Abstract
Purpose
To investigate the prognostic value of programmed death ligand-1 (PD-L1) expression in tumor-infiltrating immune cells (ICs) in men treated with adjuvant chemotherapy (AC) following radical cystectomy (RC) for muscle-invasive bladder cancer (MIBC).
Materials and Methods
We retrospectively reviewed 219 “high-risk” (≥pT3a and/or pN+) patients who underwent RC and received cisplatin-based AC for MIBC between March 2015 and September 2019. PD-L1 expression was measured using the VENTANA (SP-142) immunohistochemistry assay and categorized into the three groups according to the percentage of the tumor area covered by PD-L1 expression on ICs: IC0 (<1%), IC1 (≥1% and <5%), and IC2/3 (≥5%). Positive PD-L1 expression was defined as IC2/3 (≥5%). Kaplan–Meier survival analysis was used to assess recurrence-free survival (RFS), and Cox proportional hazard models were applied to identify factors predicting tumor recurrence.
Results
In the entire cohort, the overall prevalence of PD-L1 IC0, IC1, and IC2/3 was 13.2%, 27.4%, and 59.4%, respectively. During the mean follow-up of 32.5 months, tumor recurrence was detected in 115 (52.5%) patients. On multivariable analysis, tumor stage (≥pT3; P=0.032), positive lymph nodes (P=0.001), and positive PD-L1 on ICs (P=0.005) were independent predictors of tumor recurrence. The 3 year RFS was 54.7% in patients with negative PD-L1 and 31.7% in patients with positive PD-L1.
Conclusion
PD-L1 is widely expressed in ICs. Positive PD-L1 on ICs was significantly associated with shorter RFS in patients treated with cisplatin-based AC following RC. The present results support the use of adjuvant immunotherapy in “high-risk” patients with PD-L1-expressing ICs.
Keywords: adjuvant chemotherapy, bladder cancer, programmed death ligand-1, recurrence, tumor-infiltrating immune cell
Introduction
Radical cystectomy (RC) with pelvic lymph node dissection is the standard, potentially curative treatment option for muscle-invasive bladder cancer (MIBC).1,2 However, patients with locally advanced bladder cancer (BCa) following RC have a poor prognosis, with 5 year disease-free survival (DFS) rates ranging from 11.2% to 56.9%, depending on stage and lymph node (LN) status.3–5 Recent guidelines recommend cisplatin-based combination adjuvant chemotherapy (AC) in patients with pT3/4 and/or pN+ disease after RC who did not receive neoadjuvant chemotherapy.1 The main advantage of AC after local treatment is that there is no delay in the definite treatment, and the pathologic stage can be accurately identified.6 In addition, recent meta-analyses show that AC has some benefit in terms of overall survival (OS; hazard ratio [HR] = 0.77, P = 0.049) and DFS (HR = 0.66, P = 0.014).7 However, at least 30% of patients do not respond to AC and experience treatment-related adverse events without benefits.8 Therefore, identifying a biomarker that can predict the success of AC in an individual patient would be beneficial.
Tumor-host immune interactions are important for an effective response to chemotherapy.9,10 BCa is considered an immunogenic tumor because of its relatively high tumor mutational burden,11,12 and inhibition of programmed death-1 (PD-1)/programmed death ligand-1 (PD-L1) interaction has shown positive results in BCa by restoring T-cell mediated immune responses.13–15 However, the association between immune checkpoint molecules and outcomes of AC in MIBC remains unclear.
The identification of biomarkers for predicting the response to treatment and prognosis is necessary to select patients who may benefit from AC and to design follow-up strategies. Therefore, in this study, we investigated the prognostic value of PD-L1 expression on tumor-infiltrating immune cells (ICs) in patients treated with cisplatin-based AC following RC for MIBC.
Materials and Methods
Study Population
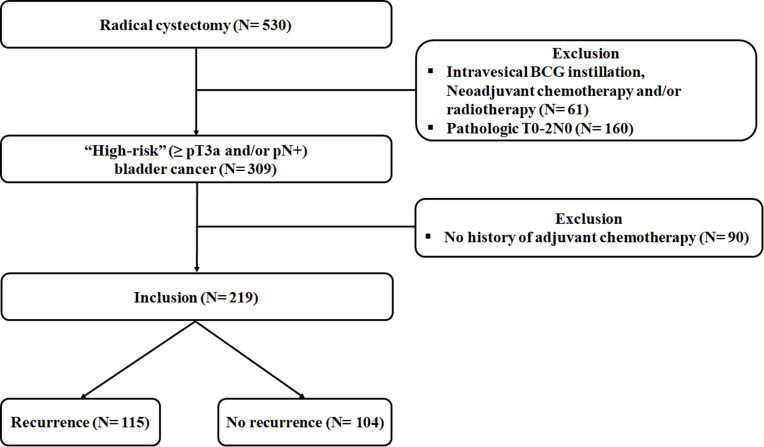
This retrospective study was approved by the Institutional Review Board of Ewha Womans University Mokdong Hospital (IRB No. 2019–04-032), and the requirement for written informed consent was waived due to the study design. All study protocols were carried out in accordance with the Declaration of Helsinki and all patient data complied with relevant data protection and privacy regulations. A prospectively maintained database of 530 patients who underwent RC for BCa between March 2015 and September 2019 by a single urologic oncology surgeon was retrospectively reviewed. In the entire cohort, patients who received intravesical instillation of Bacillus Calmette-Guerin (BCG) and chemotherapy and/or radiotherapy prior to RC (n = 61) were pathologically diagnosed with pT0-2N0 BCa (n = 160), and patients who did not receive AC following RC (n = 90) were excluded from the analysis. Ultimately, 219 patients with “high-risk” (≥pT3a and/or pN+) BCa treated with cisplatin-based AC following RC were analyzed in this study (Figure 1). All patients were staged cM0 preoperatively.
