Abstract
Cucumber mosaic virus (CMV), Turnip mosaic virus (TuMV) and Turnip crinkle virus (TCV) are important plant infecting viruses. In the present study, whole transcriptome alteration of Arabidopsis thaliana in response to CMV, TuMV and TCV, individual as well as mixed infections of CMV and TuMV/CMV and TCV were investigated using microarray data. In response to CMV, TuMV and TCV infections, a total of 2517, 3985 and 277 specific differentially expressed genes (DEGs) were up-regulated, while 2615, 3620 and 243 specific DEGs were down-regulated, respectively. The number of 1222 and 30 common DEGs were up-regulated during CMV and TuMV as well as CMV and TCV infections, while 914 and 24 common DEGs were respectively down-regulated. Genes encoding immune response mediators, signal transducer activity, signaling and stress response functions were among the most significantly upregulated genes during CMV and TuMV or CMV and TCV mixed infections. The NAC, C3H, C2H2, WRKY and bZIP were the most commonly presented transcription factor (TF) families in CMV and TuMV infection, while AP2-EREBP and C3H were the TF families involved in CMV and TCV infections. Moreover, analysis of miRNAs during CMV and TuMV and CMV and TCV infections have demonstrated the role of miRNAs in the down regulation of host genes in response to viral infections. These results identified the commonly expressed virus-responsive genes and pathways during plant–virus interaction which might develop novel antiviral strategies for improving plant resistance to mixed viral infections.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-021-00925-3.
Keywords: Arabidopsis thaliana, Defense responses, Differentially expressed genes, Microarray, Virus infection
Introduction
Plants are subjected to a variety of biotic and abiotic stresses which adversely affect the crop growth and yield (Suzuki et al. 2014; Sharma et al. 2013). Among biotic stresses, viruses are highly devastating and causing major losses to economically important host plants (Nicaise 2014; Chen and Wei 2020). Plant viruses are infectious agents that can only replicate and survive within their host cells. Due to their obligate nature, they depend on host cellular machinery to complete their reproduction cycles (replication and movement processes) (Ahlquist et al. 2003; Whitham and Wang 2004). Among viruses, Cucumber mosaic virus (CMV) (genus Cucumovirus, family Bromoviridae) is one of the most important aphids transmitted plant pathogens which infects various plant species (Palukaitis and Garcia-Arenal 2003). In addition, Turnip mosaic virus (TuMV) (genus Potyvirus, family Potyviridae) is another aphid transmitted virus that is considered as one of the most damaging plant pathogens infecting vegetable crops around the world, and is likely the most common and economically important virus infecting various species of Brassicaceae (Nguyen et al. 2013; Yasaka et al. 2015; Edwardson and Christie 1991; Walsh and Jenner 2002). Moreover, Turnip crinkle virus (TCV) (genus Carmovirus, family Tombusviridae) is a positive single-strand RNA virus that is transmitted by chrysomelid beetles with a wide host range (Broadbent and Heathcote 1958; Hollings and Stone 1972). TuMV induces various symptoms including necrosis, stunting and plant death in the host plants (Guerret et al. 2017; Edwardson and Christie 1991), resulting in yield losses and poor product quality in crops (Jiang et al. 2010; Spence et al. 2007). TuMV infections negatively affect developmental traits like flower viability in A. thaliana (Walsh and Jenner 2002; Sánchez et al. 2015). It has also been reported that CMV and TCV are considered as highly virulent pathogens on Arabidopsis plants (Van Regenmortel et al. 2000; Cohen et al. 2000). Moreover, CMV and TCV can tackle plant defense mechanisms, thus resulting in severe losses in crops around the world (Manfre and Simon 2008). CMV and TCV induce markedly different symptoms in A. thaliana. CMV infections cause small leaves and stunting, while TCV infections express chlorotic symptom in A. thaliana (Yang et al. 2010). Therefore, CMV, TuMV and TCV are capable of infecting a wide number of plants. Arabidopsis thaliana is a genetically model plant in dicots that is a susceptible host for these viruses (Sommerville and Koornneef 2002) and therefore it is a suitable model plant to study plant-pathogen interactions (Pagan et al. 2010; Bethke and Glazebrook 2019). A successful infection requires counteracting host defense mechanisms and other cellular factors (Harries and Ding 2011). Therefore, viruses recruit various strategies to utilize cellular resources of host plants to facilitate their infections. Consequently, viral infections induce a variety of specific and general changes in plant gene expression of multiple regulatory and host defense responses at host physiological and developmental processes (Golem and Culver 2003; Lecellier and Voinnet 2004; Maule et al. 2002; Whitham et al. 2006; Pesti et al. 2019). Gene expression of plants is transiently changed to help viral gene expression and to create a favorable cellular environment for viral infections (Jelitto-Van Dooren et al. 1999). Therefore, studying the global transcriptome alteration in response to virus infection and host-virus interactions using gene expression profiling analysis including DNA microarray is widely used to understand the mechanism of virus-host interaction and the host response mechanism (Moustafa and Cross 2016; Shaik and Ramakrishna 2013; Pashaiasl et al. 2016). Among differentially expresses genes, transcription factors (TFs) play a critical role in responses against environmental stresses. The TFs are DNA-binding proteins that modulate the genes expression by binding to specific DNA sequences in the genes promoter and act as transcriptional activators/repressors to regulate other gene (Seo and Choi 2015; Li et al. 2014a; Seki et al. 2002). Plant micro RNAs (miRNAs) are endogenous, noncoding and small RNAs that regulate gene expression via interactions with their specific target mRNAs (Liu et al. 2008). MiRNAs play a vital role in many biological and metabolic processes including regulation of plant growth, development and in particular response to biotic and abiotic stresses (Shukla et al. 2008; Liu et al. 2008; Jones-Rhoades and Bartel 2004; Kulshrestha et al. 2020). It has also been observed that miRNAs play pivotal roles in plant–virus interactions (Huang et al. 2016), which mediate defense responses in plants against viruses (Pacheco et al. 2012). Since, different viruses show various responses in a common host, hence we cannot ignore mixed infections which frequently occurs in nature and resulted into antagonistic or synergistic viral interactions (Senthil et al. 2005; Whitham et al. 2003; Syller 2012; Moreno and López-Moya 2020). It has also been observed that in the mixed infection, CMV leads to synergistic interaction with TuMV (Martínez et al. 2013; Takeshita et al. 2012), while antagonistic interaction with TCV in A. thaliana (Chen et al. 2014; Yang et al. 2010). It was also observed that mixed infection of CMV and TuMV cause more severe symptoms than single viral infections in Nicotiana benthamiana (Takeshita et al. 2012). Mixed infection of CMV and TCV showed symptoms similar to those caused by single TCV infection (Yang et al. 2010). TCV infection results in resistance to CMV during mixed infection of these viruses in Arabidopsis showing suppression of CMV infection by TCV (Chen et al. 2014). However, their mechanisms in terms of common responses remained unknown. The present study has been designed to discover transcriptome response of Arabidopsis to CMV and TuMV and to CMV and TCV infections to better understand the virus-host interaction mechanisms through different approaches including GO, KEGG and network analysis which were used to explain the complexity of Arabidopsis reaction to different viral infections and their common responses. The function of TFs and miRNAs during viral infections has been studied. To our knowledge, this is the first comparative study of common and specific genes of A. thaliana in response to different virus infections compared with healthy plants.
Material and methods
Virus inoculations and microarray analysis
To identify common differential expression genes against CMV and TuMV or CMV and TCV infection in Arabidopsis leaf tissues, microarray data were obtained from four independent experiments deposited in ArrayExpress database (https://www.ebi.ac.uk/arrayexpress/). The Arabidopsis plants were inoculated with CMV (E-GEOD-37921), TuMV (E-MEXP-509 and E-GEOD-20278) and TCV (E-MEXP-1218). Detailed protocol information of each project is available at the ArrayExpress. In all experiments, the inoculated plants were maintained under controlled growth chamber with 16/8 h light/dark period, 60–80% humidity at 21–25 °C temperature. All the CEL data were analyzed using FlexArray software version 1.6.3, data normalization was conducted by Robust Multiarray Average (RMA) algorithm and RMA signal values were transformed into log2 (Khojasteh et al. 2018). Moreover, the quality control of CEL data was checked. The adjusted false discovery rate with P value less than 0.05 was selected for identification of DEGs (Khojasteh et al. 2018). Consequently, a list of genes was retrieved for further analysis and a Venn graph was depicted using http://bioinformatics.psb.ugent.be/webtools/Venn/. Moreover, The DEGs over the Arabidopsis genome were shown by using circus (Cheong et al. 2015).
Functional enrichment analysis
Gene Ontology (GO) analysis of differentially expressed genes in response to each individual virus infection was studied using AgriGO tools (http://bioinfo.cau.edu.cn/agriGO/). Moreover, GO and Singular Enrichment Analysis (SEA) analysis of common up-regulated and down-regulated genes in response to CMV and TuMV and to CMV and TCV infections were studied. GO enrichment analysis of DEGs in response to virus infections were identified (Du et al. 2010). To understand the biological significance of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway, enrichment analysis of common up/down-regulated genes were performed using KOBAS 3.0 database (Xie et al. 2011). The corrected P value < 0.05 was considered as significant.
Identification of TFs and miRNA targets
The TF families of up-regulated genes in response to virus infections were determined using AGRIS database (Yilmaz et al. 2011). To identify a gene association network in individual or between virus infections and the protein interaction relationships corresponding to the DEGs regulated by the TFs, the Search Tool for the Retrieval of Interacting Genes was used (STRING v.10) (Szklarczyk et al. 2014). A STRING was used to determine relationships among TFs and to identify significant protein pairs with combined score > 0.7. Subsequently, Genevestigator (https://genevestigator.com/gv/) database was used to analyze individual and common up-regulated TF genes expression at different developmental stages and tissues of A. thaliana (Zimmermann et al. 2004). The miRNA targets were identified using default parameters in psRNATarget (http://plantgrn.noble.org/psRNATarget/) database (Dai and Zhao 2018).
