Abstract
Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) of the urinary bladder is an extremely rare entity accounting for 0.2% of all malignant urinary bladder neoplasms, and the diagnosis could be challenging. We present here a patient with urinary bladder MALT lymphoma and review of all published case reports in the literature. We summarized the reported immunophenotype of the neoplasm, ancillary studies, therapy, and follow-up for all 59 patients in the table. The median patients’ age was 57 years-old (range, 17 to 88), with female predominance in 50 of 59 patients representing a 1:5.6 ratio. Geographical distribution of the reported patients was as follows: 22 from Asia, of which more than a half (16) originated from Japan; 28 from Europe, of which 19 reported from the United Kingdom, and 3 patients were reported from the United States (including our patient). Twenty-three (77%) of 30 patients, for whom their clinical presentation was recorded, had symptoms of cystitis; Escherichia coli was the most common pathogen. We concluded that a prominent female predominance, uneven geographic distribution of urinary bladder MALT lymphoma, and a success of antibacterial therapy in selected cases suggest the link between urinary tract infection and urinary bladder MALT lymphoma.
Keywords: MALT lymphoma, urinary bladder, cystitis, urinary tract infection
Introduction
Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) is a low-grade B cell lymphoma which accounts for 7–8% of all B cell lymphomas.1 The stomach is the most common organ involved by MALT lymphoma (about 35%), followed by other anatomical locations: eyes and ocular adnexa, skin, lungs, salivary glands, breasts, and thyroid.2
The first definitive report of MALT lymphoma in urinary bladder was published in 1990 by Kuhara and colleagues.3 MALT lymphoma of the urinary bladder is an extremely rare entity and accounts to less than 1% of all non-Hodgkin lymphomas and 0.2% of all malignant urinary bladder neoplasms.4 The majority of patients present with hematuria and/or dysuria. The differential diagnosis could be very broad and includes inflammatory lesions, bladder carcinoma, and infections.5
Some authors hypothesized that chronic antigenic stimulation and lymphoid hyperplasia caused by Escherichia coli or other bacterial infections might be a precursor of bladder MALT lymphomas.6 The positive experience in antibiotics treatment of MALT lymphoma of bladder might serve as an indirect support of this hypothesis.7–11
Herein, we report and discuss a case of primary bladder MALT lymphoma and summarize other cases reported in the literature.
Case Presentation
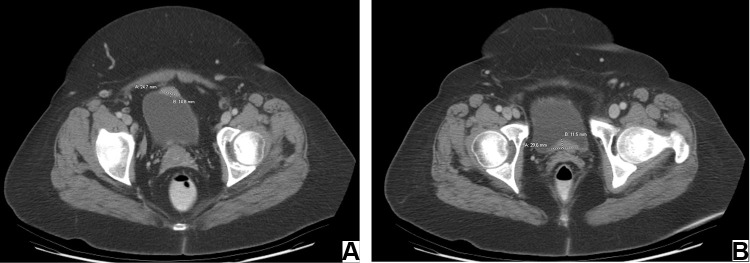
A 58-year-old Caucasian female with history of hypertension, asthma, rheumatoid arthritis, irritable bowel syndrome, colon polyps, depression, and menopausal syndrome presented to the clinic with nonspecific urinary symptoms: dysuria, nocturia, and urinary frequency. There were no B symptoms identified. The CT demonstrated multiple thickening on the anterior and posterior bladder walls (Figure 1A and B). Consequently, patient underwent a cystoscopy evaluation with transurethral resection of bladder tumor (TURBT), which revealed 2.5 x 2.5 cm mass on posterior bladder wall distally from the trigone. The mass did not have characteristic bladder cancer architecture. The patient signed the informed consent/authorization for participation in research which includes the permission to collect and use the information from medical records, imaging studies, medical photographs, pathology images, and study results for future research projects and publications. A copy of the signed consent is kept on file in the patient electronic records.
Figure 1.
Axial CT images showing multiple abnormal nodular thickening on the anterior (A) and posterior (B) bladder walls.
Pathological Findings
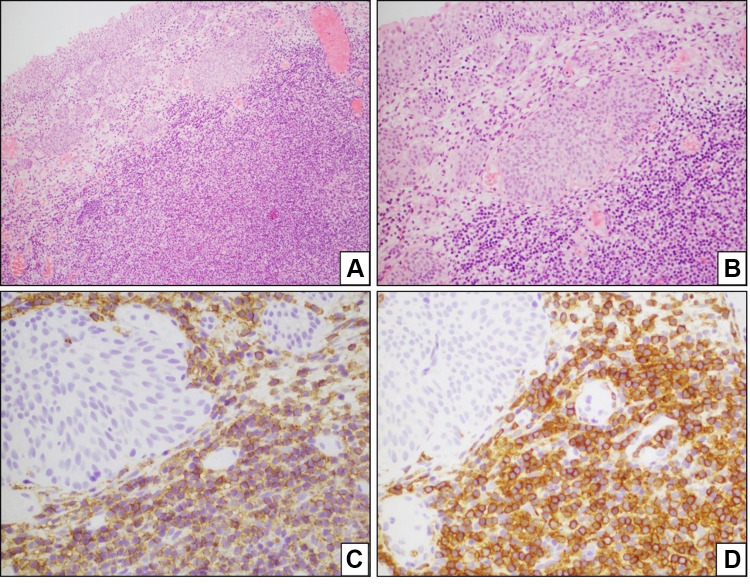
The histologic sections showed a dense lymphocytic proliferation beneath the urothelial surface of urinary bladder mucosa. Within the lymphoid proliferation, a monomorphic population of small lymphocytes having moderately abundant, pale staining cytoplasm was dominant. Regularly scattered, reactive lymphoid follicles were prominent within the mucosal lymphoid proliferation. The neoplastic lymphocytic proliferation had a perifollicular infiltration pattern.
