Abstract
Lessons Learned
Axitinib exhibited marginal activity against gemcitabine‐refractory unselected biliary tract cancer.
Pretreated soluble vascular endothelial growth factor receptor‐2 may be a useful biomarker for axitinib treatment outcome.
Ascites should be carefully monitored in patients receiving anti–vascular endothelial growth factor receptor therapy including axitinib in advanced biliary tract cancer.
Background
There are no clear options for second‐line treatment in patients with gemcitabine (GEM)‐refractory biliary tract cancer (BTC). We conducted a multicenter, single‐arm, phase II trial to confirm the efficacy and safety of axitinib, a potent selective inhibitor of vascular endothelial growth factor receptor (VEGFR)‐1/2/3, in patients with GEM‐refractory BTC.
Methods
Patients refractory or intolerant to GEM‐based chemotherapy were enrolled. Axitinib was administered orally at an initial dose of 5 mg twice daily. The primary endpoint was progression‐free survival (PFS), and the threshold and expected values were set at 2 and 3 months, respectively. The target sample size was 32 patients.
Results
Nineteen patients were enrolled. The trial was interrupted for a total of 13 months for the evaluation of adverse events. Thirteen patients were previously treated with ≥2 regimens. The median PFS was 2.8 months (95% confidence interval [CI]: 2.1–4.1). The median overall survival was 5.8 months (95% CI: 3.3–9.7). The response rate was 5.3% (95% CI: 0.0–15.3). Grade 3 ascites occurred in two patients. Baseline soluble VEGFR‐2 levels were significantly associated with PFS.
Conclusion
Axitinib exhibited marginal activity against GEM‐refractory BTC. Ascites should be carefully monitored in axitinib‐treated patients with advanced BTC.
Keywords: Axitinib, Gemcitabine, Biliary tract cancer, Chemotherapy refractory
Discussion
This study was the first clinical trial to evaluate the efficacy and safety of axitinib in patients with GEM‐refractory BTC. This study met the primary endpoint at the lower limit of 95% CI for a median PFS of 2.1 months (Fig. 1A), which was superior to the 2.0 months of the null hypothesis, with a response rate (RR) of 5.3% and median overall survival (OS) of 5.8 months (Fig. 1B). However, we consider that axitinib exhibited marginal activity against GEM‐refractory unselected BTC.
Figure 1.
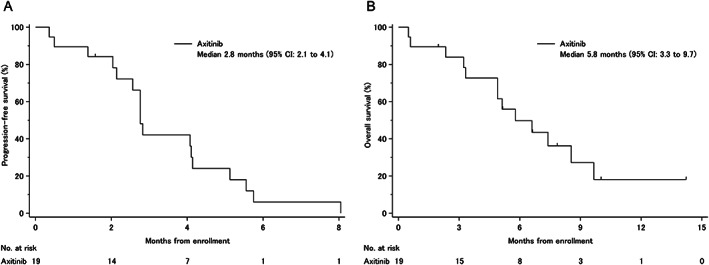
Kaplan‐Meier curves. (A): Progression‐free survival. (B): Overall survival.Abbreviation: CI, confidence interval.
In addition, this study provides important insights about biomarker analyses. Pretreated high soluble VEGFR (sVEGFR)‐2 levels were significantly associated with longer PFS. In a phase II study in Japanese patients with cytokine‐refractory metastatic renal cell carcinoma, greater reductions of sVEGFR‐2 were associated with high RR and longer PFS. Considering the mechanism of action of axitinib, sVEGFR‐2 may have an important role in patients treated with axitinib.
Although multikinase inhibitors targeting VEGFR, such as sorafenib, sunitinib, and pazopanib, previously showed modest efficacy in BTC [1, 2, 3], we hypothesized that axitinib could have greater activity, owing to stronger inhibition of VEGFR‐1/2/3, the half maximal inhibitory concentration for axitinib being 10‐fold lower than for the other kinase inhibitors [4]. However, the current study showed similar efficacy compared with other molecular targeted therapies. VEGF promotes immune suppression in the tumor microenvironment. In a preclinical study, axitinib reprogramed the immune suppressive environment; thus, the antitumor effect of axitinib not only is due to direct antiangiogenic effects but also involves important effects on tumor immunity. Axitinib plus immune checkpoint inhibitors have significantly improved the PFS compared with sunitinib for first‐line treatment in patients with advanced renal cell carcinoma. These results suggest that axitinib should be studied in combination with immune checkpoint inhibitors in selected patients with advanced BTC that exhibit high levels of sVEGFR‐2.
We found that ascites was often observed as an important adverse event (AE) in the present study. Ascites of more than moderate degree rapidly worsened in four patients. Additionally, a limited amount of ascites was observed in four patients, and peritoneal dissemination might have already developed at study enrollment in some of them. Furthermore, all four patients had gallbladder cancer (GBC) and were previously treated with two or more regimens. The indication of sequential chemotherapy should be carefully considered in patients with GBC, ascites, and multiple previous treatments. Conversely, ascites possibly related to axitinib were observed in two patients. Clinicians should be attentive to the onset of ascites in patients treated with anti‐VEGFR therapy.
