Abstract
Lessons Learned
The novel therapeutic vaccine hVEGF26–104/RFASE was found to be safe and well tolerated in patients with cancer.
hVEGF26–104/RFASE failed to induce seroconversion against native hVEGF165 and, accordingly, neither a decrease in circulating vascular endothelial growth factor (VEGF) levels nor clinical benefit was observed.
Remarkably, hVEGF26–104/RFASE induced VEGF165‐neutralizing antibodies in a nonhuman primate model. The absence of seroconversion in human calls for caution in the interpretation of efficacy of human vaccines in nonhuman primates.
Background
Targeting vascular endothelial growth factor‐A (VEGF) is a well‐established anticancer therapy. We designed a first‐in‐human clinical trial to investigate the safety and immunogenicity of the novel vaccine hVEGF26–104/RFASE.
Methods
Patients with advanced solid malignancies with no standard treatment options available were eligible for this phase I study with a 3+3 dose‐escalation design. On days 0, 14, and 28, patients received intramuscular hVEGF26–104, a truncated synthetic three‐dimensional (3D)‐structured peptide mimic covering the amino acids 26–104 of the human VEGF165 isoform, emulsified in the novel adjuvant Raffinose Fatty Acid Sulphate Ester (RFASE), a sulpholipopolysaccharide. Objectives were to determine safety, induction of VEGF‐neutralizing antibodies, and the maximum tolerated dose. Blood was sampled to measure VEGF levels and antibody titers.
Results
Eighteen of 27 enrolled patients received three immunizations in six different dose‐levels up to 1,000 μg hVEGF26–104 and 40 mg RFASE. No dose‐limiting toxicity was observed. Although in four patients an antibody titer against hVEGF26–104 was induced (highest titer: 2.77 10log), neither a reduction in VEGF levels nor neutralizing antibodies against native VEGF165 were detected.
Conclusion
Despite having an attractive safety profile, hVEGF26–104/RFASE was not able to elicit seroconversions against native VEGF165 and, consequently, did not decrease circulating VEGF levels. Deficient RFASE adjuvant activity, as well as dominant immunoreactivity toward neoepitopes, may have impeded hVEGF26–104/RFASE's efficacy in humans.
Keywords: Angiogenesis inhibitors, Peptite vaccine, Vascular Endothelial Growth Factor A
Discussion
Inhibition of vascular endothelial growth factor‐A (VEGF) is a well‐established anticancer approach in combination with cytotoxic agents. Nonetheless, the observed clinical benefit is usually rather modest, and long‐term treatment can be burdensome because of repeated intravenous administration. Therefore, neutralization of VEGF by active immunization could be an attractive alternative treatment strategy. It would offer the advantage of continuous and a potentially more pronounced inhibition of VEGF without the need for repeated antibody administrations. Here, we describe the results of a phase I trial of the novel therapeutic vaccine hVEGF26–104/RFASE [1, 2] in patients with advanced solid malignancies.
The vaccine comprised a truncated synthetic 3D‐structured peptide mimic covering the amino acids 26–104 of the human VEGF165 isoform, emulsified in the novel adjuvant Raffinos Fatty Acid Sulphate Ester (RFASE), a sulpholipopolysaccharide [3, 4]. Dose‐limiting toxicities, including related grade ≥ 3 adverse events (AEs), were not observed. None of the AEs could be associated with VEGF inhibition. No significant reduction in serum VEGF levels was found (Fig. 1A) and no clinical benefit was observed. Interestingly, in four patients, an antibody titer against hVEGF26–104 was measured (highest titer 2.77 10log), peaking four to six weeks after the first immunization (Fig. 1B). Nevertheless, cross‐reactive antibodies against native VEGF165 were not detected.
Figure 1.
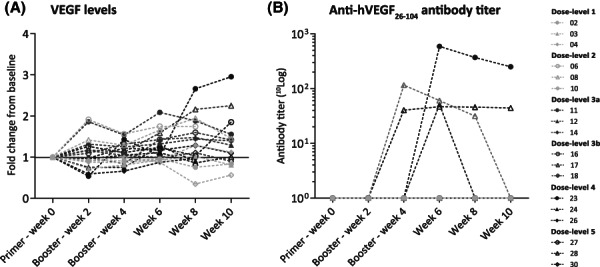
VEGF levels and anti‐hVEGF26–104 antibody titers, shown per patient, per dose level. VEGF levels in serum are shown relative to baseline in (A). Antibody titers measured in serum are shown for hVEGF26–104 in (B). Titers are in 10log scale: 2.06 for patient 08, 2.77 for patient 23, 1.71 for patient 24, and 1.67 for patient 28, respectively.Abbreviations: VEGF, vascular endothelial growth factor‐A.
There might be several explanations for the poor immunogenicity of hVEGF26–104/RFASE in humans. First, the capped N‐ and C‐terminal sequence of human VEGF165 and the dimerization‐domain (in which two cysteines were replaced by alanines) that became solvent‐exposed in the monomeric hVEGF26–104 represent potential neoepitopes. These epitopes could conceivably elicit dominant immunoreactivity and thereby interfere with reactivity to native VEGF. The fact that cross‐reactivity to native VEGF was observed in cynomolgus monkeys [2] may be related to interspecies B‐ or T‐cell receptor repertoire differences. Second, lower VEGF levels in nonhuman primates might make them more susceptible to breaking self‐tolerance. The hVEGF26–104 dose in humans might still have been below the threshold for breaking immune tolerance, since antigen‐dosing in vaccination strategies is generally not linearly correlated with the desired immune response but rather has an “on–off' effect. Finally, RFASE adjuvant might not have been sufficiently potent to induce an immune response against a self‐antigen like VEGF, especially in the context of cancer‐related immunosuppression. Proven clinically active Toll‐like receptor agonists, like Poly I:C (polyinosinic:polycytidylic acid) or CpG oligodeoxynucleotides, might stimulate a more potent immune response. However, adjuvant substitution would require not only altering the drug composition but also additional preclinical testing for drug‐combination safety, as well as conducting a new phase I trial. In view of these considerable hurdles, it was decided to terminate further development and testing of the vaccine at this point.
