Graeme Preston: Conceptualization, Formal analysis, Investigation, Writing - original draft, Visualization. Tim Emmerzaal: Formal analysis, Visualization, Writing - review & editing. Silvia Radenkovic: Writing - review & editing. Ian R. Lanza: Methodology, Formal analysis, Resources. Devin Oglesbee: Methodology, Resources, Writing - review & editing. Eva Morava: Conceptualization, Writing - review & editing. Tamas Kozicz: Conceptualization, Writing - review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
No authors have any conflict of interest to disclose, and there has been so financial support that might influence this work.
Acknowledgments
This work was made possible by the generosity of the Marriott Family and the Hayward Foundation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2021.100300.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Suppl. Fig. 1. PTSD-induction paradigm experimental outline. A. On day 1, animals performed an open field test to screen for locomotor or trait-anxiety behavior which could interfere with behavioral analyses. On day 5 and 6, animals were exposed to the PTSD-induction, which involved two sessions of inescapable electric foot shock: a relatively intense “Trauma” session on day 5, followed by a relatively less intense “Trigger” session on day 6 (B). Animals subsequently performed a series of behavioral tests for PTSD-like behaviors (dark/light transfer for risk assessment, marble burying for compulsive behavior, acoustic startle for hyperarousal, and home cage activity for disrupted sleep cycle) (A,C). The 20% of animals displaying the most “PTSD-like” behavior in each test (reduced risk assessment, increased marble burying, reduced pre-pulse inhibition and latency to peak startle amplitude, and increased light phase activity) were awarded points for that test (C). Animals scoring more than 4 points total were designated as “PTSD-like” while animals scoring no points were designated as “Resilient.” On day 22 animals performed a 25 min restraint stress test for corticosterone response (A). Finally, on day 31 animals were sacrificed via live decapitation (A).
Suppl. Fig. 2. Cerebellar choline abundance correlates with predictors of PTSD-like behavior. Cerebellar choline abundance in stressed animals was directly correlated with PTSD-symptom score (A), and inversely correlated with the activities of cerebellar mitochondrial complex I (B) and Complex IV (C), though not the activity of cerebellar succinate:cytochrome c oxidase (SCC) (D). p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), p < 0.0001 (****).
References
- Achanta Lavanya B., Rae Caroline D. 'β-Hydroxybutyrate in the brain: one molecule, multiple mechanisms. Neurochem. Res. 2017;42:35–49. doi: 10.1007/s11064-016-2099-2. [DOI] [PubMed] [Google Scholar]
- Ahn Y., Narous M., Tobias R., Rho J.M., Mychasiuk R. 'The ketogenic diet modifies social and metabolic alterations identified in the prenatal valproic acid model of autism spectrum disorder. Dev. Neurosci. 2014;36:371–380. doi: 10.1159/000362645. [DOI] [PubMed] [Google Scholar]
- Ari C., Kovács Z., Juhasz G., Murdun C., Goldhagen C.R., Koutnik A.P., Poff A.M., Kesl S.L., D'Agostino D.P. 'Exogenous ketone supplements reduce anxiety-related behavior in sprague-dawley and wistar albino glaxo/rijswijk rats. Front. Mol. Neurosci. 2016;9:137. doi: 10.3389/fnmol.2016.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artioli Guilherme Giannini, Gualano Bruno, Smith Abbie, Stout Jeffrey, Lancha Antonio Herbert. Role of beta-alanine supplementation on muscle carnosine and exercise performance. Med. Sci. Sports Exerc. 2010;42:1162–1173. doi: 10.1249/MSS.0b013e3181c74e38. [DOI] [PubMed] [Google Scholar]
