Abstract
Dysregulated motivation to consume psychoactive substances leads to addictive behaviors that often result in serious health consequences. Understanding the neuronal mechanisms that drive drug consumption is crucial for developing new therapeutic strategies. The fruit fly Drosophila melanogaster offers a unique opportunity to approach this problem with a battery of sophisticated neurogenetic tools available, but how they consume these drugs remains largely unknown. Here, we examined drug self-administration behavior of Drosophila and the underlying neuronal mechanisms. We measured the preference of flies for five different psychoactive substances using a two-choice feeding assay and monitored its long-term changes. We found that flies show acute preference for ethanol and methamphetamine, but not for cocaine, caffeine or morphine. Repeated intake of ethanol, but not methamphetamine, increased over time. Preference for methamphetamine and the long-term escalation of ethanol preference required the dopamine receptor Dop1R1 in the mushroom body. The protein level of Dop1R1 increased after repeated intake of ethanol, but not methamphetamine, which correlates with the acquired preference. Genetic overexpression of Dop1R1 enhanced ethanol preference. These results reveal a striking diversity of response to individual drugs in the fly and the role of dopamine signaling and its plastic changes in controlling voluntary intake of drugs.
Subject terms: Reward, Genetics of the nervous system, Addiction
Introduction
Substance use disorders cause severe human health problems, including more than 350 thousand direct and many more indirect deaths per year1. Contribution of genetic factors to the risk of drug addiction is estimated to be around half2, highlighting the importance of animal models that enable investigation of the genetic underpinnings.
The fruit fly Drosophila melanogaster has been established as a useful genetic model organism for decades. These flies show many characteristic responses common in humans, especially toward ethanol. Ethanol is known to target several ion channels, protein kinase C and adenylate cyclase3. Exposure of the flies to vapored ethanol make them first hyperactive, uncoordinated, and eventually sedated4–6. They develop tolerance after repeated exposure7,8 and show withdrawal-like symptoms when ethanol is suddenly withheld after chronic exposure9. These flies indeed like ethanol: food odor attraction is enhanced by the addition of ethanol10,11, and they prefer to feed or lay eggs on food containing ethanol12,13. When an odor cue coincides with ethanol exposure, they form associative reward memory14,15. Importantly, self-administration of ethanol-containing food increases after several days of drinking or acute exposure to the drug12,16–18, capturing one of the most critical steps toward the formation of alcohol use disorder19,20.
Effects of other drugs have also been examined in Drosophila. X-ray crystallography showed that methamphetamine and cocaine bind to the Drosophila dopamine transporter21, which is the primary molecular target of these drugs in mammals22. Similar to humans, methamphetamine and cocaine prevent sleep and cause hyperactivity in a dopamine-dependent manner23–25. Caffeine also causes insomnia through dopamine and the cAMP pathway26–29. Opioid, which targets several G-protein coupled receptors known as opioid receptors in mammals, affects development and lifespan of flies30–32. An opiate antagonist is reported to suppress alcohol preference in flies17, implying functional conservation. Because these drugs often cause compulsive drug seeking behavior in humans, it is tempting to hypothesize that flies may also choose to consume these drugs.
Previous studies indicated the relevance of the reward circuit in some of these behaviors. Natural reward, such as sugar or water, is conveyed by a cluster of dopamine neurons called protocerebral anterior medial (PAM) to the mushroom body (MB)33–37. Prolonged exposure of ethanol vapor activates PAM cluster neurons38, and blocking PAM neurons or the MB intrinsic or output neurons impairs the ethanol-rewarded olfactory memory14,38–40. PAM and the MB intrinsic neurons also influences ethanol preference for oviposition13. Self-administration of ethanol is regulated by neuropeptide-F41, which also mediates reward42, and a cytoskeletal regulator in the MB43. However, involvement of dopamine and the MB circuit for ethanol drinking remains to be elucidated.
In this study, we aimed to obtain a systematic overview of voluntary intake of five different drugs (ethanol, cocaine, caffeine, morphine and methamphetamine) using two-choice capillary feeding (CAFE) assay44. We found that flies prefer to drink ethanol and methamphetamine, but not cocaine, caffeine and morphine. Moreover, dopamine-Dop1R1 signaling in the mushroom body (MB) and its experience-dependent change mediate ethanol and methamphetamine preference. These results reveal diverse responses of flies toward different drugs and the underlying neuronal mechanisms.
Results
Systematic investigation of drug feeding behavior of flies
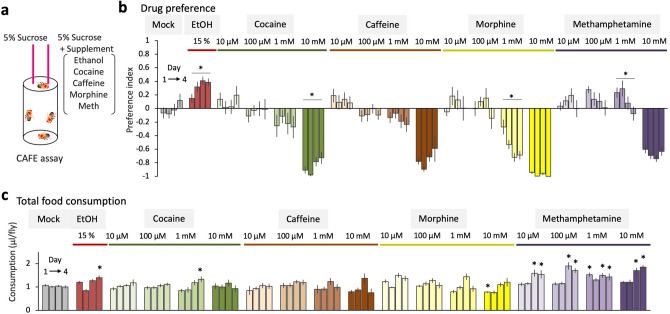
To measure the drug preference and its experience-dependent changes, we performed two-choice CAFE assay44. A group of four wild type male flies was given a choice between 5% sucrose solution and 5% sucrose solution supplemented with five different drugs in different concentrations (ethanol, cocaine, caffeine, morphine or methamphetamine) (Fig. 1a). Daily consumption of each solution was measured for 4 days, and the drug preference index was calculated (“Methods”). Consistent with the previous report12, flies showed a mild preference for ethanol on the first day, which escalated on the following days (Fig. 1b). In contrast, they showed a dose-dependent aversion to caffeine, cocaine and morphine throughout the measurement (Fig. 1b). Aversion to morphine developed over time, suggesting a negative reinforcing effect of the drug (Fig. 1b). Interestingly, methamphetamine was preferred on the first day at 100 µM or 1 mM, but the preference gradually diminished on the following days (Fig. 1b). Ethanol and methamphetamine increased the total food consumption—the sum of consumed sucrose solutions irrespective of drug presence (Fig. 1c), which might be because of the hyperactivity induced by these drugs23,25,45. Altogether, these results revealed distinct dynamics of drug intake behavior of flies.
Figure 1.
