Abstract
Background
High‐dose (HD) methotrexate (MTX) is an essential component of treatment protocols in acute lymphoblastic leukemia, aggressive lymphoma, and osteosarcoma. However, delayed MTX clearance may lead to life‐threatening toxicities. Administration of supportive therapy for HD‐MTX is complex, and insufficient supportive care increases the risk of MTX toxicity. To improve patient safety, we investigated the implementation of a checklist and urine alkalinization protocol in addition to standard supportive care during HD‐MTX therapy.
Materials and Methods
The intervention included individualized patient checklists for control of adequate supportive care for every HD‐MTX treatment cycle and a urine alkalinization protocol for documentation and guidance during urine alkalinization therapy. The impact of these tools on the rate of adverse events (acute renal injury, delayed MTX clearance) was retrospectively assessed in patients treated from April 2017 to April 2019 (intervention group) and compared with patients treated from January 2015 to March 2017 who received standard supportive care for HD‐MTX according to a standard operating procedure (SOP).
Results
In total, 118 patients received 414 HD‐MTX cycles in the intervention group compared with 108 patients with 332 treatment cycles in the SOP group. Delayed MTX clearance was observed in 2.6% of treatment cycles in the intervention cohort opposed to 15.2% of cycles in the SOP group. The rate of acute kidney injury was also significantly reduced in the intervention group (6.2%. vs. 0.7%). The use of carboxypeptidase as rescue treatment for severe renal impairment and insufficient MTX clearance was necessary in five cases in the SOP group and in only two cycles within the intervention group.
Conclusion
The use of standardized documentation for supportive care during HD‐MTX therapy is recommended to minimize the risk of adverse events.
Implications for Practice
High‐dose methotrexate (HD‐MTX) is a commonly used treatment in several cancer types. Distinct supportive measures are necessary to minimize the risk of HD‐MTX side effects, which can be life‐threatening. Supportive care consists of certain examinations and interventions before starting HD‐MTX and permanent alkalinization of the urine, as this greatly increases the elimination of MTX and decreases the risk of kidney injury. After implementing a checklist for control of supportive care and a urine alkalinization protocol to optimize urine alkalinization, a significant decrease of side effects was observed in comparison to the standard of care; therefore, the use of a safety checklist and alkalinization protocol is recommended for all patients who receive HD‐MTX.
Keywords: HD‐MTX, Methotrexate, Checklist, Carboxypeptidase, Supportive care
Short abstract
Supportive measures are necessary to minimize the risk of side effects from high‐dose methotrexate (HD‐MTX) treatment. This article reports the findings of a study that investigated the implementation of a checklist for control of supportive care and a urine alkalinization protocol to optimize urine alkalinization.
Introduction
High‐dose (HD) methotrexate (MTX; defined as MTX dose >500 mg/m2) has been used for several decades as an important backbone in treatment protocols for acute lymphoblastic leukemia, osteosarcoma, and lymphoma [1, 2, 3]. MTX acts as folate antimetabolite and inhibits DNA synthesis by blocking the enzyme dihydrofolate reductase. High doses of MTX are feasible by using folinic acid (syn, leucovorin, the reduced form of folic acid) to reduce the toxic effects upon nonmalignant cells as a “leucovorin rescue.”
Notable toxicities of HD‐MTX are mucositis, myelosuppression, renal failure, liver injury, and neurotoxicity [4]. The risk of HD‐MTX toxicity is especially increased in the case of therapy‐induced renal failure because impaired renal function leads to reduced MTX renal clearance, which is the major route (90%) of MTX elimination [5]. Prolonged exposure to toxic MTX levels can induce severe myelosuppression and mucositis and is a life‐threatening condition. Avoidance of renal injury and the consecutive risk of impaired MTX clearance is therefore paramount to prevent MTX toxicity.
HD‐MTX administration demands specialized supportive care to prevent adverse events. This includes strict urine alkalinization and hyperhydration to increase MTX elimination and to prevent kidney damage as well as administration of leucovorin to reduce mucositis and myelosuppression [4, 6].