Axitinib exhibited marginal activity against GEM‐refractory unselected BTC. Ascites should be carefully monitored in patients receiving anti‐VEGFR therapy including axitinib in advanced BTC. Baseline sVEGFR‐2 may be a useful biomarker for axitinib treatment outcome. Further development of new regimens is required for the treatment of patients with GEM‐refractory BTC.
Trial Information
| Disease | Biliary tract: Gallbladder cancer and cholangiocarcinoma |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | 1 prior regimen |
| Type of Study | Phase II, single arm |
| Primary Endpoint | Progression‐free survival |
| Secondary Endpoints | Overall survival, overall response rate, safety, correlative endpoint |
| Additional Details of Endpoints or Study Design | |
| Study design: This study was an open‐label, multicenter, single‐arm phase II trial from six participating Japanese institutions. Written informed consent was obtained from all patients before enrollment, and the study protocol was approved by the institutional review board of each participating institution. The study was conducted in accordance with the Declaration of Helsinki. | |
| Patients: The inclusion criteria for enrollment in this study were as follows: patients diagnosed with recurrent or unresectable biliary tract cancer including intrahepatic cholangiocarcinoma, extrahepatic cholangiocarcinoma, gallbladder cancer, and ampulla of Vater cancer, with histologically confirmed adenocarcinoma; age ≥20 years; Eastern Cooperative Oncology Group Performance Status of 0 or 1; refractory or intolerant to gemcitabine‐based chemotherapy; ≥1 measurable lesion; no evidence of brain metastasis; no evidence of moderate or more ascites and/or pleural effusion; and no evidence of pre‐existing uncontrolled hypertension. In addition, patients had preserved organ functions: white blood cell count ≥3,000/mm3, neutrophil count ≥1,500/mm3, hemoglobin level ≥8.0 g/dL, platelet count ≥100,000/mm3, serum creatinine level ≤1.5 mg/dL, serum total bilirubin level ≤2 mg/dL (≤3 mg/dL in patients with biliary drainage), and serum aspartate transaminase and alanine transaminase levels ≤75 IU/L (≤150 IU/L in patients with biliary drainage), and urinary protein level ≤1+ by the dipstick test or <2 g/day. Finally, patients had a life expectancy of ≥3 months and were willing to provide written informed consent. The main exclusion criteria were as follows: active hemorrhagic ulcer or active diverticulitis; surgery within 28 days prior to enrollment in the study; radiation therapy within 14 days prior to enrollment in the study; serious gastrointestinal disorder; serious complications, such as cardiac disease, renal disease, uncontrolled diabetes mellitus; active infections; pregnant or lactating women; and use of drugs known to be potent inhibitors or inducers of cytochrome P450 isoenzyme 4A. | |
| Study treatment: Axitinib was administered orally at an initial dose of 5 mg twice daily. Dose escalation of axitinib was allowed, increasing to 7 or 10 mg orally twice daily, in patients if tolerated. Treatment was continued until disease progression, emergence of intolerable adverse events (AEs), or patient refusal to continue the treatment. Up to two dose‐level modifications to 3 or 2 mg orally twice daily owing to uncontrolled nonhematological grade 3 AEs were also allowed. Dose interruption was also allowed for uncontrolled grade 4 AEs, and axitinib was restarted on dose decrease when the patients recovered to grade 2 or less. The above criteria were for AEs except hypertension and proteinuria. In patients who developed hypertension during treatment (systolic blood pressure >150 mmHg or diastolic blood pressure >100 mmHg), antihypertensive treatment was started, or if the patients were under antihypertensive treatment, doses of antihypertensive drugs were increased or other drugs were added. For patients who had persistent hypertension despite maximum antihypertensive treatment, the axitinib dose was reduced. In patients who developed hypertension during treatment (systolic blood pressure >160 mmHg or diastolic blood pressure >105 mmHg), treatment interruption and antihypertensive treatment adjustment were performed. After blood pressure recovered (systolic blood pressure <150 mmHg or diastolic blood pressure <100 mmHg), axitinib was restarted with a dose reduction. For patients with proteinuria of 2+ or more measured by dipstick, quantitative assessment was performed by 24‐hour urine collection. If the patients had proteinuria of >3.5 g/24 hours, treatment was interrupted, and after the patients recovered to ≤3.5 g/24 hours, axitinib was restarted at the same dosage or dose decrease under the investigator's judgment. | |
