Abstract
Purpose
To assess the rate of bilateral sentinel lymph node (SLN) detection with indocyanine green (ICG), to evaluate the sensitivity and the negative predictive value of cervical cancer patients undergoing open radical hysterectomy; to compare open versus minimally invasive SLN biopsy performance and to assess factors related to no/unilateral SLN mapping.
Methods
We retrospectively reviewed consecutive patients with FIGO 2018 stage IA1 with lymph-vascular space involvement to IIB and IIIC1p cervical carcinoma who underwent SLN mapping with ICG followed by systematic pelvic lymphadenectomy between 05/2017 and 06/2020. Patients were divided according to surgical approach for statistical analysis.
Results
Eighty-five patients met inclusion criteria. Twenty-seven (31.8%) underwent open and 58 (68.2%) underwent minimally invasive SLN mapping. No difference in any SLN mapping (laparotomy 92.6% and minimally invasive 91.4%) or in SLN bilateral detection (laparotomy 72.0% and minimally invasive 84.9%) (p = 0.850 and p = 0.222, respectively), in median number of SLNs mapped and retrieved (2 in both groups, p = 0.165) and in site of SLN mapping per hemi-pelvis (right side, p = 0273 and left side, p = 0.618) was evident between open and minimally invasive approach. Per-patient sensitivity of SLN biopsy in laparotomy was 83.3% (95% CI 35.9–99.6%) and the negative predictive value was 95.0% (95% CI 76.0–99.1%). No difference in per-patient sensitivity was noted between two approaches (p = 0.300). None of the analyzed variables was associated with no/unilateral SLN mapping.
Conclusion
The use of ICG to detect SLN in cervical cancer treated with open surgery allows a bilateral detection, sensitivity and negative predictive value comparable to minimally invasive surgery with potential advantages of ICG compared to other tracers.
Electronic supplementary material
The online version of this article (10.1007/s00432-020-03393-6) contains supplementary material, which is available to authorized users.
Keywords: Cervical cancer, Sentinel lymph node, Laparotomy, Minimally invasive surgery, Indocyanine green, Detection rate
Introduction
Despite the introduction of screening and vaccination has significantly reduced the incidence of cervical cancer in developed countries (Brisson 2020; Wright 2015), this tumor still represents the fourth most common cancer worldwide, with 569,847 new cases and 311,365 deaths in 2018 (Arbyn 2020). Treatment of early-stage disease (clinical International Federation of Gynecology and Obstetrics (FIGO) stage IA1-IB1 and IIA1) is represented by radical hysterectomy and pelvic lymphadenectomy with sentinel lymph node (SLN) biopsy (Cibula 2018). After the publication of a randomized controlled trial, which demonstrated an inferior survival outcome if patients with early-stage cervical cancer were treated with minimally invasive, compared to open radical hysterectomy (Ramirez 2018), multiple retrospective studies showed a survival difference between the two approaches, with consequent recommendations amendment by international societies (Koh 2019; Nitecki 2020; Querleu 2020), particularly for tumors larger than 2 cm (Pedone Anchora 2020).
SLN biopsy has been largely studied in cervical cancer and multiple evidences support its use in early-stage disease treated with primary surgery (Cibula 2018,2019); also, few studies reported the use of SLN biopsy after neo-adjuvant chemotherapy (NACT) (Slama 2012; Robova 2014). Different studies reported the adoption of SLN in open radical surgery with blue dye or radioactive tracer (Silva 2005; Wydra 2006; Altgassen 2007; Kara 2008; Dostalek 2020), but experience on the use of indocyanine green (ICG) in open surgery, is limited to few case reports or case series (Furukawa 2010; Crane 2011; van der Vorst 2011; Schaafsma 2012; Buda 2016; Rychlik 2016). On the other hand, it seems well established that ICG has been showed to be superior to blue dye or radioactive tracer in SLN bilateral detection rate in minimally invasive surgery (Di Martino 2017).
The primary objective of the present study was to assess the rate of bilateral SLN detection with ICG in cervical cancer patients undergoing open radical hysterectomy. Secondary objectives were to evaluate the sensitivity and the negative predictive value of open ICG SLN biopsy, to compare open versus minimally invasive SLN biopsy performance and to assess factors related to no/unilateral SLN mapping.
Methods
Inclusion and exclusion criteria
The present retrospective observational cohort study was approved by Institutional Review Board (Dipartimento per la salute della Donna e del Bambino e della Salute Pubblica, number DIPUSVSP-26-05-2059, date 26/05/2020). After signing consent form, consecutive patients who underwent surgery at Fondazione Policlinico A. Gemelli IRCCS (Rome, Italy), for International Federation of Gynecology and Obstetrics (FIGO) 2018 (Bhatla 2018) stage IA1 with lymph-vascular space involvement (LVSI) to IIB and IIIC1p cervical carcinoma between 05/2017 and 06/2020 and had SLN mapping attempt with systematic pelvic lymphadenectomy, were included. Data were retrieved from institution’s electronic database. All patients had pre-operative histology confirmation of cervical cancer and underwent pre-operative abdominal Magnetic Resonance Imaging (MRI) scan, pelvic ultrasound scan and chest X-ray. Patients with FIGO stage > IB1 underwent whole body positron emission tomography (PET)/computed tomography (CT) scan to exclude distant metastasis. Radicality of hysterectomy was classified according to Querleu-Morrow classification (Querleu 2017). Examination under anesthesia was performed at the same time of the surgery. Only women with no evidence of enlarged (i.e., short axis > 10 mm) pelvic and para-aortic lymph nodes at pre-operative MRI-scan were submitted to SLN mapping. Patients with pre-invasive cervical disease, who did not undergo systematic pelvic lymphadenectomy, with special histology sub-types (other than squamous cell carcinoma—SCC, adenocarcinoma or adenosquamous carcinoma), allergy to iodine or patients with SLN analyzed with standard H&E only, were excluded.
