Abstract
Background
Proportionate female representation in health research is necessary for scientific rigor and health equity. We aimed to assess the representation of women in clinical trials leading to U.S. Food and Drug Administration (FDA) cancer drug approvals.
Materials and Methods
Trials supporting FDA cancer drug approvals between July 2008 and June 2018 were sourced from PubMed and ClinicalTrials.gov. The ratio of female to male trial enrollment was compared with cancer incidence and mortality in the U.S. using International Agency for Research on Cancer data. Reproductive tract and breast cancers were excluded. Odds ratios (ORs) and 95% confidence intervals (CIs) comparing trial enrollment with population incidence and mortality were calculated.
Results
A total of 186 trials leading to 170 FDA cancer drug approvals showed slight female underrepresentation compared with overall cancer incidence in the U.S. (OR, 0.97; 95% CI, 0.95–0.98, p < .0001). Female enrollment for drugs approved between 2008–2013 and 2014–2018 was unchanged (OR, 1.02; 95% CI, 0.99–1.05, p = .25). There was slight female underrepresentation in hematological trials (OR, 0.95; 95% CI, 0.91–0.998; p = .040 for leukemia; OR, 0.95; 95% CI, 0.90–0.997; p = .040 for lymphoma) and significant female underrepresentation in colorectal (OR, 0.72; 95% CI, 0.69–0.76; p < .0001), pancreas (OR, 0.85; 95% CI, 0.78–0.93; p = .0004), lung (OR, 0.77; 95% CI, 0.75–0.80; p < .0001), kidney (OR, 0.63; 95% CI, 0.60–0.67; p < .0001), and thyroid cancer trials (OR, 0.26; 95% CI, 0.23–0.28; p < .0001) compared with U.S. incidence.
Conclusion
Female underrepresentation has persisted within solid organ tumor trials but is less notable in hematologic trials. Additional work is required to identify drivers of such disparity.
Implications for Practice
Adequate gender representation in clinical trials is a matter of health equity. This study demonstrates that women remain underrepresented in trials across hematological and solid organ trials compared with cancer incidence and mortality in women, with the disparity worse in a number of solid organ tumor types. There are thus still significant improvements to be made regarding adequate representation of women in trials. Studies exploring the reasons for ongoing disparity in gender representation are warranted to help clinicians to rectify this.
Keywords: Clinical trial, Neoplasms, Sex, Healthcare disparities
Short abstract
Disparities in health care have come into sharp focus recently. This article assesses female representation in clinical trials and reports on the effect of policy changes to address underrepresentation and whether females were adequately represented within clinical trials that have recently led to FDA cancer drug approvals.
Background
Over the past 40 years, there has been significant progression in policy surrounding accrual of women onto clinical trials. In 1977, the U.S. Food and Drug Administration (FDA) guidance initially recommended women of childbearing potential be excluded from early phase clinical studies [1]. This was reversed in 1993, and The American Congress enacted the National Institutes of Health (NIH) Revitalization Act in the same year to encourage representation of women and minority groups in health research [2]. Since the year 2000, the FDA has had the authority to place trials on hold if persons were excluded purely because of reproductive potential [3].
Underrepresentation of women has important biologic implications. In 1997, 8 of 10 drugs withdrawn by the FDA were withdrawn because of greater toxicity in women [4], and by the following year, new drug applications had to present efficacy and safety data by sex [5]. Sex‐based differences are seen in pharmacodynamics and pharmacokinetics [6, 7, 8], with a recent study demonstrating increased toxicity in women receiving adjuvant fluoropyrimidine‐based chemotherapy for colon cancer [9]. Sex differences are also seen in tumor biology [6], as well as within innate and adaptive immune pathways [10]. The microbiome, which exhibits sexual dimorphism, informs inflammatory, innate immune and adaptive immune pathways [11, 12] and therefore responses to chemotherapy [13] and immunotherapy [14]. Sexual dimorphism in immune response and microbiota may thus lead to different treatment responses and adverse event profiles between men and women [15].
Identifying and measuring disparities in health are the first steps toward achieving health equity [16]. Studies of randomized controlled or prospective clinical trials published in the early 2000s demonstrated ongoing sex‐based disparities in trial enrollment [17, 18, 19, 20]. There has been increasing attention on sex disparity and efforts to improve representation since then. Disparities in health have come into sharp focus recently, within the U.S. and globally. The question remains: has the policy intent of proportional sex representation in health research been realized in practice? We therefore designed a study to assess whether women were adequately represented more recently within clinical trials that have led to FDA cancer drug approvals over a contemporaneous 10‐year span.
Design and Methods
FDA cancer drug approvals and the respective pivotal trials that formed the basis of approval are chronicled on an online database as per information presented in the sponsors’ applications for the agency's review process [21]. Approvals between July 2008 and June 2018 were collated. The trial reports supporting these approvals were then sourced from PubMed [22] and the NIH trial registry, ClinicalTrials.gov [23]. Tumor types that were sex specific were excluded from analyses; these were defined as cancers of the reproductive tract and breast cancer (because of rarity in men). The proportion of female and male patients enrolled in each trial was recorded. Rates of enrollment by sex across all included trials was compared between the first and second half of the period reviewed. The ratio of female to male (female/male) enrollment was compared with female/male proportions for incidence and mortality data by crude number in 2018 across the entire U.S. population, as per data from the International Agency for Research on Cancer (IARC) [24].