| Assessment: Physical examination, including blood pressure measurement and laboratory tests (blood biochemistry tests and urine tests), was performed at least every 2 weeks. Furthermore, thyroid hormone levels were examined every 4 weeks for 2 months after starting treatment and every 6 weeks thereafter. Computed tomography or magnetic resonance imaging was performed every 6 weeks for the evaluation of the treatment efficacy. The exploratory analyses of potential plasma biomarkers for axitinib were performed by SRL Inc. (Tokyo, Japan). Enzyme‐linked immunosorbent assay kits for testing levels of plasma biomarkers including VEGF‐A, VEGF‐C, VEGF‐D, and sVEGFR‐2 from R&D System (Minneapolis, MN, USA), sVEGFR‐1 from Roche Diagnostics (Basel, Switzerland), and sVEGFR‐3 from IBL (Gunma, Japan) were measured at baseline and 1 month after treatment, in accordance with the manufacturer's instructions. | |
| Statistical analysis: Considering the median PFS with S‐1 for second‐line chemotherapy was 2.5 months, the null hypothesis for the threshold and the alternative hypothesis for the expected median PFS were set as 2 months and 3 months, respectively. A sample size of at least 30 patients was estimated as the requirement for the study to have a one‐sided significance level of 5% and a power of 73%. Considering dropouts, the target sample size was set as 32 patients in this study. | |
| For PFS and OS, survival curves were estimated using the Kaplan‐Meier method and compared using the log‐rank test. A Cox proportional hazards model was used to estimate hazard ratios. All statistical analyses were performed using SAS Release 9.4 (SAS Institute, Cary, NC). Statistically significant differences in plasma biomarker levels between baseline and 1 month after treatment were determined by the Wilcoxon signed‐rank test. Baseline plasma biomarkers were divided into high group and low group using median values. | |
| Investigator's Analysis | Evidence of target inhibition but no or minimal antitumor activity |
Drug Information
| Drug 1 | |
| Generic/Working Name | Axitinib |
| Trade Name | Inlyta |
| Company Name | Pfizer |
| Drug Type | Small molecule |
| Drug Class | Angiogenesis ‐ VEGF |
| Dose | 5 mg per flat dose |
| Route | p.o. |
| Schedule of Administration | 5 mg orally twice daily with dose adjustments based on tolerability |
Patient Characteristics
| Number of Patients, Male | 10 |
| Number of Patients, Female | 9 |
| Stage | Metastatic/recurrent: 10/9 |
| Age | Median (range): 67 (44–80) years |
| Number of Prior Systemic Therapies | Median (range): 2 (1–3) |
| Performance Status: ECOG |
0 — 10 1 — 9 2 — 3 — Unknown — |
| Primary tumor site, n (%) |
Gallbladder, 9 (47%) Extrahepatic bile duct, 6 (32%) Intrahepatic bile duct, 4 (21%) |
| Presence of ascites, n (%) | Yes/No: 5 (26%)/14 (74%) |
| Prior chemotherapy, n (%) |
First line: 19 GEM + cisplatin, 15 (79%) GEM + S‐1, 1 (5%) GEM monotherapy, 1 (5%) Other, 2 (11%) |
| Second line: 13 |
S‐1 monotherapy, 6 (46%) GEM + cisplatin, 2 (15%) GEM + S‐1, 2 (15%) Other, 3 (23%) |
| Third line: 1 | Clinical trial: 1 (100%) |
| Cancer Types or Histologic Subtypes | Adenocarcinoma, 19 |
Primary Assessment Method: Progression‐Free Survival
| Number of Patients Screened | 19 |
| Number of Patients Enrolled | 19 |
| Number of Patients Evaluable for Toxicity | 19 |
| Number of Patients Evaluated for Efficacy | 19 |
| Evaluation Method | RECIST 1.1 |
| (Median) Duration Assessments PFS | 2.8 months, CI: 2.1–4.1 |
| Outcome Notes | The primary endpoint was met, because the lower limit of the 95% CI was superior to the null hypothesis of 2.0 months. |
Secondary Assessment Method: Overall Survival and Overall Response Rate
| Number of Patients Screened | 19 |
| Number of Patients Enrolled | 19 |
| Number of Patients Evaluable for Toxicity | 19 |
| Number of patients Evaluated for Efficacy | 19 |
| Evaluation Method | RECIST 1.1 |
| Response Assessment CR | n = 0 (0%) |
| Response Assessment PR | n = 1 (5.3%) |
| Response Assessment SD | n = 14 (73.7%) |
| Response Assessment PD | n = 1 (5.3%) |
| Response Assessment OTHER | n = 3 (15.8%) |
| (Median) Duration Assessments OS | 5.8 months, CI: 3.3–9.7 |
| Outcome Notes | The tumor responses were evaluated in 16 patients. The RR was 5.3% (95% CI: 0.0–15.3); 12 patients showed shrinkage of target lesions from baseline (Fig. 2). |
Adverse Events
| All Cycles | |||||||
|---|---|---|---|---|---|---|---|
| Name | NC/NA | 1 | 2 | 3 | 4 | 5 | All grades |
| Anemia | 11% | 58% | 26% | 5% | 0% | 0% | 89% |
| Platelet count decreased | 31% | 53% | 16% | 0% | 0% | 0% | 69% |
| White blood cell decreased | 84% | 11% | 5% | 0% | 0% | 0% | 16% |
| Neutrophil count decreased | 84% | 5% | 11% | 0% | 0% | 0% | 16% |
| Hypertension | 10% | 11% | 37% | 42% | 0% | 0% | 90% |
