Abstract
Agents blocking BRAF and MEK produce robust responses in patients with BRAF V600‐mutated melanoma; however, more accurate clinical biomarkers are needed to predict prognosis. To explore this question, we retrospectively studied 158 patients with BRAF‐mutated melanoma treated with BRAF with or without MEK inhibitors. We found that the number of distinct tumor sites upon initiation of targeted therapy was associated with decreased progression‐free survival but had no effect on overall survival. Serum values of lactate dehydrogenase and absolute lymphocyte count to absolute neutrophil count ratio independently had the strongest association with both progression‐free survival and overall survival. Using both of these markers can help stratify prognosis of patients with metastatic melanoma receiving targeted therapy.
Short abstract
This article explores the need for more accurate clinical biomarkers to predict prognosis in patients with <i > BRAF</i > ‐mutated melanoma, reporting a retrospective study of patients with metastatic melanoma treated with BRAF +/– MEK inhibitors.
Introduction
Inhibitors of BRAF and/or MEK usually produce a clinical response in patients with BRAF‐mutated melanoma, but most patients eventually progress. These agents antagonize the mitogen‐activated protein kinase (MAPK) pathway and also augment the host immune response to melanoma [1, 2]. Preclinical and clinical studies have also shown benefit with combined BRAF/MEK inhibitors and immune checkpoint inhibitors (ICIs) [3]. Although several biomarkers correlate with response to ICIs in melanoma, less is known about the value of these clinical factors in response to BRAF/MEK inhibitors. For example, recent studies have shown that baseline tumor size, neutrophil to lymphocyte ratio, and organ sites of metastases all correlate with immunotherapy responses [4, 5, 6]. Furthermore, integrating these clinical factors in addition to known prognostic features such as lactate dehydrogenase (LDH) and American Joint Committee on Cancer metastatic (AJCC M) stage has not been done [7]. Although small studies have assessed whether similar prognostic factors apply to both ICI and BRAF inhibitors, results have been mixed [8]. Herein, we correlated these clinical parameters with outcomes in patients with metastatic melanoma treated with BRAF with or without MEK inhibitors.
Materials and Methods
Following an institutional review board waiver of consent, we reviewed records of patients treated with BRAF and/or MEK therapy. We assessed patient demographics (age, gender, and prior therapies), tumor characteristics (mutation type, number of tumors present, and maximum diameter of the largest tumor), and laboratory values (LDH, absolute neutrophil count [ANC], absolute lymphocyte count [ALC] at initiation of targeted therapy). We assessed progression‐free survival (PFS) and overall survival (OS) per RECIST version 1.1.
Kaplan‐Meier survival curves were stratified by above‐ and below‐median ALC/ANC ratio and for normal versus high LDH levels. Multivariable logistic regression was used; variables with known, published impact on response to BRAF/MEK inhibitors were forced into the model, specifically treatment class (BRAF inhibition vs. dual BRAF and MEK inhibition vs. sequential inhibition), BRAF mutation type (V600E vs. other V600 mutations), presence of prior therapies, AJCC M‐stage, and LDH level. Backward selection was performed to expand the “base” model by identifying additional predictors from number of tumors, the presence of other visceral metastases, and ALC/ANC ratio. All tests are significant at the two‐sided .05 level. Statistical analyses were performed in R‐3.6.2.
Results
We identified 158 patients treated with BRAF and/or MEK inhibitors from our center. Of these patients, 89 were men (56.3%), with median (range) age of 56 (21–90). Of our cohort, 78 (49.4%) were treated with BRAF inhibitors alone, 64 (40.5%) were treated with dual BRAF and MEK inhibition, and 16 (10.1%) were initially treated with single‐agent BRAF inhibition before changing to dual therapy at progression. For the entire cohort, median PFS and OS were 194 and 450 days, respectively.
Next, multivariable analysis was performed to determine the independent prognostic impact of these clinical variables (Table 1). The strongest effect was observed for LDH for both PFS (hazard ratio [HR], 1.17; 95% confidence interval [CI], 1.08–1.26; p < .0001) and OS (HR, 1.23; 95% CI, 1.14–1.33; p < .0001). The number of tumors (10 or more tumors) correlated with decreased PFS (HR, 2.24; 95% CI, 1.31–3.82; p = .0031) but had no impact on OS (HR, 1.30; 95% CI, 0.72–2.34; p = .39) compared with patients with one to three tumors. Tumor bulk (diameter of the largest tumor) was not associated with PFS or OS. The presence of other visceral metastases (nonliver, nonbrain, nonbone) was also associated with inferior PFS but not OS. Additionally, higher ALC/ANC ratio was strongly associated with increased PFS (HR, 0.68; 95% CI, 0.54–0.85; p = .0006) and OS (HR, 0.8; 95% CI, 0.65–0.99; p = .04). Notably, ALC/ANC ratio early in treatment (approximately 4 weeks into therapy) was not associated with clinical outcomes in univariate analyses.
