Abstract
The aim of this study was to investigate the effect of calcium nanoparticles (CaNP) and putrescine polyamine on some physiological and biochemical properties of saffron (Crocus sativus L.) under the control condition. Saffron corm was treated by different concentrations of putrescine (0, 0.25, 0.5, 1, 2 mM) and CaNP (0, 0.25, 0.5, 1, 1.5 g/l). The treatment of corm with putrescine and CaNP separately caused a significant increase in morphological parameters. Changes in biochemical parameters were also significant. Compared to other concentrations, the highest concentration of putrescine (1 mM) and CaNP (1 g/l) treatment in the plant showed the greatest effect. The combined effect of putrescine and CaNP treatment on morphological parameters was significant. The results of HPLC analysis showed that CaNP treatment alone is more effective on crocin, picrocrocin, and safranal content than the combined effect of CaNP and putrescine. The present study reported the functional potential of CaNP and putrescine combination to increase growth and phytochemical properties in Crocus sativus.
Keywords: Calcium nanoparticle, Greenhouse, Putrescine, Saffron, Phytochemistry
Introduction
Saffron (Crocus sativus L), a genus of the Iridaceae family, is known as an expensive spice with corm (Amin and Hosseinzadeh 2012). A previous study reported that the origin of saffron came from Iran (Moghaddasi 2010; Ahmed et al. 2016). One of the alternative methods for traditional saffron cultivation is planting it in a controlled environment such as greenhouse. The advantages of this method include reducing labor costs, more precise controlling of pests and diseases, reducing environmental stresses, increasing the flowering period and doubling the yield in greenhouse conditions compared to field cultivation (Madadi and Rostamza 2013). The chemical composition of saffron has been thoroughly studied by various groups over the past decades (Bagur et al. 2018; Rahmani et al. 2017; Milajerdi et al. 2014). There are three main secondary metabolites in saffron (crocin, picrocrocin, and safranal). Crocin is among one of the nature's few limited carotenoids that dissolves easily in water. This solubility is one of the reasons for its widespread uses as a colorant in food and medicine compared to other carotenoids. Picrocroin is a colorless, bitter-tasting glycoside responsible for the odor of volatile oils. Safranal is another bioactive compound in saffron that is effective in the organoleptic characteristics of saffron such as pigments, taste and smell (Rahmani et al. 2017; Bagur et al. 2018; Amin and Hosseinzadeh 2012).
Polyamines are a group of low-molecular-weight organic polypropionic compounds with two or more amine groups that are found in almost all living organisms and are involved in cellular metabolism. Polyamines include putrescine (diamine), spermidine (triamine), and spermin (tetraamine) (Killiny et al. 2012). Putrescine, with the chemical formula of C4H12N2, is one of the simplest polyamines that plays a key role in plant development, regulation of tRNA activity, stimulating cell division, DNA and protein synthesis, flowering, delay of aging, membrane stability, control rooting and embryogenesis and also in response to environmental stresses (Dever and Ivanov 2018). One of the new areas affecting the targeted changes in growing conditions of plant is the addition of low-consumption and high-consumption elements of nano size in plants (Chehrazi et al. 2015). Nanoparticles can attach to plant cell tissue, and also enter DNA and plant cells (Ramezani et al 2019a). Nanotechnology can act as a key element in crop management and development. In recent research, the application of nanoparticles in the plant growth has been considered.
Calcium (Ca) is one of the key factors in the development of saffron. It is an essentialelement and a structural component of the cell wall that affects the growth process in the plant (Mohseni Nik et al. 2012). Calcium is known as the essential nutrient for crop production and plays an important role in plant cell structure and metabolism, which affects the quantity and quality of plant secondary metabolites (Hassan 2012).
The effect of chemical inducers on the morphological parameters, yield and quality of saffron was evaluated (Cardone et al. 2020). They concluded that there was a positive correlation between saffron color and aroma of applied total calcium. They also concluded that evaluation of plant nutrition was important to achieve the best saffron yield.
Recently, the application of nanoparticles as fertilizers to the plants has been considered by researchers due to its different impacts such as faster and easier penetration into the cell membrane. Nanoparticles are smaller than normal calcium molecules and are not absorbed in competition with ionic sites (Khot et al. 2012). Through the application of Nano-fertilizers, the time and speed of the elements released are in accordance with the nutritional needs of the plant, so the plant is able to absorb the maximum amount of nutrients, and thus, while reducing elements leaching, it increases crop yield (Jafar Dokht et al. 2015). From results of the study by Liu et al. (2016), it can be concluded that nanomaterials have positive effects on plant growth and have the potential to be used as a Nano-fertilizer. Based on the Rane et al. (2015), it has been observed that calcium phosphate nanoparticles (CaPO4 NPs) showed a synergistic effect on growth improvement, root elongation and vitality along with endosymbiotic and arbuscular mycorrhizal fungi. The result of this research strengthens the hypothesis of the present study. In a previous study by Hua et al. (2015), the comparison between plants treated with Ca nanoparticle (CaNP) and Ca colloid showed more calcium content (about 13 times) in CaNP-treated leaves than in colloidal calcium treated leaves. In addition, CaNP is probably more easily absorbed by Tankan leaves than Ca colloid, which confirms the positive effects of CaNP treatment as a fertilizer on plant growth. However, in another study by Upadhyaya et al. (2017), CaNP showed better plant performance in protection, fertilization, and pest control than Ca bulk. The CaNP concentration was about one tenth of that in the calcium bulk. This study demonstrated the importance of the CaNP concentration dependence of on calcium bulk.
No literature is available on the use of both CaNP and putrescine elicitor to improve the morphological (flower, stigma, and petals of the flowers) and phytochemical properties of saffron. Therefore, the aim of this study was to investigate the combined effect of elicitors on several parameters of saffron.
Materials and methods
This experiments were conducted in 2019 (from April to September 2019) in a greenhouse located in the Lalim area of the Miandrood city in Iran. This experimental field is geographically located in the west of the Mediterranean at a 36° 28′ N latitude and 52° 23′ E longitude, and is 60 m above sea level. To measure and analyze the data, the experiments were conducted at Sana Institute of Higher Education in Sari, Iran, under greenhouse conditions. A factorial experiment was performed in a completely randomized design with five replications. Different treatments were selected based on the results of various studies that showed effective range of concentrations of putrescine and CaNP.
Experimental treatments included two treatments (putrescine and CaNP) at five concentrations, including:
Treatment a: putrescine at a concentration of 0 (the control sample without any treatment), 0.25, 0.5, 1, 2 mM.
Treatment b: CaNP at a concentration of 0 (the control sample without any treatment), 0.25, 0.5, 1, 1.5 g/L.
First, 150 uniform saffron corms, weighing 10–12 g, which were in the apparent sleep stage, were selected and then placed in a disinfectant solution (copper sulfate fungicide + acaricide) for 20 min (Silva et al. 2016).