Surgical approach was performed by minimally invasive surgery (laparoscopic or robotic) at surgeon’s discretion until October 2018 (Ramirez 2018) and for tumors < 2 cm in compliance with European Society of Gynaecological Oncology (ESGO) statement and National Comprehensive Cancer Network (NCCN) guidelines, using protecting maneuvers (no uterine manipulator and vaginal cuff formation in patients with tumor completely excised at pre-operative conization), after that date (Koh 2019; Querleu 2020). Laparotomy approach was performed at surgeon’s discretion until October 2018 (Ramirez 2018) and for tumors ≥ 2 cm after that date. Uterine surgery involved radical hysterectomy in patients who did not wish to retain fertility; and cervical conization or cervical biopsy followed by NACT [as part of a previously described clinical trial, NCT02323841—in press Fertility and Sterility (ClinicalTrials.gov Identifier: NCT02323841) (Clinicaltrial 2014)], in patients who desired to preserve fertility with tumor diameter ≤ 2 cm and > 2 cm, respectively. Patients with clinical diagnosis of FIGO stage IIB, underwent NACT followed by radical hysterectomy. All patients undergoing open surgery were treated with radical hysterectomy.
FIGO stage refers to pathologic stage for patients who underwent primary surgery and to clinical stage at diagnosis for patients undergoing NACT.
Sentinel lymph node assessment
Technique and method for SLN mapping was the same in all cases. SLN was detected after 1 ml superficial and deep cervical injections of ICG (diluted with sterile water at 1.25 mg/ml) at 3 and 9 o’clock. About 15 min after cervical injection, pelvic retroperitoneal space was opened, and lymph nodes were assessed with near infra-red (NIR) camera: Visera Elite II (Olympus, Tokyo, Japan) and Pinpoint (Stryker, Kalamazoo, Michigan, US) in case of laparoscopic or Da Vinci Xi (Intuitive, Sunnyvale, California, US) in case of robotic approach; SLN in open surgery was detected with SPY Portable Handheld Imager (SPY-PHI) camber (Stryker, Kalamazoo, Michigan, US). SLN was defined as the ICG-positive pelvic node closest to the tumor; retroperitoneal spaces were explored with the following order to assess SLN presence: external iliac, interiliac, obturator, common iliac, parametrial and pre-sacral and low para-aortic area (Marnitz 2006; Balaya 2019). All mapped SLNs were removed and analyzed with ultrastaging (Cibula 2012) or with one-step nucleic acid amplification (OSNA) (Bizzarri 2020).
Ultrastaging procedure was performed as follows: hematoxylin and Eosin (H&E) negative nodes were entirely sectioned at 150 μm intervals, until exhaustion of the lymph node, according to the length of the long axis. Each level was examined by H&E and AE1/AE3. Low volume disease was defined by the American Joint Committee on Cancer (AJCC) (Schwartz 2002): macro-metastases were defined as cancer deposits larger than 2.0 mm; micro-metastases were defined as deposits between 0.2 and 2.0 mm; and isolated tumor cells were defined as deposits no greater than 0.2 mm, including the presence of single non-cohesive cytokeratin-positive cancer cells.
OSNA analysis of SLNs was performed as per previously described protocol.
Systematic bilateral pelvic lymphadenectomy was performed in all patients, regardless SLN mapping. Non-SLNs were examined with routine H&E staining.
Statistical analysis
Standard descriptive statistics were used to evaluate the distribution of each variable. Continuous variables were reported as median and categorical variables as frequency and percentage. Comparison of each variable between the groups of patients have been performed with t test for continuous variables and Chi-square or Fisher’s exact test for categorical variables. Sensitivity, negative predictive value, and false-negative rates were calculated per patient and per hemi-pelvis. We considered findings to be false negative when lymphatic mapping showed drainage to one or more SLNs in a hemi-pelvis, biopsy of the SLN(s) revealed no metastases, and the patient had at least one metastatic non-SLN. A positive non-SLN in a hemi-pelvis with no SLN identified on lymphatic mapping was not considered to indicate a false-negative finding as our practice was to perform a complete pelvic lymphadenectomy in any case. All p values reported were two sided and a p value < 0.05 was considered statistically significant. Analysis was computed using SPSS version 26.0 (IBM Corporation 2018, Armonk, NY: IBM Corp.).
Results
Entire cohort characteristics
Eighty-five patients met inclusion criteria and underwent ICG injection to detect SLN. Twenty-seven (31.8%) underwent open and 58 (68.2%) underwent minimally invasive SLN mapping. Of these, 43 (50.6%) underwent laparoscopic and 15 (17.6%) robotic approach.
Table 1 shows characteristics of the entire cohort. Nine (10.6%) patients were submitted to NACT before radical hysterectomy. Most of patients had SCC histology (56.5%), grade 2 (51.8%), maximum tumor diameter < 4 cm (56.5%) and underwent type B or C radical hysterectomy (82.4%). Overall, 63 (74.1%) patients had bilateral SLN mapping, while 15 (17.6%) and 7 (8.2%) had unilateral and no mapping, respectively. One-hundred and seventy-six SLNs were detected and retrieved. Median number of SLNs removed was 2 (range, 1–5) per patient. The most frequent site of SLN mapping was external iliac in 76 (58.5%), followed by obturator in 38 (29.2%), internal iliac in 7 (5.4%) and common iliac in 5 (3.8%) cases. Two (1.5%) and two (1.5%) patients had para-aortic and pre-sacral mapping, respectively, but none of these was isolated. No patient presented allergic reaction to ICG in the entire cohort.