The Drosophila drug intake behavior. (a) Schematics of the two-choice CAFE assay. A group of four male flies is given a choice between 5% (w/v) sucrose solution and 5% sucrose solution supplemented with drugs. Daily consumption of each solution is measured as a descent of the meniscus for 4 days. (b) Preference indices of the wild type male flies are plotted from the day 1 to the day 4. Concentration of the drug is indicated above the bar graphs. One-way repeated-measures ANOVA or the Friedman test is performed among the indices from the day 1 to 4. 15% EtOH: F (2.654, 53.08) = 6.743, P = 0.0010, n = 21 (ANOVA); 10 µM cocaine: P = 0.3013, n = 11 (Friedman); 100 µM cocaine: F (1.610, 16.10) = 0.4848, P = 0.5845, n = 11 (ANOVA); 1 mM cocaine: F (1.433, 15.76) = 0.4714, P = 0.5700, n = 12 (ANOVA); 10 mM cocaine: P = 0.0088, n = 8 (Friedman); 10 µM caffeine: F (2.482, 27.30) = 0.4237, P = 0.7012, n = 12 (ANOVA); 100 µM caffeine: F (2.047, 18.42) = 0.7174, P = 0.5042, n = 10 (ANOVA); 1 mM caffeine: P = 0.2881, n = 11 (Friedman); 10 mM caffeine: P = 0.1904, n = 10 (Friedman); 10 µM morphine: F (1.880, 15.04) = 1.546, P = 0.2448, n = 9 (ANOVA); 100 µM morphine: F (2.025, 20.25) = 2.003, P = 0.1604, n = 11 (ANOVA); 1 mM morphine: P = 0.0011, n = 11 (Friedman); 10 mM morphine: F (1.522, 15.22) = 3.251, P = 0.0773, (ANOVA); 10 µM methamphetamine: F (2.100, 29.40) = 1.166, P = 0.3277, n = 15 (ANOVA); 100 µM methamphetamine: F (2.695, 43.12) = 1.816, P = 0.1634, n = 17 (ANOVA); 1 mM methamphetamine: F (2.450, 41.64) = 4.581, P = 0.0111, n = 18 (ANOVA); 10 mM methamphetamine: P = 0.2572, n = 15 (Friedman). Bar graphs: mean ± SEM. *: P < 0.05. (c) Daily food consumption per fly. Total consumption of the control and the drug solution is plotted. Consumption of each group is compared to that of the mock group on the same day (Two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli). 15% EtOH: q = 0.3330, 0.7398, 0.1127, 0.0031 (from the day 1 to 4); 10 µM cocaine: q = 0.3827, 0.9714, 0.8151, 0.1205; 100 µM cocaine: q = 0.6909, 0.8851, 0.8151, 0.1985; 1 mM cocaine: q = 0.2488, 0.8851, 0.3234, 0.0210; 10 mM cocaine: q = 0.8374, 0.9714, 0.4366, 0.5297; 10 µM caffeine: q = 0.8281, 0.9714, 0.3234, 0.2297; 100 µM caffeine: q = 0.3330, 0.6240, 0.0891, 0.1020; 1 mM caffeine: q = 0.8281, 0.8851, 0.2266, 0.0736; 10 mM caffeine: q = 0.2488, 0.8566, 0.0891, 0.6174; 10 µM morphine: q = 0.9166, 0.8851, 0.2495, 0.1985; 100 µM morphine: q = 0.6909, > 0.9999, 0.4768, 0.5297; 1 mM morphine: q = 0.0607, 0.8305, 0.3608, 0.5463; 10 mM morphine: q = 0.0295, 0.6240, 0.8151, 0.3114; 10 µM methamphetamine: q = 0.8281, 0.7398, 0.0099, 0.0051; 100 µM methamphetamine: q = 0.0609, 0.8244, 0.0002, 0.0001; 1 mM methamphetamine: q = 0.0295, 0.3329, 0.0099, 0.0068; 10 mM methamphetamine: q = 0.3330, 0.6240, 0.0003, 0.0001. Bar graphs: mean ± SEM. *: P < 0.05. Bar graphs: mean ± SEM. *: P < 0.05.
Repeated drug feeding underlies the preference
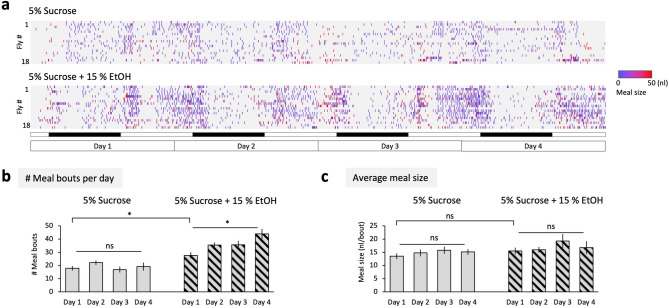
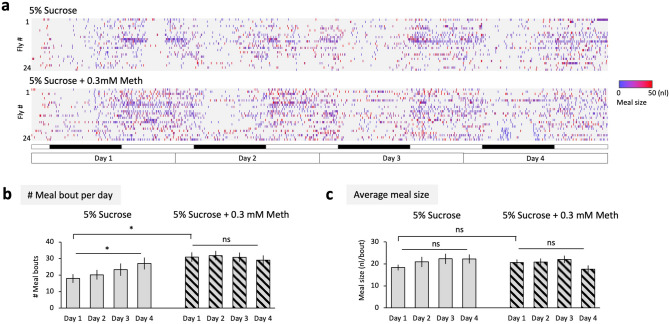
One of the hallmarks of drug addiction is repeated drug intake. We therefore next asked if the preference for ethanol or methamphetamine comes from more frequent feeding on drug-containing food or increased meal size on it. To dissociate these two possibilities, we estimated the number of feeding events and the average meal size of a single-housed fly by measuring the liquid level every 5 minutes (Figs. 2a and 3a). Interestingly, we found that the flies drank ethanol- or methamphetamine-containing solution more often than the control on the first day (Figs. 2b and 3b). The average size of each meal on the other hand was not significantly different (Figs. 2c and 3c). The frequency of ethanol intake, but not the average meal size, progressively increased over time (Fig. 2b,c). Conversely when methamphetamine was provided, flies became to consume the control sucrose solution more often while frequency of methamphetamine feeding stayed rather constant across the 4 days (Fig. 3b). Therefore, we conclude that the drug preference is shaped by repeated drug feeding, but not by different size of each meal.
Figure 2.