Dried saffron corm was treated with various concentrations of putrescine and CaNP. The corms were transferred to the greenhouse with 16 h of light (8000 to 10,000 lx light) and 8 h of darkness, 60% relative humidity, and average temperature of 30 ± 5 °C. After the flower emergence in the reproductive stage (BBCH scale: 6), all flowers were harvested and placed in separate sealed containers for being transferred to the laboratory. Flower, stigma, and petal weights were measured with a sensitive scale (Ramezani et al. 2019b).
After that, each of weighed petals and stigmas were placed in an oven at 50 °C for 12 h to dry. Upon the drying phase completion, the petals and stigmas were weighed again and then the rest of the measurements were performed (Zeka et al. 2015).
Chlorophyll content in plant leaves
Saffron treated samples were gathered and dried in the air, and then, were blended into a powder. As plant samples weighed (0.2 g), they have been extracted with 10 mL acetone. After filtering with paper, the extract was centrifuged for 10 min at 3000 rpm. The supernatant volume reached to 10 mL with 80% acetone. All the samples were analyzed at 663 nm, 645 nm, and 480 nm. The following formula was used for the calculation of chlorophyll concentrations (Arnon 1949):
Chlorophyll a (mg/mL) = 12.7 A663 − 2.69 A645
Chlorophyll b (mg/mL) = 22.9 A645 − 4.68 A663
Total Chlorophyll (mg/mL) = Chlorophyll a + Chlorophyll b
Carotenoid = {(1000*A480) − 1.82 chl a − 85.02 chl b/198)} * V/W*1000
Total flavonoid content
For the determination of total flavonoid content, the aluminum chloride colorimetric assay was used (Pal et al. 2009). Plant Samples (2 g) were homogenized in 80% aqueous ethanol at room temperature and centrifuged at 10,000 rpm for 15 min and the supernatant extract was preserved for the estimation of further analysis. An aliquot (1 ml) of extract or a standard solution of catechol (10 mg/100 ml) was added to 10 ml flask containing 4 ml of distilled water. 0.3 ml 5% NaNO2 was also added. After 5 min, 0.3 ml of 10% AlCl3 was added. After 6 min, 2 ml of 1 M NaOH was added and the total volume was made up to 10 ml with distilled water. Absorbance were recorded at 510 nm with a spectrophotometer (Biochrom WPA Biowave II).
The extraction for antioxidant enzyme assays
For the preparation of crude extract, the homogenization of frozen saffron treated samples in sodium phosphate buffer (1 mM ascorbic acid and 0.5% (w/v) polyvinyl pyrrolidone) was used. The final homogenate extract was filtered through the paper, and then, the filtrate extract was centrifuged at 5000×g for 15 min. Final supernatant was used for the next stage of the assay (Großkinsky et al. 2015).
Antioxidant enzyme activity measurement
Catalase (CAT) activity
Catalase activity was determined according to the modified method of Aebi (1983). In this method, the H2O2 decomposition by reaction mixture (sodium phosphate buffer, H2O2 and crude extract) was monitored at 240 nm absorbance by a spectrophotometer (Biochrom WPA Biowave II).
The peroxidase (POD) activity
For the POX activity determination, 4-methylcatechol as substrate was used. The oxidation of 4-methyl catechol by H2O2 in the reaction mixture (sodium phosphate buffer, 4-methyl catechol, H2O2 and crude extract), resulted in an increase in the absorption, which was monitored at 420 nm (Onsa et al. 2004) by a spectrophotometer (Biochrom WPA Biowave II).
HPLC analysis for the determination of saffron, crocins, and picocrocin
To determine the quantity of saffron plant compounds, the chemicals, saffron stigma samples, and plant materials were collected for further analysis. Three standards of safranal, crocin and picrocrocin, HPLC Methanol, Deionized water, and HPLC acetonitrile were purchased from Sigma–Aldrich (St. Louis, MO) (Lage and Cantrell 2009). Approximately 50 mg of each saffron sample was taken and saffron stigma was ground using a razor blade. For the determination of crocins, safranal, and picrocrocin in saffron plant, the extraction was done with degassed methanol, and then, sonicated for 1 h and kept overnight in darkness. The filtration of each extract was done using a Tuberculin syringe and a filter tip into an HPLC vial for HPLC analysis. The final sample (25 ml) was injected into an HPLC coupled to a diode array detector (Lage and Cantrell 2009).
The Agilent 1100 series HPLC system was used to analyse the prepared extract. It contained a degasser, quaternary pump, ALS auto-sampler, PDA detector, an Agilent Zorbax SB-C-18, and 5 mm column with a column flow of 1 mL/min. The 25 µl for all saffron plant samples, and standards were injected. A solution of H2O and ACN was used as a gradient elution during the analysis. The analyses were repeated three times for each sample. Compounds under study were detected at different absorbance. The safranal was detected at 310 nm, crocin at 440 nm and picrocrocin at 250 nm (Lage and Cantrell 2009).
Considering the quantitative analysis for each concentration of treated plant samples, a standard least-squares regression was drawn. The three standards of crocin, picrocrocin, and safranal were considered for curves calibration. The acquired chromatograms of each plant sample were compared with the standards. The final peaks were fixed by retention time and UV spectral data (Lage and Cantrell 2009).
Statistical analysis
The experimental data were analyzed by statistical analysis system 9.3.1 software (SAS Institute, Cary, NC) (Kang 2015). The randomized complete block design was employed for comparing the different treatments. The means of the examined traits were arranged according to Duncan’s multiple range tests (Neil 2010).
Results
The results of this study showed that the effect of putrescine and CaNP alone and in combination had a significant effect on the fresh weight of flowers and stigmas, but not on the dry weight of the flowers. Also, their combined effect on stigma dry weight was significant at 5% level (P < 0.05) (Table 1). Plant treated with the concentrations of 0.5, 1 and 1.5 g/l of CaNP showed the highest significant value compared to the control sample. A mean comparison of the combined effect of putrescine and CaNP on a fresh weight of the flower showed that at different levels of putrescine, and at the concentration of 1.5 g/l of Ca, it had the highest effect on fresh weight compared to the control (Fig. 1). The fresh weight of stigma at the concentrations of 0.5 g/l, 1 g/l and 1.5 g/l of CaNP were recorded 0.0368 g, 0.0413 g and 0.449 g/l respectively. The results of the data analysis showed that the highest concentration of putrescine treatment (1 mM) on plants led to an increase in fresh weight of the stigma. The mean comparison of the combined effect of putrescine and CaNP on the fresh weight of stigma showed that at different concentrations of the putrescine, CaNP at a concentration of 1.5 g/l had the highest effect on the fresh weight of the stigma compared to the control sample (Fig. 2a). The mean comparison of the stigma dry weight showed that the highest concentration of CaNP treatment resulted in the highest dry weight (0.01 g/l) (Fig. 2b).
Table 1.
