Highlights
-
•
BDNF Val66Met is linked to effect of menstrual pain severity on hippocampal volume.
-
•
Moderate PDM with Val alleles showed neuroprotective effect on hippocampal volume.
-
•
The neuroprotective effect of the BDNF Val variant is attenuated in severe PDM.
Keywords: Brain-derived neurotrophic factor, Hippocampus, Primary dysmenorrhea, Menstrual pain severity, Imaging genetics, Magnetic resonance imaging
Abstract
Primary dysmenorrhea (PDM) refers to menstrual pain of which the pathological cause(s) are unknown. This study examined the associations among BDNF Val66Met polymorphisms, menstrual pain severity, and hippocampal volume among young PDM subjects. We recruited 115 PDM subjects, including severe cases (n = 66) and moderate cases (n = 44), and 117 young females (aged 20–30 years) as a control group (CON) for BDNF Val66Met genotyping and MRI examination. The assessment of hippocampal volume involved analysis at various anatomical resolutions, i.e., whole hippocampal volume, hippocampal subfields, and voxel-based morphometry (VBM) volumetric analysis. Two-way ANOVA analyses with planned contrasts and Bonferroni correction were conducted for the assessment of hippocampal volume. Linear regression was used to test for BDNF Val66Met Val allele dosage-dependent effects. We observed no main effects of group, genotype, or group-genotype interactions on bilateral whole hippocampal volumes. Significant interactions between PDM severity and BDNF Val66Met genotype were observed in the right whole hippocampus, subiculum, and molecular layer. Post-hoc analysis revealed that the average hippocampal volume of Val/Val moderate PDM subjects was greater than that of Val/Val severe PDM subjects. Note that right hippocampal volume was greater in the Val/Val group than in the Met/Met group, particularly in the right posterior hippocampal region. Dosage effect analysis revealed a positive dosage-dependent relationship between the Val allele and volume of the right whole hippocampus, subiculum, molecular layer, and VBM-defined right posterior hippocampal region in the moderate PDM subgroup only. These findings indicate that Val/Val PDM subjects are resistant to intermittent moderate pain-related stress, whereas Met carrier PDM subjects are susceptible. When confronted with years of repeated PDM stress, the hippocampus can undergo differential structural changes in accordance with the BDNF genotype and pain severity. This triad study on PDM (i.e., combining genotype with endophenotype imaging results and clinical phenotypes), underscores the potential neurobiological consequences of PDM, which may prefigure in neuroimaging abnormalities associated with various chronic pain disorders. Our results provide evidence for Val allele dosage-dependent protective effects on the hippocampal structure; however, in cases of the Val variant, these effects were modulated in accordance with the severity of menstrual pain.
1. Introduction
Primary dysmenorrhea (PDM) refers to menstrual pain of unknown origin prevalent among women of reproductive age (Coco, 1999). The disorder is common among adolescents and may cause adaptive/maladaptive functional and structural changes in the brain as well as multidimensional psychological responses (Berkley, 2013, Tu et al., 2010, Wei et al., 2016a). Worldwide, approximately 90% of adolescent girls and more than 50% of menstruating women suffer from PDM, 10% to 20% of whom describe their suffering as severe and distressing (Berkley, 2013, Iacovides et al., 2015). In many cases, PDM can be debilitating, with effects on attendance at school and work. The literature strongly suggests that the prevalence of chronic functional pain disorders (e.g., irritable bowel syndrome (Lovell and Ford, 2012), fibromyalgia (Heidari et al., 2017)) is higher among women than among men; however, there is no explicit explanation for this from a neurological or neuroscientific perspective. PDM has been proposed as a plausible clinical precipitant for many chronic functional pain disorders preponderant among females (Berkley, 2013). Despite its high prevalence and effects on quality of life, PDM has received surprisingly little attention in the scientific community (Iacovides et al., 2015).
The hippocampus is a pivotal neural hub of the limbic structures involved in processing memory, emotion, stress, anxiety, and pain (Fortin et al., 2002, Kim et al., 2015, Liu and Chen, 2009). Stress-related glucocorticoids are known have deleterious effects in many regions of the brain; however, it appears that the hippocampus is one of the primary targets (McEwen et al., 1968). Altered hippocampal volume (usually a reduction) is a neural hallmark associated with a multitude of psychiatric disorders (e.g., post-traumatic stress disorders (PTSD), major depression, bipolar disorder, schizophrenia, and antisocial personality disorder) (Geuze et al., 2005) and chronic pain disorders (e.g., fibromyalgia, migraine) (Maleki et al., 2013, McCrae et al., 2015). The hippocampus is not a uniform structure. It is composed of several subfields with distinct morphologies and functions, including the cornu ammonis (CA) 1–4, fimbria, hippocampal fissure, hippocampal tail, dentate gyrus including a granule cell layer and a molecular layer that continuously crosses adjacent subiculum/presubiculum and CA fields, as well as others (Fanselow and Dong, 2010, O'Mara, 2005). In fact, specific subfields of the hippocampus are differentially associated with a variety of neuropathophysiological and behavioral aspects of mood disorders (Hayes et al., 2017, Huang et al., 2013). This study is based on the hypothesis that hippocampal volume could be used as a biometric indicator of vulnerability to various stress-related psychiatric and pain disorders (Gilbertson et al., 2002, Vachon-Presseau et al., 2013).