In conclusion, the therapeutic vaccine hVEGF26–104/RFASE displayed an attractive safety profile, but did not elicit an immune response strong enough to convey clinical benefit for patients with advanced solid malignancies.
Trial Information
| Disease | Advanced cancer/solid tumor only |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | No designated number of regimens |
| Type of Study | Phase I, 3+3 |
| Primary Endpoints | Safety, tolerability, maximum tolerated dose |
| Secondary Endpoint | Efficacy |
| Additional Details of Endpoints or Study Design | |
| Trial design: Patients with advanced solid malignancies with no standard treatment options available were eligible for this phase I study with a 3 + 3 dose‐escalation design. Patients were enrolled in six different dose levels (Table 1). The study medication consisted of 1.0 mL hVEGF26–104 (in escalating doses of 62.5 μg, 125 μg, 250 μg, 500 μg, 1,000 μg, 2000 μg, and 4,000 μg) combined with 1.0 mL RFASE (20 mg in dose‐levels 1, 2, and 3a and 40 mg in dose‐levels 3b, 4, and 5). The total volume that was administered was therefore 2.0 mL. Injections were administered in a split‐dose contralateral fashion, in either the left and right deltoid or gluteal muscles. The starting dose of 62.5 μg hVEGF26–104 equaled one‐eighth of the maximal dose given in animals. The starting dose of 20 mg RFASE equaled half of the maximal dose given in animals. On days 0, 14, and 28, patients received hVEGF26–104/RFASE intramuscularly, followed by an observation period of six weeks. To assess potential toxicity of RFASE, three patients enrolled in the first cohort of the study received 1.0 mL RFASE (20 mg) as a single agent 14 days prior to the first immunization with hVEGF26–104/RFASE. Another booster injection could be administered to patients showing response or stable disease on imaging without (prior) VEGF neutralization in serum at first evaluation (10 weeks). | |
| Study endpoints: The coprimary outcome measures of this study were the safety and tolerability profile of hVEGF26–104/RFASE and the effective dose of hVEGF26–104/RFASE required to neutralize VEGF in serum. Secondary outcome measures were the anti‐VEGF165 and anti‐VEGF26–104 antibody titers induced by hVEGF26–104/RFASE immunization and clinical benefit, defined by at least no signs of progression at first evaluation. | |
| Safety profile: Toxicity was graded by the National Cancer Institute CTCAE version 4.0 and recorded using electronic case record forms. Serious adverse events were reported to the Dutch Central Committee on Research Involving Human Subjects (CCMO) through the web portal “ToetsingOnline.” Dose‐limiting toxicity (DLT) was defined as any one of the following toxicities considered by the investigator to be related to hVEGF26–104/RFASE and occurring during the DLT assessment window (day 0 of week 0 to day 7 of week 9): any‐grade ≥ 3 hematological toxicity or any‐grade ≥ 3 nonhematological toxicity that was not attributable to disease progression or another clearly identifiable cause, excluding grade 3 diarrhea that responded to standard‐of‐care therapy; grade 3 nausea or vomiting, in the absence of premedication, that responded to standard‐of‐care therapy; or grade 3 infusion reaction, in the absence of premedication that responded to standard‐of‐care therapy. Patients were observed for DLTs for a minimum of 42 days after their last dose of hVEGF26–104/RFASE before any patient in the next higher dose cohort received treatment, except in cases in which there was no VEGF neutralization observed 14 days after the third and last dose of hVEGF26–104/RFASE. | |
| VEGF serum levels: VEGF protein concentration was measured in serum, frozen at the day the material was received, and stored at −80°C until analysis, using a commercially available human enzyme‐linked immunosorbent assay (ELISA) kit (Quantikine, R&D Systems, Abingdon, U.K.) according to manufacturer's instructions. Absorbance was measured using a BioTek Synergy HT plate reader with an optical density (OD) of 450 nm. VEGF levels were measured every 2 weeks in the DLT period, and VEGF neutralization was defined as a VEGF level below 9 pg/mL. | |
| VEGF serological responses: Anti‐VEGF antibody titers were measured in serum, frozen the day the material was received, and stored at −80°C until analysis, using an in‐house developed ELISA. Microplates were coated with 100 μL recombinant hVEGF165 (1 μg/mL; BioLegend, San Diego). After washing, the plates were blocked with 200 μL 4% horse serum (Sigma‐Aldrich, St. Louis, MO). Hereafter, the plates were incubated with 100 μL 1:30 diluted serum. Horseradish peroxidase (HRP)–conjugated rabbit antihuman immunoglobulin G antibodies (1:8,000 dilution; Sigma‐Aldrich, St. Louis) were applied to detect bound antibodies in the microplate wells. In the presence of chromogenic substrate TMB (R&D Systems, Abingdon, UK) color was developed by the enzymatic reaction of HRP. Absorbance was measured using a BioTek Synergy HT plate reader at an OD of 450 nm. If the OD was above a predetermined cut‐off (mean + 3 SDs of all patient serum baseline OD levels), a relevant antibody response was suspected and a dilution series was performed. The antibody titer was defined as the 10logarithm of the highest dilution which resulted in a signal above the predetermined cut‐off. A similar ELISA was performed on all samples to measure antibodies recognizing VEGF26–104. | |
| Tumor response assessment: Tumor response was assessed according to RECIST 1.1 at baseline, 10 weeks after start of treatment and every eight weeks during the follow‐up period in case of response and/or a repeated booster administration. | |
| Cytokine release assay: Peripheral blood mononuclear cells from healthy donors were isolated by standard Ficoll‐Hypaque density centrifugation. Cells were cultured for 24 hours (1 × 106 cells per mL per well) with lipopolysaccharide (1 μg/mL) or RFASE (1 μg/mL) (without squalene‐in‐water component, originally tested in a range from 0.5 to 5 μg/mL) in culture medium (Iscove's Modified Dulbecco's Media (IMDM), 10% Fetal Calf Serum (FCS), pen/strep). Dimethyl sulfoxide (DMSO) 0.1% served as negative control. A cytokine release assay for interleukin (IL)‐1β, IL‐6, IL‐10, IL‐8, and TNF‐α (CBA Human Inflammatory Cytokines Kit, Becton Dickinson, CA) was performed following the manufacturer's instructions with cell culture supernatants collected after 24 hours and temporarily stored at ‐20°. Data acquisition was performed on a FACS‐Calibur flow cytometer (Becton Dickinson, CA). Quantity (picograms per milliliter of the respective cytokines was calculated using FCAP array software (Soft Flow Hungary Ltd.). | |
| Statistical analysis: Statistical analyses were performed using IBM SPSS Statistics for Windows (Version 25.0; SPSS, Armonk, NY). Kaplan‐Meier analysis was performed to determine overall survival and progression free survival. Means of cytokine release assays were compared with a student t‐test. Median C‐reactive protein and WBC levels were compared using a Wilcoxon matched‐pairs signed rank test. A p value of <.05 was considered statistical significant | |
| Investigator's Analysis | Drug tolerable, efficacy indeterminant |
Table 1.
