Abstract
Lessons Learned
The combination of eribulin with 5‐fluorouracil, either doxorubicin or epirubicin, and cyclophosphamide (FAC/FEC) was not superior to the combination of paclitaxel with FAC/FEC and was associated with greater hematologic toxicity.
Eribulin followed by an anthracycline‐based regimen is not recommended as a standard neoadjuvant therapy in nonmetastatic operable breast cancer.
Background
Neoadjuvant systemic therapy is the standard of care for locally advanced operable breast cancer. We hypothesized eribulin may improve the pathological complete response (pCR) rate compared with paclitaxel.
Methods
We conducted a 1:1 randomized open‐label phase II study comparing eribulin versus paclitaxel followed by 5‐fluorouracil, either doxorubicin or epirubicin, and cyclophosphamide (FAC/FEC) in patients with operable HER2‐negative breast cancer. pCR and toxicity of paclitaxel 80 mg/m2 weekly for 12 doses or eribulin 1.4 mg/m2 on days 1 and 8 of a 21‐day cycle for 4 cycles followed by FAC/FEC were compared.
Results
At the interim futility analysis, in March 2015, 51 patients (28 paclitaxel, 23 eribulin) had received at least one dose of the study drug and were thus evaluable for toxicity; of these, 47 (26 paclitaxel, 21 eribulin) had undergone surgery and were thus evaluable for efficacy. Seven of 26 (27%) in the paclitaxel group and 1 of 21 (5%) in the eribulin group achieved a pCR, and this result crossed a futility stopping boundary. In the paclitaxel group, the most common serious adverse events (SAEs) were neutropenic fever (grade 3, 3 patients, 11%). In the eribulin group, nine patients (39%) had neutropenia‐related SAEs, and one died of neutropenic sepsis. The study was thus discontinued. For the paclitaxel and eribulin groups, the 5‐year event‐free survival (EFS) rates were 81.8% and 74.0% (hazard ratio [HR], 1.549; 95% confidence interval [CI], 0.817–2.938; p = .3767), and the 5‐year overall survival (OS) rates were 100% and 84.4% (HR, 5.813; 95% CI, 0.647–52.208; p = .0752), respectively.
Conclusion
We did not observe a higher proportion of patients undergoing breast conservation surgery in the eribulin group than in the paclitaxel group. The patients treated with eribulin were more likely to undergo mastectomy and less likely to undergo breast conservation surgery, but the difference was not statistically significant.
As neoadjuvant therapy for operable HER2‐negative breast cancer, eribulin followed by FAC/FEC is not superior to paclitaxel followed by FAC/FEC and is associated with a higher incidence of neutropenia‐related serious adverse events.
Keywords: Eribulin, Paclitaxel, HER2‐negative, Breast cancer, Neoadjuvant chemotherapy
Discussion
Here, we report a first randomized phase II study result that showed the lack of clear efficacy and higher toxicity when eribulin was used as a part of a neoadjuvant chemotherapy regimen in operable HER‐2 negative breast cancers. Figure 1 shows Kaplan‐Meier plots for EFS and OS by treatment groups. Table 1 shows response data. We do not think this negative result was due to smaller size of the patient groups accrued to each arm, because the study was preplanned to have interim efficacy and toxicity assessments. Although it is disappointing, given the efficacy of eribulin in the metastatic setting and the fact that a larger randomized trial confirmed our results with higher statistical power, we do not recommend a follow‐up study.
Figure 1.