Table 1.
Hazard ratio for clinical variables for progression‐free survival and overall survival
| Clinical variable | Progression‐free survival | Overall survival | ||
|---|---|---|---|---|
| Hazard ratio | p value | Hazard ratio | p value | |
| Lactate dehydrogenase | 1.166 | <.0001 | 1.230 | <.0001 |
| Absolute lymphocyte count/absolute neutrophil count ratio | 0.679 | .0006 | 0.799 | .0383 |
| Presence of visceral metastasis | 0.548 | .0059 | N/A | |
| Presence of lung metastasis | N/A | 1.768 | .0081 | |
| More than 10 tumors | 2.239 | .0031 | 1.297 | .3875 |
| Presence of 4–9 tumors | 1.426 | .1947 | 0.682 | .22 |
| Prior therapy received | 1.275 | .1823 | 1.228 | .2895 |
| Dual BRAF/MEK therapy | 0.614 | .0144 | 0.662 | .0494 |
| Sequential BRAF, MEK therapy | 0.500 | .0492 | 0.939 | .8512 |
| Presence of V600E mutation | 0.288 | <.0001 | 0.593 | .0506 |
| M‐stage IVb | 0.329 | .0092 | 0.961 | .9306 |
| M‐stage IVc | 0.577 | .1105 | 1.537 | .2195 |
| M‐stage IVd | 0.538 | .1381 | 1.434 | .4165 |
N/A, not applicable; variable was not selected on backward selection process as a potentially significant variable for inclusion in the final model.
After identifying a strong and independent effect for both LDH and ALC/ANC ratio for both PFS and OS, we sought to use the combination of the two as an additional stratification measure for prognosis. Using LDH (higher than institutional upper limit of normal vs. normal) and ALC/ANC ratio (above vs. below the median), both PFS and OS were stratified using these variables. Median PFS values were 351 versus 235 versus 147 versus 128 days, p < .0001, for low LDH, high ALC/ANC; low LDH, low ALC/ANC; high LDH, high ALC/ANC; and high LDH, low ALC/ANC, respectively (Fig. 1A). Median OS values were 960 versus 645 versus 428 versus 214 days, p < .0001, for low LDH, high ALC/ANC; low LDH, low ALC/ANC; high LDH, high ALC/ANC; and high LDH, low ALC/ANC, respectively (Fig. 1B).
Figure 1.
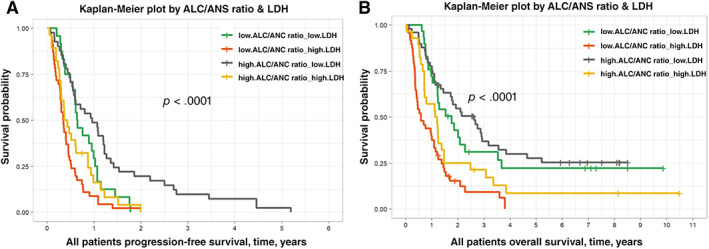
Kaplan‐Meier plots of progression‐free and overall survival. (A): Progression‐free survival stratified by serum LDH and serum ALC/ANC ratio. This figure shows the progression‐free survival was best in patients with low serum LDH values and high ALC/ANC ratio upon initiation of targeted therapy while poorest in patients with high serum LDH and low ALC/ANC ratio. The differences in these curves serve as a potentially useful stratification guide for patients beginning targeted therapy. (B): Overall survival stratified by serum LDH and serum ALC/ANC ratio. This figure shows the overall survival was best in patients with low serum LDH values and high ALC/ANC ratio upon initiation of targeted therapy while poorest in patients with high serum LDH and low ALC/ANC ratio. The differences in these curves serve as a potentially useful stratification guide for patients beginning targeted therapy.Abbreviations: ALC, absolute lymphocyte count; ANC, absolute neutrophil count; LDH, lactate dehydrogenase.