Escalation of repeated intake of ethanol. (a) Raster plots of meal-bouts of 5% sucrose solution (upper) and 5% sucrose supplemented with 15% ethanol (lower). Flies are offered with both solution similar to Fig. 1. Note that flies are measured individually not to mix up the consumption of multiple flies. Level of meniscus is measured every 5 minutes and the drop bigger than mean + 2*SD evaporation is defined as a meal bout. Rows and columns respectively indicate individual flies and time bins, and the open and filled open boxes below indicate light and dark cycle (12–12 h). Size of each meal is shown with color as shown on the right. (b) The number of detected meal bouts per day. 5% sucrose: F (2.018, 34.30) = 1.408, P = 0.2585, n = 18 (one-way repeated-measures ANOVA); 5% sucrose + 15% EtOH: F (1.669, 28.38) = 7.710, P = 0.0034, n = 18 (one-way repeated-measures ANOVA). 5% sucrose vs 5% sucrose + 15% EtOH on the day 1: t = 2.698, P = 0.0152, n = 18 (paired t-test). (c) Average meal size. 5% sucrose: F (2.452, 41.68) = 0.9641, P = 0.4047, n = 18 (one-way repeated-measures ANOVA). 5% sucrose + 15% EtOH: P = 0.0719, n = 18 (Friedman test). 5% sucrose vs 5% sucrose + 15% EtOH on the day 1: P = 0.1187, n = 18 (Wilcoxon test). Bar graphs: mean ± SEM. *: P < 0.05.
Figure 3.
Repeated intake of methamphetamine. (a) Raster plots of meal-bouts of 5% sucrose solution (upper) and 5% sucrose supplemented with 0.3 mM methamphetamine (lower). This dose is chosen because both 0.1 and 1 mM methamphetamine elicit the drug preference (see Fig. 1b). Meal events and the size are measured and shown in a similar manner to the Fig. 2. (b) The number of detected meal bouts per day. 5% sucrose: P = 0.0273, n = 24 (Friedman test); 5% sucrose + 0.3 mM methamphetamine: P = 0.7756, n = 24 (Friedman test). 5% sucrose vs 5% sucrose + 0.3 mM methamphetamine on day1: P = 0.0047, n = 24 (Wilcoxon test). (c) Average meal size. 5% sucrose: P = 0.1337, n = 24 (Friedman test); 5% sucrose + 0.3 mM methamphetamine; F (1.648, 37.89) = 2.547, P = 0.1008, n = 24 (one-way repeated-measures ANOVA). 5% sucrose vs 5% sucrose + 0.3 mM methamphetamine on the day 1: P = 0.2076, n = 24 (Wilcoxon test). Bar graphs: mean ± SEM. *: P < 0.05.
Dop1R1 signaling in the mushroom body regulates drug preference
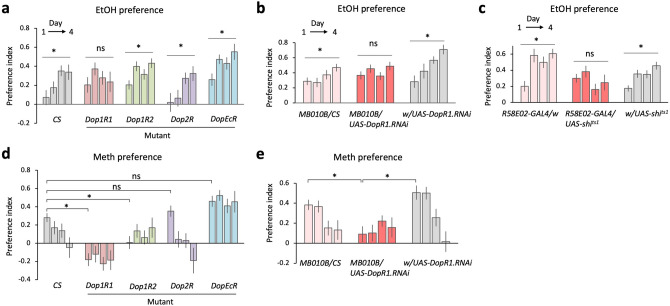
Ethanol and methamphetamine induce dopamine release in the fly brain21,38,45–47. Therefore, we tested the role of dopamine receptors in the preference for ethanol and methamphetamine by using mutants of the four dopamine receptors: Dop1R1, Dop1R2, Dop2R and DopEcR. We found that the mutant of Dop1R1, but not of the other receptors, failed to acquire the experience-dependent ethanol preference (Fig. 4a). The Dop1R1 mutant however showed a normal or slightly enhanced ethanol preference on the first day (Fig. 4a), suggesting that the acute ethanol preference is Dop1R1-independent. In contrast, the same mutation suppressed the preference for methamphetamine throughout the measurement (Fig. 4d). Dop1R2 mutation also suppressed the methamphetamine preference (Fig. 4d). In contrast, the DopEcR mutant showed continuously high preference for both drugs (Fig. 4a,d), suggesting its role in the drug aversion. Because sugar and ethanol reward is conveyed to the mushroom body (MB) in the context of associative learning33,38,48,49, we next tested the role of Dop1R1 in the MB. Genetic knock-down of Dop1R1 in the MB showed a consistent phenotype to the mutant, highlighting the importance of the MB in this behavior (Fig. 4b,e). Consistently, blockade of the reward-conveying PAM cluster dopamine neurons using shibirets150 abolished the acquired ethanol preference (Fig. 4c). Altogether, these results highlight the importance of the Dop1R1 signaling in the MB for the drug preference but in a distinct manner: acquired preference for ethanol and basal preference for methamphetamine.
Figure 4.
Dop1R1 signaling in the mushroom body mediates ethanol and methamphetamine preference. (a) Dop1R1 is necessary for acquired ethanol preference. Daily preference for 15% ethanol is plotted for the wild type (CS) and mutant strains for the four dopamine receptors. One-way repeated-measures ANOVA or the Friedman test is performed among the indices from the day 1 to 4. CS: P < 0.0001, n = 24 (Friedman); Dop1R1: P = 0.2004, n = 22, (Friedman); Dop1R2: F (2.176, 43.52) = 6.715, P = 0.0023, n = 21 (ANOVA); Dop2R: F (1.676, 38.55) = 5.037, P = 0.0155, n = 24 (ANOVA); DopEcR: F (2.021, 40.42) = 5.731, P = 0.0063, n = 21 (ANOVA). (b) Dop1R1 expression in the mushroom body is necessary for acquired ethanol preference. Dop1R1 is genetically knocked down using the MB010B-GAL4 driver. MB010B/CS: F (1.719, 56.73) = 4.093, P = 0.0271, n = 34 (ANOVA); MB010B/UAS-Dop1R1.RNAi: F (2.806, 78.57) = 2.282, P = 0.0896, n = 29 (ANOVA); w/UAS-Dop1R1.RNAi: F (1.979, 43.54) = 6.227, P = 0.0043, n = 23 (ANOVA). (c) Neurotransmission from the PAM cluster dopamine neurons is necessary for acquired ethanol preference. PAM cluster neurons are blocked throughout the measurement using the R58E02-GAL4 and the UAS-shibirets1 strains and the 15% ethanol preference is plotted. R58E02-GAL4/w: F (2.663, 47.93) = 9.746, P < 0.0001, n = 19 (ANOVA); R58E02-GAL4/UAS-shits1: F (2.382, 52.41) = 1.718, P = 0.1840, n = 23 (ANOVA); w/UAS-shits1: F (2.705, 119.0) = 6.435, P = 0.0007, n = 45 (ANOVA). Note that female flies were used for the measurement because of the high mortality when males were used (see also Supplementary Figure 1). (d) Dop1R1 is necessary for acute methamphetamine preference. Daily preference for 0.3 mM methamphetamine is plotted for the wild type (CS) and mutant strains for the four dopamine receptors. The drug preference of mutant strains is compared to that of the wild type on the first day (Dunnett’s multiple comparison). CS vs Dop1R1: P < 0.0001, CS vs Dop1R2: P = 0.0052, CS vs Dop2R: P = 0.8207, CS vs DopEcR: P = 0.2005. (e) Dop1R1 expression in the mushroom body is necessary for acute methamphetamine preference. Dop1R1 is genetically knocked down using the MB010B-GAL4 driver. The drug preference on the first day is compared among genotypes (Dunn’s multiple comparison). MB010B-GAL4/CS vs. MB10B-GAL4/UAS-DopR1.RNAi: P = 0.0193; MB010B-GAL4/UAS-DopR1/RNAi vs. w/UAS-DopR1.RNAi: P = 0.0001. Bar graphs: mean ± SEM. *: P < 0.05.