Dose‐levels
| Dose‐level | hVEGF26‐104, μg | RFASE, mg |
|---|---|---|
| Dose‐level 1 a | 62.5 | 20 |
| Dose‐level 2 | 125 | 20 |
| Dose‐level 3A | 250 | 20 |
| Dose‐level 3B | 250 | 40 |
| Dose‐level 4 | 500 | 40 |
| Dose‐level 5 | 1,000 | 40 |
The patients in dose‐level 1 received a first immunization with 20 mg RFASE alone to study the potential adverse effects of the adjuvant.
Abbreviation: RFASE, raffinose fatty acid sulphate ester.
Drug Information
| Drug 1 | |
| Generic/Working Name | hVEGF26–104/RFASE |
| Trade Name | hVEGF26–104/RFASE |
| Company Name | Immunovo |
| Drug Type | Vaccine |
| Drug Class | Angiogenesis ‐ VEGF |
| Dose | Variable per |
| Route | Other |
| Schedule of Administration | Intramuscular vaccination on day 0, 14 and 28 |
Dose‐Escalation Table
| Dose level | Dose of drug: hVEGF26–104/RFASE | Number enrolled | Number evaluable for toxicity |
|---|---|---|---|
| 1 | 62.5 μg/20 mg | 4 | 4 |
| 2 | 125 μg/20 mg | 3 | 3 |
| 3A | 250 μg/20 mg | 4 | 4 |
| 3B | 250 μg/40 mg | 4 | 4 |
| 4 | 500 μg/40 mg | 8 | 7 |
| 5 | 1,000 μg/40 mg | 4 | 4 |
Patient Characteristics
| Number of Patients, Male | 19 |
| Number of Patients, Female | 11 |
| Stage | Patients with advanced solid malignancies with no standard treatment options available were eligible |
| Age | Median (range): 65 (40–78) years |
| Number of Prior Systemic Therapies | Median (range): 3 (0–9) |
| Performance Status: ECOG |
0 — 1 1 — 24 2 — 2 3 — 0 Unknown — 3 |
| Other | One patient had both esophageal as well as oropharynx cancer, hence 31 instead of 30 cases are listed in the histologic diagnoses itemized below. |
| Cancer Types or Histologic Subtypes | Urothelial, 1; Neuroendocrine tumor (pancreas and unknown primary), 2; Squamous cell carcinoma (unknown primary), 1; Salivary duct, 1; Ovarian, 2; Pancreas, 1; Colorectal, 7; Gastric, 2; Pleiomorphic adenoma, 1; Metaplastic carcinoma, 1; Glioblastoma, 1; Tungbase, 1; Tonsil, 1; Oropharynx, 1; Esophageal, 2; Hepatocellular, 2; Hypopharynx, 1; Breast, 2; Prostate, 1. |
Primary Assessment Method
| Title | Response evaluation week 10 |
| Number of Patients Screened | 30 |
| Number of Patients Enrolled | 27 |
| Number of Patients Evaluable for Toxicity | 26 |
| Number of Patients Evaluated for Efficacy | 26 |
| Evaluation Method | RECIST 1.1 |
| Response Assessment CR | n = 0 (0%) |
| Response Assessment PR | n = 0 (0%) |
| Response Assessment SD | n = 5 (19%) |
| Response Assessment PD | n = 13 (50%) |
| Response Assessment OTHER | n = 8 (31%) |
| (Median) Duration Assessments PFS | 70 days, CI: 69–71 |
| (Median) Duration Assessments TTP | 69 days, CI: 55–85 |
| (Median) Duration Assessments OS | 157 days, CI: 117–197 |
| (Median) Duration Assessments Duration of Treatment | 70 days |
| Outcome Notes |
Other category specified: Early death from malignant disease (n = 3) Early death from other cause (n = 1) Not assessable (withdrew consent) (n = 2) Not assessable (rapid clinical deterioration) (n = 1) Not assessable (off‐study after infections) (n = 1) |
Adverse Events
| All Dose Levels, All Cycles | |||||||
|---|---|---|---|---|---|---|---|
| Name | NC/NA | 1 | 2 | 3 | 4 | 5 | All Grades |
| Injection site reaction | 38% | 62% | 0% | 0% | 0% | 0% | 62% |
| Fatigue | 57% | 31% | 12% | 0% | 0% | 0% | 43% |
| Fever | 65% | 27% | 8% | 0% | 0% | 0% | 35% |
| Nausea | 88% | 4% | 8% | 0% | 0% | 0% | 12% |
| Flu like symptoms | 88% | 12% | 0% | 0% | 0% | 0% | 12% |
| Weight loss | 92% | 4% | 4% | 0% | 0% | 0% | 8% |
| General disorders and administration site conditions, malaise | 92% | 4% | 4% | 0% | 0% | 0% | 8% |
| Pain in extremity | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
| Neck pain | 96% | 4% | 0% | 0% | 0% | 0% | 4% |
| Anorexia | 92% | 4% | 4% | 0% | 0% | 0% | 8% |
| Bone pain | 96% | 0% | 4% | 0% | 0% | 0% | 4% |
| Skin and subcutaneous tissue disorders, erythema | 96% | 4% | 0% | 0% | 0% | 0% | 4% |
| Aspartate aminotransferase increased | 96% | 0% | 4% | 0% | 0% | 0% | 4% |