Multiples immunohistochemical studies on biopsy material were performed and showed that neoplastic cells were positive for CD20, CD79a, BCL-2, and immunoglobulin kappa light chain (weak) confirming the clonality of the neoplastic cells; the neoplastic cells were negative for CD5, CD10, and immunoglobulin lambda light chain (Figure 2).
Figure 2.
Bladder MALT lymphoma. The histologic sections show a dense, abnormal lymphocytic proliferation right beneath the urothelial surface of urinary bladder mucosa (A and B) the neoplastic cells are positive for CD20 (C) and CD79a (D).
The diagnosis of MALT lymphoma of urinary bladder floor was established. No additional molecular studies were performed.
Treatment/Follow-Up
The patient received 4 cycles of Rituximab and achieved complete remission seen on the pelvic computed tomography (CT) (Figure 3). The patient showed no signs of disease with the last follow-up more than 10 years after original diagnosis.
Figure 3.
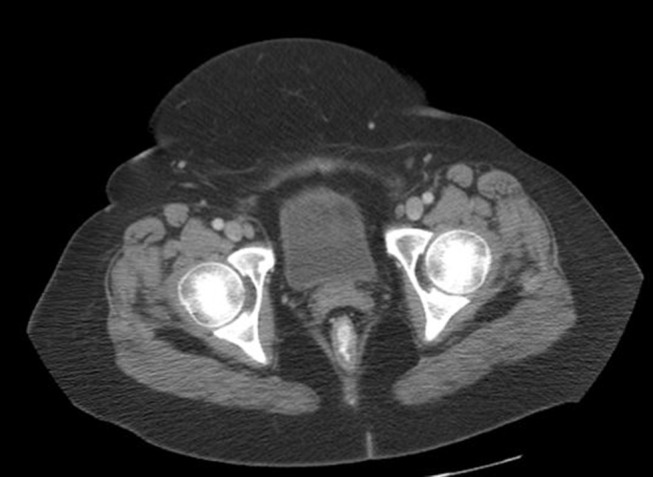
Axial CT image of depicting the bladder and showing no evidence of abnormal nodularity 3 months later.
Literature Review
The literature review was initiated starting with 1990 when Kuhara and colleagues reported what was eventually called “the first definitive report of a MALT lymphoma of the urinary bladder”.3,12 After a systemic search of the PubMed database for primary MALT lymphoma involving urinary bladder, the search identified 42 reports with a total of 58 patients.3,5–45 The available information about diagnostic immunohistochemistry, clinical manifestations, treatments, and outcomes of these cases along with a current case is summarized in Table 1.3,5–45 There was a strong female predominance with 50 of 59 patients being females with a male:female ratio of 1:5.6. The median patients’ age was 57 years-old (range, 17 to 88). A significant proportion of cases (22 total cases) was reported from in Asia, of which more than half (16) originated from Japan3,9,10,16,18,22–24,28,31,33,36,41,42,44,45 According to some of the available reports there more publications in Japanese literature which we could not find in PubMed.23,41 The cases reported from Asia showed even a higher female predominance where only one of 22 patients was a male.18 United Kingdom was another location with significant number of patients and accounted for 19 out of 28 European cases.8,12,17,20,21,25,40 Including our patient, only 3 patients were reported in the USA.32,34 All patients had some urinary symptoms at presentation which led to the diagnosis. Hematuria was the most common presenting symptom and was previously reported from 50.9% to 75%. Twenty three of 30 patients, for whom the information regarding presence or absence of cystitis was available, had cystitis; Escherichia coli was the most common pathogen.21,38 Xu and colleagues found that most patients, 76.5%, had a bladder solid mass on presentation.38 None of patients demonstrated “B” group symptoms such as weight loss, fever, or night sweats.
Table 1.
Urinary Bladder MALT Lymphoma Cases: Diagnostics and Treatment
| Case No. | Age (y.o.) | Sex | Immunohistochemistry | FISH and Molecular Study | Treatment | Follow-Up | Cystitis (Present/Absent/N/A) | Urine Culture (a Bug/Negative/N/A) | Country of Origin | Year of Report | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 56 | F | Positive: CD20 (L26), CD79a (MB1) Negative: CD43 (MT1), CD45RO (UCHL1) Lambda light chain restricted |
N/A | Total cystectomy | 9 months after surgery, negative for recurrence | Present | Enterococcus | Japan (Asia) | 1990 | Kuhara et al3 |
| 2 | 67 | F | Positive: CD20 (L26) | N/A | Chemotherapy (CHOP) | 24 months follow-up, negative for recurrence | Present | N/A | UK (Europe) | 1993 | Pawade et al12 |
| 3 | 74 | F | Positive: CD20 (L26) | N/A | Radiotherapy | Dead (no follow-up) | Present | N/A | UK (Europe) | 1993 | Pawade et al12 |
| 4 | 22 | F | Positive: CD20 (L26) | N/A | Chemotherapy and radiotherapy | 46 months follow-up, negative for recurrence | N/A | N/A | UK (Europe) | 1993 | Pawade et al12 |
| 5 | 83 | F | Positive: CD20 (L26) | N/A | Radiotherapy | 20 months follow-up, negative for recurrence | N/A | N/A | UK (Europe) | 1993 | Pawade et al12 |
| 6 | 80 | M | Positive: CD20 (L26) | N/A | Not treated | Died in 30 months after diagnosis of ischemic heart disease | Present | N/A | UK (Europe) | 1993 | Pawade et al12 |
| 7 | 73 | F | Positive: CD45 (LCA), CD20 (L26), MB2 Negative: CD79a (MB1), CD45RO (UCHL1) | N/A | Chemotherapy | Died in 8 months due to unrelated to lymphoma cause. No lymphoma recurrence in bladder. Lymphoma of the thyroid. | N/A | N/A | Spain (Europe) | 1996 | Fernandez et al13 |