The hippocampus is one cardinal location of brain-derived neurotrophic factor (BDNF) activity (Hofer et al., 1990). BDNF plays key roles in synaptic plasticity, neuronal growth, and neuronal survival, and is associated with stress regulation (Huang and Reichardt, 2001). BDNF expression is higher in the hippocampus than in any other region of the brain (Murer et al., 1999). Stress has been shown to decrease BDNF expression via inhibitory gamma-aminobutyric acid (GABA)-ergic interneurons in limbic structures, particularly in the hippocampus (Duman and Monteggia, 2006). It has been observed that anxiety levels in Met/Met PDM subjects exceed those in Val carrier PDM subjects, which suggests that the BDNF Val66Met polymorphism is an important regulator of emotions related to menstrual pain (Lee et al., 2014). It has been posited that functional engagement of the hippocampus and other limbic structures can make a critical contribution to the chronicity of pain (Hashmi et al., 2013) and the future development of chronic pain (Wei et al., 2016b). Our recent study discovered that Val/Val homozygosity may provide protective benefits against the effects of PDM, whereas Met/Met homozygosity may render individuals vulnerable to PDM. This may be explained by the fact that the former primarily engages sensory regions of the pain matrix, whereas the later engages limbic structures, such as the hippocampus (Wei et al., 2016b). Furthermore, particularly severe cases of PDM appear to be more strongly associated with the Met/Met genotype (Wei et al., 2016b). Nonetheless, the avenues by which the BDNF Val66Met polymorphism interacts structurally with the complex hippocampus, as modulated by clinical PDM severity, remain hitherto unexplored.
In the current imaging genetics study, we investigated the means by which BDNF Val66Met polymorphisms contribute to the structural plasticity of the hippocampus and its subfields, and how these effects are modulated by pain severity in PDM subjects. Our objective was to elucidate genotype-specific morphometric dynamics that might shed light on individual differences in hippocampal plasticity induced by long-term stress. This study posits that interactions between the BNDF Val66Met polymorphism and PDM severity and their effects on hippocampal volume could be used as a foundation upon which to investigate mechanisms predisposing individuals to chronic pain disorder. This was achieved by adopting a strategy that encompasses hippocampal volumetry at various levels of structural resolution; i.e., whole hippocampal volume, hippocampal subfields, and voxel-based morphometry (VBM) volumetric analysis.
2. Materials and methods
2.1. Participants and study design
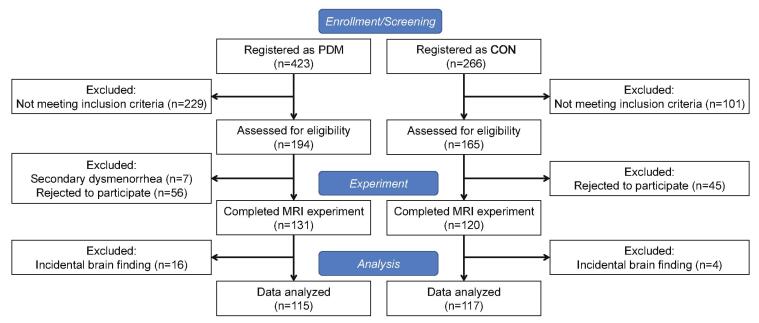
In this imaging genetics study, 423 PDM and 266 control (CON) subjects were enrolled via internet advertisements. The inclusion criteria were as follows: 1) 20–30 year-old right-handed Taiwanese female; 2) regular menstrual cycle (27–32 days); 3) history of PDM exceeding 6 months; 4) average menstrual pain level rated ≧ 4 on a numerical rating scale (NRS; 0 = no pain, 10 = the worst pain imaginable) over the previous 6 months. The inclusion criteria for control subjects were similar to those for PDM. The primary exception was the experience of menstrual pain level rated from none to mild (defined as NRS < 3). CON subjects reported few or no pain-related symptoms, experienced no pain-related limitations in the performance of daily activities, and did not consume NSAIDs or opioids. The exclusion criteria for PDM and CON subjects were a history of head injury, pelvic disease, pituitary gland disease, psychiatric or neurological disorders, a positive pregnancy test or plans for pregnancy, a history of childbirth, claustrophobia, and pacemaker/metal implants. The PDM subjects underwent pelvic sonography to rule out secondary dysmenorrhea with known pathological cause(s). All PDM subjects were screened and diagnosed by the same gynecologist (H.-T.C.). Following rigorous screening in accordance with the above criteria, 194 PDM subjects and 165 CON subjects were included in the study. Among the PDM subjects, 7 were excluded due to secondary dysmenorrhea (e.g., endometriosis, hysteromyoma) in pelvic sonography results, and 56 PDM were excluded due to personal reasons. Among the CON subjects, 45 were excluded for personal reasons. The participants were required not to have used oral contraceptives within 6 months or analgesics within 24 h prior to MRI scanning. We also excluded 16 PDM subjects and 3 CON subjects with incidental conditions observed during the MRI examinations (e.g., mega cisterna magna, cavum septum pellucidum, or arachnoid cysts; for more details, see (Li et al., 2015)). The final number of subjects eligible for neuroimaging analyses included 115 PDM and 117 CON subjects (Fig. 1). PDM cases were partitioned into moderate pain (NRS = 4–6) and severe pain (NRS = 7–10) subgroups in accordance with common practice in both research and clinical settings (Ameade and Mohammed, 2016, Breivik et al., 2008). We exclude 5 PDM subjects in pain severity subgroups grouping due to NRS data loss. All participants provided written informed consent and the Institutional Review Board of Taipei Veterans General Hospital approved this study. All participants completed data acquisition on the same day, including blood collection, psychological/clinical assessments, and MRI scanning, during the periovulatory phase visit (non-pain stage; days 12–16 of the menstrual cycle).