- Baldaçara Leonardo, Borgio João Guilherme Fiorani, Araújo Célia, Nery-Fernandes Fabiana, Lacerda Acioly Luiz Taveres, Moraes Walter André Dos Santos, Montaño Maria Beatriz Marcondes Macedo, Rocha Marlos, Quarantini Lucas C., Schoedl Aline, Pupo Mariana, Mello Marcelo F., Andreoli Sergio B., Miranda-Scippa Angela, Roberto Ramos Luiz, Mari Jair J., Bressan Rodrigo Affonseca, Jackowski Andrea Parolin. Relationship between structural abnormalities in the cerebellum and dementia, posttraumatic stress disorder and bipolar disorder. Dementia Neuropsychol. 2012;6:203–211. doi: 10.1590/S1980-57642012DN06040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjet C., Bromet E., Karam E.G., Kessler R.C., McLaughlin K.A., Ruscio A.M., Shahly V., Stein D.J., Petukhova M., Hill E., Alonso J., Atwoli L., Bunting B., Bruffaerts R., Caldas-de-Almeida J.M., de Girolamo G., Florescu S., Gureje O., Huang Y., Lepine J.P., Kawakami N., Kovess-Masfety V., Medina-Mora M.E., Navarro-Mateu F., Piazza M., Posada-Villa J., Scott K.M., Shalev A., Slade T., ten Have M., Torres Y., Viana M.C., Zarkov Z., Koenen K.C. The epidemiology of traumatic event exposure worldwide: results from the World Mental Health Survey Consortium. Psychol. Med. 2016;46:327–343. doi: 10.1017/S0033291715001981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersani F.S., Morley C., Lindqvist D., Epel E.S., Picard M., Yehuda R., Flory J., Bierer L.M., Makotkine I., Abu-Amara D., Coy M., Reus V.I., Lin J., Blackburn E.H., Marmar C., Wolkowitz O.M., Mellon S.H. 'Mitochondrial DNA copy number is reduced in male combat veterans with PTSD'. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2016;64:10–17. doi: 10.1016/j.pnpbp.2015.06.012. [DOI] [PubMed] [Google Scholar]
- Boldyrev A.A., Koldobski A., Kurella E., Maltseva V., Stvolinski S. 'Natural histidine-containing dipeptide carnosine as a potent hydrophilic antioxidant with membrane stabilizing function. A biomedical aspect', Mol Chem Neuropathol. 1993;19 doi: 10.1007/BF03160178. 185-192. [DOI] [PubMed] [Google Scholar]
- Brownlow Milene L., Jung Seung H., Moore Raquel J., Bechmann Naomi, Ryan Jankord. 'Nutritional ketosis affects metabolism and behavior in sprague-dawley rats in both control and chronic stress environments. Front. Mol. Neurosci. 2017;10 doi: 10.3389/fnmol.2017.00129. 129-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan I.S., Ginsburg G.S. 'Personalized medicine: progress and promise. Annu. Rev. Genom. Hum. Genet. 2011;12:217–244. doi: 10.1146/annurev-genom-082410-101446. [DOI] [PubMed] [Google Scholar]
- Chen T., Wang Y., Zhang J., Wang Z., Xu J., Li Y., Yang Z., Liu D. 'Abnormal concentration of GABA and glutamate in the prefrontal cortex in schizophrenia.-an in vivo 1H-MRS study. Shanghai Arch Psychiatr. 2017;29:277–286. doi: 10.11919/j.issn.1002-0829.217004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherix Antoine, Larrieu Thomas, Grosse Jocelyn, Rodrigues João, McEwen Bruce, Nasca Carla, Gruetter Rolf, Sandi Carmen. 'Metabolic signature in nucleus accumbens for anti-depressant-like effects of acetyl-L-carnitine. eLife. 2020;9 doi: 10.7554/eLife.50631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong J., Wishart D.S., Xia J. 'Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr Protoc Bioinform. 2019;68:e86. doi: 10.1002/cpbi.86. [DOI] [PubMed] [Google Scholar]
- D'Antona G., Ragni M., Cardile A., Tedesco L., Dossena M., Bruttini F., Caliaro F., Corsetti G., Bottinelli R., Carruba M.O., Valerio A., Nisoli E. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metabol. 2010;12:362–372. doi: 10.1016/j.cmet.2010.08.016. [DOI] [PubMed] [Google Scholar]
- El-Hattab, Ayman W., Almannai Mohammed, Scaglia Fernando. 'Arginine and citrulline for the treatment of MELAS syndrome. J. Inborn Errors Metabol. Screen. 2017;5 doi: 10.1177/2326409817697399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterhuizen Karien, van der Westhuizen Francois H., Louw Roan. 'Metabolomics of mitochondrial disease. Mitochondrion. 2017;35:97–110. doi: 10.1016/j.mito.2017.05.012. [DOI] [PubMed] [Google Scholar]