Discussion
In this study, we retrospectively studied the role of several clinical and radiographic markers on the prognosis of patients receiving BRAF with or without MEK inhibitors for melanoma. We identified several associations, including the number of tumors and other visceral metastases on PFS. However, the most robust association was ALC/ANC ratio, with a strong effect on both PFS and OS and independent of LDH. This was strongly correlated with outcomes even when adjusted for metastatic stage, type of BRAF mutation, tumor size and numbers, and other known factors.
Targeting BRAF and MEK remains an important cornerstone of melanoma therapies in addition to ICI. Although the biology driving response to targeted therapy largely stems from MAPK pathway signaling abrogation, recent work has shown the effects of BRAF inhibition on increased T‐cell infiltration and improving the tumor microenvironment, suggesting a role for dual therapy [1, 9]. As such, there is rationale for studying biomarkers that have correlated with responses to ICI in the context of targeted therapy. A number of recent papers have studied the prognostic role of the absolute lymphocyte count or neutrophil lymphocyte ratio in patients receiving ICI, with much less data on the BRAF/MEK inhibitor‐treated population [8, 10, 11]. We observe this association as well in the context of BRAF with or without MEK inhibition. This could relate to specific immune mechanisms, or it simply could reflect another aspect of disease aggressiveness.
We acknowledge that there are several limitations to our study. First, this study was conducted retrospectively at a single center, which may result in region‐specific biases. Second, the radiologic data was assessed retrospectively and not in a controlled, prospective fashion. Finally, many patients received BRAF inhibitor monotherapy, which is rarely used currently. However, we observed similar correlations between outcomes and ALC/ANC irrespective of therapy type.
Ultimately, this study identified the value of using blood‐based biomarkers such as LDH and ALC/ANC ratio as prognostic markers for patients receiving targeted therapy for metastatic melanoma. These findings may be useful to inform selection of therapy for patients. Further research is needed to understand the biological mechanisms, and a combination of clinical and molecular markers (as opposed to a single factor) will likely aid in prognostication for these patients.
Disclosures
Douglas B. Johnson: Array Biopharma, Bristol‐Myers Squibb, Jansen, Merck, Novartis (SAB‐ advisory boards), Bristol‐Myers Squibb, Incyte (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
Grants for research support: National Cancer Institute/National Institutes of Health K23 CA204726 (D.B.J.).
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Frederick DT, Piris A, Cogdill AP et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res 2013;19:1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Knight DA, Ngiow SF, Li M et al. Host immunity contributes to the anti‐melanoma activity of BRAF inhibitors. J Clin Invest 2013;123:1371–1381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3. Cooper ZA, Juneja VR, Sage PT et al. Response to BRAF inhibition in melanoma is enhanced when combined with immune checkpoint blockade. Cancer Immunol Res 2014;2:643–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Warner AB, Postow MA. Bigger is not always better: tumor size and prognosis in advanced melanoma. Clin Cancer Res 2018;24:4915–4917. [DOI] [PubMed] [Google Scholar]
- 5. Ferrucci P, Gandini S, Battaglia A et al. Baseline neutrophil‐to‐lymphocyte ratio is associated with outcome of ipilimumab‐treated metastatic melanoma patients. Br J Cancer 2015;112:1904–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tumeh PC, Hellmann MD, Hamid O et al. Liver metastasis and treatment outcome with anti‐PD‐1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res 2017;5:417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Robert C, Grob JJ, Stroyakovskiy D et al. Five‐year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med 2019;381:626–636. [DOI] [PubMed] [Google Scholar]
- 8. Cassidy MR, Wolchok RE, Zheng J et al. Neutrophil to lymphocyte ratio is associated with outcome during ipilimumab treatment. EBioMedicine 2017;18:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wilmott JS, Long GV, Howle JR et al. Selective BRAF inhibitors induce marked T‐cell infiltration into human metastatic melanoma. Clin Cancer Res 2012;18:1386–1394. [DOI] [PubMed] [Google Scholar]
- 10. Postow MA, Chasalow SD, Kuk D et al. Absolute lymphocyte count as a prognostic biomarker for overall survival in patients with advanced melanoma treated with ipilimumab. Melanoma Res 2020;30:71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Capone M, Giannarelli D, Mallardo D et al. Baseline neutrophil‐to‐lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer 2018;6:74. [DOI] [PMC free article] [PubMed] [Google Scholar]