We performed a sensitivity analysis using two population comparisons within the IARC: very high–human development index (HDI) countries and the global population. HDI is a composite measure of life expectancy, education, and income. Very high–HDI countries include Canada, Australia, and northern and western European countries. It was chosen because it represents the broader population in which international trial enrollment commonly occurs. Trials were subgrouped by common tumor types within the U.S. where IARC data on incidence and mortality were available.
Comparisons between female trial participation, population incidence, and population mortality were performed using Fisher's exact test or χ2 with Yates’ correction for larger samples. Odds ratios (ORs) for female trial enrollment with 95% confidence intervals (CIs) were assessed using the Baptista‐Pike method or Woolf logit interval for larger samples. The p values were two‐sided and considered statistically significant when unadjusted p < .05. Statistical analyses were performed using GraphPad Prism, v8.2.1 (La Jolla, CA).
Results
Female/Male Enrollment Across All trials
Baseline approval and trial characteristics have been reported previously [25]. Of 228 reported trials leading to 204 FDA cancer drug approvals involving 108 different drugs between July 2008 and June 2018, there were 222 trials involving 109,987 patients in which information on sex of participants was available (Table 1). Excluding 36 sex‐specific trials and 145 patients from 10 trials who had missing sex data, there were 186 trials associated with 170 FDA drug approvals, enrolling 78,840 patients. A total of 31,743 (40.3%) of these were female. Lung cancer trials accounted for the largest proportion of patients (17,079), and thyroid cancer trials accounted for the smallest proportion (1,486).
Table 1.
Proportion of women and menenrolled on trials leading to U.S. Food and Drug Administration cancer drug approvals between July 2008 and June 2018
| Cancer type | Trials, n | Women enrolled, n (%) | Men enrolled, n (%) | Total enrolled, n |
|---|---|---|---|---|
| All cancers a | 222 | 51,847 (47.2) | 57,995 (52.8) | 109,842 |
| All cancers, sex‐specific excluded | 186 | 31,743 (40.3) | 47,097 (59.7) | 78,840 |
| Leukemia | 28 | 3,431 (40.4) | 5,054 (59.6) | 8,485 |
| Lymphoma | 27 | 3,079 (43.7) | 3,965 (56.3) | 7,044 |
| Multiple myeloma | 11 | 2,330 (43.2) | 3,065 (56.8) | 5,395 |
| Lung b | 35 | 6,877 (40.3) | 10,202 (59.7) | 17,079 |
| Colorectal | 8 | 2,235 (39.9) | 3,362 (60.1) | 5,597 |
| Pancreas c | 4 | 863 (43.6) | 1,115 (56.4) | 1,978 |
| Kidney | 11 | 1,529 (27.3) | 4,062 (72.7) | 5,591 |
| Bladder | 6 | 559 (28.9) | 1,373 (71.1) | 1,932 |
| Melanoma | 18 | 4,175 (42.0) | 5,760 (58.0) | 9,935 |
| Thyroid | 5 | 670 (45.1) | 816 (54.9) | 1,486 |
145 patients and 6 trials with missing sex data have been excluded.
There were no small cell lung cancer trials in this trial cohort.
Includes adenocarcinoma and neuroendocrine tumors of the pancreas.
Of the 170 non sex‐specific FDA drug approvals, 74 of those approvals were drawn from trials that did not include sex in their assessment of treatment response in their main publication. Thirty‐eight of 66 hematological drug approvals involving 10,013 (46%) of 21,816 hematologic patients within the cohort and 35 of 104 solid organ approvals involving 15,970 (28%) of 57,024 patients with solid tumors were based on trials results that did not demonstrate an assessment of the effect of sex on treatment response. There were thus 25,983 patients, of which 11,595 (44.6%) were female, involved in approvals for whom efficacy by sex were unavailable.
Distribution of female and male enrollment in non–sex‐specific cancer trials across the study period is shown in Figure 1. A total of 35,008 patients were enrolled in trials leading to FDA approvals for cancer drugs between 2008 and 2013; 14,174 (40.5%) were female. A total of 17,569 (40.1%) of the 43,832 patients enrolled between 2014 and 2018 were female. The rate of female trial enrollment between these two periods was unchanged (OR, 1.02; 95% CI, 0.99–1.05; p = .25). Across all non–sex‐specific cancers, there was underrepresentation of women across trials compared with both U.S. incidence (OR, 0.97; 95% CI, 0.95–0.98; p < .0001) and U.S. mortality (OR, 0.91; 95% CI, 0.90–0.93; p < .0001).
Figure 1.