Urine alkalinization has been identified as a crucial aspect of MTX clearance and prevention of renal toxicity. At a urine pH of 5, the solubility of MTX is very low, and it can precipitate within renal tubuli, which induces severe kidney damage with the consequence of acute renal failure. In some cases, this condition will require hemodialysis. By raising the urine pH from 5 to 7.5, solubility of MTX increases about 20‐fold [4, 5, 6, 7]. Therefore, permanent and effective urine alkalinization and hyperhydration are absolutely mandatory for safe HD‐MTX treatment.
As MTX can accumulate in third‐space fluids like ascites and pleural effusions, thereby leading to prolonged MTX clearance due to distribution processes, the presence of such third‐space fluids prior to HD‐MTX needs to be ruled out. This is usually performed by sonography.
Furthermore, pharmacokinetic interactions between several drugs (i.e., proton‐pump inhibitors, β‐lactam antibiotics, nonsteroidal anti‐inflammatory drugs like indomethacin and naproxen) and MTX have been previously identified as reason for delayed MTX elimination and subsequent toxicity [4, 8, 9, 10, 11]. Avoidance of such interactions is another important part of supportive care during HD‐MTX therapy.
Even slight deviations from optimal supportive therapy may lead to profound toxicities of HD‐MTX. Therefore, strict adherence to supportive measures during HD‐MTX is vital for patient safety. As HD‐MTX is often used in curative treatment settings (i.e., acute lymphoblastic leukemia, lymphoma), toxicity of HD‐MTX needs to be minimized to prevent treatment delays as this may impair the overall prognosis.
We hypothesized that supportive care for HD‐MTX can be significantly improved by implementing standardized protocols for all relevant elements of supportive treatment.
Materials and Methods
Additional tools for supportive care during HD‐MTX therapy including a checklist for supportive care and a urine alkalinization protocol were implemented in our clinic, a tertiary comprehensive cancer center.
The checklist contains several elements of supportive care and treatment preparation during HD‐MTX (Fig. 1). Important items of the checklist are sonographic detection of third‐space fluids, correct intravenous fluid administration, proper urine alkalinization before the start of MTX infusion, preparation of a leucovorin rescue protocol, and evaluation of possible pharmacokinetic interactions.
Figure 1.

Checklist for high‐dose MTX therapy. Abbreviation: ASS, acetylsalicyclic acid; MTX, methotrexate; NSAID, nonsteroidal anti‐inflammatory drug.
A protocol for measurement and recording of urine pH values as well as documentation of alkalinization therapy was designed to ensure proper urinary alkalinization (Fig. 2).
Figure 2.
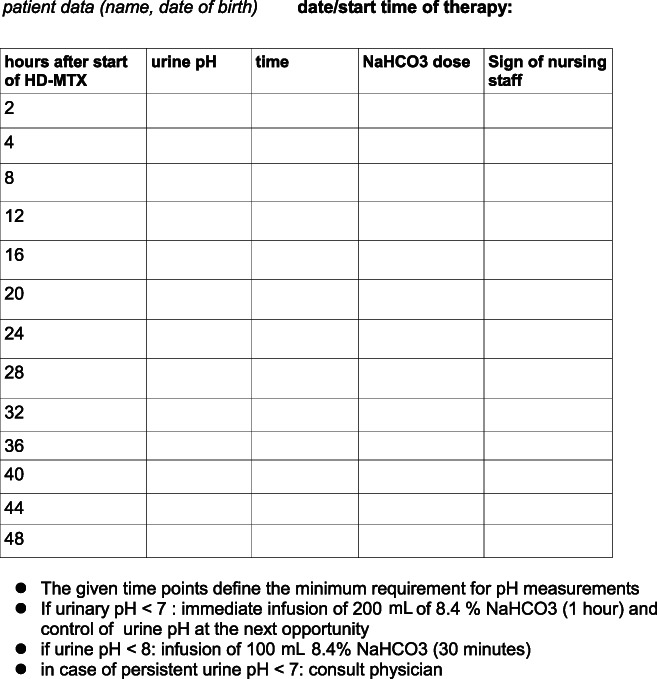
Protocol for urine pH measurement and urine alkalinization during HD‐MTX. Abbreviations: HD‐MTX, high‐dose methotrexate.
The use of both the checklist and the alkalinization protocol was implemented in all patients receiving HD‐MTX starting in April 2017. These two documents were individually used for every single HD‐MTX treatment course in every patient. Both physicians and the nursing staff were responsible for using this documentation system. To assess the efficacy of this intervention, treatment data of 118 patients receiving HD‐MTX from April 2017 to April 2019 were analyzed (intervention group).