Fig. 1.
Flow-chart showing process of subject selection.
2.2. BDNF Val66Met genotyping and hormone measurements
Genotyping for the BDNF Val66Met polymorphism (rs6265) and hormone measurements were conducted using the methods described in previous studies (Lee et al., 2014). Briefly, whole blood collected in 10 mL EDTA tubes was subjected to DNA extraction using the Puregene kit (Gentra Systems, Minneapolis, MN). Genotyping was performed using the TaqMan SNP Genotyping Assays in an ABI 7900HT Fast Real-Time Polymerase Chain Reaction System (Applied Biosystems, Foster City, CA, USA). Allele calling was performed using the SDS 2.2 software package. Polymerase chain reaction conditions were as follows: 50 °C for 2 min, 95 °C for 10 min, and 40 cycles of 94 °C for 15 sec and 62 °C for 1 min. Subjects were assigned to the BDNF Val66Met genotype subgroups by two technicians working independently.
Estradiol concentrations were measured via chemiluminescence immunoassay using the UniCel DxC 800 Synchron Clinical Systems (Beckman Coulter, Brea, CA, USA). Fluctuations in estradiol concentrations throughout the menstrual cycle have been shown to affect changes in hippocampal volume (Lisofsky et al., 2015). In the current study, estradiol concentration was used as a covariate to avoid the influence of individual variations on estimates of hippocampal volume.
2.3. Psychological/clinical assessments
Prior to MRI scanning, the participants filled out the self-rated Spielberger State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), Beck Depression Inventory (BDI), Pain Catastrophizing Scale (PCS), Basic Personality Inventory (BPI), and Short Form (36) Health Survey (SF-36) to evaluate their psychological state and quality of life. Note that we used only the hypochondriasis and anxiety scale of BPI in this study. The PDM subjects completed the McGill pain questionnaire (MPQ) to characterize menstrual pain events and assess pain intensity.
2.4. MRI image acquisition
High-resolution T1-weighted brain images were obtained using a 3-Tesla MRI system (MAGNETOM Trio, A Tim System; Siemens Medical Solution, Erlangen, Germany) with a three-dimensional magnetization-prepared rapid gradient echo (3D-MPAGE) sequence (repetition time (TR) = 2530 ms, echo time (TE) = 3.03 ms, inversion time (TI) = 1100 ms, flip angle = 7°, matrix size = 224 * 256, field of view (FOV) = 224 * 256, 192 contiguous images, 1 mm thickness, voxel size = 1*1*1 mm3) with a 12-channel head coil.
2.5. Image processing and analysis
2.5.1. Atlas-based volumetric analyses of the whole hippocampus and hippocampal subfields
Image processing was implemented as follows: head motion correction, non-brain tissue removal, automated Talairach space transformation, image intensity normalization, segmentation of subcortical white matter and deep gray matter, tessellation of gray matter and white matter boundaries, automated topology correction, and surface deformation to ensure the optimal placement of gray/white and gray/cerebrospinal fluid boundaries. We employed automated parcellation using the FreeSurfer software package (version 6.0, available at http://sutfer.nmr.mgh.harvard.edu/) to segment the hippocampus and its subfields. Volumes of hippocampal subfields (i.e., parasubiculum, presubiculum, subiculum, CA1, CA2/3, CA4, granule cells in the molecular layer of the dentate gyrus (GC-ML-DG), hippocampal-amygdaloid transitional area (HATA), fimbria, molecular layer of the hippocampus, hippocampal fissure, and hippocampal tail) were estimated in accordance with a refined probabilistic atlas, which built upon manual delineations of the hippocampus from 15 ultra-high ex-vivo scans.
2.5.2. Voxel-based morphometry (VBM) analysis of the whole hippocampus
Statistical Parametric Mapping 12 (SPM12, Welcome Department of Cognitive Neurology, Institute of Neurology, University College London, London, UK) was employed for VBM analysis. The skull and non-brain tissue were first stripped using the BET (Brain Extraction Tool) included in the FSL package (FMRIB Software Library, Oxford, UK). MR image-intensity non-uniformity correction was implemented using the Bias Field Corrector in the BrainSuite package (http://brainsuite.usc.edu). We then segmented the MRIs into images showing gray matter (GM), white matter (WM), and cerebrospinal fluid. The segmented GM images were spatially normalized to a customized study-specific GM template via diffeomorphic anatomical registration using the exponential Lie algebra (DARTEL) algorithm. Individual GM images were normalized to a study-specific template and then transformed to the Montreal Neurological Institute (MNI) space. The normalized GM images were modulated and smoothed using an 8-mm FWHM Gaussian kernel. Finally, we applied a voxel threshold to a GM probability value exceeding 0.2 to avoid partial volume effects in subsequent statistical analysis. Hippocampal masks derived using the automated anatomical labeling (AAL) atlas were applied in voxel-wise volumetric comparisons.