| Dyspnea | 96% | 4% | 0% | 0% | 0% | 0% | 4% |
| Myalgia | 96% | 0% | 4% | 0% | 0% | 0% | 4% |
| Skin and subcutaneous tissue disorders, rash | 96% | 4% | 0% | 0% | 0% | 0% | 4% |
| Dizziness | 96% | 4% | 0% | 0% | 0% | 0% | 4% |
| Edema limbs | 96% | 4% | 0% | 0% | 0% | 0% | 4% |
| Alkaline phosphatase increased | 96% | 4% | 0% | 0% | 0% | 0% | 4% |
| Blood and lymphatic system disorders, venous stasis | 96% | 4% | 0% | 0% | 0% | 0% | 4% |
| Blood bilirubin increased | 96% | 4% | 0% | 0% | 0% | 0% | 4% |
| Diarrhea | 96% | 4% | 0% | 0% | 0% | 0% | 4% |
| Headache | 96% | 4% | 0% | 0% | 0% | 0% | 4% |
All adverse events listed are possible, probable, or certainly related. See also Table 5.
Abbreviation: NC/NA, no change from baseline/no adverse event
Serious Adverse Events
| Name | Grade | Attribution |
|---|---|---|
| Fever | 1 | Probable |
| Fever | 2 | Possible |
| Fever | 2 | Possible |
| Pain in extremity | 3 | Unrelated |
| Pain in extremity | 3 | Unlikely |
| Tumor pain | 3 | Unrelated |
| Anemia | 3 | Unrelated |
| Confusion | 2 | Unrelated |
| Urinary tract infection | 3 | Unrelated |
| Sepsis | 4 | Unrelated |
| Somnolence | 3 | Unrelated |
| Thromboembolic event | 3 | Unrelated |
| Abdominal pain | 2 | Unlikely |
| Upper GI hemorrhage | 3 | Unrelated |
| Malaise | 2 | Possible |
| Nausea | 2 | Possible |
| Vomiting | 2 | Unrelated |
See also Table 6. Abbreviation: GI, gastrointestinal.
Dose‐Limiting Toxicities
| Dose level | Dose of drug: hVEGF26–104/RFASE | Number enrolled | Number evaluable for toxicity | Number with a dose‐limiting toxicity |
|---|---|---|---|---|
| 1 | 62.5 μg/20 mg | 4 | 3 | 0 |
| 2 | 125 μg/20 mg | 3 | 3 | 0 |
| 3A | 250 μg/20 mg | 4 | 4 | 0 |
| 3B | 250 μg/40 mg | 4 | 4 | 0 |
| 4 | 500 μg/40 mg | 8 | 7 | 0 |
| 5 | 1,000 μg/40 mg | 4 | 4 | 0 |
Assessment, Analysis, and Discussion
| Completion | Study terminated before completion |
| Terminated Reason | Company stopped development |
| Investigator's Assessment | Drug tolerable, efficacy indeterminant |
Vascular endothelial growth factor‐A (VEGF) is an angiogenic growth factor involved in normal physiology (such as embryogenesis) and disease (such as cancer) [5]. VEGF is produced by several cell types in the human body, including cancer cells and megakaryocytes [6]. Four isoforms are detected in the human body, of which VEGF165 and VEGF121 circulate and are detectable by the VEGF enzyme‐linked immunosorbent assay (ELISA) as used. VEGF in serum is largely derived from platelets, which secrete VEGF upon wounding and in the tumor vasculature to stimulate angiogenesis (i.e., the growth of new blood vessels from preexisting capillaries) [7]. Upon treatment with the anti‐VEGF monoclonal antibody bevacizumab, VEGF is neutralized and no longer exerts biological activity [8].
Antiangiogenic therapy is mostly combined with cytotoxic agents, although there is mounting interest to combine it with other forms of anticancer treatment, such as immunotherapy and radiotherapy [9, 10, 11]. Nonetheless, the clinical benefit observed from antiangiogenic therapy is usually modest, and treatment withdrawal has been associated with rebound growth, possibly due to compensatory pathways activated by other proangiogenic factors and cytokines [12, 13]. Therefore, neutralization of VEGF by active immunization could be an attractive alternative [14]. VEGF inhibition might not only be more durable but also more pronounced due to the induction of a polyclonal antibody response, resulting in higher avidity binding. Furthermore, tumor‐associated plasma cells might ensure that endogenous antibodies have a better tumor‐penetrating capacity, as compared with exogenously administered antibodies [15]. In addition, continued VEGF suppression beyond progressive disease might convey a survival benefit, as demonstrated in metastatic colorectal cancer [16, 17]. Finally, active immunization could lead to a notable reduction in hospital visits and treatment costs, as compared with monoclonal antibody therapy.