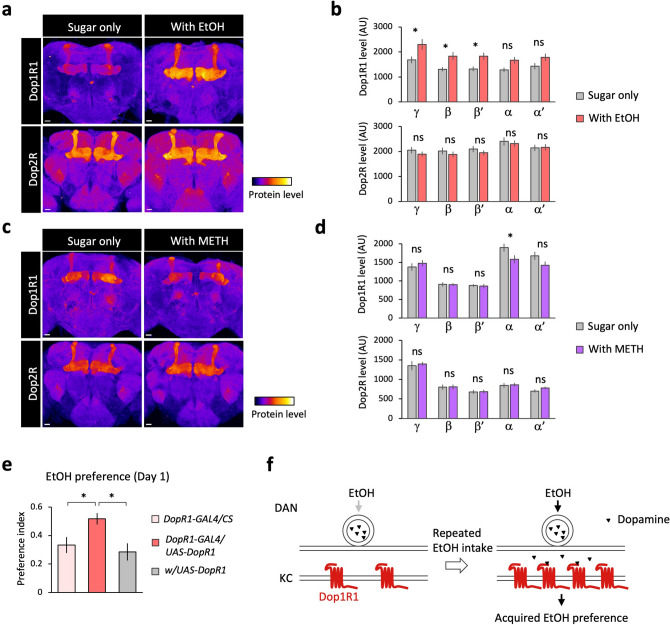
The selective requirement of Dop1R1 for acquired, but not acute, ethanol preference prompted us to hypothesize that voluntary intake of ethanol might change the Dop1R1 signaling. To test this hypothesis, we quantified the protein level of Dop1R1 and Dop2R, which mediate the opposite effects51, in the brain using the Venus tagged endogenous dopamine receptors52. Strikingly, when the flies were given an access to the ethanol-containing food for 3 days, the protein level of Dop1R1, but not Dop2R, was significantly increased in the medial lobe of the MB (Fig. 5a,b). In contrast, addition of methamphetamine did not lead to the Dop1R1 augmentation (Fig. 5c,d). We then hypothesized that the increased Dop1R1 level is sufficient to enhance ethanol preference. Indeed, we found that genetic overexpression of Dop1R1 enhanced ethanol preference (Fig. 5e). Therefore, these results suggest that voluntary ethanol intake increases protein level of Dop1R1, which further mediates the acquired ethanol preference (Fig. 5f).
Figure 5.
Experience-dependent increase of Dop1R1 protein mediates acquired preference for ethanol. (a) Ethanol intake increases the protein level of Dop1R1-Venus, but not Dop2R-Venus. Representative images of the z-projection are shown. Flies are offered with only 5% sucrose solution (left, “Sugar only”) or a choice of 5% sucrose and 5% sucrose with 15% ethanol (right, “With EtOH”) for 3 days in the CAFE assay. (b) Increased Dop1R1 (up) or Dop2R (down) protein in the medial lobe of the MB. Mean fluorescent intensity of Dop1R1- or Dop2R-Venus at the slice of the mushroom body lobes is compared between the groups (Sidak’s multiple comparison). Dop1R1; γ lobe: P = 0.0075, β lobe: P = 0.0350, β′ lobe: P = 0.0405, α lobe: P = 0.2044, α′ lobe: P = 0.2809. n = 16 and 14 for “Sugar only” and “With EtOH”, respectively. Dop2R; γ lobe: P = 0.9008, β lobe: P = 0.9467, β′ lobe: P = 0.9151, α lobe: P = 0.9928, α′ lobe: P > 0.9999. n = 8 and 11 for “Sugar only” and “With EtOH”, respectively. (c) Methamphetamine does not increase the protein level of Dop1R1 or Dop2R. Flies were offered with only 5% sucrose solution or a choice of 5% sucrose and 5% sucrose with 0.3 mM methamphetamine for 3 days in the CAFE assay. (d) Quantification of level of Dop1R1 (up) and Dop2R (down) in the MB lobes. Dop1R1; γ lobe: P = 0.9226, β lobe: P > 0.9999, β′ lobe: P > 0.9999, α lobe: P = 0.0479, α′ lobe: P = 0.1819 (Sidak’s multiple comparison). n = 10 for each group. Dop2R; γ lobe: P = 0.9899, β lobe: P > 0.9999, β′ lobe: P > 0.9999, α lobe: P = 0.9999, α′ lobe: P = 0.8845 (Sidak’s multiple comparison). n = 5 for each group. (e) Genetic overexpression of Dop1R1 enhances ethanol preference. Dop1R1 is overexpressed using the Dop1R1 knock-in 2A-GAL4 driver and 15% ethanol preference is measured for one day. F (2, 41) = 5.712, P = 0.0065 (one-way ANOVA), Dop1R1-GAL4/w vs. Dop1R1-GAL4/UAS-Dop1R1: P = 0.0302, Dop1R1-GAL4/UAS-Dop1R1 vs. w/UAS-Dop1R1, P = 0.0055, n = 14, 16, 14 for Dop1R1-GAL4/w, Dop1R1-GAL4/UAS-Dop1R1 and w/UAS-Dop1R1, respectively (Dunnett’s multiple comparison). (f) Model. Repeated ethanol intake alters the dopamine synapses by elevating the protein level of Dop1R1, thereby promotes further intake. DAN: dopamine neurons. KC: Kenyon cells. Bar graphs: mean ± SEM. *: P < 0.05.