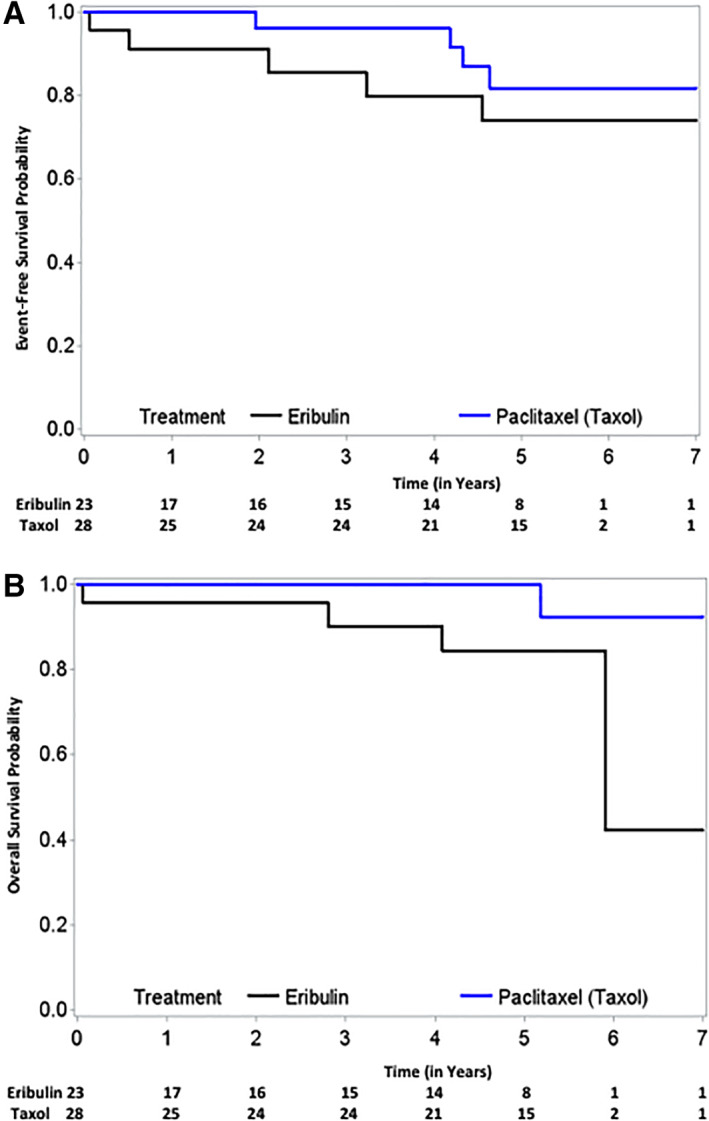
A total of 23 patients in the eribulin arm and 28 patients in the taxol arm were available for long‐term clinical outcome measurement. Five‐year event‐free survival for the eribulin‐ and paclitaxel‐based arms was 74.0% and 81.8%, respectively (A). Five‐year overall survival of eribulin‐ and paclitaxel‐based arms, was 84.4% and 100%, respectively (B).
Table 1.
Pathologic response and type of surgery per treatment group
| Treatment group | Paclitaxel and FAC/FEC (n = 26) | Eribulin and FAC/FEC (n = 21) |
|---|---|---|
| Residual cancer burden category a | ||
| 0 (pCR) | 7 (27) | 1 (5) |
| I | 7 (27) | 0 (0) |
| II | 8 (31) | 8 (38) |
| III | 4 (15) | 12 (57) |
| Type of surgery | ||
| Mastectomy | 17 (65) | 16 (76) |
| Breast conserving surgery | 9 (35) | 5 (24) |
Residual cancer burden (RCB) was calculated by the RCB calculator (by pathologists: http://www3.mdanderson.org/app/medcalc/index.cfm?pagename=jsconvert3).
Abbreviations: FAC/FEC, 5‐fluorouracil, either doxorubicin or epirubicin, and cyclophosphamide; pCR, pathologic complete response.
Trial Information
| Disease | Breast cancer |
| Stage of Disease/Treatment | Neoadjuvant |
| Prior Therapy | None |
| Type of Study | Phase II, randomized |
| Primary Endpoint | Complete response rate |
| Secondary Endpoint | Event‐free survival |
| Additional Details of Endpoints or Study Design | |
| At the time of the first interim futility analysis, 8 (30.8%) of the 26 patients in the paclitaxel group and 1 (4.8%) of the 21 patients in the eribulin group had achieved a pCR (Table 2). The test statistic was , where p = (1 + 8)/(21 + 26) and it crossed the futility stopping boundary. In an exploratory analysis (not preplanned) comparing outcomes by breast cancer molecular subtype, we found that among the 34 patients with hormone receptor–positive disease, 3 of 18 (17%) in the paclitaxel group and 0 of 16 (0%) in the eribulin group achieved a pCR. Among the 13 patients with triple‐negative breast cancer, 4 of 8 (50%) in the paclitaxel group and 1 of 5 (20%) in the eribulin group achieved a pCR. For each disease subtype, the difference in pCR rate between the paclitaxel and eribulin groups was not significant. | |
| Investigator's Analysis | |
| The combination of eribulin and FAC/FEC was not superior to paclitaxel and FAC/FEC and was associated with higher hematological toxicity; therefore, we do not recommend eribulin/FAC/FEC as a standard neoadjuvant therapy in early‐stage breast cancer. | |
Drug Information
| Drug 1 | |
| Generic/Working Name | Eribulin |
| Trade Name | Halavan |
| Drug Type | Other |
| Drug Class | Microtubule‐targeting agent |
| Dose | 1.4 mg/m2 |
| Route | IV, per push |
| Schedule of Administration | Day 1, day 8, every 21 days × 4 cycles |
| Drug 2 | |
| Generic/Working Name | Paclitaxel |
| Trade Name | Taxol |
| Drug Class | Microtubule‐targeting agent |
| Dose | 80 mg/m2 |
| Route | IV |
| Schedule of Administration | Weekly x 12 weeks |
Patient Characteristics: Paclitaxel Arm
| Number of Patients, Male | 0 |
| Number of Patients, Female | 28 |
| Stage | II A — 5 |
| II B — 12 | |
| III A — 7 | |
| III B — 1 | |
| III C — 3 | |
| Age | Median: 48 years |
| Number of Prior Systemic Therapies | Median: 0 |
| Performance Status: ECOG |
0 — 27 1 — 1 2 — 3 — Unknown — |
| Other | |
| Receptor status | ER/PR‐postive — 15 |
| ER positive/PR negative — 4 | |
| ER neg/PR positive — 1 | |
| ER/PR negative — 8 | |
| Nuclear grade | Grade 1 — 3 |
| Grade 2 — 10 | |
| Grade 3 — 15 |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor.