hVEGF26–104 is a three‐dimensional (3D)‐structured truncated peptide antigen derived of the endogenous protein human VEGF165 that perfectly mimics the 3D structure of the cysteine knot motif of VEGF165. Immunization with hVEGF26–104 is thus expected to result in antibodies that can cross‐react with and neutralize VEGF165. Biological activity of the (monomeric) peptide hVEGF26–104 itself is prohibited by the substitution of two cysteines, vital for the formation of the VEGF165 homodimer and consequent receptor binding capacities, for alanines. hVEGF26–104 is mixed 1:1 with RFASE adjuvant, a sulpholipopolysaccharide in a squalane‐in‐water emulsion with polysorbate 80 as emulsifier [3, 4]. Immunization of nonhuman primates with hVEGF26–104/RFASE resulted in an RFASE dependent antibody titer against hVEGF26–104 and cross‐reactive antibodies against VEGF165 28 days after primer immunization. Anti‐VEGF165 antibodies were able to inhibit the binding of bevacizumab with VEGF165 in a competition ELISA. Moreover, the biological activity of VEGF165 could be inhibited by the addition of immunized monkey serum in a VEGF‐specific bioassay [18].
In the current phase I clinical trial, 18 out of 27 enrolled patients received all three immunizations and completed the dose‐limiting toxicity observation period (Table 2); reasons for not completing treatment are listed in Table 3. Comparison of median difference in C‐reactive protein (CRP) and white blood cell (WBC) count before and after vaccination suggests a possible, but very weak, innate immune response (Fig. 2). In four patients a body temperature of 38.5°C or higher was observed (Fig. 2); in only one of these patients an antibody titer against hVEGF26–104 was detected. However, a correlation between dosage and these parameters was not observed. In 44% of all administrations a grade 1 local reaction was observed, mostly warmth, pain, and swelling (Table 4). Neither clinically significant toxicity (Table 5, 6) nor clinical benefit was observed at any of the dose‐levels. At a first evaluation by computed tomography scan, stable disease (SD) was observed in five patients. Nevertheless, four of these patients had clinical progression (progressive disease; PD) and went off‐study. One patient in the first dose level received an additional booster vaccine after 10 weeks (optional for patients with SD and no signs of VEGF suppression); she progressed at evaluation 10 weeks later. In total, 13 patients showed PD. Four patients succumbed before first evaluation; three because malignant disease and one because of pneumonia. Finally, four patients were not assessable. Median overall survival was 157 days (95% confidence interval [CI], 117–197), and median progression free survival was 70 days (95% CI, 69–71; Table 3).
Table 2.
Baseline characteristics
| Dose‐Level | Sex | Age | ECOG status | Prior systemic therapies | Tumor type | Enrolled | Completed DLT period |
|---|---|---|---|---|---|---|---|
| 1 | F | 51 | 1 | 2 | Urothelial | Yes | No |
| 1 | M | 77 | 1 | 3 | NET a of pancreas | Yes | Yes |
| 1 | F | 49 | 1 | 3 | SCC b of unknown primary | Yes | Yes |
| 1 | M | 70 | 1 | 2 | Salivary duct | Yes | Yes |
| 2 | M | 56 | NA | 7 | NETa of unknown primary | No | Screen failure |
| 2 | F | 56 | 1 | 4 | Ovarian | Yes | Yes |
| 2 | F | 59 | NA | 1 | Pancreas | No | Screen failure |
| 2 | M | 69 | 1 | 0 | Colorectal | Yes | Yes |
| 2 | M | 59 | NA | 0 | Gastric | No | Screen failure |
| 2 | M | 70 | 1 | 4 | Gastric | Yes | Yes |
| 3A | F | 67 | 1 | 3 | Pleiomorphic adenoma | Yes | Yes |
| 3A | F | 68 | 1 | 4 | Metaplastic carcinoma | Yes | Yes |
| 3A | M | 68 | 2 | 3 | Glioblastoma | Yes | No |
| 3A | M | 67 | 1 | 3 | Tongue base | Yes | Yes |
| 3B | F | 59 | 1 | 2 | Colorectal | Yes | No |
| 3B | M | 60 | 1 | 6 | Colorectal | Yes | Yes |
| 3B | F | 78 | 1 | 2 | Colorectal | Yes | Yes |
| 3B | M | 55 | 1 | 3 | Tonsil | Yes | Yes |
| 4 | M | 54 | 1 | 1 | Colorectal | Yes | No |
| 4 | M | 64 | 2 | 3 | Oropharynx and esophageal | Yes | No |
| 4 | F | 62 | 1 | 1 | Ovarian | Yes | No |
| 4 | M | 66 | 1 | 6 | Hepatocellular | Yes | No |
| 4 | M | 70 | 1 | 1 | Colorectal | Yes | Yes |
| 4 | M | 63 | 1 | 1 | Hypopharynx | Yes | Yes |
| 4 | F | 40 | 1 | 3 | Breast | Yes | No |
| 4 | M | 77 | 1 | 5 | Esophageal | Yes | Yes |
| 5 | M | 69 | 1 | 9 | Hepatocellular | Yes | Yes |
| 5 | M | 60 | 0 | 2 | Colorectal | Yes | Yes |
| 5 | M | 71 | 1 | 3 | Prostate | Yes | No |
| 5 | F | 72 | 1 | 3 | Breast | Yes | Yes |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; F, female; M, male; NA, not applicable; NET.
neuroendocrine tumor.
squamous cell carcinoma.
Table 3.