Table 1.
Patients’ characteristics
| Characteristic | N = 85, median (range, %) |
|---|---|
| Age (years) | 44 (27–80) |
| BMI (kg/m2) | 23.0 (17.3–36.0) |
| Neo-adjuvant chemotherapy | |
| No | 76 (89.4) |
| Yes | 9 (10.6) |
| Pre-operative conization | |
| No | 58 (68.2) |
| Yes | 27 (31.8) |
| Approach | |
| Laparoscopy | 43 (50.6) |
| Robot | 15 (17.6) |
| Laparotomy | 27 (31.8) |
| Type of surgery on uterus/cervix at time of SLN biopsy | |
| Conization | 7 (8.2) |
| Biopsy only | 2 (2.4) |
| Type A RH | 6 (7.1) |
| Type B RH | 31 (36.5) |
| Type C RH | 39 (45.9) |
| SLN mapping | |
| No | 7 (8.2) |
| Unilateral | 15 (17.6) |
| Bilateral | 63 (74.1) |
| SLN analysis | |
| No mapping | 7 (8.2) |
| Ultra staging | 49 (57.6) |
| OSNA | 29 (34.1) |
| Median number SLN | 2 (1–5) |
| Histology | |
| SCC | 48 (56.5) |
| Adenocarcinoma | 34 (40.0) |
| Adenosquamous | 3 (3.5) |
| Grade | |
| 1 | 9 (10.6) |
| 2 | 44 (51.8) |
| 3 | 21 (87.1) |
| Unknown | 11 (12.9) |
| LVSI | |
| Negative | 45 (52.9) |
| Positive | 35 (41.2) |
| Unknown | 5 (5.9) |
| Depth of stromal infiltration | 5 (0.5–25) |
| Maximum tumor diameter (mm) | 20 (1–65) |
| Tumor diameter | |
| ≤ 20 mm | 45 (52.9) |
| > 20 mm | 40 (47.1) |
| FIGO stage 2018 | |
| IA1 | 7 (8.2) |
| IA2 | 4 (4.7) |
| IB1 | 19 (22.4) |
| IB2 | 20 (23.5) |
| IB3 | 6 (7.1) |
| IIA1 | 4 (4.7) |
| IIB | 5 (5.9) |
| IIIC1p | 20 (23.5) |
| Number or removed lymph nodes | 18 (5–86) |
| SLN largest dimension metastasis | |
| No mapping | 7 (8.2) |
| No | 60 (70.6) |
| ITC | 3 (3.5) |
| Micro | 13 (15.3) |
| Macro | 2 (2.4) |
BMI body mass index, SLN sentinel lymph node, OSNA one-step nucleic acid amplification, SCC squamous cell carcinoma, LVSI lymph-vascular space involvement, FIGO international federation of gynecology and obstetrics
Overall, 20 (23.5%) patients had metastatic lymph nodes, and 18 (21.2%) had metastatic SLN. Of these, 3 (3.5%) had ITCs, 13 (15.3%) had micro- and 2 (2.4%) had macro-metastases.
In the entire cohort, when calculated per-patient, the sensitivity of SLN biopsy was 90.0% (95% CI 68.3–98.8%) and the negative predictive value was 96.7% (95% CI 88.6–99.1%). When calculated per hemi-pelvis, the sensitivity of SLN biopsy was 97.1% (95% CI 85.1–99.9%) and the negative predictive value was 99.1% (95% CI 93.9–99.9%) (Supplementary Table 1).
Comparison of open and minimally invasive approach
Comparison of characteristics of minimally invasive (58, 68.2%) and open approach (27, 31.8%) is reported in Table 2. Patients who underwent open approach reported higher rate of grade 3 (p = 0.028) and positive LVSI (p = 0.003), deeper stromal infiltration (median 8 mm vs. 5 mm, p = 0.016), larger maximum tumor diameter (median 25 mm vs. 15 mm, p = 0.003) and higher FIGO stage (p = 0.039). Despite these differences, no difference in any SLN mapping or in SLN bilateral detection was evident between open and minimally invasive approach (p = 0.850 and p = 0.222, respectively). Moreover, there was no difference in median number of SLNs mapped and retrieved between the two approaches (2 in both groups, p = 0.165) and in site of SLN mapping per hemi-pelvis (right side, p = 0273 and left side, p = 0.618). Figures 1 and 2 show two examples of SLN mapping in open and minimally invasive surgery, respectively.
Table 2.