Prior to implementation of this standardized documentation, supportive care for HD‐MTX in our clinic was performed as recommended by international guidelines [4, 6], with modifications according to local practice following a dedicated standard operating procedure (SOP). This included urine alkalinization and hyperhydration, leucovorin rescue, detection of third‐space fluids, and avoidance of pharmacokinetic interactions. Urine alkalinization was achieved by intravenous application of sodium bicarbonate, and a urine pH of 8 was defined as target value. Fluid administration and leucovorin rescue were performed according to standardized chemotherapy treatment protocols. Documentation of supportive care was done in electronic patient records but was not standardized.
Predefined adverse events (acute kidney injury, prolongation of MTX clearance) were retrospectively assessed in a cohort of 108 patients treated from January 2015 to March 2017. In this control group, supportive care was delivered following the SOP but without standardized documentation tools.
Delayed MTX clearance was defined as failure to reach prespecified thresholds (<0.2 μmol/L at 72 hours after therapy) and if prolonged leucovorin rescue was necessary.
Acute kidney injury was defined according to the AKIN classification [12]. The threshold for definition of acute kidney injury was AKIN stage 1. By definition, this finding is made in patients with an absolute serum creatinine increase of 0.3 mg/dL or greater, an increase of 1.5‐fold or greater above the baseline serum creatinine, or onset of oliguria (urine output <0.5 mL/kg per hour lasting 6–12 hours).
For statistical comparison of adverse events between the treatment groups, the Fisher‐Yates test was used. A p value < .05 was defined as statistically significant.
This study was conducted after review by the local ethics committee of the Hamburg chamber of physicians, Germany (Ref. no. WF106‐20).
Results
In the intervention group, 118 patients were treated with 414 cycles of HD‐MTX. The SOP group consisted of 108 patients who received 332 cycles of HD‐MTX (Table 1). The most common indications for HD‐MTX in both groups were central nervous system lymphoma (43 patients in both groups), acute lymphoblastic leukemia (36 patients in the intervention and 23 in the SOP group), and non‐Hodgkin lymphoma (16 patients in the intervention and 18 in the SOP group).
Table 1.
Characteristics of SOP and intervention groups and frequency of adverse events
| Characteristic | SOP group | Intervention group | p value |
|---|---|---|---|
| No. of patients | 108 | 118 | |
| No. of treatment cycles | 332 | 414 | |
| Mean age (range), yr | 53 (18–85) | 52 (18–83) | |
| Burkitt lymphoma, n | 16 | 19 | |
| CNS lymphoma, n | 43 | 43 | |
| Osteosarcoma, n | 8 | 2 | |
| Choriocarcinoma, n | 0 | 2 | |
| Acute lymphoblastic leukemia, n | 23 | 36 | |
| Non‐Hodgkin Lymphoma, n | 18 | 16 | |
| MTX dose, mean (range), g/m2 | 2.7 (0.5–12) | 2.1 (0.5–12) | |
| No. of cycles with delayed MTX clearance (%) | 51 (15.2) | 11 (2.6) | <.001 |
| Acute kidney injury, n (%) | 21 (6.2.) | 3 (0.7) | <.001 |
|
No. of cycles with use of carboxypeptidase (%) |
5 (1.47) | 2 (0.48) | .25 |
Abbreviations: CNS, central nervous system; MTX, methotrexate; SOP, standard operating procedure.
The average MTX dose per treatment cycle was 2.7 g/m2 in the SOP group and 2.1 g/m2 in the intervention group. All patients with acute lymphoblastic leukemia received HD‐MTX as a continuous infusion over 24 hours, whereas in all other indications, MTX was administered as an infusion over 3–4 hours.
In 51 of 332 HD‐MTX cycles (15.2%) within the SOP group, MTX clearance was delayed. In contrast, delayed MTX clearance was only observed in 11 of 414 treatment cycles of the intervention group (2.6%, p < .001)
Acute kidney injury occurred in 6.3% of cycles (n = 21) within the SOP group and in only 0.7% of cycles (n = 3) in the intervention group (p < .001).
One patient with acute renal failure in the SOP group underwent hemodialysis. In all patients experiencing acute kidney injury, kidney damage was reversible and did not induce chronic renal impairment.
