Abstract
Individual differences in attentional control may explain null findings and inconsistent patterns of threat-related attentional bias (ABT) that are common in the posttraumatic stress disorder (PTSD) literature. At Time 1 (T1), trauma-exposed community participants (N = 89) completed a clinical interview, self-report measures, and an eye-tracking task developed to assess ABT. Participants completed follow-up assessments online 6 (T2) and 12 (T3) months later. Those with higher PTSD symptoms and deficits in attentional control exhibited a pattern of undercontrol, characterized by attention maintenance on threat and increased arousal. In contrast, those with higher PTSD symptoms and relatively better attentional control exhibited a pattern of overcontrol, characterized by threat avoidance and reduced arousal. These effects were specific to threat stimuli. Among PTSD symptom clusters, symptoms of hyperarousal were of central importance to the observed effects. Results from the longitudinal analysis indicate that both of these patterns of ABT are maladaptive, resulting in symptom maintenance at T2 and T3. These results have implications for (a) reconciling tensions between disparate models of ABT (i.e., vigilance-avoidance vs. attention maintenance), (b) precision medicine based approaches to targeting PTSD-related ABT, and (c) understanding the transdiagnostic role that attentional control may play in influencing ABT expression.
Keywords: eye tracking, attentional bias, attentional control, posttraumatic stress, longitudinal, arousal
1. Introduction
Threat-related attentional bias (ABT) is conceptualized as a core vulnerability factor for the development of posttraumatic stress disorder (PTSD; Beck, Emery, & Greenberg, 1985; Williams, Watts, MacLeod, & Matthews, 1997). However, findings regarding the degree to which individuals with PTSD exhibit ABT have been equivocal. In addition to null findings (Kimble, Frueh, & Marks, 2009; Van Bockstaele et al., 2014), inconsistent patterns of ABT have been observed in relation to PTSD, including sustained attention on threat (e.g., Pineles, Shipherd, Welch, & Yovel, 2007) and threat-related attentional avoidance (e.g., Bardeen & Daniel, 2017; Wald et al., 2013; and see Dennis-Tiwar, Roy, Denefrio, & Myruski, 2019, for a review). Individual differences in attentional control (i.e., the strategic control of higher-order executive attention in regulating bottom-up, stimulus driven responses to prepotent stimuli; Sarapas, Weinberg, Langenecker, & Shankman, 2017) may help to explain these discrepant findings. Specifically, evidence suggests that those with PTSD and relatively worse attentional control are more likely to maintain attention on threat, while those with relatively better attentional control are more likely to exhibit threat-related attentional avoidance (Bardeen & Daniel, 2017; Bardeen & Orcutt, 2011; Bardeen, Tull, Daniel, Evenden, & Stevens, 2016). While these findings have improved our understanding PTSD-related ABT, some important questions remain unanswered. For example, the effect of attentional control on the relationship between PTSD and ABT may be more relevant to certain types of PTSD symptoms within this heterogeneous disorder (i.e., hyperarousal symptoms), which may have implications for bridging the anxiety/ABT and PTSD/ABT literatures. Additionally, determining the long-term impact of rigid threat avoidance and maintenance among those with greater PTSD symptoms may aid in the identification of distinct risk profiles for predicting symptom chronicity.
1.1. Attentional Control: The Key to Understanding Heterogeneity in PTSD-related ABT
Theory (e.g., attentional control theory: Eysenck, Derakshan, Santos, & Calvo, 2007) and empirical evidence suggest that, among individuals with PTSD, those with relatively better attentional control can disengage and shift attention from perceived threat by drawing on reserve attentional control resources through active effort (i.e., a pattern of threat avoidance). In contrast, those with PTSD and relatively poorer attentional control appear to have greater difficulty disengaging from threat stimuli because they lack the requisite resources to do so (i.e., a pattern of threat maintenance; Bardeen & Daniel, 2017; Bardeen & Orcutt, 2011; Bardeen et al., 2016). In theory, both of these patterns of PTSD-related ABT (i.e., maladaptive under- and overcontrol; Dennis-Tiwary et al., 2019), when used chronically and rigidly, should result in the maintenance and exacerbation of PTSD symptoms (Weierich, Treat, & Hollingsworth, 2008). Preliminary evidence partially supports this proposition.
Bardeen and Daniel (2017) conducted a longitudinal study in which trauma-exposed undergraduate participants completed self-report measures of attentional control, trauma history, and posttraumatic stress symptoms at an initial laboratory session (T1: N = 116) and again at a six month follow-up session (T2: n = 49). Additionally, participants completed a performancebased task that assessed attentional inhibition and an eye-tracking task (i.e., a free-viewing task) in which eye movements and pupillary response were recorded in response to viewing pictorial stimuli (i.e., threat and neutral images) over the course of 60 trials. Bardeen and Daniel (2017) found that the relationship between attentional control (both self-reported attentional control and behaviorally assessed attentional inhibition) and ABT was moderated by posttraumatic stress (PTS) symptoms. Specifically, among those with relatively higher PTS symptoms, those with better attentional control disengaged from threat and shifted attention to the neutral stimulus, while those with worse attentional control maintained attention on threat. Additionally, those with higher PTS symptoms and relatively better attentional control who avoided threat stimuli exhibited significantly lower pupillary reactivity to threat stimuli (i.e., down-regulation of sympathetic nervous system arousal) in comparison to those with higher PTS symptoms and relatively worse attentional control who maintained attention on threat.
Results of the longitudinal portion of this study suggest that using attentional control to shift attention away from threat stimuli and down-regulate emotional arousal maintains, and perhaps exacerbates, PTS symptoms over a six-month period. This pattern of threat avoidance suggests a rigid fear-based approach to reducing emotional arousal. As described in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5; American Psychiatric Association [APA], 2013), the use of avoidance to provide short-term relief from aversive internal experiences (i.e., trauma-related bodily sensations, emotions, memories, thoughts) is a symptom of PTSD. Over time, the chronic use of attentional avoidance prevents disconfirmation of faulty threat appraisals, thus resulting in the continued use of maladaptive avoidance behaviors and the maintenance of PTSD.
Bardeen and Daniel (2017) did not find that a pattern of undercontrol at T1 (i.e., relatively higher PTS symptoms + lower attentional control + attention maintenance on threat) predicted symptom maintenance at T2. This is inconsistent with the attention maintenance model of ABT (Weierich et al., 2008), which postulates that maintenance of attention on threat stimuli by fearful individuals prolongs emotional distress and increases the likelihood that fear-related pathology will develop. However, methodological limitations should be considered before drawing strong conclusions based on the results of the longitudinal portion of this study. First, the relatively small T2 sample size (n = 49), with its limited power, may have decreased the likelihood of detecting the hypothesized three-way interaction (i.e., increased Type II error). Additionally, the low retention rate (i.e., 45%) is a significant limitation that may have obscured the proposed effect. Although not uncommon in this literature, the majority of participants in this study were relatively asymptomatic, with only 5-11% of the sample reporting symptom levels indicative of PTSD. Replication of these findings in a more symptomatic sample, with substantially larger follow-up retention, would increase confidence in these results. One additional limitation is worth noting. Much like the majority of studies in this line of research, Bardeen and Daniel (2017) compared negatively valenced to neutral stimuli without considering the possibility that emotionally arousing stimuli (both positively- and negatively-valenced) may provoke biased attentional processing among individuals with PTS symptoms.
1.2. The Moderating Role of Attentional Control may be Specific to Hyperarousal Symptoms
The modulatory role of attentional control on ABT does not appear to be specific to PTSD. Derryberry and Reed (2002) used a spatial cueing task with an undergraduate student sample (N = 114) to examine attention to threat cues (i.e., symbols indicating that a trial would likely result in failure). They found that individuals high in trait anxiety and high in self-reported attentional control showed significantly faster disengagement from threat cues in comparison to participants high in trait anxiety and low in attentional control. Similar moderation effects were observed when attentional control was assessed via self-report and a modified dot-probe task was used to assess ABT in relation to (a) trait anxiety in a sample of 109 high school students (Ho, Yueng, & Mak, 2016) and (b) social anxiety in a sample of 75 undergraduate students (Taylor, Cross, & Amir, 2015). These results, in combination with those from the PTSD-ABT literature (Bardeen & Daniel, 2017; Bardeen & Orcutt, 2011; Bardeen et al., 2016), suggest that individual differences in attentional control may influence the expression of ABT across fear- and anxiety-related disorders.