Figure 1.
Flow chart.
All RCs were performed as open procedures and included removal of the prostate and seminal vesicle in men, and removal of the uterus and ovaries in women. Standard bilateral pelvic lymphadenectomy was performed in all patients.16 AC was performed approximately 1 month following RC, and patients received three to six courses of a combined gemcitabine/cisplatin (GC) regimen (1,000 mg/m2 gemcitabine on days 1 and 8, and 15 and 70 mg/m2 cisplatin on day 2) every 4 weeks.
Data Collection
Clinical and pathological characteristics of patients, including age at surgery, gender, pathologic tumor stage and LN involvement at RC, number of resected LNs, concomitant carcinoma in situ (CIS), lymphovascular invasion (LVI), surgical margin status, type of urinary diversion, and underlying histology, were obtained from medical records at the time of surgery.
Disease recurrence was defined as high-grade, upper tract primary tumors, local recurrence at the surgical bed or regional LNs and/or distant metastasis.17 Recurrence-free survival (RFS) was measured from the date of RC to the date of the first documented recurrence or the date of the last follow-up when the patient had not yet experienced disease recurrence.
Histologic Assessment
RC specimens were processed on formalin-fixed, paraffin-embedded sections and reviewed by an experienced pathologist specialized in genitourinary cancer. Pathologic staging and tumor grading were determined according to the 2010 TNM classification from the American Joint Committee on Cancer (AJCC)18 and the 2004 World Health Organization (WHO)/International Society of Urologic Pathology consensus classification.19
Immunohistochemistry (IHC) Assay for PD-L1 Expression and Quantification
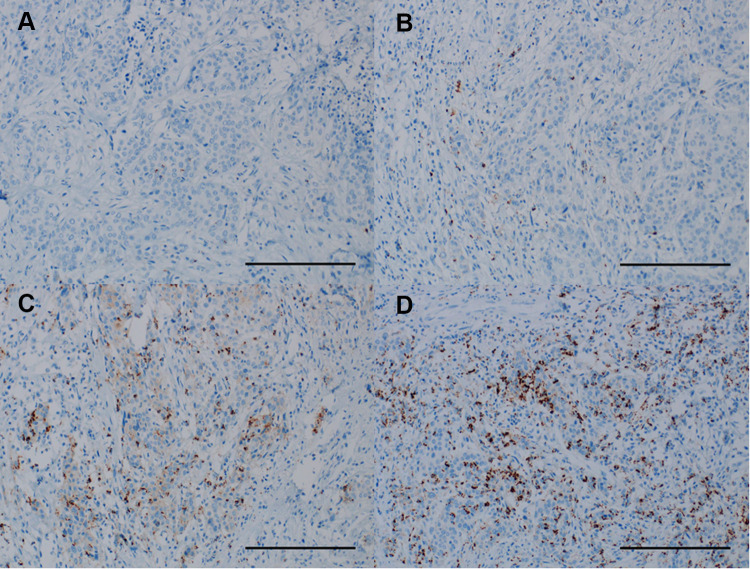
The VENTANA PD-L1 (SP142) rabbit monoclonal primary antibody (Ventana Medical Systems, Tucson, AZ, USA) was used as a fully automated IHC assay on the BenchMark ULTRA (Ventana Medical Systems) staining platform to measure PD-L1 expression on tumor-infiltrating ICs following routine protocols and specific manufacturer instructions. This assay was optimized for the detection of PD-L1 expression in urothelial carcinoma, where IC is predictive.20 The results were analyzed by a pathologist specialized in genitourinary cancer who was blinded to the clinicopathologic and survival data. PD-L1 expression level was categorized into the following three groups based on the percentage of the tumor area covered by PD-L1 expression on ICs: IC0 (<1%), IC1 (≥1% and <5%), and IC2/3 (≥5%).21,22 In addition, PD-L1 expression was dichotomized as positive (≥5%) or negative (<5%) using a 5% cutoff value. Representative images of PD-L1 expression on ICs are shown in Figure 2.
Figure 2.
Representative images showing immunohistochemical staining of programmed death ligand-1 on tumor-infiltrating immune cells in radical cystectomy specimens. (A) IC0 (×200), (B) IC1 (×200), (C) IC2 (×200), and (D) IC3 (×200) (bar scale 100 μm).
