Abstract
The coronavirus disease 2019 (COVID‐19) pandemic may have affected cancer management. We aimed to evaluate changes in every oncology care pathway essential step, from screening to treatment, during the pandemic.
Monthly oncological activity differences between 2019 and 2020 (screening tests, histopathological analyzes, multidisciplinary tumor board meetings (MTBMs), diagnostic announcement procedures (DAPs), and treatments were calculated in two French areas experiencing different pandemic intensity (Reims and Colmar).
COVID‐19 has had a dramatic impact in terms of screening (−86% to −100%), diagnosis (−39%), and surgical treatment (−30%). This global decrease in all essential oncology care pathway steps contrasted with the relative stability of chemotherapy (−9%) and radiotherapy use (−16%). Outbreak occurred earlier and with more intensity in Colmar but had a comparable impact in both areas regarding MTMBs and DAPs.
The current ONCOCARE‐COV study is still in progress and with a longer follow‐up to analyze postlockdown situation.
Short abstract
The COVID‐19 pandemic may have affected cancer management. This article evaluates the changes in oncological care pathways during the COVID‐19 crisis, based on results of the ONCOCARE‐COV study.
Introduction
The coronavirus disease 2019 (COVID‐19) pandemic led to prioritizing emergency care dedicated to infection management. Other conditions, such as cancer management, may have been affected during the sanitary lockdown [1]. Consequences of this “distraction effect” are suspected, but the immediate impact of this pandemic is still unknown [2]. The ONCOCARE‐COV study evaluated changes in oncological care pathways during the COVID‐19 crisis.
Materials and Methods
Monthly oncological activity indicators were extracted using electronic files and nation‐wide procedure codes Classification commune des actes médicaux (CCAM) from January 1, 2019, to May 31, 2020, in a French area of high COVID‐19 incidence (Grand East region), in a tertiary care center (University Hospital and Godinot Cancer Institute in Reims), and in a general hospital (in the first national outbreak epicenter in Colmar). The daily number of infected and deceased inpatients with COVID‐19 and the monthly number of different steps of oncological care pathways (screening, diagnosis, multidisciplinary tumor board meeting [MTBM], diagnosis announcement procedure [DAP] and treatment) were collected (Fig. 1). We calculated monthly activity differences between 2019 and 2020, focusing on the 3‐month COVID‐19 pandemic period, to identify changes and to compare DAP and MTBM between both areas (Reims and Colmar). Trends were visually compared using temporal curves. Graphic representation and statistical analyses were performed using R (R Development Core Team, version 1.2.5019) and Excel (Microsoft, version 2018). This study has been registered on ClinicalTrials.gov (NCT04445870).
Figure 1.
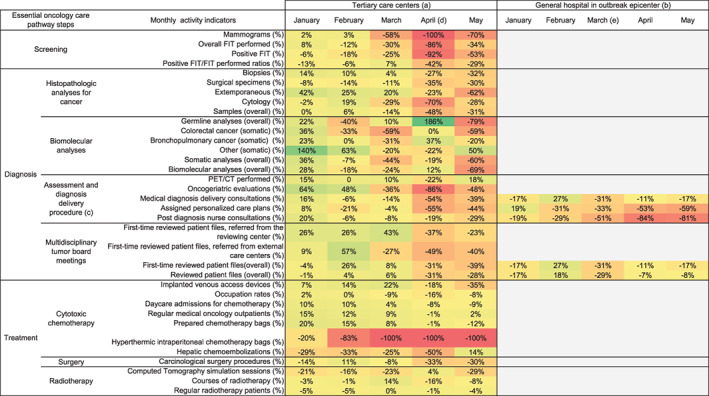
Monthly oncological activity volume difference between 2019 and 2020. Monthly changes in volume of oncological activities (%) are calculated with (2020 activity – 2019 activity) / 2019 activity and are illustrated through color variation from green (rising activity) to red (decreasing activity). Grey areas show unavailable data for the first analyses of the study. Further data collection is still in progress. (a) Reims University Hospital and Cancer Institute (Grand East region in France). (b) Colmar General Hospital (Grand East region in France). (c) Diagnosis announcement procedure (3 steps) is a measure of the first French cancer plan (2003‐2007). (d) Overall inpatients peak in Reims (April 5, 2020). (e) Overall inpatients peak in Colmar (March 24, 2020)
Abbreviations: FIT, fecal immunochemical test; PET/CT, Positron emission tomography with computed tomography.
Results
Compared with the same trimester in 2019, oncological activity decreased dramatically on all essential oncological care pathway steps during the COVID‐19 pandemic. The trends and comparisons of monthly activity volume are depicted in Figures 1 and 2.
Figure 2.
