Abstract
Faba bean (Vicia faba L.) is the major food legume crop in Tunisia. However, its growth and yield is strongly affected by water‐limited environments. In this study, osmotic stress exhibited a negative effect on Bachar and Badii cultivar. Nevertheless, the deteriorating effects of osmotic stress were relatively low on studied parameters of Bachar due to its better efficiency to reduce oxidative damage by increasing enzymatic activities such as catalase (CAT), superoxide dismutase (SOD) and ascorbate peroxidase (APX), accumulation of total chlorophyll (Chlt), soluble sugars and leaf relative water content (RWC). GC–MS analysis determined a total of 11 soluble carbohydrates induced by osmotic stress and differentially accumulated in the both cultivars. Bachar showed elevated levels of mannose, glucose, galactose, ribose, rhamnose and myo-inositol which might help to maintain osmotic adjustment, membranes and proteins protection from the damaging effect of reactive oxygen species. Sugar metabolism related genes (VfNINV3, VfPHS2, VfFRK4, VfHXK1, VfGPI1, VfSTP1.1, VfpGlcT1.1, VfSTP5.1, VfpGlcT1.2, VfSWEET2.1, VfVINV2, VfSUS1, VfPGM1, VfSUT1.1, VfGPT1, VfSPS1, VfSPP1, VfPHS1, VfSUT4.1 and VfTMT1.1) were differentially expressed in both cultivars demonstrating their important roles in sugar accumulation. Most of these genes were upregulated in the leaves of Bachar under moderate and severe stress, which could lead to increase glycolysis and tricarboxylic acid cycle in order to accelerate energy production, necessary to increase osmotic regulation and consequently enhancing the osmotic stress tolerance in that cultivar. Overall, sugars accumulation ability can be used as a useful indicator for the osmotic stress tolerant potential in faba bean breeding programs.
Supplementary Information
The online version contains supplementary material available at (10.1007/s12298-021-00935-1).
Keywords: Faba bean, Gene expression, GC–MS, Osmotic stress, Sugar metabolism, qRT-PCR
Introduction
Faba bean (Vicia faba L.) is a major food legume crop grown and consumed in Tunisia because it represents an important source of proteins for animal feed and human nutrition. It is also incorporated in crop rotation systems with cereals in semi-arid regions in order to provide substantial benefits such as increasing and maintaining soil fertility due to its high capacity for nitrogen fixation. This could lead to reducing fertilizer input, pests and soil-borne disease incidence in cereals and consequently a potential increases in the yield (Karkanis et al. 2018). In 2018, the amount of land dedicated to faba bean was 54,907 ha with 64,508 tons of yield (FAOSTAT 2018). Indeed, the national average faba bean production is low (1.12 t/ha) and unstable, characterized by wide fluctuations compared to the worldwide average harvest (2.26 t/ha) (FAOSTAT 2018) due to the lack of suitable genetic material with stable yield and reasonable tolerance to biotic (prevalent diseases and pests) and abiotic (mainly water deficit) stresses. Recently, the production of faba bean in Tunisian semi-arid regions is likely to be drastically affected by the gradual increase of global temperature and the reduced precipitation, due to the negative effect of hot and dry climate conditions on the growth of this crop, thus reducing the interest of the farmers to produce it (Ouji et al. 2017). It is well known that Vicia faba is more sensitive to heat and, in greater manner, to water deficit compared to other grain legumes like common bean, pea and chickpea (Khan et al. 2010).
It is well known that carbohydrates such as starch, glucose, sucrose, fructose, trehalose, raffinose, stachyose and inositol are commonly synthesized in plants by photosynthesis. These carbohydrates are essential for many processes including energy transference, signal transduction, osmoprotection and tolerance (Sami et al. 2016). Soluble carbohydrates (sugars) are considered as important metabolites and osmolytes in plants under environmental stresses including drought (Arabzadeh 2012). The accumulation of soluble sugars under abiotic stresses (mainly drought and salt stress) plays not only a leading role in osmoprotection and osmotic adjustment but also helps in stabilizing membranes, regulation of gene expression and signaling (Rosa et al. 2009). Moreover, the roles of sugars as signaling molecules, especially in plant microbe interactions and systemic role as modulators of plant immunity are reported (Trouvelot et al. 2014).
Several reports on sugars accumulation in different parts of plant during drought stress in various species such as maize (Mohammadkhani and Heidari 2008), soybean (Sarkar et al. 2015), pepper (Okunlola et al. 2016), faba bean (Abid et al. 2017) and wheat (Abid et al. 2018) have been published. In this context, soluble sugar accumulation was strongly positively correlated with productivity of common bean (Andrade et al. 2016) and wheat (Marček et al. 2019) under drought conditions, making this component potentially useful indicator for drought tolerance and biochemical marker for selection of genotypes for breeding programs. Interestingly, previous studies reported that soluble sugars content increased as a result of drought stress for all studied faba bean genotypes, however, the highest increases were observed mainly in drought-tolerant genotypes (Abid et al. 2017). Similar results have been reported by other authors in wheat (Yang et al. 2007). Moreover, drought-resistant genotypes of rice were characterized by higher soluble sugar accumulation compared to the susceptible ones (Xu et al. 2015). These data suggest a crucial role of sugars in plant drought stress tolerance.
It has been reported that diverse carbohydrates accumulation depends on the level of drought stress, plant species, and plant organs and, also, depends on the used genotype or cultivar. For example, application of drought stress to Luohan7 (LH7), a higher wheat drought resistant cultivar and Xinong979 (XN979), a lower drought resistant cultivar, resulted in the differential accumulation of total soluble sugars (sucrose, glucose, fructose, and fructan) in peduncle, penultimate and lower internodes (Hou et al. 2018). In the leaves and roots of soybean cultivars Shennong8 and Shennong12, drought induced the accumulation of soluble sugars, but reduction in starch content observed (Du et al. 2020). In potato, drought challenge led to the accumulation of sucrose in the source leaves of cultivars with contrasting drought responses (Aliche et al. 2020). Moreover, Legay et al. (2011) found that the potato drought tolerant accession (397,077.16) and the sensitive variety (Canchan) exhibited an increase in galactinol, raffinose, galactose and inositol content in leaves. However, drought reduced the level of fructose, galactose and sucrose.
It is worth to notice that several members of various gene families encoding key enzymes including invertase (INV), cell wall invertase (CWINV) sucrose synthase (SUS), sucrose phosphate synthase (SPS), phosphoglucoisomerase (GPI), phosphoglucomutase (PGM) and UDPG-pyrophosphorylase (UGP) are key determinants involved in sugar metabolism, biosynthesis and composition. Moreover, transporters like sucrose-will-eventually-be-exported-transporter (SWEET), sucrose transporter (SUT) and monosaccharide transporter (MST) are implicated in sugar accumulation (Li et al. 2012). According to Kang et al. (2019), MST family comprises seven groups such as sugar transport protein (STP), polyol/monosaccharide transporter (PLT), inositol transporter (INT), vacuolar glucose transporter (VGT), tonoplast membrane transporter (TMT), plastidic glucose transporter (pGlcT) and early-responsive to dehydration six-like (ESL).
The aim of this research was to study the effect of osmotic stress on soluble carbohydrates (sugars) and expression profiles of twenty corresponding metabolism-related genes in leaf tissues under osmotic stress in two faba bean genotypes (Bachar and Badii) with contrasting levels of drought tolerance. These findings will provide useful information for genetic improvement of faba bean to environmental stress challenge.
Materials and methods
Plant material, growth conditions, treatments and experimental design
The research was conducted in a growth chamber under controlled conditions (temperature of 23 ± 2 °C, relative humidity 55–65%, light 270 μmol of photons m−2 s−1 photosynthetic active radiations and a 14/10 h day/night photoperiod) during the period of 2019–2020 at the Experimental Station of Biotechnology Center of Borj Cedria (35 km South-east of Tunis; 36° 42′ N, 10° 28′ E). Two faba bean (Vicia faba L. var. minor) cultivars Bachar and Badii were used in the study. A previous field experiment was conducted by Ouji et al. (2017) in Tunisian semi-arid zone in order to determine drought tolerant and sensitive genotypes on the basis of faba bean yield. The obtained results showed that Bachar has low drought susceptibility index (DSI) value than Badii and consequently has a better ability to tolerate drought stress. The seeds, obtained from plants grown in the National Institute of Agricultural Research in Tunisia, were surface sterilized with 5% sodium hypochlorite (NaClO) solution for 5 min, thoroughly washed with sterile distilled water and germinated on perlite at 23 ± 2 °C. Two weeks old seedlings (corresponding to four fully expanded leaves) were uprooted from the perlite and transplanted in plastic boxes (height 10 cm, width 17 cm, length 40 cm) filled with 5L of Hoagland nutrient solution (Hoagland and Arnon 1950) and oxygenated by oxygen pumps. Therefore after the transfer, faba bean seedlings were grown hydroponically with density of 15 seedlings per box for one week (acclimatization period) before subjecting to three different levels of osmotic stress namely, mild osmotic stress (Mild OS), moderate osmotic (Moderate OS) and severe osmotic stress (Severe OS) induced by polyethylene glycol (PEG-6000), corresponding to final osmotic potentials of − 0.30 MPa (10% PEG), − 0.51 MPa (15% PEG) and − 0.80 MPa (21% PEG), respectively (Muscolo et al. 2014). Untreated seedlings in nutrient solution were used as controls. Three replicates (3 boxes) for each treatment were executed and repeated three times under the same conditions. Two days after treatments, the seedlings were harvested and all physiological, biochemical and chemical parameters were measured. For molecular analyses, leaf tissues were snap frozen in liquid N2, and stored at − 80 °C until further analysis.