Mean comparison of the putrescine and Ca nanoparticle (CaNP) effect on saffron reproductive characters
| Traits | Source of variation | Degrees of freedom | Sum of squares | Mean square | F-value |
|---|---|---|---|---|---|
| Fresh weight of stigma | Putrescine | 4 | 0.008 | 0.002* | 2.55 |
| CaNP | 4 | 0.025 | 0.0060** | 7.91 | |
| Putrescine × CaNP | 16 | 0.028 | 0.002* | 2.21 | |
| Error | 50 | 0.039 | 0.001 | 0.001 | |
| Dry weight of stigma | Putrescine | 4 | 0.032 | 0.008ns | 1.01 |
| CaNP | 4 | 0.0480 | 0.012ns | 1.50 | |
| Putrescine × CaNP | 16 | 0.112 | 0.007ns | 0.88 | |
| Error | 50 | 0.39 | 0.008 | 0.008 | |
| Fresh weight of flower | Putrescine | 4 | 0.001 | 0** | 8.37 |
| CaNP | 4 | 0.001 | 0** | 1.53 | |
| Putrescine × CaNP | 16 | 0.001 | 0** | 3.49 | |
| Error | 50 | 0.001 | 0 | 0 | |
| Dry weight of flower | Putrescine | 4 | 0.001 | 0ns | 1.22 |
| CaNP | 4 | 0.001 | 0ns | 0.93 | |
| Putrescine × CaNP | 16 | 0.006 | 0* | 2.21 | |
| Error | 50 | 0.009 | 0 | 0 |
*, **Significant effect and ns shows no significance effect at probability level of 5% and 1%
Fig. 1.
The effect of different concentrations of Ca nanoparticle (Ca 0, 0.25, 0.5, 1 and 1.5 g/l) × Putrescine (P 0, 0.25, 0.5, 1 and 2 mM) combination on flower fresh weight of saffron
Fig. 2.
The effect of different concentrations of Ca nanoparticle (Ca 0, 0.25, 0.5, 1 and 1.5 g/l) × Putrescine (P 0, 0.25, 0.5, 1 and 2 mM) combination on stigma fresh weight (A) and dry weight (B) of saffron
The variance analysis of the effect of CaNP, and putrescine separately and its combined effect on chlorophyll pigments (a, b, total and carotenoids) was significant at the 1% level (P < 0.01). The highest amount of chlorophyll a was at a concentration of 1 g/l (0.5 mg/g FW) of the treated plant. The combined effect of putrescine and CaNP treatment showed that at concentrations of 1 and 2 mM of putrescine and at a concentration of 0.5 g/l of CaNP, the highest chlorophyll a content was observed (Fig. 3a). The highest chlorophyll b content compared to control (0.552 mg/g fresh weight) was observed when the plants were treated with CaNP at a concentration of 1 g/l (1.03 mg/g fresh weight) /g). Similarly, high chlorophyll b content compared to control was observed in the plant treated with putrescine at the concentrations of 1 and 2 mM (1.1 mg/g fresh weight). The combined effect of both treatments showed that putrescine at concentration of 1 and 2 mM and CaNP at a concentration of 0.5 g/l showed the highest chlorophyll b content (Fig. 3b). Results showed the highest total chlorophyll content was reported in a CaNP-treated plant at a concentration of 1 g/l (1.6 mg/g fresh weight) compared to the control sample (0.826 mg/g fresh weight). Plants treated with putrescine had higher total chlorophyll content than the control group at a concentration of 1 mm. The combined effect of CaNP at a concentration of 0.5 g/l and putrescine at a concentration of 1 and 2 mM revealed the highest total chlorophyll content (Fig. 3c). Results indicated that CaNP and putrescine alone had a significant effect on the carotenoid content in the treated plants. The highest content of carotenoid was observed in plants treated with 1 g/l CaNP (0.297 mg/g fresh weight) compared to the control (0.118 mg/g fresh weight). Plants treated with putrescine at a concentration of 1 mM showed the highest carotenoid content compared to control samples (0.35 mg/g fresh weight). The combined effect of putrescine and CaNP showed that highest level of carotenoids was observed at a concentration of 1 mM of putrescine and a concentration of 1 g/l of CaNP (Fig. 3d, Table 2).
Fig. 3.
The effect of different concentrations of Ca nanoparticle (Ca 0, 0.25, 0.5, 1 and 1.5 g/l) × Putrescine (P 0, 0.25, 0.5, 1 and 2 mM) combination on A chlorophyll a, B chlorophyll b and C total chlorophyll, D carotenoids of saffron
Table 2.
The mean comparison of the putrescine and Ca nanoparticle (CaNP) effect on saffron reproductive characters
| Traits | Source of variation | Degrees of freedom | Sum of squares | Mean square | F-value |
|---|---|---|---|---|---|
| Chlorophyll a | Putrescine | 4 | 0.059 | 0.015** | 5.22 |
| CaNP | 4 | 0.058 | 0.014** | 5.13 | |
| Putrescine × CaNP | 16 | 1.163 | 0.01** | 3.61 | |
| Error | 50 | 0.1410 | 0.003 | 0.003 | |
| Chlorophyll b | Putrescine | 4 | 0.0208 | 0.052** | 4.47 |
| CaNP | 4 | 0.0253 | 0.06** | 5.43 | |
| Putrescine × CaNP | 16 | 0.558 | 0.035** | 3.003 | |
| Error | 50 | 0.58 | 0.012 | 0.012 | |
| Total chlorophyll | Putrescine | 4 | 0.472 | 0.118** | 4.95 |
| CaNP | 4 | 0.542 | 0.13** | 5.69 | |
| Putrescine × CaNP | 16 | 1.264 | 0.079** | 3.32 | |
| Error | 50 | 1.18 | 0.024 | 0.024 | |
| Carotenoid | Putrescine | 4 | 0.015 | 0.004** | 6.15 |
| CaNP | 4 | 0.024 | 0.006** | 10.19 | |
| Putrescine × CaNP | 16 | 0.059 | 0.004** | 6.20 | |
| Error | 50 | 0.029 | 0.001 | 0.001 |
*, **Significant effect and ns shows no significance effect at probability level of 5% and 1%
The analysis of variance of the effect of putrescine and CaNP on saffron showed that plants treated with putrescine and CaNP alone had a significant effect on antioxidant activity at 1% level (P < 0.01) and their combined effect on catalase activity at 5% level was significant (P < 0.05) but its effect on the total flavonoid content was significant at the 1% level and their effect on peroxidase enzymes was not significant (Table 3).
Table 3.