Discussion
In this study, we systematically tested the preference and its experience-dependent changes of five different drugs using a two-choice feeding assay (Fig. 1). Flies showed a robust preference to ethanol, which escalated over time. A previous study has shown that it occurs in a wide range of the ethanol concentration12. In contrast, they avoided cocaine, caffeine and morphine, all of which are plant alkaloid. They even developed an aversion to morphine (Fig. 1b). This diversity might be explained by their evolutionally background: many of the Drosophila species are naturally feeding on fruits, and melanogaster particularly prefers fermented rotten fruits53, which is the major source of ethanol in the wild54. Therefore, ethanol might function as an indicator of a good food resource, which urges them to repeatedly consume (Fig. 2). Plant alkaloids on the other hand are mainly defensive compounds against herbivore insects55. Indeed, cocaine and caffeine prevent the larvae of Manduca sexta from eating plant leaves and eventually kill them56,57. Also for flies, cocaine shortens the lifespan and disrupts oogenesis58, and caffeine prevents food intake59. Our result revealed that Drosophila can readily avoid these plant alkaloids, supporting an idea that drug intake behavior is shaped by the adaptive evolution60,61.
Methamphetamine is an artificial compound and was preferred at the beginning of the measurement (Figs. 1, 3). Analysis of the mutants and the genetic knock-down suggests that the preference is dependent on Dop1R1 and Dop1R2, reminiscent of sugar-rewarded appetitive memory33,62,63 (Fig. 4d). A recent genome wide association study consistently identified the Dop1R1 gene as a critical regulator of consumption of methamphetamine64. Interestingly for ethanol, the Dop1R1 mutant showed normal or slightly enhanced preference on the first day of the measurement but no significant increase on the following days (Fig. 4a). These observations clearly indicate the involvement of the dopamine system, but with different temporal dynamics among these two drugs. Because methamphetamine directly induces a robust dopamine release, primarily by induction of reverse transport through monoamine transporters21,46,65, it may be strong enough to stimulate Dop1R1 to promote the drug intake from the beginning. Ethanol on the other hand does not strongly activate the rewarding dopamine neurons at the first exposure but the response develops afterwards38. We found that self-administration of ethanol, but not methamphetamine, enhanced the Dop1R1 protein level (Fig. 5). These lines of evidence support an idea that repeated ethanol intake, but not methamphetamine, sensitizes the dopamine-Dop1R1 signaling, possibly by increasing both the dopamine release and the receptor. The sensitized reward signaling might escalate self-administration of ethanol (Fig. 5f). A recently reported switch of Dop2R isoforms in the MB after repeated ethanol exposure40 might also contribute to the behavioral change. It would be therefore important in the future to investigate at which level, i.e. transcription, translation or protein turnover, the experience-dependent changes take place and how they are regulated.
DopEcR mutant on the other hand showed a higher preference for both ethanol and methamphetamine (Fig. 4a,d). The DopEcR mutant flies are less sensitive to ethanol sedation66,67, and show defects in dealing with various stressors such as starvation, heat and sexual rejection68–70. Therefore, DopEcR might mediate aversive effects of drugs, which include ethanol sedation, thereby function as a ‘brake’ for the further drug intake. Consistently, a prospective clinical study in humans has shown that lower sensitivity to ethanol sedation predicted a greater number of alcohol use disorders afterwards71. Closer look at the interaction of the accel and the brake would help understanding the neural mechanisms of the drug intake behavior.
Methods
Flies
Flies were reared in a mass culture on standard cornmeal food at 24 °C under 12–12 h light–dark cycles. Canton-S was used as the wild-type. Following dopamine receptor mutants were used: dumb248, Dop1R2attP72, Dop2R∆173 and DopEcRGAL466. Following transgenic strains were used: w1118;;R58E02-GAL433 (BDSC #41347), w1118;;UAS-Shibirets1 (pJFRC100)74, w1118;MB010B-GAL449 (BDSC #68293), y1v1;UAS-Dop1R1.RNAi (P{TRiP.HMC02344})75 (BDSC #55239), w1118;;Dop1R1-GAL452, y1w1118;;Dop1R1-Venus52, y1w1118,Dop2R-Venus52 and w1118,UAS-Dop1R176.
CAFE assay
CAFE assay was performed referring to the original study44 with some modifications. Male and female flies were separated within 1 day after eclosion and 4–7 days old flies were used for the measurement. A group of four male flies (Figs. 1, 4, 5) or a single male fly (Figs. 2, 3) was placed in a column-shaped plastic container, to which two capillaries were inserted. Female flies were used for the shibirets1 blockade (Fig. 4c), because of the high mortality when male flies were measured at the high temperature. Capillaries with inner diameter of 0.5 mm (BF100-50–15, Sutter Instrument, CA, USA) or 0.021 mm (BR708707, BRAND GMBH, Germany) were used for grouped or single flies, respectively. Sucrose (S9378, Sigma-Aldrich, St. Louis, MO, USA), ethanol (09-0851-5, Sigma-Aldrich), caffeine (C8960, Sigma-Aldrich), cocaine hydrochloride (Takeda Pharmaceutical, Tokyo, Japan), methamphetamine hydrochloride (Sumitomo Dainippon Pharma, Osaka, Japan) and morphine hydrochloride (Daiichi-Sankyo, Tokyo, Japan) were dissolved into the evian mineral water (Danon, Paris, France) with indicated concentrations. The sucrose concentration (5%) was chosen following previous studies that measured ethanol preference12,41,43,44. Sulforhodamine B sodium salt (S1402, Sigma-Aldrich) was added by 0.005% (w/v, Figs. 1, 4, 5) or 0.02% (Figs. 2, 3) to stain the solution. n-Octyl Acetate (0.001%, A6042, Toyo Chemical Industry, Tokyo, Japan) and vanillin (0.0001%, H0264, Tokyo Chemical Industry) were reciprocally added to the two capillaries to give an odor cue for the flies: half of the groups were presented with the drugs flavored with n-Octyl Acetate and the other half with vanillin. Experiments were performed in a plexiglas box containing wet tissue paper to minimize evaporation in 12 h-12 h light–dark cycles. Temperature was set to 24 °C, except for the shibirets1 blockade experiment (Fig. 4c), which was performed at 30 °C. Capillaries were pictured every day (Figs. 1, 4, 5) or every 5 min (Figs. 2, 3) using the Pentax Q-S1 camera (Ricoh, Tokyo, Japan). Evaporation was measured for each drug and dose in parallel by imaging the capillaries without flies.