Validation analysis
In order to validate the results of our analysis, a meta-analysis was initially conducted with “metaSeq” package in Rstudio (Tsuyuzaki and Nikaido 2020). In this step, we confirmed the presence of the hub genes in our database through Fisher and Stouffer methods in this package (Fig. S2A). Subsequently, a cross-validation analysis based on Support Vector Machine (SVM) was carried out through “e1071” package on expression values of selected genes (Dimitriadou et al. 2009; Tahmasebi et al. 2019).
Results and discussion
Identification and functional enrichment analysis of differentially expressed genes
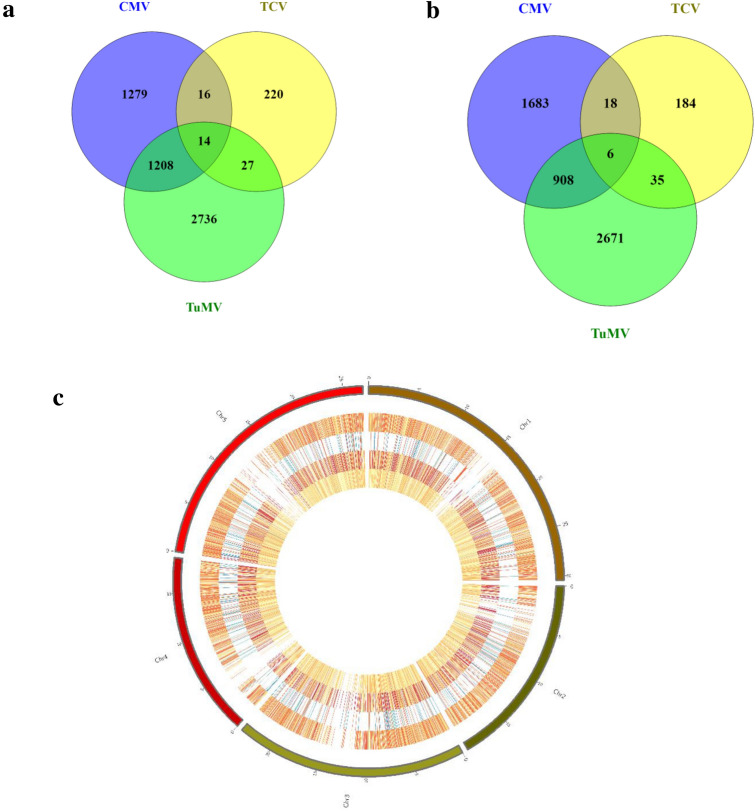
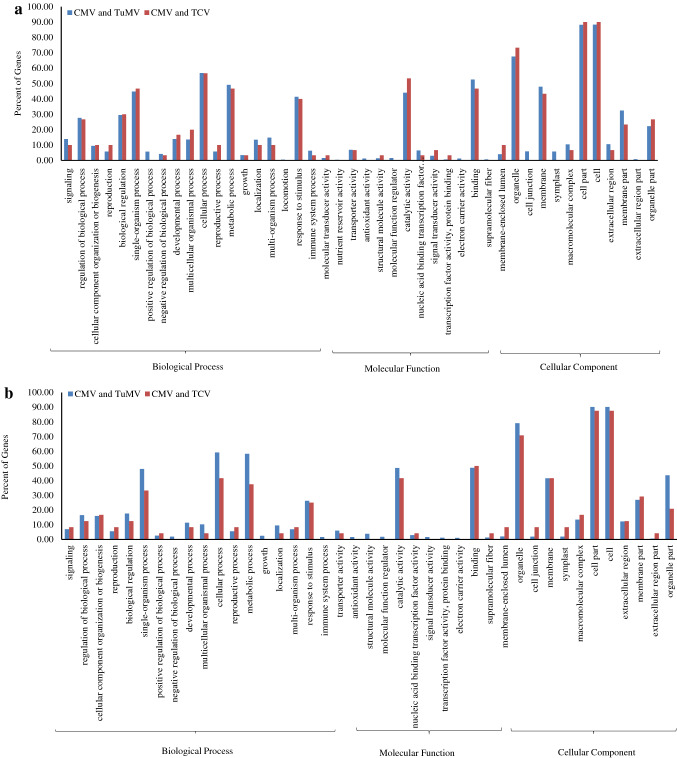
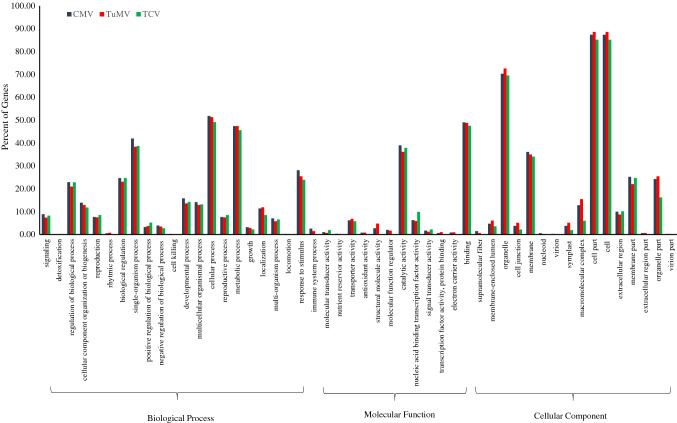
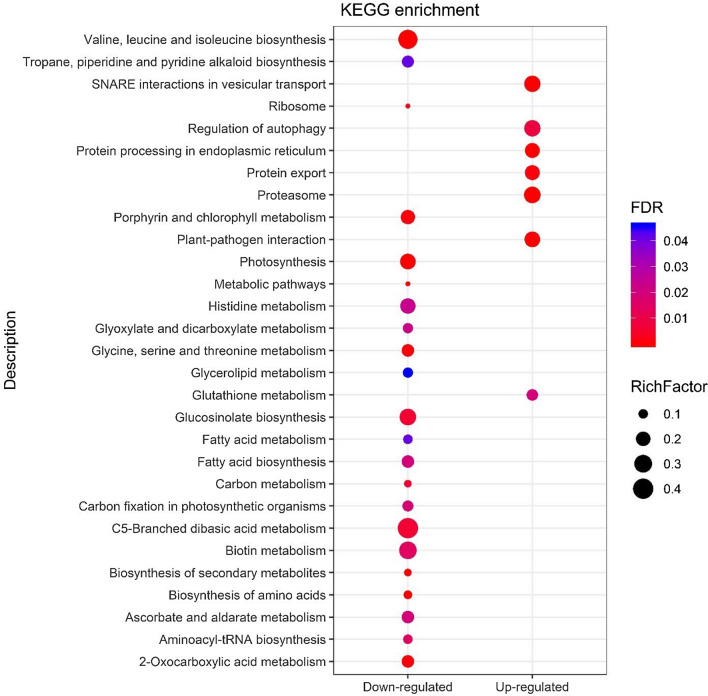
The experiments were designed to dissect the mechanism of transcriptional regulation of Arabidopsis genes in response to viral infections. All virus infections in A. thaliana have substantially stimulated plant gene expression. Single virus infection by CMV, TuMV and TCV resulted in up/down-regulation of 2517/2615, 3985/3620 and 277/243 genes, respectively (Fig. 1). In CMV and TuMV or CMV and TCV treatments, a total of 1222/914 and 30/24 genes were up/down-regulated, respectively (Fig. 1). The quality of array was checked (Fig. S1) and full description of up and down-regulated genes has been mentioned in Tables S1 and S2. The viral infections significantly offer the expression of common genes which are potential candidates for the genetic manipulation of plants to improve resistance to mixed viral infections. The information of common genes during viral infections might be an effective approach for the identification of important pathways involved in plant-virus interaction as well as virus to virus interactions. This study has further provided insight into the common gene expression profiling in Arabidopsis during viral infections. GO distribution of the common up/down-regulated genes and DEGs response to each virus signaled the biological process, cellular component and molecular function categories (Figs. 2, 3). Genes encoding immune response mediators, signal transducer activity, signaling and stress response functions were among the most significantly upregulated genes (Fig. 2a), which were also indicated in cellular components including cell (88.3 and 90%), cell part (88.22 and 90%) and organelles (67.59 and 73.33%), during CMV and TuMV or CMV and TCV infections, respectively in upregulated genes (Fig. 2a). Whereas, most of the down-regulated genes were related to cellular components including cell (90.1 and 87.5%), cell part (90.1 and 87.5%) and organelles (79.08 and 70.83%), respectively during CMV and TuMV or CMV and TCV infections (Fig. 2b). Viruses rely on host cell machinery and their infection can fine-tune gene expression in cells, which might cause upregulation and downregulation of the genes. It has also been observed that viruses disrupt a few processes in plant cells leading to changes in metabolic responses (Alazem and Lin 2015). Furthermore, the presence of organelles like mitochondria (Tables S1 and S2) in GO analysis of differentially expressed genes during viral infections might provide energy balance required for defense responses including programmed cell death (Rojas et al. 2014; Lam et al. 2001), which may play pivotal roles in Arabidopsis's response to the viruses. In molecular function process, the largest group of up-regulated genes has belonged to the binding with 52.63 and 46.66% followed by catalytic activity with 44.03 and 53.33% for CMV and TuMV or CMV and TCV infections, respectively (Fig. 2a). The GO results revealed up/down-regulated genes related to plant defense responses and plant-virus interaction during CMV, TuMV and TCV infections. As compared to common DEGs between CMV and TCV, some functional categories such as positive regulation of biological process, locomotion, nutrient reservoir activity, molecular function regulator, electron carrier activity, supramolecular fiber, cell junction, symplast, extracellular region parts were up-regulated between CMV and TuMV (Fig. 2a), while growth, negative regulation of biological process, immune system process, antioxidant activity, structural molecule activity, molecular function regulator, signal transducer activity, TF activity, protein binding related DEGs were down-regulated (Fig. 2b). Their down-regulation in the Arabidopsis in response to viral infections could suggest a functional part for these factors in biotic stresses. These transcriptome alterations were distinctly different between two viral interactions, and it might activate different host pathways resulting in various viral interactions. In this study, the responsive genes to biotic and abiotic stresses, immune and defense responses, response to hormones including abscisic acid, salicylic acid (SA) and jasmonic acid (JA) and leaf senescence were upregulated in Arabidopsis against the CMV and TuMV infections (Table S3). Plant hormones play a major role in various defense signaling pathways, and some viruses interact with these signaling pathways to enhance their infection process (Culver and Padmanabhan 2007; Bari and Jones 2009). Chemical signal compounds, such as SA and methyl jasmonate (MeJA), which have defensive and senescence-promoting functions, regulate the expression of certain senescence and pathogen-associated genes (Schenk et al. 2005). Abscisic acid is a key signaling molecule that mediates plant stress response by activating many stress-related genes (Cheng et al. 2016). Genes encoding photosynthesis and metabolism processes were downregulated in response to the viral infections (Table S4). Previous studies showed that plant photosynthetic activity is negatively regulated by virus infection (Rahoutei et al. 2000). The viral restrictions on photosynthesis, chlorophyll and metabolism processes might be due to nutrient deprivation as a common outcome of both viral infections (Bazzini et al. 2011). In addition, both viral infections can perturb metabolic process of Arabidopsis and activate plant-pathogen interaction, resulting in alterations of gene expression and cellular metabolism. As compared to individual infections of CMV or TuMV, positive regulation of biological process, reproductive process, molecular and signal transducer activity and nucleic acid binding transcription factor (TF) activity showed the highest percentage in TCV infection (Fig. 3). While, activities like cell killing, growth, immune system process, nutrient reservoir activity, antioxidant activity, molecular function regulator, TF activity and protein binding indicated lower percentage in TCV than those in individual infections of CMV and TuMV (Fig. 3). Some processes including developmental process, response to stimulus, immune system process, signaling, cellular component organization or biogenesis and catalytic activity showed the highest value in CMV infection compared to others infections in this study. The highest percentage of some processes like structural molecule activity, organelle, cell junction, symplast, cell and macromolecular complex was also observed in TuMV infection (Fig. 3). These findings revealed that immune system process contained the lowest and highest extent in response to TCV and CMV infections, respectively. Eight significant pathways were enriched with up-regulated DEGs in KEGG analysis, including glutathione metabolism, regulation of autophagy, protein export, protein processing in endoplasmic reticulum, proteasome, SNARE interactions in vesicular transport and plant-virus interaction during CMV and TuMV (Fig. 4). On the other hand, down-regulated DEGs exhibited the enrichment of some pathways including metabolic processes, photosynthesis and biosynthesis and metabolism of amino acids and fatty