| 8 | 50 | F | Positive: CD20 (L26), MB2 Kappa light chain restricted | N/A | Chemotherapy | 60 months follow-up, negative for recurrence | N/A | N/A | Spain (Europe) | 1996 | Fernandez et al13 |
| 9 | 75 | F | Positive: CD20 (L26), MB2 | N/A | Chemotherapy | 9 months follow-up, negative for recurrence | N/A | N/A | Spain (Europe) | 1996 | Fernandez et al13 |
| 10 | 70 | F | N/A | N/A | Chemotherapy and radiotherapy | 48 months follow-up, negative for recurrence | N/A | N/A | Chile (America) | 1998 | Gallardo et al15 |
| 11 | 80 | F | Positive: CD20 (L26), CD79a Negative: CD3, CD5, CD10, CD23, CD43 (MT1) | N/A | Radiotherapy | 16 months follow-up, negative for recurrence | N/A | N/A | UK (Europe) | 1998 | Yuille et al40 |
| 12 | 77 | F | Positive: CD19, CD20, Negative: CD3, CD5, CD10, CD21, CD23, CD45RO Lambda light chain restriction | Clonal heavy-chain IgH gene rearrangement (PCR) | Transurethral resection (TUR) x 2 | 36 months follow-up after last TUR, negative for recurrence | Present | N/A | Japan (Asia) | 1999 | Ando et al16 |
| 13 | 27 | M | N/A | N/A | Chemotherapy and radiotherapy | 18 months follow-up, negative for recurrence | N/A | N/A | Japan (Asia) | 2000 | Kawakami et al18 |
| 14 | 75 | F | N/A | N/A | Chemotherapy and radiotherapy | 36 months follow-up, negative for recurrence | N/A | N/A | France (Europe) | 2000 | Tasu et al19 |
| 15 | 66 | F | Positive: CD20 | N/A | N/A | 12 months follow-up, negative for recurrence | N/A | N/A | UK (Europe) | 2000 | Bates et al17 |
| 16 | 79 | F | Positive: CD20 | N/A | N/A | No follow-up | N/A | N/A | UK (Europe) | 2000 | Bates et al17 |
| 17 | 59 | F | Positive: CD20, CD43 | N/A | N/A | 36 months follow-up, positive for recurrence | N/A | N/A | UK (Europe) | 2000 | Bates et al17 |
| 18 | 64 | F | Positive: CD20, CD45 Negative: CD43 Light chain restriction: inconclusive | Clonal heavy-chain IgH gene rearrangement (PCR) | Radiotherapy | 156 months follow-up, negative for recurrence | Present | Staphylococci, Streptococci, Escherichia coli, Diphtheroid bacillus | Canada (America) | 2001 | Al-Maghrabi et al6 |
| 19 | 69 | F | Positive: CD20, CD45 Negative: CD5, CD10, CD43, CD45RO Lambda light chain restriction | Clonal heavy-chain IgH gene rearrangement (PCR) | Radiotherapy | 60 months follow-up, negative for recurrence | Present | Escherichia coli | Canada (America) | 2001 | Al-Maghrabi et al6 |
| 20 | 72 | F | Positive: CD20, CD45 Negative: CD5, CD10, CD43, CD45RO Kappa light chain restriction | Inconclusive results of heavy-chain IgH gene rearrangement (PCR) | Radiotherapy | 36 months follow-up, negative for recurrence | Present | Escherichia coli | Canada (America) | 2001 | Al-Maghrabi et al6 |
| 21 | 62 | M | Positive: CD19, CD20, CD43 (focal) Negative: CD5, CD10, CD23, CD45RO Kappa light chain restriction | Clonal heavy-chain IgH gene rearrangement (PCR) | Radiotherapy | 24 months follow-up, negative for recurrence | Present | Staphylococcus aureus | Canada (America) | 2001 | Al-Maghrabi et al6 |
| 22 | 65 | F | Positive: CD20, CD79 Negative: IgD, CD43 | N/A | Chemotherapy (CHOP) | 36 months follow-up, negative for recurrence | Present | Coliform Bacteria | UK (Europe) | 2001 | Wazait et al20 |
| 23 | 70 | F | Positive: CD20, CD79 Negative: CD43 | N/A | Chemotherapy (Chlorambucil) | 60 months follow-up, negative for recurrence | N/A | N/A | UK (Europe) | 2001 | Wazait et al20 |
| 24 | 70 | F | Positive: CD20 Negative: CD43 | N/A | Chemotherapy and radiotherapy | 48 months follow-up, negative for recurrence | N/A | N/A | Chile (America) | 2001 | Painemal Duarte et al43 |
| 25 | 59 | M | Positive: CD20, CD79a Negative: CD3, CD5, CD10, CD23, CD43, CD45RO, BCL6, cyclin D1 | N/A | Antibiotics (First paper to use HP eradication therapy which works; H. Pilori test was positive) | 36 months follow-up, negative for recurrence | Absent | Negative | Netherland (Europe) | 2002 | van den Bosch et al7 |
| 26 | 57 | M | Positive: CD20 Negative: CD3, CD5, CD10, CD23, CD43, cyclin D1 Ki-67 - low | Negative for t(14;18) Trisomy of Chromosome 3 | Antibiotics treatment against H. Pilori (even H. Pilori test was negative) | 36 months follow-up, negative for recurrence | N/A | N/A | Germany (Europe) | 2002 | Krober et al11 |
| 27 | 78 | F | Positive: CD20, CD79 | Clonal heavy-chain IgH gene rearrangement (PCR) | Antibiotics (trimethoprim, nitrofurantoin, and cephradine) |
19 months follow-up, negative for recurrence | Present | Escherichia coli | UK (Europe) | 2002 | Oscier et al8 |
| 28 | 82 | F | N/A | N/A | Chemotherapy (ChlVP) | Died, negative for recurrence | N/A | N/A | UK (Europe) | 2005 | Hughes et al21 |
| 29 | 81 | F | N/A | N/A | Diathermy | 12 months follow-up, negative for recurrence | N/A | N/A | UK (Europe) | 2005 | Hughes et al21 |
| 30 | 28 | M | N/A | N/A | Chemotherapy (ChlVP) | 120 months follow-up, negative for recurrence | N/A | N/A | UK (Europe) | 2005 | Hughes et al21 |