Measurement of gas exchange parameters
The net CO2 assimilation rate (A), stomatal conductance (gs), transpiration rate (E), and intercellular CO2 concentration (Ci) of the youngest fully expanded attached leaves and uniform in terms of age were measured using a Portable Photosynthesis System (LCpro+ , Inc., UK). The measurements were taken between 10 and 12 AM using five leaves for each treatment. The photosynthetically active radiation in the leaf chamber was set at 980 µmol m−2 s−1 during the monitoring.
Measurement of relative water content (RWC)
The third fully developed leaf from the top was used for RWC measurement. Leaf RWC was determined according to the method proposed by Barrs and Weatherleyt (1962). The RWC was calculated through the formula: RWC (%) = (fresh weight − dry weight)/(turgid weight − dry weight) × 100.
Measurement of proline, soluble carbohydrates, chlorophyll (Chl), hydrogen peroxide (H2O2) and malondialdehyde (MDA) content and leaf electrolyte leakage (EL) level
To determine the Chl content, 0.1 g of fresh leaf samples were ground in 80% acetone to extract total Chl. After centrifugation at 12,000 rpm for 15 min, the absorbance was read spectrophotometrically at 663 and 646 nm. Total chlorophyll (Chlt), Chl a and Chl b were calculated according to Lichtenthaler (1987).
Proline content was determined according to the method of Bates et al. (1973). Dry leaf samples (0.1 g) were extracted in 3% (w/v) sulfosalicylic acid solution. After centrifugation at 12,000 rpm for 10 min, 2 ml of supernatant was homogenized with 2 ml of glacial acetic acid and ninhydrin reagent. The mixture was incubated at 100 °C in water bath for 1 h and then cooled on ice. The homogenate was extracted with 4 ml of toluene and the upper phase was read spectrophotometrically at 520 nm. Calibrations were made with l-proline.
H2O2 concentration was measured using the method of Velikova et al. (2000). Briefly, leaf samples (0.5 g) were extracted with 5 ml of 0.1% (w/v) trichloroacetic acid (TCA) and the centrifuged at 12,000 rpm for 10 min. One ml of 10 mM potassium phosphate buffer (pH 7.0) and 2 ml of 1 M KI was added to 1 ml of the supernatant. The absorbance of supernatant was read at 390 nm and the H2O2 level was calculated using a standard calibration curve and expressed as µmol g−1 FW.
The concentration of MDA was determined based on the protocol described by Dhindsa et al. (1981). Leaf samples (0.5 g) were homogenized in 5 ml of 0.1% (w/v) trichloroacetic acid (TCA). After centrifugation at 12,000 rpm for 10 min, 4 ml of 0.5% (w/v) thiobarbituric acid (TBA) containing 20% (w/v) TCA was added to 1 ml of supernatant. The absorbance of the supernatant was recorded at 532 and 600 nm. MDA was quantified by its extinction coefficient of 155 mM–1 cm–1 and expressed as nmol g−1 FW.
For electrolyte leakage (EL) level, the method of Murray et al. (1989) was applied. Leaf samples (0.1 g) were introduced in distilled water and initial conductivity (Ci) was measured. Samples were later autoclaved to determine the final conductivity (Cf). Electrolyte leakage rate was then calculated by the following formula: EL (%) = (Ci/Cf) × 100%.
Soluble sugars were analyzed by the method of phenolsulfuric acid (Dubois et al. 1956). Dry leaf samples (0.1 g) were homogenized with deionized water. One ml of extract was treated with 0.5 ml of 5% phenol solution and 2.5 ml of 98% sulphuric acid. Samples were incubated for 1 h and then absorbance at 490 nm was determined by spectrophotometer. Content of sugars was expressed as µg g−1 DW.
Antioxidant enzyme activities
Enzyme extract was prepared by homogenizing 1 g of fresh leaf tissues under ice-cold condition in 1 ml of 50 mM potassium phosphate buffer (0.1% (v/v) triton X-100, 1% (w/w) polyvinylpyrrolidone (PVP), 1 mM phenylmethylsulfonyl fuoride (PMSF) and 2 mM EDTA, pH 7.8). APX was extracted in the same manner except the buffer contained 2 mM ascorbic acid. The homogenates were centrifuged at 12,000 rpm for 15 min at 4 °C and the supernatant was used for enzymatic assays. Protein content was determined according to the Bradford (1976).
The activity of CAT was assayed as described by Cakmak and Marschner (1992) by measuring the decrease in absorbance at 240 nm due to the consumption of H2O2 for 2 min calculated using an extinction coefficient of (ε = 39.4 mM−1 cm−1) and was expressed in µmol H2O2 min−1 mg−1 protein.
Activity of SOD was estimated based on the inhibition of nitro blue tetrazolium (NBT) photoreduction at 560 nm according to the method of Del Longo et al. (1993). One unit of SOD was defined as the amount of enzyme which caused a 50% decrease in the SOD-inhibited NBT reduction at 25 °C. The reaction mixture (3 ml) comprises 1 μM riboflavin, 12 mM L-methionine, 0.1 mM EDTA, 75 μM NBT, 50 mM K phosphate buffer (pH 7.8) and 20 µl of plant homogenate. The activity of SOD has expressed in Units SOD min−1 mg−1 protein.
The activity of APX was determined using Nakano and Asada (1981) protocol. The decrease in absorbance at 290 nm during 1 min was recorded using an extinction coefficient of (ε = 2.8 mM−1 cm−1). The reaction mixture contained 1 mM H2O2 in 50 mM phosphate buffer (pH 7.8), 1 mM EDTA-Na2 and 20 µl of enzyme extract. APX activity was expressed in µmol H2O2 min−1 mg−1 protein.
Activity of GPX was determined as described by Polle et al. (1994). The increase in absorbance at 470 nm during 1 min due to guaiacol oxidation was recorded using an extinction coefficient of (ε = 25.5 mM−1 cm−1). The reaction mixture contained 10 mM H2O2 in 100 mM phosphate buffer (pH 7.8), 16 mM guaiacol and 40 µl of enzyme extract and GPX activitywas expressed in µmol guaiacol min−1 mg−1 protein.
Sample preparation for mass spectrometry analysis of sugars
Frozen-ground leaf samples (5 g) were transferred to 50 ml plastic tubes and 10 ml sterile deionized water was added. The homogenate was heated at 70 °C on water bath for 100 min. The precipitates that formed were collected by centrifugation at 4000 rpm for 15 min. The supernatant of each sample was transferred to a new tube and evaporated in a Speed-Vac concentrator until approximately 1/3 of initial volume remained in the tube. The samples were deproteinized using Sevag reagent (1:4 n-butanol/chloroform, v/v). Two volumes of cold absolute ethanol were added to the aqueous phase, and were incubated at 4 °C overnight. Then, the samples were centrifuged for 15 min at 4000 rpm. After washing with acetone and centrifugation at 4000 rpm for 10 min, the organic layer containing total soluble sugars was recovered and dried under a stream of nitrogen. Total soluble sugars extraction was performed in triplicate. The dried extract was derivatized for 2 h at 37 °C with 40 µl of freshly prepared methoxyamine hydrochloride in anhydrous pyridine (20 mg ml−1), followed by incubation with 50 µl of N-methyl-N-(trimethylsilyl)trifluoroacetamide (MSTFA) for 30 min at 30 °C. Each derivatized sample was then transferred into a GC vial, capped with moisture-proof parafilm and directly stored at − 20 °C prior to analysis. A sample volume of 1 µl was injected into the gas chromatograph column for analysis.
GC–MS analysis
Sugars were analyzed using Agilent GC–MS system (GC with 7890A, mass detector 5975C with Triple-Axis, insert XL MSD). The temperature of the oven was programmed at 70 °C for 2 min, raised to 230 °C for 20 min and raised again to 270 °C for 25 min. Electron ionization (EI) mass spectra were collected at 70 eV and mass spectra were recorded at 4 scans per second with an m/z 50–550 scanning range. The identification of sugars was done with Wiley 09 NIST2011 library. The percentage determination was based on peak area normalization without using correction factors (El Ayed et al. 2017).
Total RNA extraction and cDNA synthesis
Total RNA was extracted according to the protocol of Chang et al. (1993). The residual genomic DNA was removed by RNase-free DNase I (Biomatik) according to the manufacturer’s recommendations. RNA integrity and concentration were confirmed using gel electrophoresis and Nanodrop spectrophotometer. The first strand cDNAs were synthesized using a First Strand cDNA Synthesis Kits (Biomatik) following manufacturer’s protocol.
Real-time reverse transcription-PCR (qRT-PCR)
Gene transcript abundance was quantified with Maxima SYBR Green/ROX qPCR Master Mix (2X) kit (Biomatik) in a 7300 Real-Time PCR Detection System (Applied Biosystems, Foster City, USA). The 30 µl reaction mixture contained 15 μl Maxima SYBR Green/ROX qPCR Master Mix (2X), 200 µM each of gene specific primers (Supplementary file Table S1), 2 µl (100 ng) of the template (reverse transcription reaction product) and 12 μl DEPC-treated sterile H2O. Thermocycler conditions for all real-time analyses were 95 °C for 10 min, followed by 40 cycles of 95 °C for 30 s, 60 °C for 1 min. All reactions were performed in triplicate. Melting curves were obtained by slow heating from 65 to 95 °C at 0.5 °C/s and continuous monitoring of the fluorescence signal. The VfELFA-1 gene from Vicia faba was used as an internal control to normalize amounts of template cDNA. Relative expression was calculated using the 2−ΔΔCt method (Schmittgen and Livak 2008). The heat map was generated using R package (http://www.r-project.org/) based on the log2 fold change (log2(FC)) values (treated/control) to compare the expression profiling of the transcriptome in both faba bean cultivars under different stress treatments.