HD‐MTX therapy was permanently discontinued because of toxicity in five patients of the SOP group and in one patient of the intervention group. Five patients of the SOP group and two patients within the intervention group received carboxypeptidase because of acute renal failure and insufficient MTX clearance. The indication for carboxypeptidase was evaluated according to international recommendations [6, 13]. There were no treatment‐related deaths.
A review of potential reasons for complications in the SOP group by analysis of all treatment cycles with acute kidney injury and/or delayed MTX clearance revealed potential pharmacokinetic drug–drug interactions in 17 treatment cycles and inadequate control of urine alkalinization in 5 treatment cycles.
In the 21 treatment cycles of the SOP group that resulted in acute kidney injury, a potential drug–drug interaction was present in eight cases and inadequate urine alkalinization was present in five cycles. Other causes for nephrotoxicity were one case of tumor lysis syndrome and two patients with pretherapeutic renal impairment.
In the intervention cohort, administration of potentially interacting drugs could be successfully avoided. Specifically, in the three cases of acute kidney injury in the intervention group, no interfering medication was given. Table 2 summarizes the analysis of underlying causes for acute kidney injury in both treatment groups.
Table 2.
Analysis of patients with acute kidney injury after high‐dose MTX
| Potential reason for MTX toxicity | Intervention group (n = 3) | SOP group (n = 21) |
|---|---|---|
| Concomitant medication | 0 | 8 |
| Tumor lysis syndrome | 0 | 1 |
| Urinary retention due to bladder obstruction | 1 | 0 |
| Insufficient urine alkalinization | 0 | 5 |
| Preexisting renal impairment | 0 | 2 |
| Unknown | 2 | 12 |
Abbreviations: MTX, methotrexate; SOP, standard operating procedure.
Discussion
Herein, we demonstrate that standardized documentation protocols for supportive care significantly reduce the rate of adverse events during HD‐MTX therapy by using a simplified and structured guidance consisting of only two single‐page documents (checklist and urine alkalinization protocol). Implementation of these measures was performed with high acceptance by physicians and nurses, as both the checklist and the alkalinization protocol were successfully applied in all patients within the intervention group for every treatment course.
Even slight deviations in supportive care may lead to acute kidney failure and subsequent toxicities of HD‐MTX. In clinical practice, strict adherence to these requirements can be challenging, and errors may occur. Optimal supportive care has been previously demonstrated to reduce the risk of HD‐MTX toxicity [4, 6, 13, 14].
Pharmacokinetic interactions between concomitant medications and MTX with significantly delayed MTX elimination have been previously described [8, 9, 10, 11, 15]. Therefore, avoidance of such pharmacokinetic interactions is imperative to administer HD‐MTX safely. The use of a checklist containing information on potential pharmacokinetic interactions can assist in preventing these adverse events.
For instance, we observed a case of acute renal failure necessitating hemodialysis in our SOP group after intravenous administration of a single dose of 40 mg pantoprazole only a few hours after infusion of HD‐MTX. As this example demonstrates, supportive care prior, during, and after HD‐MTX administration has to follow strict rules to avoid serious toxicity.
The important role of pharmacokinetic interactions for HD‐MTX toxicity is also supported by the finding of potentially interacting medication in about one third of the 21 cycles leading to acute kidney injury within the SOP group. In contrast, no patient within the intervention group had received interacting medications.
Previously, the rate of renal failure after HD‐MTX despite adequate, nonstandardized supportive care was reported to be 1.8% [6]. In another retrospective analysis in patients with lymphoma receiving HD‐MTX, the rate of renal injury was about 9% [16]. The reason for these different findings are presumably patient‐related factors like age and comorbidities.
In our intervention group, the overall rate of any degree of acute kidney injury (according to the AKIN classification) could be reduced from 6.3% (SOP group) to 0.7%. This finding demonstrates the high efficacy of standardized supportive care documentation to prevent acute renal failure during HD‐MTX treatment. The low rate of nephrotoxicity in our intervention group also shows the importance of effective and continuous urine alkalinization to prevent kidney damage during HD‐MTX treatment. The crucial role of urine alkalinization for adequate HD‐MTX clearance was also reported by other studies [7, 14].