PTSD is a complex and heterogeneous disorder (Galatzer-Levy & Bryant, 2013). DSM-5 PTSD criteria include 20 symptoms divided into four clusters: intrusion, avoidance, negative alterations in cognition and mood (cognition), and hyperarousal (APA, 2013). Results from the studies described above suggest that the interaction between fear- and anxiety-related distress and attentional control on ABT may be more relevant to certain types of PTS symptoms. For example, symptoms within the hyperarousal cluster cut across fear- and anxiety-related disorders, exhibiting large magnitude associations with symptom measures of panic disorder, social phobia, and generalized anxiety disorder, whereas associations between symptoms from the intrusion cluster and these anxiety disorder symptom measures are significantly smaller in magnitude (Zelazny & Simms, 2015). Moreover, hypervigilance toward threat information, a hallmark symptom of the hyperarousal cluster (APA, 2013), suggests that individuals with PTSD should preferentially process threat- and trauma-related stimuli. Accordingly, it may be that individuals with relatively higher hyperarousal symptoms preferentially process threat stimuli and individual differences in attentional control determine whether this biased processing will be maintained or interrupted (i.e., avoidance). An analysis examining domain-specific interaction effects in the context of PTS cluster scores has yet to be published, but such an analysis may help explain the potential transdiagnostic nature of the moderation effect of interest. This would help bridge the anxiety/ABT and PTSD/ABT literatures and reconcile tensions between models of ABT that presuppose either attention maintenance or avoidance (Weirich et al., 2008).
1.4. Present study
The purpose of this study was to replicate and extend the results of Bardeen and Daniel (2017) by (a) recruiting a trauma-exposed community sample and prescreening participants to ensure the presence of substantial PTS symptomatology, (b) using the gold-standard structured clinical interview to identify baseline symptoms, (c) examining attentional biases to both positively and negatively valenced stimuli, (d) adding an additional one-year follow-up assessment and retaining large follow-up samples to increase confidence in the stability and longevity of longitudinal effects, and (e) examining the specificity of the described modulatory effect to the four PTSD symptom clusters. As recommended (Wald et al., 2013), eye-tracking technology was used in the present study because, unlike stimulus-response tasks that use button press to make inferences about covert attention, with eye tracking, the construct of interest (overt attention) is directly assessed via eye movements. As such, eye-tracking indices are less susceptible to alternate explanations than attentional bias scores from stimulus-response tasks. Additionally, indices of attentional bias obtained via eye tracking (e.g., proportion of viewing time on threat vs. neutral stimuli) have been shown to have adequate reliability (Bardeen & Daniel, 2017; 2018; Waechter, Nelson, Wright, Hyatt, & Oakman, 2014).
Following from Bardeen and Daniel (2017), we predicted that attentional control would moderate the association between PTS symptoms and (a) ABT (i.e., dwell time on threat) and (b) sympathetic arousal (pupillary response; Bradley, Miccoli, Escrig, & Lang, 2008; Partala & Surakka, 2003). Specifically, among participants with higher PTS symptoms, those with worse attentional control would dwell longer on threat compared to neutral stimuli and exhibit greater sympathetic nervous system arousal (assessed via pupillary response) to threat stimuli (i.e., maintenance/undercontrol) compared to those with relatively better attentional control (i.e., avoidance/overcontrol). Additionally, based on the rationale presented above, we predicted that these effects would be specific to the hyperarousal symptom cluster (versus the three other cluster scores) and negatively (versus positively) valenced stimuli.
The goal of our longitudinal analysis was to determine the long-term impact of rigid threat avoidance and maintenance among those who exhibited these patterns of under- and overcontrol at baseline. Based on the rationale presented above, we predicted that PTS symptoms would be maintained at 6- and 12-month follow-up sessions among those who reported relatively higher PTS symptoms at baseline and exhibited patterns of under- (i.e., relatively lower attentional control and attention maintenance on, and relatively higher pupillary reactivity to, threat stimuli) and overcontrol (i.e., relatively higher attentional control and attentional avoidance of, and relatively lower pupillary reactivity to, threat stimuli) at baseline. We predicted that these effects would be specific to hyperarousal symptoms and negatively valenced stimuli. Confirmation of the proposed hypotheses may further reconcile tensions between models of ABT (i.e., vigilance-avoidance vs. attention maintenance), identify distinct risk profiles for chronicity of PTS symptoms, and pave the way for precision medicine-based approaches to targeting PTS-related ABT.
2. Method
2.1. Participants
Participants were recruited by advertising in local newspapers and posting flyers in public places (e.g., hospitals, mental health clinics, local coffee shops). These flyers targeted individuals who had experienced one or more “stressful life events”. Study staff were contacted by approximately 350 individuals who underwent an initial phone screen. Participants were deemed eligible to participate in the laboratory session (T1) if they (a) were between the ages of 18-65, (b) had normal or corrected vision, (c) were native English speakers, (d) reported experiencing at least one potentially traumatic event defined as per Criterion A of the DSM-5 (APA, 2013), and (e) obtained a score on the Primary Care PTSD Screen for DSM-5 (PC-PTSD-5; Prins et al., 2016) < 2 or ≥ 4. Of the 350 people who were screened over the phone, 130 met these eligibility criteria and 109 completed the T1 session. However, twenty participants were removed from the final sample for having comorbid conditions for which deficits or abnormalities in top-down attentional control are central (e.g., Li, Lin,Chang, & Hung, 2004; Wynn, Breitmeyer, Nuechterlein,& Green, 2006). These included reporting a diagnosis of attention-deficit/hyperactivity disorder, diagnosis of bipolar disorder, current psychosis (First et al., 2015), or cognitive impairment (Folstein, Folstein, & McHugh, 1975).
The average age of the T1 sample (N = 89; 73% female) was 31.9 years (SD = 13.8). In terms of race, 75% self-identified as White, 21% as Black, 1% as Asian, and 3% endorsed “other”. Additionally, 8% of the T1 sample reported being of Hispanic or Latino/a ethnicity. With regard to educational attainment, 99% of the sample had received their high school diploma or GED, 89% reported the completion of at least some higher education, and 44% reported the completion of at least a 4-year college degree. The majority of participants were single (54%), with a household income of less than $50,000 (56%), and were either currently employed (49%) or full-time students (35%).
2.2. Self-report and Interview Measures
Screening measures.
The trauma screening questions from the PTSD module of the Structured Clinical Interview for DSM-5 (SCID-5; First et al., 2015) were used during the phone screen to ensure that potential participants had experienced at least one traumatic event as defined by Criterion A of the DSM-5 (APA, 2013). A score ≥ 4 on PC-PTSD, a 5-item measure designed as a screening tool for PTSD, was identified as the best threshold for maximizing efficiency in identifying probable cases of PTSD (Prins et al., 2016). For the present study, once 50 phone screen-eligible participants with a score on the PC-PTSD-5 < 2 (i.e., low symptoms) were scheduled to participate in the T1 session, participants were required to have a score ≥ 4 (i.e., high symptoms) on the PC-PTSD-5 to be scheduled for the T1 session. This approach was used to ensure that a substantial proportion of the final sample had relatively high levels of posttraumatic stress. In addition, the psychosis screener from the SCID-5 (First et al., 2015) and the Mini Mental Status Exam (MMSE; Folstein et al., 1975) were administered at T1 to ensure that participants did not have a current psychotic disorder or were not cognitively impaired.
Life Events Checklist for DSM-5 (LEC-5; Weathers et al., 2013a).
The LEC-5 assesses lifetime trauma exposure. It consists of a list of 17 potentially traumatic events (e.g., sexual assault, motor vehicle accident, physical assault). For each event, respondents are asked to indicate whether the event happened to them, they witnessed it, they learned about it, it was part of their job, they are unsure, or the event did not apply to them. In the present study, the LEC-5 was used in conjunction with the CAPS-5 and PCL-5 (see below) to identify the Criterion A event that would serve as the index event when assessing current PTS symptoms.
Clinician-Administered PTSD Scale for DSM-5 (CAPS-5; Weathers et al., 2013b).
The CAPS-5 is the gold-standard clinical interview used to assess the 20 DSM-5 PTSD symptoms. The CAPS-5 has exhibited adequate psychometric properties (Weathers et al., 2018). In the present study, the CAPS-5 was administered by graduate level clinical psychology students under the guidance of the last author, a licensed clinical psychologist. The last author independently reviewed 20% of the recorded CAPS-5 interviews for evaluation of interrater reliability. Excellent interrater reliability was observed with an intraclass correlation for total severity of .97 (Cicchetti, 1994). Based on evidence that PTSD is not a discreet clinical syndrome, but rather a dimensional construct (Forbes, Haslam, Williams, & Creamer, 2005; Ruscio, Ruscio, & Keane, 2002), the 20 items of the CAPS-5 were summed to create an overall total score, as well as individual symptom cluster scores, for use as continuous variables. In the present study, 85% of the sample reported at least one symptom that met DSM-5 criteria for PTSD and considerable variability in symptom expression was observed (M symptoms = 5.76 [SD = 4.90], range = 0 -16).
PTSD Checklist-5-Civilian Version (PCL-5; Weathers et al., 2013c).