Statistical analysis
All the data are presented as means ± standard deviation (SD) of at least three independent experiments. Statistical significance between different treatments was examined by one-way analysis of variance (ANOVA) and Tukey’s honestly significant difference (HSD) test (p < 0.05) using Statistical Package for Social Sciences (SPSS 16.0) software (SPSS Inc., Chicago, Illinois, USA).
Results
Leaf gas exchange
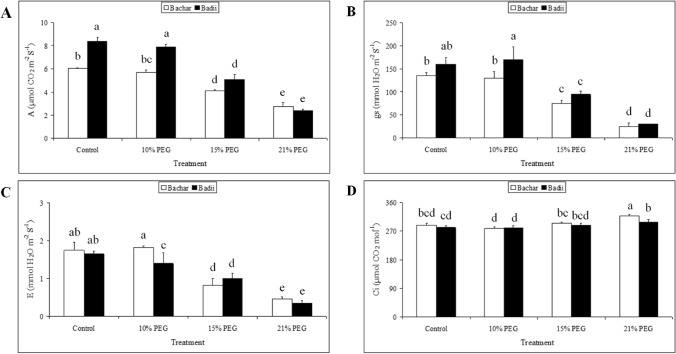
Leaf physiological traits like net CO2 assimilation rate (A), stomatal conductance (gs), transpiration rate (E) and intercellular CO2 concentration (Ci) contents were significantly altered in osmotic stress-treated plants compared to control (Fig. 1) and varied significantly between cultivars (Bachar and Badii). Under osmotic stress, A significantly decreased by 32% and 55% in Bachar and by 40% and 72% in Badii for moderate (15% PEG) and severe osmotic stress (21% PEG), respectively, when compared to control plants (Fig. 1A). Decrease in A was accompanied by sharp decreases in gs, ranging from 45 to 81% in Bachar and 41 to 82% in Badii under 15% PEG and 21% PEG, respectively (Fig. 1B). In parallel with the A and gs decreases, remarkable reductions in E by 53% and 75% in Bachar and by 40% and 79% in Badii were observed in water-stressed plants for 15% PEG and 21% PEG, respectively (Fig. 1C). Indeed, the gs variations were more intense when comparing with the E and A data under 21% PEG. In general, Ci did not show statistical differences between the treatments (Fig. 1D).
Fig. 1.
Effects of drought stress on net CO2 assimilation rate (A), stomatal conductance (B), transpiration rate (C) and intercellular CO2 concentration (D). Values are means ± SD of three replicates. The same letter indicates no significant difference (p ≤ 0.05)
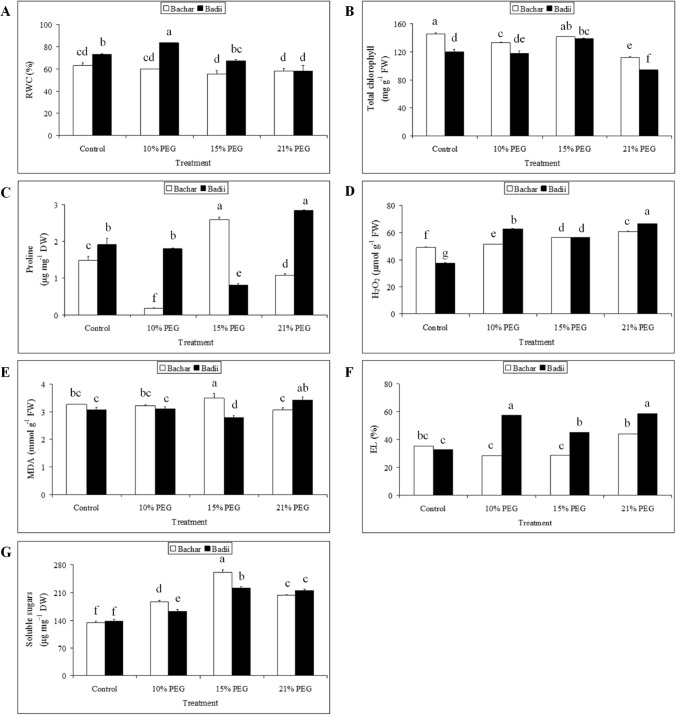
Leaf relative water (RWC), total chlorophyll (Chlt), proline, hydrogen peroxide (H2O2), malondialdehyde (MDA) and soluble sugars content and electrolyte leakage (EL) level
RWC was significantly reduced under moderate and severe osmotic stress (Fig. 2A). RWC decreased by about 12% and 9% in Bachar and by 8% and 21% in Badii under 15% PEG and 21% PEG, respectively. However, mild stress (10% PEG) increased by 15% RWC in Badii osmotic-stressed plants compared to controls. On the other hand, 10% PEG could not affect RWC in Bachar.
Fig. 2.
Effects of drought stress on RWC (A), total chlorophyll (B), proline (C), H2O2 (D), MDA (E), EL (F) and soluble sugars (G). Values are means ± SD of three replicates. The same letter indicates no significant difference (p ≤ 0.05)
Total chlorophyll (Chlt) content decreased significantly at 21% PEG by 24% and 22% in Bachar and Badii, respectively (Fig. 2B). The results showed that the difference in Chlt content between controls and osmotic-stressed plants under 15% PEG treatment in Bachar and 10% PEG treatment in Badii was not significant. Treatment with 15% PEG increased significantly by 16% Chlt content in Badii plants. However, a smaller decrease by 9% was showed in Bachar under 10% PEG challenge.
Leaf proline level significantly decreased by 89% and 27% in Bachar plants at 10% PEG and 21% PEG, respectively, when compared to the controls (Fig. 2C). In Badii, 21% PEG significantly increased proline content by 50%, while 10% PEG could not affect proline content in Badii. Proline accumulation in Bachar was found to be more significant than Badii under moderate osmotic stress. An increase of 77% was observed in Bachar in response to 15% PEG, whereas proline content decreased by 59% in Badii when compared to the control plants.
Hydrogen peroxide (H2O2) content augmented in the studied genotypes in response to osmotic treatments (Fig. 2D) with higher increase observed in Badii. Consistently, osmotic stress elevated leaf H2O2 concentration by 5%, 25% and 26% in Bachar and by 68%, 51% and 78% in Badii at 10% PEG, 15% PEG and 21% PEG, respectively.
In Bachar, no significant difference was found in leaf malondialdehyde (MDA) level between the osmotic-stressed plants under 10% and 21% PEG and controls (Fig. 2E). However, the leaf MDA content was 7% higher under 15% PEG. Similar to Bachar, 10% PEG could not induce MDA accumulation in the leaves of Badii. On the other hand, MDA was significantly decreased by 10% under 15% PEG and increased by 12% under 21% PEG.
The electrolyte leakage (EL) level showed significant elevation in Badii plants by 76%, 38% and 80% under 10%, 15% and 21% PEG, respectively, as compared to controls (Fig. 2F). The EL level was also significantly augmented by 25% in Bachar only at 21% PEG. Statistical analysis showed that the difference in EL level between controls and Bachar osmotic-stressed plants under 10% and 15% PEG was not significant.
Imposition of different PEG treatments to the studied faba bean genotypes significantly increased total sugar content in their leaves (Fig. 2G). There was no significant difference in leaf soluble sugar content between Bachar and Badii control plants. However, increase of soluble sugar content in Bachar osmotic-stressed plants was higher than in leaf tissues of Badii. Mild, moderate and severe osmotic stress significantly increased leaf soluble sugar of Bachar by 39%, 95% and 51% compared to control, respectively. Similarly, osmotic stress lead to a remarkable augmentation of soluble sugars in leaves of Badii by 18%, 60% and 56%.
Leaf antioxidant enzyme activities
CAT activity increased dramatically with increasing PEG concentration in Bachar and Badii, and it was much higher at 21% PEG treatment than in the others (Fig. 3A). In general, CAT activity was higher in Badii than in Bachar under all osmotic stress treatments. CAT activity increased in Bachar osmotic challenged plants by 68%, 80% and 242% at 10%, 15% and 21% PEG respectively compared to controls. However, this increase was by 195%, 283% and 445% in Badii osmotic-stressed plants.
Fig. 3.
Effects of drought stress on the activities of CAT (A), SOD (B), APX (C) and GPX (D). Values are means ± SD of three replicates. The same letter indicates no significant difference (p ≤ 0.05)
Osmotic pressure resulted in lower SOD activity in leaves of Bachar (Fig. 3B). The decrease in SOD activity was 54%, 64% and 24% in the 10%, 15%, and 21% PEG treatment, respectively, as compared to controls. Similarly, a significant reduction in SOD activity was observed in Badii leaf tissues only under 10% and 15% PEG treatment. However, Badii leaves showed increased SOD activity under 21% PEG treatment by 91%.
PEG treatments significantly raised APX activity in leaves of the two genotypes (Fig. 3C). But, the APX activity of Bachar leaves was higher compared to Badii. In Bachar, 10%, 15% and 21% PEG treatment increased APX activity by 150%, 23% and 89%, respectively. However, it increased by 21%, 83% and 71%, respectively, in Badii.