Patient Characteristics: Eribulin Arm
| Number of Patients, Male | 0 |
| Number of Patients, Female | 21 |
| Stage | II A — 4 |
| II B — 6 | |
| III A — 6 | |
| III B — 0 | |
| III C — 5 | |
| Age | Median: 51 years |
| Number of Prior Systemic Therapies | Median: 0 |
| Performance Status: ECOG |
0 — 20 1 — 1 2 — 3 — Unknown — |
| Other | ER/PR positive — 13 |
| ER positive/PR negative — 3 | |
| ER negative/PR positive — 0 | |
| ER/PR negative — 5 |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor.
Primary Assessment Method
| Title | Response: paclitaxel arm |
| Number of Patients Screened | 28 |
| Number of Patients Enrolled | 28 |
| Number of Patients Evaluable for Toxicity | 28 |
| Number of Patients Evaluated for Efficacy | 26 |
| Evaluation Method | RECIST 1.0 |
| Response Assessment CR | n = 7 (27%) |
| Response Assessment PR | n = 19 (73%) |
| (Median) Duration Assessments OS | 61 months |
| Title | Survival: paclitaxel arm |
| Number of Patients Screened | 28 |
| Number of Patients Enrolled | 28 |
| Number of Patients Evaluable for Toxicity | 28 |
| Number of Patients Evaluated for Efficacy | 26 |
| Response Assessment CR | n = 8 (31%) |
| Response Assessment PR | n = 18 (69%) |
| Title | Response: eribulin arm |
| Number of Patients Screened | 26 |
| Number of Patients Enrolled | 24 |
| Number of Patients Evaluable for Toxicity | 23 |
| Number of Patients Evaluated for Efficacy | 21 |
| Evaluation Method | RECIST 1.0 |
| Response Assessment CR | n = 1 (5%) |
| Response Assessment PR | n = 20 (95%) |
| (Median) Duration Assessments OS | 61 months |
| Title | Survival: eribulin arm |
| Number of Patients Screened | 28 |
| Number of Patients Enrolled | 28 |
| Number of Patients Evaluable for Toxicity | 28 |
| Number of Patients Evaluated for Efficacy | 26 |
| Response Assessment CR | n = 8 (31%) |
| Response Assessment PR | n = 18 (69%) |
| Outcome Notes | |
| The median follow‐up was 5 years. The median EFS was not reached in either arm, but 5‐year event‐free survival for the eribulin‐based regimen and the paclitaxel‐based regimen was 74.0% and 81.8%, respectively. The median OS was 5.9 years for eribulin and was not reached for the paclitaxel arm, and the 5‐year overall survival for the eribulin‐based regimen and the paclitaxel‐based regimen was 84.4% and 100%, respectively. | |
Adverse Events: Paclitaxel Arm
| Name | NC/NA | 1 | 2 | 3 | 4 | 5 | All grades |
|---|---|---|---|---|---|---|---|
| Alopecia | 46% | 0% | 54% | 0% | 0% | 0% | 54% |
| Fatigue | 64% | 4% | 25% | 7% | 0% | 0% | 36% |
| Nausea | 81% | 4% | 11% | 4% | 0% | 0% | 19% |
| Neutrophil count decreased | 83% | 0% | 4% | 13% | 0% | 0% | 17% |
| Paresthesia | 78% | 0% | 18% | 4% | 0% | 0% | 22% |
| Skin and subcutaneous tissue disorders | 82% | 0% | 18% | 0% | 0% | 0% | 18% |
| Constipation | 85% | 0% | 4% | 11% | 0% | 0% | 15% |
| Mucositis oral | 96% | 0% | 4% | 0% | 0% | 0% | 4% |
| Myalgia | 96% | 0% | 4% | 0% | 0% | 0% | 4% |
| Diarrhea | 96% | 0% | 4% | 0% | 0% | 0% | 4% |
| Fever | 96% | 0% | 4% | 0% | 0% | 0% | 4% |
| Vomiting | 93% | 0% | 7% | 0% | 0% | 0% | 7% |
| Alanine aminotransferase increased | 93% | 0% | 7% | 0% | 0% | 0% | 7% |
| Nasal congestion | 92% | 0% | 4% | 4% | 0% | 0% | 8% |
| Pain | 96% | 0% | 4% | 0% | 0% | 0% | 4% |
| Rash acneiform | 93% | 0% | 7% | 0% | 0% | 0% | 7% |
| White blood cell decreased | 96% | 0% | 4% | 0% | 0% | 0% | 4% |
| Abdominal pain | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Arthralgia | 96% | 0% | 4% | 0% | 0% | 0% | 4% |
| Aspartate aminotransferase increased | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Bladder infection | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Dizziness | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Edema limbs | 96% | 0% | 4% | 0% | 0% | 0% | 4% |
| Headache | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Hyperglycemia | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Infections and infestations | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Insomnia | 96% | 0% | 4% | 0% | 0% | 0% | 4% |
| Left ventricular systolic dysfunction | 96% | 0% | 0% | 4% | 0% | 0% | 4% |
| Memory impairment | 96% | 0% | 4% | 0% | 0% | 0% | 4% |
| Nail loss | 96% | 0% | 4% | 0% | 0% | 0% | 4% |
| Neutropenic sepsis | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Skin infection | 96% | 0% | 4% | 0% | 0% | 0% | 4% |
| Vaginal infection | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Vaginal inflammation | 96% | 0% | 4% | 0% | 0% | 0% | 4% |
Adverse Events: Eribulin Arm