- Evangeliou A., Vlachonikolis I., Mihailidou H., Spilioti M., Skarpalezou A., Makaronas N., Prokopiou A., Christodoulou P., Liapi-Adamidou G., Helidonis E., Sbyrakis S., Smeitink J. 'Application of a ketogenic diet in children with autistic behavior: pilot study. J. Child Neurol. 2003;18:113–118. doi: 10.1177/08830738030180020501. [DOI] [PubMed] [Google Scholar]
- Farber S.A., Slack B.E., Blusztajn J.K. 'Acceleration of phosphatidylcholine synthesis and breakdown by inhibitors of mitochondrial function in neuronal cells: a model of the membrane defect of Alzheimer's disease. Faseb. J. 2000;14:2198–2206. doi: 10.1096/fj.99-0853. [DOI] [PubMed] [Google Scholar]
- Farine L., Niemann M., Schneider A., Bütikofer P. 'Phosphatidylethanolamine and phosphatidylcholine biosynthesis by the Kennedy pathway occurs at different sites in Trypanosoma brucei. Sci. Rep. 2015;5:16787. doi: 10.1038/srep16787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaquer A., Baumbach C., Ladwig K.H., Kriebel J., Waldenberger M., Grallert H., Baumert J., Meitinger T., Kruse J., Peters A., Emeny R., Strauch K. Mitochondrial genetic variants identified to be associated with posttraumatic stress disorder. Transl. Psychiatry. 2015;5:e524. doi: 10.1038/tp.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flory J.D., Yehuda R. 'Comorbidity between post-traumatic stress disorder and major depressive disorder: alternative explanations and treatment considerations. Dialogues Clin. Neurosci. 2015;17:141–150. doi: 10.31887/DCNS.2015.17.2/jflory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han F., Yan S., Shi Y. 'Single-prolonged stress induces endoplasmic reticulum-dependent apoptosis in the hippocampus in a rat model of post-traumatic stress disorder. PloS One. 2013;8 doi: 10.1371/journal.pone.0069340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri A.R. The emerging importance of the cerebellum in broad risk for psychopathology. Neuron. 2019;102:17–20. doi: 10.1016/j.neuron.2019.02.031. [DOI] [PubMed] [Google Scholar]
- Hollis F., van der Kooij M.A., Zanoletti O., Lozano L., Canto C., Sandi C. 'Mitochondrial function in the brain links anxiety with social subordination. Proc. Natl. Acad. Sci. U. S. A. 2015;112:15486–15491. doi: 10.1073/pnas.1512653112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes Sophie E., Scheinost Dustin, DellaGioia Nicole, Davis Margaret T., Matuskey David, Pietrzak Robert H., Hampson Michelle, Krystal John H., Esterlis Irina. vol. 2. 2018. (Cerebellar and Prefrontal Cortical Alterations in PTSD: Structural and Functional Evidence). Chronic stress (Thousand Oaks, Calif.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong Chian Ju, Ito Takashi, Prentice Howard, Wu Jang-Yen, Schaffer Stephen W. Role of mitochondria and endoplasmic reticulum in taurine-deficiency-mediated apoptosis. Nutrients. 2017;9:795. doi: 10.3390/nu9080795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kainulainen H., Hulmi J.J., Kujala U.M. 'Potential role of branched-chain amino acid catabolism in regulating fat oxidation. Exerc. Sport Sci. Rev. 2013;41:194–200. doi: 10.1097/JES.0b013e3182a4e6b6. [DOI] [PubMed] [Google Scholar]
- Karam E.G., Friedman M.J., Hill E.D., Kessler R.C., McLaughlin K.A., Petukhova M., Sampson L., Shahly V., Angermeyer M.C., Bromet E.J., de Girolamo G., de Graaf R., Demyttenaere K., Ferry F., Florescu S.E., Haro J.M., He Y., Karam A.N., Kawakami N., Kovess-Masfety V., Medina-Mora M.E., Browne M.A., Posada-Villa J.A., Shalev A.Y., Stein D.J., Viana M.C., Zarkov Z., Koenen K.C. 'Cumulative traumas and risk thresholds: 12-month PTSD in the World Mental Health (WMH) surveys. Depress. Anxiety. 2014;31:130–142. doi: 10.1002/da.22169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwaya Yoshihiro, Bergman Christian, Lee Jong-Hwan, Wan Ruiqian, Todd King M., Mughal Mohamed R., Okun Eitan, Clarke Kieran, Mattson Mark P., Veech Richard L. 'A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer's disease. Neurobiol. Aging. 2013;34:1530–1539. doi: 10.1016/j.neurobiolaging.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura N., Shinoda K., Sato H., Sasaki K., Suzuki M., Yamaki K., Fujimori T., Yamamoto H., Osei-Hyiaman D., Ohashi Y. Plasma metabolome analysis of patients with major depressive disorder. Psychiatr. Clin. Neurosci. 2018;72:349–361. doi: 10.1111/pcn.12638. [DOI] [PubMed] [Google Scholar]