Daily consumption was calculated as descent of liquid level subtracted by evaporation, divided by the number of living flies. Preference index was calculated as
To detect each meal event (Figs. 2, 3), a threshold was calculated for each time bin as mean evaporation + (standard deviation of evaporation) * 2 using measurements of evaporation without flies. A descent of liquid level bigger than the threshold was defined as a meal bout.
Immunohistochemistry
Male flies harboring the Venus-tagged dopamine receptors52 were presented with 5% sucrose solution (“Sugar only”) or 5% sucrose solution and 5% sucrose solution supplemented with 15% ethanol (“With ethanol”) in the CAFE assay chamber for 3 days. Fly brains were then dissected in PBS, immediately fixed in 2% paraformaldehyde in PBS for 1 h at room temperature, and washed three times with PBST (0.1% Triton X-100 in PBS). The brains were then blocked with 3% goat serum in PBST for 30 min at room temperature and incubated in the primary antibody solution (rabbit anti-GFP (1:1000; catalog #A11122, Thermo Fisher Scientific, MA, USA) and 1% goat serum in PBST) and in the secondary antibody solution (Alexa Fluor 488 goat antirabbit (1:1000; catalog #A11034, Thermo Fisher Scientific) and 1% goat serum in PBST) at 4 °C for two nights respectively. Brains were then washed for three times with PBST and mounted in SeeDB2G77. Images were obtained using the Olympus FV1200 confocal microscope (Olympus, Tokyo, Japan) with the 20x, 0.85NA oil objective lens (UPLSAPO20XO, Olympus). Images of the two groups were acquired at the same time periods under the identical microscope settings.
Statistics
Statistical tests were performed on GraphPad Prism 8 for macOS (GraphPad Software, CA, USA). One-sample t-test, repeated measures one-way analysis of variance (ANOVA) or ordinary one-way ANOVA followed by Dunnett’s or Sidak’s multiple comparison was performed when the assumption of normal distribution (D'Agostino & Pearson test) was not violated. Two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli was performed to compare the consumption of the wild type flies (Fig. 1c). Otherwise nonparametric statistics, i.e. Wilcoxon test, Friedman test or Kruskal–Wallis test followed by Dunn’s multiple comparison was performed. The significance level of statistical tests was set to 0.05.
Supplementary Information
Acknowledgements
We thank Chi U Seak, Sibel Jakob, Tomomi Onodera, Noboru Fujii, Kazuma Saito, Katrina Lynn, Atsushi Sugie and Kazuhiko Kume for technical and/or instructional supports. We also thank the Janelia FlyLight Project Team, the Bloomington Drosophila Stock Center and the TRiP at Harvard Medical School for the transgenic strains used in this study. This study was supported by Ministry of Education, Culture, Sports, Science and Technology—Japan/JSPS KAKENHI (19K16265 and 16K20919 to TI, 17H01378 and 26250001 to HT) and Naito Foundation (HT).
Author contributions
H.T. and T.I. conceived and designed the project. M.K., S.H., and T.I. carried out the experiments and analyzed data. S.K. provided unpublished reagents. T.I. wrote the manuscript with the help of all the other authors.
Data availability
The datasets generated during the current study are available from the corresponding author T.I. on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-82813-0.
References
- 1.Roth GA, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ducci F, Goldman D. The genetic basis of addictive disorders. Psychiatr. Clin. N. Am. 2012;35:495–519. doi: 10.1016/j.psc.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abrahao KP, Salinas AG, Lovinger DM. Alcohol and the brain: Neuronal molecular targets, synapses, and circuits. Neuron. 2017;96:1223–1238. doi: 10.1016/j.neuron.2017.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore MS, et al. Ethanol intoxication in Drosophila: Genetic and pharmacological evidence for regulation by the cAMP signaling pathway. Cell. 1998;93:997–1007. doi: 10.1016/S0092-8674(00)81205-2. [DOI] [PubMed] [Google Scholar]
- 5.Singh CM, Heberlein U. Genetic control of acute ethanol-induced behaviors in Drosophila. Alcohol. Clin. Exp. Res. 2000;24:1127–1136. doi: 10.1111/j.1530-0277.2000.tb02075.x. [DOI] [PubMed] [Google Scholar]
- 6.Wolf FW, Rodan AR, Tsai LT-Y, Heberlein U. High-resolution analysis of ethanol-induced locomotor stimulation in Drosophila. J. Neurosci. Off. J. Soc. Neurosci. 2002;22:11035–11044. doi: 10.1523/JNEUROSCI.22-24-11035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger KH, Heberlein U, Moore MS. Rapid and chronic: Two distinct forms of ethanol tolerance in Drosophila. Alcohol. Clin. Exp. Res. 2004;28:1469–1480. doi: 10.1097/01.ALC.0000141817.15993.98. [DOI] [PubMed] [Google Scholar]
- 8.Scholz H, Ramond J, Singh CM, Heberlein U. Functional ethanol tolerance in Drosophila. Neuron. 2000;28:261–271. doi: 10.1016/S0896-6273(00)00101-X. [DOI] [PubMed] [Google Scholar]
- 9.Robinson BG, Khurana S, Kuperman A, Atkinson NS. Neural adaptation leads to cognitive ethanol dependence. Curr. Biol. 2012;22:2338–2341. doi: 10.1016/j.cub.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giang T, He J, Belaidi S, Scholz H. Key odorants regulate food attraction in Drosophila melanogaster. Front. Behav. Neurosci. 2017;11:160. doi: 10.3389/fnbeh.2017.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneider A, et al. Neuronal basis of innate olfactory attraction to ethanol in Drosophila. PLoS ONE. 2012;7:e52007. doi: 10.1371/journal.pone.0052007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devineni AV, Heberlein U. Preferential ethanol consumption in Drosophila models features of addiction. Curr. Biol. 2009;19:2126–2132. doi: 10.1016/j.cub.2009.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azanchi R, Kaun KR, Heberlein U. Competing dopamine neurons drive oviposition choice for ethanol in Drosophila. Proc. Natl. Acad. Sci. 2013;110:21153–21158. doi: 10.1073/pnas.1320208110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaun KR, Azanchi R, Maung Z, Hirsh J, Heberlein U. A Drosophila model for alcohol reward. Nat. Neurosci. 2011;14:612–619. doi: 10.1038/nn.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nunez KM, Azanchi R, Kaun KR. Cue-induced ethanol seeking in Drosophila melanogaster is dose-dependent. Front. Physiol. 2018;9:438. doi: 10.3389/fphys.2018.