The PCL-5 is a 20-item self-report measure designed to assess DSM-5 PTSD symptom criteria (APA, 2013). Participants rate each item on a 5-point scale (0 = not at all to 4 = extremely), indicating how much they have been bothered by each symptom in the past month in relation to the potentially traumatic event that they identified as most distressing on the LEC-5. PCL-5 items were summed to create an overall total score and cluster scores for use as continuous variables in our longitudinal analyses. In the present sample, internal consistency for the PCL Total score was adequate at all time points (T1 α = .92, T2 α = .94, T3 α = .95). Additionally, internal consistency for the PCL cluster scores was adequate at all time points (α’s from .72 to .89). Considerable variability in symptom expression was reported on the PCL-5 at all time points (T1 M = 28.53 [SD = 17.19], T2 M = 26.71 [SD = 15.46], and T3 M = 23.52 [SD = 15.59]). Also, it is important to note that a considerable proportion of the sample reported the presence of clinically relevant PTS symptoms at each time point (i.e., 53% at T1, 41% at T2, and 33% at T3 using the more liberal PCL-5 cut score of 28, and 31% at T1, 27% at T2, and 18% at T3 using the most conservative PCL-5 cut score of 37; Blevins, Weathers, Davis, Witte, & Domino, 2015).
Attentional Control Scale (ACS; Derryberry & Reed, 2002).
The ACS is a 20-item self-report measure that assesses one’s ability to flexibly control attention. ACS items are rated on a 4-point scale (1 = Almost never to 4 = Always). Participants were asked to rate how often, or how much, each statement applies to them in general (e.g., “I can quickly switch from one task to another”). The ACS total score has exhibited adequate psychometric properties, including good internal consistency and concurrent validity (Derryberry & Reed, 2002). In the present sample, internal consistency of the ACS total score was adequate at T1 (α = .83, M = 52.96, SD = 8.10).
2.3. Equipment
Participants completed self-report measures and the eye-tracking task on a Hewlett Packard Z230 desktop computer with a 17-inch monitor with a resolution of 1024×768 pixels and a refresh rate of 60 Hz. A computer keyboard and mouse were used to respond to questionnaires. Self-report masures were presented via Qualtrics (http://www.qualtrics.com/). A desktop-mounted EyeLink 1000 Plus eye-tracking system was used to record eye movements and pupil diameter. Recording and stimulus presentation were controlled using SR Research software (SR Research Ltd, Mississauga, Canada). During tracking, the EyeLink 1000 Plus eye-tracking system uses pupil center and corneal reflection to record monocular gaze position at 1000 Hz (1000 samples per second), with up to 0.25° accuracy and 0.01° spatial resolution. Participants were seated at a viewing distance of 60 cm and their heads were secured in a chinrest throughout the task.
2.4. Eye-Tracking Task
The set of images used in the present study included 40 threat (e.g., vicious dog, car accident), 40 positive (e.g., puppies, ice cream), and 80 neutral images (e.g., broom, busy pedestrian sidewalk) from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 1999). IAPS images were originally rated on dimensions of valence and arousal using 9-point rating scales (Lang et al., 1999). General threat images had negative valence (M = 2.2) and high arousal (M = 6.5), positive images had positive valence (M = 7.3) and high arousal (M = 6.1), and neutral images had neither negative nor positive valence (M = 5.1) and low arousal (M = 3.0; Lang et al., 1999).
After a 9-point calibration, participants were instructed that they should view task images freely during the task, as “the purpose of this experiment is to measure parts of your eye, such as your pupil, while you view different pictures on the computer screen” (Bardeen & Daniel, 2017; Buckner, Maner, & Schmidt, 2010). Gaze contingency was used such that the stimuli for each trial were only presented after the participant had attended to a central fixation cross for a duration of at least 500ms at the beginning of each trial. Following the fixation cross, two images appeared side-by-side on the screen (i.e., neutral-neutral, threat-neutral [block 1], or positive-neutral [block 2]) for 3,000 ms (Armstrong, Bilsky, Zhao, & Olatunji, 2013). Neutral-neutral image pairings were presented in both blocks (60 trials in each block) to reduce the expectancy of seeing an emotionally arousing image. After providing corrective feedback on practice trials, a research assistant (RA) sat behind a room divider and communicated with the participant though the use of an intercom system. The order of image type was randomized within each block across participants.
Eye-movements and pupil diameter were recorded over the entire 3,000ms trial interval for threat-neutral and positive-neutral pairings, as well as during the presentation of the fixation cross. The proportion of time attending to threat versus neutral (dwell threat: M = .53 [SD = .21], Spearman r = .88), or positive versus neutral (dwell positive: M = .65 [SD = .10], Spearman r = .77), stimuli for threat-neutral and positive-neutral presentations was calculated as our measures of biased processing.1 Attending was defined as fixations of at least 100ms on either one of the images in each trial pair (Buckner et al., 2010). Pupillary response was calculated by subtracting baseline pupil diameter (i.e., while viewing the pre-trial fixation cross) from pupil diameter during fixation on threat or positive stimul (threat pupillary response: M = 64.43 [SD = 93.67], Spearman r = .87 and positive pupillary response: M = 61.87 [SD = 57.95], Spearman r = .94; Duque, Sanchez, & Vazquez, 2014).
2.5. Procedure
At a single laboratory session (T1), participants completed self-report measures, the CAPS-5 clinical interview, and the eye-tracking task described above. Participants were debriefed before leaving the session and received $90 compensation. All participants consented to follow-up contact, and thus received an invitation (i.e., via e-mail, postal mail, and telephone depending on whether or not a timely response was received) to complete an online battery of questionnaires 6- (M = 190 days [SD = 10.2]) and 12- (M = 373 days [SD = 7.7]) months (T2 & T3) after completing T1. Thirty dollars compensation was provided for completing each online session and these sessions could be completed from any computer with internet access. A bonus payment of $20 was provided for completing all three sessions. Of the 89 participants in the final T1 sample, 85 completed T2 (96% retention) and 83 completed T3 (93% retention). Study procedures were approved by the local institutional review board.
3. Results
3.1. Predicting Dwell Time and Pupillary Response
Total Posttraumatic Stress Symptoms Predicting Dwell Time.
A variable representing the amount of time between the index event and the baseline assessment of symptoms (i.e., time since event) was included as a covariate in all analyses because evidence suggests that differences in this time duration are associated with PTS symptom severity (Amir, Kaplan, & Kotler, 1996; Maercker, Michael, Fehm, Becker, & Margraf, 2004). Hierarchical regression was used to examine total PTS symptoms (i.e., CAPS symptom count) as a moderator of the relationship between attentional control and dwell time on threat. Predictor variables (i.e., PTS symptoms, attentional control, and time since event) were mean centered and entered into the first step of the model (Aiken & West, 1991). Dwell threat served as the outcome variable. An interaction term (PTS symptoms by attentional control) was entered as a predictor variable in the second step of the model.
In the first step of the regression model (R2 = .11, p = .02), attentional control (β = −.12, p = .28) and PTS symptoms (β = .01, p =.95) did not predict dwell threat, while time since event did (β = −.30, p = .004). In the second step of the model (ΔR2 = .03, p =.08), the interaction term (PTS symptoms by attentional control) did not predict dwell threat at the traditional threshold of p < .05 (β = −18, p = .08). However, the size of the interaction was consistent with that which was observed by Bardeen and Daniel (i.e., β = −.19; 2017) in their larger sample (N = 116). As such, we chose to conduct a follow-up simple slopes analysis to determine whether the pattern of the interaction was consistent with that observed by Bardeen and Daniel (2017).
Simple slopes analysis consists of constructing two simple regression equations in which the relationship between the predictor variable and the outcome variable is tested at both high (+1 SD) and low (−1 SD) levels of the moderating variable (i.e., PTS symptoms). Results of the simple slopes analysis indicated that the association between attentional control trended toward significance at high (β = −.25, p < .075), but not low (β = .10, p = .52), levels of PTS symptoms. The nature of the interaction was such that as attentional control increased, dwell threat decreased (avoidance rather than maintenance), but only among those with higher PTS symptoms. The pattern of this interaction is consistent with the hyperarousal by attentional control interaction described below and presented in Figure 1.
Figure 1.
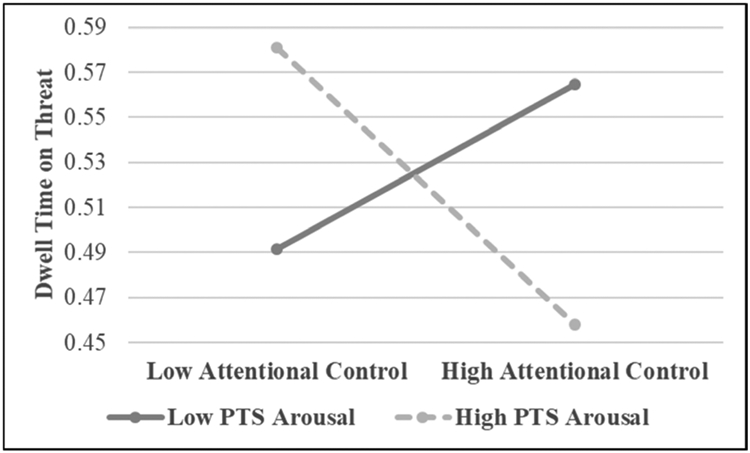
The interaction between posttraumatic stress (PTS) Arousal symptoms and attentional control predicting dwell time on threat.