- Kazi A.I., Oommen A. Chronic noise stress-induced alterations of glutamate and gamma-aminobutyric acid and their metabolism in the rat brain. Noise Health. 2014;16:343–349. doi: 10.4103/1463-1741.144394. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Sonnega A., Bromet E., Hughes M., Nelson C.B. 'Posttraumatic stress disorder in the national comorbidity survey'. Arch. Gen. Psychiatr. 1995;52 doi: 10.1001/archpsyc.1995.03950240066012. 1048-1060. [DOI] [PubMed] [Google Scholar]
- Klein Matthias S., Shearer Jane. ’Metabolomics and type 2 diabetes: translating basic research into clinical application. J. Diabet. Res. 2016;2016:1–10. doi: 10.1155/2016/3898502. 3898502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohen Roni, Yamamoto Yorihiro, Cundy K.C., Ames B.N. 'Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc. Natl. Acad. Sci. U. S. A. 1988;85:3175–3179. doi: 10.1073/pnas.85.9.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraeuter A.K., Loxton H., Lima B.C., Rudd D., Sarnyai Z. 'Ketogenic diet reverses behavioral abnormalities in an acute NMDA receptor hypofunction model of schizophrenia. Schizophr. Res. 2015;169:491–493. doi: 10.1016/j.schres.2015.10.041. [DOI] [PubMed] [Google Scholar]
- Kraeuter Ann-Katrin, van den Buuse Maarten, Sarnyai Zoltán. 'Ketogenic diet prevents impaired prepulse inhibition of startle in an acute NMDA receptor hypofunction model of schizophrenia. Schizophr. Res. 2019;206:244–250. doi: 10.1016/j.schres.2018.11.011. [DOI] [PubMed] [Google Scholar]
- Kraft Bryan D., Westman Eric C. 'Schizophrenia, gluten, and low-carbohydrate, ketogenic diets: a case report and review of the literature. Nutr. Metab. 2009;6:10. doi: 10.1186/1743-7075-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebow M., Neufeld-Cohen A., Kuperman Y., Tsoory M., Gil S., Chen A. 'Susceptibility to PTSD-like behavior is mediated by corticotropin-releasing factor receptor type 2 levels in the bed nucleus of the stria terminalis. J. Neurosci. 2012;32:6906–6916. doi: 10.1523/JNEUROSCI.4012-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.M., Han F., Liu D.J., Shi Y.X. 'Single-prolonged stress induced mitochondrial-dependent apoptosis in hippocampus in the rat model of post-traumatic stress disorder. J. Chem. Neuroanat. 2010;40:248–255. doi: 10.1016/j.jchemneu.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Liriano F., Hatten C., Schwartz T.L. 'Ketamine as treatment for post-traumatic stress disorder: a review. Drugs Context. 2019;8:212305. doi: 10.7573/dic.212305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalouf M., Sullivan P.G., Davis L., Kim D.Y., Rho J.M. 'Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience. 2007;145:256–264. doi: 10.1016/j.neuroscience.2006.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally Melanie A., Hartman Adam L. 'Ketone bodies in epilepsy. J. Neurochem. 2012;121:28–35. doi: 10.1111/j.1471-4159.2012.07670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon S.H., Bersani F.S., Lindqvist D., Hammamieh R., Donohue D., Dean K., Jett M., Yehuda R., Flory J., Reus V.I., Bierer L.M., Makotkine I., Abu Amara D., Henn Haase C., Coy M., Doyle F.J., 3rd, Marmar C., Wolkowitz O.M. 'Metabolomic analysis of male combat veterans with post traumatic stress disorder. PloS One. 2019;14 doi: 10.1371/journal.pone.0213839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhoff Dieter J., Anderson Mon, Metzler Thomas, Neylan Thomas C. Cortical gamma-aminobutyric acid and glutamate in posttraumatic stress disorder and their relationships to self-reported sleep quality. Sleep. 2014;37:893–900. doi: 10.5665/sleep.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modica-Napolitano J.S., Renshaw P.F. 'Ethanolamine and phosphoethanolamine inhibit mitochondrial function in vitro: implications for mitochondrial dysfunction hypothesis in depression and bipolar disorder. Biol. Psychiatr. 2004;55:273–277. doi: 10.1016/s0006-3223(03)00784-4. [DOI] [PubMed] [Google Scholar]