Comparison of characteristics of patients undergoing SLN mapping by laparotomy (LPT) and minimally invasive surgery (MIS)
| Characteristic | LPT = 27, % | MIS = 58, % | p value |
|---|---|---|---|
| Age (years) | 45 (27–80) | 34.5 (29–77) | 0.421 |
| BMI (kg/m2) | 23.0 (18.7–35.0) | 23.3 (17.3–36.0) | 0.718 |
| Neo-adjuvant chemotherapy | |||
| No | 23 (85.2) | 53 (91.4) | 0.456 |
| Yes | 4 (14.8) | 5 (8.6) | |
| Histology | 0.483 | ||
| SCC | 16 (59.3) | 32 (55.2) | |
| Adenocarcinoma | 11 (40.7) | 23 (39.7) | |
| Adenosquamous | 0 | 3 (5.2) | |
| Gradea | 0.028 | ||
| 1 | 2 (7.4) | 7 (12.1) | |
| 2 | 11 (40.7) | 33 (56.9) | |
| 3 | 12 (44.4) | 9 (15.5) | |
| Unknown | 2 (7.4) | 9 (15.5) | |
| LVSIa | 0.003 | ||
| Negative | 8 (29.6) | 37 (63.8) | |
| Positive | 17 (63.0) | 18 (31.0) | |
| Unknown | 2 (7.4) | 3 (5.2) | |
| Depth of stromal infiltration | 8 (0.4–25) | 5 (0.6–17) | 0.016 |
| Maximum tumor diameter (mm) | 25 (5–65) | 15 (1–50) | 0.003 |
| Tumor diameter | 0.005 | ||
| ≤ 20 mm | 8 (29.6) | 37 (63.8) | |
| > 20 mm | 19 (70.4) | 21 (36.2) | |
| FIGO stage 2018 | 0.039 | ||
| IA1 | 0 | 7 (12.1) | |
| IA2 | 0 | 4 (6.9) | |
| IB1 | 5 (18.5) | 14 (24.1) | |
| IB2 | 7 (25.9) | 13 (22.4) | |
| IB3 | 5 (18.5) | 1 (1.7) | |
| IIA1 | 1 (3.7) | 3 (5.2) | |
| IIB | 3 (11.1) | 2 (3.4) | |
| IIIC1p | 6 (22.2) | 14 (24.1) | |
| SLN mapping | 0.850 | ||
| No | 2 (7.4) | 5 (8.6) | |
| Yes | 25 (92.6) | 53 (91.4) | |
| SLN detectionb | 0.222 | ||
| Unilateral | 7 (28.0) | 8 (15.1) | |
| Bilateral | 18 (72.0) | 45 (84.9) | |
| SLN analysisb | 0.133 | ||
| Ultrastaging | 19 (76.0) | 30 (56.6) | |
| OSNA | 6 (24.0) | 23 (43.4) | |
| Number of SLNb | 0.476 | ||
| 1 | 5 (20.0) | 7 (13.2) | |
| 2 | 17 (68.0) | 30 (56.6) | |
| 3 | 1 (4.0) | 9 (17.0) | |
| 4 | 1 (4.0) | 5 (9.4) | |
| 5 | 1 (4.0) | 1 (1.9) | |
| 6 | 0 | 1 (1.9) | |
| Median number of SLN | 2 (1–5) | 2 (1–6) | 0.165 |
| Median number of removed lymph nodes | 23 (8–57) | 18 (5–86) | 0.197 |
| Site of mapping of first Right SLNc | 0.273 | ||
| Obturator | 6 (26.1) | 13 (31.0) | |
| Internal iliac | 0 | 4 (9.5) | |
| External iliac | 16 (69.6) | 21 (50.0) | |
| Common iliac | 0 | 3 (7.1) | |
| Para-aortic | 1 (4.3) | 1 (2.4) | |
| Site of mapping of first Left SLNd | 0.618 | ||
| Obturator | 6 (30.0) | 13 (31.0) | |
| Internal iliac | 0 | 3 (7.1) | |
| External iliac | 13 (65.0) | 25 (59.5) | |
| Common iliac | 1 (5.0) | 1 (2.4) | |
| SLN metastasisb | 0.801 | ||
| No | 20 (80.0) | 40 (75.5) | |
| ITC | 1 (4.0) | 2 (3.8) | |
| Micro | 4 (16.0) | 9 (17.0) | |
| Macro | 0 | 2 (3.8) | |
Bold significance values are p < 0.05
BMI body mass index, SCC squamous cell carcinoma, LVSI lymph-vascular space involvement, FIGO international federation of gynecology and obstetrics, SLN sentinel lymph node, OSNA one-step nucleic acid amplification, ITC isolated tumor cell
a Analysis performed on cases with available data only
b Analysis performed on 78 cases with uni/bilateral mapping
c analysis performed on 65 right hemipelvis mapping, 7 patients had pelvic mapping but site not specified; total number of SLNs retrieved: 176
d analysis performed on 62 left hemipelvis mapping, 7 patients had pelvic mapping but site not specified; total number of SLNs retrieved: 176
Fig. 1.
Right external iliac SLN detected with ICG green mode (1a) and color-segmented fluorescence (1b) during open surgery
Fig. 2.