Follow-Up
Each patient was followed up according to the institutional protocol and recommendations. In general, after RC, patients were scheduled for a follow-up at 1 month postoperatively and then every 3 months for the first 2 years, every 6 months for the next 3 years, and annually thereafter. During the follow-up period, a physical examination with laboratory tests, urine analysis with cytology, chest radiography, and radiologic evaluation including computed tomography (CT) or magnetic resonance imaging (MRI) of the chest, abdomen, and pelvis were performed at every visit to identify local recurrence and/or distant metastasis. A bone scintigraphy scan was performed when clinically indicated. In cases of orthotopic urinary diversion, cystoscopy was performed in patients showing abnormal findings in urine cytology or when symptoms (irritative voiding or hematuria) were present.
Statistical Analysis
Descriptive statistics were obtained for demographic variables. Quantitative variables are presented as median (range) or mean (standard deviation, SD), and qualitative variables are presented as an absolute value (percentage). An independent t-test was used to compare continuous variables, and Pearson’s chi-square test was used to compare categorical clinicopathologic characteristics. Kaplan–Meier survival analysis was used to estimate RFS, and differences were assessed with the Log rank test. Cox proportional hazard models were used to identify predictive factors associated with tumor recurrence. All statistical analyses were performed using IBM SPSS statistics for Windows, version 23.0 (IBM Corp. Armonk, NY, USA). Two-sided P-values <0.05 were considered statistically significant.
Results
Patient Characteristics
The baseline clinicopathologic characteristics of the 219 “high-risk” patients who underwent cisplatin-based combination AC following RC for BCa are summarized in Table 1. In the entire cohort, the median (range) age at RC was 65.0 (37.0–74.0) years, and the male:female ratio was 4.2:1. Pure urothelial carcinoma was identified in 76.3% (167/219) of patients, and 190 (86.8%) patients had a locally advanced tumor stage (≥pT3) at RC. The median (range) number of resected LNs was 20.0 (3.0–52.0), and pathologic analysis demonstrated LN involvement in 117 (53.4%) patients. The overall prevalence of PD-L1 IC0, IC1, and IC2/3 on ICs was 13.2%, 27.4%, and 59.4%, respectively. After dividing patients into two groups according to tumor recurrence (Yes vs No), there were significant differences in pathologic T and N stages, number of resected LNs (22.0 vs 18.0), number of AC (4.0 vs 3.0), and PD-L1 score on ICs (each P < 0.05). However, there were no significant differences in age at surgery, gender, concomitant CIS, LVI, surgical margin status, type of urinary diversion, and histologic subtype between the groups.
Table 1.
Clinicopathologic Characteristics of 219 “High-Risk” Patients Treated with Cisplatin-Based Combination Adjuvant Chemotherapy Following Radical Cystectomy
| Parameters | Total | Recurrence | P | |
|---|---|---|---|---|
| Yes | No | |||
| No. of patients | 219 (100.0) | 115 (52.5) | 104 (47.5) | |
| Age at surgery, years | 0.504 | |||
| Median (range) | 65.0 (37.0–74.0) | 65.0 (37.0–71.0) | 67.0 (41.0–74.0) | |
| Mean (SD) | 63.6 (10.3) | 63.0 (10.6) | 63.8 (10.9) | |
| Gender, n (%) | 0.717 | |||
| Male | 177 (80.8) | 94 (81.7) | 83 (79.8) | |
| Female | 42 (19.2) | 21 (18.3) | 21 (20.2) | |
| Pathologic T stage at RC, n (%) | 0.001 | |||
| pT2 | 29 (13.2) | 7 (6.1) | 22 (21.2) | |
| pT3 | 146 (66.7) | 72 (62.6) | 74 (71.1) | |
| pT4 | 44 (20.1) | 36 (31.3) | 8 (7.7) | |
| Concomitant CIS at RC, n (%) | 0.106 | |||
| Yes | 122 (55.7) | 70 (66.7) | 52 (50.0) | |
| No | 97 (44.3) | 45 (33.3) | 52 (50.0) | |
| LVI at RC, n (%) | 0.467 | |||
| Yes | 142 (64.8) | 72 (62.6) | 70 (67.3) | |
| No | 77 (35.2) | 43 (37.4) | 34 (32.7) | |
| No. of resected LNs at RC | 0.001 | |||
| Median (range) | 20.0 (3.0–52.0) | 22.0 (4.0–52.0) | 18.0 (3.0–52.0) | |
| Mean (SD) | 20.6 (10.4) | 23.8 (10.5) | 18.8 (11.0) | |
| Pathologic N status at RC, n (%) | 0.001 | |||
| Negative | 102 (46.6) | 38 (33.0) | 64 (61.5) | |
| Positive | 117 (53.4) | 77 (67.0) | 40 (38.5) | |
| Histologic subtype, n (%) | 0.293 | |||
| Pure UC | 167 (76.3) | 91 (79.1) | 76 (73.1) | |
| Mixed UC | 52 (23.7) | 24 (20.9) | 28 (26.9) | |
| Surgical margin status, n (%) | 0.727 | |||
| Negative | 179 (81.7) | 93 (80.9) | 86 (82.7) | |
| Positive | 40 (18.3) | 22 (19.1) | 18 (17.3) | |
| No. of adjuvant chemotherapy, n (%) | 0.001 | |||
| Median (range) | 4.0 (3.0–6.0) | 4.0 (3.0–6.0) | 3.0 (3.0–6.0) | |
| Mean (SD) | 4.2 (1.3) | 4.6 (1.3) | 3.8 (1.2) | |
| Type of urinary diversion, n (%) | 0.545 | |||
| Ileal conduit | 24 (11.0) | 14 (12.2) | 10 (9.6) | |
| Orthotopic neobladder | 195 (89.0) | 101 (87.8) | 94 (90.4) | |
| PD-L1 score on ICs in RC specimens | 0.001 | |||
| IC0 (<1%) | 29 (13.2) | 8 (7.0) | 21 (20.2) | |
| IC1 (≥1% and <5%) | 60 (27.4) | 26 (22.6) | 34 (32.7) | |
| IC2/3 (≥5%) | 130 (59.4) | 81 (70.4) | 49 (47.1) | |
| Follow-up, months | 0.531 | |||
| Median (range) | 26.2 (3.1–66.6) | 26.2 (3.1–66.6) | 26.4 (3.6–60.8) | |
| Mean (SD) | 32.5 (24.4) | 32.7 (24.8) | 32.2 (24.2) | |
Abbreviations: SD, standard deviation; RC, radical cystectomy; CIS, carcinoma in situ; LVI, lymphovascular invasion; LN, lymph node; UC, urothelial carcinoma; PD-L1, programmed death-ligand 1; IC, immune cell.