2.6. Statistical analyses
In BDNF Val66Met genotyping, the Hardy-Weinberg equilibrium was assessed using a Chi-square test. For demographic data and psychological and clinical assessments, we conducted two two-way ANOVA analyses using group (PDM vs. CON) /severity (severe PDM vs. moderate PDM) and the BDNF Val66Met genotype (Val/Val vs. Val/Met vs. Met/Met) as independent variables. In cases where we observed the main effects of genotype or interactions (group–genotype/severity–genotype), post-hoc analysis with Bonferroni adjustment was performed. To test for BDNF Val66Met Val allele dosage-dependent effects, we constructed a linear regression model using phenotypes as the dependent variable and Val66Met genotype coded according to the number of Val alleles (Val/Val = 2, Val/Met = 1, and Met/Met = 0) as the independent variable. Missing data were excluded from the analysis.
In our volumetric analysis of the whole hippocampus and hippocampal subfields, we applied a statistical model similar to the one mentioned above; however, age, concentration of estradiol, and total GM volume were used as covariates. We conducted a two-tailed partial correlation analysis using age, concentration of estradiol, and total GM volume as covariates in order to characterize the relationships between psychological/clinical data and the GM volumetric related to the hippocampus. To avoid multiple comparison problem, the Bonferroni correction was applied by lowering the significance levels to P = 0.025 (P = 0.05/2) for whole hippocampus volumetric analysis and P = 0.002 (P = 0.05/24) for hippocampal subfields volumetric analysis. Because the study was exploratory, a less strict threshold of uncorrected p < 0.01 was considered in this study. All statistical analysis was performed using SPSS Statistics 25.0 (IBM Corporation, Software Group, Somers, New York, USA) or the MATLAB statistics toolbox.
VBM analysis was based on a general linear model with age, concentration of estradiol, and total GM volume used as covariates. Here, we adopted the same two-way ANOVA model described above. We adopted a planned contrast approach (Brooks and Johanson, 2011, Wu and Slakter, 1990) to characterize between-group differences in BDNF Val66Met genotypes (using a two-sample t-test model) and within-group differences in BDNF Val66Met genotypes (using a one-way ANOVA model). Planned contrasts pose specific questions as opposed to the null vs. alternative hypotheses of conventional ANOVA (Seltman, 2018). They are commonly used in functional neuroimaging studies to detect subtle but potentially significant neuroscientific findings (Lee et al., 2018, Pazmany et al., 2017, Wu et al., 2016). A stringent threshold of p < 0.05 with FWE correction was applied. Mean GM values of all voxels within each surviving cluster obtained from VBM analysis were extracted for analysis of BDNF Val66Met allele dosage effects and correlation analysis.
3. Results
3.1. Using behavioral and genetic information for grouping
Based on our previous works in imaging genetics (Wei et al., 2016b) and behavior (Lee et al., 2014), we did not expect to find strong single SNPs (single nucleotide polymorphisms) associated with complex pain behaviors in a small sample. Note that the sample size in the current study would be considered small for a behavioral study, but relatively large for an imaging study. Rather, our aim was to group genetic and behavioral information for use in subsequent imaging analyses. Following the exclusion of problematic data (e.g., defective MRI scans, brain anomalies, overt head motions, incomplete experiment), we enrolled 115 young PDM subjects (23.5 ± 2.2 y/o) and 117 young control subjects (24.1 ± 2.5 y/o), who were grouped for the imaging genetics study as follows (PDM, CON): Val/Val (30, 35), Val/Met (47, 57) and Met/Met (38, 25). The BDNF Val66Met genotype distributions in both groups complied with the Hardy-Weinberg equilibrium (PDM: X2 = 3.67, p = 0.16; CON: X2 = 0.004, p = 0.98). Among the 115 PDM subjects, 66 graded their pain as severe (NRS = 7–10) and 44 females graded their pain as moderate (NRS = 4–6). The participant distributions among the genetic subgroups in terms of pain severity (severe, moderate) were as follows: Val/Val (17, 13), Val/Met (28, 16), and Met/Met (21, 15). The demographic data did not reveal any factors with significant effects other than age distribution (i.e., group, genotype, or group–genotype interaction) (Table 1). The scores pertaining to psychological assessments of pain-laden emotions were significantly higher in the PDM group than in the CON group: State-Trait Anxiety Inventory (STAI)-Trait, Beck Depression Inventory (BDI), Beck Anxiety Inventory (BAI), Pain Catastrophizing Scale (PCS), hypochondriasis and the anxiety scales of Basic Personality Inventory (BPI), and Short Form-36 Health Survey (SF-36) (Supplementary Table 1). The main effect of genotype on STAI-State scores was significant, and the post-hoc Bonferroni test revealed significant genotypic differences between the Val/Val and Met/Met subgroups (Supplementary Table 1). The PCS level was higher among severe PDM subjects than among moderate PDM subjects, as were the scores on the Hypochondriasis Scale of BPI, SF-36, and recalled pain rating score of the McGill pain questionnaire (MPQ). The main effects of genotype and severity–genotype interactions in PDM subjects were non-significant (Supplementary Table 2).