The mean comparison of the putrescine and Ca nanoparticle (CaNP) effect on saffron reproductive characters
| Traits | Source of variation | Degrees of freedom | Sum of squares | Mean square | F-value |
|---|---|---|---|---|---|
| Catalase (µg/g plant) | Putrescine | 4 | 2.2 | 0.55** | 9.90 |
| CaNP | 4 | 3.15 | 0.78** | 14.03 | |
| Putrescine × CaNP | 16 | 2.058 | 0.12** | 2.28 | |
| Error | 50 | 2.81 | 0.05 | 0.05 | |
| Peroxidase (µg protein/min) | Putrescine | 4 | 7.8 | 1.95** | 8.98 |
| CaNP | 4 | 5.33 | 1.33** | 6.13 | |
| Putrescine × CaNP | 16 | 3.81 | 0.23ns | 1.097 | |
| Error | 50 | 10.86 | 0.21 | 0.21 | |
| Total flavonoid (mg Quercetin/g fresh weight) | Putrescine | 4 | 136.1 | 34.04* | 39.63 |
| CaNP | 4 | 96.6 | 24.15** | 28.12 | |
| Putrescine × CaNP | 16 | 94.27 | 5.89* | 6.85 | |
| Error | 50 | 42.95 | 0.850 | 0.85 |
*, **Significant effect and ns shows no significance effect at probability level of 5% and 1%
CaNP and putrescine separately had a significant effect on the induction of CAT activity, so that the highest CAT activity was obtained in plants treated with a concentration of 1 g/l CaNP and 1 mM of putrescine separately. It has been reported that the combined effects of CaNP (0.5 g/l) and putrescine (0.5 mM) on plants showed the highest CAT activity (Fig. 4).
Fig. 4.
The effect of different concentrations of Ca nanoparticle (Ca 0, 0.25, 0.5, 1 and 1.5 g/l) × Putrescine (P 0, 0.25, 0.5, 1 and 2 mM) combination on catalase activity of saffron
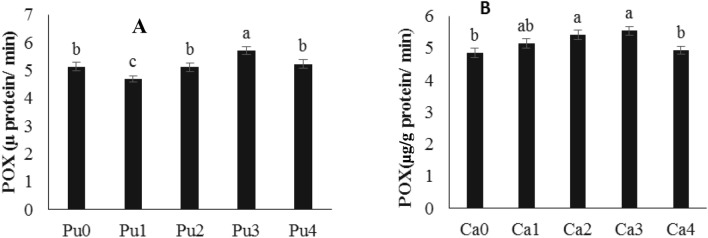
The results showed that with increasing putrescine concentration, the POX activity increased in the treated plants. The highest POX activity was observed at a concentration of 1 mM putrescine compared to the control sample. Also, the results showed that with increasing CaNP concentration, the Pox activity increased; the highest POX activity was reported in plants treated with CaNP at the concentrations of 0.5 g/l and 1.5 g/l (Fig. 5).
Fig. 5.
The effect of different concentrations of A Ca nanoparticle (Ca 0, 0.25, 0.5, 1 and 1.5 g/l) × B Putrescine (P 0, 0.25, 0.5, 1 and 2 mM) combination on the POX activity of saffron
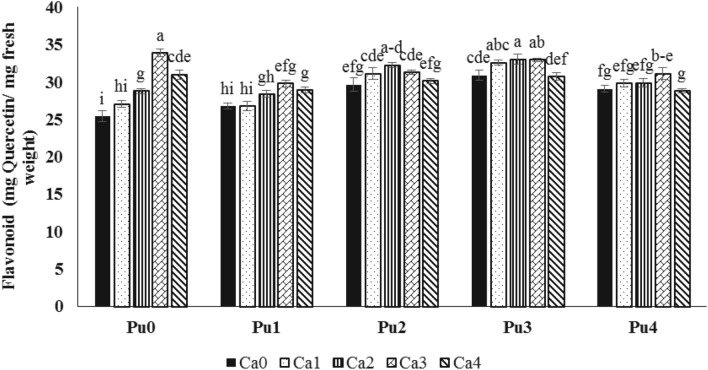
Results of flavonoid analysis revealed that the highest flavonoid content (34 mg quercetin/g fresh weight) was observed in plant treated with 1 g/l CaNP compared to the control. Plants treated with putrescine at 0.5, 1 and, 2 mM concentration showed higher flavonoid content than control samples. The combined effect of CaNP and putrescine showed that at all concentrations of putrescine, flavonoid content increased with increase of CaNP concentration. The highest flavonoid content was related to plants treated with putrescine at a concentration of 1 mM and CaNP at a concentration of 0.5 g/l (Fig. 6).
Fig. 6.
The effect of different concentrations of Ca nanoparticle (Ca 0, 0.25, 0.5, 1 and 1.5 g/l) × Putrescine (P 0, 0.25, 0.5, 1 and 2 mM) combination on flavonoid content of saffron
HPLC results analysis
The quantitative extraction of the saffron by HPLC showed a good appearance for the peaks of safranal, picrocrocin and, crocin. The mean safranal recovery was 26 ± 4.4% and 15 ± 2.0% for crocin and 16 ± 6.0% for picrocrocin at the different levels of the spike (Fig. 7).
Fig. 7.
HPLC chromatogram of leaves extract from saffron: 1-picocrocin; 2-crocin; 3-safranal
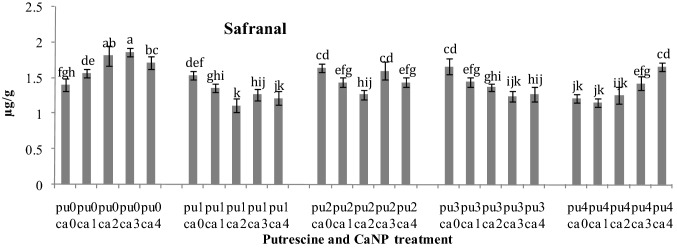
HPLC results reported that with increasing CaNP concentration, safranal level showed an increasing trend, so that the highest safranal level was reported in the plants treated with 1 g/l CaNP compared to the control (1.8 µg/g). The results showed that putrescine treatment alone at the concentrations of 0.25, 0.5 and 1 mM resulted in an increase in safranal level in the treated plants (1.66 µg/g). The combined effect of putrescine and CaNP showed that at the highest level of putrescine treatment (2 mM), with increase of the CaNP concentration, safranal content showed an increasing trend (1.6 µg/g) (Fig. 8).
Fig. 8.
The effect of applying various concentrations of Ca nanoparticles (Ca0, Ca1, Ca2, Ca3, Ca4 mM) and various concentrations of putrescin on (Pu0, Pu1, Pu2, Pu3, Pu4) Safranal level (µgr/g) in tissues of Crocus sativus L. The mean values were the results of three separate experiments. Data represent the mean ± SD, n = 3; LSD: least significant difference; significance level: P < 0.05. Each column of means that have at least one letter in common were not significantly different
The results of HPLC analysis showed that the effect of different concentrations of CaNP and putrescine treatment alone on the crocin level had an increasing trend. Thus, the highest level of crocin (10.45 µg/g) was observed in CaNPtreated plants at the concentration of 1 g/l. Putrescine at concentrations of 0.25, 0.5, 1 mM had the greatest effect on crocin compared to control plants. Putrescine in the high concentration of (2 mM) showed a decreasing effect on the crocin level in the treated plants (6.9 µg/g). The combined effect of putrescine and CaNP was significant, so that at different concentrations of putrescine, with increasing CaNP concentration, crocin level decreased. (Fig. 9).
Fig. 9.