Response evaluation
| Dose‐level 1 | Dose‐level 2 | Dose‐level 3a | Dose‐level 3b | Dose‐level 4 | Dose‐level 5 | All dose‐levels (95% CI) | |
|---|---|---|---|---|---|---|---|
| Screened | 4 | 6 | 4 | 4 | 8 | 4 | 30 |
| Enrolled | 4 | 3 | 4 | 4 | 8 | 4 | 27 |
| Evaluable for toxicity | 4 | 3 | 4 | 4 | 7a | 4 | 26 |
| Evaluable for efficacyb | 4 | 3 | 4 | 4 | 7a | 4 | 26 |
| Stable disease | 2 | 0 | 1 | 2 | 0 | 0 | 5 |
| Progressive disease | 1 | 2 | 2 | 1 | 4 | 3 | 13 |
| Otherc | 1 | 1 | 1 | 1 | 3 | 1 | 8 |
| Median PFS, (days) | 140 | 70 | 70 | NA | 68 | 69 | 70 (69–71) |
| Median TTP, (days) | 112 | 68 | 70 | 69 | 62 | 69 | 69 (55–85) |
| Median OS, (days) | 146 | 151 | 500 | 125 | 137 | 174 | 157 (117–197) |
| Median response duration, (days) | NA | NA | NA | NA | NA | NA | NA |
| Median treatment duration, (days) | 84 | 70 | 69 | 74 | 34 | 77 | 70 |
One patient in dose‐level 4 did not commence treatment because of pulmonary embolism and was therefore excluded from efficacy and toxicity evaluations.
First response evaluation (wk 10) using RECIST 1.1.
Other category specified per dose‐level (DL): DL 1: early death from malignant disease (1×); DL 2: not assessable (rapid clinical deterioration) (1×); DL 3A: early death from malignant disease (1×); DL 3B: not assessable (withdrew consent) (1×); DL 4: early death from malignant disease (1×), early death from other cause (1×), not assessable (off‐study after infections) (1x); DL 5: not assessable (withdrew consent) (1×).
Abbreviations: CI, confidence interval; OS, overall survival; NA, not available; PFS, progression‐free survival; TTP, time to progression.
Figure 2.
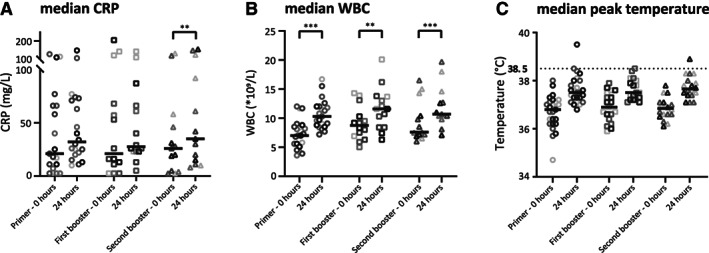
Median rise in CRP (A) and WBC (B) 24 hours after vaccination as compared with baseline was 7.91 mg/l (95% confidence interval [CI], 0.78–11.55, p = .030) and 2.87*109/L (95% CI, 2.30–3.83, p < .001), respectively. Median peak body temperature within 24 hours after vaccination (C) was 0.90°C (95% CI, 0.67–1.22), 0.50°C (95% CI, 0.32–1.07), and 0.95°C (95% CI, 0.52–1.28) higher as compared with baseline, for primer, first, and second booster, respectively. In four cases, a body temperature of 38.5°C or higher was observed. *P < 0.05, **P < 0.01, ***P < 0.001.Abbreviations: CRP, C‐reactive protein; WBC, white blood cell.
Table 4.
Local injection site reactions
| Dose‐level 1 | Dose‐level 2 | Dose‐level 3A | Dose‐level 3B | Dose‐level 4 | Dose‐level 5 | All dose‐levels, n (%) | |
|---|---|---|---|---|---|---|---|
| Reactionsa | 3 | 6 | 6 | 9 | 3 | 1 | 28 (44) |
| Primer | 1 | 1 | 3 | 3 | 2 | 0 | 10 (40) |
| First booster | 1 | 2 | 3 | 3 | 0 | 1 | 10 (50) |
| Second booster | 1 | 3 | 0 | 3 | 1 | 0 | 8 (42) |
| Typeb | |||||||
| Abscess | 0 | 0 | 0 | 0 | 0 | 0 | 0 (0) |
| Cellulitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 (0) |
| Nodule | 0 | 0 | 1 | 0 | 0 | 0 | 1 (4) |
| Induration | 0 | 4 | 0 | 2 | 0 | 0 | 6 (21) |
| Swelling | 2 | 4 | 1 | 5 | 0 | 0 | 12 (43) |
| Pain | 2 | 0 | 3 | 7 | 1 | 1 | 14 (50) |
| Erythema | 1 | 1 | 0 | 0 | 1 | 0 | 3 (11) |
| Warmth | 1 | 4 | 4 | 6 | 2 | 0 | 17 (61) |
Number of local injection site reactions observed in 64 vaccine administrations in 26 patients.
Specification of local reaction type (multiple reaction types possible per reaction).
Table 5.
Adverse events
| Adverse event a | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Total | NC/NA, % |
|---|---|---|---|---|---|---|---|
| Injection site reaction | 16 | 0 | 0 | 0 | 0 | 16 | 38.5 |
| Fatigue | 8 | 3 | 0 | 0 | 0 | 11 | 57.7 |
| Fever | 7 | 2 | 0 | 0 | 0 | 9 | 65.4 |
| Nausea | 1 | 2 | 0 | 0 | 0 | 3 | 88.5 |
| Flu like symptoms | 3 | 0 | 0 | 0 | 0 | 3 | 88.5 |
| Weight loss | 1 | 1 | 0 | 0 | 0 | 2 | 92.3 |
| Malaise | 1 | 1 | 0 | 0 | 0 | 2 | 92.3 |
| Anorexia | 1 | 1 | 0 | 0 | 0 | 2 | 92.3 |
| Pain in extremity | 2 | 0 | 0 | 0 | 0 | 2 | 92.3 |
| Neck pain | 1 | 0 | 0 | 0 | 0 | 1 | 96.2 |
| Bone pain | 0 | 1 | 0 | 0 | 0 | 1 | 96.2 |
| Erythema | 1 | 0 | 0 | 0 | 0 | 1 | 96.2 |
| Aspartate aminotransferase increased | 0 | 1 | 0 | 0 | 0 | 1 | 96.2 |
| Dyspnea | 1 | 0 | 0 | 0 | 0 | 1 | 96.2 |
| Myalgia | 0 | 1 | 0 | 0 | 0 | 1 | 96.2 |
| Rash | 1 | 0 | 0 | 0 | 0 | 1 | 96.2 |
| Dizziness | 1 | 0 | 0 | 0 | 0 | 1 | 96.2 |
| Edema limbs | 1 | 0 | 0 | 0 | 0 | 1 | 96.2 |
| Alkaline phosphatase increased | 1 | 0 | 0 | 0 | 0 | 1 | 96.2 |
| Venous stasis | 1 | 0 | 0 | 0 | 0 | 1 | 96.2 |
| Blood bilirubin increased | 1 | 0 | 0 | 0 | 0 | 1 | 96.2 |
| Diarrhea | 1 | 0 | 0 | 0 | 0 | 1 | 96.2 |
| Headache | 1 | 0 | 0 | 0 | 0 | 1 | 96.2 |
| Total | 51 | 13 | 0 | 0 | 0 | 64 |
Listed adverse events are possible, probable, or certainly related.