| Name | NC/NA | 1 | 2 | 3 | 4 | 5 | All grades |
|---|---|---|---|---|---|---|---|
| Alopecia | 60% | 11% | 29% | 0% | 0% | 0% | 40% |
| Fatigue | 68% | 7% | 21% | 4% | 0% | 0% | 32% |
| Nausea | 75% | 7% | 18% | 0% | 0% | 0% | 25% |
| Neutrophil count decreased | 71% | 0% | 0% | 18% | 11% | 0% | 29% |
| Paresthesia | 96% | 0% | 4% | 0% | 0% | 0% | 4% |
| Skin and subcutaneous tissue disorders | 93% | 0% | 7% | 0% | 0% | 0% | 7% |
| Constipation | 92% | 4% | 4% | 0% | 0% | 0% | 8% |
| Mucositis oral | 82% | 4% | 14% | 0% | 0% | 0% | 18% |
| Myalgia | 81% | 11% | 4% | 4% | 0% | 0% | 19% |
| Diarrhea | 89% | 4% | 7% | 0% | 0% | 0% | 11% |
| Fever | 89% | 4% | 7% | 0% | 0% | 0% | 11% |
| Vomiting | 92% | 0% | 4% | 4% | 0% | 0% | 8% |
| Alanine aminotransferase increased | 96% | 0% | 0% | 4% | 0% | 0% | 4% |
| Nasal congestion | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Pain | 96% | 0% | 4% | 0% | 0% | 0% | 4% |
| Rash acneiform | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| White blood cell decreased | 96% | 0% | 0% | 0% | 4% | 0% | 4% |
| Abdominal pain | 96% | 4% | 0% | 0% | 0% | 0% | 4% |
| Arthralgia | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Aspartate aminotransferase increased | 96% | 0% | 0% | 4% | 0% | 0% | 4% |
| Bladder infection | 96% | 0% | 4% | 0% | 0% | 0% | 4% |
| Dizziness | 96% | 0% | 0% | 4% | 0% | 0% | 4% |
| Edema limbs | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Headache | 96% | 0% | 4% | 0% | 0% | 0% | 4% |
| Hyperglycemia | 96% | 4% | 0% | 0% | 0% | 0% | 4% |
| Infections and infestations | 96% | 0% | 4% | 0% | 0% | 0% | 4% |
| Insomnia | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Left ventricular systolic dysfunction | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Memory impairment | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Nail loss | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Neutropenic sepsis | 96% | 0% | 0% | 0% | 0% | 4% | 4% |
| Skin infection | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Vaginal infection | 96% | 0% | 4% | 0% | 0% | 0% | 4% |
| Vaginal inflammation | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
Serious Adverse Events
| Name | Grade | Attribution |
|---|---|---|
| Eribulin, neutropenic sepsis | 4 | Definite |
| Eribulin, neutropenic sepsis | 4 | Definite |
| Eribulin, neutropenic sepsis | 4 | Definite |
| Eribulin, neutropenia | 3 | Definite |
| Eribulin, neutropenia | 3 | Definite |
| Eribulin, neutropenia | 3 | Definite |
| Eribulin, neutropenia | 3 | Definite |
| Eribulin, neutropenia | 3 | Definite |
| Paclitaxel, neutropenia | 3 | Definite |
| Paclitaxel, neutropenia | 3 | Definite |
| Paclitaxel, neutropenia | 3 | Definite |
| Eribulin, fatigue | 3 | Probable |
| Paclitaxel, fatigue | 3 | Probable |
| Paclitaxel, fatigue | 3 | Probable |
| Eribulin, AST abnormality | 3 | Probable |
| Eribulin, ALT abnormality | 3 | Probable |
| Eribulin, dizziness | 3 | Probable |
| Paclitaxel, LVEF abnormality | 3 | Probable |
| Eribulin, myalgia | 3 | Definite |
| Paclitaxel, nasal congestion | 3 | Probable |
| Paclitaxel, nausea | 3 | Probable |
| Eribulin, neutropenic sepsis and death | 5 | Probable |
| Paclitaxel, paresthesia | 3 | Probable |
| Eribulin, vomiting | 3 | Probable |
| Eribulin, white blood cell decreased | 4 | Definite |
If patients received at least one dose of study drug, they were deemed to be evaluable for toxicity. Adverse events including laboratory results were graded according to the National Cancer Institute's CTCAE, version 4.0. Dose‐limiting toxicity was defined as occurrence of adverse events that were attributed as possibly, probably, or definitely related to each study drug and occurring within 2 cycles after the first dose: grade 4 thrombocytopenia or grade 4 neutropenia lasting >1 week or any febrile neutropenia; greater than grade 3 nonhematologic toxic effect; or > 14 days of treatment delay due to any grade of therapy‐related toxic effects (grade 1–2). For patients with multiple instances of the same adverse event and different grades at different instances, we counted the adverse event only once and assigned the highest grade experienced for that event. Toxicity was evaluated on days 8 and 15 for the first 2 cycles and at the end of each cycle thereafter. Dose modification followed standard care for each taxol and eribulin per U.S. Food and Drug Administration package insert and left up to the treating physician's discretion.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; LVEF, left ventricular ejection fraction.