Association of Clinicopathologic Characteristics with PD-L1 Expression
The associations between PD-L1 expression and clinicopathologic characteristics are summarized in Table 2. The prevalence of PD-L1 IC0, IC1, and IC2/3 on ICs was 6.0%, 29.1%, and 64.9%, respectively, in patients with positive LNs, and the rates were significantly different from those in patients with negative LNs (P = 0.003). However, there was no association between PD-L1 expression on ICs and any remaining clinicopathologic parameters, including age, gender, tumor stage, concomitant CIS, LVI, surgical margin status, type of urinary diversion, and histologic subtype.
Table 2.
Association of Programmed Death-Ligand 1 Expression and Clinicopathologic Characteristics
| Parameters | Total | PD-L1 Expression on ICs | P | ||
|---|---|---|---|---|---|
| IC0 | IC1 | IC2/3 | |||
| Age | 0.654 | ||||
| <65.0 | 109 | 13 (11.9) | 28 (25.7) | 68 (62.4) | |
| ≥65.0 | 110 | 16 (14.5) | 32 (29.1) | 62 (56.4) | |
| Gender | 0.569 | ||||
| Male | 177 | 22 (12.4) | 47 (26.6) | 108 (61.0) | |
| Female | 42 | 7 (16.7) | 13 (30.9) | 22 (52.4) | |
| Tumor stage | 0.640 | ||||
| pT2 | 29 | 5 (17.3) | 9 (31.0) | 15 (51.7) | |
| pT3–4 | 190 | 24 (12.6) | 51 (26.9) | 115 (60.5) | |
| Concomitant CIS | 0.324 | ||||
| Yes | 122 | 18 (14.8) | 37 (30.3) | 67 (54.9) | |
| No | 97 | 11 (11.3) | 23 (23.7) | 63 (65.0) | |
| LVI | 0.154 | ||||
| Yes | 142 | 21 (14.8) | 33 (23.2) | 88 (62.0) | |
| No | 77 | 8 (10.4) | 27 (35.1) | 42 (54.5) | |
| Lymph node positivity | 0.003 | ||||
| Negative | 102 | 22 (21.6) | 26 (25.5) | 54 (52.9) | |
| Positive | 117 | 7 (6.0) | 34 (29.1) | 76 (64.9) | |
| Surgical margin status | 0.462 | ||||
| Negative | 179 | 24 (13.4) | 52 (29.1) | 103 (57.5) | |
| Positive | 40 | 5 (12.5) | 8 (20.0) | 27 (67.5) | |
| Histologic subtype | 0.333 | ||||
| Pure UC | 167 | 19 (11.4) | 46 (27.5) | 102 (61.1) | |
| Mixed UC | 52 | 10 (19.3) | 14 (26.9) | 28 (53.8) | |
| Type of urinary diversion | 0.786 | ||||
| Ileal conduit | 24 | 3 (12.5) | 8 (33.3) | 13 (54.2) | |
| Orthotopic neobladder | 195 | 26 (13.3) | 52 (26.7) | 117 (60.0) | |
Abbreviations: CIS, carcinoma in situ; LVI, lymphovascular invasion; UC, urothelial carcinoma; PD-L1, programmed death-ligand 1; IC, immune cell.