The effect of applying various concentrations of CaNP (Ca0, Ca1, Ca2, Ca3, Ca4 mM) and various concentrations of putrescine (Pu0, Pu1, Pu2, Pu3, Pu4) on crocin level (µg/g) in tissues of Crocus sativus L. The mean values were the result of three separate experiments. Data represent the mean ± SD, n = 3; LSD: least significant difference; significance level: P < 0.05. Each column of means that have at least one letter in common were not significantly different
Different concentrations of CaNP and putrescine alone in treated plants showed a significant increase in picrocrocin level. The highest amount of picrocrocin (11.6 µg/g) has been reported at a concentration of 1 g/l CaNP in the treated plant. Plants treated with putrescine at concentrations of 0.25, 0.5 and 1 mM showed the highest picrocrocin level, while at a concentration of 2 mM putrescine, the picrocrocin level decreased (6.8 µg/g). The combined effect of putrescine and CaNP showed that at different concentrations of putrescine, with increase of CaNP concentration, the picrocrocin level decreased. So that, the putrescine at a concentration of 2 mM, and CaNP at concentrations of 0.25, 0.5, 1 and 1.5 showed an increasing trend on picrocrocin level in treated plants (Fig. 10).
Fig. 10.
The effect of applying various concentrations of Ca nanoparticles (Ca0, Ca1, Ca2, Ca3, Ca4 mM) and various concentrations of putrecinon (Pu0, Pu1, Pu2, Pu3, Pu4) and picrocrocin level (µg/g) in tissues of Crocus sativus L. The mean values were the result of three separate experiments. Data represent the mean ± SD, n = 3; LSD: least significant difference; significance level: P < 0.05. Each column of means that have at least one letter in common were not significantly different
Discussion
Different factors can affect the function of the elicitor (biomass and secondary metabolite production) such as concentrations, type, duration of exposure, culture, stage of the culture, and medium composition (Kaur 2018; Dhiman et al. 2018; Naik and Al-Khayri 2016). Previous researchers have reported that a combination of more than one elicitor in different culture media can increase plant yields more compared to a single elicitor (Wang et al. 2009; Zheng et al. 2008; Gangopadhyay et al. 2011; Ramezani et al. 2017).
In recent years, the role of polyamines regulators such as putrescine in physiological and cellular processes during plant growth has been considered (Yunjun et al. 2016; Dever and Ivanov 2018). Polyamines protect the membrane through ionic bonding to the thylakoid membrane, and so they are indirectly involved in the maintaining of photosynthesis (Gupta et al. 2003). The increase in chlorophyll content due to putrescine is because of their antioxidant properties, which prevent the destruction of the chloroplast membrane structure. The positive effect of putrescine is probably related to the role of this hormone in increasing cell division, increasing plant hormones (gibberellin and auxin), and reducing acyclic acid, which led to improved plant growth (Cohen et al. 2004). In the present study, the highest amount of chlorophyll was observed in the plants treated with putrescine. It can be stated that the polyamines treatment maintains the stability of chloroplast membrane and prevent the chlorophyll decomposition. The effect of putrescine on the enhancement of photosynthetic pigments in cucumber (El-Bassiouny et al. 2008), beans (He et al. 2002) and mulberry (Nassar et al. 2003) has been reported. Previous research on wheat has also shown that the external use of putrescine induces the internal stimulation of cytokinin, which results in chlorophyll biosynthesis and chloroplast differentiation (El-Bassiouny et al. 2008). Since magnesium is one of the main components of the chlorophyll structure, and putrescine increases the absorption of elements such as potassium and magnesium, it improves the photosynthetic pigments in the plant (El-Bassiouny et al. 2008; Mahgoub et al. 2011). Unal et al. (2007) has observed that polyamines act as protectors for chlorophyll and protein, and then stabilized the membranes by reducing lipid peroxidation. Chloroplasts contain large amounts of biosynthetic enzymes of polyamine, which played an important role in regulating stomatal activity and improving photosynthesis (Rosmana et al. 2016).
According to the previous studies, at the end of the saffron growing season and before the corm becomes dormant, the amount of phenolic compounds increases, which further cause dormancy in the saffron and protects it from stress. As corm enters in the dormant phase, the content of these compounds decreased (Esmaeili et al. 2011). Polyamines are usually found in combination with phenolic compounds in the cell wall (Gorecka et al. 2007). One of the reasons for the decrease in total phenolic content in corm after the polyamines treatment could be the binding of polyamines to phenolic compounds. The observed difference between phenolic, and flavonoid contents in response to the treatments could be a consequence of the division of the phenylpropanoid pathway into several branches, with an increase in one branch (phenol production) and decrease in another branch (flavonoid production).
Polyamines consists nitrogen in their structure and it can acts as plant growth regulator as a source of nitrogen to the plants and thus improves plant growth. The most important role of putrescine is the storage of nitrogen during the flowering stage in plant (Pritsa and Demetios 2005). The possible reason for the increase in the plant yield is because putrescine helps in reducing the destruction of cell membranes and intracellular tissues as well as the production of secondary metabolites by plants such as proline, which further increases the osmotic potential of the plant, and increases the adsorption power of water in adverse environmental conditions. The foliar application of putrescine increases fresh and dry weights of aerial parts in Dahlia pinnata (Mahgoub et al. 2011). The reason for the increase in plant fresh and dry weights in the current study, as well as the improvement of other traits studied in this plant by putrescine, can be attributed to the fact that the use of putrescine increases cell division in the plant, and this increase improves the plant metabolism and increases photosynthetic pigmentation, and antioxidant activity in the plant (Gupta et al. 2003; Mahgoub et al. 2011). Mahros et al. (2011) showed that putrescine application increased carotenoid content in Chrysanthemum indicum, which is consistent with the results of the present study. The mechanism of polyamines action to increase the activity of antioxidant enzyme is likely to be for the point that polyamines, as signaling molecules, trigger a set of defensive pathways that induce the activity of antioxidant enzyme (Tomi et al. 2010). Amraeitabar et al. (2016) reported that the use of putrescine increased the activity of catalase and peroxidase enzyme in Prunus dulcis and Prunus persica.
With nano-fertilizers, the time and rate of element's release is adjusted according to the plant's nutritional requirements, enabling the plants to absorb the maximum amount of nutrients. and thus, while reducing the element leaching, the plant yield increases (Derosa et al. 2010; Naderi and Danesh-Shahraki 2013). The results of the present study showed that the treatment of different concentrations of CaNP increased all the studied traits compared to the control. Thus, the highest levels of chlorophyll, carotenoids, flavonoids, catalase and peroxidase were observed in the plants treated with 1 g/l of CaNP.