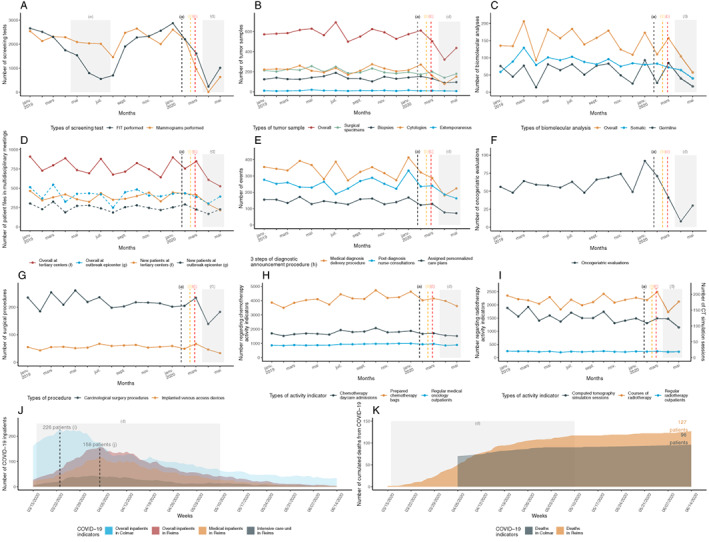
Temporal curves of monthly oncological activity volume (between January 2019 and May 2020) and of daily COVID‐19 pandemic indicators (between March 16, and June 15, 2020). (A): Screening activity in the Marne county. (B): Carcinologic histopathological analyses depending on tumor samples. (C); Biomolecular analyses (somatic and germline). (D): Multidisciplinary board meeting activity (comparison between Reims and Colmar centers). (E): Diagnostic announcement procedures (h). (F): Oncogeriatric evaluations. (G): Oncological surgical activity. (H): Cytotoxic chemotherapy activity. (I): Radiotherapy activity. (J): Daily COVID‐19 inpatients. (K): Daily cumulated deaths from COVID‐19. (a) The black dashed vertical line marks timeline of the first diagnosed COVID‐19 patient in France (January 24, 2020). (b) The orange dashed vertical line marks timeline of the first COVID‐19 deceased patient in France (February 15, 2020). (c) The red dashed vertical line marks timeline of the first COVID‐19 admitted patient in Reims (February 27, 2020). (d) The gray rectangular area marks the lockdown period (from March 17, 2020, to May 11, 2020). (e) The gray rectangular area marks a period of FIT stock shortage (from April 15, 2019, to July 25, 2019). (f) University Hospital and Godinot Cancer Institute, Reims, France. (g) Pasteur General Hospital, Colmar, France. (h) Diagnosis announcement procedure (3 steps) is a measure of the first French cancer plan (2003–2007). (i) The black dashed vertical line marks timeline of the overall inpatients peak (226 inpatients) in Colmar (March 24, 2020). (j) The black dashed vertical line marks timeline of the overall inpatients peak (158 inpatients) in Reims (April 5, 2020).
Abbreviations: COVID‐19, coronavirus disease 2019; CT, computed tomography; FIT, fecal immunochemical test.
Colon and breast cancer screening test fell by 86% to 100%, respectively. All activities linked to sampling, histopathological (−48%), and biomolecular analyses (−69%) were drastically reduced. A decrease in medical announcement consultations (−54%) and oncogeriatric evaluations (−86%) was also observed; fewer medical patient files (−31%; including those of new patients; −39%) were reviewed in MTBM. Regarding treatment, systemic chemotherapy (−9%) and radiotherapy (−16%) experienced a lighter decline, whereas oncological surgical procedures were heavily impacted (−30%) over a 2‐month period. All clinical research trials were stopped for 3 months (data not shown), and all hyperthermic intraperitoneal chemotherapies were postponed.
Although the COVID‐19 outbreak occurred earlier and with more intensity in Colmar, it had a comparable impact in both areas regarding MTBM and DAP.