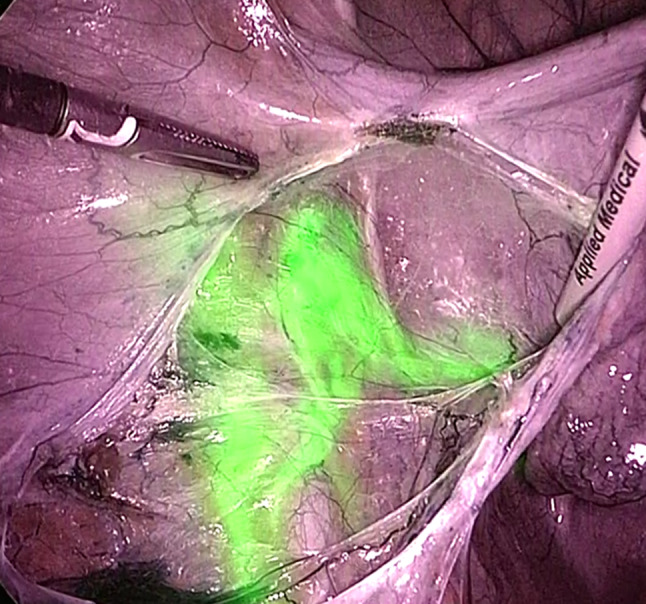
Left external iliac SLN detected with ICG during laparoscopic surgery
In the sub-group of laparotomy patients, per-patient sensitivity of SLN biopsy was 83.3% (95% CI 35.9–99.6%) and negative predictive value was 95.0% (95% CI 76.0–99.1%). When calculated by hemi-pelvis, the sensitivity of SLN biopsy was 88.9% (95% CI 51.7–99.7%) and the negative predictive value was 97.1% (95% CI 84.3–99.5%) (Supplementary Table 1). For what concerns minimally invasive group, the sensitivity of SLN biopsy was 92.9% (95% CI 66.1–99.8%) and the negative predictive value was 97.5% (95% CI 85.5–99.6%) in the per-patients and they were 100.0% (95% CI 86.8–100.0%) and 100.0% respectively, in the per-hemipelvis calculation (Supplementary Table 1). No difference in per-patient sensitivity was noted between laparotomy and minimally invasive surgery (p = 0.300).
Comparison of no/unilateral and bilateral SLN detection
Analysis of variables associated with bilateral SLN detection, compared with no/unilateral SLN detection within the entire cohort, is reported in Table 3. None of the analyzed characteristics was significantly different between the two groups. In particular, no surgery-related, or tumor-related variable was associated with higher bilateral SLN mapping. When we divided the study period in two parts, considering the first as “learning period” (33 patients, until 12/2018) and the second “experienced period” (52 patients, from 01/2019), no difference was observed in bilateral SLN detection rate (p = 0.450).
Table 3.
Factors associated with bilateral detection in the entire cohort
| Characteristic | No/unilateral mapping (N = 22) | Bilateral mapping (N = 63) | p value |
|---|---|---|---|
| Age (years) | 48 (29–80) | 42 (27–75) | 0.121 |
| BMI (kg/m2) | 24.2 (18.1–33.2) | 23 (17.3–36) | 0.905 |
| Histology | 0.618 | ||
| SCC | 11 (50.0) | 37 (58.7) | |
| Non-SCC | 11 (50.0) | 26 (41.3) | |
| Gradea | 0.737 | ||
| 1 | 3 (13.6) | 6 (9.5) | |
| 2 | 11 (50.0) | 33 (52.4) | |
| 3 | 7 (31.8) | 14 (22.2) | |
| Unknown | 1 (4.5) | 10 (15.9) | |
| LVSIa | 0.444 | ||
| Negative | 10 (45.5) | 35 (55.5) | |
| Positive | 11 (50.0) | 24 (38.1) | |
| Unknown | 1 (4.5) | 4 (6.3) | |
| Maximum tumor diameter (mm) | 20.5 (1–57) | 19 (1–65) | 0.634 |
| Tumor diameter | 0.464 | ||
| ≤ 20 mm | 10 (45.5) | 35 (55.6) | |
| > 20 mm | 12 (54.5) | 28 (44.4) | |
| Depth of stromal infiltration | 5 (0.6–19) | 5 (0.4–25) | 0.953 |
| FIGO stage 2018 | 0.602 | ||
| IA-IB | 16 (72.7) | 40 (63.5) | |
| IIA-IIB/IIIc1p | 6 (27.3) | 23 (36.5) | |
| Pathologic parametrial infiltration | 0.332 | ||
| No | 22 (100.0) | 57 (90.5) | |
| Yes | 0 | 6 (9.5) | |
| Pathologic lymph node metastasis | 0.918 | ||
| No | 17 (77.3) | 48 (76.2) | |
| Yes | 5 (22.7) | 15 (23.8) | |
| Neo-adjuvant treatment | 0.435 | ||
| No | 21 (95.5) | 55 (87.3) | |
| Yes | 1 (4.5) | 8 (12.7) | |
| Approach | 0.299 | ||
| Laparotomy | 9 (40.9) | 18 (28.6) | |
| MIS | 13 (59.1) | 63 (71.4) | |
| SLN metastasisb | 0.739 | ||
| No | 11 (73.3) | 49 (77.8) | |
| Yes | 4 (26.7) | 14 (22.2) | |
| SLN metastasisb | 0.798 | ||
| No | 11 (73.3) | 49 (77.8) | |
| ITC | 1 (6.7) | 2 (3.2) | |
| Micro | 3 (20.0) | 10 (15.9) | |
| Macro | 0 | 2 (3.2) | |
BMI body mass index, SCC squamous cell carcinoma, LVSI lymph-vascular space involvement, FIGO international federation of gynecology and obstetrics SLN sentinel lymph node, ITC isolated tumor cell
a Analysis performed on cases with available data only
b 7 patients did not have SLN mapping therefore no information on SLN metastasis available
Discussion
The aim of this study was to compare the bilateral SLN detection rate with ICG of patients undergoing open and minimally invasive radical hysterectomy for cervical cancer. Following the publication of the LACC trial (Ramirez 2018), which demonstrated survival advantage in patients operated with laparotomy approach, international societies have changed their recommendations in terms of surgical approach for radical hysterectomy in early-stage cervical cancer (Koh 2019, Querleu 2020). Multiple subsequent observational studies have confirmed LACC trial results, particularly for patients with tumors larger than 2 cm (Nitecki 2020, Pedone Anchora 2020). SLN has become essential part of the operative management of early-stage cervical cancer, as it provides important information on lymph nodes at highest probability of metastasis and it allows a thorough analysis of these nodes by ultrastaging (Cibula 2012) or OSNA (Bizzarri 2020). Such analysis allows to detect low-volume metastases, which represents an important information, having some authors described the negative prognostic impact of micrometastases (Kocian 2020); nevertheless, not all studies support the negative impact of micrometastases in cervical cancer (Guani 2020). Moreover, ICG has been demonstrated to be superior to other tracers alone (such as blue dye or radioactive tracers), and comparable with combination of blue and radioactive tracer in detecting SLNs in cervical cancer (Di Martino 2017). With this background, we intended to assess whether ICG provides such a high bilateral SLN detection rate in open surgery as in minimally invasive surgery, in view of the clinical practice change that many centers across the world had with regards to the surgical approach.