acids during CMV and TuMV infections (Fig. 4). The DEGs of pathways including glutathione metabolism and autophagy were significantly induced in response to CMV and TuMV infections. Glutathione as an antioxidant and important regulator of redox signaling plays key role in modulating plant defense by the activation of defense genes following virus infections (Foyer and Noctor 2009). Autophagy as a conserved intracellular degradation pathway is also induced against various stresses like viral infections (Batoko et al. 2017; Hafren et al. 2017). In addition, autophagy has also been related to regulation of defense hormone signaling and host cell death (Kabbage et al. 2013; Dagdas et al. 2016). Protein export-related DEGs were also up-regulated during CMV and TuMV infections. Under stress conditions, nuclear import of certain proteins can be critical for the plant to reprogram cellular processes to combat the stress (Zhao et al. 2007), which can show protein export activity in response to viral infections. In this study, the DEGs of endoplasmic reticulum (ER) were up-regulated which is a membrane-bound compartment that plays important roles in many cellular processes such as calcium homeostasis, protein processing, synthesis of protein and membrane lipid (Verchot 2014; Hetz 2012). Many viruses commandeer ER resident chaperones to contribute to virus replication and intercellular movement (Verchot 2014). Viruses make these changes on ER to create a cellular rich environment which is an essential step for viral infection. We have identified up-regulated proteasome-related DEGs that were responsive to the viral infections. Ubiquitin-26S proteasome system (UPS) is an important mechanism for protein removal (Vierstra 2009). UPS is also involved in the plant defense and in pathogen virulence program and plants-pathogens interactions (Citovsky et al. 2009; Dielen et al. 2010; Trujillo and Shirasu 2010), and the regulation of viral infection and plant-virus interactions (Alcaide-Loridan and Jupin 2012). Contrarily, UPS degradation of viral or cellular proteins is a major mechanism regulating viral infection (Citovsky et al. 2009; Alcaide-Loridan and Jupin 2012) which promotes viral replication and movement (Verchot 2016). The responsive up-regulated DEGs related to soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor (SNARE) proteins were identified. SNARE proteins are involved in facilitating vesicle traffic to ensure efficient targeting and delivery of specific membrane proteins (Rand and Parsegian 1989; Bock et al. 2001). Plant viruses utilize host endomembrane system and some viral proteins interact with SNARE-interacting proteins to promote virus systemic infection, replication and movement (Schoelz et al. 2011; Lewis and Lazarowitz 2010).
Fig. 1.
Global transcription of differentially expressed genes in Arabidopsis thaliana. a Venn diagram of up-regulated genes in response to CMV, TCV and TuMV viruses and b down-regulated genes. c Transcriptional responses of genes shown in circos on 5 chromosomes
Fig. 2.
Gene Ontology (GO) analysis of a the common up-regulated genes in response to CMV and TuMV and to CMV and TCV and b the common down-regulated genes in response to CMV and TuMV and to CMV and TCV in Arabidopsis. The x-axis represented GO terms classify based on three functional categories and the y-axis represented the percent of incorporated genes
Fig. 3.
Gene Ontology (GO) analysis of differentially expressed genes (DEGs) in response to CMV, TuMV and TCV in Arabidopsis. The x-axis represented GO terms classification based on three functional categories and the y-axis represented the percent of incorporated genes
Fig. 4.
KEGG pathway enrichment analysis of DEGs in response to CMV and TuMV in Arabidopsis (FDR rate < 0.05). The y-axis shows the enriched KEGG pathways. The color and the size of pathways descriptions represent the FDR and the Rich Factor, respectively. Rich factor is the ratio of the gene number to the total gene number in that specific pathway
Responsive TFs
TF activity is one of the essential parts of molecular function among up-regulated genes in response to virus infections. It has been observed that TFs play a vital role in plant innate immunity (Seo and Choi 2015; Ashrafi-Dehkordi et al. 2018). TFs play pivotal roles in plant innate immunity by regulating genes related to pathogen-associated molecular pattern-triggered immunity and effector-triggered immunity (Seo and Choi 2015). Therefore, TFs role was investigated in response to individual virus or CMV and TuMV or CMV and TCV infections. The results indicated specific TFs including ARID, GeBP, C2C2-CO-like and CCAAT-HAP5 in TuMV, ARR-B and VOZ-9 in CMV, and BBR/BPC in TCV infection. TFs including WRKY, MYB and C3H in CMV, and C2H2, HSF, Homeobox and MADS in TuMV infection showed the highest TF numbers compared to those in the individual and common viral infections (Table 1). The highly expressed TFs might play a vital and distinct role in response to viral infections. In this study, twenty-six and two TF families were significantly up-regulated in response to CMV and TuMV or CMV and TCV infections, respectively (Table 1). The NAC, C3H, C2H2, WRKY and bZIP were the most commonly presented TF families in CMV and TuMV infection, while AP2-EREBP and C3H were the TF families involved in CMV and TCV infections (Table 1). The functional analysis of interactions between TFs and other proteins is very important for elucidating the role of these transcriptional regulators in different signaling cascades (Alves et al. 2014). Seven up-regulated basic leucine zipper (bZIP) TFs were identified during CMV and TuMV infections. bZIPs regulate disease resistance through interaction with other proteins in defense responses (Kaminaka et al. 2006). Furthermore, the number of twenty-three and eight Cys2/His2 (C2H2)-type zinc finger TF have been identified during TuMV and CMV and TuMV infections, respectively. C2H2 TFs are important components in the regulation of plant growth, development, hormone responses, defense responses to biotic and abiotic stresses (Jiang and Pan 2012; Ciftci-Yilmaz and Mittler 2008; Kiełbowicz-Matuk 2012). Moreover, C3H-type zinc finger TFs were identified among the most up-regulated TF families in response to CMV, CMV and TuMV and CMV and TCV infections. C3H TFs have been involved in multiple processes, including leaf senescence (Asad et al. 2013), and abiotic and biotic responses (Wang et al. 2008; Sui et al. 2012; Zhang et al. 2011). In addition, some C3H proteins are involved in multiple stress responses and hormonal pathways (Jan et al. 2013; Guo et al. 2009). The results revealed twelve and eight up-regulated WRKY TFs during CMV and CMV and TuMV infections. WRKY TFs are a large family of regulatory proteins forming such a network and are involved in diverse biological processes including biotic/abiotic stress-induced defense responses and senescence (Eulgem and Somssich 2007; Rushton et al. 2010; Jiang et al. 2014). WRKY transcription factor genes are also responsive to viral infections (Chen et al. 2013). The number of fourteen up-regulated NAC TFs were detected in response to CMV and TuMV infections. NAC TFs are one of the largest families of transcriptional regulators in plants, and have been suggested to play important roles in the regulation of plant stress responses (McLellan et al. 2013; Nuruzzaman et al. 2013; Jensen et al. 2010). Some NAC TFs can be targeted by various pathogens to enhance disease susceptibility and as negative regulators of the plant defense responses (McLellan et al. 2013). For instance, in rice seedlings, NAC genes were up regulated after virus infections (Nuruzzaman et al. 2010), and increases in the expression level of NAC genes have been monitored in response to virus infections (Ren et al. 2000; Selth et al. 2005).
Table 1.