Assessment, Analysis, and Discussion
| Completion | Study terminated before completion |
| Terminated Reason | Toxicity |
| Investigator's Assessment | Eribulin/AC as a standard neoadjuvant therapy in early‐stage breast cancer is not recommended. |
We report the first randomized phase II study comparing eribulin and paclitaxel followed by 5‐fluorouracil, either doxorubicin or epirubicin, and cyclophosphamide (FAC/FEC) as neoadjuvant chemotherapy for HER2‐negative early‐stage breast cancer (Fig. 2). The primary efficacy measure of pathological complete response (pCR) [1, 2, 3] assessed by residual cancer burden showed that eribulin was not superior to paclitaxel based on the interim analysis (pCR rate: 4.8% with eribulin and 26.9% with paclitaxel). Because of this lack of superiority at the interim analysis, the study was closed early.
Figure 2.
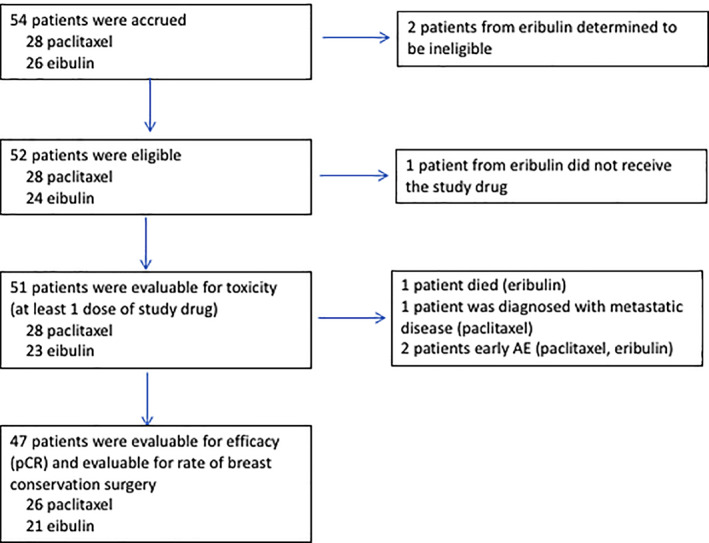
Flow diagram.Abbreviations: AE, adverse event; pCR, pathological complete response.
To our surprise, toxicity was greater in the eribulin arm, which was not expected from the metastatic treatment data [4, 5]. Eribulin is approved in metastatic breast cancer, and the increase in toxicity reported for this drug was mainly attributed to the later line introduction of the therapy. However, in our study in early breast cancer, eribulin was associated with higher toxicity.
Several groups have studied eribulin as part of neoadjuvant systemic therapy regimen, especially in triple‐negative breast cancer. Kaklamani et al. conducted a phase II trial of carboplatin and eribulin as neoadjuvant treatment in patients with early‐stage triple‐negative breast cancer. In this study, the combination of carboplatin and eribulin produced a pCR rate of 43%, with mostly grade 1 and 2 toxic effects [6]. Cadoo et al. conducted a phase II trial of the feasibility (defined as the percentage of patients who completed the regimen) of dose‐dense doxorubicin/cyclophosphamide (AC) followed by eribulin with and without prophylactic filgrastim in patients with stage I–III, HER2‐nonamplified early‐stage breast cancer, and showed that eribulin along with AC combination in neoadjuvant therapy for stage I–III patients was feasible in only 72.9% when pegfilgrastim was used and in only 60% when pegfilgrastim was not used [7]. This is in line with the toxicity that was observed in our study. Kaufman et al. conducted a phase III randomized clinical trial of eribulin or capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane [8]. In that trial, eribulin was not superior to capecitabine in terms of either of the coprimary endpoints: median overall survival (15.9 months for eribulin and 14.5 months for capecitabine; hazard ratio [HR], 088; 95% confidence interval [CI], 0.77–1.00; p = .06) or median progression‐free survival (4.1 months for eribulin and 4.2 months for capecitabine; HR, 1.08; 95% CI, 0.93–1.25; p = .30).