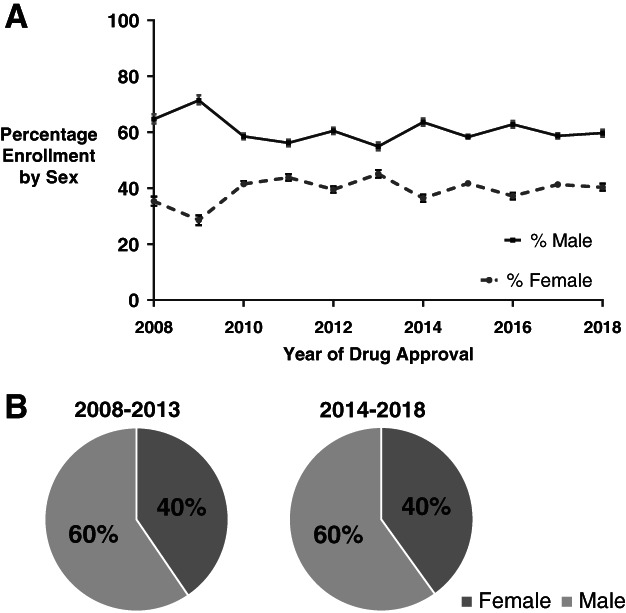
Distribution of female and male trial enrollment between July 2008 and June 2018. (A): Distribution of female and male enrollment for trials leading to cancer drug approvals between July 2008 and June 2018. Sex‐specific cancers have been excluded. (B): Distribution of female and male enrollment in trials associated with drug approvals that occurred between 2008 and 2013 and 2014 and 2018 did not differ (odds ratio, 1.02; 95% confidence interval, 0.99–1.05; p = .25).
Results for comparisons with the two international reference populations can be found in supplemental online Tables 2 and 3 and supplemental online Figures 1 and 2. Women were overrepresented when trial enrollment was compared with cancer mortality for very high–HDI countries (OR, 1.02; 95% CI, 1.01–1.04; p = .0023) and compared with global incidence and global mortality (OR, 1.04; 95% CI, 1.03–1.06; p < .0001 and OR, 1.15; 95% CI, 1.13–1.17; p < .0001).
Female/Male Enrollment Across Hematological Trials
Common hematological malignancies in which clinical trials leading to FDA approvals occurred during the specified time frame and in which IARC data were available were leukemia, lymphoma, and multiple myeloma. Female individuals were slightly underrepresented within leukemia (OR, 0.95; 95% CI, 0.91–0.998; p = .040) and lymphoma trials (OR, 0.95; 95% CI, 0.90–0.997; p = .040) when compared with U.S. population incidence; all other comparisons with U.S. incidence and mortality were nonsignificant (Figs. 2, 3; supplemental online Table 1).
Figure 2.
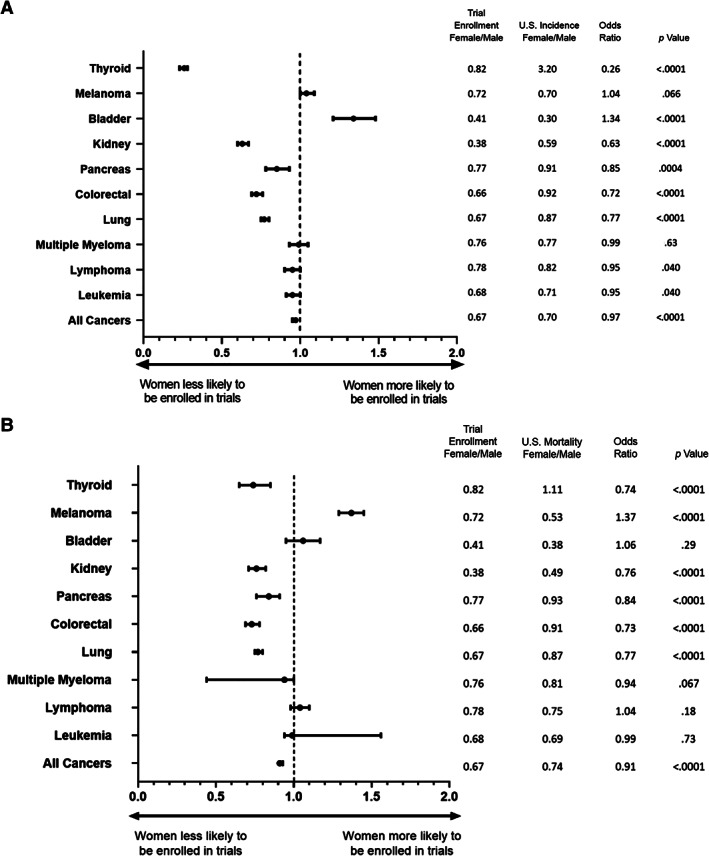
Odds ratio for female trial enrollment versus U.S. incidence or mortality. (A): Odds ratio (OR) with 95% confidence intervals for female trial enrollment compared with U.S. incidence by tumor type. “All cancers” includes all non–sex‐specific cancer types. Female to male ratios (female/male) for trial enrollment and US incidence, ORs, and p values are shown on the right. (B): OR for female trial enrollment compared with U.S. mortality by tumor type.
Figure 3.