The up-regulated TF families in response to CMV, TCV, TuMV, and to both CMV and TuMV and to CMV and TCV infection in Arabidopsis
| TF families | Genes number | ||||
|---|---|---|---|---|---|
| CMV | TCV | TuMV | CMV and TuMV | CMV and TCV | |
| ABI3VP1 | ND | ND | 1 | ND | ND |
| Alfin-like | ND | ND | ND | 3 | ND |
| AP2-EREBP | 7 | ND | 9 | 5 | 1 |
| ARF | 2 | 1 | 4 | 1 | ND |
| ARID | ND | ND | 1 | ND | ND |
| ARR-B | 1 | ND | ND | ND | ND |
| BBR/BPC | ND | 1 | ND | ND | ND |
| bHLH | 7 | 3 | 9 | 1 | ND |
| bZIP | 8 | 1 | 9 | 7 | ND |
| BZR | 1 | ND | ND | 1 | ND |
| C2C2-CO-like | ND | ND | 2 | ND | ND |
| C2C2-Dof | 1 | ND | 2 | ND | ND |
| C2C2-Gata | 1 | ND | 3 | ND | ND |
| C2H2 | 8 | 2 | 23 | 8 | ND |
| C3H | 15 | 2 | 6 | 9 | 1 |
| CAMTA | 1 | ND | ND | ND | ND |
| CCAAT-DR1 | ND | ND | 1 | 1 | ND |
| CCAAT-HAP2 | 4 | ND | 2 | 2 | ND |
| CCAAT-HAP3 | 1 | ND | 2 | ND | ND |
| CCAAT-HAP5 | ND | ND | 4 | ND | ND |
| CPP | 1 | ND | ND | ND | ND |
| E2F-DP | ND | ND | 1 | ND | ND |
| EIL | ND | ND | ND | 1 | ND |
| G2-like | 1 | 1 | 1 | 2 | ND |
| GeBP | ND | ND | 4 | ND | ND |
| GRAS | 3 | ND | 7 | 1 | ND |
| GRF | 1 | ND | 3 | ND | ND |
| Homeobox | 3 | 1 | 10 | 1 | ND |
| HSF | 1 | 1 | 5 | 3 | ND |
| JUMONJI | ND | ND | 1 | 1 | ND |
| MADS | ND | 1 | 3 | 1 | ND |
| MYB | 8 | ND | 4 | 3 | ND |
| NAC | 4 | 2 | 6 | 14 | ND |
| NLP | ND | ND | 1 | ND | ND |
| PHD | 1 | ND | ND | ND | ND |
| RAV | ND | ND | ND | 2 | ND |
| REM | ND | 1 | 3 | 1 | ND |
| SBP | 2 | ND | 1 | 1 | ND |
| TCP | 1 | ND | 2 | 1 | ND |
| Trihelix | 3 | 1 | 3 | 1 | ND |
| TUB | 1 | ND | 1 | 1 | ND |
| VOZ-9 | 1 | ND | ND | ND | ND |
| Whirly | ND | ND | 1 | ND | ND |
| WRKY | 12 | ND | 4 | 8 | ND |
| ZF-HD | ND | ND | 1 | ND | ND |
ND not detected
TF's network
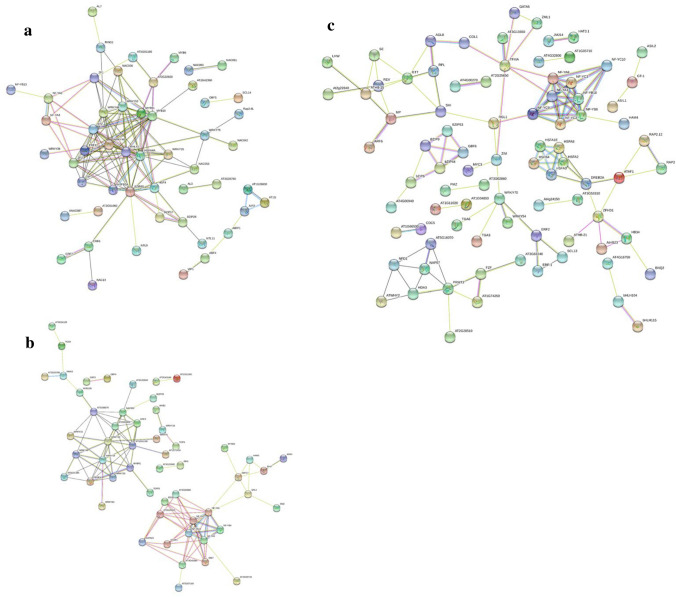
TFs including WRKY and NF-YA in CMV, and NF-YC, NF-YA and NF-YB in TuMV showed the nodes with the score higher than 0.7 (Fig. 5b, c). Recently, it has been shown that plant NF-Y TFs play important roles in plant–microbe interactions and adaptation to stresses (Zanetti et al. 2017). To uncover transcriptional regulatory mechanisms by TFs in host innate immune responses of Arabidopsis, a TFs network was constructed by common differentially expressed TFs between CMV and TuMV interaction including about 40 nodes in A. thaliana (Fig. 5) MYB15 (AT3G23250), MYB51 (AT1G18570), STZ (AT1G27730), WRKY40 (AT1G80840), RHL41 (AT5G59820), HSFA4A (AT4G18880) and ERF11 (AT1G28370) have revealed most interactions between differentially expressed genes during CMV and TuMV infections (Fig. 5a). The RHL41 and WRKY40 were primarily affected by other TFs such as, STZ, MYB15, ERF11, WRKY46 (AT2G46400) and HSFA4A (Fig. 5a). We aimed to construct a gene network among differentially expressed TFs. Thus, it is suggested that MYB15, MYB51, STZ, WRKY40, RHL41, HSFA4A and ERF11 play main role in compatible interaction during CMV and TuMV infection in Arabidopsis.
Fig. 5.
Gene network of up-regulated TFs in response to a both CMV and TuMV, b CMV and c TuMV in Arabidopsis. The lines represent interaction between differentially expressed genes
These TFs might play important roles in response to these viral infections. Additionally, several other signaling components, such as MYB, ERF and WRKY TFs, contribute in regulation of defense response against virus infection. Previous studies have shown the importance of ERF in biotic and abiotic stress tolerance mechanisms (Sharma et al. 2010). Nevertheless, MYBs essentially contribute in plant growth, development, primary and secondary metabolism, and response to biotic and abiotic stresses (Oh et al. 2003; Van der Ent et al. 2008). WRKY TF positively regulates defense response upon Tobacco mosaic virus infection in Capsicum annuum and is involved in resistance via transcriptional reprogramming of pathogenesis-related (PR) gene expression (Huh et al. 2015). In conclusion, TFs and their networks are important regulators of biological processes that provide insight into the regulation of defense mechanisms which are useful in plant breeding to enhance virus resistance.
Expression pattern of TFs in different developmental stages and tissues
Genevestigator database analysis showed maximum expression of the majority of TFs genes occurred at the senescence stage in leaf protoplast (Fig. 6a–e and Fig. S3). It indicates that senescence rates are influenced by different mechanisms upon viral infections that might be due to different viral infections by inducing nutrient competition. Leaf senescence is a fine-tuned and natural developmental process that involves cell death for recycling and reuse of valuable resources (Gan and Amasino 1997), and which has also been induced by abiotic and biotic stresses (Haffner et al. 2015; Navabpour et al. 2003; Xiong et al. 2005). Pathogen infection influence leaf senescence via modulation of the plant metabolite status directly affecting primary metabolism or by regulating levels of plant hormones (Masclaux-Daubresse et al. 2010; Fagard et al. 2014; Diaz-Mendoza et al. 2016). The expression of senescence-associated genes was also increased during compatible interactions between plants and viruses (Espinoza et al. 2007). Senescence-related genes may play key roles in the modulation of plant susceptibility to virus infection by supporting virus infections in systemically infected host plants (Fernandez-Calvino et al. 2016). Where, NAC and WRKY showed the common presented TF families in A. thaliana during CMV and TuMV infection. NAC and WRKY families are among the largest groups of senescence-related TFs (Balazadeh et al. 2008; Breeze et al. 2011), which play important roles in leaf senescence. Thus, our results support the notion that senescence may be closely related to NAC-mediated stress responses (Nuruzzaman et al. 2013). Moreover, further studies are needed to study the convergence between senescence and pathogen defense in viral infections.
Fig. 6.
Heat map representation for expression pattern of up-regulated TFs in 10 different developmental stages in a CMV and TuMV, b CMV, c TuMV, d TCV and e CMV and TCV
Identification of miRNA targeting down-regulated genes
Earlier findings revealed that plant miRNAs are involved in response to stresses (Gao et al. 2011; Sunkar and Jagadeeswaran 2012). Analysis of miRNAs during CMV and TuMV and CMV and TCV infections demonstrated eleven and five miRNA families, respectively indicating the role of these miRNAs in the down regulation of host genes in response to viral infections (Table 2 and Table S5). We also detected the miRNA's functional roles in growth, metabolism and biotic and abiotic stress responses. The ath-miR5021, ath-miR854, ath-miR5658, ath-miR172 and ath-miR414 were predicted to have the highest number of gene targets with 43, 35, 23, 19 and 17 targets among down-regulated genes, respectively, in CMV and TuMV infection, whereas the ath-miR5021 and ath-miR393 were identified with the highest targets (four and two targets) during CMV and TCV infection that were significantly higher than the other miRNAs (Table 2 and S3). There was ath-miR5021 miRNA with 20 nt in length, while the other miRNAs including ath-miR838, ath-miR414, ath-miR4239 with 21 nt were identified in CMV and TCV infections. The ath-miR5021 and ath-miR156 miRNAs represented 20 nt in length, while other identified miRNAs with frequencies more than five showed 21 nt in length during CMV and TuMV infections. The ath-miR5021 was recently found to have role in biotic stress responses and targets a diverse set of genes including global transcription factors and disease resistance proteins (Das et al. 2015; Kharonov 2013). The role of miR854 in the plant’s response against arsenic (As) stress was already shown (Srivastava et al. 2012). The miR5658 targets various transcription factors, the nucleotide binding site leucine-rich repeat (NBS-LRR) resistance protein, proteins involved in signal transduction, molecular chaperone regulators, growth regulators, protein ligases, WRKY transcription factor, argonaute protein etc. (Han et al. 2014; Li et al. 2014b; Joshi 2018). Some miRNAs such as miRNA172 and miR393 are involved in pathogen defense (Sunkar and Jagadeeswaran 2012). MiR393 affects various processes including plant response to biotic stress and can fine-tune plant defense responses (Etemadi et al. 2014; Wong et al. 2014; Robert-Seilaniantz et al. 2011). Responsive abscisic acid genes were up-regulated during both viral infections. Accordingly, miRNAs such as miR172a and miR5658 may imply a complex crosstalk between the global regulation of miRNA metabolism and abscisic acid signaling functions which enable the fine-tuning of stress response in plants (Matsui et al. 2013). MiR414 targets transcriptional factors including the bZIP, WRKY, MYB, B3, scarecrow, heat shock proteins and TCP that play roles in plant growth, development, physiological and morphological changes, metabolism defense responses and abiotic stress regulation (Li et al. 2014b; Palatnik et al. 2003; Guo et al. 2007; Romanel et al. 2009). In addition, genes involved in proteasome degrading pathway such as ubiquitin conjugating enzyme are regulated by miRNAs including miR414 (Das and Mondal 2010). Therefore, the results of this study showed that these miRNAs could affect some common genes and pathways in the viral infections. In addition, the identification of miRNAs and target genes have a great capacity to progress plant genetic improvement that can result in transgenic plants with improved productivity.