In contrast to APX activity, PEG treatment significantly dropped GPX activity in leaves of Bachar and Badii as compared to control plants (Fig. 3D). GPX activity decreased in leaf tissues of Bachar by 15%, 30% and 6% in the 10% PEG, 15% PEG and 21% PEG treatment, respectively. Similarly, it was decreased by 31%, 40% and 43% in Badii.
Carbohydrate changes in response to osmotic stress treatments
Under osmotic stress treatments, 11 and 10 sugars in leaves of Bachar and Badii were significantly changed (Table 1). Chromatographic compounds analysis showed that the major sugars in the leaves of unstressed Bachar and Badii plants, respectively, are α-d-mannopyranose (40.10% and 23%), β-d-(+)-mannopyranose (3.50% and 19.20%), D-glucopyranose (10.50% and 47%) and β-d-galactopyranose (8.20%). Osmotic stress increased the accumulation of most sugars (α-d-mannopyranose, β-d-(+)-mannopyranose, d-glucopyranose, d-(-)-ribofuranose and myo-inositol) in leaves of Bachar, whereas were decreased in leaves of Badii. Some sugars (β-d-(-)-ribopyranose, rhamnose and α-l-mannofuranose) showed elevated levels in leaves Bachar and Badii. However, α-d-(-)-ribopyranose and D-xylose were diminished. On the other hand, β-d-galactopyranose which was detected only in Bachar leaves was markedly increased under osmotic stress challenge.
Table 1.
GC–MS data of carbohydrates compounds found in leaves of Bachar and Badii under drought stress treatments
| Carbohydrates | Relative abundance (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Bachar | Badii | |||||||
| Control | 10% PEG | 15% PEG | 21% PEG | Control | 10% PEG | 15% PEG | 21% PEG | |
| α-d-Mannopyranose | 40.10 | 56.90 | 47.40 | 43.30 | 23.00 | 16.60 | 18.90 | 11.30 |
| β-d-(+)-Mannopyranose | 3.50 | 21.90 | 32.30 | 33.50 | 19.20 | 13.50 | 18.00 | 10.20 |
| d-Glucopyranose | 10.50 | 34.60 | 32.40 | 41.40 | 47.00 | 27.50 | 23.50 | 23.50 |
| β-d-Galactopyranose | 8.20 | 31.10 | 24.90 | 34.00 | ND | ND | ND | ND |
| α-d-(-)-Ribopyranose | 3.00 | ND | ND | 0.70 | 0.60 | ND | ND | ND |
| d-(-)-Ribofuranose | ND | ND | 2.20 | 1.00 | 2.50 | 0.60 | 0.60 | ND |
| d-Xylose | 1.63 | ND | ND | ND | 2.00 | ND | ND | ND |
| β-d-(-)-Ribopyranose | 0.56 | ND | 2.33 | 0.99 | ND | 0.60 | 0.66 | 0.41 |
| Myo-Inositol | ND | 2.10 | ND | 2.00 | 4.5 | 1.70 | 2.30 | 0.90 |
| Rhamnose | ND | 0.70 | 0.80 | 0.80 | 0.50 | 0.20 | 2.10 | 1.60 |
| α-l-Mannofuranose | ND | 0.70 | 0.71 | 0.70 | 0.50 | ND | 2.20 | 1.10 |
Candidate genes involved sugars metabolism
An in silico analysis was performed in order to identify genes involved in faba bean soluble carbohydrates (sugar) metabolism. Using the BLASTn program, the coding regions of the different genes from Medicago truncatula, Pisum sativum, Medicago sativa and Cicer arietinum were used as queries to search on the Transcriptome Shotgun Assembly (TSA) database available from Vicia faba for homology. A total of 20 genes showed significant homology with their homologs in Medicago truncatula (NINV3, PHS2, FRK4, HXK1, GPI1, STP1.1, pGlcT1.1, STP5.1, pGlcT1.2 and SWEET2.1), Pisum sativum (VINV2, SUS1, PGM1, SUT1.1 and GPT1), Medicago sativa (SPS1) and Cicer arietinum (SPP1, PHS1, SUT4.1, TMT1.1) were identified. Specific primers were designed for each of the 20 selected genes (Table S1).
Expression patterns of faba bean genes involved in sugars metabolism
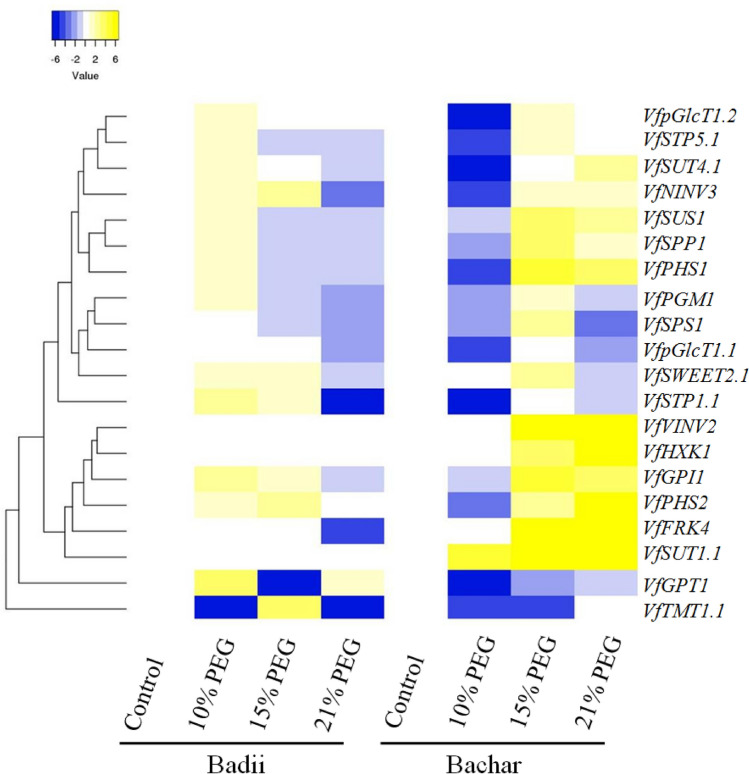
In order to elucidate the possible roles of faba bean identified genes involved in soluble carbohydrate metabolism in osmotic stress tolerance, the expression profiles of VfNINV3, VfPHS2, VfFRK4, VfHXK1, VfGPI1, VfSTP1.1, VfpGlcT1.1, VfSTP5.1, VfpGlcT1.2, VfSWEET2.1, VfVINV2, VfSUS1, VfPGM1, VfSUT1.1, VfGPT1, VfSPS1, VfSPP1, VfPHS1, VfSUT4.1 and VfTMT1.1 in response to osmotic stress treatments (10%, 15% and 21% PEG) were conducted in leaf tissues of Bachar and Badii by qRT-PCR. The expression analysis of the selected genes showed differential transcript accumulation between the two contrasting genotypes and between PEG treatments (Fig. 4) and according to the expression profiles they could be grouped into six clusters. Cluster 1 contains 7 (35%) members which are divided into 2 sub-clusters. The first sub-cluster (VfNINV3, VfSTP5.1, VfSUT4.1 and VfpGlcT1.2) showed upregulation in Badii and significantly downregulation in Bachar under the 10% PEG treatment. Under 15% PEG and 21% PEG treatment, these genes were downregulated in leaves of Badii, while upregulated in Bachar. The gene members of the second sub-cluster, which comprises VfSUT1.1, VfSPP1 and VfPHS1, were upregulated in Badii and downregulated in Bachar under the 10% PEG treatment. The mRNA accumulation of these genes showed an upregulation in Bachar and downregulation in Badii under the 15% and 21% PEG challenge. Cluster 2 mainly consists of 4 (VfPGM1, VfSPS1, VfpGlcT1.1 and VfSWEET2.1, 20%) genes, which were upregulated in Badii, but in contrast, downregulated in Bachar under the 10% PEG treatment. On the other hand, these genes were widely upregulated by 15% PEG treatment in Bachar and weakly downregulated in Badii. All members of this cluster showed markedly low transcript abundance profiles in leaf tissues of Bachar and Badii under severe osmotic stress. Cluster 3 has only 1 (VfSTP1.1, 5%) member showing upregulation in Badii and downregulation in Bachar at 10% PEG application. Moderate osmotic stress weakly induced the expression of VfHXT1 in the leaves of Badii, however the expression of VfSTP1.1 was not affected by 15% PEG treatment. Under severe osmotic stress VfSTP1.1 showed weak downregulation in Bachar and more significant downregulation in Badii.
Fig. 4.
Heat map representation of the effects of drought stress on the genes expression in the leaf tissues of Bachar and Badii. The heat map was generated based on the log2 fold change (log2(FC)) values (treated/control). Yellow and blue indicate higher and lower expression values, respectively. Intensity of the colors is proportional to the absolute value of log2 of the fold difference in expression
Cluster 4 contains six genes (VfPHS2, VfFRK4, VfHXK1, VfGPI1, VfVINV2 and VfSUT1.1, 30%), which were significantly upregulated in leaf tissues of Bachar under the 15% and 21% PEG treatment. However, in this genotype, these transcripts were unaffected or downregulated under the 10% PEG. Under mild and moderate osmotic stress, members of cluster 4 were slightly upregulated, while under severe osmotic stress, their expression was unchanged or downregulated. Cluster 5 has one member (VfGPT1, 5%), which was mainly downregulated in Bachar under osmotic stress treatments. The expression of VfGPT1 was downregulated in leaf tissues of Badii only under moderate stress but was upregulated under mid and severe stress. The VfTMT1.1 of cluster 6 (5%) was downregulated in Badii and Bachar under mild stress. VfTMT1.1 mRNA was significantly accumulated in the leaves of Badii under moderate stress and it was downregulated in Bachar. The VfTMT1.1 gene showed no variation in expression in the leaves of Bachar under severe stress as compared to control. On the other hand, it showed markedly low transcript abundance profiles in Badii.