| Anorexia | 37% | 26% | 32% | 5% | 0% | 0% | 63% |
| Fatigue | 63% | 21% | 11% | 5% | 0% | 0% | 37% |
| Palmar‐plantar erythrodysesthesia syndrome | 68% | 16% | 11% | 5% | 0% | 0% | 32% |
| Mucositis oral | 74% | 5% | 16% | 5% | 0% | 0% | 26% |
| Nausea | 79% | 21% | 0% | 0% | 0% | 0% | 21% |
| Diarrhea | 85% | 5% | 5% | 5% | 0% | 0% | 15% |
| Vomiting | 89% | 11% | 0% | 0% | 0% | 0% | 11% |
| Dysgeusia | 89% | 11% | 0% | 0% | 0% | 0% | 11% |
| Alkaline phosphatase increased | 0% | 37% | 32% | 26% | 5% | 0% | 100% |
| Hypoalbuminemia | 0% | 53% | 42% | 5% | 0% | 0% | 100% |
| Hyperglycemia | 10% | 56% | 17% | 17% | 0% | 0% | 90% |
| Aspartate aminotransferase increased | 21% | 47% | 11% | 21% | 0% | 0% | 79% |
| Alanine aminotransferase increased | 32% | 42% | 5% | 21% | 0% | 0% | 68% |
| Proteinuria | 32% | 26% | 37% | 5% | 0% | 0% | 68% |
| Hyperkalemia | 58% | 32% | 5% | 5% | 0% | 0% | 42% |
| Blood bilirubin increased | 63% | 5% | 16% | 16% | 0% | 0% | 37% |
| Hypocalcemia | 74% | 26% | 0% | 0% | 0% | 0% | 26% |
| Creatinine increased | 79% | 11% | 5% | 5% | 0% | 0% | 21% |
| Hypercalcemia | 79% | 21% | 0% | 0% | 0% | 0% | 21% |
| Hypokalemia | 84% | 16% | 0% | 0% | 0% | 0% | 16% |
| Adverse Events Legend | |||||||
| The median dose was 8.75 (2.44–9.44) mg/day. No patient required dose escalation. Seven patients (37%) needed at least one dose reduction, which was due to hypertension in three patients and to hypertension and proteinuria, oral mucositis, malaise, and palmar‐plantar erythrodysesthesia syndrome in one patient each, respectively. Although the most common treatment‐related grade 3/4 AE was hypertension (42%), this AE was controllable. There was no treatment‐related death owing to close follow‐up, appropriate dose interruption, and/or dose decrease. The data collection cutoff date was August 2018. At the time of data cutoff, all patients discontinued protocol treatment, and 13 of 19 (68%) patients had died. Reasons for protocol treatment discontinuation were disease progression in 13 patients (68%), AEs in 4 patients (21%), patient refusal associated with AE in 1 patient (5%), and death due to gastrointestinal bleeding because of complications of disease progression in one patient (5%), respectively. This death was evaluated as not treatment related based on results of postmortem pathology. One patient had missing data for blood glucose determination. Abbreviation: NC/NA, no change from baseline/no adverse event. | |||||||
Serious Adverse Events
| Name | Grade | Attribution |
|---|---|---|
| Gastrointestinal bleeding | 5 | Unrelated |
| Ascites | 3 | Possible |
| Ascites | 3 | Possible |
| Ascites | 3 | Unrelated |
| Ascites | 3 | Unrelated |
| Jaundice | 3 | Unrelated |
| Serious Adverse Events Legend | ||
| Ascites of more than moderate entity was observed in four patients during the study treatment. These four patients died as a result of tumor progression: three patients had early death, which was defined as death within 30 days after completing the study treatment, and one patient without early death experienced rapidly increasing ascites during study treatment. Two of the four patients were determined to have experienced ascites as a treatment‐related grade 3 AE on discussion with the Data and Safety Monitoring Committee. Among the two patients presenting treatment‐related grade 3 ascites (one patient experienced early death, and the other patient did not), one patient had evidence of positive ascites cytology, but the AE was attributed to be possibly due to axitinib, because the degree of increasing ascites exceeded the assumption of increasing ascites as tumor progression. The patient died of liver failure due to progression of liver metastasis. In the other patient without early death, ascites was possibly due to axitinib because of negative ascites cytology. Once the patient's condition had recovered by palliative treatment for ascites, he ultimately died as a result of tumor progression. For the other two patients, ascites was attributed to peritoneal dissemination due to tumor progression, and both also died from progression. All four patients had GBC with ascites at enrollment, and three of these patients had previously undergone two or three chemotherapeutic regimens. | ||
Pharmacokinetics/Pharmacodynamics
Plasma biomarkers at baseline and 1 month after treatment were evaluated in 16 patients. Axitinib was associated with significant increases in VEGF‐A and decreases in sVEGFR‐1/2/3 (Table 1). Of the baseline biomarkers, high sVEGFR‐2 and sVEGFR‐3 were significantly associated with longer PFS and OS, respectively. High sVEGFR‐3 and low VEGF‐D tended to increase with PFS, and high sVEGFR‐2 and low VEGF‐D tended to increase with OS (Table 2).