In terms of the type of surgery, we did not observe an improvement in breast conservation surgery. The patients who were treated with eribulin were more likely to undergo mastectomy and less likely to undergo breast conservation surgery; however, the difference was not statistically significant.
In summary, the combination of eribulin and FAC/FEC was not superior to paclitaxel and FAC/FEC and was associated with higher hematological toxicity; therefore, we do not recommend eribulin/FAC/FEC as a standard neoadjuvant therapy in early‐stage breast cancer.
Disclosures
Mariana Chavez‐MacGregor: Roche, Pfizer, AstraZeneca, Novartis, Abbott (C/A), Novartis (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Figures and Tables
Table 2.
Sum of individual adverse events by grade and relationship to study treatment
| Grade and relationship | Number of events | |
|---|---|---|
| Paclitaxel group (n = 28) | Eribulin group (n = 23) | |
| Grade 5 | ||
| Probable | 0 | 1 |
| Total | 0 | 1 |
| Grade 4 | ||
| Definite | 0 | 2 |
| Probable | 0 | 2 |
| Total | 0 | 4 |
| Grade 3 | ||
| Definite | 6 | 4 |
| Probable | 2 | 5 |
| Possible | 1 | 1 |
| Unlikely | 0 | 1 |
| Total | 9 | 11 |
| Grade 2 | ||
| Definite | 35 | 19 |
| Possible | 6 | 2 |
| Probable | 13 | 11 |
| Unlikely | 3 | 4 |
| Unrelated | 2 | 2 |
| Total | 59 | 38 |
| Grade 1 | ||
| Definite | 0 | 8 |
| Probable | 3 | 4 |
| Possible | 0 | 2 |
| Unlikely | 0 | 1 |
| Unrelated | 0 | 1 |
| Total | 3 | 16 |
Table 3.
Severe adverse events by maximum grade experienced a in the paclitaxel and eribulin arms
| SAE | Grade of toxicity, n | |||
|---|---|---|---|---|
| 3 (Severe) | 4 (Life threatening) | 5 (Lethal) | Total | |
| Neutrophil count decreased | ||||
| Paclitaxel | 3 | 0 | 0 | 3 |
| Eribulin | 5 | 3 | 0 | 8 |
| Fatigue | ||||
| Paclitaxel | 2 | 0 | 0 | 2 |
| Eribulin | 1 | 0 | 0 | 1 |
| Alanine aminotransferase increased | ||||
| Paclitaxel | 0 | 0 | 0 | 0 |
| Eribulin | 1 | 0 | 0 | 1 |
| Aspartate aminotransferase increased | ||||
| Paclitaxel | 0 | 0 | 0 | 0 |
| Eribulin | 1 | 0 | 0 | 1 |
| Dizziness | ||||
| Paclitaxel | 0 | 0 | 0 | 0 |
| Eribulin | 1 | 0 | 0 | 1 |
| Left ventricular systolic dysfunction | ||||
| Paclitaxel | 1 | 0 | 0 | 1 |
| Eribulin | 0 | 0 | 0 | 0 |
| Myalgia | ||||
| Paclitaxel | 0 | 0 | 0 | 0 |
| Eribulin | 1 | 0 | 0 | 1 |
| Nasal congestion | ||||
| Paclitaxel | 1 | 0 | 0 | 1 |
| Eribulin | 0 | 0 | 0 | 0 |
| Nausea | ||||
| Paclitaxel | 1 | 0 | 0 | 1 |
| Eribulin | 0 | 0 | 0 | 0 |
| Neutropenic sepsis | ||||
| Taxol | 0 | 0 | 0 | 0 |
| Eribulin | 0 | 0 | 1 | 1 |
| Paresthesia | ||||
| Paclitaxel | 1 | 0 | 0 | 1 |
| Eribulin | 0 | 0 | 0 | 0 |
| Vomiting | ||||
| Paclitaxel | 0 | 0 | 0 | 0 |
| Eribulin | 1 | 0 | 0 | 1 |
| White blood cell decreased | ||||
| Paclitaxel | 0 | 0 | 0 | 0 |
| Eribulin | 0 | 1 | 0 | 1 |
For patients with multiple instances of the same adverse event and different grades at different instances, we counted the adverse event only once and assigned the highest grade experienced for that event.
No grade 4 or 5 adverse events were observed in the paclitaxel group.