Discussion
Plants have evolved various mechanisms to cope with osmotic stress induced by environmental constraints such as water-deficit including drought escape via a short life cycle, enhanced water uptake and reduced water loss, osmotic adjustment, increased antioxidant capacity and desiccation tolerance (Fang and Xiong 2015). The current study carried out physiological, biochemical, molecular and metabolic analyses to investigate sugars metabolism regulation in response to osmotic stress in Vicia faba leaf tissues which are considered the most drought sensitive part of the plant (Kang et al. 2019). Moreover, according to Lemoine et al. (2013) as much as 80% of the CO2 assimilated during photosynthesis is employed for synthesis of sugars mainly sucrose which is the major transport form of organic carbon exported from the photosynthetic source (leaves) to sink organs (roots and seeds).
Osmotic stress treatments decreased photosynthetic parameters (A, E and gs), photosynthetic pigments (Chlt) and leaf relative water content (RWC) in faba bean plants compared to controls. These data are in agreement with previously published results (Abid et al. 2017), further supporting that photosynthesis, photosynthetic capacity and RWC decrease in Vicia faba undergoing water deficit stress. Therefore, the RWC level, the amount of Chlt content and photosynthetic capacities strongly depend on the species’ physiological responses and their ability to tolerate stress. According to Marček et al. (2019) a higher RWC accompanied with better photosynthetic activity, more open stomata and higher transpiration rate indicated occurrence of active metabolic re-arrangements in osmotic-stressed plants, which enabled the plants to maintain normal cellular function and growth. The relationship between physiological traits such as RWC, Chlt, photosynthetic parameters and osmotic stress tolerance has already been reported for various species including wheat and barley (Sallam et al. 2019). Indeed, in drought-tolerant wheat cultivar exhibited a lower reduction of A, RWC and Chlt after exposure to drought compared to drought-sensitive cultivar (Marček et al. 2019). Similar results were reported in faba bean (Ali et al. 2013), chickpea (Saglam et al. 2014) and apple (Bai et al. 2019). In this study, A, E, gs, Chlt and RWC of leaves of Bachar and Badii significantly decreased under osmotic stress treatments to avoid water loss. Comparing the two genotypes, the most pronounced decrease of RWC, Chlt, A, E, and gs was observed in Badii, particularly under the 21% PEG treatment. On the other hand, a low gs in Bachar which might cause low A under control condition compared to Badii is probably a way to conserve water when plants under osmotic stress (Kholová et al. 2010). Thus, we suggested that Bachar with a low decrease in leaf RWC has a higher photosynthetic rate under osmotic stress and may have better ability to resist osmotic stress compared to Badii.
In Bachar and Badii, EL level and content of MDA and H2O2 increased under different PEG treatments compared to controls. However, all the three oxidative stress indicators were found to be almost lower in osmotic-stressed Bachar plants compared to osmotic-stressed Badii, especially under the 21% PEG. This indicated that Bachar showed better tolerance to osmotic stress than Badii. These data are in agreement with the results of Ali et al. (2013), Siddiqui et al. (2015) and Abid et al. (2017). The lowest level of EL and content of H2O2 in Bachar osmotic-stressed plants under the 15% PEG could be due to the highest accumulation of proline that acts as an antioxidant and reduces the generation of free radicals and lipid peroxidation. Moreover, tolerance of Bachar under severe osmotic stress could be positively related to leaf RWC and Chlt accumulation (Jiang and Huang 2001; Siddiqui et al. 2015).
The formation of reactive oxygen species (ROS) under osmotic stress is prevented by an antioxidant system that includes antioxidant enzymes which lead to overcome oxidative damage (Laxa et al. 2019). In the present study, activities of antioxidant enzymes (CAT and APX) were significantly augmented under PEG treatments in Bachar and Badii compared to controls. The highest APX activity was observed in Bachar, while the highest CAT activity was measured in Badii. On the other hand, the activities of SOD and GPX were decreased in Bachar and Badii under osmotic stress. Moreover, the magnitude of decrease in GPX activity in Bachr was lower than Badii and SOD activity in Bachar osmotic-stressed plants at the 10% and 15% PEG displayed higher rate than Badii. Overall, activities of CAT, SOD, APX and GPX suggested that antioxidant protection in faba bean plants under osmotic stress conditions could be linked mainly to CAT and APX and the highest activity of these enzymes might help Bachar to cope better with osmotic stress than Badii.
Several authors (Sharma et al. 2019) reported the accumulation of soluble carbohydrates (sugars) under drought stress. Sugar accumulation acts as an important osmolyte to maintain the turgidity of leaves, leaf water content and osmotic potential during drought conditions (Fabregas and Fernie 2019). This could lead to stabilizing membrane integrity by scavenging ROS and cell turgidity in drought-stressed plants (Kozminska et al. 2019). Moreover, sugars activate expression of stress-linked genes and also functions as an energy source in plants to stand the drought stress (Sheen et al. 1999). The accumulation of sugars (such as sucrose, fructose, glucose, mannitol, sorbitol, and trehalose) has been reported widely in many plant species exposed to drought like wheat (Hou et al. 2018; Marček et al. 2019), potato (Aliche et al. 2020; Legay et al. 2011) and soybean (Du et al. 2020). According to Bohnert and Jensen (1996), accumulation of sugars in various plant species is related to high tolerance to drought stress. Various genes are associated with sugars biosynthesis, transport and accumulation (Wang et al. 2016). Several transgenic plants were produced with genes of mannitol, fructan, trehalose and sorbitol biosynthesis and showed more tolerance to drought stress than wild type control (Khan et al. 2015). Ectopic overexpression of apple MdSWEET17, a sugar transporter, promotes the accumulation of soluble sugar and enhances drought tolerance in tomatoes (Jing et al. 2019). Moreover, overexpression of AtTPPF, a trehalose-6-phosphate phosphatase family gene show increased levels of soluble sugars in transgenic plants and improves the drought tolerance of Arabidopsis thaliana (Lin et al. 2019).
Soluble sugars rate increased in Bachar and Badii plants in response to PEG treatments and these biochemical changes induced osmotic adjustment in osmotic-stressed plants (Amede and Schubert 2003). Under mild and moderate osmotic stress, soluble sugar content augmented with increasing the level of the osmotic stress. However, the highest soluble sugar content was reported in Bachar. In this study, some sugars including mannose remarkably increased in Bachar under osmotic stress. But, this sugar decreased in response to osmotic stress in Badii. Changes in mannose have been reported in drought-stressed cotton (Loka and Oosterhuis 2014). Moreover, Guo et al. (2018) reported that mannose content increased in wheat drought tolerant genotype (JD17) while decreased in drought sensitive genotype (JD8). Mannose was increased in the leaves of Bachar subjected to osmotic stress mainly because of the enhanced VfSTP1.1 expression (Wenzel et al. 2015).
Glucose plays a key role in stomatal closure and enhances plant adaptability under drought conditions (Osakabe et al. 2014). Indeed, the sugar content such as glucose, galactose, myo-inositol and rhamnose was increased in leaves in Bachar under osmotic stress. It is evident that the drought-tolerant plant species have more carbohydrates than the sensitive species. However, up-regulated sugar transporters (VfSTP1.1, VfpGlcT1.1, VfSTP5.1, VfpGlcT1.2, VfSWEET2.1, VfSUT1.1 and VfSUT4.1) in Bachar could enhance phloem loading, and more soluble sugar segregation are the important mechanisms behind the osmotic stress tolerance (Ferner et al. 2012). On the other hand, ribose and xylose in leaves of Bachar were decreased. It is possible that those sugars are hydrolyzed to fructose, which is used more in glycolysis and TCA cycle as plant endogenous antioxidants to enhance plant metabolism and resist drought stress (Bogdan and Zagdanska 2006). Here, we found that under severe osmotic stress, various carbohydrates in leaves of Badii were significantly decreased compared to other treatments. Yang et al. (2017) suggested that carbohydrates have an osmotic regulatory role in plants under mild and moderate stress; however, under severe stress, carbohydrates are not the main osmotic regulators, and more carbohydrates are used to synthesize amino acids, organic acids, and other substances that are more effective for regulating osmotic balance.
In general, sugars were differentially accumulated in leaves of Bachar and Badii under osmotic stress treatments. This could indicate that the changes are mainly associated with adaptation of faba bean plants to osmotic stress which leads to gain in synthetic activity, carbohydrate content and other changes associated with them.
A large number of stress responsive genes and genes corresponding to enzymes of carbohydrate metabolism have been reported to be induced by sugars like glucose, sucrose and fructose under abiotic stresses including drought, indicating the role of sugars in plant metabolism and drought stress responses (Gupta and Kaur 2005).