Table 2.
A list of miRNA families potentially targeting down-regulated genes in response to both (CMV and TuMV) viruses (where frequency > 5 and all miRNA families are related to CMV and TCV viruses)
| Response to viruses | MiRNA family | Number of down-regulated target genes with the miRNA motif | Mean UPE | Mean expectation | Inhibition mechanism |
|---|---|---|---|---|---|
| CMV and TuMV | ath-miR5021 | 43 | 10.24 | 2.51 | Cleavage/translation |
| ath-miR854 | 35 | 7.65 | 3 | Cleavage/translation | |
| ath-miR5658 | 23 | 9.34 | 2.15 | Cleavage/translation | |
| ath-miR172 | 19 | 16.77 | 2.31 | Cleavage/translation | |
| ath-miR414 | 17 | 11.79 | 2.55 | Cleavage/translation | |
| ath-miR157 | 9 | 16.99 | 3 | Cleavage/translation | |
| ath-miR395 | 9 | 19.81 | 2.16 | Cleavage | |
| ath-miR838 | 9 | 15.21 | 2.88 | Cleavage/translation | |
| ath-miR156 | 8 | 13.83 | 2.75 | Cleavage/translation | |
| ath-miR1886 | 7 | 9.52 | 2.78 | Cleavage/translation | |
| ath-miR159 | 6 | 12.94 | 2.75 | Cleavage/translation | |
| CMV and TCV | ath-miR5021 | 4 | 9.42 | 2.25 | Cleavage/translation |
| ath-miR393 | 2 | 10.68 | 2.5 | Cleavage | |
| ath-miR4239 | 1 | 19.93 | 2.5 | Cleavage | |
| ath-miR414 | 1 | 10.44 | 3 | Cleavage | |
| ath-miR838 | 1 | 9.92 | 3 | Translation |
UPE: maximum energy to unpair the target site
Cross-validation analysis
The validation analysis of our data was implemented through two approaches, a metaSeq package in Rstudio was conducted to confirm the accuracy of our data. All of the common genes in our database were confirmed through this package in Fisher and Stouffer methods (Fig. S2A). Then, SVM method in Cross-validation analysis was used to determine the accuracy of our data between samples under normal and stressful circumstances. Therefore, this analysis was correctly categorized as the expression level of selected genes with the accuracy of 77.5% with area under curve (AUC) value of 0.96 (Fig. S2B and C).
Conclusion
The dissection of the molecular network of virus-host interactions will help us to better understand the molecular mechanisms and regulatory cellular pathways of defense in plants, identify essential host gene pathways required for resistance to viruses, clarify the interactions among different TFs, and determine responsive miRNAs and identify candidate genes during viral infections. These observations will be important to identify differentially and commonly expressed virus-responsive genes and unique and conserved pathways in plant-virus interaction. The study of important conserved genes between CMV and turnip viruses might be an effective approach for the understanding of host responses during synergistic and antagonistic interactions of viruses which might provide common targets and develop novel plant antiviral strategies for improving plant resistance to mixed viral infections using genetic engineering approaches.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
No funding was received for conducting this study.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Consent for publication
The manuscript has been read and approved by all named authors.
Ethical approval
This study did not include any experiments with human participants or animals performed by all the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahlquist P, Noueiry AO, Lee WM, Kushner DB, Dye BT. Host factors in positive-strand RNA virus genome replication. J Virol. 2003;77(15):8181–8186. doi: 10.1128/JVI.77.15.8181-8186.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alazem M, Lin NS. Roles of plant hormones in the regulation of host–virus interactions. Mol Plant Pathol. 2015;16(5):529–540. doi: 10.1111/mpp.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaide-Loridan C, Jupin I. Ubiquitin and plant viruses, let's play together! Plant Physiol. 2012;160(1):72–82. doi: 10.1104/pp.112.201905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves M, Dadalto S, Gonçalves A, de Souza G, Barros V, Fietto L. Transcription factor functional protein-protein interactions in plant defense responses. Proteomes. 2014;2(1):85–106. doi: 10.3390/proteomes2010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asad J, Maruyama K, Todaka D, Kidokoro S, Abo M, Yoshimura E, Shinozaki K, Nakashima K, Yamaguchi-Shinozaki K. OsTZF1, a CCCH-tandem zinc finger protein, confers delayed senescence and stress tolerance in rice by regulating stress-related genes. Plant Physiol. 2013;161(3):1202–1216. doi: 10.1104/pp.112.205385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafi-Dehkordi E, Alemzadeh A, Tanaka N, Razi H. Meta-analysis of transcriptomic responses to biotic and abiotic stress in tomato. PeerJ. 2018;6:e4631. doi: 10.7717/peerj.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazadeh S, Riaño-Pachón DM, Mueller-Roeber B. Transcription factors regulating leaf senescence in Arabidopsis thaliana. Plant Biol. 2008;10:63–75. doi: 10.1111/j.1438-8677.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Bari R, Jones JD. Role of plant hormones in plant defence responses. Plant Mol Biol. 2009;69(4):473–488. doi: 10.1007/s11103-008-9435-0. [DOI] [PubMed] [Google Scholar]
- Batoko H, Dagdas Y, Baluska F, Sirko A. Understanding and exploiting autophagy signaling in plants. Essays Biochem. 2017;61(6):675–685. doi: 10.1042/EBC20170034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzini AA, Manacorda CA, Tohge T, Conti G, Rodriguez MC, Nunes-Nesi A, Villanueva S, Fernie AR, Carrari F, Asurmendi S. Metabolic and miRNA profiling of TMV infected plants reveals biphasic temporal changes. PLoS ONE. 2011;6(12):e28466. doi: 10.1371/journal.pone.0028466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethke G, Glazebrook J. Measuring pectin properties to track cell wall alterations during plant-pathogen interactions. In: Gassmann W, editor. Plant innate immunity. Methods in molecular biology. New York, NY: Humana; 2019. [DOI] [PubMed] [Google Scholar]
- Bock JB, Matern HT, Peden AA, Scheller RH. A genomic perspective on membrane compartment organization. Nature. 2001;409(6822):839. doi: 10.1038/35057024. [DOI] [PubMed] [Google Scholar]
- Breeze E, Harrison E, McHattie S, Hughes L, Hickman R, Hill C, Kiddle S, Kim YS, Penfold CA, Jenkins D, Zhang C. High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell. 2011;23(3):873–894. doi: 10.1105/tpc.111.083345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent L, Heathcote GD. Properties and host range of turnip crinkle, rosette and yellow mosaic viruses. Ann Appl Biol. 1958;46(4):585–592. [Google Scholar]
- Chen Q, Wei T. Cell biology during infection of plant viruses in insect vectors and plant hosts. Mol Plant Microbe Interact. 2020;33(1):18–25. doi: 10.1094/MPMI-07-19-0184-CR. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhang L, Li D, Wang F, Yu D. WRKY8 transcription factor functions in the TMV-cg defense response by mediating both abscisic acid and ethylene signaling in Arabidopsis. Proc Natl Acad Sci USA. 2013;110(21):E1963–E1971. doi: 10.1073/pnas.1221347110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, Zhang J, Liu J, Deng XG, Zhang P, Zhu T, Chen LJ, Bao WK, Xi DH, Lin HH. The capsid protein p38 of turnip crinkle virus is associated with the suppression of cucumber mosaic virus in Arabidopsis thaliana co-infected with cucumber mosaic virus and turnip crinkle virus. Virology. 2014;462:71–80. doi: 10.1016/j.virol.2014.05.031. [DOI] [PubMed] [Google Scholar]
- Cheng HY, Wang Y, Tao X, Fan YF, Dai Y, Yang H, Ma XR. Genomic profiling of exogenous abscisic acid-responsive microRNAs in tomato (Solanum lycopersicum) BMC Genom. 2016;17(1):423. doi: 10.1186/s12864-016-2591-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong WH, Tan YC, Yap SJ, Ng KP. ClicO FS: an interactive web-based service of Circos. Bioinformatics. 2015;31(22):3685–3687. doi: 10.1093/bioinformatics/btv433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciftci-Yilmaz S, Mittler R. The zinc finger network of plants. Cell Mol Life Sci. 2008;65:1150–1160. doi: 10.1007/s00018-007-7473-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citovsky V, Zaltsman A, Kozlovsky SV, Gafni Y, Krichevsky A. Proteasomal degradation in plant–pathogen interactions. Semin Cell Dev Biol. 2009;20(9):1048–1054. doi: 10.1016/j.semcdb.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Cohen Y, Gisel A, Zambryski PC. Cell-to-cell and systemic movement of recombinant green fluorescent protein-tagged turnip crinkle viruses. Virology. 2000;273(2):258–266. doi: 10.1006/viro.2000.0441. [DOI] [PubMed] [Google Scholar]
- Culver JN, Padmanabhan MS. Virus-induced disease: altering host physiology one interaction at a time. Annu Rev Phytopathol. 2007;45:221–243. doi: 10.1146/annurev.phyto.45.062806.094422. [DOI] [PubMed] [Google Scholar]
- Dagdas YF, Belhaj K, Maqbool A, Chaparro-Garcia A, Pandey P, Petre B, Tabassum N, Cruz-Mireles N, Hughes RK, Sklenar J, Win J. An effector of the Irish potato famine pathogen antagonizes a host autophagy cargo receptor. Elife. 2016;5:e10856. doi: 10.7554/eLife.10856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Zhao PX. psRNATarget: a plant small RNA target analysis server. Nucl Acids Res. 2018;46(W1):W49–W54. doi: 10.1093/nar/gky316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Mondal TK. Computational identification of conserved microRNAs and their targets in tea (Camellia sinensis) Am J Plant Sci. 2010;1(02):77. [Google Scholar]