Association of PD-L1 Expression with RFS
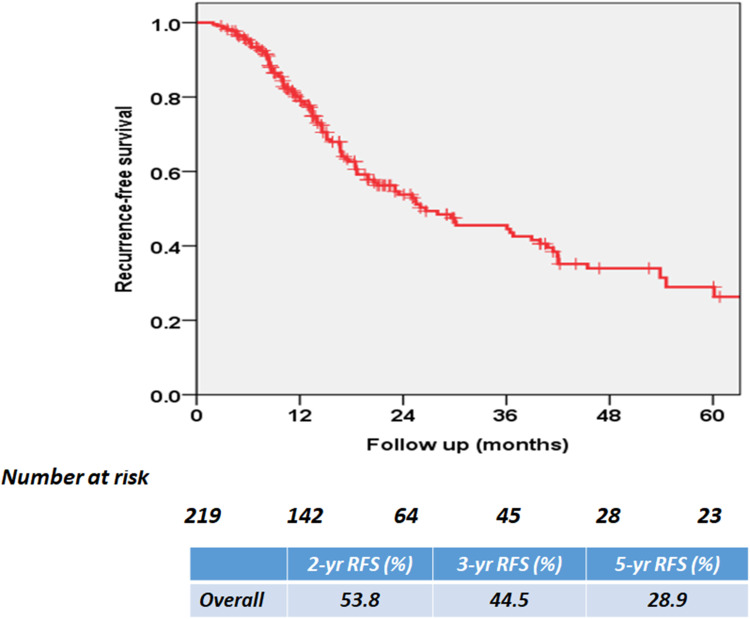
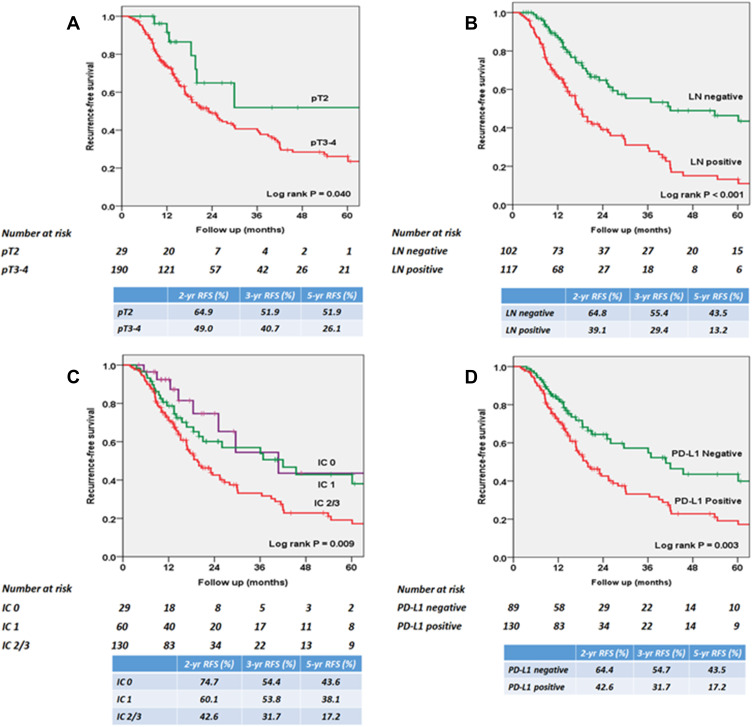
During the mean (SD) follow-up of 32.5 (24.4) months, local recurrence and/or distant metastasis was identified in 115 (52.5%) patients. Figure 3 shows the overall RFS rates estimated using the Kaplan–Meier method. The 2 and 3 year overall RFS rates were 53.8% and 44.5%, respectively. After stratification according to pathologic parameters, extra-vesical tumor stage (≥pT3) and positive LNs were associated with poor RFS (P = 0.040 and P < 0.001, respectively; Figure 4A and B). After stratification according to PD-L1 expression on ICs, RFS was statistically significantly poorer in patients with IC2/3 than in patients with IC0 (P = 0.020) and those with IC1 (P = 0.021; Figure 4C). When PD-L1 expression was dichotomized using a 5% cutoff value, PD-L1 expression on ICs significantly affected RFS (P = 0.003; Figure 4D). The 3 year RFS rate was 54.7% in PD-L1-negative patients and 31.7% in PD-L1-positive patients.
Figure 3.
Kaplan–Meier survival curve for overall recurrence-free survival (RFS).
Figure 4.
Kaplan–Meier survival curves for recurrence-free survival (RFS) according to (A) pathologic T stage, (B) pathologic N stage, (C) PD-L1 expression score, and (D) PD-L1 expression positivity.
The outcomes of Cox proportional hazard regression analysis of prognostic factors for tumor recurrence after RC are presented in Table 3. Multivariable Cox regression analyses revealed that tumor stage ≥pT3 (hazard ratio [HR] = 1.63; 95% confidence interval [CI]: 1.04–2.55; P = 0.032), positive LNs (HR = 2.27; 95% CI: 1.46–3.52; P = 0.001), and positive PD-L1 expression on ICs (HR = 1.68; 95% CI: 1.17–2.43; P = 0.005) were independently associated with a significantly increased risk of tumor recurrence.
Table 3.