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engel GL, et al. Sir2/Sirt1 links acute inebriation to presynaptic changes and the development of alcohol tolerance, preference, and reward. J. Neurosci. 2016;36:5241–5251. doi: 10.1523/JNEUROSCI.0499-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koyyada R, et al. Naltrexone reverses ethanol preference and protein kinase C activation in Drosophila melanogaster. Front. Physiol. 2018;9:175. doi: 10.3389/fphys.2018.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peru Y, Colón de Portugal RL, et al. Long-lasting, experience-dependent alcohol preference in Drosophila. Addict. Biol. 2014;19:392–401. doi: 10.1111/adb.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devineni AV, Heberlein U. Addiction-like behavior in Drosophila. Commun. Integr. Biol. 2010;3:357–359. doi: 10.4161/cib.3.4.11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petruccelli E, Kaun KR. Insights from intoxicated Drosophila. Alcohol Fayettev. N. 2019;74:21–27. doi: 10.1016/j.alcohol.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang KH, Penmatsa A, Gouaux E. Neurotransmitter and psychostimulant recognition by the dopamine transporter. Nature. 2015;521:322–327. doi: 10.1038/nature14431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu J, Reith MEA. Role of the dopamine transporter in the action of psychostimulants, nicotine, and other drugs of abuse. CNS Neurol. Disord. Drug Targets. 2008;7:393–409. doi: 10.2174/187152708786927877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andretic R, van Swinderen B, Greenspan RJ. Dopaminergic modulation of arousal in Drosophila. Curr. Biol. 2005;15:1165–1175. doi: 10.1016/j.cub.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 24.Andretic R, Kim Y-C, Jones FS, Han K-A, Greenspan RJ. Drosophila D1 dopamine receptor mediates caffeine-induced arousal. Proc. Natl. Acad. Sci. USA. 2008;105:20392–20397. doi: 10.1073/pnas.0806776105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bainton RJ, et al. Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr. Biol. 2000;10:187–194. doi: 10.1016/S0960-9822(00)00336-5. [DOI] [PubMed] [Google Scholar]
- 26.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 27.Hendricks JC, et al. Rest in Drosophila is a sleep-like state. Neuron. 2000;25:129–138. doi: 10.1016/S0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 28.Nall AH, et al. Caffeine promotes wakefulness via dopamine signaling in Drosophila. Sci. Rep. 2016;6:20938. doi: 10.1038/srep20938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu MN, et al. The effects of caffeine on sleep in drosophila require PKA activity, but not the adenosine receptor. J. Neurosci. 2009;29:11029–11037. doi: 10.1523/JNEUROSCI.1653-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santoro C, Hall LM, Zukin RS. Characterization of two classes of opioid binding sites in Drosophila melanogaster head membranes. J. Neurochem. 1990;54:164–170. doi: 10.1111/j.1471-4159.1990.tb13297.x. [DOI] [PubMed] [Google Scholar]
- 31.Tekieh E, et al. Effects of oral morphine on the larvae, pupae and imago development in Drosophila melanogaster. Cell J. 2011;13:149–154. [PMC free article] [PubMed] [Google Scholar]
- 32.Dubiley TA, Rushkevich YE, Koshel NM, Voitenko VP, Vaiserman AM. Life span extension in Drosophila melanogaster induced by morphine. Biogerontology. 2011;12:179–184. doi: 10.1007/s10522-010-9308-1. [DOI] [PubMed] [Google Scholar]
- 33.Liu C, et al. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature. 2012;488:512–516. doi: 10.1038/nature11304. [DOI] [PubMed] [Google Scholar]
- 34.Lin S, et al. Neural correlates of water reward in thirsty Drosophila. Nat. Neurosci. 2014;17:1536–1542. doi: 10.1038/nn.3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burke CJ, et al. Layered reward signalling through octopamine and dopamine in Drosophila. Nature. 2012;492:433–437. doi: 10.1038/nature11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamagata N, et al. Distinct dopamine neurons mediate reward signals for short- and long-term memories. Proc. Natl. Acad. Sci. 2015;112:578–583. doi: 10.1073/pnas.1421930112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huetteroth W, et al. Sweet taste and nutrient value subdivide rewarding dopaminergic neurons in Drosophila. Curr. Biol. 2015;25:751–758. doi: 10.1016/j.cub.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scaplen KM, et al. Circuits that encode and guide alcohol-associated preference. eLife. 2020;9:e48730. doi: 10.7554/eLife.48730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aso Y, et al. Mushroom body output neurons encode valence and guide memory-based action selection in Drosophila. eLife. 2014;3:e04580. doi: 10.7554/eLife.04580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petruccelli E, et al. Alcohol activates scabrous-notch to influence associated memories. Neuron. 2018;100:1209–1223.e4. doi: 10.1016/j.neuron.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shohat-Ophir G, Kaun KR, Azanchi R, Mohammed H, Heberlein U. Sexual deprivation increases ethanol intake in Drosophila. Science. 2012;335:1351–1355. doi: 10.1126/science.1215932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shao L, et al. Dissection of the Drosophila neuropeptide F circuit using a high-throughput two-choice assay. Proc. Natl. Acad. Sci. 2017;114:E8091–E8099. doi: 10.1073/pnas.1710552114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ojelade SA, et al. Rsu1 regulates ethanol consumption in Drosophila and humans. Proc. Natl. Acad. Sci. 2015;112:E4085–E4093. doi: 10.1073/pnas.1417222112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ja WW, et al. Prandiology of Drosophila and the CAFE assay. Proc. Natl. Acad. Sci. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kong EC, et al. A pair of dopamine neurons target the D1-like dopamine receptor DopR in the central complex to promote ethanol-stimulated locomotion in Drosophila. PLoS ONE. 2010;5:e9954. doi: 10.1371/journal.pone.0009954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freyberg Z, et al. Mechanisms of amphetamine action illuminated through optical monitoring of dopamine synaptic vesicles in Drosophila brain. Nat. Commun. 2016;7:10652. doi: 10.1038/ncomms10652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim Y-C, Lee H-G, Han K-A. D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J. Neurosci. Off. J. Soc. Neurosci. 2007;27:7640–7647. doi: 10.1523/JNEUROSCI.1167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vogt K, et al. Shared mushroom body circuits underlie visual and olfactory memories in Drosophila. eLife. 2014;3:e02395. doi: 10.7554/eLife.02395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J. Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- 51.Mustard JA, Beggs KT, Mercer AR. Molecular biology of the invertebrate dopamine receptors. Arch. Insect Biochem. Physiol. 2005;59:103–117. doi: 10.1002/arch.20065. [DOI] [PubMed] [Google Scholar]