Abbreviation: NC/NA, no change from baseline/no adverse event.
Table 6.
Serious adverse events
| SAE | Grade 1 | Related | Grade 2 | Related | Grade 3 | Related | Grade 4 | Related | Grade 5 | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Fever | 1 | Probable | 2 | Possible | 0 | 0 | 0 | 3 | ||
| Pain in extremity | 0 | 0 | 2 | Unlikely 1×; Unrelated 1× | 0 | 0 | 2 | |||
| Tumor pain | 0 | 0 | 1 | Unrelated | 0 | 0 | 1 | |||
| Anemia | 0 | 0 | 1 | Unrelated | 0 | 0 | 1 | |||
| Confusion | 0 | 1 | Unrelated | 0 | 0 | 0 | 1 | |||
| Urinary tract infection | 0 | 0 | 1 | Unrelated | 0 | 0 | 1 | |||
| Sepsis | 0 | 0 | 0 | 1 | Unrelated | 0 | 1 | |||
| Somnolence | 0 | 0 | 1 | Unrelated | 0 | 0 | 1 | |||
| Thromboembolic event | 0 | 0 | 1 | Unrelated | 0 | 0 | 1 | |||
| Abdominal pain | 0 | 1 | Unlikely | 0 | 0 | 0 | 1 | |||
| Upper GI hemorrhage | 0 | 0 | 1 | Unrelated | 0 | 0 | 1 | |||
| Malaise | 0 | 1 | Possible | 0 | 0 | 0 | 1 | |||
| Nausea | 0 | 1 | Possible | 0 | 0 | 0 | 1 | |||
| Vomiting | 0 | 1 | Unrelated | 0 | 0 | 0 | 1 | |||
| Total | 1 | 7 | 8 | 1 | 0 | 17 |
Abbreviations: GI, gastrointestinal; SAE, serious adverse event.
Despite the encouraging results in nonhuman primates, hVEGF26–104/RFASE did not elicit the formation of VEGF165‐cross‐reactive antibodies in patients with cancer. The lack of seroconversion against native VEGF calls for caution in the interpretation of human vaccine efficacies in nonhuman primates. There might be several explanations for the apparent poor antigenicity of hVEGF26–104/RFASE in humans. Besides the earlier mentioned possibilities of diversion of the immune response by dominant neoepitopes in the hVEGF26–104 domain, suboptimal dosing or insufficient adjuvant potency provided by RFASE in humans might have played a role.
To break immunosuppression and self‐tolerance, a powerful adjuvant is a key component of any cancer vaccine. Most cancer peptide vaccines have relied on adjuvants such as incomplete Freund's adjuvant (IFA) or Montanide ISA‐51, both water‐in‐oil emulsions with the antigen forming a depot for slow release purpose. RFASE is an oil‐in‐water emulsion designed to function as an antigen depot and to induce local inflammation and activation of Toll‐like receptor (TLR)‐4 signaling. Interestingly, evidence is emerging that Toll‐like receptor ligands, such as CpG oligonucleotides (TLR‐9 agonist) [19] and Poly I:C polyinosinic:polycytidylic acid; (TLR‐3 agonist) [20] used as vaccine adjuvants, show more effective immune responses after peptide vaccination as compared with IFA or Montanide ISA‐51 [21, 22, 23]. In our in vitro models, stimulation of healthy control human peripheral blood mononuclear cells with lipopolysaccharide (LPS) showed a significant increase in (inflammatory) cytokine release, whereas stimulation with RFASE failed to induce any detectable cytokine release over background levels (Fig. 3). This is a clear indication that RFASE, which is related to LPS, does not have the capacity for induction of an immune response in humans.
Figure 3.
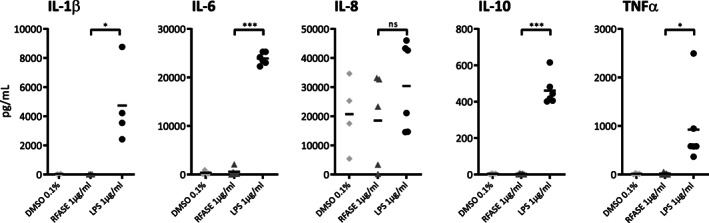
Cytokine levels of IL‐1β, IL‐6, IL‐8, IL‐10, and TNFα in human peripheral blood mononuclear cell supernatants of healthy controls are shown after incubation for 24 hours with either RFASE (1 μg/mL) or LPS (1 μg/mL). DMSO 0.1% served as negative control. *P < 0.05, **P < 0.01, ***P < 0.001.Abbreviations: DMSO, Dimethyl sulfoxide; IL, interleukin; LPS, lipopolysaccharide; ns, not significant; RFASE, raffinose fatty acid sulphate ester; TNF, tumor necrosis factor.