- Das A, Chaudhury S, Kalita MC, Mondal TK. In silico identification, characterization and expression analysis of miRNAs in Cannabis sativa L. Plant Gene. 2015;2:17–24. [Google Scholar]
- Diaz-Mendoza M, Velasco-Arroyo B, Santamaria ME, González-Melendi P, Martinez M, Diaz I. Plant senescence and proteolysis: two processes with one destiny. Genet Mol Biol. 2016;39(3):329–338. doi: 10.1590/1678-4685-GMB-2016-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dielen AS, Badaoui S, Candresse T, German-Retana SYLVIE. The ubiquitin/26S proteasome system in plant–pathogen interactions: a never-ending hide-and-seek game. Mol Plant Pathol. 2010;11(2):293–308. doi: 10.1111/j.1364-3703.2009.00596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriadou E, Hornik K, Leisch F, Meyer D, Weingessel A, Leisch MF (2009) Package ‘e1071’. R Software package. http://cran.rproject.org/web/packages/e1071/index.html
- Du Z, Zhou X, Ling Y, Zhang Z, Su Z. AgriGO: a GO analysis toolkit for the agricultural community. Nucle Acids Res. 2010;38:W64–W70. doi: 10.1093/nar/gkq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwardson JR, Christie RG (1991) The Potyvirus group, Florida agricultural experiment station monograph series, Gainesville, FL, University of Florida, No. 16
- Espinoza C, Medina C, Somerville S, Arce-Johnson P. Senescence-associated genes induced during compatible viral interactions with grapevine and Arabidopsis. J Exp Bot. 2007;58(12):3197–3212. doi: 10.1093/jxb/erm165. [DOI] [PubMed] [Google Scholar]
- Etemadi M, Gutjahr C, Couzigou JM, Zouine M, Lauressergues D, Timmers A, Audran CB, M, Bécard G, Combier JP, Auxin perception is required for arbuscule development in arbuscular mycorrhizal symbiosis. Plant Physiol. 2014;166(1):281–292. doi: 10.1104/pp.114.246595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulgem T, Somssich IE. Networks of WRKY transcription factors in defense signaling. Curr Opin Plant Biol. 2007;10(4):366–371. doi: 10.1016/j.pbi.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Fagard M, Launay A, Clément G, Courtial J, Dellagi A, Farjad M, Krapp A, Soulié MC, Masclaux-Daubresse C. Nitrogen metabolism meets phytopathology. J Exp Bot. 2014;65(19):5643–5656. doi: 10.1093/jxb/eru323. [DOI] [PubMed] [Google Scholar]
- Fernandez-Calvino L, Guzmán-Benito I, Del Toro FJ, Donaire L, Castro-Sanz AB, Ruíz-Ferrer V, Llave C. Activation of senescence-associated Dark-inducible (DIN) genes during infection contributes to enhanced susceptibility to plant viruses. Mol Plant Pathol. 2016;17(1):3–15. doi: 10.1111/mpp.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxid Redox Signal. 2009;11(4):861–905. doi: 10.1089/ars.2008.2177. [DOI] [PubMed] [Google Scholar]
- Gan S, Amasino RM. Making sense of senescence (molecular genetic regulation and manipulation of leaf senescence) Plant Physiol. 1997;113(2):313. doi: 10.1104/pp.113.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Bai X, Yang L, Lv D, Pan X, Li Y, Cai H, Ji W, Chen Q, Zhu Y. osa-MIR393: a salinity-and alkaline stress-related microRNA gene. Mol Biol Rep. 2011;38(1):237–242. doi: 10.1007/s11033-010-0100-8. [DOI] [PubMed] [Google Scholar]
- Golem S, Culver JN. Tobacco mosaic virus induced alterations in the gene expression profile of Arabidopsis thaliana. Mol Plant Microbe Interact. 2003;16(8):681–688. doi: 10.1094/MPMI.2003.16.8.681. [DOI] [PubMed] [Google Scholar]
- Guerret MG, Nyalugwe EP, Maina S, Barbetti MJ, Van Leur JA, Jones RA. Biological and molecular properties of a Turnip mosaic virus (TuMV) strain that breaks TuMV resistances in Brassica napus. Plant Dis. 2017;101(5):674–683. doi: 10.1094/PDIS-08-16-1129-RE. [DOI] [PubMed] [Google Scholar]
- Guo Q, Xiang A, Yang Q, Yang Z. Bioinformatic identification of microRNAs and their target genes from Solanum tuberosum expressed sequence tags. Chin Sci Bull. 2007;52(17):2380–2389. [Google Scholar]
- Guo YH, Yu YP, Wang D, Wu CA, Yang GD, Huang JG, Zheng CC. GhZFP1, a novel CCCH-type zinc finger protein from cotton, enhances salt stress tolerance and fungal disease resistance in transgenic tobacco by interacting with GZIRD21A and GZIPR5. New Phytol. 2009;183(1):62–75. doi: 10.1111/j.1469-8137.2009.02838.x. [DOI] [PubMed] [Google Scholar]
- Haffner E, Konietzki S, Diederichsen E. Keeping control: The role of senescence and development in plant pathogenesis and defense. Plants. 2015;4(3):449–488. doi: 10.3390/plants4030449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafren A, Macia JL, Love AJ, Milner JJ, Drucker M, Hofius D. Selective autophagy limits cauliflower mosaic virus infection by NBR1-mediated targeting of viral capsid protein and particles. Proc Natl Acad Sci USA. 2017;114(10):E2026–E2035. doi: 10.1073/pnas.1610687114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Xie H, Kong ML, Sun QP, Li RZ, Pan JB. Computational identification of miRNAs and their targets in Phaseolus vulgaris. Genet Mol Res. 2014;13(1):310–322. doi: 10.4238/2014.January.17.16. [DOI] [PubMed] [Google Scholar]
- Harries P, Ding B. Cellular factors in plant virus movement: at the leading edge of macromolecular trafficking in plants. Virology. 2011;411(2):237–243. doi: 10.1016/j.virol.2010.12.021. [DOI] [PubMed] [Google Scholar]
- Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13(2):89. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- Hollings M, Stone OM (1972) Turnip crinkle virus. CMI/AAB Descriptions of Plant Viruses, No 109
- Huang J, Yang M, Lu L, Zhang X. Diverse functions of small RNAs in different plant-pathogen communications. Front Microbiol. 2016;7:1552. doi: 10.3389/fmicb.2016.01552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh SU, Lee GJ, Jung JH, Kim Y, Kim YJ, Paek KH. Capsicum annuum transcription factor WRKYa positively regulates defense response upon TMV infection and is a substrate of CaMK1 and CaMK2. Sci Rep. 2015;5:7981. doi: 10.1038/srep07981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan A, Maruyama K, Todaka D, Kidokoro S, Abo M, Yoshimura E, Shinozaki K, Nakashima K, Yamaguchi-Shinozaki K. OsTZF1, a CCCH-tandem zinc finger protein, confers delayed senescence and stress tolerance in rice by regulating stress-related genes. Plant Physiol. 2013;161(3):1202–1216. doi: 10.1104/pp.112.205385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelitto-Van Dooren EP, Vidal S, Denecke J. Anticipating endoplasmic reticulum stress: a novel early response before pathogenesis-related gene induction. Plant Cell. 1999;11(10):1935–1943. doi: 10.1105/tpc.11.10.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MK, Kjaersgaard T, Nielsen MM, Galberg P, Petersen K, O'Shea C, Skriver K. The Arabidopsis thaliana NAC transcription factor family: structure–function relationships and determinants of ANAC019 stress signalling. Biochem J. 2010;426(2):183–196. doi: 10.1042/BJ20091234. [DOI] [PubMed] [Google Scholar]
- Jiang L, Pan LJ. Identification and expression of C2H2 transcription factor genes in Carica papaya under abiotic and biotic stresses. Mol Biol Rep. 2012;39(6):7105–7115. doi: 10.1007/s11033-012-1542-y. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Wang JH, Yang H, Xu MY, Yuan S, Sun W, Xu WL, Xi DH, Lin HH. Identification and sequence analysis of Turnip mosaic virus infection on cruciferous crops in southwest China. J Plant Pathol. 2010;92:241–244. [Google Scholar]
- Jiang Y, Duan Y, Yin J, Ye S, Zhu J, Zhang F, Lu W, Fan D, Luo K. Genome-wide identification and characterization of the Populus WRKY transcription factor family and analysis of their expression in response to biotic and abiotic stresses. J Exp Bot. 2014;65(22):6629–6644. doi: 10.1093/jxb/eru381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Rhoades MW, Bartel DP. Computational identification of plant microRNAs and their targets, including a stress-induced miRNA. Mol Cell. 2004;14(6):787–799. doi: 10.1016/j.molcel.2004.05.027. [DOI] [PubMed] [Google Scholar]
- Joshi H. In silico identification and target prediction of microRNAs in Sesame (sesamum indicum L.) expressed sequence tags. Genet Mol Res. 2018;17(2):gmr16039911. [Google Scholar]
- Kabbage M, Williams B, Dickman MB. Cell death control: the interplay of apoptosis and autophagy in the pathogenicity of Sclerotinia sclerotiorum. PLoS Pathog. 2013;9(4):e1003287. doi: 10.1371/journal.ppat.1003287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminaka H, Näke C, Epple P, Dittgen J, Schütze K, Chaban C, Holt BF, III, Merkle T, Schäfer E, Harter K, Dangl JL. bZIP10-LSD1 antagonism modulates basal defense and cell death in Arabidopsis following infection. EMBO J. 2006;25(18):4400–4411. doi: 10.1038/sj.emboj.7601312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharonov Y. Computational prediction and qPCR validation of miRNA in Ceratopteris richardii. New York: CUNY Academic Works; 2013. [Google Scholar]
- Khojasteh M, Khahani B, Taghavi M, Tavakol E. Identification and characterization of responsive genes in rice during compatible interactions with pathogenic pathovars of Xanthomonas oryzae. Eur J Plant Pathol. 2018;151(1):141–153. [Google Scholar]