With our results, we showed no difference in any SLN and bilateral SLN detection between patients undergoing SLN mapping with open surgery compared to laparoscopic or robotic surgery. Moreover, with an overall and bilateral detection rate of 92.6% and 72.0%, SLN mapping with ICG in laparotomy is in line with previously reported pooled data from a diagnostic review, showing overall and bilateral detection rate of 91% and 60%, respectively (Tax 2015).
Differences in tumor characteristics between patients undergoing open and minimally invasive approach is likely to be related to the approach selection. Even though not significant, the difference in bilateral detection rate between the two approaches (72.0% in open versus 84.9% in minimally invasive), could be explained by the tumor characteristics of patients undergoing open approach. In fact, as previously described by (Dostálek 2018), bilateral SLN detection in large volume cervical cancer was lower, compared to smaller tumors.
Regarding sensitivity and negative predictive value in the per-patient analysis, our results demonstrated that there was no significant difference between patients undergoing open ICG SLN (83.3–95.0%, respectively) and minimally invasive ICG SLN (92.9–97.5%, respectively). The same applies for the per-hemipelvis analysis. When compared with other literature studies, we found sensitivity from open ICG SLN in the present series to be slightly lower compared with other sensitivities reported in the literature (ranging from 91.0 to 96.4%), with a negative predictive value concordant with other series (ranging from 91.0 to 100%) (Cibula 2012; Tax 2015; Salvo 2017).
This is not the first study reporting the use of ICG to detect SLN in open surgery for cervical cancer. However, to date, only few case reports or small series were reported (Furukawa 2010; Crane 2011; van der Vorst 2011; Schaafsma 2012; Buda 2016; Rychlik 2016; Snyman 2018; Bedyńska 2019). Table 4 shows the characteristics of the studies which reported on the use of ICG to detect SLN in laparotomy. On the other hand, SLN in open surgery has been largely described with the use of other tracers such as blue dye, radioactive tracer (technetium) or combined technique (Silva 2005; Wydra 2006; Altgassen 2007; Kara 2008; Dostalek 2020). Nevertheless, ICG shows some advantages compared to other tracers: the low-risk of allergic reaction compared to blue dye (Papadia 2017); possibility of performing SLN biopsy in one-step at the time of surgery and the absence of patients exposure to ionizing radiation compared to radioactive tracer; lastly, it allows a real-time visualization of lymphatic channels.
Table 4.
Literature review showing previous cases of SLN detected with ICG in open surgery for cervical cancer
| Authors | Year | Total number of cervical cancer cases | Number of open cases with ICG | Tracer to detect SLN | Setting | Overall detection rate | Bilateral detection rate | SLN metastasis | Sensitivity | NPV |
|---|---|---|---|---|---|---|---|---|---|---|
| Crane et al. | 2010 | 10 | 10 | Methylene blue + ICG | Primary surgery | 60% | 30% | 11% | 100% | 100% |
| Furukawa et al. | 2010 | 12 | 12 | ICG | Primary surgery | 83% | 83% | 16.7% | 100% | 100% |
| Van der Vorst et al. | 2011 | 9 | 9 | ICG |
Primary surgery: 8 No uterine surgery: 1 |
100% | 88.9% | 22.2% | 100% | 100% |
| Schaafsma et al. | 2012 | 18 | 17 | ICG |
Primary surgery: 17 After NACT: 1 |
78% | 61% | 33% | 83.3% | 92.3% |
| Buda et al. | 2016 | 2 | 2 | ICG | Primary surgery | 100% | 100% | 0 | 100% | 100% |
| Rychlik et al. | 2016 | 1 | 1 | ICG | Primary surgery | 100% | 100% | 0 | 100% | 100% |
| Snyman et al. | 2018 | 72 | NS | Methylene blue + ICG | Primary surgery | 87.5%a | 30.5%a | NS | 85.7%a | 100%a |
| Bedyńska et al. | 2019 | 6 | NS | ICG | Primary surgery | 67.0% | 56.0% | 0 | NS | NS |
| Present study | 2020 | 85 | 27 | ICG |
Primary surgery: 23 After NACT: 4 |
92.6% | 72.0% | 18.5% | 83.3% | 95.0% |
SLN sentinel lymph node, NIR near infrared, NPV negative predictive value, ICG indocyanine green, NS not stated in the original article, NACT neo-adjuvant chemotherapy
a Not only in open-ICG cases
The main limitation of the present study is the possible selection bias for patients submitted to open surgery; on the other hand, we have to acknowledge that this is the first study reporting the use of ICG in open surgery in a relatively large number of patients with cervical cancer, with a novel near infrared technology.