Table 1.
Demographic data and clinical assessment results in PDM and CON groups.
| PDM (n = 115) | CON (n = 117) | p-value |
|||
|---|---|---|---|---|---|
| Main effect |
Interaction | ||||
| Group | Genotype | ||||
| Age, y/o | 23.5 (2.2) | 24.1 (2.5) | 0.03* | 0.13 | 0.10 |
| Val/Val | 22.8 (1.9) | 24.2 (2.7) | |||
| Val/Met | 24.2 (2.5) | 24.1 (2.6) | |||
| Met/Met | 23.1 (1.7) | 24.0 (2.4) | |||
| Age of menarche, y/o | 12.1 (1.2) | 12.2 (1.2) | 0.44 | 0.88 | 0.82 |
| Val/Val | 12.1 (1.3) | 12.1 (1.3) | |||
| Val/Met | 12.0 (1.2) | 12.3 (1.1) | |||
| Met/Met | 12.2 (1.1) | 12.3 (1.3) | |||
| Gynecological age, years | 11.4 (2.5) | 11.8 (2.7) | 0.12 | 0.18 | 0.08 |
| Val/Val | 10.7 (2.4) | 12.0 (2.7) | |||
| Val/Met | 12.2 (2.9) | 11.8 (2.7) | |||
| Met/Met | 10.9 (1.9) | 11.7 (3.0) | |||
| Menstrual cycle, days | 29.5 (1.5) | 29.7 (1.5) | 0.32 | 0.57 | 0.82 |
| Val/Val | 29.4 (1.6) | 29.5 (1.0) | |||
| Val/Met | 29.5 (1.4) | 29.7 (1.7) | |||
| Met/Met | 29.5 (1.4) | 29.9 (1.5) | |||
| Estradiol (pg/mL) | 139.7 (100.4) | 125.5 (106.7) | 0.33 | 0.07 | 0.70 |
| Val/Val | 106.8 (75.5) | 104.4 (95.7) | |||
| Val/Met | 145.8 (101.9) | 138.2 (117.5) | |||
| Met/Met | 157.4 (111.5) | 125.5 (94.1) | |||
| Age of onset, y/o | 15.1 (2.2) | – | 0.12 | ||
| Val/Val | 15.8 (2.0) | – | |||
| Val/Met | 14.9 (2.2) | – | |||
| Met/Met | 14.8 (2.2) | – | |||
| Pain history, years | 8.3 (3.1) | – | 0.004* | ||
| Val/Val | 7.0 (2.8) | – | |||
| Val/Met | 9.3 (3.5) | – | |||
| Met/Met | 8.2 (2.2) | – | |||
| Pain duration, days | 1.8 (0.8) | – | 0.12 | ||
| Val/Val | 1.8 (0.8) | – | |||
| Val/Met | 1.7 (0.8) | – | |||
| Met/Met | 2.0 (0.8) | – | |||
| Recall numeric pain rating scale | 6.9 (1.5) | – | 0.93 | ||
| Val/Val | 6.8 (1.7) | – | |||
| Val/Met | 6.9 (1.5) | – | |||
| Met/Met | 6.9 (1.3) | – | |||
| MPQ-recalled pain rating scale | 35.4 (13.9) | – | 0.70 | ||
| Val/Val | 33.5 (14.6) | – | |||
| Val/Met | 35.8 (13.9) | – | |||
| Met/Met | 36.2 (13.6) | – | |||
Data are expressed as mean (standard deviation) and *, p < 0.05.
Abbreviations: PDM, primary dysmenorrhea; CON, control; BDNF, brain-derived neurotrophic factor; Val, Valine; Met, Methionine; MPQ, McGill Pain Questionnaire.
3.2. Association of global hippocampal volume and genotypes as modulated by PDM severity
PDM tends to be cyclic, acute, and repetitive with a relatively short duration; therefore, we did not expect to observe prominent volumetric alterations at the whole-hippocampus level, which is commonly observed in sustained intense distress accompanied with eminent emotional problems (e.g., post-traumatic stress disorder) (Woon et al., 2010). We first conducted a whole hippocampal volume analysis from a global perspective. As anticipated, we observed no main effects of group, genotype, or group-genotype interactions on bilateral whole hippocampal volumes (Supplementary Table 3). As a biometric measurement, the sensitivity of neuroimaging is greater than that of behavioral presentations (Rose and Donohoe, 2013); therefore, we next looked for subtle interactions between genotypes and volume morphometrics as modulated by clinical PDM severity.