The use of a standardized urine alkalinization protocol is an effective approach to optimize urine alkalinization during HD‐MTX therapy.
We decided to use a checklist for control of supportive care because previously, the usage of checklist‐based interventions was proven to be highly effective for improving the quality of routine clinical care and to ensure adherence to guidelines in clinical practice [17]. The low rate of adverse events in our intervention group demonstrates that the checklist‐based control of supportive care can improve the safety of HD‐MTX therapy. As outlined before, the most important element of this checklist is presumably the prevention of pharmacokinetic drug–drug interactions.
Another advantage of the checklist‐based documentation for supportive care during HD‐MTX therapy is that this approach enables a comprehensive, transparent overview of all important supportive care measures. Thus, the risk of insufficient supportive care is minimized while clinicians and nurses require less time and effort to control whether all necessary preparations for HD‐MTX are present.
Regarding the risk profile for adverse events in the SOP and intervention groups, the average age in both groups was about 53 years. The average MTX dose was slightly higher in the SOP cohort (2.7 vs. 2.1 g/m2). There was also a higher proportion of patients receiving 24‐hour infusional MTX within the intervention cohort. However, although both lower MTX dose and longer infusion times may reduce the overall risk of MTX toxicity, insufficient supportive care is still a major risk factor for adverse events in such circumstances. In comparison with the findings in our intervention group, other studies have reported comparable or even significantly higher rates of acute kidney injury for HD‐MTX in patients with childhood acute lymphoblastic leukemia [18, 19].
Limitations of our study are its retrospective, nonrandomized design and the evaluation within a single treatment center. However, our intervention of standardized supportive care documentation for HD‐MTX was effective in a broad, nonselected real‐world patient population, which warrants confirmation in further prospective clinical trials.
Our drug–drug interaction list did not include tyrosine kinase inhibitors like imatinib and dasatinib. However, it has been recently reported that such drugs may reduce MTX clearance and should therefore also be avoided during HD‐MTX treatment [20, 21].
Conclusion
The use of a standardized, checklist‐based documentation for supportive care significantly improves the safety of HD‐MTX treatment. These tools are able to minimize the risk of pharmacokinetic drug–drug interactions and insufficient urine alkalinization, leading to a low rate of adverse events. We therefore highly recommend implementation of such adjunctive measures for all patients receiving HD‐MTX. In our experience, the use of a checklist and urine alkalinization protocol is very efficient and can be easily implemented into clinical practice.
Author Contributions
Conception/design: Winfried H. Alsdorf, Panagiotis Karagiannis, Claudia Langebrake, Carsten Bokemeyer, Christian Frenzel
Provision of study material or patients: Winfried H. Alsdorf, Claudia Langebrake, Carsten Bokemeyer
Collection and/or assembly of data: Claudia Langebrake, Winfried Alsdorf, Christian Frenzel
Data analysis and interpretation: Winfried H. Alsdorf, Panagiotis Karagiannis, Claudia Langebrake, Carsten Bokemeyer, Christian Frenzel
Manuscript writing: Winfried H. Alsdorf, Claudia Langebrake, Carsten Bokemeyer
Final approval of manuscript: Winfried H. Alsdorf, Panagiotis Karagiannis, Claudia Langebrake, Carsten Bokemeyer, Christian Frenzel
Disclosures
Panagiotis Karagiannis: Abbvie Inc. (OI); Carsten Bokemeyer: Sanofi Aventis, Novartis, Merck Sharpe & Dohme, Bristol‐Myers Squibb, Eli Lilly & Co/Imclone, GSO Hamburg, AOK Health Insurance, Merck Darmstadt, Roche, Bayer Healthcare (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Rowe JR, Goldstone AH. How I treat acute lymphocytic leukemia in adults. Blood 2007;110:2268–2275. [DOI] [PubMed] [Google Scholar]