If same patient had more than one episode of toxicity observed during study period, each time was counted as one.
Abbreviation: SAE, serious adverse event.
Table 4.
Severe adverse events by maximum grade experienced a
| Arm | Grade of toxicity | Total | |||||
|---|---|---|---|---|---|---|---|
| 1 (Mild) | 2 (Moderate) | 3 (Severe) | 4 (Life threatening) | 5 (Lethal) | |||
| Alopecia | Taxol | 0 | 15 | 0 | 0 | 0 | 15 |
| Alopecia | Eribulin | 3 | 8 | 0 | 0 | 0 | 11 |
| Fatigue | Taxol | 1 | 7 | 2 | 0 | 0 | 10 |
| Fatigue | Eribulin | 2 | 6 | 1 | 0 | 0 | 9 |
| Nausea | Taxol | 1 | 3 | 1 | 0 | 0 | 5 |
| Nausea | Eribulin | 2 | 5 | 0 | 0 | 0 | 7 |
| Neutrophil count decreased | Taxol | 0 | 1 | 3 | 0 | 0 | 4 |
| Neutrophil count decreased | Eribulin | 0 | 0 | 5 | 3 | 0 | 8 |
| Paresthesia | Taxol | 0 | 5 | 1 | 0 | 0 | 6 |
| Paresthesia | Eribulin | 0 | 1 | 0 | 0 | 0 | 1 |
| Skin and subcutaneous tissue disorders | Taxol | 0 | 5 | 0 | 0 | 0 | 5 |
| Skin and subcutaneous tissue disorders | Eribulin | 0 | 2 | 0 | 0 | 0 | 2 |
| Constipation | Taxol | 1 | 3 | 0 | 0 | 0 | 4 |
| Constipation | Eribulin | 1 | 1 | 0 | 0 | 0 | 2 |
| Mucositis oral | Taxol | 0 | 1 | 0 | 0 | 0 | 1 |
| Mucositis oral | Eribulin | 1 | 4 | 0 | 0 | 0 | 5 |
| Myalgia | Taxol | 0 | 1 | 0 | 0 | 0 | 1 |
| Myalgia | Eribulin | 3 | 1 | 1 | 0 | 0 | 5 |
| Diarrhea | Taxol | 0 | 1 | 0 | 0 | 0 | 1 |
| Diarrhea | Eribulin | 1 | 2 | 0 | 0 | 0 | 3 |
| Fever | Taxol | 0 | 1 | 0 | 0 | 0 | 1 |
| Fever | Eribulin | 1 | 2 | 0 | 0 | 0 | 3 |
| Vomiting | Taxol | 0 | 2 | 0 | 0 | 0 | 2 |
| Vomiting | Eribulin | 0 | 1 | 1 | 0 | 0 | 2 |
| Alanine aminotransferase increased | Taxol | 0 | 2 | 0 | 0 | 0 | 2 |
| Alanine aminotransferase increased | Eribulin | 0 | 0 | 1 | 0 | 0 | 1 |
| Nasal congestion | Taxol | 0 | 1 | 1 | 0 | 0 | 2 |
| Nasal congestion | Eribulin | 0 | 0 | 0 | 0 | 0 | 0 |
| Pain | Taxol | 0 | 1 | 0 | 0 | 0 | 1 |
| Pain | Eribulin | 0 | 1 | 0 | 0 | 0 | 1 |
| Rash acneiform | Taxol | 0 | 2 | 0 | 0 | 0 | 2 |
| Rash acneiform | Eribulin | 0 | 0 | 0 | 0 | 0 | 0 |
| White blood cell decreased | Taxol | 0 | 1 | 0 | 0 | 0 | 1 |
| White blood cell decreased | Eribulin | 0 | 0 | 0 | 1 | 0 | 1 |
| Abdominal pain | Taxol | 0 | 0 | 0 | 0 | 0 | 0 |
| Abdominal pain | Eribulin | 1 | 0 | 0 | 0 | 0 | 1 |
| Arthralgia | Taxol | 0 | 1 | 0 | 0 | 0 | 1 |
| Arthralgia | Eribulin | 0 | 0 | 0 | 0 | 0 | 0 |
| Aspartate aminotransferase increased | Taxol | 0 | 0 | 0 | 0 | 0 | 0 |
| Aspartate aminotransferase increased | Eribulin | 0 | 0 | 1 | 0 | 0 | 1 |
| Bladder infection | Taxol | 0 | 0 | 0 | 0 | 0 | 0 |
| Bladder infection | Eribulin | 0 | 1 | 0 | 0 | 0 | 1 |
| Dizziness | Taxol | 0 | 0 | 0 | 0 | 0 | 0 |
| Dizziness | Eribulin | 0 | 0 | 1 | 0 | 0 | 1 |
| Edema limbs | Taxol | 0 | 1 | 0 | 0 | 0 | 1 |
| Edema limbs | Eribulin | 0 | 0 | 0 | 0 | 0 | 0 |
| Headache | Taxol | 0 | 0 | 0 | 0 | 0 | 0 |
| Headache | Eribulin | 0 | 1 | 0 | 0 | 0 | 1 |
| Hyperglycemia | Taxol | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyperglycemia | Eribulin | 1 | 0 | 0 | 0 | 0 | 1 |
| Infections and infestations (other), specify | Taxol | 0 | 0 | 0 | 0 | 0 | 0 |
| Infections and infestations (other), specify | Eribulin | 0 | 1 | 0 | 0 | 0 | 1 |
| Insomnia | Taxol | 0 | 1 | 0 | 0 | 0 | 1 |
| Insomnia | Eribulin | 0 | 0 | 0 | 0 | 0 | 0 |
| Left ventricular systolic dysfunction | Taxol | 0 | 0 | 1 | 0 | 0 | 1 |
| Left ventricular systolic dysfunction | Eribulin | 0 | 0 | 0 | 0 | 0 | 0 |
| Memory impairment | Taxol | 0 | 1 | 0 | 0 | 0 | 1 |