- Kiełbowicz-Matuk A. Involvement of plant C2H2-type zinc finger transcription factors in stress responses. Plant Sci. 2012;185:78–85. doi: 10.1016/j.plantsci.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Kulshrestha C, Pathak H, Kumar D, Dave S, Sudan J. Elucidating micro RNAs role in different plant–pathogen interactions. Mol Biol Rep. 2020;47:8219–8227. doi: 10.1007/s11033-020-05810-y. [DOI] [PubMed] [Google Scholar]
- Lam E, Kato N, Lawton M. Programmed cell death, mitochondria and the plant hypersensitive response. Nature. 2001;411(6839):848–853. doi: 10.1038/35081184. [DOI] [PubMed] [Google Scholar]
- Lecellier CH, Voinnet O. RNA silencing: no mercy for viruses? Immunol Rev. 2004;198(1):285–303. doi: 10.1111/j.0105-2896.2004.00128.x. [DOI] [PubMed] [Google Scholar]
- Lewis JD, Lazarowitz SG. Arabidopsis synaptotagmin SYTA regulates endocytosis and virus movement protein cell-to-cell transport. Proc Natl Acad Sci USA. 2010;107(6):2491–2496. doi: 10.1073/pnas.0909080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Gaudinier A, Tang M, Taylor-Teeples M, Nham NT, Ghaffari C, Benson DS, Steinmann M, Gray JA, Brady SM, Kliebenstein DJ. Promoter based integration in plant defense regulation. Plant Physiol. 2014;166(4):1803–1820. doi: 10.1104/pp.114.248716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Hou Y, Zhang L, Zhang W, Quan C, Cui Y, Bian S. Computational identification of conserved microRNAs and their targets from expression sequence tags of blueberry (Vaccinium corybosum) Plant Signal Behav. 2014;9(9):e29462. doi: 10.4161/psb.29462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HH, Tian X, Li YJ, Wu CA, Zheng CC. Microarray-based analysis of stress-regulated microRNAs in Arabidopsis thaliana. RNA. 2008;14(5):836–843. doi: 10.1261/rna.895308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfre AJ, Simon AE. Importance of coat protein and RNA silencing in satellite RNA/virus interactions. Virology. 2008;379(1):161–167. doi: 10.1016/j.virol.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Martínez F, Elena SF, Daròs JA. Fate of artificial microRNA-mediated resistance to plant viruses in mixed infections. Phytopathology. 2013;103(8):870–876. doi: 10.1094/PHYTO-09-12-0233-R. [DOI] [PubMed] [Google Scholar]
- Masclaux-Daubresse C, Daniel-Vedele F, Dechorgnat J, Chardon F, Gaufichon L, Suzuki A. Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Ann Bot. 2010;105(7):1141–1157. doi: 10.1093/aob/mcq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui A, Nguyen A, Nakaminami K, Seki M. Arabidopsis non-coding RNA regulation in abiotic stress responses. Int J Mol Sci. 2013;14(11):22642–22654. doi: 10.3390/ijms141122642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maule A, Leh V, Lederer C. The dialogue between viruses and hosts in compatible interactions. Curr Opin Plant Biol. 2002;5(4):279–284. doi: 10.1016/s1369-5266(02)00272-8. [DOI] [PubMed] [Google Scholar]
- McLellan H, Boevink PC, Armstrong MR, Pritchard L, Gomez S, Morales J, Whisson SC, Beynon JL, Birch PR. An RxLR effector from Phytophthora infestans prevents re-localisation of two plant NAC transcription factors from the endoplasmic reticulum to the nucleus. PLoS Pathog. 2013;9(10):e1003670. doi: 10.1371/journal.ppat.1003670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno AB, López-Moya JJ. When viruses play team sports: Mixed infections in plants. Phytopathology. 2020;110(1):29–48. doi: 10.1094/PHYTO-07-19-0250-FI. [DOI] [PubMed] [Google Scholar]
- Moustafa K, Cross J. Genetic approaches to study plant responses to environmental stresses: an overview. Biology. 2016;5(2):20. doi: 10.3390/biology5020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navabpour S, Morris K, Allen R, Harrison E, A-H-MackernessBuchanan-Wollaston SV. Expression of senescence-enhanced genes in response to oxidative stress. J Exp Bot. 2003;54(391):2285–2292. doi: 10.1093/jxb/erg267. [DOI] [PubMed] [Google Scholar]
- Nguyen HD, Tomitaka Y, Ho SY, Duchêne S, Vetten HJ, Lesemann D, Walsh JA, Gibbs AJ, Ohshima K. Turnip mosaic potyvirus probably first spread to Eurasian brassica crops from wild orchids about 1000 years ago. PLoS ONE. 2013;8(2):e55336. doi: 10.1371/journal.pone.0055336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicaise V. Crop immunity against viruses: outcomes and future challenges. Front Plant Sci. 2014;5:660. doi: 10.3389/fpls.2014.00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuruzzaman M, Manimekalai R, Sharoni AM, Satoh K, Kondoh H, Ooka H, Kikuchi S. Genome-wide analysis of NAC transcription factor family in rice. Gene. 2010;465(1):30–44. doi: 10.1016/j.gene.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Nuruzzaman M, Sharoni AM, Kikuchi S. Roles of NAC transcription factors in the regulation of biotic and abiotic stress responses in plants. Front Microbiol. 2013;4:248. doi: 10.3389/fmicb.2013.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S, Park S, Han KH. Transcriptional regulation of secondary growth in Arabidopsis thaliana. J Exp Bot. 2003;54(393):2709–2722. doi: 10.1093/jxb/erg304. [DOI] [PubMed] [Google Scholar]
- Pacheco R, Garcıa-Marcos A, Barajas D, Martianez J, Tenllado F. PVX-potyvirus synergistic infections differentially alter microRNA accumulation in Nicotiana benthamiana. Virus Res. 2012;165:231–235. doi: 10.1016/j.virusres.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Pagan I, Fraile A, Fernandez-Fueyo E, Montes N, Alonso-Blanco C, García-Arenal F. Arabidopsis thaliana as a model for the study of plant-virus co-evolution. Philos Trans R Soc Lond B Biol Sci. 2010;365(1548):1983–1995. doi: 10.1098/rstb.2010.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palatnik JF, Allen E, Wu X, Schommer C, Schwab R, Carrington JC, Weigel D. Control of leaf morphogenesis by microRNAs. Nature. 2003;425(6955):257. doi: 10.1038/nature01958. [DOI] [PubMed] [Google Scholar]
- Palukaitis P, García-Arenal F. Cucumoviruses. Adv Virus Res. 2003;62:241–323. doi: 10.1016/s0065-3527(03)62005-1. [DOI] [PubMed] [Google Scholar]
- Pashaiasl M, Khodadadi K, Kayvanjoo AH, Pashaei-asl R, Ebrahimie E, Ebrahimi M. Unravelling evolution of Nanog, the key transcription factor involved in self-renewal of undifferentiated embryonic stem cells, by pattern recognition in nucleotide and tandem repeats characteristics. Gene. 2016;578(2):194–204. doi: 10.1016/j.gene.2015.12.023. [DOI] [PubMed] [Google Scholar]
- Pesti R, Kontra L, Paul K, Vass I, Csorba T, Havelda Z, Várallyay É. Differential gene expression and physiological changes during acute or persistent plant virus interactions may contribute to viral symptom differences. PLoS ONE. 2019;14(5):e0216618. doi: 10.1371/journal.pone.0216618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahoutei J, García-Luque I, Barón M. Inhibition of photosynthesis by viral infection: effect on PSII structure and function. Physiol Plant. 2000;110(2):286–292. [Google Scholar]
- Rand RP, Parsegian VA. Hydration forces between phospholipid bilayers. Biochim Biophys Acta Rev Biomembr. 1989;988(3):351–376. [Google Scholar]
- Ren T, Qu F, Morris TJ. HRT gene function requires interaction between a NAC protein and viral capsid protein to confer resistance to turnip crinkle virus. Plant Cell. 2000;12(10):1917–1925. doi: 10.1105/tpc.12.10.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Seilaniantz A, MacLean D, Jikumaru Y, Hill L, Yamaguchi S, Kamiya Y, Jones JD. The microRNA miR393 re-directs secondary metabolite biosynthesis away from camalexin and towards glucosinolates. Plant J. 2011;67(2):218–231. doi: 10.1111/j.1365-313X.2011.04591.x. [DOI] [PubMed] [Google Scholar]
- Rojas CM, Senthil-Kumar M, Tzin V, Mysore K. Regulation of primary plant metabolism during plant–pathogen interactions and its contribution to plant defense. Front Plant Sci. 2014;5:17. doi: 10.3389/fpls.2014.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanel EA, Schrago CG, Couñago RM, Russo CA, Alves-Ferreira M. Evolution of the B3 DNA binding superfamily: new insights into REM family gene diversification. PLoS ONE. 2009;4(6):e5791. doi: 10.1371/journal.pone.0005791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton PJ, Somssich IE, Ringler P, Shen QJ. WRKY transcription factors. Trends Plant Sci. 2010;15(5):247–258. doi: 10.1016/j.tplants.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Sánchez F, Manrique P, Mansilla C, Lunello P, Wang X, Rodrigo G, López-González S, Jenner C, González-Melendi P, Elena SF, Walsh J. Viral strain-specific differential alterations in Arabidopsis developmental patterns. Mol Plant Microbe Interact. 2015;28(12):1304–1315. doi: 10.1094/MPMI-05-15-0111-R. [DOI] [PubMed] [Google Scholar]
- Schenk PM, Kazan K, Rusu AG, Manners JM, Maclean DJ. The SEN1 gene of Arabidopsis is regulated by signals that link plant defence responses and senescence. Plant Physiol Biochem. 2005;43:997–1005. doi: 10.1016/j.plaphy.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Schoelz JE, Harries PA, Nelson RS. Intracellular transport of plant viruses: finding the door out of the cell. Mol Plant. 2011;4(5):813–831. doi: 10.1093/mp/ssr070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki M, Narusaka M, Ishida J, Nanjo T, Fujita M, Oono Y, Kamiya A, Nakajima M, Enju A, Sakurai T, Satou M. Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray. Plant J. 2002;31(3):279–292. doi: 10.1046/j.1365-313x.2002.01359.x. [DOI] [PubMed] [Google Scholar]