Also, through increasing the CaNP concentration in plant treatment, the fresh weight of flowers and stigmas showed an increasing trend, so that the concentrations of 0.5, 1, and 1.5 g/l of this nanoparticle showed the highest significant value compared to the control. Ca affects the growth process in plants (Mohseni Nik et al. 2012). Ca is beneficial for stabilizing cell membrane surfaces, maintaining photosynthesis at the normal level, which induces activation of different enzymes, and biochemical functions, thus affecting the plant's growth (Ting et al. 2019). According to Ghahremani et al. (2013), the foliar application of CaNP increased the growth index and chlorophyll content in basil, which was due to the improved photosynthesis and nutrients uptake by plants, followed by the increased vegetative and reproductive growth. Salehi et al. (2017) has also observed that the highest quantity of chlorophyll was observed after CaNP treatments in Tropaeolum majus.
The results show that CaNP and putrescine separately had an increasing effect on crocin, picrocrocin, and safranal content, but the combined effect of both elicitors on the saffron plant led to a decrease in crocin, picrocrocin, and safranal content. The elicitor concentration plays an essential role in the elicitation process. The optimum concentration was determinating for the elicitor induction (Zheng et al. 2008; Ramezani et al. 2019a; Nokandeh et al. 2020). In the present study, plants' treatment with a high concentration of CaNP and putrescine combination decreased the level of crocin, picrocrocin, and safranal, respectively.
A previous study reported that the combined effect of two or more elicitor's agent in the plant culture medium induced the production of secondary metabolite compared to treatment with one elicitor agent (Wang et al. 2009; Zheng et al. 2008; Gangopadhyay et al. 2011). The metabolic engineering in combination with two elicitations led to an increase in the production of flavonoids in hairy roots of Glycyrrhiza uralensis (Zhang et al. 2009).
The polyamines can increase the growth, yield, and stress tolerance of medicinal plants through exogenous treatment or genetic manipulation; however, these properties depend on the concentration level, type of polyamine and plant species.
In the present study, crocin, picrocrocin, and safranal content increased in putrescine treated plants with concentrations of 0.25, 0.5, and 1 mM, but decreased in the plants treated with a high concentration of putrescine (2 mM), crocin level, picrocrocin and safranal.
The absorption of nanoparticles in plants is dependent on various factors including the exceptional properties of nanoparticles, the combination of the nanoparticles with the different elicitors, and the physiological parameters of the plant (Yadav et al. 2014; Ma et al. 2015; Zahed et al. 2015; Ramezani et al. 2017; Majlesi et al. 2018). In the present study, the use of CaNP at 0.25, 0.5 and 1 g/l on saffron increased the levels of crocin, picrocrocin, and safranal, but a high concentration of CaNP decreased them. Similarly, Oloumi et al. (2015) reported that the presence of 1 and 10 µM CuO and ZnO nanoparticles in Hoagland nutrient solution, in Glycyrrhiza glabra seedlings, increased the content of glycyrrhizin and phenolic compounds compared to their non-treated samples. In the study by Khan et al. (2016) the effect of metal and non-metal nanoparticles (Ag, Au, Cu, AgCu (1:3), AgCu (3:1), AuCu (1:3), AuCu (3:1), AgAu (1:3), AgAu (3:1)) was reported on the total amount of phenolic and flavonoid in Silybum marianum plant. The duration of the exposure significantly affected the total flavonoids and phenolic content of Silybum marianum. Mainly different factors (surface area, size, and composition of nanoparticles) play an important role in the single or in mixed treatment. The direct biophysical and/or biochemical interactions of the nanoparticles-biological interfaces/systems are not yet widely known.
Conclusion
In the current study, each treatment alone may serve as a new growth stimulant rather than a combinatorial treatment of both elicitors (CaNP and putrescine) on Crocus sativus. Higher concentrations of both elicitors evoked better responses in phytochemical properties and growth in the plants. Both elicitors act in a dose-dependent manner. It can be concluded that because both elicitors are enhancing the phytochemical yield this may contribute to the formulation of new nano-growth inducers for agricultural, food, and pharmaceutical industries. Therefore, it can help to reduce fertilizer wastage, and in turn, environmental contamination due to the agricultural mismanagement. However, the details of the physiological and molecular mechanisms of CaNP and putrescine and their effect on Crocus sativus L or other plants are unclear that should be investigated in the future. On the other hand, the higher concentration of applied elicitors can be the subject of future research to improve plant products.
Acknowledgements
This paper was supported by Sana Institute of Higher Education, Iran. We are thankful from Sana Institute.
Author contributions
LB and MR wrote the manuscript and performed the experiments. MS, MG and DA designed the experiment, analyzed the data, and helped to draft the manuscript. LB, MR, MS, MG and DA revised the manuscript. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The author declares that the study has no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aebi HE. Catalase. In: Bergmeyer HU, editor. Methods of enzymatic analysis. Weinhem: Verlag Chemie; 1983. pp. 273–286. [Google Scholar]
- Ahmed N, Anwar S, Al-Sokari SS, Ansari SY, Wagih ME. Essential oils in food preservation, flavor and safety. New York: Academia Press; 2016. pp. 705–713. [Google Scholar]
- Amin B, Hosseinzadeh H. Evaluation of aqueous and ethanolic extracts of saffron, Crocus sativus L., and its constituents, safranal and crocin in allodynia and hyperalgesia induced by chronic constriction injury model of neuropathic pain in rats. Fitoterapia. 2012;83(5):888–895. doi: 10.1016/j.fitote.2012.03.022. [DOI] [PubMed] [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagur MJ, Salinas GLA, Jiménez-Monreal AM. Saffron: an old medicinal plant and a potential novel functional food. Molecules. 2018;23(1):30. doi: 10.3390/molecules23010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardone L, Castronuovo D, Perniola M, Scrano L, Cicco N, Candido V. The influence of soil physical and chemical properties on saffron (Crocus sativus L.) growth, yield and quality. Agronomy. 2020;10:1154. [Google Scholar]
- Chehrazi M, Pourghasemi D, Khoshbakht M. Effects of Ascorbic acid on quantitative and qualitative characteristic of two alternanthera repens genotypes (“Entire leaf and undulated leaf”) under salinity stress. Sci J Agric. 2015;38(2):65–76. [Google Scholar]