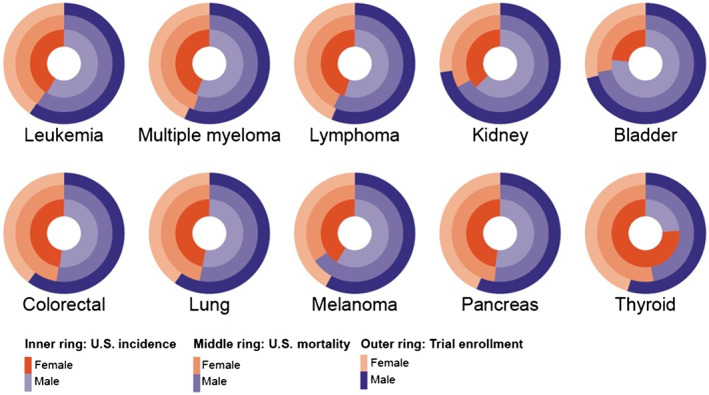
Female and male distribution across U.S. incidence, U.S. mortality, and trialenrollment across 10 tumor types. Graphical representation of the ratio of female to male (female/male) within trials leading to U.S. Food and Drug Administration drug approvals during the period reviewed for the 10 most common tumor types in the US. The inner ring denotes the female/male U.S. incidence for the tumor type denoted below the circle, and the middle ring denotes female/male U.S mortality for that tumor type. The outer ring of each circle represents female/male enrollment across all trials for that tumor type.
Given the significant difference in treatment setting for acute versus chronic leukemia, with the former more frequently occurring in an inpatient, tertiary center setting and the latter being predominantly outpatient care, an exploratory analysis comparing enrollment between these two subsets was carried out. A total of 45.6% of patients in acute leukemia trials and 38.1% of patients with chronic leukemia trials were female. The OR for acute versus chronic leukemia female trial enrollment was 1.36 (95% CI, 1.24–1.49; p < .0001), indicating that women were more frequently enrolled in acute rather than chronic leukemia trials. IARC data on population incidence and mortality specifically for acute and chronic leukemia was unavailable, and therefore the OR for population comparisons could not be performed.
Comparisons of female/male trial enrollment with very high–HDI countries and global population sex distribution by tumor type for incidence and mortality are shown in supplemental Tables 2 and 3 and supplemental Figures 1 and 2. All three hematologic cancer trial types reviewed underenrolled women compared with incidence and mortality in very high–HDI countries, with the exception of lymphoma when compared with mortality. Within the global population, only leukemia trials underenrolled women compared with incidence, whereas both leukemia and multiple myeloma underenrolled compared with mortality.
Female/Male Enrollment Across Solid Organ Trials
Common solid tumor types with FDA approvals and IARC data available were lung, colorectal, pancreas, kidney, bladder, melanoma, and thyroid cancers. This covered 87 trials enrolling 43,958 patients, with 16,908 (38.5%) being female. Within lung cancer trials, women were underenrolled compared with both U.S. incidence and mortality (OR, 0.77; 95% CI, 0.75–0.80; p < .0001 for both). The same underenrollment for women was noted in colorectal cancer trials (OR, 0.72; 95% CI, 0.69–0.76; p < .0001 and OR, 0.73; 95% CI, 0.69–0.78; p < .0001), pancreatic cancer trials (OR, 0.85; 95% CI, 0.78–0.93; p = .0004 and OR, 0.84; 95% CI, 0.76–0.91; p < .0001), kidney cancer trials (OR, 0.64, 95% CI, 0.60–0.67; p < .0001 and OR, 0.76; 95% CI, 0.71–0.82; p < .0001), and thyroid cancer trials (OR, 0.26; 95% CI, 0.23–0.28; p < .0001 and OR, 0.74; 95% CI, 0.65–0.85; p < .0001) relative to U.S. incidence and mortality, respectively.
The bladder cancer trials reviewed overenrolled women compared with U.S. incidence (OR, 1.34; 95% CI, 1.21–1.48; p < .0001), but there was no significant difference when compared with mortality. Within melanoma, women were overenrolled compared with mortality only (OR, 1.37; 95% CI, 1.29–1.45; p < .0001), but no difference was noted relative to U.S. incidence.
Comparisons of trial enrollment by sex with very high–HDI countries and global population sex distribution for solid tumors can be found in the supplemental Tables 2 and 3 and supplemental Figures 1 and 2. Findings were comparable with the U.S. population with the exception of melanoma, lung, and bladder cancer. Women were underrepresented in melanoma trials (OR, 0.83; 95% CI, 0.79–0.86; p < .0001 and OR, 0.80; 95% CI, 0.77–0.83; p < .0001) and overrepresented in lung cancer trials compared with incidence in very high–HDI countries and the global population (OR, 1.08; 95% CI, 1.05–1.12; p < .0001 and OR, 1.27; 95% CI, 1.23–1.31; p < .0001). With mortality as the comparator, women were overrepresented in bladder cancer trials (OR, 1.14; 95% CI, 1.03–1.26; p = .0098 and OR, 1.17; 95% CI, 1.06–1.29; p = .0022) and lung cancer trials (OR, 1.21; 95% CI, 1.17–1.25; p < .0001 and OR, 1.39; 95% CI, 1.35–1.43; p < .0001) in very high–HDI countries and the global population.