Table 1.
Plasma biomarkers at baseline and at 1 month after treatment (n = 16)
| Biomarleftker | Baseline, median (range) | 1 month later, median (range) | Change amount, median (range) | p value |
|---|---|---|---|---|
| VEGF‐A, pg/mL | 61.5 (30 to 251) | 133.5 (49 to 395) | 48.0 (–22 to 311) | <.001 |
| VEGF‐C, pg/mL | 3,075.0 (1,680 to 5,870) | 3,080.0 (1,990 to 7,140) | –290.0 (–2,030 to 5,350) | .140 |
| VEGF‐D, pg/mL | 394.5 (320 to 647) | 446.5 (325 to 675) | 31.0 (–152 to 278) | .130 |
| VEGFR‐1, pg/mL | 514.0 (340 to 800) | 405.5 (293 to 592) | –92.0 (–273 to 71) | .002 |
| VEGFR‐2, pg/mL | 8,195.0 (6,450 to 11,100) | 5,240.0 (4,020 to 7,410) | –2,730.0 (–5,060 to –850) | <.001 |
| VEGFR‐3, pg/mL | 57.6 (37.6 to 89.4) | 35.5 (21.6 to 54.8) | –20.8 (–34.6 to –3.2) | <.001 |
Abbreviations: VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Table 2.
Association between baseline plasma biomarkers and survival (n = 16)
| Biomarker | Baseline | Median PFS (95% CI), months | Log‐rank p value | Median OS (95% CI), months | Log‐rank p value |
|---|---|---|---|---|---|
| VEGF‐A, pg/mL | High | 2.8 (1.4–4.1) | .102 | 5.5 (2.3–9.7) | .144 |
| Low | 4.1 (2.0–5.7) | 7.4 (4.9–NA) | |||
| VEGF‐C, pg/mL | High | 2.8 (2.0–4.1) | .777 | 7.4 (3.3–NA) | .331 |
| Low | 3.4 (1.4–5.6) | 5.8 (2.3–8.5) | |||
| VEGF‐D, pg/mL | High | 2.7 (1.4–4.1) | .095 | 5.0 (2.3–9.7) | .076 |
| Low | 4.1 (2.8–5.6) | 8.5 (3.2–NA) | |||
| VEGFR‐1, pg/mL | High | 4.1 (2.1–5.1) | .806 | 8.5 (3.2–NA) | .513 |
| Low | 2.8 (1.4–5.7) | 6.2 (2.3–9.7) | |||
| VEGFR‐2, pg/mL | High | 4.1 (2.6–5.7) | .028 | NA (3.3–NA) | .081 |
| Low | 2.8 (1.4–4.1) | 5.3 (2.3–8.5) | |||
| VEGFR‐3, pg/mL | High | 4.1 (2.6–5.6) | .125 | 8.5 (3.2–NA) | .047 |
| Low | 2.8 (1.4–4.1) | 5.3 (2.3–7.4) |
Abbreviations: CI, confidence interval; NA, not available; OS, overall survival; PFS, progression‐free survival; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Assessment, Analysis, and Discussion
| Completion | Study terminated before completion |
| Terminated Reason | Did not fully accrue |
| Investigator's Assessment | Evidence of target inhibition but no or minimal antitumor activity |
Overexpression of vascular endothelial growth factor (VEGF) has been identified as an important indicator of poor prognosis in patients with advanced biliary tract cancer (BTC) [5, 6]. Axitinib, an orally administered selective inhibitor of VEGF receptor (VEGFR)‐1/2/3, strongly inhibits VEGFR [4]. We have previously reported the promising efficacy of axitinib against gemcitabine (GEM)‐refractory BTC in a preclinical study and our clinical experience in five patients [7, 8].
This study was the first clinical trial to evaluate the efficacy and safety of axitinib in patients with GEM‐refractory BTC. As there are currently no clear second‐line treatment options, this study was designed as single‐arm trial, and the primary endpoint threshold and expected progression‐free survival (PFS) were set based on historical data [9]. As a result, this study met the primary endpoint at the lower limit of 95% confidence interval for a median PFS of 2.1 months, which was superior to the 2.0 months of the null hypothesis, with a response rate (RR) of 5.3% and median overall survival (OS) of 5.8 months.
Although this study had planned to enroll 32 patients, we did not extend the enrollment period after 19 patients. Although we did not perform integrated analysis, the efficacy of axitinib based on the clinical experience of a total 24 patients, including five patients from a previous study [8], treated with this drug was marginal. We considered that this marginal efficacy would not change, even if the remaining 13 patients were enrolled in this study. Furthermore, as mentioned below, the development of VEGF inhibition and immune checkpoint inhibitor combination therapy is expected to be introduced for patients with selected GEM‐refractory BTC such as renal cell carcinoma.