- Cohen AS, Popovic RB, Zalik S. Effects of polyamines on chlorophyll and protein content, photochemical activity and chloroplast ultrastructure of barley leaf discs during senescence. Plant Physiol. 2004;64:717–720. doi: 10.1104/pp.64.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derosa MR, Monreal C, Schnitzer M, Walsh R, Sultan Y. Nanotechnology in fertilizers. Natural. Nanotechnology. 2010;5:91–92. doi: 10.1038/nnano.2010.2. [DOI] [PubMed] [Google Scholar]
- Dever TE, Ivanov IP. Roles of polyamines in translation. J Biol Chem. 2018;293:18719–18729. doi: 10.1074/jbc.TM118.003338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhiman N, Patial V, Bhattacharya A. The current status and future applications of hairy root cultures. In: Kumar N, editor. Biotechnological approaches for medicinal and aromatic plants. Singapore: Springer; 2018. pp. 87–155. [Google Scholar]
- El-Bassiouny HM, Mostafa HA, El-Khawas SA, Hassanein RA, Khalil SI, Abd El- Monem AA. Physiological responses of wheat plant to foliar treatments with arginine or putrescine. Aust J Basic Appl Sci. 2008;2:1390–1403. [Google Scholar]
- Esmaeili N, Ebrahimzadeh H, Abdi K, Safarian S. Determination of some phenolic compounds in Crocus sativus L. corms and its antioxidant activities study. Pharmacognosy Mag. 2011;7(25):74–80. doi: 10.4103/0973-1296.75906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangopadhyay M, Dewanjee S, Bhattacharya S. Enhanced plumbagin production in elicited Plumbago indica hairy root cultures. J Biosci Bioeng. 2011;111:706–710. doi: 10.1016/j.jbiosc.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Ghahremani A, Akbari K, Yusof poor MR (2013) The effect of nano potassium and calcium chelate fertilizers on quantitative and qualitative characteristics of basil (Ocimum basilicum), the first national conference on nanotechnology applications in industry, mining, agriculture and medicine, Karaj (In Persian)
- Gorecka K, Cvikrová M, Kowalska U, Eder J, Szafrańska K, Górecki R, Janas KM. The impact of Cu treatment on phenolic and polyamine levels in plant material regenerated from embryos obtained in anther culture of carrot. Plant Physiol Biochem. 2007;45(1):54–61. doi: 10.1016/j.plaphy.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Großkinsky DK, Svensgaard J, Christensen S, Roitsch T. Plant phenomics and the need for physiological phenotyping across scales to narrow the genotype-to-phenotype knowledge gap. J Exp Bot. 2015;66:5429–5440. doi: 10.1093/jxb/erv345. [DOI] [PubMed] [Google Scholar]
- Gupta S, Sharma ML, Gupta NK, Kumar A. Productivity enhancement by putrescine in wheat (Triticum aestivum L.) Physiol Mol Biol Plants. 2003;9:279–282. [Google Scholar]
- Hassan A. Effects of mineral nutrients on physiological and biochemical processes related to secondary metabolites production in medicinal herbs. Med Arom Plant Sci Biotechnol. 2012;6:105–110. [Google Scholar]
- He L, Nada K, Tachibana S. Effects of spermidine pretreatment through the roots on growth and photosynthesis of chilled cucumber plants (Cucumis sativus L.) J Jpn Soc Horticult Sci. 2002;71:490–498. [Google Scholar]
- Hua KH, Wang HC, Chung RS, Hsu JC. Calcium carbonate nanoparticles can enhance plant nutrition and insect pest tolerance. J Pesticide Sci. 2015;40(4):208–213. [Google Scholar]
- Jafar Dokht R, Mosavi Nik SM, Mehraban A, Basiri M. Effect of water stress and foliar micronutrient application on physiological characteristics and nutrient uptake in mung bean. Electron J Crop Product. 2015;8:121–141. [Google Scholar]
- Kang MS. Efficient SAS programs for computing path coefficients and index weights for selection indices. J Crop Improv. 2015;29(1):6–22. [Google Scholar]
- Khan MS, Zaka M, Abbasi BH, Rahman LU, Shah A. Seed germination and biochemical profile of Silybum marianum exposed to monometallic and bimetallic alloy nanoparticles. IET Nanobiotechnol. 2016;10(6):359–366. doi: 10.1049/iet-nbt.2015.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khot LR, Sankaran S, Mari JJ, Schuster EW. Applications of nanomaterials in agricultural production and crop protection: a review. Crop Prot. 2012;35(C):64–70. [Google Scholar]
- Killiny N, Khot LR, Sankaran S, Maja JM, Ehsani R, Schuster EW. Applications of nanomaterials in agricultural production and crop protection: a review. Crop Protection. 2012;35:64–70. [Google Scholar]
- Lage M, Cantrell CL. Quantification of saffron (Crocus sativus L.) metabolites crocins, picrocrocin and safranal for quality determination of the spice grown under different environmental Moroccan conditions. Sci Hortic. 2009;121:366–373. [Google Scholar]
- Liu R, Zhang H, Lal R. Effects of stabilized nanoparticles of copper, zinc, manganese, and iron oxides in low concentrations on lettuce (Lactuca sativa) seed germination: nanotoxicants or nanonutrients? Water Air Soil Pollut. 2016;227:1–14. [Google Scholar]
- Ma X, Wang Q, Rossi L, Zhang W. Cerium Oxide nanoparticles and bulk cerium oxide leading to different physiological and biochemical responses in Brassica rapa. Environ Sci Technol. 2015;50:6793–6802. doi: 10.1021/acs.est.5b04111. [DOI] [PubMed] [Google Scholar]
- Madadi A, Rostamza AM. Production of saffron greenhouses and the possibility of harvesting more than once a year, the first regional conference of medicinal plants in the north of the country. Gorgan: Golestan Agricultural and Natural Resources Research Center; 2013. [Google Scholar]
- Mahgoub MH, Abd-El Aziz NG, Mazhar AMA. Response of Dahlia pinnata L. plant to foliar spray with putrescine and thimine on growth, flowering and photosynthetic. Am Eurasian J Agric Environ Sci. 2011;10(5):769–775. [Google Scholar]
- Mahros KM, Badawy EM, Mahgoub MH, Habib AM, El-Sayed IM. Effect of putrescine and uniconazole treatments on flower characters and photosynthetic pigments of Chrysanthemum indicum L. Am J Plant Sci. 2011;7:399–408. [Google Scholar]
- Majlesi Z, Ramezani M, Gerami M. Investigation on some main glycosides content of Stevia rebaudian B under different concentration of commercial and synthesized silver nanoparticles. Pharmaceut Biomed Res. 2018;4(1):1–10. [Google Scholar]
- Milajerdi A, Bitarafan V, Mahmoudi M. A review on the effects of saffron extract and its constituents on factors related to neurologic, cardiovascular and gastrointestinal diseases. J Med Plants. 2014;14:1–20. [Google Scholar]
- Moghaddasi MS. Saffron chemicals and medicine usage. J Med Plants Res. 2010;4(6):427–430. [Google Scholar]
- Mohseni Nik N, Zabihi H, Asgharzadeh A. Evaluate the response to cut flower rose to biofertilizer application in hydroponic system. Sci Technol Greenhouse Cult. 2012;2(8):57–69. [Google Scholar]