- 52.Kondo S, et al. Neurochemical organization of the Drosophila brain visualized by endogenously tagged neurotransmitter receptors. Cell Rep. 2020;30:284–297.e5. doi: 10.1016/j.celrep.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 53.Karageorgi M, et al. Evolution of multiple sensory systems drives novel egg-laying behavior in the fruit pest Drosophila suzukii. Curr. Biol. 2017;27:847–853. doi: 10.1016/j.cub.2017.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dudley R. Ethanol, fruit ripening, and the historical origins of human alcoholism in primate frugivory. Integr. Comp. Biol. 2004;44:315–323. doi: 10.1093/icb/44.4.315. [DOI] [PubMed] [Google Scholar]
- 55.Rattan RS. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot. 2010;29:913–920. doi: 10.1016/j.cropro.2010.05.008. [DOI] [Google Scholar]
- 56.Nathanson JA. Caffeine and related methylxanthines: Possible naturally occurring pesticides. Science. 1984;226:184–187. doi: 10.1126/science.6207592. [DOI] [PubMed] [Google Scholar]
- 57.Nathanson JA, Hunnicutt EJ, Kantham L, Scavone C. Cocaine as a naturally occurring insecticide. Proc. Natl. Acad. Sci. USA. 1993;90:9645–9648. doi: 10.1073/pnas.90.20.9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sedore Willard S, Koss CM, Cronmiller C. Chronic cocaine exposure in Drosophila: Life, cell death and oogenesis. Dev. Biol. 2006;296:150–163. doi: 10.1016/j.ydbio.2006.04.448. [DOI] [PubMed] [Google Scholar]
- 59.Keebaugh ES, Park JH, Su C, Yamada R, Ja WW. Nutrition influences caffeine-mediated sleep loss in Drosophila. Sleep. 2017;40:zsx146. doi: 10.1093/sleep/zsx146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saah T. The evolutionary origins and significance of drug addiction. Harm. Reduct. J. 2005;2:8. doi: 10.1186/1477-7517-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sullivan RJ, Hagen EH. Psychotropic substance-seeking: Evolutionary pathology or adaptation? Addict. Abingdon Engl. 2002;97:389–400. doi: 10.1046/j.1360-0443.2002.00024.x. [DOI] [PubMed] [Google Scholar]
- 62.Musso P-Y, Tchenio P, Preat T. Delayed dopamine signaling of energy level builds appetitive long-term memory in Drosophila. Cell Rep. 2015;10:1023–1031. doi: 10.1016/j.celrep.2015.01.036. [DOI] [PubMed] [Google Scholar]
- 63.Sun H, et al. Dopamine receptor Dop1R2 stabilizes appetitive olfactory memory through the Raf/MAPK pathway in Drosophila. J. Neurosci. Off. J. Soc. Neurosci. 2020;40:2935–2942. doi: 10.1523/JNEUROSCI.1572-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Highfill CA, Baker BM, Stevens SD, Anholt RRH, Mackay TFC. Genetics of cocaine and methamphetamine consumption and preference in Drosophila melanogaster. PLoS Genet. 2019;15:e1007834. doi: 10.1371/journal.pgen.1007834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: A review. Prog. Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 66.Petruccelli E, Li Q, Rao Y, Kitamoto T. The unique dopamine/ecdysteroid receptor modulates ethanol-induced sedation in Drosophila. J. Neurosci. Off. J. Soc. Neurosci. 2016;36:4647–4657. doi: 10.1523/JNEUROSCI.3774-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aranda GP, Hinojos SJ, Sabandal PR, Evans PD, Han K-A. Behavioral sensitization to the disinhibition effect of ethanol requires the dopamine/ecdysone receptor in Drosophila. Front. Syst. Neurosci. 2017;11:56. doi: 10.3389/fnsys.2017.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petruccelli E, Lark A, Mrkvicka JA, Kitamoto T. Significance of DopEcR, a G-protein coupled dopamine/ecdysteroid receptor, in physiological and behavioral response to stressors. J. Neurogenet. 2020;34:55–68. doi: 10.1080/01677063.2019.1710144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Inagaki HK, et al. Visualizing neuromodulation in vivo: TANGO-mapping of dopamine signaling reveals appetite control of sugar sensing. Cell. 2012;148:583–595. doi: 10.1016/j.cell.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ishimoto H, Wang Z, Rao Y, Wu C-F, Kitamoto T. A novel role for ecdysone in Drosophila conditioned behavior: Linking GPCR-mediated non-canonical steroid action to cAMP signaling in the adult brain. PLoS Genet. 2013;9:e1003843. doi: 10.1371/journal.pgen.1003843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.King AC, McNamara PJ, Hasin DS, Cao D. Alcohol challenge responses predict future alcohol use disorder symptoms: A 6-year prospective study. Biol. Psychiatry. 2014;75:798–806. doi: 10.1016/j.biopsych.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Keleman K, et al. Dopamine neurons modulate pheromone responses in Drosophila courtship learning. Nature. 2012;489:145–149. doi: 10.1038/nature11345. [DOI] [PubMed] [Google Scholar]
- 73.Scholz-Kornehl S, Schwärzel M. Circuit analysis of a drosophila dopamine type 2 receptor that supports anesthesia-resistant memory. J. Neurosci. Off. J. Soc. Neurosci. 2016;36:7936–7945. doi: 10.1523/JNEUROSCI.4475-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pfeiffer BD, Truman JW, Rubin GM. Using translational enhancers to increase transgene expression in Drosophila. Proc. Natl. Acad. Sci. 2012;109:6626–6631. doi: 10.1073/pnas.1204520109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ni J-Q, et al. A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat. Methods. 2011;8:405–407. doi: 10.1038/nmeth.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qin H, et al. Gamma neurons mediate dopaminergic input during aversive olfactory memory formation in Drosophila. Curr. Biol. 2012;22:608–614. doi: 10.1016/j.cub.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ke M-T, et al. Super-resolution mapping of neuronal circuitry with an index-optimized clearing agent. Cell Rep. 2016;14:2718–2732. doi: 10.1016/j.celrep.2016.02.057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available from the corresponding author T.I. on reasonable request.