Discussion
COVID‐19 has had a dramatic impact on all aspects of the cancer care pathway, particularly in terms of screening, diagnosis, and surgical treatment. To the best of our knowledge, this is the first study assessing the overall management of cancers from screening to treatment. Screening and a drop in the number of screening‐related samples were observed in Belgium [3]. Reduction in cancer diagnoses has been noted in other European countries, particularly for colon and skin cancers [4, 5]. Primary care was also impacted, with urgent cancer referrals falling by 60% in the U.K. [6]. We experienced a similar decrease in new oncological referrals. In Spain, outpatient visits decreased, and remote visits using phones or internet became a standard [7]. As in a U.S. tertiary care cancer center (MD Anderson Cancer Center), oncological surgical care was drastically reduced because of limited availability of health personnel, logistical resources, and available beds [8]. Facing this resource scarcity, an international collaborative group recommended a fair and consistent prioritization to maximize health benefits, considering the patient, its disease, and its prognosis [9]. This global decrease in all essential oncology care pathway steps contrasts with the relative stability of chemotherapy and radiotherapy use. Patients anteriorly diagnosed with cancers continued to be treated. Limitations of the present study include lack of information on patient characteristics and prognosis. The consequences of delay in diagnosis and treatment have only been estimated in model‐based analysis [10]. Complementary qualitative studies are warranted to estimate the real impact on cancer outcomes. The ongoing CAPANCOVID‐19 study aims to evaluate the impact of the COVID‐19 pandemic on management and outcomes of patients with exocrine pancreatic cancer (https://clinicaltrials.gov/ct2/show/NCT04406571). The current ONCOCARE‐COV study is still in progress, and with a longer follow‐up, we will be able to analyze the postlockdown volume of oncological activity and the impact of a possible second COVID‐19 epidemic wave in relation to types of cancer.
Disclosures
Christine Essner: Novartis (C/A‐board, treatment of Breast Cancer), Pfizer (ET‐formation for agents visiting doctors); Olivier Bouché: Merck KGaA, Roche Genentech, Bayer, Astra‐Zeneca, Grunenthal, Merck Sharpe & Dohme, Amgen (SAB), Servier, Amgen, Pierre Fabre (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
The authors would like to acknowledge the hard and accurate work of the following contributors, especially in collecting all the data: F. Arnold, M.D. (from Dépistage des Cancers‐Centre de Coordination Grand‐Est); C. Boulagnon‐Rombi, M.D., A. Marchal, M.D., Ph.D., S. Gallois, V. Dalstein, Pharm.D., Ph.D., C. Clavel, M.D. Ph.D., J.B. Oudart, M.D., Ph.D., R. Mahmoudi, M.D., Ph.D., J.C. Merol, M.D., O. Hilbig, A. Marechal, Pharm.D., F. Slimano, Pharm.D., Ph.D., F. Clère, M.D. and S. Deguelte, M.D. (all from Reims University Hospital); and C. Garbar, M.D., Ph.D., F. Brayotel, M.D., H. Larbre, Pharm.D., S. Perin, M.D., C. Schvartz, M.D., J.B. Rey, Pharm.D., Ph.D., D. Parent, Pharm.D., L. Laurent, resident, D. Papathanassiou, M.D., Ph.D., and D. Morland, M.D., Ph.D., (all from Godinot Cancer Institute, Reims).
The authors wish to thank A. Le Guillou, resident, and C. Barbe, M.D., Ph.D., (both from Reims University Hospital) for providing their assistance in data analysis.
None of these individuals were compensated for their contribution beyond their salaries. The authors also thank D. Pellot, as she helped with the English language revision of the manuscript. Pellot was not compensated for her work.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Reynolds KL, Klempner SJ, Parikh A et al. The art of oncology: COVID era. The Oncologist 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cortiula F, Pettke A, Bartoletti M et al. Managing COVID‐19 in the oncology clinic and avoiding the distraction effect. Ann Oncol 2020;31:553–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Pelsemaeker M‐C, Guiot Y, Vanderveken J, Galant C, Van Bockstal MR. The impact of the COVID‐19 pandemic and the associated Belgian governmental measures on cancer screening, surgical pathology and cytopathology. Pathobiology 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dinmohamed AG, Visser O, Verhoeven RHA et al. Fewer cancer diagnoses during the COVID‐19 epidemic in the Netherlands. Lancet Oncol. 2020;21:750–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Vincentiis L, Carr RA, Mariani MP et al. Cancer diagnostic rates during the 2020 ‘lockdown’, due to COVID‐19 pandemic, compared with the 2018–2019: An audit study from cellular pathology. J Clin Pathol 2020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 6. Mahase E. Covid‐19: Urgent cancer referrals fall by 60%, showing “brutal” impact of pandemic. BMJ 2020;369:m2386. [DOI] [PubMed] [Google Scholar]
- 7. Manso L, De Velasco G, Paz‐Ares L. Impact of the COVID‐19 outbreak on cancer patient flow and management: Experience from a large university hospital in Spain. ESMO Open. 2020;4(suppl 2):e000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang EI, Liu JJ. Flattening the curve in oncologic surgery: Impact of Covid‐19 on surgery at tertiary care cancer center. J Surg Oncol 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Al‐Shamsi HO, Alhazzani W, Alhuraiji A et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID‐19) pandemic: An international collaborative group. The Oncologist 2020;25:e936–e945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sud A, Torr B, Jones ME et al. Effect of delays in the 2‐week‐wait cancer referral pathway during the COVID‐19 pandemic on cancer survival in the UK: A modelling study. Lancet Oncol 2020;21:1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]