Notwithstanding promising activity in nonhuman primate studies, hVEGF26–104/RFASE did not elicit cross‐reactive neutralizing antibodies against native VEGF and did not show any hint of clinical activity in patients with advanced solid malignancies. We propose that in future studies, addition or substitution of RFASE by an alternative adjuvant with proven efficacy should be considered to break self‐tolerance, induce cross‐reactive antibodies against VEGF165, and consequently, decrease VEGF serum levels.
Disclosures
Hans J. van der Vliet: CSO Lava Therapeutics (E), Lava Therapeutics (IP), Lava Therapeutics, Glycostem (RF), Lava Therapeutics (OI); Tanja D. de Gruijl: DCPrime BV (C/A), Idera Pharmaceuticals (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Figures and Tables
Footnotes
- ClinicalTrials.gov Identifier: NCT02237638
- Sponsor: Immunovo
- Principal Investigator: Henk M.W. Verheul
- IRB Approved: Yes
References
- 1. Wentink MQ, Hackeng TM, Tabruyn SP et al. Targeted vaccination against the bevacizumab binding site on VEGF using 3D‐structured peptides elicits efficient antitumor activity. Proc Natl Acad Sci 2016;113:12532–12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wentink MQ, Verheul HMW, Griffioen AW et al. A safety and immunogenicity study of immunization with hVEGF 26‐104 /RFASE in cynomolgus monkeys. Vaccine 2018;36:2025–2032. [DOI] [PubMed] [Google Scholar]
- 3. Blom AG, Hilgers LAT. Sucrose fatty acid sulphate esters as novel vaccine adjuvants: effect of the chemical composition. Vaccine 2004;23:743–754. [DOI] [PubMed] [Google Scholar]
- 4. Hilgers LA, Blom AG. Sucrose fatty acid sulphate esters as novel vaccine adjuvant. Vaccine 2006;24:S81–S82. [DOI] [PubMed] [Google Scholar]
- 5. Griffioen AW, Angiogenesis Molema G.: Potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol Rev 2000;52:237–268. [PubMed] [Google Scholar]
- 6. Möhle R, Green D, Moore MA et al. Constitutive production and thrombin‐induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc Natl Acad Sci USA 1997;94:663–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Verheul HM, Hoekman K, Luykx‐de Bakker S et al. Platelet: Transporter of vascular endothelial growth factor. Clin Cancer Res 1997;3:2187–2190. [PubMed] [Google Scholar]
- 8. Verheul HMW, Lolkema MPJ, Qian DZ et al. Platelets take up the monoclonal antibody bevacizumab. Clin Cancer Res 2007;13:5341–5347. [DOI] [PubMed] [Google Scholar]
- 9. Goedegebuure RSA, de Klerk LK, Bass AJ et al. Combining radiotherapy with anti‐angiogenic therapy and immunotherapy; A therapeutic triad for cancer? Front Immunol 2019;9:3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ramjiawan RR, Griffioen AW, Duda DG. Anti‐angiogenesis for cancer revisited: Is there a role for combinations with immunotherapy? Angiogenesis 2017;20:185–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dings RPM, Vang KB, Castermans K et al. Enhancement of T‐cell‐mediated antitumor response: angiostatic adjuvant to immunotherapy against cancer. Clin Cancer Res 2011;17:3134–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haemmerle M, Bottsford‐Miller J, Pradeep S et al. FAK regulates platelet extravasation and tumor growth after antiangiogenic therapy withdrawal. J Clin Invest 2016;126:1885–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Griffioen AW, Mans LA, de Graaf AMA et al. Rapid angiogenesis onset after discontinuation of sunitinib treatment of renal cell carcinoma patients. Clin Cancer Res 2012;18:3961–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rahat MA. Targeting angiogenesis with peptide vaccines. Front Immunol 2019;10:1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wentink MQ, Huijbers EJM, de Gruijl TD et al. Vaccination approach to anti‐angiogenic treatment of cancer. Biochim. Biophys. Acta 2015;1855:155–171. [DOI] [PubMed] [Google Scholar]
- 16. Grothey A, Sugrue MM, Purdie DM et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol 2008;26:5326–5334. [DOI] [PubMed] [Google Scholar]
- 17. Bennouna J, Sastre J, Arnold D et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): A randomised phase 3 trial. Lancet Oncol 2013;14:29–37. [DOI] [PubMed] [Google Scholar]
- 18. Wentink MQ, Broxterman HJ, Lam SW et al. A functional bioassay to determine the activity of anti‐VEGF antibody therapy in blood of patients with cancer. Br J Cancer 2016;115:940–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Valmori D, Souleimanian NE, Tosello V et al. Vaccination with NY‐ESO‐1 protein and CpG in Montanide induces integrated antibody/Th1 responses and CD8 T cells through cross‐priming. Proc Natl Acad Sci USA 2007;104:8947–8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ammi R, De Waele J, Willemen Y et al. Poly(I:C) as cancer vaccine adjuvant: Knocking on the door of medical breakthroughs. Pharmacol Ther 2015;146:120–131. [DOI] [PubMed] [Google Scholar]
- 21. Bonam SR, Partidos CD, Halmuthur SKM et al. An overview of novel adjuvants designed for improving vaccine efficacy. Trends Pharmacol Sci 2017;38:771–793. [DOI] [PubMed] [Google Scholar]
- 22. Dubensky TW, Reed SG. Adjuvants for cancer vaccines. Semin Immunol 2010;22:155–161. [DOI] [PubMed] [Google Scholar]
- 23. Khong H, Overwijk WW. Adjuvants for peptide‐based cancer vaccines. J Immunother cancer 2016;4:56. [DOI] [PMC free article] [PubMed] [Google Scholar]