| 31 | 76 | F | N/A | N/A | Radiotherapy | 24 months follow-up, negative for recurrence | N/A | N/A | UK (Europe) | 2005 | Hughes et al21 |
| 32 | 77 | M | N/A | N/A | Chemotherapy (ChlD) | 48 months follow-up, negative for recurrence | N/A | N/A | UK (Europe) | 2005 | Hughes et al21 |
| 33 | 66 | F | N/A | N/A | Radiotherapy | Died, negative for recurrence | Present | N/A | UK (Europe) | 2005 | Hughes et al21 |
| 34 | 85 | F | N/A | N/A | Radiotherapy | Negative for recurrence | N/A | N/A | Japan (Asia) | 2005 | Takahara et al22 |
| 35 | 84 | F | N/A | N/A | Chemotherapy (R-CHOP) | N/A | N/A | N/A | Japan (Asia) | 2006 | Kakuta et al41 |
| 36 | 84 | F | N/A | N/A | Radiotherapy | 14 months follow-up, negative for recurrence | Present | Escherichia coli | Japan (Asia) | 2007 | Hatano et al23 |
| 37 | 64 | F | Positive: CD20, BCL2 Negative: CD5, CD10, cyclin D1 Ki-67 - low | Clonal heavy-chain IgH gene rearrangement (PCR) | Transurethral resection (TUR) and radiotherapy | 19 months later recurred in stomach | Absent | Negative | Japan (Asia) | 2007 | Ueno et al24 |
| 38 | 69 | F | Positive: CD20, CD79a Negative: CD5, CD10 | N/A | Antibiotics | 25 months follow-up, negative for recurrence | Present | N/A | Japan (Asia) | 2008 | Fujimura et al9 |
| 39 | 64 | F | N/A | N/A | Radiotherapy | 14 months follow-up, negative for recurrence | Present | Escherichia coli | Japan (Asia) | 2008 | Terasaki et al42 |
| 40 | 31 | F | Ki-67 ~ 20–25% | N/A | Chemotherapy (CHOP) | N/A | N/A | N/A | UK (Europe) | 2010 | Sen et al25 |
| 41 | 88 | F | Positive: CD5, CD20, CD79a, CD45, BCL2, p53 Negative: CD10, CD15, CD23, CD30, CD34, CD43, CD56, cyclin D1, TdT Lambda light chain restriction Ki-67 ~ 50% | N/A | Antibiotics | 5 months follow-up, negative for recurrence | N/A | N/A | Japan (Asia) | 2011 | Terada et al10 |
| 42 | 65 | F | Positive: CD20, BCL2 Negative: CD3, CD5, CD10, cyclin D1, CD21, CD23, CD15, CD30 Ki-67 ~ 5–10% | N/A | Chemotherapy (R-CHOP) | Recur 12 months after treatment; died of septicemic shock secondary to a bladder abscess | Absent | N/A | Malaysia (Asia) | 2011 | Maninderpal et al26 |
| 43 | 17 | F | Positive: CD20, BCL2, CD45 Ki-67 ~ 25% | N/A | Transurethral resection (TUR) and chemotherapy | 24 months follow-up, negative for recurrence | N/A | N/A | Poland (Europe) | 2011 | Szopinski et al27 |
| 44 | 68 | F | Positive: CD20, CD79a Negative: CD3, CD5, CD10 Kappa light chain restriction | Clonal heavy-chain IgH gene rearrangement (PCR) | Chemotherapy (rituximab) | Negative for recurrence | Absent | N/A | Japan (Asia) | 2012 | Morita et al28 |
| 45 | 72 | F | N/A | N/A | Chemotherapy (rituximab) and radiotherapy | N/A | Present | N/A | Japan (Asia) | 2013 | Mizumo et al44 |
| 46 | 71 | F | Positive: CD20 Negative: CD3 | N/A | Transurethral resection (TUR) | N/A | Present | N/A | Japan (Asia) | 2013 | Takahashi et al31 |
| 47 | 48 | M | Positive: CD20, BCL2 Negative: CD5, CD23, CD43, cyclin D1 Ki-67 ~ 5% Kappa light chain restriction | N/A | Chemotherapy (R-CHOP), radiotherapy, antibiotics | N/A | Present | Escherichia coli | Croatia (Europe) | 2013 | Bacalja et al30 |
| 48 | 72 | F | Positive: CD20, CD79a, BCL2, IgD Negative: CD5, CD10, BCL6, cyclin D1 Ki-67 ~ 15% | Clonal heavy-chain IgH gene rearrangement (PCR); FISH - t(11;18)(q21;q21); Trisomy of Chromosome 3 and 18 | Antibiotics (ciprofloxacinfor 6 weeks) | 6 months follow-up, negative for recurrence | Present | Escherichia coli | Italy (Europe) | 2013 | Lucioni et al29 |
| 49 | 63 | F | Positive: CD20, BCL2 Negative: CD3, CD5, CD10, cyclin D1 Ki-67 ~ 20% Lambda light chain restriction | Clonal heavy-chain IgH gene rearrangement (PCR) | Radiotherapy | 11 months follow-up, negative for recurrence | Absent | Negative | Taiwan (Asia) | 2014 | Chen et al39 |
| 50 | 54 | M | Positive: CD20 Negative: CD3, CD10, BCL6, cyclin D1 |
N/A | Radiotherapy | 36 months follow-up, negative for recurrence | Absent | Negative | USA (America) | 2014 | Haddad-Lacle et al32 |
| 51 | 78 | F | Positive: CD20, CD79a, BCL2 Negative: CD3, CD10, CD23, cyclin D1 Ki-67 ~ 20% Kappa light chain restriction | N/A | Chemotherapy (rituximab) | N/A | Present | Escherichia coli | Japan (Asia) | 2014 | Matsuda et al33 |
| 52 | 76 | F | Positive: CD20, BCL2 Negative: CD3, CD10 |
N/A | Radiotherapy | 3 months follow-up, negative for recurrence | N/A | N/A | Taiwan (Asia) | 2015 | Hsu et al35 |
| 53 | 65 | F | Positive: CD20, PAX5 Negative: CD5, CD10 |
N/A | A transurethral resection of the bladder tumor (TURBT) and radiotherapy | 3 months follow-up, negative for recurrence | N/A | N/A | USA (America) | 2015 | Vempati et al34 |
| 54 | 53 | F | Positive: CD20, CD45 Negative: CD3, CD5, CD10, BCL2 |