Recently, the ABC‐06 trial, conducted in the U.K., was the first randomized phase III trial comparing FOLFOX (5‐fluorouracil, folinic acid, and oxaliplatin) plus active symptom control (ASC) and ASC alone in patients with GEM plus cisplatin (GC)‐refractory BTC. FOLFOX demonstrated an improved OS as the primary endpoint in the trial. However, the improvement in OS was only of approximately 1 month (6.2 months vs. 5.3 months) [10]. In addition, in two large retrospective studies, fluoropyrimidine‐platinum or fluoropyrimidine‐based combination chemotherapy has not shown any survival benefit after GC or GEM‐platinum combination chemotherapy [11, 12]. Therefore, development of second‐line treatment in patients with advanced BTC is urgently needed.
Although multikinase inhibitors targeting VEGFR such as sorafenib, sunitinib, and pazopanib showed modest efficacy in BTC [1, 2, 3], axitinib more strongly inhibits VEGFR‐1/2/3, as the half maximal inhibitory concentration for axitinib is 10‐fold lower than for these kinase inhibitors [4]. However, the current study showed similar efficacy compared with other molecular targeted therapies [1, 2, 3, 13, 14, 15, 16, 17, 18].
This study provides important findings about biomarker analyses. In the current study, pretreated soluble VEGFR (sVEGFR)‐2 and sVEGFR‐3 levels may be predictive biomarkers of axitinib treatment outcome. High sVEGFR‐2 levels were significantly associated with longer PFS, and high sVEGFR‐3 levels were significantly associated with longer OS. In a phase II study in Japanese patients with cytokine‐refractory metastatic renal cell carcinoma, greater reductions of sVEGFR‐2 were associated with high RR and longer PFS [19]. In addition, plasma biomarker analyses confirmed the antiangiogenic effects of axitinib in GEM‐refractory BTC because sVEGFR‐1/2/3 decreased after administration of axitinib. Considering the mechanism of action of axitinib, the results of our study, and those of a phase II trial of metastatic renal cell carcinoma, sVEGFR‐2 may have an important role in patients treated with axitinib.
Conversely, immune checkpoint inhibitor monotherapy has shown limited efficacy in previously treated BTC, with an RR of 3.3%–22%, median PFS of 1.4–3.7 months, and median OS of 5.2–14.2, respectively [20, 21, 22]. Importantly, the usefulness of programmed death ligand‐1 expression as a predictive biomarker has been controversial in patients with previously treated BTC [20, 21, 22]. According to a review of the cancer‐immunity cycle, VEGF promotes immune suppression in the tumor microenvironment [23]. In a preclinical study, axitinib reprogramed the immune suppressive environment; thus, the antitumor effect of axitinib not only is due to direct antiangiogenic effects but also may involve important effects on tumor immunity [24]. Indeed, axitinib plus immune checkpoint inhibitors have significantly improved the PFS compared with sunitinib for first‐line treatment in patients with advanced renal cell carcinoma [25, 26]. These results suggest that the development of axitinib plus immune checkpoint inhibitors against selected advanced BTC, which have high sVEGFR‐2 and/or sVEGFR‐3, will be used in the future.
In addition, we found that ascites was often observed as an important adverse event in the present study. Ascites of more than moderate degree rapidly worsened in four patients, and on consultation with the investigators, the Data and Safety Monitoring Committee meeting was held to investigate the causes. A limited amount of ascites was observed in the four patients, and peritoneal dissemination might have already developed at study enrollment in some of them. Furthermore, all four patients had gallbladder cancer (GBC) and were previously treated with two or more regimens. Patients with advanced GBC with peritoneal dissemination are known to have an extremely poor prognosis [27]. The indication of sequential chemotherapy should be carefully considered in patients with GBC, ascites, and multiple previous treatments. Conversely, ascites possibly related to axitinib was observed in two patients. Clinicians should be attentive to the onset of ascites in patients treated with anti‐VEGFR therapy.
There were some limitations in this study. First, the sample size was small; only 19 patients were enrolled despite the planned enrollment of 32 patients. Second, previous treatments varied with two to three regimens and 13 of 19 patients had previously received two regimens at least, because the S‐1 containing regimen was available in Japan.
In conclusion, axitinib exhibited marginal activity against GEM‐refractory unselected BTC. Ascites should be carefully monitored in patients receiving anti‐VEGFR therapy including axitinib in advanced BTC. Baseline sVEGFR‐2, in particular, and sVEGFR‐3 may be useful biomarkers for axitinib treatment outcome. Further development of new regimens is required for the treatment of selected patients with GEM‐refractory BTC.