A significant correlation between PDM severity and BDNF Val66Met genotype was observed in the right hippocampus (F(2,101) = 4.85, p = 0.01, Cohen's f = 0.31; Supplementary Table 4) and a non-significant interaction was observed in the left hippocampus (F(2,101) = 2.12, p = 0.13, Cohen's f = 0.20; Supplementary Table 4). Simple post-hoc analysis revealed that the right hippocampal volumes of Val/Val moderate PDM subjects were greater than those of Val/Val severe PDM subjects (left: F(1,101) = 3.88, p = 0.052; right: F(1,101) = 7.63, p = 0.007). We observed no differences in PDM severity between Val/Met carriers (left: p = 0.54; right: p = 0.23) and Met/Met carriers (left: p = 0.67; right: p = 0.40). Post-hoc analysis on the simple effects of genotype in each hippocampus hemisphere revealed significant differences only among subjects with moderate PDM (left: F(2,101) = 3.00, p = 0.054; right: F(2,101) = 4.82, p = 0.01). Note that the right hippocampal volume in the Val/Val group was greater than those in the Val/Met group (left: p = 0.063; right: p = 0.019) and Met/Met group (left: p = 0.20; right p = 0.029) (Fig. 2A).
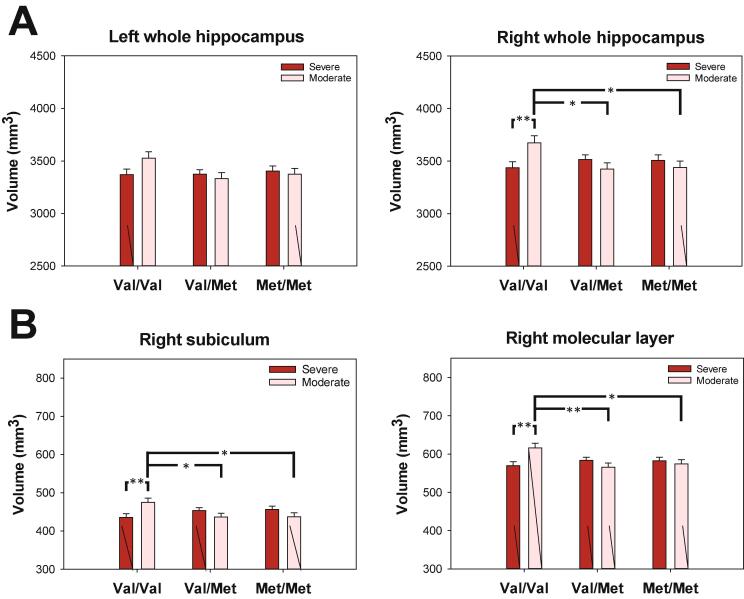
Fig. 2.
Post-hoc genotype analysis of PDM severity on global (A) and subfield (B) hippocampal volumes. Neuroprotective effect of the Val variant of BDNF Val66Met polymorphism on hippocampus in subjects with moderate menstrual pain. Post-hoc genotype analysis on global and subfield hippocampal volumes revealed that only subjects with moderate PDM demonstrated a positive Val allele dosage effect on the hippocampus. Note that the effects were particularly evident in the right whole hippocampus, subiculum, and molecular layer subfields. All volumetric data were adjusted by age, concentration of estradiol, and total brain gray matter volume. Bar graphs show means and SEM. *p < 0.05; **p < 0.01, post-hoc Bonferroni test.
Volumetric analysis of the right hippocampus revealed a positive Val allele dosage effect only among subjects with moderate PDM (viz. negative Met allele dosage effect) (moderate PDM: r = 0.56, beta = 0.36, t = 2.65, p = 0.012, Cohen's f2 = 0.45; severe PDM: r = 0.63, beta = -0.08, t = -0.78, p = 0.44, Cohen's f2 = 0.66; Fig. 2A).
Based on the fact that the hippocampus is involved in the regulation of stress-related emotions, we then investigated whether the volumetric changes were related to psychological phenomena (Phelps, 2004). Among Val/Met moderate PDM subjects, there was a negative correlation between right hippocampal volume and scores on the PCS (r = -0.56, p = 0.048). Among Met/Met severe PDM subjects, there were negative correlations between bilateral hippocampal volumes and scores on the BPI anxiety scale (left: r = -0.62, p = 0.008; right: r = -0.51, p = 0.035). Among all Met/Met PDM subjects, there was a negative correlation between left hippocampal volume and scores on the BPI-anxiety scale (r = -0.35, p = 0.044).
It was interesting to observe that among Met/Met CON subjects, the left and right hippocampal volumes were positively correlated with the BPI-anxiety scale (left: r = 0.54, p = 0.009; right: r = 0.63, p = 0.002). Such discordant relationships imply that the hippocampus of Met/Met CON subjects responded to ordinary anxiety in a normal reactive manner, whereas the hippocampus of Met/Met PDM subjects responded to distressed pain anxiety in a deranged manner.
Collectively, these results provide initial confirmation of our hypothesis that the Val allele exerts neuroprotective effects on hippocampal structures and clinical manifestations (PDM severity and negative emotions), whereas the Met allele exerts deleterious effects. In addition, our proposition that hippocampal volume could be used as an objective indicator inversely reflecting the degree of pain-laden negative emotions (pain as a stress) is also basically confirmed (Mutso et al., 2012). It appears that the neural protective (Val allele) or deleterious (Met allele) effects manifest in an allele dosage-dependent manner. Our findings revealed that Met/Met homozygous individuals were limited in their capacity to cope with pain-related stress. Note however that the protective effects of Val were only viable in cases of moderate PDM, as indicated by the relative reduction in volume observed in Val/Val subjects with severe PDM. This implies that repeated overt painful stress may consume the protective reservoir available to Val/Val homozygous individuals.