Cox Proportional Hazard Regression Analyses to Predict Tumor Recurrence After Cisplatin-Based Combination Adjuvant Chemotherapy Following Radical Cystectomy
| Variables | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | ||||||
| <65.0 | Ref | |||||
| ≥65.0 | 1.01 | 0.99–1.03 | 0.263 | |||
| Gender | ||||||
| Male | Ref | |||||
| Female | 1.03 | 0.64–1.63 | 0.812 | |||
| Tumor stage | ||||||
| pT2 | Ref | Ref | ||||
| pT3–4 | 1.78 | 1.12–2.78 | 0.015 | 1.63 | 1.04–2.55 | 0.032 |
| Concomitant CIS | ||||||
| No | Ref | |||||
| Yes | 1.24 | 0.71–1.97 | 0.510 | |||
| LVI | ||||||
| No | Ref | Ref | ||||
| Yes | 1.55 | 1.16–2.08 | 0.003 | 1.31 | 0.96–1.78 | 0.092 |
| Lymph node positivity | ||||||
| Negative | Ref | Ref | ||||
| Positive | 2.13 | 1.42–3.17 | 0.001 | 2.27 | 1.46–3.52 | 0.001 |
| Histologic subtype | ||||||
| Pure UC | Ref | |||||
| Mixed UC | 1.29 | 0.74–2.36 | 0.288 | |||
| Surgical margin status | ||||||
| Negative | Ref | |||||
| Positive | 1.68 | 0.83–3.40 | 0.152 | |||
| No. of adjuvant chemotherapy | ||||||
| 3 | Ref | |||||
| 4–6 | 1.27 | 0.85–1.90 | 0.212 | |||
| Type of urinary diversion | ||||||
| Ileal conduit | Ref | |||||
| Orthotopic neobladder | 0.84 | 0.56–1.29 | 0.423 | |||
| PD-L1 on ICs | ||||||
| Negative | Ref | Ref | ||||
| Positive | 1.84 | 1.23–2.70 | 0.004 | 1.68 | 1.17–2.43 | 0.005 |
Abbreviations: CIS, carcinoma in situ; LVI, lymphovascular invasion; UC, urothelial carcinoma; PD-L1, programmed death-ligand 1; IC, immune cell.
Discussion
In this study, among 219 “high-risk” patients who were treated with cisplatin-based combination AC following RC for BCa, tumor recurrence was identified in 115 (52.5%) patients. However, after stratifying patients according to pathologic parameters, RFS differed significantly according to extra-vesical tumor stage, positive LNs, and positive PD-L1 expression on ICs. In addition to the pathologic T and N stages, positive PD-L1 expression on ICs was a significant prognostic factor for predicting RFS after cisplatin-based combination AC following RC. The present results may help to establish therapeutic strategies for patients with “high-risk” (≥ pT3a and/or pN+) BCa. To the best of our knowledge, this is the first study to evaluate the prognostic value of PD-L1 expression on ICs in this patient population.
Several studies have examined the prognostic value of PD-L1 on ICs as a biomarker in BCa,23–25 and high PD-L1 expression on ICs is associated with both unfavorable23,25 and favorable24 results. These conflicting results may be attributed to the use of different PD-L1 antibodies and inhomogeneous cutoff values to define positive PD-L1 expression.26 In our study, we only used the VENTANA assay with the SP 142 antibody for measuring PD-L1 expression in tumor-infiltrating ICs because, in the past, atezolizumab treatment was only reimbursed by the government for second line treatment for metastatic BCa based on the results of VENTANA test. In addition, we used 5% as the cutoff for positive PD-L1 expression to minimize the impact of heterogeneities between PD-L1 IHC assays. Furthermore, PD-L1 expression was measured only in RC specimens from patients without a history of BCG instillation, chemotherapy, and/or radiotherapy prior to RC. As a result, positive PD-L1 expression on ICs was identified in 59.4% (130/219) of patients, which is similar to the value reported previously.23
We examined the relationship between PD-L1 expression on ICs and clinicopathologic characteristics, and the results indicated that positive PD-L1 expression on ICs was closely associated with LN positivity. This result is consistent with that of a previous study reporting that positive PD-L1 expression on ICs is positively associated with tumor stage, tumor size, histologic grade, and nodal status.23 Taken together, these findings suggest that positive PD-L1 expression on ICs is associated with aggressive pathologic features in BCa.
Pichler et al25 reviewed 83 high-risk (≥pT3a and/or pN+) patients who underwent RC without cisplatin-based AC. In that study, PD-L1 (≥1%) expression was identified in 61.4% (51/83) of patients, and RFS was significantly shorter in patients with positive PD-L1 expression on ICs than in those with negative PD-L1 expression (P = 0.015). The authors hypothesized that high-risk patients with positive PD-L1 expression on ICs might benefit from adjuvant immunotherapy after RC. In our study, we analyzed patients who received cisplatin-based AC following RC, and the results support the need for adjuvant immunotherapy in high-risk patients with positive PD-L1 expression on ICs.
On the other hand, Bellmunt et al24 reviewed 143 urothelial carcinoma samples and detected positive PD-L1 expression (≥5%) on tumor-infiltrating mononuclear cells (TIMCs) in 58 (40.6%) patients. They reported that positive PD-L1 expression in TIMCs was significantly associated with longer survival in patients who developed metastasis and subsequently received platinum-based chemotherapy (P = 0.0007). However, in the entire cohort, 51.9% of patients were T2 or less. In addition, because platinum-based chemotherapy was performed after metastasis was identified, the association between PD-L1 expression and recurrence was not confirmed.