The same hierarchical regression model was run a second time with the only difference between the models being that dwell positive replaced dwell threat as the outcome variable. In the first step of the regression model (R2 = .05, p = .05), time since event (β = −.17, p = .11) and attentional control (β = .06, p =.59) did not predict dwell positive, while PTS symptoms did (β = −.25, p = .02). In the second step of the model (ΔR2 = .02, p =.16), the interaction term (PTS symptoms by attentional control) did not predict dwell positive (β = −15, p = .16).
Posttraumatic Stress Cluster Scores Predicting Dwell Time.
Regression analysis was used to examine the PTSD symptom clusters as moderators of the relationship between attentional control and dwell time on threat and positive stimuli in separate models (see Table 2). In the first step of the model in which dwell threat served as the outcome variable, time since event was the only main effects variable to be a significant predictor (p = .005). The only interaction term to significantly predict dwell threat in the second step of the model was hyperarousal by attentional control (p = .01; see Figure 1). None of the main effects variables or interaction variables significantly predicted dwell positive in the second model (see Table 2).
Table 2.
PTSD symptom clusters and attentional control predicting pupillary response to threat and positive stimuli
| Pupillary Response to Threat Stimuli |
Pupillary Response to Positive Stimuli |
|||||
|---|---|---|---|---|---|---|
| ΔR2 | Step 1 β |
Step 2 β |
ΔR2 | Step 1 β |
Step 2 β |
|
| Step 1 | .09 | .09 | ||||
| Time since event | −.02 | −.05 | −.10 | −.12 | ||
| Intrusion | .10 | .13 | −.05 | −.04 | ||
| Avoidance | −.20 | −.17 | .05 | .07 | ||
| Cognition | .06 | .02 | .01 | −.02 | ||
| Arousal | −.13 | −.14 | −.17 | −.17 | ||
| AC | −.25* | −.21 | −.24* | −.23^ | ||
| Step 2 | .06 | .02 | ||||
| Intrusion x AC | .08 | .08 | ||||
| Avoidance x AC | .10 | .06 | ||||
| Cognition x AC | −.01 | .01 | ||||
| Arousal x AC | −.32^ | −.18 | ||||
Note. N = 89. AC = attentional control.
p < .06.
p < .05.
p < .01.
p < .001.
Simple slopes analysis was used to further examine the significant interaction between hyperarousal by attentional control in predicting dwell threat (Aiken & West, 1991). A significant negative association was observed between attentional control and dwell threat at high (β = −.39, p =.02), but not low (β = .29, p = .12), levels of hyperarousal. More specifically, as attentional control increased, dwell threat decreased (avoidance rather than maintenance), but only among those with higher hyperarousal symptoms.
Total Posttraumatic Stress Symptoms Predicting Pupillary Response.
Pupillary response to threat and positive stimuli served as outcome variables in separate hierarchical regression models. Predictor variables were consistent with those above (i.e., time since event, PTS symptoms, attentional control, PTS symptoms by attentional control). In the first step of the model in which pupillary response to threat served as the outcome variable (R2 = .09, p = .24), attentional control was a significant predictor (β = −.26, p = .02), while time since event (β = −.02, p =.86) and PTS symptoms were not (β = −.12, p =.27). In the second step of the model (ΔR2 = .02, p = .20), the interaction term did not predict pupillary response to threat (β = −.14, p = .20). In the first step of the model in which pupillary response to positive stimuli served as the outcome variable (R2 = .08, p = .07), attentional control was a significant predictor (β = −.25, p = .03), while time since event (β = −.09, p =.37) and PTS symptoms were not (β = −.15, p =.16). In the second step of the model (ΔR2 = .001, p = .78), the interaction term did not predict positiverelated pupillary response (β = −.04, p = .75).
Posttraumatic Stress Cluster Scores Predicting Pupillary Response.
Regression analysis was used to examine the PTSD symptom clusters as moderators of the relationship between attentional control and pupillary response to threat and positive stimuli in separate models (see Table 2). In the first step of the model in which pupillary response to threat stimuli served as the outcome variable, attentional control was the only main effects variable to be a significant predictor (p = .03). The only interaction term to approach significance as a predictor in the second step of the model was hyperarousal by attentional control (p = .059; see Figure 2). In the first step of the model in which pupillary response to positive stimuli served as the outcome variable, attentional control was the only main effects variable to be a significant predictor (p = .03). In the second step of the model, none of the interaction terms approached significance in predicting pupillary response to positive stimuli (see Table 2).
Figure 2.
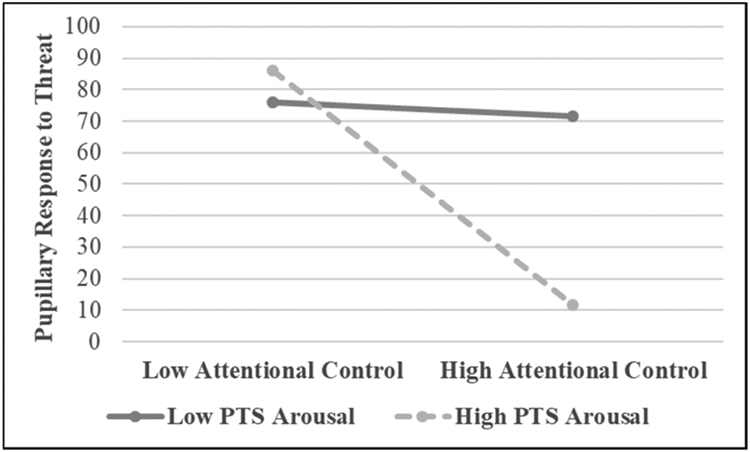
The interaction between posttraumatic stress (PTS) Arousal symptoms and attentional control predicting pupillary response to threat stimuli.
Simple slopes analysis was used to further examine the marginally significant interaction effect between hyperarousal and attentional control in predicting pupillary response to threat stimuli (Aiken & West, 1991). A significant negative association was observed between attentional control and pupillary response to threat at high (β = −.49, p =.006), but not low (β = .06, p = .76), levels of hyperarousal. More specifically, as attentional control increased, pupillary response to threat increased, but only among those with higher hyperarousal symptoms.
3.2. Prospectively predicting Posttraumatic Stress Symptoms
Total Posttraumatic Stress Symptoms as a Longitudinal Predictor.
A series of hierarchical regression models was used to examine the interactions of interest (T1 attentional control x T1 PTS symptoms x T1 eye tracking indices [dwell threat or pupillary response to threat]) predicting T2 and T3 PTS symptoms. Time since event served as a covariate in all models. In the first model, T1 PTS symptoms, T1 attentional control, and T1 dwell threat, as well as the two- and three-way interactions between these variables served as predictor variables (see Table 3). The only difference between the first and second model is that T3 PTS symptoms replaced T2 PTS symptoms as the outcome variable. These models were run a second time with pupillary response to threat replacing dwell threat as the eye-tracking variable in each model (i.e., models three and four).
Table 3.
Time 1 variables predicting posttraumatic stress symptoms at Times 2 and 3
| ET = Time 2 Total Dwell Time to Threat |
ET = Time 2 Pupillary Response to Threat |
|||||||
|---|---|---|---|---|---|---|---|---|
| ΔR1 | Step 1 β |
Step 2 β |
Step 3 β |
ΔR1 | Step 1 β |
Step 2 β |
Step 3 β |
|
| Step 1 | .40*** | .40*** | ||||||
| TSE | .13 | .12 | .12 | .13 | .14 | .13 | ||
| PTSS | .63*** | .64*** | .60*** | .62*** | .63*** | .58*** | ||
| AC | −.01 | −.01 | .03 | −.02 | −.03 | −.01 | ||
| ET | −.03 | −.05 | −.07 | .04 | −.01 | −.01 | ||
| Step 2 | .01 | .01 | ||||||
| PTSS x AC | −.04 | −.01 | −.03 | .13 | ||||
| PTSS x ET | −.07 | −.02 | −.08 | −.00 | ||||
| AC x ET | .10 | .16 | −.01 | .12 | ||||
| Step 3 | .05** | .05** | ||||||
| PTSS x AC x ET | −.25** | −.32** | ||||||
| ET = Time 3 Total Dwell Time to Threat |
ET = Time 3 Pupillary Response to Threat |
|||||||
| ΔR2 | Step 1 β |
Step 2 β |
Step 3 β |
ΔR2 | Step 1 β |
Step 2 β |
Step 3 β |
|
| Step 1 | .35*** | .35*** | ||||||
| TSE | .14 | .13 | .13 | .15 | .13 | .12 | ||
| PTSS | .50*** | .50*** | .44*** | .50*** | .49*** | .44*** | ||
| AC | −.19* | −.18 | −.13 | −.21* | −.20* | −.19* | ||
| ET | .07 | .06 | .03 | −.09 | −.00 | −.01 | ||
| Step 2 | .01 | .02 | ||||||
| PTSS x AC | .01 | .05 | −.02 | .14 | ||||
| PTSS x ET | .06 | .12 | −.10 | −.02 | ||||
| AC x ET | .01 | .09 | −.12 | .02 | ||||
| Step 3 | .08*** | .05** | ||||||
| PTSS x AC x ET | −.31*** | −.33** | ||||||
Note. N = 89. TSE = time since event; PTSS = posttraumatic stress symptoms; AC = attentional control; ET = eye tracking variable.
p < .05.
p < .01.
p < .001.