N/A | Chemotherapy (R-CHOP) | 9 months follow-up, negative for recurrence | Present | Negative | India (Asia) | 2016 | Jitani et al5 |
| 55 | 72 | F | N/A | N/A | Transurethral resection of bladder tumors (TURBT) | 13 months follow-up, negative for recurrence | N/A | N/A | Japan (Asia) | 2018 | Ozawa et al45 |
| 56 | 77 | F | Positive: CD20, CD79a, BCL2 | N/A | Radiotherapy | 60 months follow-up, negative for recurrence | Present | Escherichia coli | Japan (Asia) | 2018 | Isono et al36 |
| 57 | 74 | F | Positive: CD20 | N/A | Radiotherapy | N/A | Present | Negative | Singapore (Asia) | 2019 | Kadam et al37 |
| 58 | 77 | F | Positive: CD20, PAX5, BCL2, CD21 Negative: CD10, MUM1, TDT, cyclin D1 |
N/A | Transurethral resection of bladder tumors (TURBT) | The patient was alive and healthy at the 15-month follow-up | N/A | N/A | China (Asia) | 2020 | Xu et al38 |
| 59 | 58 | F | Positive: CD20, CD79a, BCL2 Negative: CD5, CD10 |
N/A | Chemotherapy (rituximab) | 120 months follow-up, negative for recurrence | Absent | N/A | USA (America) | 2020 | Lyapichev et al. |
Abbreviations: F, female; M, male; y.o., years old; N/A, not available or provided not in English; CHOP, cyclophosphamide, doxorubicin, vincristine and prednisolone; R-CHOP, rituximab with CHOP; ChlVP, Chl, chlorambucil; V, vincristine; P, prednisolone; ChlD, Chl, chlorambucil; D, dexamethasone; FISH, fluorescent in situ hybridization; PCR, polymerase chain reaction.
Only one case of MALT lymphoma with CD5 expression was reported.10 The clonality for heavy-chain IgH gene rearrangement by PCR was studied in 10 cases and was found to be clonally rearranged.6,8,16,24,28,29,39
Cytogenetic studies were reported in two cases.11,29 Krober and colleagues found that the first case was negative for t(14:18) and positive for trisomy3, while second case showed translocation t(11;18)(q21;q21), trisomy 3, and trisomy 18.29
Treatment approaches were variable including surgical excision, antibiotics, chemotherapy, radiation, or combined modality. Six patients were successfully treated with antibiotics.7–9,11,29 Eight patients had some surgical procedures with or without consequent radiotherapy and/or chemotherapy.3,16,24,27,31,34,38,45 Hughes and colleagues reported one patient who was successfully treated by diathermy.21 Majority of the patients (24) were treated with chemotherapy either alone (16) or in combination with radiation (8) (Table 1). Three cases did not have any information about therapeutic approach.17 Overall the outcome of the treatment was good with median follow-up time of 64.5 months (from 3 to 156 months).
It is important to acknowledge the limitations of our review. It is possible that some reports did not specify the urinary bladder as an involvement site, so we cannot exclude the possibility of missing a significant number of cases. This is a retrospective study, and patients have not been studied and treated uniformly; therefore, it is impossible to draw a definitive conclusion regarding the pathogenesis of MALT lymphoma of the urinary bladder.
The patient we report has a history of rheumatoid arthritis (RA). The relationship between RA and MALT lymphoma in this patient remains unclear. An increased risk of malignant lymphoma has been reported in patients with RA;46,47 however, patients with RA usually develop diffuse large B-cell lymphoma.48 While MALT lymphoma is common in patients suffering from primary Sjogren syndrome,49 MALT lymphoma in patients with RA are exceedingly rare; we identified only 11 single case reports in the literature.50–60
Conclusion
In summary, MALT lymphoma of the urinary bladder is a rare low-grade extranodal B-cell lymphoma which predominantly affects elderly women of Asian origin. The disease often presents with nonspecific symptoms and is strongly associated with cystitis.14,34 The prognosis is generally excellent. Tissue biopsy with immunohistochemistry is crucial to reach the final diagnosis. A prominent female predominance, uneven geographic distribution of the cases, dramatic prevalence of cystitis among affected patients, and a success of antibacterial therapy in selected cases suggest the role of urinary tract infection, particularly E. coli, in the pathogenesis of urinary bladder MALT lymphoma.
Ethics Approval and Informed Consent
The patient reported in the manuscript signed the informed consent/authorization for participation in research (MD Anderson Cancer Center protocol LAB01-473) which includes the permission to use data collected in future research projects including presented case details and images used in this manuscript. A copy of the signed consent is kept on file in the patient electronic records.
Disclosure
The authors declare no conflicts of interest for this work and that there are no conflicts of interest regarding the publication of this article.