The PD-1/PD-L1 signaling pathway constitutes a mechanism of escape from an antitumor immune response.27 In this study, when patients were stratified by PD-L1 expression on ICs, patients with positive PD-L1 expression on ICs showed a statistically significantly poorer RFS than those with negative PD-L1 on ICs. Although the exact mechanism underlying the regulation of PD-L1 expression in patients with BCa remains to be elucidated, possible mechanisms are as follows: PD-L1 expression in the tumor environment is controlled by the tumor-related stroma in a process called “adaptive immune resistance”28 In this process, PD-L1 is upregulated by interferon-gamma in the tumor niche, and the PD pathway acts to suppress tumor immunity as a negative feedback mechanism.29 However, an exhausted immune state called “immune privilege” is a feature of the tumor microenvironment.30 In this condition, the antitumor function is restricted by several mechanisms including regulation of the spatial distribution of T-cells, promotion of T-cell apoptosis, or impairment of T-cell activation.30,31
Recently, potential role of adjuvant immunotherapy has been examined. In a Phase III IMvigor010 trial, adjuvant atezolizumab in patients with high-risk muscle invasive BCa did not improve DFS (HR = 0.89; P = 0.2446).32 However, in a phase III CheckMate-274 trial, adjuvant nivolumab in patients with high-risk muscle invasive BCa improved DFS in both all randomized patients and in patients whose tumor cells express PD-L1 ≥1%.33 In addition, in a phase III JAVELIN Bladder 100 trial, the addition of maintenance avelumab to best supportive care significantly prolonged OS (HR = 0.56; P < 0.001) in PD-L1 positive patients with unresectable locally advanced or metastatic BCa who had not progressed with first-line chemotherapy.34 Collectively, these trials will hopefully provide guidance for further treatment strategy.
Despite the potential clinical implications of our study, there were several limitations. First, this study had a non-randomized, retrospective design and was conducted at a single, tertiary referral center, which raises concerns for inherent selection bias. Nonetheless, this study used a prospectively maintained database and reflects real-world clinical practice. Second, the mean follow-up period was relatively short, which limited the analysis to one end-point (RFS); other clinically significant oncologic outcomes such as cancer-specific survival and OS were not analyzed. Third, in our study, we only used the VENTANA assay with the SP 142 antibody for measuring PD-L1 expression to avoid heterogeneities between IHC diagnostic assays. However, when interpreting PD-L1 status, discrepancies according to different antibody clones, staining platforms, and scoring algorithms should be considered.35 Finally, data for PD-L1 expression on tumor cells were not available; we could not assess the clinical impact of PD-L1 expression on tumor cells. A large, prospective randomized study is warranted to confirm the results and their clinical implications.
In conclusion, in “high-risk” BCa patients, PD-L1 is widely expressed in ICs from RC specimens. Positive PD-L1 expression on ICs was closely associated with LN positivity and significantly associated with shorter RFS in patients treated with cisplatin-based combination AC following RC for BCa. The present results support the need for adjuvant immunotherapy in “high-risk” patients with positive PD-L1 expression on ICs. Further prospective studies are needed to clarify the role of PD-L1 expression in ICs as a biomarker for BCa.
Funding Statement
This research was supported by the Basic Science Research Program through a National Research Foundation of Korea grant funded by the Ministry of Science, ICT & Future Planning (NRF-2018R1C1B6007678).
Disclosure
All the authors have approved the manuscript and agree with submission to your esteemed journal. The authors declare that they have no conflicts of interest.
References
- 1.Witjes JA, Bruins HM, Cathomas R, et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol. 2020. [DOI] [PubMed] [Google Scholar]
- 2.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19(3):666–675. doi: 10.1200/JCO.2001.19.3.666 [DOI] [PubMed] [Google Scholar]
- 3.Ghoneim MA, Abdel-Latif M, el-Mekresh M, et al. Radical cystectomy for carcinoma of the bladder: 2,720 consecutive cases 5 years later. J Urol. 2008;180(1):121–127. doi: 10.1016/j.juro.2008.03.024 [DOI] [PubMed] [Google Scholar]
- 4.Galsky MD, Stensland KD, Moshier E, et al. Effectiveness of adjuvant chemotherapy for locally advanced bladder cancer. J Clin Oncol. 2016;34(8):825–832. doi: 10.1200/JCO.2015.64.1076 [DOI] [PubMed] [Google Scholar]
- 5.Ploussard G, Shariat SF, Dragomir A, et al. Conditional survival after radical cystectomy for bladder cancer: evidence for a patient changing risk profile over time. Eur Urol. 2014;66(2):361–370. doi: 10.1016/j.eururo.2013.09.050 [DOI] [PubMed] [Google Scholar]
- 6.Sternberg CN, Skoneczna I, Kerst JM, et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised Phase 3 trial. Lancet Oncol. 2015;16(1):76–86. doi: 10.1016/S1470-2045(14)71160-X [DOI] [PubMed] [Google Scholar]