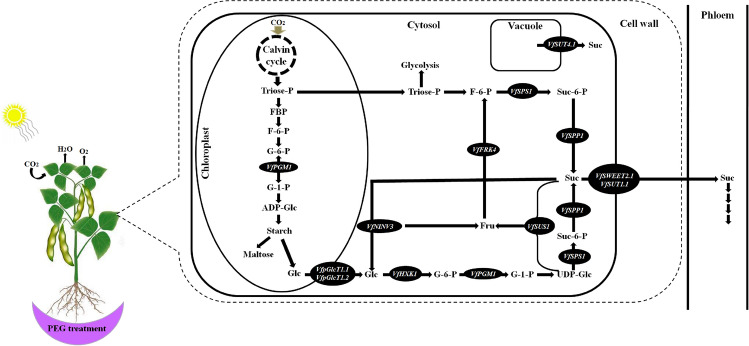
Sucrose (Suc) is the main photosynthetic product in higher plants and is the principal carbohydrates translocated from source leaves to sink organs such as developing seeds. Suc can be brought into sink cells by sucrose transporters (SUTs and SWEETs) or enter through plasmodesmata (Stein and Granot 2019). Inside the cell, Suc can be stored in the vacuole or hydrolyzed by VINV. In the cytosol, Suc can be hydrolyzed by cytosolic INV to yield glucose (Glc) and fructose (Fru), or cleaved by cytosolic SUS to yield Fru and UDP-glucose (UDPG). Wang et al. (2020) found that in the cell wall space Suc can be converted to Fru and Glc by cell wall invertase (CWINV), and then transported into the parenchyma cells by monosaccharide transporters (MSTs). Moreover, according to Claeyssen et al. (2013) the hexoses (Fru and Glc) can be phosphorylated to hexose phosphates: glucose 6-phsophate (G6P) and fructose 6-phosphate (F6P) by monosaccharide transporter (MST) and fructokinase (FK, specific for Fru). The conversions between F6P, G6P, G1P, and UDPG can be catalyzed by phosphoglucoisomerase (GPI), phosphoglucomutase (PGM), and UDPG-pyrophosphorylase (UGP) in readily reversible reactions (Rolland et al., 2006). UDPG can be used for cellulose synthesis or combined to re-synthesize Suc via sucrose phosphate synthase (SPS) and sucrose phosphate phosphatase (SPP). According to Li et al. (2012), most of the Fru, Glc and Suc that have not been metabolized are transported by special tonoplast transporters (TMTs) into vacuole for storage.
The effects of osmotic stress on sugar metabolism were investigated using qRT-PCR analysis of genes involved in sugar metabolism in leaves. This study showed that there are differences between the expression patterns of the tested genes of two faba bean cultivars under osmotic stress. In general, compared to the control, osmotic stress significantly increased the expression levels of VfNINV3, VfPHS2, VfFRK4, VfHXK1, VfGPI1, VfSTP1.1, VfpGlcT1.1, VfSTP5.1, VfpGlcT1.2, VfSWEET2.1, VfVINV2, VfSUS1, VfPGM1, VfSUT1.1, VfSUT4.1, VfSPS1, VfSPP1 and VfPHS1 in Bachar leaves, especially under moderate (15% PEG) and severe (21% PEG) osmotic stress, while the transcripts of these genes were downregulated or unchanged in leaves of Badii. On the other hand, all these genes were downregulated in leaves of Bachar under mild stress (10% PEG). However, the expression levels of these genes were slightly upregulated in leaves of Badii. Interestingly, this result indicated that osmotic stress enhanced the sucrose metabolism related-genes in Bachar leaves. Therefore, we suggest that the sucrose metabolism pathway in leaves is involved in osmotic stress response in Vicia faba. Previous studies have shown that the capacity of sucrose transport in higher plants was correlated by the expression levels of sucrose transporter genes (such as SWEETs and SUTs) in source and sink organs (Durand et al. 2016).
Osmotic stress significantly upregulated the expression levels of VfSUT1.1, VfSUT4.1 and VfSWEET2.1 in Bachar leaves, which suggests that the capacity of sucrose loading in leaves was enhanced under osmotic stress. Enhancing the sucrose transport from leaves to other tissues could lead to maintaining faba bean plant growth under osmotic stress. Similar results were also reported by Du et al. (2020), which reported that in soybean drought stress significantly upregulated the expression levels of GmSUT2, GmSWEET6, and GmSWEET15. Moreover, drought stress upregulated the expression levels of AtSUT2 and AtSWEET11/12 in Arabidopsis leaves and promoted carbon flow from leaves to sink tissues (Durand et al. 2016). Here, we found that that osmotic stress upregulated the expression VfSUS1, VfVINV2, VfNINV3 and VfSPS1 in Bachar leaf tissues at the 15% and 21% PEG. These results suggest that upregulation of these genes in osmotic-stressed leaves could be considered as a mechanism for increase in osmotic stress-induced accumulation of soluble sugars such as glucose and fructose. These results were in agreement with previous studies, which reported that drought stress upregulated the expression levels of TaSPSIIa, TaSPSIIb, TaSPSIIc, TaSPSV, TaSPSVI, TaSuS2, TaSuS4, TaSuS5, TaSuS10, TaNInv1 and TaNInv2 in wheat leaves, and promoted accumulation of glucose and fructose (Xue et al. 2008). Among the studied genes, VfpGlcT1.2, VfSTP1.1, VfpGlcT1.1 and VfSTP5.1 are coding for hexose transporters, showed differentially expressed under osmotic stress in Bachar and Badii. These data were in accordance with previous studies reporting that VvSTP1 and VvSTP5 are differentially expressed under osmotic stress in grapevine leaves (Medici et al. 2014). These facts suggested the prevalence of sugar signaling in the transcriptional regulation of these glucose transporters by their proper substrate, the glucose. The significant increase in VfFRK4 expression in Bachar under moderate and severe stress could be important to ensure the phosphorylation of high levels of fructose and that more carbon is allocated to TCA or glycolysis for energy production (Yang et al. 2019). In this study, several key enzymes in glycolysis/gluconeogenesis metabolism were differentially expressed in Bachar and Badii under osmotic stress. Indeed, genes encoding VfHXK1, VfGPI1 and VfPGM1 showed upregulation in Bachar under osmotic stress. The upregulation of these genes is expected to be critical to the cellular levels of glucose and fructose, and the reactions catalyzed by these genes lead to hexoses entering the glycolytic pathway. In cereal crops, it has been shown that HXK is upregulated in maize (Hayano-Kanashiro et al. 2009) under drought, but downregulated in wheat and rice (Lenka et al. 2011; Xue et al. 2008). Osmotic stress induced starch recycling and promoted soluble sugar accumulation in leaves of Bachar by upregulating VfPHS1and VfPHS2 expression levels under moderate and severe osmotic stress which degraded starch to glucose-1-phosphate (G1P) and then indirectly converted to trehalose-6-phosphate (T6P) by trehalose phosphatase (TPP). According to Krasensky and Jonak (2012), by action of trehalose phosphate synthase (TPS) the T6P was converted to trehalose which plays important roles in plant osmotic adjustment under abiotic stresses. VfGPT1 encoding a glucose-6-phosphate/phosphate translocator responsible for the transport of Glucose 6-phosphate (G6P) into plastids and it can be used as a carbon source for starch biosynthesis (Kunz et al. 2010). VfGPT1 expression levels were significantly reduced in the Bachar osmotic-stressed leaves. This result indicated that osmotic stress diminished starch biosynthesis and faba bean plants could remobilize starch to provide energy and carbon at times when photosynthesis may be potentially limited. Leaf starch content was reported to decrease in response to abiotic stress, independently of the analyzed species such as barley, broad bean, soybean (Thalmann and Santelia 2017) and rice (Dien et al. 2019). VfTMT1.1 showed a marked reduction in expression in Bachar osmotic-stressed leaves, indicating a reduction in the total VfTMT1.1 mRNA level. Thus indicating a reduced accumulation of sucrose and suggests that osmotic stress induced a general increase of sucrose conversion into glucose and fructose in faba bean osmotic-stressed plants which can lead to improving the osmotic regulation ability under osmotic stress.
Conclusion
This study showed that Bachar has a better ability to tolerate osmotic stress compared to Badii. Both cultivars showed genotypic variations due to their differential responses for physiological, biochemical and molecular characteristics under osmotic stress. Bachar displayed a better capacity to retain more RWC and accumulation of total chlorophyll and soluble sugars. Also, Bachar was relatively more efficient to reduce oxidative damage by increasing activities of CAT, APX and SOD. The comparison of sugars in Bachar and Badii under control and osmotic stress conditions showed that the levels of some sugars differed between the two genotypes. Indeed, leaves of Badii contained lower levels of mannose, glucose, ribose, galactose and myo-inositol under osmotic stress. The results indicated that Bachar revealed higher capability of sugars synthesis than Badii. Sugar biosynthesis and accumulation-related genes showed differentially expressed in response to osmotic stress in Bachar and Badii with different expression levels. In conclusion, VfNINV3, VfPHS2, VfFRK4, VfHXK1, VfGPI1, VfSTP1.1, VfpGlcT1.1, VfSTP5.1, VfpGlcT1.2, VfSWEET2.1, VfVINV2, VfSUS1, VfPGM1, VfSUT1.1, VfGPT1, VfSPS1, VfSPP1, VfPHS1, VfSUT4.1 and VfTMT1.1 play important roles in sugar metabolism in faba bean. Moreover, higher content of sugars in leaves of Bachar, as well as higher expression of sugars biosynthesis and accumulation genes enhance osmotic stress tolerance of faba bean cultivar Bachar (Fig. 5).
Fig. 5.