- Naderi MR, Danesh-Shahraki AR. Nanofertilizers and their roles in sustainable agriculture. Int J Agric Crop Sci. 2013;19:2229–2232. [Google Scholar]
- Naik PM, Al-Khayri JM. Abiotic and biotic elicitors–role in secondary metabolites production through in vitro culture of medicinal plant. In: Shanker AK, Shanker C, editors. Abiotic and biotic stress in plants-recent advances and future perspectives. Rijeka: In Tech; 2016. pp. 247–277. [Google Scholar]
- Nassar AH, El-Tarabily KA, Sivasithamparam K. Growth promotion of bean (Phaseolus vulgaris L.) by a polyamine producing isolate of Streptomyces griseoluteus. Plant Growth Regul. 2003;40:97–106. [Google Scholar]
- Neil S. Encyclopedia of research design. Thousand Oaks: SAGE; 2010. [Google Scholar]
- Nokandeh S, Ramezani M, Gerami M. The physiological and biochemical responses to engineered green graphene/metal nanocomposites in Stevia rebaudiana. J Plant Biochem Biotechnol. 2020 doi: 10.1007/s13562-020-00630-4. [DOI] [Google Scholar]
- Oloumi H, Soltaninejad R, Baghizadeh A. The comparative effects of nano and bulk size particles of CuO and ZnO on glycyrrhizin and phenolic compounds contents in Glycyrrhiza glabra L. seedlings. Indian J Plant Physiol. 2015;20:157–161. [Google Scholar]
- Onsa GH, Saari N, Selamat J, Bakar J. Purification and characterization of membrane-bound peroxidases from Metroxylon sagu. Food Chem. 2004;85:365–376. [Google Scholar]
- Pal D, Sannigrahi S, Mazumder U. Analgesic and anticonvulsant effects of saponin isolated from the leaves of Clerodendrum infortunatum Linn. in mice. Indian J Exp Biol. 2009;47:743–747. [PubMed] [Google Scholar]
- Pritsa TS, Demetios GV. Correlation of ovary and leaf spermidine and spermine content with the alternate bearing habit of olive. J Plant Physiol. 2005;162:1284–1291. doi: 10.1016/j.jplph.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Rahmani AH, Rahmani AH, Amjad AAK, Yousef AK, Yousef A, Aldebasi H. Saffron (Crocus sativus) and its Active Ingredients: Role in the Prevention and Treatment of Disease. Pharmacognosy J. 2017;9(6):873–879. [Google Scholar]
- Ramezani M, Rahmani F, Dehestani A. Comparison between the effects of potassium phosphite and chitosan on changes in the concentration of Cucurbitacin E and on antibacterial property of Cucumis sativus. BMC Complement Altern Med. 2017;17(1):1–6. doi: 10.1186/s12906-017-1808-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramezani M, Gerami M, Majlesi Z. Comparison between various concentrations of commercial and synthesized silver nanoparticles on biochemical parameters and growth of Stevia rebaudian B. Plant Physiol Rep. 2019;24:1–12. [Google Scholar]
- Ramezani M, Asghari S, Gerami M, Ramezani F, Karimi Abdolmaleki M. Effect of silver nanoparticle treatment on the expression of key genes involved in glycosides biosynthetic pathway in Stevia rebaudiana B. Plant Sugar Tech. 2019;22:518–527. [Google Scholar]
- Rane M, Bawskar M, Rathod D, Nagaonkar D, Rai M. Influence of calcium phosphate nanoparticles, Piriformospora indica and Glomus mosseae on growth of Zea mays. Adv Nat Sci Nanosci Nanotechnol. 2015;6:045014. [Google Scholar]
- Rosmana A, Nasaruddin N, Hendarto H, Hakkar AA, Agriansyah N. Endophytic association of Trichoderma asperellum within Theobroma cacao suppresses vascular streak dieback incidence and Promotes Side Graft Growth. Mycobiology. 2016;44(3):180–186. doi: 10.5941/MYCO.2016.44.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi L, Chehrazi M, Mousavi S. Effect of nano-calcium and humei potash fertilizers on morphological and biochemical properties of Lade (Tropaeolum majus) J Agric Knowl Sustain Prod. 2017;72:192–202. [Google Scholar]
- Silva JAT, Kulus D, Zhang X, Zeng S, Ma G, Piqueras A. Disinfection of explants for saffron (Crocus sativus) tissue culture. Environ Exp Biol. 2016;14:183–198. [Google Scholar]
- Ting L, An Y, Neha B, Alphan A, Eldad A, Pauline D, Tarr PT, Schroeder JI. Calcium signals are necessary to establish auxin transporter polarity in a plant stem cell niche. Nat Commun. 2019;10:726. doi: 10.1038/s41467-019-08575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomi I, Moschou PN, Paschalids KA, Bouamama B, Salem-Fnayu AB, Ghirbel A, Mliki WA, Roubelakis Angelakis KA. Abscisic acid signals reorieatiation of polyamine metabolism to orchestrate stress responses via the polyamine exodus pathway in grapevine. J Plant Physiol. 2010;167:519–525. doi: 10.1016/j.jplph.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Unal D, Tuney I, Sukatar A. The role of external polyamines on photosynthetic responses, lipid peroxidation, protein and chlorophyll a content under the UV-A (352 nm) stress in Physica semipinnata. J Photochem Phytobiol. 2007;90(1):64–68. doi: 10.1016/j.jphotobiol.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Upadhyaya H, Begum L, Dey B, Nath PK, Panda SK. Impact of calcium phosphate nanoparticles on rice plant. J Plant Sci Phytopathol. 2017;1:001–010. [Google Scholar]
- Wang JW, Zheng LP, Zhang B, Zou T. Stimulation of artemisinin synthesis by combined cerebroside and nitric oxide elicitation in Artemisia annua hairy roots. Appl Microbiol Biotechnol. 2009;85:285–292. doi: 10.1007/s00253-009-2090-9. [DOI] [PubMed] [Google Scholar]
- Yadav T, Mungray AA, Mungray AK. Fabricated nanoparticles: current status and potential phytotoxic threats. In: Whitacre DM, editor. Reviews of environmental contamination and toxicology. Cham: Springer Verlag; 2014. pp. 83–110. [DOI] [PubMed] [Google Scholar]
- Yunjun F, Bo X, Man Y, Qiong D, Wei T. MicroRNAs, polyamines, and the activities antioxidant enzymes are associated with in vitro rooting in white pine (Pinus strobus L.) Springer Plus. 2016;5:416. doi: 10.1186/s40064-016-2080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahed H, Ghazala M, Setsuko K. Plant Responses to Nanoparticle Stress. Int J Mol Sci. 2015;16:26644–26653. doi: 10.3390/ijms161125980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeka K, Ruparelia K, Ruparelia K, Continenza MA. Petals of Crocus sativus L. as a potential source of the antioxidants crocin and kaempferol. Fitoterapia. 2015;107:128–134. doi: 10.1016/j.fitote.2015.05.014. [DOI] [PubMed] [Google Scholar]
- Zhang HC, Liu JM, Lu HY, Gao SL. Enhanced flavonoid production in hairy root cultures of Glycyrrhiza uralensis Fisch by combining the over-expression of chalcone isomerase gene with the elicitation treatment. Plant Cell Report. 2009;28:1205–1213. doi: 10.1007/s00299-009-0721-3. [DOI] [PubMed] [Google Scholar]
- Zheng LP, Guo YT, Wan JW, Tan RX. Nitric oxide potentiates oligosaccharide-induced artemisinin production in Artemisia annua hairy roots. J Integr Plant Biol. 2008;50:49–55. doi: 10.1111/j.1744-7909.2007.00589.x. [DOI] [PubMed] [Google Scholar]