References
- 1.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The non-Hodgkin’s lymphoma classification project. Blood. 1997;89(11):3909–3918. [PubMed] [Google Scholar]
- 2.Khalil MO, Morton LM, Devesa SS, et al. Incidence of marginal zone lymphoma in the United States, 2001–2009 with a focus on primary anatomic site. Br J Haematol. 2014;165(1):67–77. doi: 10.1111/bjh.12730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuhara H, Tamura Z, Suchi T, Hattori R, Kinukawa T. Primary malignant lymphoma of the urinary bladder. A case report. Acta Pathol Jpn. 1990;40(10):764–769. doi: 10.1111/j.1440-1827.1990.tb01541.x [DOI] [PubMed] [Google Scholar]
- 4.Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer. 1972;29(1):252–260. [DOI] [PubMed] [Google Scholar]
- 5.Jitani AK, Mishra J, Sailo SL, Raphael V. Primary urinary bladder mucosa associated lymphoid tissue type lymphoma presenting as a close mimic for genitourinary tuberculosis: case report and review of literature. Urol Ann. 2016;8(1):108–110. doi: 10.4103/0974-7796.171491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Maghrabi J, Kamel-Reid S, Jewett M, Gospodarowicz M, Wells W, Banerjee D. Primary low-grade B-cell lymphoma of mucosa-associated lymphoid tissue type arising in the urinary bladder: report of 4 cases with molecular genetic analysis. Arch Pathol Lab Med. 2001;125(3):332–336. doi: [DOI] [PubMed] [Google Scholar]
- 7.van den Bosch J, Kropman RF, Blok P, Wijermans PW. Disappearance of a mucosa-associated lymphoid tissue (MALT) lymphoma of the urinary bladder after treatment for Helicobacter pylori. Eur J Haematol. 2002;68(3):187–188. doi: 10.1034/j.1600-0609.2002.01649.x [DOI] [PubMed] [Google Scholar]
- 8.Oscier D, Bramble J, Hodges E, Wright D. Regression of mucosa-associated lymphoid tissue lymphoma of the bladder after antibiotic therapy. J Clin Oncol. 2002;20(3):882. doi: 10.1200/JCO.2002.20.3.882 [DOI] [PubMed] [Google Scholar]
- 9.Fujimura M, Chin K, Sekita N, et al. [Regression of mucosa-associated lymphoid tissue lymphoma of the bladder after antibiotic therapy: a case report]. Hinyokika Kiyo. 2008;54(12):783–786. Japanese. [PubMed] [Google Scholar]
- 10.Terada T. Primary CD5-positive mucosa-associated lymphoid tissue lymphoma of the urinary bladder. Ann Diagn Pathol. 2011;15(5):382–384. doi: 10.1016/j.anndiagpath.2011.02.006 [DOI] [PubMed] [Google Scholar]
- 11.Krober SM, Aepinus C, Ruck P, Muller-Hermelink HK, Horny HP, Kaiserling E. Extranodal marginal zone B cell lymphoma of MALT type involving the mucosa of both the urinary bladder and stomach. J Clin Pathol. 2002;55(7):554–557. doi: 10.1136/jcp.55.7.554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pawade J, Banerjee SS, Harris M, Isaacson P, Wright D. Lymphomas of mucosa-associated lymphoid tissue arising in the urinary bladder. Histopathology. 1993;23(2):147–151. doi: 10.1111/j.1365-2559.1993.tb00472.x [DOI] [PubMed] [Google Scholar]
- 13.Fernandez Acenero MJ, Martin Rodilla C, Lopez Garcia-Asenjo J, Coca Menchero S, Sanz Esponera J. Primary malignant lymphoma of the bladder. Report of three cases. Pathol Res Pract. 1996;192(2):160–165. doi: 10.1016/S0344-0338(96)80211-1 [DOI] [PubMed] [Google Scholar]
- 14.Kempton CL, Kurtin PJ, Inwards DJ, Wollan P, Bostwick DG. Malignant lymphoma of the bladder: evidence from 36 cases that low-grade lymphoma of the MALT-type is the most common primary bladder lymphoma. Am J Surg Pathol. 1997;21(11):1324–1333. doi: 10.1097/00000478-199711000-00007 [DOI] [PubMed] [Google Scholar]
- 15.Gallardo J, Gamargo C, Fodor M, Comparini B, Salman P, Yanez M. [MALT lymphoma of the bladder: report of a case]. Rev Med Chil. 1998;126(2):199–201. Spanish. [PubMed] [Google Scholar]
- 16.Ando K, Matsuno Y, Kanai Y, et al. Primary low-grade lymphoma of mucosa-associated lymphoid tissue of the urinary bladder: a case report with special reference to the use of ancillary diagnostic studies. Jpn J Clin Oncol. 1999;29(12):636–639. doi: 10.1093/jjco/29.12.636 [DOI] [PubMed] [Google Scholar]
- 17.Bates AW, Norton AJ, Baithun SI. Malignant lymphoma of the urinary bladder: a clinicopathological study of 11 cases. J Clin Pathol. 2000;53(6):458–461. doi: 10.1136/jcp.53.6.458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawakami K, Oka K, Kato M, Shiku H. Whole-bladder irradiation and doxorubicin-containing chemotherapy as successful treatment for a primary mucosa-associated lymphoid tissue lymphoma of the bladder. Int J Hematol. 2000;72(3):346–348. [PubMed] [Google Scholar]
- 19.Tasu JP, Geffroy D, Rocher L, et al. Primary malignant lymphoma of the urinary bladder: report of three cases and review of the literature. Eur Radiol. 2000;10(8):1261–1264. doi: 10.1007/s003300000343 [DOI] [PubMed] [Google Scholar]
- 20.Wazait HD, Chahal R, Sundurum SK, Rajkumar GN, Wright D, Aslam MM. MALT-type primary lymphoma of the urinary bladder: clinicopathological study of 2 cases and review of the literature. Urol Int. 2001;66(4):220–224. doi: 10.1159/000056619 [DOI] [PubMed] [Google Scholar]