3.3. Association between subfield volume and genotypes, as modulated by PDM severity
We adopted the atlas-based subfield approach to unveil possible fine-field changes, which might otherwise escape detection using the global volumetric approach. In the between-group subfield comparisons, only the volumes of the left and right presubiculum were smaller in PDM subjects than in CON subjects (left: F(1,223) = 15.39, p < 0.001, Cohen's f = 0.26; right: F(1,223) = 28.08, p < 0.001, Cohen's f = 0.36; Supplementary Table 5, Supplementary Table 6). We then sought to determine whether these decreases in volume were influenced by the severity of menstrual pain. Our results revealed no PDM severity effect on left or right presubiculum volumes (left: F(1,101) = 0.11, p = 0.74, Cohen's f = 0.03; right: F(1,101) = 1.3, p = 0.26, Cohen's f = 0.11), indicating that the observed changes in volume were unrelated to PDM severity.
Interaction analysis revealed notable PDM severity–genotype interactions in the right subiculum (F(2, 101) = 5.5, p = 0.006, Cohen's f = 0.33) and the right molecular layer of the hippocampus (F(2,106) = 5.3, p = 0.006, Cohen's f = 0.33; Supplementary Table 7, Supplementary Table 8). Subsequent post-hoc severity analysis revealed that among Val/Val subjects, the field volumes of the right subiculum and molecular layer were greater among those with moderate PDM than among those with severe PDM (p = 0.008 and 0.004, respectively). Post-hoc genotype analysis revealed that among subjects with moderate PDM, the field volumes of the right subiculum and right molecular layer were greater among Val/Val than among Val/Met subjects (p = 0.036 and 0.007, respectively) and Met/Met subjects (p = 0.041 and 0.032, respectively) (Fig. 2B).
Our next exam revealed a marginally significant positive dosage-dependent relationship between the volume of these subfields and the number of Val alleles (viz. negative dosage-dependent relationship with the number of Met alleles); however, this effect was observed only in the moderate PDM subgroup (right subiculum: moderate PDM: r = 0.45, beta = 0.38, t = 2.59, p = 0.013, Cohen's f2 = 0.25; severe PDM: r = 0.52, beta = -0.18, t = -1.57, p = 0.12, Cohen's f2 = 0.37 and the right molecular layer: moderate PDM: r = 0.54, beta = 0.36, t = 2.59, p = 0.013, Cohen's f2 = 0.41; severe PDM: r = 0.61, beta = -0.09, t = -0.85, p = 0.40, Cohen's f2 = 0.59; Fig. 2B).
The results of subfield analysis indicated that most of the dynamic structural plasticity occurred in the subiculum, presubiculum, and molecular layer. These findings provide further support for the assertion that BDNF Val66Met polymorphisms contribute to the structural plasticity of the hippocampus, as modulated by pain severity in PDM subjects. Subfield analysis provided a coherent indication that the BDNF Val66Met polymorphisms substantially modulated the variations in volume; however, the protective effects of Val worked only under conditions of moderate pain; i.e., the effects were overridden in cases of severe menstrual pain.
3.4. Association between VBM volume and genotypes, as modulated by PDM severity
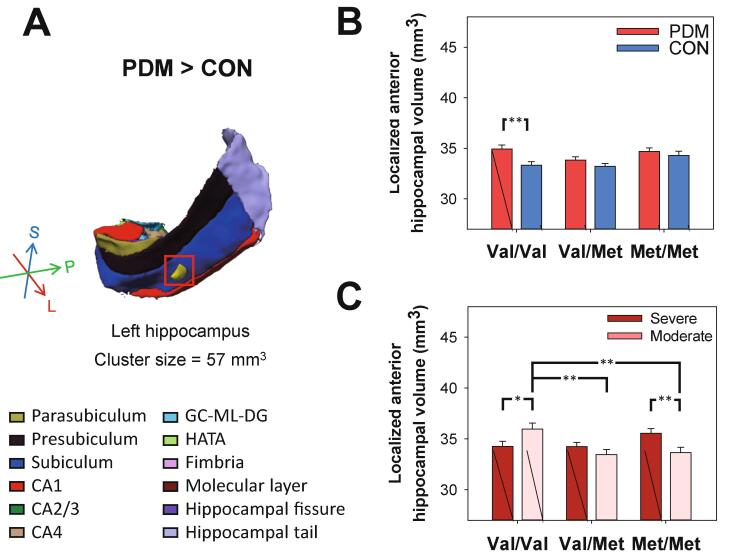
To elucidate the subtle changes in hippocampal volume, we adopted a fine-grained VBM approach to facilitate anatomical localization. We first examined potential differences between the entire PDM and CON groups. Interestingly, we observed that the VBM-defined focal gray matter (GM) volume in the left anterior hippocampus was larger in the PDM group than in the CON group (T (1, 227) = 3.28, family-wise error rate (FWE) corrected p < 0.05, cluster size = 57 mm3, peak MNI coordinates: [-30, −18, −21], Cohen’s d = 0.42; Fig. 3A). This finding suggests that PDM stress is associated almost entirely with the anterior hippocampus. Furthermore, this finding is in line with the aforementioned caudal/ventral-rostral/dorsal functional organization of the hippocampus. The localized increase in volume may partially be attributed to genotype-specific alterations in volume. Among Val/Val subjects, the local volume of the anterior hippocampus was greater among PDM subjects than among CON subjects (F(1,223) = 9.12, p = 0.003; Fig. 3B).