- Morava E., Kozicz T. 'Mitochondria and the economy of stress (mal)adaptation. Neurosci. Biobehav. Rev. 2013;37:668–680. doi: 10.1016/j.neubiorev.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Murphy P., Likhodii S., Nylen K., Burnham W.M. 'The antidepressant properties of the ketogenic diet. Biol. Psychiatr. 2004;56:981–983. doi: 10.1016/j.biopsych.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Murphy Patricia, Burnham W.M. 'The ketogenic diet causes a reversible decrease in activity level in Long–Evans rats. Exp. Neurol. 2006;201:84–89. doi: 10.1016/j.expneurol.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Newman John C., Verdin Eric. 'Ketone bodies as signaling metabolites. Trends Endocrinol. Metabol. 2014;25:42–52. doi: 10.1016/j.tem.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer C.M. 'Ketogenic diet in the treatment of schizoaffective disorder: two case studies. Schizophr. Res. 2017;189:208–209. doi: 10.1016/j.schres.2017.01.053. [DOI] [PubMed] [Google Scholar]
- Palmer C.M., Gilbert-Jaramillo J., Westman E.C. The ketogenic diet and remission of psychotic symptoms in schizophrenia: two case studies. Schizophr. Res. 2019;208:439–440. doi: 10.1016/j.schres.2019.03.019. [DOI] [PubMed] [Google Scholar]
- Parmeggiani B., Vargas C.R. 'Oxidative stress in urea cycle disorders: findings from clinical and basic research. Clin. Chim. Acta. 2018;477:121–126. doi: 10.1016/j.cca.2017.11.041. [DOI] [PubMed] [Google Scholar]
- Pei L., Wallace D.C. Mitochondrial etiology of neuropsychiatric disorders. Biol. Psychiatr. 2018;83:722–730. doi: 10.1016/j.biopsych.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps J.R., Siemers S.V., El-Mallakh R.S. The ketogenic diet for type II bipolar disorder. Neurocase. 2013;19:423–426. doi: 10.1080/13554794.2012.690421. [DOI] [PubMed] [Google Scholar]
- Preston G., Kirdar F., Kozicz T. 'The role of suboptimal mitochondrial function in vulnerability to post-traumatic stress disorder. J. Inherit. Metab. Dis. 2018;41:585–596. doi: 10.1007/s10545-018-0168-1. [DOI] [PubMed] [Google Scholar]
- Preston Graeme, Tim Emmerzaal, Kirdar Faisal, Schrader Laura, Henckens Marloes, Morava Eva, Kozicz Tamas. Cerebellar mitochondrial dysfunction and concomitant multi-system fatty acid oxidation defects are sufficient to discriminate PTSD-like and resilient male mice. Brain, Behavior, & Immunity - Health. 2020;6:100104. doi: 10.1016/j.bbih.2020.100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabellino D., Densmore M., Theberge J., McKinnon M.C., Lanius R.A. 'The cerebellum after trauma: resting-state functional connectivity of the cerebellum in posttraumatic stress disorder and its dissociative subtype. Hum. Brain Mapp. 2018;39:3354–3374. doi: 10.1002/hbm.24081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin E., Rottenberg H. 'Ethanol-induced injury and adaptation in biological membranes. Fed. Proc. 1982;41:2465–2471. [PubMed] [Google Scholar]
- Ruskin D.N., Fortin J.A., Bisnauth S.N., Masino S.A. 'Ketogenic diets improve behaviors associated with autism spectrum disorder in a sex-specific manner in the EL mouse. Physiol. Behav. 2017;168:138–145. doi: 10.1016/j.physbeh.2016.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Kashiwaya Y., Keon C.A., Tsuchiya N., King M.T., Radda G.K., Chance B., Clarke K., Veech R.L. 'Insulin, ketone bodies, and mitochondrial energy transduction. Faseb. J. 1995;9:651–658. doi: 10.1096/fasebj.9.8.7768357. [DOI] [PubMed] [Google Scholar]