In the first two models, the 3-way interaction term (T1 PTS symptoms by attentional control by dwell threat) significantly predicted T2 (β = −.25, p = .008) and T3 PTS symptoms (β = −.31, p = .001). These 3-way interactions were examined using the PROCESS macro for SPSS (Hayes, 2013). PROCESS generates simple slopes between the predictor (i.e., T1 PTS symptoms) and outcome variable (T2 or T3 PTS symptoms) at high and low levels (± 1 SD) of both moderators (i.e., attentional control, dwell threat). The association between T1 PTS symptoms and T2 PTS symptoms was significant at the combination of high attentional control + low dwell threat (t = 5.19, SE = .15, p < .001) and low attentional control + high dwell threat (t = 5.18, SE = .15, p < .001). The relationship between T1 PTS symptoms and T2 PTS symptoms was not significant for those with the combinations of low attentional control and low dwell threat (t = 1.73, SE = .19, p = .09) and high attentional control and high dwell threat (t = 1.69, SE = .16, p = .10). The same pattern of effects was observed for the 3-way interaction predicting T3 PTS symptoms. The association between T1 PTS symptoms and T3 PTS symptoms was significant at the combination of high attentional control + low dwell threat (t = 4.00, SE = .16, p < .001) and low attentional control + high dwell threat (t = 5.04, SE = .15, p < .001), but not at combinations of low attentional control and low dwell threat (t = −0.24, SE = .20, p = .81) and high attentional control and high dwell threat (t = 1.56, SE = .17, p = .12). Models one and two were run a second time with the only difference between models being that dwell threat was replaced with dwell positive. Neither of the 3-way interactions significantly predicted PTS symptoms at T2 or T3 (ps = .33 and .43, respectively).
For models three and four (see Table 3), the 3-way interaction term (T1 PTS symptoms by attentional control by pupillary response to threat) significantly predicted T2 (β = −.32, p = .01) and T3 PTS symptoms (β = −.33, p = .01). Simple slopes analysis revealed that the association between T1 PTS symptoms and T2 PTS symptoms was significant at the combination of high attentional control + low pupillary reactivity to threat (t = 4.94, SE = .18, p < .001) and low attentional control + high pupillary reactivity to threat (t = 4.94, SE = .14, p < .001), but not at the combinations of low attentional control and low pupillary reactivity to threat (t = 0.55, SE = .26, p = .59) and high attentional control and high pupillary reactivity to threat (t = 1.30, SE = .16, p = .22). The same pattern of effects was observed for the 3-way interaction predicting T3 PTS symptoms. The association between T1 PTS symptoms and T3 PTS symptoms was significant at the combination of high attentional control + low pupillary reactivity to threat (t = 4.28, SE = .19, p < .001) and low attentional control + high pupillary reactivity to threat (t = 3.82, SE = .15, p < .001), but not at combinations of low attentional control and low pupillary reactivity to threat (t = 0.02, SE = .27, p = .98) and high attentional control and high pupillary reactivity to threat (t = 1.28, SE = .16, p = .20).
Models three and four were run a second time with pupillary reactivity to positive replacing pupillary reactivity to threat in both models. The 3-way interaction term (T1 PTS symptoms by attentional control by pupillary response to positive) significantly predicted T2 (β = −.24, p = .03) and T3 PTS symptoms (β = −.24, p = .03). Specifically, the conditional effect of T1 PTS symptoms by attentional control on T2 PTS symptoms was significant at low pupillary reactivity to positive (t = 2.05, SE = .02, p = .04) , but not at high levels of pupillary reactivity to positive (t = −1.41, SE = .02, p = .16). Among those who exhibited low pupillary reactivity to positive stimuli, the association between T1 PTS symptoms and T2 PTS symptoms was significant for those with high (t = 4.22, SE = .13, p < .001), but no low attentional control (t = .51, SE = .21, p = .61). The same pattern of effects was observed for the 3-way interaction predicting T3 PTS symptoms. The conditional effect of T1 PTS symptoms by attentional control on T3 PTS symptoms was significant at low pupillary reactivity to positive (t = 2.04, SE = .02, p = .045) , but not at high levels of pupillary reactivity to positive (t = −1.14, SE = .01, p = .25). Specifically, among those who exhibited low pupillary reactivity to positive stimuli, the association between T1 PTS symptoms and T3 PTS symptoms was significant for those with high (t = 3.86, SE = .15, p < .001), but not low (t = .27, SE = .21, p = .79), attentional control.
T1 Hyperarousal as a Longitudinal Predictor.
Results from our cross-sectional analyses suggest that among the PTSD symptom clusters, hyperarousal symptoms appear to be driving the moderation effects of interest. It would be ideal to examine the three-way interactions described above while simultaneously entering all four PTSD symptoms cluster scores, as well the two- and three-way interactions among these cluster scores and attentional control and our eye-tracking indices, into each model. Unfortunately, this study, with a sample size of 89, is underpowered to detect the three-way interaction effect of interest with such a large number of predictors in the model. Instead, we replaced total T1 PTS symptoms with T1 hyperarousal symptoms and ran the four models a second time. The three other cluster scores and time since event were included as covariates in all four models. While controlling for the other three symptom clusters and time since event, the associations between the three-way interaction terms ([1] T1 PTS hyperarousal symptoms by attentional control by dwell threat and [2] T1 PTS hyperarousal symptoms by attentional control by pupillary reactivity to threat) and T2 (β = −.20, p = .02 and β = −.25, p = .085, respectively) and T3 PTS symptoms (β = −.35, p <.001 and β = −.41, p = .006, respectively) either trended toward significance with small-medium effects or were significant at p < . 05 with medium-large effects. All of these models were run a second time with the only difference between models being that each threat-related eye-tracking variable was replaced with its positive stimuli counterpart. None of the 3-way interactions in these models significantly predicted PTS symptoms at T2 or T3 (ps from .32 to .50).
For the two models that included dwell threat in the three-way interaction terms, results of simple slopes analysis were consistent with our primary longitudinal findings. For the first model, the association between T1 hyperarousal symptoms and T2 PTS symptoms was significant at the combination of high attentional control + low dwell threat (t = 4.23, SE = .49, p < .001) and low attentional control + high dwell threat (t = 4.45, SE = .52, p < .001). The relationship between T1 PTS symptoms and T2 PTS symptoms was not significant for those with the combinations of low attentional control and low dwell time (t = 1.27, SE = .63, p = .21) and high attentional control and high dwell time (t = 1.67, SE = .54, p = .10). For the second model, the association between T1 hyperarousal symptoms and T3 PTS symptoms was significant at the combination of high attentional control + low dwell threat (t = 4.77, SE = .46, p < .001) and low attentional control + high dwell threat (t = 4.77, SE = .49, p < .001). The relationship between T1 PTS symptoms and T3 PTS symptoms was not significant for those with the combinations of low attentional control and low dwell threat (t = −.16, SE = .60, p = .88) and high attentional control and high dwell threat (t = 1.25, SE = .04, p = .24).
For the two models that included pupillary reactivity in the three-way interaction terms, results of simple slopes analysis were largely consistent with our primary longitudinal findings. For the first model, the association between T1 hyperarousal symptoms and T2 PTS symptoms was significant at the combinations of high attentional control + low pupillary reactivity to threat (t = 3.37, SE = .56, p = .001), low attentional control + high pupillary reactivity to threat (t = 3.98, SE = .51, p < .001), and high attentional control + high pupillary reactivity to threat (t = 2.52, SE = .53, p = .01). The relationship between T1 PTS symptoms and T2 PTS symptoms was not significant for those with the combination of low attentional control and low pupillary reactivity to threat (t = .45, SE = .92, p = .66). For the second model, the association between T1 hyperarousal symptoms and T3 PTS symptoms was significant at the combinations of high attentional control + low pupillary reactivity to threat (t = 4.26, SE = .53, p < .001) and low attentional control + high pupillary reactivity to threat (t = 3.81, SE = .49, p < .001). The relationship between T1 PTS symptoms and T3 PTS symptoms was not significant for those with the combinations of low attentional control and low pupillary reactivity to threat (t = −.16, SE = .86, p = .88) and high attentional control + high pupillary reactivity to threat (t = 1.49, SE = .50, p = .14).