Proposed model of the sugar regulation in faba bean leaves in response to osmotic stress. Upregulated sugar metabolism related-genes are displayed in black ellipses
Supplementary Information
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abid G, Hessini K, Aouida M, Aroua I, Baudoin JP, Muhovski Y, Mergeai G, Sassi K, Machraoui M, Souissi F, Jebara M. Agro-physiological and biochemical responses of faba bean (Vicia faba L. var. ‘minor’) genotypes to water deficit stress. Biotechnol Agron Soc Environ. 2017;21:146–159. [Google Scholar]
- Abid M, Ali S, Qi KL, Zahoor R, Tian Z, Jiang D, Snider JL, Da T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.) Sci Rep. 2018;8:4615. doi: 10.1038/s41598-018-21441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali HM, Siddiqui MH, Al-Whaibi MH, Basalah MO, Sakran AM, El-Zaidy M. Effect of proline and abscisic acid on the growth and physiological performance of faba bean under water stress. Pak J Bot. 2013;45:933–940. [Google Scholar]
- Aliche EB, Theeuwena TPJM, Oortwijn M, Visser RGF, van der Linden CG. Carbon partitioning mechanisms in POTATO under drought stress. Plant Physiol Biochem. 2020;146:211–219. doi: 10.1016/j.plaphy.2019.11.019. [DOI] [PubMed] [Google Scholar]
- Amede T, Schubert S. Mechanisms of drought resistance in seed legumes. I.-Osmotic adjustment. Ethiop J Sci. 2003;26:37–46. [Google Scholar]
- Andrade ER, Ribeiro VN, Azevedo CVG, Chiorato AF, Williams TCR, Carbonell SAM. Biochemical indicators of drought tolerance in the common bean (Phaseolus vulgaris L.) Euphytica. 2016;210:277–289. [Google Scholar]
- Arabzadeh N. The effect of drought stress on soluble carbohydrates (sugars) in two species of Haloxylon persicum and Haloxylon aphyllum. Asian Journal of Plant Sciences. 2012;11:44–51. [Google Scholar]
- Bai T, Li Z, Song C, Song S, Jiao J, Liu Y, Dong Z, Zheng X. Contrasting drought tolerance in two apple cultivars associated with difference in leaf morphology and anatomy. American Journal of Plant Sciences. 2019;10:709–722. [Google Scholar]
- Barrs HD, Weatherleyt PE. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci. 1962;15:413–428. [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- Bogdan J, Zagdanska B. Changes in the pool of soluble sugars induced by dehydration at the heterotrophic phase of growth of wheat seedlings. Plant Physiol Biochem. 2006;44:787–794. doi: 10.1016/j.plaphy.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Bohnert HJ, Jensen RG. Strategies for engineering water-stress tolerance in plants. Trends Biotechnol. 1996;14:89–97. [Google Scholar]
- Bradford MM. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chang S, Puryear J, Cairney J. A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep. 1993;11:113–116. [Google Scholar]
- Cakmak I, Marschner H. Magnesium deficiency and highlight intensity enhance activities of superoxide dismutase ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992;98:1222–1227. doi: 10.1104/pp.98.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claeyssen E, Dorion S, Clendenning A, He JZ, Wally O, Chen J, Auslender EL, Moisan MC, Jolicoeur M, Rivoal J. The futile cycling of hexose phosphates could account for the fact that hexokinase exerts a high control on glucose phosphorylation but not on glycolytic rate in transgenic potato (Solanum tuberosum) roots. PLoS ONE. 2013;8:e53898. doi: 10.1371/journal.pone.0053898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Longo OT, Gonzalez CA, Pastori GM, Trippi VS. Antioxidant defenses under hyperoxygenic and hyperosmotic conditions in leaves of two lines of maize with differential sensitivity to drought. Plant Cell Physiol. 1993;34:1023–1028. [Google Scholar]
- Dien DC, Mochizuki T, Yamakaw T. Effect of various drought stresses and subsequent recovery on proline, total soluble sugar and starch metabolisms in rice (Oryza sativa L.) varieties. Plant Prod Sci. 2019;22:530–545. [Google Scholar]
- Du Y, Zhao Q, Chen L, Yao X, Zhang W, Zhang B, Xie F. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol Biochem. 2020;146:1–12. doi: 10.1016/j.plaphy.2019.11.003. [DOI] [PubMed] [Google Scholar]
- DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- Durand M, Porcheron B, Hennion N, Maurousset L, Lemoine R, Pourtau N. Water deficit enhances C export to the roots in Arabidopsis thaliana plants with contribution of sucrose transporters in both shoot and roots. Plant Physiol. 2016;170:1460–1479. doi: 10.1104/pp.15.01926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Ayed M, Kadri S, Smine S, Elkahoui S, Limam F, Aouani E. Protective effects of grape seed and skin extract against high-fat-diet-induced lipotoxicity in rat lung. Lipids Health Dis. 2017;16:174. doi: 10.1186/s12944-017-0561-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabregas N, Fernie AR. The metabolic response to drought. J Expt Bot. 2019;70:1077–1085. doi: 10.1093/jxb/ery437. [DOI] [PubMed] [Google Scholar]
- Fang Y, Xiong L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol Life Sci. 2015;72:673–689. doi: 10.1007/s00018-014-1767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAOSTAT (2018) http://www.fao.org/faostat/en/#data/QC
- Ferner E, Rennenberg H, Kreuzwieser J. Effect of flooding on C metabolism of flood-tolerant (Quercus robur) and non-tolerant (Fagus sylvatica) tree species. Tree Physiol. 2012;32:135–145. doi: 10.1093/treephys/tps009. [DOI] [PubMed] [Google Scholar]
- Guo R, Shi LX, Jiao Y, Li MX, Zhong XL, Gu FX, Liu Q, Xia X, Li HR. Metabolic responses to drought stress in the tissues of drought-tolerant and drought-sensitive wheat genotype seedlings. AOB Plants. 2018 doi: 10.1093/aobpla/ply016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Kaur N. Sugar signalling and gene expression in relation to carbohydrate metabolism under abiotic stresses in plants. J Biosci. 2005;30:761–776. doi: 10.1007/BF02703574. [DOI] [PubMed] [Google Scholar]
- Hayano-Kanashiro C, Calderón-Vázquez C, Ibarra-Laclette E, Herrera-Estrella L, Simpson J. Analysis of gene expression and physiological responses in three Mexican maize landraces under drought stress and recovery irrigation. PLoS ONE. 2009;4:e7531. doi: 10.1371/journal.pone.0007531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. Calif Agric Exp Station. 1950;347:1–32. [Google Scholar]
- Hou J, Huang X, Sun W, Du C, Wang C, Xie Y, Ma Y, Ma D. Accumulation of water-soluble carbohydrates and gene expression in wheat stems correlates with drought resistance. J Plant Physiol. 2018;231:182–191. doi: 10.1016/j.jplph.2018.09.017. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Huang B. Drought and heat stress injury to two cool-season turfgrasses in relation to antioxidant metabolism and lipid peroxidation. Crop Sci. 2001;41:436–442. [Google Scholar]
- Jing L, Mei-hong S, Qi-jun M, Hui K, Ya-jing L, Yu-jin H, Chun-xiang Y. MdSWEET17, a sugar transporter in apple, enhances drought tolerance in tomato. J Integr Agric. 2019;18:2041–2051. [Google Scholar]
- Kang Z, Babar MA, Khan N, Guo J, Khan J, Islam S, Shrestha S, Shahi D. Comparative metabolomic profiling in the roots and leaves in contrasting genotypes reveals complex mechanisms involved in post-anthesis drought tolerance in wheat. PLoS ONE. 2019;14:e0213502. doi: 10.1371/journal.pone.0213502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkanis A, Ntatsi G, Lepse L, Fernández JA, Vågen IM, Rewald B, Alsina I, Kronberga A, Balliu A, Olle M, Bodner G, Dubova L, Rosa E, Savvas D. Faba bean cultivation–revealing novel managing practices for more sustainable and competitive European cropping systems. Front Plant Sci. 2018 doi: 10.3389/fpls.2018.01115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan HR, Paull JG, Siddique KHM, Stoddard FL. Faba bean breeding for drought-affected environments: a physiological and agronomic perspective. Field Crops Res. 2010;115:279–286. [Google Scholar]
- Khan MS, Ahmad D, Khan MA. Utilization of genes encoding osmoprotectants in transgenic plants for enhanced abiotic stress tolerance. Electron J Biotechnol. 2015;18:257–266. [Google Scholar]
- Kholová J, Hash CT, Kakkera A, Kočová M, Vadez V. Constitutive water conserving mechanisms are correlated with the terminal drought tolerance of pearl millet (Pennisetum glaucum (L.) R. Br.) J Exp Bot. 2010;61:369–377. doi: 10.1093/jxb/erp314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminska A, Wiszniewska A, Hanus-Fajerska E, Boscaiu M, Al Hassan M, Halecki W, Vicente O. Identification of salt and drought biochemical stress markers in several Silene vulgaris populations. Sustainability. 2019;11:800. doi: 10.3390/su11030800. [DOI] [Google Scholar]