- 2. Marina NM, Smeland S, Bielack S et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high‐grade osteosarcoma (EURAMOS‐1): An open‐label, international, randomised controlled trial. Lancet Oncol 2016;17:1396–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferreri AJ, Cwynarski K, Pulczynski E et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: Results of the first randomisation of the International Extranodal Lymphoma Study Group‐32 (IELSG32) phase 2 trial. Lancet Haematol 2016;3:e217–e227. [DOI] [PubMed] [Google Scholar]
- 4. Howard SC, Mccormick J et al. Preventing and managing toxicities of high dose methotrexate. The Oncologist 2016;21:1471–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bleyer W. Methotrexate: Clinical pharmacology, current status and therapeutic guidelines. Cancer Treat Rev 1977;4:87–101. [DOI] [PubMed] [Google Scholar]
- 6. Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. The Oncologist 2006;11:694–703. [DOI] [PubMed] [Google Scholar]
- 7. Mir O, Ropert S, Babinet A et al. Hyper‐alkalinizaton without hyper‐hydration for the prevention of high‐dose methotrexate acute nephrotoxicity in patients with osteosarcoma. Cancer Chemother Pharmacol 2010;66:1059–1063. [DOI] [PubMed] [Google Scholar]
- 8. Suzuki K, Doki K, Homma M et al. Coadministration of proton pump inhibitors delays elimination of plasma methotrexate in high‐dose methotrexate therapy. Br J Clin Pharmacol 2009;67:44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Miguel D, García‐Suárez J, Martín Y et al. Severe acute renal failure following high‐dose methotrexate therapy in adults with haematological malignancies: A significant number results from unrecognized co‐administration of several drugs. Nephrol Dial Transplant. 2008; 23:3762–3766. [DOI] [PubMed] [Google Scholar]
- 10. Santucci R, Levêque D, Kemmel V et al. Severe intoxication with methotrexate possibly associated with concomitant use of proton pump inhibitors. Anticanc Res 2010;30:693–695. [PubMed] [Google Scholar]
- 11. Zarychanski R, Wlodarczyk K, Ariano R et al. Pharmacokinetic interaction between methotrexate and piperacillin/tazobactam resulting in prolonged toxic concentrations of methotrexate. J Antimicrob Chemother 2006;58:228–230. [DOI] [PubMed] [Google Scholar]
- 12. Lopes JA, Jorge S. The RIFLE and AKIN classifications for acute kidney injury: A critical and comprehensive review. Clin Kid J 2013;6:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ramsey LB, Balis FM, O'Brien MM et al. Consensus guideline for use of glucarpidase in patients with high‐dose methotrexate induced acute kidney injury and delayed methotrexate clearance. The Oncologist 2018;23:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Relling MV, Fairclough D, Ayers D et al. Patient characteristics associated with high‐risk methotrexate concentrations and toxicity. J Clin Oncol 1994;12:1667–1672. [DOI] [PubMed] [Google Scholar]
- 15. Drost SA, Wentzell JR, Giguere P et al. Outcomes associated with reducing the urine alkalinization threshold in patients receiving high‐dose methotrexate. Pharmacotherapy 2017;37:684–691. [DOI] [PubMed] [Google Scholar]
- 16. May J, Carson KR, Butler S et al. High incidence of methotrexate associated renal toxicity in patients with lymphoma: A retrospective analyis. Leuk Lymphoma 2014;55:1345–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Provonost P, Needham D, Berenholtz S et al. An intervention to decrease catheter‐related bloodstream infections in the ICU. New Engl J Med 2006;355:2725–2732. [DOI] [PubMed] [Google Scholar]
- 18. Christensen AM, Pauley JL, Molinelli AR et al. Resumption of high‐dose methotrexate after acute kidney injury and glucarpidase use in pediatric oncology patients. Cancer 2012;118:4321–4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cheng DH, Lu H, Liu TT et al. Identification of risk factors in high‐dose methotrexate‐induced acute kidney injury in childhood acute lymphoblastic leukemia. Chemotherapy 2018;63:101–107. [DOI] [PubMed] [Google Scholar]
- 20. Ramsey LB, Mizuno T, Vinks AA et al. Delayed methotrexate clearance in patients with acute lymphoblastic leukemia concurrently receiving dasatinib. Pediatr Blood Cancer 2019;66:e27618. [DOI] [PubMed] [Google Scholar]
- 21. Pommert L, Liberio N, Ng JS et al. Concurrent imatinib dosing with high‐dose methotrexate leads to acute kidney injury and delayed methotrexate clearance in pediatric patients with philadelphia chromosome‐positive B‐cell acute lymphoblastic leukemia. J Pediatr Hematol Oncol 2020. [Epub head of print]. [DOI] [PubMed] [Google Scholar]