- Shao L., Martin M.V., Watson S.J., Schatzberg A., Akil H., Myers R.M., Jones E.G., Bunney W.E., Vawter M.P. Mitochondrial involvement in psychiatric disorders. Ann. Med. 2008;40:281–295. doi: 10.1080/07853890801923753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth C., Prescot A.P., Legarreta M., Renshaw P.F., McGlade E., Yurgelun-Todd D. 'Reduced gamma-amino butyric acid (GABA) and glutamine in the anterior cingulate cortex (ACC) of veterans exposed to trauma. J. Affect. Disord. 2019;248:166–174. doi: 10.1016/j.jad.2019.01.037. [DOI] [PubMed] [Google Scholar]
- Sussman D., Germann J., Henkelman M. 'Gestational ketogenic diet programs brain structure and susceptibility to depression & anxiety in the adult mouse offspring. Brain Behav. 2015;5 doi: 10.1002/brb3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebani Abdellah, Abily-Donval Lenaig, Afonso Carlos, Marret Stéphane, Bekri Soumeya. 'Clinical metabolomics: the new metabolic window for inborn errors of metabolism investigations in the post-genomic era. Int. J. Mol. Sci. 2016;17:1167. doi: 10.3390/ijms17071167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson Legault J., Strittmatter L., Tardif J., Sharma R., Tremblay-Vaillancourt V., Aubut C., Boucher G., Clish C.B., Cyr D., Daneault C., Waters P.J., Vachon L., Morin C., Laprise C., Rioux J.D., Mootha V.K., Des Rosiers C. A metabolic signature of mitochondrial dysfunction revealed through a monogenic form of leigh syndrome. Cell Rep. 2015;13:981–989. doi: 10.1016/j.celrep.2015.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio A., D'Antona G., Nisoli E. Branched-chain amino acids, mitochondrial biogenesis, and healthspan: an evolutionary perspective. Aging (Albany NY) 2011;3:464–478. doi: 10.18632/aging.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanItallie T.B., Nufert T.H. 'Ketones: metabolism's ugly duckling. Nutr. Rev. 2003;61:327–341. doi: 10.1301/nr.2003.oct.327-341. [DOI] [PubMed] [Google Scholar]
- Wan J., Liu D., Zhang J., Shi Y., Han F. 'Single-prolonged stress induce different change in the cell organelle of the hippocampal cells: a study of ultrastructure. Acta Histochem. 2016;118:10–19. doi: 10.1016/j.acthis.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Wilkins J., Sakrikar D., Petterson X.M., Lanza I.R., Trushina E. A comprehensive protocol for multiplatform metabolomics analysis in patient-derived skin fibroblasts. Metabolomics. 2019;15:83. doi: 10.1007/s11306-019-1544-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B., Han F., Shi Y.X. Dysfunction of Ca2+/CaM kinase IIalpha cascades in the amygdala in post-traumatic stress disorder. Int. J. Mol. Med. 2009;24:795–799. doi: 10.3892/ijmm_00000294. [DOI] [PubMed] [Google Scholar]
- Xiao B., Yu B., Wang H.T., Han F., Shi Y.X. 'Single-prolonged stress induces apoptosis by activating cytochrome C/caspase-9 pathway in a rat model of post-traumatic stress disorder. Cell. Mol. Neurobiol. 2011;31:37–43. doi: 10.1007/s10571-010-9550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing G., Barry E.S., Benford B., Grunberg N.E., Li H., Watson W.D., Sharma P. 'Impact of repeated stress on traumatic brain injury-induced mitochondrial electron transport chain expression and behavioral responses in rats. Front. Neurol. 2013;4:196. doi: 10.3389/fneur.2013.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Chen T., Sun L., Zhao Z., Qi X., Zhou K., Cao Y., Wang X., Qiu Y., Su M., Zhao A., Wang P., Yang P., Wu J., Feng G., He L., Jia W., Wan C. Potential metabolite markers of schizophrenia. Mol. Psychiatr. 2013;18:67–78. doi: 10.1038/mp.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Li H., Hu X., Benedek D.M., Fullerton C.S., Forsten R.D., Naifeh J.A., Li X., Wu H., Benevides K.N., Le T., Smerin S., Russell D.W., Ursano R.J. Mitochondria-focused gene expression profile reveals common pathways and CPT1B dysregulation in both rodent stress model and human subjects with PTSD. Transl. Psychiatry. 2015;5 doi: 10.1038/tp.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.