Other Populations
There were 34 trials that were not included in the subgroup analyses of common hematologic or solid organ tumor types described above because of small numbers or mixed tumor types. Within five hematologic trials, there were 892 patients, of which 353 (39.6%) were female. A total of 29 solid tumor trials that were not specifically reviewed above enrolled 14,318 patients, 5,995 (41.9%) of whom were female.
Discussion
This study of 78,840 trial participants reveals that rates of female trial enrollment have been static across the decade reviewed. Rates are similar to earlier studies of female representation in clinical research [17, 18]. At first glance, when reproductive tract and breast cancers are excluded from analyses, women appear only slightly underrepresented, with an OR of 0.97 (95% CI, 0.95–0.98; p < .0001) for female enrollment across trials compared with U.S. incidence. Although the magnitude of difference is small, this equates to there being nearly 1,000 less women enrolled than expected within the trial cohort reviewed here. The disparity becomes greater when the comparator is U.S. mortality (OR, 0.91; 95% CI 0.90–0.93; p < .0001). Additionally, we noted significant underrepresentation in select tumor types, so although the overall picture shows only minimal disparity, certain cancers were noted to have dramatic differences. The greater disparity in ORs for U.S. mortality points to there being imbalances in trial enrollment of other sex‐specific cancers within the cohort that were not specifically reviewed here.
We demonstrate significant underrepresentation of women across most solid organ cancer trials when compared with either U.S. incidence or U.S. mortality. This includes lung, colorectal, pancreatic, kidney, and thyroid cancer trials. These findings suggest that despite increasing awareness of sex disparity and numerous policy statements from organizations such as the FDA, little has changed in the past 20 years, and disparity in trial enrollment is still an important area for improvement in our society. This finding is consistent with recent data on representation of women in lung and colorectal trials, as well as less common solid organ cancers [26, 27, 28, 29]. We also show that a significant number of trial publications do not consider sex in their analysis of treatment efficacy, with this omission occurring more frequently in the hematological trials in this cohort.
Given the broader range of tumor types samples reviewed here, this study is able to provide a more nuanced picture of trial enrollment by sex and tumor stream than previous literature in this field. We note that although underenrollment was seen in many tumor types, significant variation in magnitude of difference between hematologic and solid cancer trials exists. Notably, leukemia, lymphoma, and multiple myeloma trials showed small to no difference in female trial enrollment compared with U.S. incidence and mortality. However, women were more frequently enrolled in acute rather than chronic leukemia trials.
A recent meta‐analysis involving numerous tumor types has shown that lack of trial availability and patient ineligibility account for 77% of trial nonenrollment [30]. It is unknown whether there is a significant difference by sex in the number of patients approached for trial enrollment versus the number who ultimately enroll in a trial. The predominantly centralized, inpatient care of acute leukemia in tertiary centers versus the decentralized outpatient care of chronic leukemia may explain differences in recruitment between acute and chronic leukemia trials and, indeed, between hematologic and solid organ cancer trials overall. Previous studies on barriers to clinical trial participation have cited lack of awareness, transport difficulties, economic considerations, and interference with family responsibilities [31, 32, 33]. Such barriers may disproportionately affect women. Additionally, in the outpatient setting, care giving obligations or other social determinants of health that disproportionately affect women may amplify determinants of trial enrollment relative to care that is provided in an inpatient setting.
In this study, Female representation differed depending on whether U.S. incidence or mortality was the comparator. This is due to sex‐based differences in mortality. Men have worse overall cancer mortality compared with women [34, 35, 36, 37], particularly with respect to melanoma [38] and thyroid cancer [39]. Female thyroid cancer is overdiagnosed in high‐income and some middle‐income countries [40, 41] because of the increasing sophistication of diagnostic imaging and increased uptake of health surveillance. This explains the difference in magnitude of underrepresentation of women within thyroid cancer trials that was seen, depending on whether the comparator was U.S. incidence (OR, 0.26) or U.S. mortality (OR, 0.74). The same trends were seen in thyroid cancer incidence and mortality within the two international populations reviewed.
For bladder cancer, the reverse was seen, with women overrepresented in trials compared with U.S. incidence (OR, 1.34; 95% CI, 1.21–1.48; p < .0001) but no difference seen when U.S. mortality was the comparator (OR, 1.06; 95% CI, 0.95–1.17; p = .29). This is because female bladder cancer mortality is higher than male bladder cancer mortality [37]. Women are more frequently diagnosed with bladder cancer at a later stage with higher grade lesions [42, 43].
It remains unclear whether there are truly differential treatment effects between men and women, with conflicting data on chemotherapy and immunotherapy outcomes by sex [44, 45, 46, 47]. This study must be interpreted in the context of several strengths and weaknesses. Although we comprehensively evaluated all trials leading to FDA approvals over an entire decade, creating an extremely large population of patients to evaluate overall, pancreas, bladder, and thyroid cancer trials accounted for a smaller proportion of patients, potentially limiting the applicability of findings within those tumor streams. Trials that did not lead to new FDA drug approvals were not evaluated and may have different representation. We focused on trials leading to drug registration, as these are the pivotal trials that led to a change in practice.