4. Discussion
Theory (e.g., attentional control theory: Eysenck et al., 2007) and empirical evidence (Bardeen & Daniel, 2017; Bardeen & Orcutt, 2011; Bardeen et al., 2016) suggest that, among those with higher levels of PTS symptomatology, those with relatively worse attentional control are more likely to maintain attention on threat compared to those with relatively better attentional control. While our test of this interaction (PTS symptoms by attentional control) was not statistically significant at p < .05 (i.e., p = .08), the size of the interaction effect (i.e., β = −.18) was almost identical to that which was observed by Bardeen and Daniel (i.e., β = −.19; 2017) in their larger sample (N = 116). Additionally, the pattern of the interaction effect is also consistent with Bardeen and Daniel (2017). This pattern is consistent with the attention maintenance hypothesis and has been described elsewhere as a pattern of undercontrol in which one has difficulty disengaging attention from threat due to relative deficits in attentional control (Dennis-Tiwary et al., in press; Weierich et al., 2008). In the present study, these individuals exhibited greater emotional arousal (i.e., sympathetic nervous system arousal) when attending to threat stimuli in comparison to those with higher PTS symptoms and relatively better attentional control who appear to have used attentional control to shift attention from threat to neutral stimuli, thus resulting in the down-regulation of emotional arousal (i.e., maladaptive overcontrol; Dennis-Tiwary et al., 2019). These effects were specific to threat stimuli; they did not generalize to images that were arousing and positively valenced.
Results from the PTSD cluster analysis suggest that hyperarousal symptoms are the driving force behind the moderation effects of interest. This is important not only because these symptoms are present across anxiety and related disorders, but also because when examined prospectively, hyperarousal symptoms (compared to other symptom clusters) have the greatest impact on recovering from PTSD (Marshall, Schell, Glynn, & Shetty, 2006; Schell, Marshall, & Jaycox, 2004). In fact, of the PTSD symptom clusters, hyperarousal symptoms have been shown to be the strongest predictor of functional impairment and overall symptom severity and distress across a number of studies (Heir, Piatigorsky, & Weisaeth, 2010; Maguen, Stalnaker, McCaslin, & Litz, 2009; Shea, Vujanovic, Mansfield, Sevin, & Liu, 2010). Moreover, physiological indicators of hyperarousal (e.g., elevated heart rate) measured shortly after a traumatic event predict the development of PTSD (Bryant, Harvey, Guthrie, & Moulds, 2000). In future research, it will be important to examine associations among ABT, attentional control, and clinical hyperarousal symptoms in larger samples of individuals with anxiety and related disorders to determine the transdiagnostic status of the results of the present study. If study findings generalize across anxiety and related disorders based on hyperarousal symptoms, attention training interventions may be developed, based on maladaptive patterns of ABT (i.e., under- and overcontrol), that could be used for a substantial percent of the population that suffers from fear- and anxiety-related pathology.
Results from the longitudinal analysis suggest that two specific patterns of PTS-related ABT (i.e., under- and overcontrol) put one at risk for symptom maintenance for at least one year. Although both patterns of ABT put one at risk for symptom maintenance, they likely do so through different mechanisms. Information processing theories of emotion regulation suggest that the flexible use of attentional control is important for maintaining psychological well-being (Gross, 2015). Although those who exhibit a pattern of overcontrol have sufficient ability (i.e., attentional control), they appear unwilling to experience the fluctuations in emotional arousal that are commonly experienced upon contact with threat cues. As a result, attentional control is used in rigid fear-based manner to reduce emotional arousal in the short-term, thus paradoxically maintaining symptoms over time.
In contrast, those who exhibit a pattern of undercontrol may be willing to experience the fluctuations in emotional arousal associated with contact with trauma reminders and threat cues, at least to some degree, but lack the ability to use attentional control in a flexible manner. Foa and Kozak (1986) hypothesized that emotional processing, a necessary component of successful exposure therapy for PTSD, is unlikely when a client’s level of fear is too high. With this in mind, an individual with PTSD and relative deficits in attentional control should have greater difficulty using attentional control to bring their level of emotional arousal into a range in which emotional processing can occur and symptoms are subsequently reduced. As such, it may be important to consider the combination of attentional control and willingness to experience short-term emotional distress when trying to determine whether one’s symptoms will be maintained over time. These individual difference factors are also important when considering how to go about treating individuals with PTSD who exhibit maladaptive patterns of ABT.
Automated attention bias modification (ABM) interventions have been developed to train attention (implicitly) away from threat and toward non-threat stimuli (i.e., threat avoidance). These interventions do not consistently alter ABT or reduce PTSD symptoms (Badura-Brack et al., 2015; Schoor, Putman, & Van Der Does, 2013). These findings are less perplexing when one considers that the basic assumption of ABM, that individuals with fear- and anxiety-related pathology (i.e., PTSD) preferentially process threat, appears to be incorrect. Results from the present study, as well as previous research (Bardeen & Daniel, 2017; Bardeen & Orcutt, 2011; Bardeen et al., 2016; Derryberry & Reed, 2002; Ho et al., 2017), suggest that it is important to account for individual differences in attentional control to understand patterns of threat processing among those with fear- and anxiety-related pathology. Results of the present study suggest that traditional ABM programs, designed to train attention away from threat, may still hold value for alleviating PTS symptoms among those that lack the ability to maintain threat disengagement (i.e., undercontrollers).
Importantly, the present results suggest that ABM interventions may be contraindicated for those with PTS symptomatology and relatively better attentional control who exhibit chronic and inflexible threat avoidance. These individuals may be better served by participating in interventions that promote flexibility of attentional control (e.g., the attention training component of Emotion Regulation Therapy: Renna et al., 2018; the Attention Training Technique: Wells, 1990; see Fergus & Bardeen, 2016, for a review). Additionally, treatments that have been shown to be effective in increasing willingness to stay in contact with uncomfortable emotions and related internal experiences may be beneficial for individuals who exhibit rigid threat-avoidance (e.g., Acceptance and Commitment Therapy: Hayes, Luoma, Bond, Masuda, & Lillis, 2006; Mindfulness-Based Stress Reduction: Kabat-Zinn, 1990). It seems likely that larger and more stable effect sizes will be observed for the efficacy of ABM and treatments that promote attentional flexibility by identifying individuals with two distinct maladaptive patterns of ABT (i.e., under- and overcontrollers) at the outset of treatment and differentially assigning them to the type of treatment that corresponds to their respective patterns of ABT.
Study limitations must be acknowledged. As described, our test of this theoretically based interaction (total PTS symptoms by attentional control) was not statistically significant atp < .05 (i.e., p = .08). The value of interpreting the magnitude of effects rather than relying solely on p values has been noted (e.g., Cohen, 1994; Denis, 2003; Thompson, 1996). This is especially important when sample sizes are relatively small. Whereas statistical significance is in part a function of the size of the sample, effect sizes remain consistent across studies with different sample sizes. For example, the size of the interaction between total PTS symptoms and attentional control in predicting dwell time on threat in the present study (i.e., β = −.18) was almost identical to that which was observed by Bardeen and Daniel (i.e., β = −.19; 2017) in their larger sample. Had our sample size been larger, the above small to medium effect in all likelihood would have reached statistical significance.
Although eye-tracking indices of ABT are largely reliable, indices used to capture reflexive orienting toward threat (e.g., dwell time on threat in the first 500ms, latency to first fixation on threat) tend to exhibit poor reliability (Waechter et al., 2014). As such, study findings should be replicated using covert (as opposed to overt) measures of ABT that consistently exhibit adequate reliability throughout the entire time course of threat processing. Some evidence suggests that overt attention (i.e., eye movements) does not always follow covert attention (Heyman, Montemayor, & Grisanzio, 2017). As such, it is possible that individuals with higher PTS symptoms covertly, but not overtly, orient toward threat and then subsequently avoid or maintain attention on such stimuli (overtly) as a function of individual differences in attentional control. Additionally, because ABT and pupillary reactivity were not assessed at the online follow-up sessions, we cannot determine temporal relations among these constructs. Study results suggest that differential change in ABT indices over time may mediate hyperarousal symptom maintenance in overcontrollers (i.e., decrease in ABT) and undercontrollers (i.e., increase in ABT). It will be important in future research to use experimental and longitudinal study designs with an assessment of ABT at all time points to clarify the temporal nature of relations among these constructs and to infer causality.