Conclusion
In conclusion, the use of ICG to detect SLN in cervical cancer treated with open surgery allows a bilateral detection, sensitivity and negative predictive value comparable to minimally invasive surgery or with open surgery using other tracers. Performance of open SLN biopsy with ICG hits the standards described for cervical cancer. The advantages of ICG compared to other tracers make it a promising tool to detect SLN in an era where open surgery and SLN biopsy has become essential part of the personalized treatment for cervical cancer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the surgical team and the scrub nurses at Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Luigi Pedone Anchora, Gabriella Ferrandina, Gian Franco Zannoni, Maria Vittoria Carbone, Camilla Fedele, Elena Teodorico, Valerio Gallotta, Salvatore Gueli Alletti, Vito Chiantera, Anna Fagotti, Francesco Fanfani, Giovanni Scambia. The first draft of the manuscript was written by Nicolò Bizzarri, Francesco Fanfani and Giovanni Scambia and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Open access funding provided by Università Cattolica del Sacro Cuore within the CRUI-CARE Agreement. This study was not supported by funds.
Data availability
All data and materials as well as software application or custom code support their published claims and comply with field standards.
Compliance with ethical standards
Conflicts of interest
All authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Altgassen C, Paseka A, Urbanczyk H et al (2007) Dilution of dye improves parametrial SLN detection in patients with cervical cancer. Gynecol Oncol 105(2):329–334 [DOI] [PubMed] [Google Scholar]
- Arbyn M, Weiderpass E, Bruni L et al (2020) Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health 8(2):e191–e203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaya V, Guani B, Magaud L et al (2019) Surgical algorithm for sentinel lymph nodes detection in early-stage cervical cancer. Int J Gynecol Cancer. 10.1136/ijgc-2019-ESGO.3(Nov 29 (Suppl 4) A3) [Google Scholar]
- Bedyńska M, Szewczyk G, Klepacka T et al (2019) Sentinel lymph node mapping using indocyanine green in patients with uterine and cervical neoplasms: restrictions of the method. Arch Gynecol Obstet 299(5):1373–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R (2018) Cancer of the cervix uteri. Int J Gynaecol Obstet 143(Suppl 2):22–36 [DOI] [PubMed] [Google Scholar]
- Bizzarri N, Pedone Anchora L, Zannoni GF et al (2020) Role of one-step nucleic acid amplification (OSNA) to detect sentinel lymph node low-volume metastasis in early-stage cervical cancer. Int J Gynecol Cancer 30(3):364–371 [DOI] [PubMed] [Google Scholar]
- Brisson M, Kim JJ, Canfell K et al (2020) Impact of HPV vaccination and cervical screening on cervical cancer elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet 395(10224):575–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buda A, Dell’Anna T, Vecchione F, Verri D, Di Martino G, Milani R (2016) Near-infrared sentinel lymph node mapping with indocyanine green using the VITOM II ICG exoscope for open surgery for gynecologic malignancies. J Minim Invasive Gynecol 23(4):628–632 [DOI] [PubMed] [Google Scholar]
- Cibula D, McCluggage WG (2019) Sentinel lymph node (SLN) concept in cervical cancer: current limitations and unanswered questions. Gynecol Oncol 152(1):202–207 [DOI] [PubMed] [Google Scholar]
- Cibula D, Abu-Rustum NR, Dusek L et al (2012) Bilateral ultrastaging of sentinel lymph node in cervical cancer: lowering the false-negative rate and improving the detection of micrometastasis. Gynecol Oncol 127(3):462–466 [DOI] [PubMed] [Google Scholar]
- Cibula D, Pötter R, Planchamp F et al (2018) The European society of gynaecological oncology/European society for radiotherapy and oncology/European society of pathology guidelines for the management of patients with cervical cancer. Int J Gynecol Cancer 28(4):641–6558 [DOI] [PubMed] [Google Scholar]
- ClinicalTrials. Feasibility study: conservative treatment in cervical cancer. ClinicalTrials.gov Identifier: NCT02323841. https://clinicaltrials.gov/ct2/show/NCT02323841?term=scambia&draw=2&rank=1. Accessed 26 Sept 2020
- Crane LM, Themelis G, Pleijhuis RG et al (2011) Intraoperative multispectral fluorescence imaging for the detection of the sentinel lymph node in cervical cancer: a novel concept. Mol Imaging Biol 13(5):1043–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino G, Crivellaro C, De Ponti E et al (2017) Indocyanine green versus radiotracer with or without blue dye for sentinel lymph node mapping in stage >IB1 cervical cancer (>2 cm). J Minim Invasive Gynecol 24(6):954–959 [DOI] [PubMed] [Google Scholar]
- Dostálek L, Zikan M, Fischerova D et al (2018) SLN biopsy in cervical cancer patients with tumors larger than 2cm and 4cm. Gynecol Oncol 148(3):456–460 [DOI] [PubMed] [Google Scholar]
- Dostalek L, Slama J, Fisherova D et al (2020) Impact of sentinel lymph node frozen section evaluation to avoid combined treatment in early-stage cervical cancer. Int J Gynecol Cancer 30(6):744–748 [DOI] [PubMed] [Google Scholar]
- Furukawa N, Oi H, Yoshida S, Shigetomi H, Kanayama S, Kobayashi H (2010) The usefulness of photodynamic eye for sentinel lymph node identification in patients with cervical cancer. Tumori 96(6):936–940 [PubMed] [Google Scholar]