Although we performed tumor site‐specific analysis for many cancer types, we were unable to perform this for all cancers because of small numbers of approvals for certain cancer types. Previous analyses of this cohort have, however, shown significant discrepancies exist at additional tumor sites, such as hepatocellular carcinoma and gastric cancer [48]. In some instances, the discrepancy was larger when trial enrollment was compared with U.S. mortality rather than U.S. incidence, contributing to the OR of 0.91 when overall trial enrollments were compared with overall U.S. mortality.
The IARC database used for population comparisons currently does not facilitate comparison of mortality and incidence rates across the entire reviewed period, which is a potential limitation of this study. Reassuringly, differences in overall cancer incidence trends over time by sex in the U.S. predominantly reflect the change in rate of prostate cancer diagnoses, and there were no differences in overall cancer mortality trends by sex [49]. We also did not review reporting of adverse event data by sex, which remains an important area of ongoing research but is minimally commented on in conference proceedings or manuscripts. Finally, this study did not report on gender minority representation (i.e., transgender and nonbinary gendered persons). Such data is currently unavailable, both at a trial and a population level, and represents an important group requiring improved trial representation in the future.
Notwithstanding these limitations, we demonstrate that despite a number of policy changes over the past decade, female representation continues to be an issue in the U.S., in line with numerous other publications. We also compared our results with very high–HDI and global populations to provide more generalizability to our findings and because many of these trials recruited in a global manner.
Conclusion
Clinical trial demographics do not reflect real world practice. Here, we show that female representation in trials could improve in many solid tumor types, and differences were less marked in hematologic malignancies. Such disparities reflect ongoing health inequities between men and women, a significant issue in itself, and may also call into question the applicability of results to the underrepresented sex.
Author Contributions
Study concept/design: Shehara Mendis, Seerat Anand, Arvind Dasari, Joseph M. Unger, Scott Koptez, Michael J. Overman, Kanwal Raghav, Jonathan M. Loree
Acquisition, analysis, or interpretation of data: Shehara Mendis, Seerat Anand, Joanna M. Karasinska, Arvind Dasari, Joseph M. Unger, Anirudh Gothwal, Lee M. Ellis, Gauri Varadhachary, Scott Kopetz, Michael J. Overman, Kanwal Raghav, Jonathan M. Loree
Statistical analysis: Shehara Mendis, Seerat Anand, Joanna M. Karasinska, Arvind Dasari, Joseph M. Unger, Anirudh Gothwal, Michael J. Overman, Kanwal Raghav, Jonathan M. Loree
Administrative, technical, or material support: Joanna M. Karasinska, Arvind Dasari, Jonathan M. Loree
Study supervision: Kanwal Raghav, Jonatahn M. Loree
Drafting of the manuscript: Shehara Mendis, Seerat Anand, Kanwal Raghav, Jonathan M. Loree
Critical revision of the manuscript for important intellectual content: Seerat Anand, Arvind Dasari, Joseph M. Unger, Lee M. Ellis, Gauri Varadhachary, Scott Kopetz, Michael J. Overman, Kanwal Raghav, Jonathan M. Loree
Disclosures
The authors indicated no financial relationships.
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Figure 1 Odds Ratio for female trial enrollment versus very high human development index country incidence or mortality
Supplementary Figure 2: Odds Ratio for female trial enrollment versus global incidence or mortality
Supplementary Figure 3: Female and male distribution across US incidence, US mortality and 153 trials for 10 tumor types
Supplementary Table 1 Ratio of females to males (female:male) in trials reviewed by tumor type, for incidence and for mortality in the US population.
Supplementary Table 2: Ratio of females to males (female:male) in trials reviewed by tumor type, for incidence and mortality in very high human development index (HDI) countries.
Supplementary Table 3: Ratio of females to males (female:male) for incidence and mortality compared to a global population.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Disclosures of potential conflicts of interest may be found at the end of this article.
Footnotes
For Further Reading: Kelsey L. Corrigan, Walker Mainwaring Austin B. Miller et al. Exclusion of Men from Randomized Phase III Breast Cancer Clinical Trials. The Oncologist 2020;25:e990–e992.
Abstract: Male breast cancer treatment regimens are often extrapolated from female‐based studies because of a paucity of literature analyzing male breast cancer. Using ClinicalTrials.gov, we analyzed breast cancer randomized clinical trials (RCTs) to determine which factors were associated with male‐gender inclusion. Of 131 breast cancer RCTs identified, male patients represented 0.087% of the total study population, which is significantly less than the proportion of male patients with breast cancer in the U.S. (0.95%; p < .001). Twenty‐seven trials included male patients (20.6%). Lower rates of male inclusion were seen in trials that randomized or mandated hormone therapy as part of the trial protocol compared with trials that did not randomize or mandate endocrine therapy (2.5% vs. 28.6% male inclusion; p < .001). It is imperative for breast cancer clinical trials to include men when allowable in order to improve generalizability and treatment decisions in male patients with breast cancer.