Disclosures
Naohiro Okano: Taiho Pharmaceutical, Eli Lilly Japan, Eisai, Bayer Yakuhin, Chugai Pharmaceutical (H); Junji Furuse: Ono Pharmaceutical, Fuji Film, Merck Sharp & Dohme, Taiho Pharmaceutical, Takara Bio, Chugai Pharma, Astellas Pharma, AstraZeneca, Merck Bio, Mudi Pharma, Onco Therapy Science (C/A), Bayer, Ono Pharmaceutical, Eisai, Eli Lilly Japan, Merck Sharp & Dohme, Yakult Honsha, Chugai Pharma, Astellas Pharma, Novartis, AstraZeneca, Kyowa Hakko Kirin, Pfizer, Daiichi Sankyo, Takeda, Taiho Pharmaceutical, Sanofi, EA pharma, Mylan EPD (H), Ono Pharmaceutical, Merck Sharp & Dohme, Merck Bio, J‐Pharma, Eisai, Taiho Pharmaceutical, Takeda, Chugai Pharma, AstraZeneca, Daiichi Sankyo, Yakult Honsha, Mochida (RF); Makoto Ueno: Taiho Pharmaceutical, Yakult Honsha, AstraZeneca, Ono Pharmaceutical, Merck Biopharma, Merck Sharp & Dohme (H), Taiho Pharmaceutical, Daiichi Sankyo, Eisai, AstraZeneca, Ono Pharmaceutical, Merck Sharp & Dohme, Merck Biopharma, Dainippon Sumitomo Pharma, Incyte, Yakult Honsha, Astellas (RF); Satoshi Kobayashi: Taiho Pharmaceutical, Bayer (H); Fumio Nagashima: Takeda Pharmaceutical Co. LTD, Chugai Pharmaceutical Co., Ltd., Yakult Honsha Co., Ltd. (H), Astellas Pharma Inc, AstraZeneca, Eisai, Merck Sharp & Dohme, Ono Pharmaceutical Co., J‐Pharma, Daiichi Sankyo Company Co., Sumitomo Dainippon Pharma Co., Taiho Pharmaceutical Co., Takeda Pharmaceutical Co. LTD, Chugai Pharmaceutical Co., Ltd, Merck Biopharma Co., Mochida Pharmaceutical Co., Ltd., Yakult Honsha Co., Ltd. (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Figure and Tables
Figure 2.
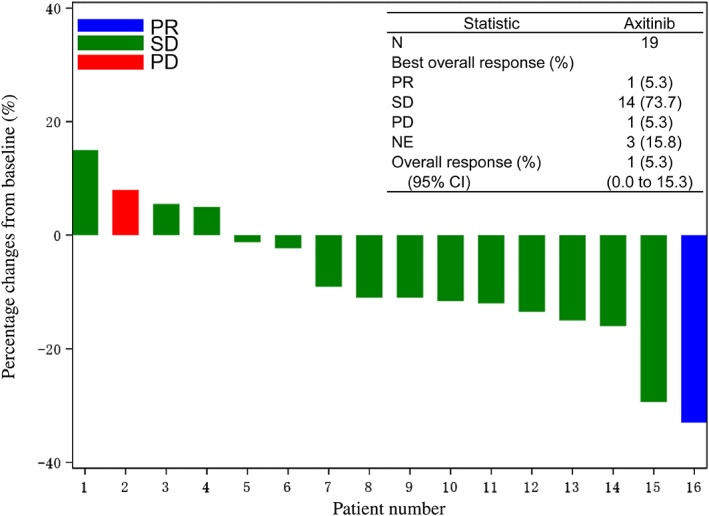
Waterfall plot of change from baseline to the maximum tumor shrinkage of the target lesions.Abbreviations: CI, confidence interval; NE, not evaluated; PD, progressive disease; PR, partial response; SD, stable disease.
Acknowledgments
This research was partially supported by the Practical Research for Innovative Cancer Control from the Japan Agency for Medical Research and Development, AMED. Axitinib was provided by Pfizer. We thank Hirofumi Fujii, Akira Fukutomi, and Shoji Nakamori of the Data and Safety Monitoring Committee. We also thank Akemi Egami for her support in study management.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
- Trial Identifier: UMIN000023014
- Sponsors: Japan Agency for Medical Research and Development, AMED
- Principal Investigator: Junji Furuse
- IRB Approved: Yes
References
- 1. Bengala C, Bertolini F, Malavasi N et al. Sorafenib in patients with advanced biliary tract carcinoma: A phase II trial. Br J Cancer 2010;102:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yi JH, Thongprasert S, Lee J et al. A phase II study of sunitinib as a second‐line treatment in advanced biliary tract carcinoma: A multicentre, multinational study. Eur J Cancer 2012;48:196–201. [DOI] [PubMed] [Google Scholar]
- 3. Shroff RT, Yarchoan M, O'Connor A et al. The oral VEGF receptor tyrosine kinase inhibitor pazopanib in combination with the MEK inhibitor trametinib in advanced cholangiocarcinoma. Br J Cancer 2018;118:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Escudier B, Gore M. Axitinib for the management of metastatic renal cell carcinoma. Drugs R D 2011;11:113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hida Y, Morita T, Fujita M et al. Vascular endothelial growth factor expression is an independent negative predictor in extrahepatic biliary tract carcinomas. Anticancer Res 1999;19:2257–2260. [PubMed] [Google Scholar]
- 6. Yoshikawa D, Ojima H, Iwasaki M et al. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer 2008;98:418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takahashi H, Ojima H, Shimizu H et al. Axitinib (AG‐013736), an oral specific VEGFR TKI, shows potential therapeutic utility against cholangiocarcinoma. Jpn J Clin Oncol 2014;44:570–578. [DOI] [PubMed] [Google Scholar]
- 8. Okano N, Kasuga A, Kawai K et al. Axitinib for gemcitabine‐refractory advanced biliary tract cancer: Report of 5 cases. Anticancer Res 2017;37:3711–3715. [DOI] [PubMed] [Google Scholar]