Because some have suggested that the Attentional Control Scale (Derryberry & Reed, 2002) may measure beliefs about attentional control rather than actual attentional control ability (Spada, Georgiou, & Wells, 2010), it will be important to use objective measures of attentional control in this line of research in the future. Much like commonly used measures of ABT, stimulus-response tasks that require button press are often used as objective measures of attentional control. While these measures have benefits over self-report, button-press introduces additional error variance because individual differences in motor speed contribute to the scores that are calculated from these tasks. Fortunately, attentional control can be reliably assessed through the use of eye-tracking technology (i.e., the antisaccade task; Hallett, 1978). As described, eye-tracking is less susceptible to alternate explanations than stimulus response tasks that require button press.
The degree to which task stimuli were unrelated to participant-specific traumatic experiences is unknown because participants did not provide ratings of trauma relevance for the images that were used in the study. Because our goal was to use general threat stimuli, it would have been ideal to identify task images that were completely unrelated to each participant’s trauma history. However, removing all images that were potentially trauma-related was not feasible because the large majority of participants reported experiencing multiple traumatic events. Moreover, study results would have been confounded had participants viewed stimuli for the purpose of making ratings prior to completing the free-viewing task because repeated exposure to the stimuli may have reduced image-related arousal during the task. Instead, to reduce trauma-specific responding, we identified images with a wide variety of content that were pre-tested elsewhere on dimensions of valence and arousal (IAPS; Lang et al., 1999).
To our knowledge, the present study is the first to provide evidence that two distinct profiles of PTS-related ABT predict symptom maintenance over the course of one year. Results from this study may have profound treatment implications in terms of precision medicine, suggesting that the use of a one-size-fits-all attention modification intervention for PTS symptomatology is contraindicated for those with higher PTS symptoms and relatively better attentional control who exhibit chronic and inflexible threat avoidance. These individuals may be better served by interventions that promote flexibility of attentional control, whereas traditional attention bias modification interventions may still hold value for treating individuals with relatively worse attentional control who exhibit attention maintenance on threat. Additionally, among PTS symptom clusters, hyperarousal symptoms appear to be central to the moderation effects of interest. Hyperarousal symptoms are common across anxiety and related disorders, and thus, may explain similar findings from studies in which other forms of pathology were assessed. Finally, because individual differences in attentional control predict distinct patterns of ABT among those with relatively higher symptoms (i.e., avoidance vs. maintenance), it is critical that we independently assess this construct in future studies in which fear- and anxiety-related ABT is examined. Failing to do so, may mask potentially important findings and further hinder progress in this area of research.
Table 1.
PTSD symptom clusters and attentional control predicting dwell time on threat and positive stimuli
| Dwell Time on Threat Stimuli |
Dwell Time on Positive Stimuli |
|||||
|---|---|---|---|---|---|---|
| ΔR2 | Step 1 β |
Step 2 β |
ΔR2 | Step 1 β |
Step 2 β |
|
| Step 1 | .15* | .11 | ||||
| Time since event | .29** | −.33** | −.17 | −.17 | ||
| Intrusion | .14 | .14 | −.05 | −.02 | ||
| Avoidance | −.25 | −.24 | −.18 | .18 | ||
| Cognition | .17 | .16 | −.19 | −.20 | ||
| Arousal | −.07 | −.09 | .08 | .08 | ||
| AC | −.09 | −.04 | −.08 | −.02 | ||
| Step 2 | .09^ | .03 | ||||
| Intrusion x AC | .13 | −.14 | ||||
| Avoidance x AC | .00 | .11 | ||||
| Cognition x AC | −.06 | .04 | ||||
| Arousal x AC | −.40* | −.17 | ||||
Note. N = 89. AC = attentional control.
p < .05.
p < .01.
p < .001.
Highlights.
Assessed attentional control (AC), attentional bias to threat (ABT), and posttraumatic stress (PTS) symptoms
Higher PTS + Higher AC (overcontrollers) predicted less ABT and arousal at baseline (T1)
Higher PTS + Lower AC (undercontrollers) predicted more ABT and arousal at baseline (T1)
PTS hyperarousal symptoms were of central importance to these effects
Under- and Over-controllers were at high risk of symptom maintenance 6- and 12-months later
Acknowledgements
This research was supported by a grant from the National Institute of Mental Health awarded to the first, second, fourth, and fifth authors (R21MH112929). We thank Natasha Benfer, Kate Clauss, Kelsey Thomas, Victoria Swaine, Natalie Conboy, and Kaylin Farmer for their assistance with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Consistent with previous research (Armstrong et al., 2013; Bardeen & Daniel, 2017; Buckner et al., 2010), dwell threat and dwell positive were also calculated for each 500ms epoch interval within the larger 3000ms presentation duration to examine differences in dwell time across early and later stages of information processing. Mean differences in dwell time, based on the four combinations of each cluster score and attentional control (i.e., high and low values of each ± 1 SD), were not significantly different at any of the epoch intervals, thus suggesting that differences in the slope of threat bias over time, based on these individual differences, was likely of relatively little substantive value in this study. As such, and based on the advice of an Reviewer, we removed these analyses from the manuscript to improve the readability of the results section.
References
- Aiken LS, & West SG (1991). Multiple Regression: Testing and interpreting interactions. Newbury Park, CA: Sage. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Amir M, Kaplan Z, & Kotler M (1996). Type of trauma, severity of posttraumatic stress disorder core symptoms, and associated features. The Journal of General Psychology, 123, 341–351. [DOI] [PubMed] [Google Scholar]
- Armstrong T, Bilsky SA, Zhao M, & Olatunji BO (2013). Dwelling on potential threat cues: an eye movement marker for combat-related PTSD. Depression and Anxiety, 30, 497–502. [DOI] [PubMed] [Google Scholar]
- Badura-Brack AS, Naim R, Ryan TJ, Levy O, Abend R, Khanna MM, Bar-Haim Y (2015). Effect of attention training on attention bias variability and PTSD symptoms: Randomized controlled trials in Israeli and U.S. combat Veterans. The American Journal of Psychiatry, 172, 1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardeen JR, & Daniel TA (2017). An eye-tracking examination of emotion regulation difficulties, cognitive reappraisal, and expressive suppression as predictors of attentional bias and pupillary reactivity to threat stimuli. Cognitive Therapy & Research, 41, 853–866. [Google Scholar]
- Bardeen JR, Tull MT, Daniel TA, Evenden J, & Stevens EN (2016). A preliminary investigation of the time course of attention bias variability in posttraumatic stress disorder: The moderating role of attentional control. Behaviour Change, 33, 94–111. [Google Scholar]
- Bardeen JR, & Orcutt HK (2011). Attentional control as a moderator of the relationship between posttraumatic stress symptoms and attentional threat bias. Journal of Anxiety Disorders, 25, 1008–1018. [DOI] [PubMed] [Google Scholar]
- Beck AT, Emery G, & Greenberg R (1985). Anxiety disorders and phobias: A cognitive perspective. New York, NY: Basic Books [Google Scholar]
- Blevins CA, Weathers FW, Davis MT, Witte TK, & Domino JL (2015). The posttraumatic stress disorder checklist for DSM-5 (PCL-5): Development and initial psychometric evaluation. Journal of Traumatic Stress, 28(6), 489–498. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Miccoli L, Escrig MA, & Lang PJ (2008). The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology, 45, 602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RA, Harvey AG, Guthrie RM, & Moulds ML (2000). A prospective study of psychophysiological arousal, acute stress disorder, and posttraumatic stress disorder. Journal of Abnormal Psychology, 109, 341–344. [PubMed] [Google Scholar]
- Buckner JD, Maner JK, & Schmidt NB (2010). Difficulty Disengaging Attention from Social Threat in Social Anxiety. Cognitive Therapy and Research, 34, 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti Domenic V. (1994). "Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology". Psychological Assessment. 6, 284–290. [Google Scholar]
- Cohen J (1994). The earth is round (p < .05). American Psychologist, 49, 997–1003. [Google Scholar]
- Denis DJ (2003). Alternatives to null hypothesis significance testing. Theory and Science, 4, 1–19. [Google Scholar]
- Dennis-Tiwary TA, Roy AK, Denefrio S, & Myruski S (2019). Heterogeneity of the anxiety-related attention bias: A review and working model for future research. Clinical Psychological Science, 7, 879–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derryberry D, & Reed MA (2002). Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology, 111, 225–236. [DOI] [PubMed] [Google Scholar]