- Guani B, Balaya V, Magaud L, Lecuru F, Mathevet P (2020) The clinical impact of low-volume lymph nodal metastases in early-stage cervical cancer: the senticol 1 and senticol 2 trials. Cancers (Basel) 12(5):1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara PP, Ayhan A, Caner B et al (2008) Sentinel lymph node detection in early stage cervical cancer: a prospective study comparing preoperative lymphoscintigraphy, intraoperative gamma probe, and blue dye. Ann Nucl Med 22(6):487–494 [DOI] [PubMed] [Google Scholar]
- Kocian R, Slama J, Fischerova D et al (2020) Micrometastases in sentinel lymph nodes represent a significant negative prognostic factor in early-stage cervical cancer: a single-institutional retrospective cohort study. Cancers (Basel) 12(6):E1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh WJ, Abu-Rustum NR, Bean S et al (2019) Cervical cancer version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 17(1):64–84 [DOI] [PubMed] [Google Scholar]
- Marnitz S, Köhler C, Bongardt S et al (2006) Topographic distribution of sentinel lymph nodes in patients with cervical cancer. Gynecol Oncol 103(1):35–44 [DOI] [PubMed] [Google Scholar]
- Nitecki R, Ramirez PT, Frumovitz M et al (2020) Survival after minimally invasive vs. open radical hysterectomy for early-stage cervical cancer: a systematic review and meta-analysis. JAMA Oncol 6(7):1–9 (published online ahead of print, 2020 Jun 11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadia A, Gasparri ML, Mueller MD (2017) Are allergic reactions to indocyanine green really that uncommon? A single institution experiences. Obstet Gynecol Rep 1(2):1–2 [Google Scholar]
- Pedone Anchora L, Turco LC, Bizzarri N et al (2020) How to select early-stage cervical cancer patients still suitable for laparoscopic radical hysterectomy: a propensity-matched study. Ann Surg Oncol 27(6):1947–1955 [DOI] [PubMed] [Google Scholar]
- Querleu D, Cibula D, Abu-Rustum NR (2017) 2017 update on the querleu-morrow classification of radical hysterectomy. Ann Surg Oncol 24(11):3406–3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querleu D, Cibula D, Concin N et al (2020) Laparoscopic radical hysterectomy: a European society of gynaecological oncology (ESGO) statement. Int J Gynecol Cancer 30(1):15 [DOI] [PubMed] [Google Scholar]
- Ramirez PT, Frumovitz M, Pareja R et al (2018) Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med 379(20):1895–1904 [DOI] [PubMed] [Google Scholar]
- Robova H, Halaska MJ, Pluta M et al (2014) Oncological and pregnancy outcomes after high-dose density neoadjuvant chemotherapy and fertility-sparing surgery in cervical cancer. Gynecol Oncol 135(2):213–216 [DOI] [PubMed] [Google Scholar]
- Rychlik A, Marin S, De Santiago J, Zapardiel I (2016) Utility of laparoscopic indocyanine green-guided sentinel node biopsy in open cervical cancer surgery. Int J Gynecol Cancer 26(7):1288–1289 [DOI] [PubMed] [Google Scholar]
- Salvo G, Ramirez PT, Levenback CF et al (2017) Sensitivity and negative predictive value for sentinel lymph node biopsy in women with early-stage cervical cancer. Gynecol Oncol 145(1):96–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaafsma BE, van der Vorst JR, Gaarenstroom KN et al (2012) Randomized comparison of near-infrared fluorescence lymphatic tracers for sentinel lymph node mapping of cervical cancer. Gynecol Oncol 127(1):126–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz GF, Giuliano AE, Veronesi U (2002) Proceedings of the consensus conference on the role of sentinel lymph node biopsy in carcinoma of the breast, April 19–22, 2001, Philadelphia, Pennsylvania. Cancer 94:2542–2551 [DOI] [PubMed] [Google Scholar]
- Silva LB, Silva-Filho AL, Traiman P et al (2005) Sentinel node detection in cervical cancer with (99m)Tc-phytate. Gynecol Oncol 97(2):588–595 [DOI] [PubMed] [Google Scholar]
- Slama J, Dundr P, Dusek L et al (2012) Sentinel lymph node status in patients with locally advanced cervical cancers and impact of neoadjuvant chemotherapy. Gynecol Oncol 125(2):303–306 [DOI] [PubMed] [Google Scholar]
- Snyman LC, Bryant EP, Wethmar EI et al (2018) Use of a sentinel lymph node biopsy algorithm in a south African population of patients with cervical cancer and high prevalence of human immunodeficiency virus infection. Int J Gynecol Cancer 28(7):1432–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tax C, Rovers MM, de Graaf C, Zusterzeel PL, Bekkers RL (2015) The sentinel node procedure in early stage cervical cancer, taking the next step; a diagnostic review. Gynecol Oncol 139(3):559–567 [DOI] [PubMed] [Google Scholar]
- van der Vorst JR, Hutteman M, Gaarenstroom KN et al (2011) Optimization of near-infrared fluorescent sentinel lymph node mapping in cervical cancer patients. Int J Gynecol Cancer 21(8):1472–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TC, Stoler MH, Behrens CM et al (2015) Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol 136(2):189–197 [DOI] [PubMed] [Google Scholar]
- Wydra D, Sawicki S, Wojtylak S, Bandurski T, Emerich J (2006) Sentinel node identification in cervical cancer patients undergoing transperitoneal radical hysterectomy: a study of 100 cases. Int J Gynecol Cancer 16(2):649–654 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and materials as well as software application or custom code support their published claims and comply with field standards.