- 9. Suzuki E, Ikeda M, Okusaka T et al. A multicentre phase II study of S‐1 for gemcitabine‐refractory biliary tract cancer. Cancer Chemother Pharmacol 2013;71:1141–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lamarca A, Palmer DH, Wasan HS et al. ABC‐06: A randomised phase III, multi‐centre, open‐label study of active symptom control (ASC) alone or ASC with oxaliplatin/5‐FU chemotherapy (ASC+mFOLFOX) for patients (pts) with locally advanced/metastatic biliary tract cancers (ABC) previously‐treated with cisplatin/gemcitabine (CisGem) chemotherapy. J Clin Oncol 2019;37(suppl 15):4003a. [Google Scholar]
- 11. Brieau B, Dahan L, De Rycke Y et al. Second‐line chemotherapy for advanced biliary tract cancer after failure of the gemcitabine‐platinum combination: A large multicentre study by the Association des Gastro‐Enterologues Oncologues. Cancer 2015;121:3290–3297. [DOI] [PubMed] [Google Scholar]
- 12. Kim BJ, Yoo C, Kim KP et al. Efficacy of fluoropyrimidine‐based chemotherapy in patients with advanced biliary tract cancer after failure of gemcitabine plus cisplatin: Retrospective analysis of 321 patients. Br J Cancer 2017;116:561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lamarca A, Hubner RA, David Ryder W et al. Second‐line chemotherapy in advanced biliary cancer: A systematic review. Ann Oncol 2014;25:2328–2338. [DOI] [PubMed] [Google Scholar]
- 14. Bekaii‐Saab T, Phelps MA, Li X et al. Multi‐institutional phase II study of selumetinib in patients with metastatic biliary cancers. J Clin Oncol 2011;29:2357–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goyal L, Zheng H, Yurgelun MB et al. A phase 2 and biomarker study of cabozantinib in patients with advanced cholangiocarcinoma. Cancer 2017;123:1979–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ikeda M, Ioka T, Fukutomi A et al. Efficacy and safety of trametinib in Japanese patients with advanced biliary tract cancers refractory to gemcitabine. Cancer Sci 2018;109:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sun W, Patel A, Normolle D et al. A phase 2 trial of regorafenib as a single agent in patients with chemotherapy‐refractory, advanced, and metastatic biliary tract adenocarcinoma. Cancer 2019;125:902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim RD, McDonough S, El‐Khoueiry AB et al. Randomised phase II trial (SWOG S1310) of single agent MEK inhibitor trametinib versus 5‐fluorouracil or capecitabine in refractory advanced biliary cancer. Eur J Cancer 2020;130:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tomita Y, Uemura H, Fujimoto H et al. Key predictive factors of axitinib (AG‐013736)‐induced proteinuria and efficacy: A phase II study in Japanese patients with cytokine‐refractory metastatic renal cell carcinoma. Eur J Cancer 2011;47:2592–2602. [DOI] [PubMed] [Google Scholar]
- 20. Ueno M, Ikeda M, Morizane C et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: A non‐randomised, multicentre, open‐label, phase 1 study. Lancet Gastroenterol Hepatol 2019;4:611–621. [DOI] [PubMed] [Google Scholar]
- 21. Piha‐Paul SA, Oh DY, Ueno M et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE‐158 and KEYNOTE‐028 studies. Int J Cancer 2020;147:2190–2198. [DOI] [PubMed] [Google Scholar]
- 22. Kim RD, Chung V, Alese OB et al. A phase 2 multi‐institutional study of nivolumab for patients with advanced refractory biliary tract cancer. JAMA Oncol 2020;6:888–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen DS, Mellman I. Oncology meets immunology: The cancer‐immunity cycle. Immunity 2013;39:1–10. [DOI] [PubMed] [Google Scholar]
- 24. Du Four S, Maenhout SK, De Pierre K et al. Axitinib increases the infiltration of immune cells and reduces the suppressive capacity of monocytic MDSCs in an intracranial mouse melanoma model. Oncoimmunology 2015;4:e998107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Motzer RJ, Penkov K, Haanen J et al. Avelumab plus axitinib versus sunitinib for advanced renal‐cell carcinoma. N Engl J Med 2019;380:1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rini BI, Plimack ER, Stus V et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal‐cell carcinoma. N Engl J Med 2019;380:1116–1127. [DOI] [PubMed] [Google Scholar]
- 27. Furuse J, Okusaka T, Ohkawa S et al. A phase II study of uracil‐tegafur plus doxorubicin and prognostic factors in patients with unresectable biliary tract cancer. Cancer Chemother Pharmacol 2009;65:113–120. [DOI] [PubMed] [Google Scholar]