- 21.Hughes M, Morrison A, Jackson R. Primary bladder lymphoma: management and outcome of 12 patients with a review of the literature. Leuk Lymphoma. 2005;46(6):873–877. doi: 10.1080/10428190500079829 [DOI] [PubMed] [Google Scholar]
- 22.Takahara Y, Kawashima H, Han YS, et al. [Primary mucosa-associated lymphoid tissue (MALT) lymphoma of the urinary bladder]. Hinyokika Kiyo. 2005;51(1):45–48. Japanese. [PubMed] [Google Scholar]
- 23.Hatano K, Sato M, Tsujimoto Y, et al. [Primary mucosa-associated lymphoid tissue (MALT) lymphoma of the urinary bladder associated with left renal pelvic carcinoma: a case report]. Hinyokika Kiyo. 2007;53(1):57–60. Japanese. [PubMed] [Google Scholar]
- 24.Ueno Y, Sakai H, Tsuruta T, Wajiki M. Mucosa-associated lymphoma of the bladder with relapse in the stomach after successful local treatment. Hinyokika Kiyo. 2007;53(8):575–579. [PubMed] [Google Scholar]
- 25.Sen S, Macaulay JH, Allford SL. A case of cerebral arteriovenous malformation in pregnancy associated with MALT lymphoma. J Obstet Gynaecol. 2010;30(3):308–310. doi: 10.3109/01443610903585234 [DOI] [PubMed] [Google Scholar]
- 26.Maninderpal KG, Amir FH, Azad HA, Mun KS. Imaging findings of a primary bladder maltoma. Br J Radiol. 2011;84(1005):e186–e190. doi: 10.1259/bjr/66130737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szopinski TR, Sudol-Szopinska I, Dzik T, Borowka A, Dembowska-Baginska B, Perek D. Incidental sonographic detection of mucosa-associated lymphoid tissue lymphoma of the urinary bladder found in a very young woman: report of a case. J Clin Ultrasound. 2011;39(4):233–235. doi: 10.1002/jcu.20786 [DOI] [PubMed] [Google Scholar]
- 28.Morita K, Nakamura F, Nannya Y, et al. Primary MALT lymphoma of the urinary bladder in the background of interstitial cystitis. Ann Hematol. 2012;91(9):1505–1506. doi: 10.1007/s00277-012-1419-0 [DOI] [PubMed] [Google Scholar]
- 29.Lucioni M, Nicola M, Riboni R, et al. Antibiotic therapy-induced remission of bladder mucosa-associated lymphoid tissue (MALT) lymphoma carrying t(11;18)(q21;q21) apoptosis inhibitor 2-MALT1. J Clin Oncol. 2013;31(19):e304–e306. doi: 10.1200/JCO.2012.46.4800 [DOI] [PubMed] [Google Scholar]
- 30.Bacalja J, Ulamec M, Rako D, et al. Persistence of primary MALT lymphoma of the urinary bladder after rituximab with CHOP chemotherapy and radiotherapy. In Vivo (Brooklyn). 2013;27(4):545–549. [PubMed] [Google Scholar]
- 31.Takahashi H, Shimazaki H, Oda T, et al. Malignant lymphoma case with urinary cytology mimicking that of urothelial carcinoma. Cytopathology. 2013;24(6):412–414. doi: 10.1111/cyt.12026 [DOI] [PubMed] [Google Scholar]
- 32.Haddad-Lacle JE, Haddad CJ, Villas B. A rare urinary bladder tumour. BMJ Case Rep. 2014;2014:bcr2013202994–bcr2013202994. doi: 10.1136/bcr-2013-202994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuda I, Zozumi M, Tsuchida YA, et al. Primary extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue type with malakoplakia in the urinary bladder: a case report. Int J Clin Exp Pathol. 2014;7(8):5280–5284. [PMC free article] [PubMed] [Google Scholar]
- 34.Vempati P, Knoll MA, Alqatari M, Strauchen J, Malone AK, Bakst RL. MALT lymphoma of the bladder: a case report and review of the literature. Case Rep Hematol. 2015;2015:934374. doi: 10.1155/2015/934374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsu JS, Lin CC, Chen YT, Lee YC. Primary mucosa-associated lymphoid tissue lymphoma of the urinary bladder. Kaohsiung J Med Sci. 2015;31(7):388–389. doi: 10.1016/j.kjms.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 36.Isono M, Sato A, Kimura F, Asano T. A case of mucosa-associated lymphoid tissue lymphoma of the bladder successfully treated with radiotherapy. Urol Case Rep. 2018;16:1–3. doi: 10.1016/j.eucr.2017.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kadam PD, Han HC, Kwok JL. An uncommon case of mucosa-associated lymphoid tissue (MALT) tumor of the bladder. Int Urogynecol J. 2019;30(6):1017–1018. doi: 10.1007/s00192-018-3813-1 [DOI] [PubMed] [Google Scholar]
- 38.Xu H, Chen Z, Shen B, Wei Z. Primary bladder mucosa-associated lymphoid tissue lymphoma: a case report and literature review. Medicine (Baltimore). 2020;99(28):e20825. doi: 10.1097/MD.0000000000020825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen YR, Hung LY, Chang KC. Mucosa-associated lymphoid tissue-type lymphoma presenting as a urethral caruncle with urinary bladder involvement. Int J Urol. 2014;21(10):1073–1074. doi: 10.1111/iju.12507 [DOI] [PubMed] [Google Scholar]
- 40.Yuille FA, Angus B, Roberts JT, Vadanan BS. Low grade MALT lymphoma of the urinary bladder. Clin Oncol (R Coll Radiol). 1998;10(4):265–266. doi: 10.1016/S0936-6555(98)80016-2 [DOI] [PubMed] [Google Scholar]
- 41.Kakuta Y, Katoh T, Saitoh J, Yazawa K, Hosomi M, Itoh K. [A case of primary mucosa-associated lymphoid tissue lymphoma of the bladder regressed after rituximab in combination with CHOP chemotherapy]. Hinyokika Kiyo. 2006;52(12):951–954. Japanese. [PubMed] [Google Scholar]