- Duque A, Sanchez A, & Vazquez C (2014). Gaze-fixation and pupil dilation in the processing of emotional faces: the role of rumination. Cognition and Emotion, 28, 1347–1366. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, & Calvo MG (2007). Anxiety and cognitive performance: Attentional control theory. Emotion, 7, 336–353. [DOI] [PubMed] [Google Scholar]
- Fergus TA, & Bardeen JR (2016). The Attention Training Technique: A review of a neurobehavioral therapy for anxiety and related disorders. Cognitive and Behavioral Practice, 23, 502–516. [Google Scholar]
- First MB, Williams JBW, Karg RS, & Spitzer R (2015). Structured Clinical Interview for DMS-5, Research Version. Arlington, VA: American Psychiatric Association. [Google Scholar]
- Foa EB, & Kozak MJ (1986). Emotional processing of fear: Exposure to corrective information. Psychological Bulletin, 99, 20–35. [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Forbes D, Haslam N, Williams BJ, & Creamer M (2005). Testing the latent structure of posttraumatic stress disorder: a taxometric study of combat veterans. Journal of Traumatic Stress, 18, 647–656. [DOI] [PubMed] [Google Scholar]
- Galatzer-Levy IR, & Bryant RA (2013). 636,120 ways to have posttraumatic stress disorder. Perspectives on Psychological Science, 8, 651–662. [DOI] [PubMed] [Google Scholar]
- Gross JJ (2015). Emotion regulation: Current status and future prospects. Psychological Inquiry, 26, 1–26. [Google Scholar]
- Hallett PE (1978). Primary and secondary saccades to goals defined by instructions. Vision Research, 18, 1279–1296. [DOI] [PubMed] [Google Scholar]
- Hayes SC, Luoma JB, Bond FW, Masuda A, & Lillis J (2006). Acceptance and Commitment Therapy: Model, processes and outcomes. Behaviour Research and Therapy, 44, 1–25. [DOI] [PubMed] [Google Scholar]
- Heir T, Piatigorsky A, & Weisaeth L (2010). Posttraumatic stress symptom clusters associations with psychopathology and functional impairment. Journal of Anxiety Disorders, 24, 936–940. [DOI] [PubMed] [Google Scholar]
- Heymen GM, Montemayor J, & Grisanzio KA (2017). Dissociating attention and eye movements in a quantitative analysis of attention allocation. Frontiers in Psychology, 8, 715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SMY, Yeung D, & Mak CWY (2017). The interaction effect of attentional bias and attentional control on dispositional anxiety among adolescents. British Journal of Psychology, 108, 564–582. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J (1990). Full catastrophe living: using the wisdom of your body and mind to face stress, pain, and illness. New York: Delacourt. [Google Scholar]
- Kimble MO, Frueh BC, & Marks L (2009). Does the modified Stroop effect exist in PTSD? Evidence from dissertation abstracts and the peer reviewed literature. Journal of Anxiety Disorders, 23, 650–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert BN (1999). International affective picture system (IAPS): Technical Manual and Affective Ratings. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida. [Google Scholar]
- Li CR, Lin W, Chang FL, & Hung Y (2004). A psychophysical measure ofattention deficit in children with attention-deficit/hyperactivity disorder. Journal of Abnormal Psychology, 113, 228–236. [DOI] [PubMed] [Google Scholar]
- Maercker A, Michael T, Fehm L, Becker ES, & Margraf J (2004). Age of traumatisation as a predictor of post-traumatic stress disorder or major depression in young women. British Journal of Psychiatry, 184, 482–487. [DOI] [PubMed] [Google Scholar]
- Maguen S, Stalnaker M, McCaslin S, & Litz BT (2009). PTSD subclusters and functional impairment in Kosovo peacekeepers. Military Medicine, 174, 779–785. [DOI] [PubMed] [Google Scholar]
- Marshall GN, Schell TL, Glynn SM, & Shetty V (2006). The role of hyperarousal in the manifestation of posttraumatic psychological distress following injury. Journal of Abnormal Psychology, 115, 624–628. [DOI] [PubMed] [Google Scholar]
- Partala T, & Surakka V (2003). Pupil size variation as an indication of affective processing. International Journal of Human-Computer Studies, 59, 185–198. [Google Scholar]
- Pineles SL, Shipherd JC, Welch LP & Yovel I (2007). The role of attentional biases in PTSD: Is it interference or facilitation? Behaviour Research and Therapy, 45, 1903–1913. [DOI] [PubMed] [Google Scholar]
- Prins A, Bovin MJ, Smolenski DJ, Marx BP, Kimerling R, Jenkins-Guarnieri MA, … Tiet QQ (2016). The Primary Care PTSD Screen for DSM-5 (PC-PTSD-5): Development and Evaluation Within a Veteran Primary Care Sample. Journal of General Internal Medicine, 31, 1206–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renna ME, Seeley SH, Heimberg RG, Etkin A, Fresco DM, & Mennin DS (2018). Increased attention regulation from emotion regulation therapy for generalized anxiety disorder. Cognitive Therapy and Research, 42, 121–134. [Google Scholar]
- Ruscio AM, Ruscio J, & Keane TM (2002). The latent structure of posttraumatic stress disorder: a taxometric investigation of reactions to extreme stress. Journal of Abnormal Psychology, 111, 290–301. [PubMed] [Google Scholar]
- Sarapas C, Weinberg A, Langenecker SA, & Shankman SA (2016). Relationships among attention networks and physiological responding to threat. Brain and Cognition, 111, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell TL, Marshall GN, & Jaycox LH (2004). All symptoms are not created equal: The prominent role of hyperarousal in the natural course of posttraumatic psychological distress. Journal of Abnormal Psychology, 113, 189–197. [DOI] [PubMed] [Google Scholar]
- Schoorl M, Putman P, & Van Der Does W (2013). Attentional bias modification in posttraumatic stress disorder: a randomized controlled trial. Psychother Psychosom, 82, 99–105. [DOI] [PubMed] [Google Scholar]
- Shea MT, Vujanovic AA, Mansfield AK, Sevin E, & Liu F (2010) Posttraumatic stress disorder symptoms and functional impairment among OEF and OIF National Guard and reserve veterans. Journal of Traumatic Stress, 23, 100–107 [DOI] [PubMed] [Google Scholar]
- Spada MM, Georgiou GA, & Wells A (2010). The relationship among metacognitions, attentional control, and state anxiety. Cognitive Behaviour Therapy, 39, 64–71. [DOI] [PubMed] [Google Scholar]
- Taylor CT, Cross K, & Amir N (2016). Attentional control moderates the relationship between social anxiety symptoms and attentional disengagement from threatening information. Journal of Behavior Therapy and Experimental Psychiatry, 50, 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B (1996). AERA editorial policies regarding statistical significance testing: Three suggested reforms. Educational Researcher, 25, 26–30. [Google Scholar]
- Van Bockstaele B, Verschuere B, Tibboel H, De Houwer J, Crombez G, & Koster EH (2014). A review of current evidence for the causal impact of attentional bias on fear and anxiety. Psychological Bulletin, 140, 682–721. [DOI] [PubMed] [Google Scholar]
- Wald I, Schechner T, Bitton S, Holoshitz Y, Charney DS, Muller D, … & Bar-Haim Y (2013). Attention bias away from threat during life threatening danger predicts PTSD symptoms at one-year follow-up. Depression and Anxiety, 28, 406–411. [DOI] [PubMed] [Google Scholar]
- Waechter S, Nelson AL, Wright C, Hyatt A, & Oakman J (2014). Measuring attentional bias to threat: Reliability of dot probe and eye movement indices. Cognitive Therapy and Research, 38, 313–333. [Google Scholar]
- Weathers FW, Blake DD, Schnurr PP, Kaloupek DG, Marx BP, & Keane TM (2013a). The Life Events Checklist for DSM-5 (LEC-5). Instrument available from the National Center for PTSD. www.ptsd.va.gov. [Google Scholar]
- Weathers FW, Blake DD, Schnurr PP, Kaloupek DG, Marx BP, & Keane TM (2013b). The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5). Interview available from the National Center for PTSD at www.ptsd.va.gov. [Google Scholar]
- Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, & Schnurr PP (2013c). The PTSD Checklist for DSM-5 (PCL-5). Scale available from the National Center for PTSD at www.ptsd.va.gov. [Google Scholar]
- Weathers FW, Bovin MJ, Lee DJ, Sloan DM, Schnurr PP, Kaloupek DG, … Marx BP (2018). The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychological Assessment, 30, 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weierich MR, Treat TA, & Hollingworth A (2008). Theories and measurement of visual attentional processing in anxiety. Cognition & Emotion, 22, 985–1018. [Google Scholar]
- Wells A (1990). Panic disorder in association with relaxation induced anxiety: An attentional training approach to treatment. Behavior Therapy, 21, 273–280. [Google Scholar]
- Williams JMG, Watts FN, MacLeod C, & Mathews A (1997). Cognitive psychology and the emotional disorders (2nd ed.). New York, NY: Wiley. [Google Scholar]
- Wynn JK, Breitmeyer B, Nuechterlein KH, & Green MF (2006). Exploring the short-term visual store in schizophrenia using the attentional blink. Journal of Psychiatric Research, 40, 599–605. [DOI] [PubMed] [Google Scholar]
- Zelazny K, & Simms LJ (2015). Confirmatory factor analyses of DSM-5 posttraumatic stress disorder symptoms in psychiatric samples differing in Criterion A status. Journal of Anxiety Disorders, 34, 15–23. [DOI] [PubMed] [Google Scholar]