- 7.Leow JJ, Martin-Doyle W, Rajagopal PS, et al. Adjuvant chemotherapy for invasive bladder cancer: a 2013 updated systematic review and meta-analysis of randomized trials. Eur Urol. 2014;66(1):42–54. doi: 10.1016/j.eururo.2013.08.033 [DOI] [PubMed] [Google Scholar]
- 8.Sternberg CN, Bellmunt J, Sonpavde G, et al. ICUD-EAU international consultation on bladder cancer 2012: chemotherapy for urothelial carcinoma—neoadjuvant and adjuvant settings. Eur Urol. 2013;63(1):58–66. doi: 10.1016/j.eururo.2012.08.010 [DOI] [PubMed] [Google Scholar]
- 9.Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res. 2015;3(5):436–443. doi: 10.1158/2326-6066.CIR-15-0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21(1):15–25. doi: 10.1038/cdd.2013.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Wilpe S, Gerretsen ECF, van der Heijden AG, de Vries IJM, Gerritsen WR, Mehra N. Prognostic and predictive value of tumor-infiltrating immune cells in urothelial cancer of the bladder. Cancers (Basel). 2020;12(9):2692. doi: 10.3390/cancers12092692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gubin MM, Zhang X, Schuster H, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515(7528):577–581. doi: 10.1038/nature13988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–562. doi: 10.1038/nature13904 [DOI] [PubMed] [Google Scholar]
- 15.Horn T, Grab J, Schusdziarra J, et al. Antitumor T cell responses in bladder cancer are directed against a limited set of antigens and are modulated by regulatory T cells and routine treatment approaches. Int J Cancer. 2013;133(9):2145–2156. doi: 10.1002/ijc.28233 [DOI] [PubMed] [Google Scholar]
- 16.Song W, Yoon HS, Kim KH, et al. Role of bowel suspension technique to prevent early intestinal obstruction after radical cystectomy with ileal orthotopic neobladder: a retrospective cohort study. Int J Surg. 2018;55:9–14. doi: 10.1016/j.ijsu.2018.04.044 [DOI] [PubMed] [Google Scholar]
- 17.Apolo AB, Milowsky MI, Kim L, et al. Eligibility and radiologic assessment in adjuvant clinical trials in bladder cancer. JAMA Oncol. 2019:1–9. 10.1001/jamaoncol.2019.4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edge SB BD, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2010. [Google Scholar]
- 19.Eble JN SG, Epstein JI. Pathology & Genetics of Tumors of the Urinary System and Male Genital Organs. Lyon, France: IARC Press; 2004:89–123. [Google Scholar]
- 20.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, Phase 2 trial. Lancet. 2016;387(10031):1909–1920. doi: 10.1016/S0140-6736(16)00561-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powles T, Duran I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391(10122):748–757. doi: 10.1016/S0140-6736(17)33297-X [DOI] [PubMed] [Google Scholar]
- 22.Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67–76. doi: 10.1016/S0140-6736(16)32455-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang B, Pan W, Yang M, et al. Programmed death ligand-1 is associated with tumor infiltrating lymphocytes and poorer survival in urothelial cell carcinoma of the bladder. Cancer Sci. 2019;110(2):489–498. doi: 10.1111/cas.13887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellmunt J, Mullane SA, Werner L, et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann Oncol. 2015;26(4):812–817. doi: 10.1093/annonc/mdv009 [DOI] [PubMed] [Google Scholar]
- 25.Pichler R, Fritz J, Lackner F, et al. Prognostic value of testing PD-L1 expression after radical cystectomy in high-risk patients. Clin Genitourin Cancer. 2018;16(5):e1015–e1024. doi: 10.1016/j.clgc.2018.05.015 [DOI] [PubMed] [Google Scholar]
- 26.Powles T, Walker J, Andrew Williams J, Bellmunt J. The evolving role of PD-L1 testing in patients with metastatic urothelial carcinoma. Cancer Treat Rev. 2020;82:101925. doi: 10.1016/j.ctrv.2019.101925 [DOI] [PubMed] [Google Scholar]
- 27.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–461. doi: 10.1016/j.ccell.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125(9):3384–3391. doi: 10.1172/JCI80011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14(4):847–856. doi: 10.1158/1535-7163.MCT-14-0983 [DOI] [PubMed] [Google Scholar]
- 30.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348(6230):74–80. doi: 10.1126/science.aaa6204 [DOI] [PubMed] [Google Scholar]
- 31.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–1355. doi: 10.1126/science.aar4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hussain M, Powles T, Albers P, et al. IMvigor010: Primary Analysis from a Phase III Randomized Study of Adjuvant Atezolizumab versus Observation in High-Risk Muscle-Invasive Urothelial Carcinoma. ASCO; 2020:5000. [Google Scholar]
- 33.Squibb BM. Opdivo (nivolumab) Significantly Improves Disease Free-Survival vs. Placebo as Adjuvant Therapy for Patients with High-Risk, Muscle-Invasive Urothelial Carcinoma in Phase 3 CheckMate −274 Trial 2020.
- 34.Powles T, Park SH, Voog E, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218–1230. doi: 10.1056/NEJMoa2002788 [DOI] [PubMed] [Google Scholar]
- 35.Rijnders M, van der Veldt AAM, Zuiverloon TCM, et al. PD-L1 antibody comparison in urothelial carcinoma. Eur Urol. 2019;75(3):538–540. doi: 10.1016/j.eururo.2018.11.002 [DOI] [PubMed] [Google Scholar]