References
- 1. U.S. Department of Health and Human Services, Food and Drug Administration . General Considerations for the Clinical Evaluation of Drugs. Rockville, MD: U.S. Food and Drug Administration; 1977. [Google Scholar]
- 2. U.S. Congress Public Law. National Institutes of Health Revitalization Act of 1993: Act to Amend the Public Health Service Act to Revise and Extend the Programs of the National Institutes of Health, and for Other Purposes, S. 1, 103rd Cong (1993).
- 3. Investigational new drug applications ; Amendment to clinical hold regulations for products intended for life‐threatening diseases and conditions. Food and Drug Administration, HHS. Final rule. Fed Regist 2000;65:34963–34971. [PubMed] [Google Scholar]
- 4. U.S. Government Accountability Office . GAO‐01‐286R: Drug Safety: Most Drugs Withdrawn in Recent Years Had Greater Health Risks for Women. Washington, DC: U.S. Government Accountability Office; 2001.
- 5. Investigational new drug applications and new drug applications–FDA . Final rule. Fed Regist 1998;63:6854–6862. [PubMed] [Google Scholar]
- 6. Özdemir BC, Csajka C, Dotto GP et al. Sex differences in efficacy and toxicity of systemic treatments: An undervalued issue in the era of precision oncology. J Clin Oncol 2018;36:2680–2683. [DOI] [PubMed] [Google Scholar]
- 7. Wagner AD, Oertelt‐Prigione S, Adjei A et al. Gender medicine and oncology: Report and consensus of an ESMO workshop. Ann Oncol 2019;30:1914–1924. [DOI] [PubMed] [Google Scholar]
- 8. Schmetzer O, Flörcken A. Sex differences in the drug therapy for oncologic diseases In: Regitz‐Zagrosek V, ed. Sex and Gender Differences in Pharmacology. Berlin, Heidelberg: Springer Berlin Heidelberg; 2012:411–442. [DOI] [PubMed] [Google Scholar]
- 9. Wagner AD, Grothey A, Andre T et al. Sex and adverse events of adjuvant chemotherapy in colon cancer: An analysis of 34,640 patients in the ACCENT database. J Natl Cancer Inst 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016;16:626–638. [DOI] [PubMed] [Google Scholar]
- 11. Vemuri R, Sylvia KE, Klein SL et al. The microgenderome revealed: Sex differences in bidirectional interactions between the microbiota, hormones, immunity and disease susceptibility. Semin Immunopathol 2019;41:265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nicholson JK, Holmes E, Kinross J et al. Host‐gut microbiota metabolic interactions. Science 2012;336:1262–1267. [DOI] [PubMed] [Google Scholar]
- 13. Iida N, Dzutsev A, Stewart CA et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013;342:967–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gopalakrishnan V, Spencer CN, Nezi L et al. Gut microbiome modulates response to anti‐PD‐1 immunotherapy in melanoma patients. Science 2018;359:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin P‐Y, Sun L, Thibodeaux SR et al. B7‐H1–dependent sex‐related differences in tumor immunity and immunotherapy responses. J Immunol 2010;185:2747–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Braveman PA, Kumanyika S, Fielding J et al. Health disparities and health equity: The issue is justice. Am J Public Health 2011;101(suppl 1):S149–S155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Geller SE, Koch A, Pellettieri B et al. Inclusion, analysis, and reporting of sex and race/ethnicity in clinical trials: Have we made progress? J Womens Health (Larchm) 2011;20:315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jagsi R, Motomura AR, Amarnath S et al. Under‐representation of women in high‐impact published clinical cancer research. Cancer 2009;115:3293–3301. [DOI] [PubMed] [Google Scholar]
- 19. Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trialsrace‐, sex‐, and age‐based disparities. JAMA 2004;291:2720–2726. [DOI] [PubMed] [Google Scholar]
- 20. Ramasubbu K, Gurm H, Litaker D. Gender bias in clinical trials: Do double standards still apply? J Womens Health Gend Based Med 2001;10:757–764. [DOI] [PubMed] [Google Scholar]
- 21. U.S. Department of Health and Human Services Food & Drug Administration . Drugs@FDA: FDA‐approved drugs. 2018. Available at https://www.accessdata.fda.gov/scripts/cder/daf/. Accessed January 2, 2019.
- 22. U.S. National Library of Medicine: National Institutes of Health . PubMed.gov. 2019. Available at https://www.ncbi.nlm.nih.gov/pubmed/. Accessed January 2, 2019.
- 23. U.S. National Library of Medicine: National Institutes of Health . ClinicalTrials.gov. 2019. Available at https://clinicaltrials.gov/. Accessed January 2, 2019.
- 24. Cancer Today . 2018. Available at https://gco.iarc.fr/today/online‐analysis. Accessed March 26, 2020.