- Krasensky J, Jonak C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J Exp Bot. 2012;63:1593–1608. doi: 10.1093/jxb/err460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz HH, Hausler RE, Fettke J, Herbst K, Niewiadomski P, Gierth M, Bell K, Steup M, Flugge UI, Schneider A. The role of plastidial glucose-6-phosphate/phosphate translocators in vegetative tissues of Arabidopsis thaliana mutants impaired in starch biosynthesis. Plant Biol. 2010;12:115–128. doi: 10.1111/j.1438-8677.2010.00349.x. [DOI] [PubMed] [Google Scholar]
- Laxa M, Liebthal M, Telman W, Chibani K, Dietz KJ. The role of the plant antioxidant system in drought tolerance. Antioxidants. 2019;8:94. doi: 10.3390/antiox8040094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legay S, Lefevre I, Lamoureux D, Barreda C, Luz RT, Gutierrez R, Quiroz R, Hoffmann L, Hausman JF, Bonierbale M, Evers D, Schafleitner R. Carbohydrate metabolism and cell protection mechanisms differentiate drought tolerance and sensitivity in advanced potato clones (Solanum tuberosum L) Funct Integr Genom. 2011;11:275–291. doi: 10.1007/s10142-010-0206-z. [DOI] [PubMed] [Google Scholar]
- Lemoine R, La Camera S, Atanassova R, Dédaldéchamp F, Allario T, Pourtau N, Bonnemain JL, Laloi M, Coutos-Thévenot P, Maurousset L, Faucher M, Girousse C, Lemonnier P, Parrilla J, Durand M. Source-to-sink transport of sugar and regulation by environmental factors. Front Plant Sci. 2013;4:272. doi: 10.3389/fpls.2013.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenka SK, Katiyar A, Chinnusamy V, Bansal KC. Comparative analysis of drought-responsive transcriptome in Indica rice genotypes with contrasting drought tolerance. Plant Biotechnol J. 2011;9:315–327. doi: 10.1111/j.1467-7652.2010.00560.x. [DOI] [PubMed] [Google Scholar]
- Li M, Feng F, Cheng L. Expression patterns of genes involved in sugar metabolism and accumulation during apple fruit development. PLoS ONE. 2012;7:e33055. doi: 10.1371/journal.pone.0033055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–382. [Google Scholar]
- Lin Q, Yang J, Wang Q, Zhu H, Chen Z, Dao Y, Wang K. Overexpression of the trehalose-6-phosphate phosphatase family gene AtTPPF improves the drought tolerance of Arabidopsis thaliana. BMC Plant Biol. 2019;19:381. doi: 10.1186/s12870-019-1986-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loka DA, Oosterhuis DM. Water-deficit stress effects on pistil biochemistry and leaf physiology in cotton (Gossypium hirsutum L.) S Afr J Bot. 2014;93:131–136. [Google Scholar]
- Marček T, Hamow KA, Vegh B, Janda T, Darko E. Metabolic response to drought in six winter wheat genotypes. PLoS ONE. 2019;14:e0212411. doi: 10.1371/journal.pone.0212411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici A, Laloi M, Atanassova R. Profiling of sugar transporter genes in grapevine coping with water deficit. FEBS Lett. 2014;588:3989–3997. doi: 10.1016/j.febslet.2014.09.016. [DOI] [PubMed] [Google Scholar]
- Mohammadkhani N, Heidari R. Drought-induced accumulation of soluble sugars and proline in two maize varieties. World Appl Sci J. 2008;3:448–453. [Google Scholar]
- Murray MB, Cape JN, Fowler D. Quantification of frost damage in plant tissues by rates of electrolyte leakage. New Phytol. 1989;113:307–311. doi: 10.1111/j.1469-8137.1989.tb02408.x. [DOI] [PubMed] [Google Scholar]
- Muscolo A, Sidari M, Anastasi U, Santonoceto C, Maggio A. Effect of PEG-induced drought stress on seed germination of four lentil genotypes. J Plant Interact. 2014;9:354–363. [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Okunlola GO, Akinwale RO, Adelusi AA. Proline and soluble sugars accumulation in three pepper species (Capsicum spp) in response to water stress imposed at different stages of growth. Sci Cold Arid Reg. 2016;8:205–211. [Google Scholar]
- Osakabe Y, Yamaguchi-Shinozaki K, Shinozaki K, Tran LSP. ABA control of plant macroelement membrane transport systems in response to water deficit and high salinity. New Phytol. 2014;202:35–49. doi: 10.1111/nph.12613. [DOI] [PubMed] [Google Scholar]
- Ouji A, Naouari M, Mouelhi M, Ben Younes M. Yield and yield components of faba bean (Vicia faba L.) as influenced by supplemental irrigation under semi-arid region of Tunisia. World J Agric Res. 2017;5:52–57. [Google Scholar]
- Polle A, Otter T, Seifert F. Apoplastic peroxidases and lignification in needles of norway spruce Picea abies L. Plant Physiol. 1994;106:53–60. doi: 10.1104/pp.106.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland F, Baena-Gonzalez E, Sheen J. Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol. 2006;57:675–709. doi: 10.1146/annurev.arplant.57.032905.105441. [DOI] [PubMed] [Google Scholar]
- Rosa M, Prado C, Podazza G, Interdonato R, González JA, Hilal M, Prado FE. Soluble sugars-Metabolism, sensing and abiotic stress. Plant Signal Behav. 2009;4:388–393. doi: 10.4161/psb.4.5.8294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglam A, Terzi R, Demiralay M. Effect of polyethylene glycol induced drought stress on photosynthesis in two chickpea genotypes with different drought tolerance. Acta Biol Hung. 2014;65:178–188. doi: 10.1556/ABiol.65.2014.2.6. [DOI] [PubMed] [Google Scholar]
- Sallam A, Alqudah AM, Dawood MFA, Baenziger PS, Börner A. Drought stress tolerance in wheat and barley: advances in physiology, breeding and genetics research. Int J Mol Sci. 2019;20:3137. doi: 10.3390/ijms20133137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sami F, Yusuf M, Faizan M, Faraz A, Hayat S. Role of sugars under abiotic stress. Plant Physiol Biochem. 2016;109:54–61. doi: 10.1016/j.plaphy.2016.09.005. [DOI] [PubMed] [Google Scholar]
- Sarkar KK, Mannan MA, Haque MM, Ahmed JU. Physiological basis of water stress tolerance in soybean. Bangladesh Agron J. 2015;18:71–78. [Google Scholar]
- Sharma A, Shahzad B, Kumar V, Kohli SK, Sidhu GPS, Bali AS, Handa N, Kapoor D, Bhardwaj R, Zheng B. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules. 2019;9:285. doi: 10.3390/biom9070285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Sheen J, Zhou L, Jang JC. Sugars as signaling molecules. Curr Opin Plant Biol. 1999;2:410–418. doi: 10.1016/s1369-5266(99)00014-x. [DOI] [PubMed] [Google Scholar]
- Siddiqui MH, Al-Khaishany MY, Al-Qutami MA, Al-Whaibi MH, Grover A, Ali HM, Al-Wahibi MS, Bukhari NA. Response of different genotypes of faba bean plant to drought stress. Int J Mol Sci. 2015;16:10214–10227. doi: 10.3390/ijms160510214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein O, Granot D. An overview of sucrose synthases in plants. Front Plant Sci. 2019;10:95. doi: 10.3389/fpls.2019.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalmann M, Santelia D. Starch as a determinant of plant fitness under abiotic stress. New Phytol. 2017;214:943–951. doi: 10.1111/nph.14491. [DOI] [PubMed] [Google Scholar]
- Trouvelot S, Héloir MC, Poinssot B, Gauthier A, Paris F, Guillier C, Combier M, Trdá L, Daire X, Adrian M. Carbohydrates in plant immunity and plant protection: roles and potential application as foliar sprays. Front Plant Sci. 2014;5:592. doi: 10.3389/fpls.2014.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid raintreated bean plants. Plant Sci. 2000;151:59–66. [Google Scholar]
- Wang W, Zhou H, Ma H, Owiti A, Korban SS, Han Y. Divergent evolutionary pattern of sugar transporter genes is associated with the difference in sugar accumulation between Grasses and Eudicots. Sci Rep. 2016;6:29153. doi: 10.1038/srep29153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Wei X, Yang J, Li H, Ma B, Zhang K, Zhang Y, Cheng L, Ma F, Li M. Heterologous expression of the apple hexose transporter MdHT2.2 altered sugar concentration with increasing cell wall invertase activity in tomato fruit. Plant Biotechnol J. 2020;18:540–552. doi: 10.1111/pbi.13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel A, Frank T, Reichenberger G, Herz M, Engel KH. Impact of induced drought stress on the metabolite profiles of barley grain. Metabolomics. 2015;11:454–467. [Google Scholar]
- Xue GP, McIntyre CL, Glassop D, Shorter R. Use of expression analysis to dissect alterations in carbohydrate metabolism in wheat leaves during drought stress. Plant Mol Biol. 2008;67:197–214. doi: 10.1007/s11103-008-9311-y. [DOI] [PubMed] [Google Scholar]
- Xu W, Cui K, Xu A, Nie L, Huang J, Peng S. Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol Plant. 2015;37:1–11. [Google Scholar]
- Yang DL, Jing RL, Chang XP, Li W. Identification of quantitative trait loci and environmental interactions for accumulation and remobilization of water-soluble carbohydrates in wheat (Triticum aestivum L.) stems. Genetics. 2007;176:571–584. doi: 10.1534/genetics.106.068361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Zhang J, Li M, Shi L. Metabolomics analysis reveals the salt-tolerant mechanism in Glycine soja. J Plant Growth Regul. 2017;36:460–471. [Google Scholar]
- Yang J, Zhang J, Li C, Zhang Z, Ma F, Li M. Response of sugar metabolism in apple leaves subjected to short-term drought stress. Plant Physiol Biochem. 2019;141:164–171. doi: 10.1016/j.plaphy.2019.05.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.