We read with interest the work by Weber et al 1 reporting that severe liver failure was observed in a patient during SARS-CoV-2 infection. They suggested that close monitoring of liver function is necessary, and further investigation is required to elucidate the risk factors for liver failure in patients with COVID-19. Previous studies have indicated that liver injury could affect the prognosis of patients with COVID-19, and mortality rate was significantly increased in patients with severe liver injury.2 3 However, the risk factors in patients with COVID-19 developing severe liver injury during hospitalisation have not been thoroughly investigated. Thus, in this study, patients with COVID-19 were recruited to identify the risk factors in patients with COVID-19 with severe liver injury.
A total of 192 patients diagnosed with COVID-19 were consecutively hospitalised at Chongqing Public Health Medical Center from January to March 2020, and 12 patients with existing liver disease had been excluded in this study. Liver injury was detected in 75 (39.06%) enrolled patients at admission and 133 (69.27%) during hospitalisation, respectively. Interestingly, liver injury was observed in 25 out of 29 (86.21%) patients with severe COVID-19. In consistence with our findings, Qi et al 4 revealed that 45.71% of the patients had liver injury at admission; furthermore, Fan et al 5 reported that 48.4% of the patients with normal liver function developed liver injury during hospitalisation, suggesting that a high percentage of patients with COVID-19 have liver injury.
Therefore, identification of the risk factors contributing to severe liver injury during hospitalisation is essential for the treatment of COVID-19. The results indicated that the number of T lymphocyte subsets (CD3+, CD4+ and CD8+ T cells) were significantly reduced in patients with severe liver injury, while the proportion of ritonavir, the number of neutrophils and monocytes, and the production of IL-6 and IL-10 were remarkably increased (online supplementary table 1). Univariate analysis indicated that ritonavir, CD3+, CD4+ and CD8+ T cells, IL-6 and IL-10 were potential risk factors in patients with COVID-19 with severe liver injury during hospitalisation (p<0.05, table 1); multivariate analysis revealed that ritonavir (OR 5.63, 95% CI 2.86 to 18.63, p<0.001), IL-6 (OR 2.21, 95% CI 1.09 to 4.67, p=0.006), IL-10 (OR 1.78, 95% CI 1.08 to 3.12, p=0.014) and CD4+ T cells (OR 3.24, 95% CI 1.05 to 6.38, p<0.001) were independent risk factors in patients with COVID-19 with severe liver damage (table 1), suggesting that the progression of liver injury was associated with medication, T lymphocyte subsets and inflammatory cytokines.
gutjnl-2020-321913supp001.pdf (77.5KB, pdf)
Table 1.
Identification of putative risk factors in patients with COVID-19 developing severe liver injury during hospitalisation
| Variable | Univariable OR (95% CI) | P value | Multivariable OR (95% CI) | P value |
| Ritonavir | 5.24 (0.69 to 16.39) | <0.001 | 5.63 (2.86 to 18.63) | <0.001 |
| IL-6, pg/mL | 2.13 (1.08 to 3.21) | <0.001 | 2.21 (1.09 to 4.67) | 0.006 |
| IL-10, pg/mL | 1.82 (1.03 to 2.85) | 0.004 | 1.78 (1.08 to 3.12) | 0.014 |
| CD4+ T cell, per μL | 2.90 (1.85 to 5.96) | <0.001 | 3.24 (1.05 to 6.38) | <0.001 |
| CD8+ T cell, per μL | 1.88 (1.03 to 3.15) | 0.005 | ||
| CD4+ T/CD8+ T cell | 1.08 (0.99 to 1.35) | 0.163 | ||
| CD3+ T cell, per μL | 0.43 (0.19 to 0.88) | 0.034 | ||
| Neutrophil count, ×109/L | 1.11 (1.02 to 1.25) | 0.077 | ||
| Monocyte count, ×109/L | 3.41 (1.05 to 17.99) | 0.106 |
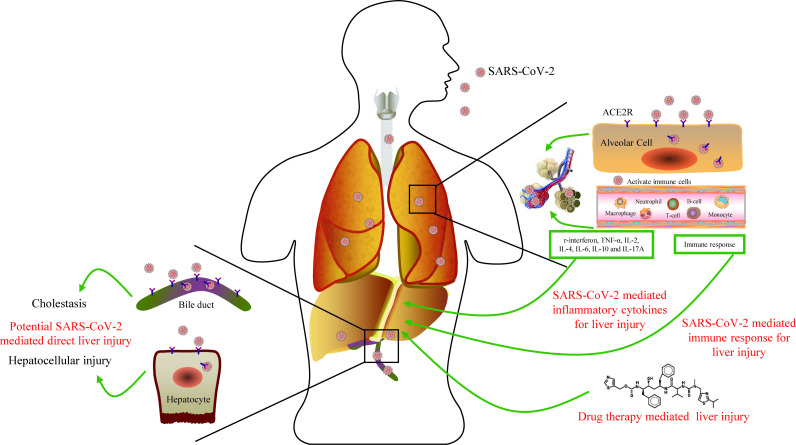