- 42.Terasaki Y, Okumura H, Ishiura Y, et al. [Primary mucosa-associated lymphoid tissue lymphoma of the urinary bladder successfully treated by radiotherapy and rituximab]. Rinsho Ketsueki. 2008;49(1):30–34. Japanese. [PubMed] [Google Scholar]
- 43.Painemal Duarte C, Gallardo J, Valdebenito JP, Gamargo C, Rubio B, Harbst H. [MALT lymphoma of the bladder. Report of a case]. Arch Esp Urol. 2001;54(10):1138–1140. Spanish. [PubMed] [Google Scholar]
- 44.Mizuno K, Nakanishi S, Sakatani T, et al. [A case of primary mucosa-associated lymphoid tissue-type lymphoma of the urinary bladder that progressed after antibiotic therapy]. Hinyokika Kiyo. 2013;59(4):239–242. Japanese. [PubMed] [Google Scholar]
- 45.Ozawa M, Suenaga S, Ishii T, Suzuki H, Tsuchiya N, Ohtake H. [Primary malignant lymphoma of the bladder diagnosed by transurethral bladder tumor resection: a case report]. Nihon Hinyokika Gakkai Zasshi. 2018;109(1):45–49. Japanese. doi: 10.5980/jpnjurol.109.45 [DOI] [PubMed] [Google Scholar]
- 46.Baecklund E, Askling J, Rosenquist R, Ekbom A, Klareskog L. Rheumatoid arthritis and malignant lymphomas. Curr Opin Rheumatol. 2004;16(3):254–261. doi: 10.1097/00002281-200405000-00014 [DOI] [PubMed] [Google Scholar]
- 47.Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch Intern Med. 2005;165(20):2337–2344. doi: 10.1001/archinte.165.20.2337 [DOI] [PubMed] [Google Scholar]
- 48.Baecklund E, Sundstrom C, Ekbom A, et al. Lymphoma subtypes in patients with rheumatoid arthritis: increased proportion of diffuse large B cell lymphoma. Arthritis Rheum. 2003;48(6):1543–1550. doi: 10.1002/art.11144 [DOI] [PubMed] [Google Scholar]
- 49.Kauppi M, Pukkala E, Isomaki H. Elevated incidence of hematologic malignancies in patients with Sjogren’s syndrome compared with patients with rheumatoid arthritis (Finland). Cancer Causes Control. 1997;8(2):201–204. doi: 10.1023/A:1018472213872 [DOI] [PubMed] [Google Scholar]
- 50.Magnoli F, Cimetti L, Bernasconi B, et al. Primary extranodal marginal cell lymphoma, MALT type, of the endometrium arising in a patient with rheumatoid arthritis: report of a case. Int J Gynecol Pathol. 2016;35(4):327–332. doi: 10.1097/PGP.0000000000000244 [DOI] [PubMed] [Google Scholar]
- 51.Kobayashi Y, Kimura K, Fujitsu Y, Shinkawa K, Muta H, Sonoda KH. Methotrexate-associated orbital lymphoproliferative disorder in a patient with rheumatoid arthritis: a case report. Jpn J Ophthalmol. 2016;60(3):212–218. doi: 10.1007/s10384-016-0439-z [DOI] [PubMed] [Google Scholar]
- 52.Gonzalez-Murillo EA, Castro-Rodriguez A, Sanchez-Venegas JC, Pena-Ruelas CI. Subglottic MALT lymphoma of the larynx in a patient with rheumatoid arthritis. Acta Otorrinolaringol Esp. 2014;65(5):317–319. doi: 10.1016/j.otoeng.2013.03.001 [DOI] [PubMed] [Google Scholar]
- 53.Go H, Cho HJ, Paik JH, et al. Thymic extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue: a clinicopathological and genetic analysis of six cases. Leuk Lymphoma. 2011;52(12):2276–2283. doi: 10.3109/10428194.2011.596968 [DOI] [PubMed] [Google Scholar]
- 54.Mikolaenko I, Listinsky CM. Systemic CD5+ MALT lymphoma: presentation with Waldenstrom syndrome. Ann Diagn Pathol. 2009;13(4):272–277. doi: 10.1016/j.anndiagpath.2008.04.010 [DOI] [PubMed] [Google Scholar]
- 55.Sordet C, Mrabet D, Ardizzone M, Marcellin L, Hirschorn P, Sibilia J. A centrofacial B lymphoma in a rheumatoid arthritis patient. Eur J Intern Med. 2007;18(1):71–73. doi: 10.1016/j.ejim.2006.04.019 [DOI] [PubMed] [Google Scholar]
- 56.Douglas KM, Raza K, Stevens R, Erb N, Jones EL, Kitas GD. Bronchial MALT lymphoma in longstanding rheumatoid arthritis. Rheumatology (Oxford). 2005;44(5):687–689. doi: 10.1093/rheumatology/keh545 [DOI] [PubMed] [Google Scholar]
- 57.Kim JM. Primary extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue-type in the thymus of a patient with Sjogren’s syndrome and rheumatoid arthritis. J Korean Med Sci. 2003;18(6):897–900. doi: 10.3346/jkms.2003.18.6.897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sutcliffe N, Smith C, Speight PM, Isenberg DA. Mucosa-associated lymphoid tissue lymphomas in two patients with rheumatoid arthritis on second-line agents, and secondary Sjogren’s syndrome. Rheumatology (Oxford). 2000;39(2):185–188. doi: 10.1093/rheumatology/39.2.185 [DOI] [PubMed] [Google Scholar]
- 59.Yokose T, Kodama T, Matsuno Y, Shimosato Y, Nishimura M, Mukai K. Low-grade B cell lymphoma of mucosa-associated lymphoid tissue in the thymus of a patient with rheumatoid arthritis. Pathol Int. 1998;48(1):74–81. doi: 10.1111/j.1440-1827.1998.tb03832.x [DOI] [PubMed] [Google Scholar]
- 60.Serefhanoglu S, Tapan U, Ertenli I, Kalyoncu U, Uner A. Primary thyroid marginal zone B-cell lymphoma MALT-type in a patient with rheumatoid arthritis. Med Oncol. 2010;27(3):826–832. doi: 10.1007/s12032-009-9293-x [DOI] [PubMed] [Google Scholar]