| Memory impairment | Eribulin | 0 | 0 | 0 | 0 | 0 | 0 |
| Nail loss | Taxol | 0 | 1 | 0 | 0 | 0 | 1 |
| Nail loss | Eribulin | 0 | 0 | 0 | 0 | 0 | 0 |
| Neutropenic sepsis | Taxol | 0 | 0 | 0 | 0 | 0 | 0 |
| Neutropenic sepsis | Eribulin | 0 | 0 | 0 | 0 | 1 | 1 |
| Skin infection | Taxol | 0 | 1 | 0 | 0 | 0 | 1 |
| Skin infection | Eribulin | 0 | 0 | 0 | 0 | 0 | 0 |
| Vaginal infection | Taxol | 0 | 0 | 0 | 0 | 0 | 0 |
| Vaginal infection | Eribulin | 0 | 1 | 0 | 0 | 0 | 1 |
| Vaginal inflammation | Taxol | 0 | 1 | 0 | 0 | 0 | 1 |
| Vaginal inflammation | Eribulin | 0 | 0 | 0 | 0 | 0 | 0 |
For patients with multiple instances of the same adverse event and different grades at different instances, we counted the adverse event only once and assigned the highest grade experienced for that event.
No grade 4 or 5 adverse events were observed in the paclitaxel group.
If same patient had more than one episode of toxicity observed during study period, each time was counted as one.
Acknowledgments
We acknowledge the patients who participated in this clinical trial. Eisai Inc. sponsored the trial but did not participate in the design or conduct of the study. We also thank Stephanie Deming of Editing Services, Research Medical Library, MD Anderson Cancer Center, for editing the manuscript.
Footnotes
- ClinicalTrials.gov Identifier: NCT01593020
- Sponsor: Eisai
- Principal Investigator: Vicente Valero
- IRB Approved: Yes
References
- 1. Wu K, Yang Q, Liu Y, Wu A et al. Meta‐analysis on the association between pathologic complete response and triple‐negative breast cancer after neoadjuvant chemotherapy. World J Surg Oncol 2014;12:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bonnefoi H, Litiere S, Piccart M et al. Pathological complete response after neoadjuvant chemotherapy is an independent predictive factor irrespective of simplified breast cancer intrinsic subtypes: A landmark and two‐step approach analyses from the EORTC 10994/BIG 1‐00 phase III trial. Ann Oncol 2014;25:1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cortazar P, Zhang L, Untch M et al. Pathological complete response and long‐term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014;384:164–172. [DOI] [PubMed] [Google Scholar]
- 4. Donoghue M, Lemery SJ, Yuan W et al. Eribulin mesylate for the treatment of patients with refractory metastatic breast cancer: Use of a "physician's choice" control arm in a randomized approval trial. Clin Cancer Res 2012;18:1496–1505. [DOI] [PubMed] [Google Scholar]
- 5. Yoshida T, Ozawa Y, Kimura T et al. Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial‐mesenchymal transition (EMT) to mesenchymal‐epithelial transition (MET) states. Br J Cancer 2014;110:1497–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaklamani VG, Jeruss JS, Hughes E et al. Phase II neoadjuvant clinical trial of carboplatin and eribulin in women with triple negative early‐stage breast cancer (NCT01372579). Breast Cancer Res Treat 2015;151:629–638. [DOI] [PubMed] [Google Scholar]
- 7. Cadoo KA, Kaufman PA, Seidman AD et al. Phase 2 study of dose‐dense doxorubicin and cyclophosphamide followed by eribulin mesylate with or without prophylactic growth factor for adjuvant treatment of early‐stage human epidermal growth factor receptor 2‐negative breast cancer. Clin Breast Cancer 2018;18:433–440.e431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaufman PA, Awada A, Twelves C et al. Phase III open‐label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol 2015;33:594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]