SARS-CoV-2–mediated liver injury might be a key factor in liver damage.6 SARS-CoV-2 may directly target ACE2-positive cholangiocytes and hepatocytes, further leading to liver cell damage and bile duct cell dysfunction, consequently aggravating liver damage. Dysregulated immune response was observed in patients with COVID-19.7 Furthermore, previous studies have indicated that the SARS-CoV-2 infection may primarily affect T lymphocytes, particularly CD4+ and CD8+ cells, which are involved in pro-inflammatory responses.7 8 At present, no specific treatment is available for patients with COVID-19. The commonly used antiviral drugs lopinavir/ritonavir are mainly metabolised in the liver but exhibit side effects such as liver dysfunction. In addition, overdose of ribavirin induces hemolysis and exacerbates tissue hypoxia, leading to the elevation of serum liver enzymes.9 10 A recent study revealed higher proportion in patients with liver dysfunction following the treatment with lopinavir/ritonavir during hospitalisation.5
In conclusion, potential risk factors in patients with COVID-19 developing severe liver injury were ritonavir, elevated IL-6 and IL-10, and reduced CD4+ T cells. In addition, the underlying mechanisms of liver injury in patients with COVID-19 involve immune response, cytokine production, drug-induced liver injury and potential SARS-CoV-2–mediated liver damage (figure 1). Therefore, during the treatment of COVID-19, liver function, inflammatory cytokines and T lymphocyte subsets should be closely monitored, and drug-induced liver damage could be considered in clinical practice.
Figure 1.
Host immune responses and potential liver injury during the viral infection of SARS-CoV-2.
Footnotes
Contributors: ZM, AZ, GY and SL initiated and designed the study. KZ, JL, LL, KY, LQ and CL collected the data. KZ, SL and YB analysed the data. KZ, SL and JL wrote the letter. SL drafted the figure. ZM, AZ and GY reviewed and edited the letter. All authors read and approved the final version for publication.
Funding: This work was supported by the Chongqing Special Research Project for Prevention and Control of COVID-19 (grant no. cstc2020jscx-fyzx0103).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Ethics approval: Chongqing Public Health Medical Center (2020-025-KY).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Weber S, Mayerle J, Irlbeck M, et al. Severe liver failure during SARS-CoV-2 infection. Gut 2020;69:1365–7. 10.1136/gutjnl-2020-321350 [DOI] [PubMed] [Google Scholar]
- 2. Qi X, Liu Y, Wang J, et al. Clinical course and risk factors for mortality of COVID-19 patients with pre-existing cirrhosis: a multicentre cohort study. Gut 2021;70:433–6. 10.1136/gutjnl-2020-321666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lei F, Liu Y-M, Zhou F, et al. Longitudinal association between markers of liver injury and mortality in COVID-19 in China. Hepatology 2020. 10.1002/hep.31301. [Epub ahead of print: 02 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Qi X, Liu C, Jiang Z, et al. Multicenter analysis of clinical characteristics and outcomes in patients with COVID-19 who develop liver injury. J Hepatol 2020. 10.1016/j.jhep.2020.04.010. [Epub ahead of print: 17 Apr 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fan Z, Chen L, Li J, et al. Clinical features of COVID-19-related liver functional abnormality. Clin Gastroenterol Hepatol 2020;18:1561–6. 10.1016/j.cgh.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol 2020;5:529–30. 10.1016/S2468-1253(20)30084-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ong EZ, Chan YFZ, Leong WY, et al. A dynamic immune response shapes COVID-19 progression. Cell Host Microbe 2020;27:879–82. 10.1016/j.chom.2020.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maggi E, Canonica GW, Moretta L. COVID-19: unanswered questions on immune response and pathogenesis. J Allergy Clin Immunol 2020. 10.1016/j.jaci.2020.05.001. [Epub ahead of print: 08 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hung IF-N, Lung K-C, Tso EY-K, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet 2020;395:1695–704. 10.1016/S0140-6736(20)31042-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cai Q, Huang D, Yu H, et al. COVID-19: abnormal liver function tests. J Hepatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2020-321913supp001.pdf (77.5KB, pdf)