- 25. Loree JM, Anand S, Dasari A et al. Disparity of race reporting and representation in clinical trials leading to cancer drug approvals from 2008 to 2018. JAMA Oncol 2019;5:e191870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ludmir EB, Fuller CD, Moningi S et al. Sex‐based disparities among cancer clinical trial participants. J Natl Cancer Inst 2020;112:211–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pang HH, Wang X, Stinchcombe TE et al. Enrollment trends and disparity among patients with lung cancer in national clinical trials, 1990 to 2012. J Clin Oncol 2016;34:3992–3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Benchetrit L, Torabi SJ, Tate JP et al. Gender disparities in head and neck cancer chemotherapy clinical trials participation and treatment. Oral Oncol 2019;94:32–40. [DOI] [PubMed] [Google Scholar]
- 29. Duma N, Aguilera JV, Paludo J et al. Representation of minorities and women in oncology clinical trials: Review of the past 14 years. J Oncol Pract 2018;14:e1–e10. [DOI] [PubMed] [Google Scholar]
- 30. Unger JM, Vaidya R, Hershman DL et al. Systematic review and meta‐analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. J Natl Cancer Inst 2019;111:245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brown DR, Fouad MN, Basen‐Engquist K et al. Recruitment and retention of minority women in cancer screening, prevention, and treatment trials. Ann Epidemiol 2000;10(Suppl 1):S13–S21. [DOI] [PubMed] [Google Scholar]
- 32. Borno HT, Zhang L, Siegel A et al. At what cost to clinical trial enrollment? A retrospective study of patient travel burden in cancer clinical trials. The oncologist. 2018;23:1242–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Young RC. Cancer clinical trials — A chronic but curable crisis. N Engl J Med 2010;363:306–309. [DOI] [PubMed] [Google Scholar]
- 34. Torre LA, Bray F, Siegel RL et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 35. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- 36. Radkiewicz C, Johansson ALV, Dickman PW et al. Sex differences in cancer risk and survival: A Swedish cohort study. Eur J Cancer 2017;84:130–140. [DOI] [PubMed] [Google Scholar]
- 37. Cook MB, McGlynn KA, Devesa SS et al. Sex disparities in cancer mortality and survival. Cancer Epidemiol BiomarkersPrev 2011;20:1629–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gamba CS, Clarke CA, Keegan TH et al. Melanoma survival disadvantage in young, non‐Hispanic white males compared with females. JAMA Dermatol 2013;149:912–920. [DOI] [PubMed] [Google Scholar]
- 39. Sipos JA, Mazzaferri EL. Thyroid cancer epidemiology and prognostic variables. Clin Oncol 2010;22:395–404. [DOI] [PubMed] [Google Scholar]
- 40. Vaccarella S, Franceschi S, Bray F et al. Worldwide thyroid‐cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med 2016;375:614–617. [DOI] [PubMed] [Google Scholar]
- 41. Mathew IE, Mathew A. Rising thyroid cancer incidence in Southern India: An epidemic of overdiagnosis? J Endocr Soc 2017;1:480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Scosyrev E, Noyes K, Feng C et al. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer 2009;115:68–74. [DOI] [PubMed] [Google Scholar]
- 43. Mungan NA, Kiemeney LA, van Dijck JA et al. Gender differences in stage distribution of bladder cancer. Urology 2000;55:368–371. [DOI] [PubMed] [Google Scholar]
- 44. Wheatley‐Price P, Blackhall F, Lee SM et al. The influence of sex and histology on outcomes in non‐small‐cell lung cancer: A pooled analysis of five randomized trials. Ann Oncol 2010;21:2023–2028. [DOI] [PubMed] [Google Scholar]
- 45. Fresneau B, Hackshaw A, Hawkins DS et al. Investigating the heterogeneity of alkylating agents’ efficacy and toxicity between sexes: A systematic review and meta‐analysis of randomized trials comparing cyclophosphamide and ifosfamide (MAIAGE study). Pediatr Blood Cancer 2017;64:e26457. [DOI] [PubMed] [Google Scholar]
- 46. Wallis CJD, Butaney M, Satkunasivam R et al. Association of patient sex with efficacy of immune checkpoint inhibitors and overall survival in advanced cancers: A systematic review and meta‐analysis. JAMA Oncol 2019;5:529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Conforti F, Pala L, Bagnardi V et al. Cancer immunotherapy efficacy and patients' sex: A systematic review and meta‐analysis. Lancet Oncol 2018;19:737–746. [DOI] [PubMed] [Google Scholar]
- 48. Mendis SR, Anand S, Dasari A et al. Female representation in clinical trials leading to FDA cancer drug approvals for gastrointestinal (GI) cancers between 2008 to 2018. J Clin Oncol 2020;38(suppl):809a. [Google Scholar]
- 49. Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Figure 1 Odds Ratio for female trial enrollment versus very high human development index country incidence or mortality
Supplementary Figure 2: Odds Ratio for female trial enrollment versus global incidence or mortality
Supplementary Figure 3: Female and male distribution across US incidence, US mortality and 153 trials for 10 tumor types
Supplementary Table 1 Ratio of females to males (female:male) in trials reviewed by tumor type, for incidence and for mortality in the US population.
Supplementary Table 2: Ratio of females to males (female:male) in trials reviewed by tumor type, for incidence and mortality in very high human development index (HDI) countries.
Supplementary Table 3: Ratio of females to males (female:male) for incidence and mortality compared to a global population.