- Selth LA, Dogra SC, Rasheed MS, Healy H, Randles JW, Rezaian MA. A NAC domain protein interacts with tomato leaf curl virus replication accessory protein and enhances viral replication. Plant Cell. 2005;17(1):311–325. doi: 10.1105/tpc.104.027235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senthil G, Liu H, Puram VG, Clark A, Stromberg A, Goodin MM. Specific and common changes in Nicotiana benthamiana gene expression in response to infection by enveloped viruses. J Gen Virol. 2005;86(9):2615–2625. doi: 10.1099/vir.0.81043-0. [DOI] [PubMed] [Google Scholar]
- Seo E, Choi D. Functional studies of transcription factors involved in plant defenses in the genomics era. Brief Funct Genom. 2015;14(4):260–267. doi: 10.1093/bfgp/elv011. [DOI] [PubMed] [Google Scholar]
- Shaik R, Ramakrishna W. Genes and co-expression modules common to drought and bacterial stress responses in Arabidopsis and rice. PLoS ONE. 2013;8(10):e77261. doi: 10.1371/journal.pone.0077261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma MK, Kumar R, Solanke AU, Sharma R, Tyagi AK, Sharma AK. Identification, phylogeny, and transcript profiling of ERF family genes during development and abiotic stress treatments in tomato. Mol Genet Genom. 2010;284(6):455–475. doi: 10.1007/s00438-010-0580-1. [DOI] [PubMed] [Google Scholar]
- Sharma R, De Vleesschauwer D, Sharma MK, Ronald PC. Recent advances in dissecting stress-regulatory crosstalk in rice. Mol Plant. 2013;6(2):250–260. doi: 10.1093/mp/sss147. [DOI] [PubMed] [Google Scholar]
- Shukla LI, Chinnusamy V, Sunkar R. The role of microRNAs and other endogenous small RNAs in plant stress responses. Biochim Biophys Acta Gene Regul Mech. 2008;1779(11):743–748. doi: 10.1016/j.bbagrm.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Sommerville C, Koornneef M. A fortunate choice: the history of Arabidopsis as a model plant. Nat Rev Genet. 2002;3(11):883–889. doi: 10.1038/nrg927. [DOI] [PubMed] [Google Scholar]
- Spence NJ, Phiri NA, Hughes SL, Mwaniki A, Simons S, Oduor G, Chacha D, Kuria A, Ndirangu S, Kibata GN, Marris GC. Economic impact of Turnip mosaic virus, Cauliflower mosaic virus and Beet mosaic virus in three Kenyan vegetables. Plant Pathol. 2007;56:317–323. [Google Scholar]
- Srivastava S, Srivastava AK, Suprasanna P, D’souza SF, Identification and profiling of arsenic stress-induced microRNAs in Brassica juncea. J Exp Bot. 2012;64(1):303–315. doi: 10.1093/jxb/ers333. [DOI] [PubMed] [Google Scholar]
- Sui S, Luo J, Ma J, Zhu Q, Lei X, Li M. Generation and analysis of expressed sequence tags from Chimonanthus praecox (Wintersweet) flowers for discovering stress-responsive and floral development-related genes. Comp Funct Genom. 2012;2012:134596. doi: 10.1155/2012/134596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkar R, Li YF, Jagadeeswaran G. Functions of microRNAs in plant stress responses. Trends Plant Sci. 2012;17(4):196–203. doi: 10.1016/j.tplants.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Rivero RM, Shulaev V, Blumwald E, Mittler R. Abiotic and biotic stress combinations. New Phytol. 2014;203(1):32–43. doi: 10.1111/nph.12797. [DOI] [PubMed] [Google Scholar]
- Syller J. Facilitative and antagonistic interactions between plant viruses in mixed infections. Mol Plant Pathol. 2012;13(2):204–216. doi: 10.1111/j.1364-3703.2011.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M. STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucl Acids Res. 2014;43(D1):D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmasebi A, Ashrafi-Dehkordi E, Shahriari AG, Mazloomi SM, Ebrahimie E. Integrative meta-analysis of transcriptomic responses to abiotic stress in cotton. Prog Biophys Mol Biol. 2019;1(146):112–122. doi: 10.1016/j.pbiomolbio.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Takeshita M, Koizumi E, Noguchi M, Sueda K, Shimura H, Ishikawa N, Matsuura H, Ohshima K, Natsuaki T, Kuwata S, Furuya N. Infection dynamics in viral spread and interference under the synergism between Cucumber mosaic virus and Turnip mosaic virus. Mol Plant Microbe Interact. 2012;25(1):18–27. doi: 10.1094/MPMI-06-11-0170. [DOI] [PubMed] [Google Scholar]
- Trujillo M, Shirasu K. Ubiquitination in plant immunity. Curr Opin Plant Biol. 2010;13(4):402–408. doi: 10.1016/j.pbi.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Tsuyuzaki K, Nikaido I (2020) MetaSeq: meta-analysis of RNA-Seq count data in multiple studies. R package version 1.30.0
- Van der Ent S, Verhagen BW, Van Doorn R, Bakker D, Verlaan MG, Pel MJ, Joosten RG, Proveniers MC, Van Loon LC, Ton J, Pieterse CM. MYB72 is required in early signaling steps of rhizobacteria-induced systemic resistance in Arabidopsis. Plant Physiol. 2008;146(3):1293–1304. doi: 10.1104/pp.107.113829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Regenmortel MH, Fauquet CM, Bishop DH, Carstens EB, Estes MK, Lemon SM, Maniloff J, Mayo MA, McGeoch DJ, Pringle CR, Wickner RB (2000) Virus taxonomy: classification and nomenclature of viruses. In: Seventh report of the International Committee on Taxonomy of Viruses. Academic Press
- Verchot J. The ER quality control and ER associated degradation machineries are vital for viral pathogenesis. Front Plant Sci. 2014;5:66. doi: 10.3389/fpls.2014.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verchot J. Plant virus infection and the ubiquitin proteasome machinery: arms race along the endoplasmic reticulum. Viruses. 2016;8(11):314. doi: 10.3390/v8110314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierstra RD. The ubiquitin-26S proteasome system at the nexus of plant biology. Nat Rev Mol Cell Biol. 2009;10(6):385. doi: 10.1038/nrm2688. [DOI] [PubMed] [Google Scholar]
- Walsh JA, Jenner CE. Turnip mosaic virus and the quest for durable resistance. Mol Plant Pathol. 2002;3(5):289–300. doi: 10.1046/j.1364-3703.2002.00132.x. [DOI] [PubMed] [Google Scholar]
- Wang D, Guo Y, Wu C, Yang G, Li Y, Zheng C. Genome-wide analysis of CCCH zinc finger family in Arabidopsis and rice. BMC Genom. 2008;9(1):44. doi: 10.1186/1471-2164-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham SA, Wang Y. Roles for host factors in plant viral pathogenicity. Curr Opin Plant Biol. 2004;7(4):365–371. doi: 10.1016/j.pbi.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Whitham SA, Quan S, Chang HS, Cooper B, Estes B, Zhu T, Wang X, Hou YM. Diverse RNA viruses elicit the expression of common sets of genes in susceptible Arabidopsis thaliana plants. Plant J. 2003;33(2):271–283. doi: 10.1046/j.1365-313x.2003.01625.x. [DOI] [PubMed] [Google Scholar]
- Whitham SA, Yang C, Goodin MM. Global impact: elucidating plant responses to viral infection. Mol Plant Microbe Interact. 2006;19(11):1207–1215. doi: 10.1094/MPMI-19-1207. [DOI] [PubMed] [Google Scholar]
- Wong J, Gao L, Yang Y, Zhai J, Arikit S, Yu Y, Duan S, Chan V, Xiong Q, Yan J, Li S. Roles of small RNA s in soybean defense against Phytophthora sojae infection. Plant J. 2014;79(6):928–940. doi: 10.1111/tpj.12590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, Mao X, Huang J, Ding Y, Wu J, Dong S, Kong L, Gao G, Li CY, Wei L. KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucl Acids Res. 2011;39:W316–W322. doi: 10.1093/nar/gkr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Contento AL, Bassham DC. AtATG18a is required for the formation of autophagosomes during nutrient stress and senescence in Arabidopsis thaliana. Plant J. 2005;42(4):535–546. doi: 10.1111/j.1365-313X.2005.02397.x. [DOI] [PubMed] [Google Scholar]
- Yang H, Wang S, Xi D, Yuan S, Wang J, Xu M, Lin H. Interaction between Cucumber mosaic virus and Turnip crinkle virus in Arabidopsis thaliana. J Phytopathol. 2010;158(11–12):833–836. [Google Scholar]
- Yasaka R, Ohba K, Schwinghamer MW, Fletcher J, Ochoa-Corona FM, Thomas JE, Ho SY, Gibbs AJ, Ohshima K. Phylodynamic evidence of the migration of turnip mosaic potyvirus from Europe to Australia and New Zealand. J Gen Virol. 2015;96(3):701–713. doi: 10.1099/jgv.0.000007. [DOI] [PubMed] [Google Scholar]
- Yilmaz A, Mejia-Guerra MK, Kurz K, Liang X, Welch L, Grotewold E. AGRIS: The Arabidopsis gene regulatory information server, an update. Nucl Acids Res. 2011;39:D1118–D1122. doi: 10.1093/nar/gkq1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti ME, Rípodas C. Niebel A (2017) Plant NF-Y transcription factors: key players in plant-microbe interactions, root development and adaptation to stress. Biochim Biophys Acta Gene Regul Mech. 1860;5:645–654. doi: 10.1016/j.bbagrm.2016.11.007. [DOI] [PubMed] [Google Scholar]
- Zhang W, Gao S, Zhou X, Chellappan P, Chen Z, Zhou X, Zhang X, Fromuth N, Coutino G, Coffey M, Jin H. Bacteria-responsive microRNAs regulate plant innate immunity by modulating plant hormone networks. Plant Mol Biol. 2011;75(1–2):93–105. doi: 10.1007/s11103-010-9710-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Zhang W, Zhao Y, Gong X, Guo L, Zhu G, Wang X, Gong Z, Schumaker KS, Guo Y. SAD2, an importin β-like protein, is required for UV-B response in Arabidopsis by mediating MYB4 nuclear trafficking. Plant Cell. 2007;19(11):3805–3818. doi: 10.1105/tpc.106.048900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W. GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol. 2004;136(1):2621–2632. doi: 10.1104/pp.104.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.