Fig. 3.
Post-hoc analysis on VBM-defined hippocampal volumes for PDM vs. CON and PDM severity. (A) The volume of the VBM-defined localized left anterior hippocampus was significantly greater in the overall PDM group than in the overall CON group (indicated by the yellow sphere in the 3D visualization of the left hippocampal subfield segmentation). (B) Among Val/Val subjects, the volume of this region was greater among PDM subjects than among CON subjects (p = 0.003). (C) This region also manifested a significantly positive dosage-dependent relationship with the number of Val alleles in subjects with moderate PDM (r = 0.60, beta = 0.33, t = 2.53, p = 0.015). Note that among Val/Val subjects, the volume of this region was greater in cases of moderate PDM than in cases of severe PDM (p = 0.027). Note also that within-severity group post-hoc analysis revealed that the volume of this region was greater in the Val/Val group than in the Val/Met group (p = 0.006) and Met/Met group (p = 0.012). Among Met/Met subjects, the volume of the VBM-defined anterior hippocampus was greater in subjects with severe PDM than in subjects with moderate PDM (p = 0.007); however, we observed neither genotype main effects nor between-genotype differences in the severe PDM subgroup. All volumetric data were adjusted by age, concentration of estradiol, and total gray matter volume. Bar graphs show means and SEM. *p < 0.05; **p < 0.01, post-hoc Bonferroni test. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Post-hoc analysis of PDM severity revealed that among Val/Val subjects, the volume of VBM-defined anterior hippocampus was greater among those with moderate PDM than among those with severe PDM (F(1,101) = 5.01, p = 0.027; Fig. 3C). Post-hoc analyses of genotype revealed that the volume of the VBM-defined anterior hippocampus was greater in the Val/Val group than in the Val/Met group (p = 0.005) and Met/Met group (p = 0.008), which indicates that the main contribution was from moderate PDM subjects (Fig. 3C). Despite the fact that among Met/Met subjects, the volume was greater in cases of severe PDM than in cases of moderate PDM (F(1,101) = 7.48, p = 0.007; Fig. 3C), we did not observe genotype main effects nor between-genotype differences in the severe PDM subgroup, which implies that volume protection from the Val allele was insignificant. Furthermore, the VBM-defined anterior hippocampus presented a significantly positive dosage-dependent relationship with the number of Val alleles in moderate PDM subjects (r = 0.60, beta = 0.33, t = 2.53, p = 0.015, Cohen's f2 = 0.56; Fig. 3C). We provide an evidence for the Val variants of the BDNF Val66Met polymorphism protective effects on hippocampal volume under repeated exposure to PDM pain-related stress of moderate degree.
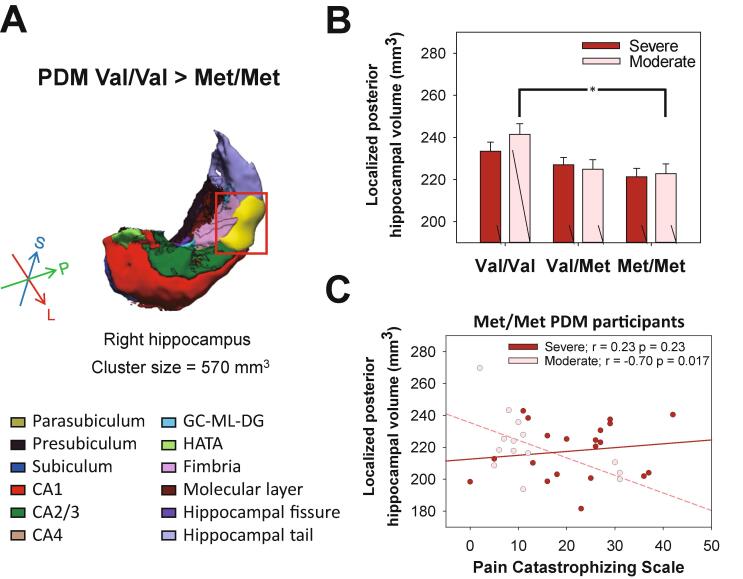
We next looked for subtle differences between Val/Val PDM and Met/Met subjects overall. Our analysis revealed that the VBM-defined localized volume of the right posterior hippocampus was larger among Val/Val PDM subjects than among Met/Met PDM subjects (T(1,109) = 3.64, FWE corrected p < 0.05, cluster size = 570 mm3, peak MNI coordinates: [35, −35, −5], Cohen’s d = 0.61; Fig. 4A). The Val/Val VBM-defined right posterior hippocampus demonstrated a simple genotype effect in moderate PDM subjects (F(2,101) = 4.40, p = 0.015; Fig. 4B). Post-hoc analysis also revealed that the volume of the VBM-defined right posterior hippocampus was greater in the Val/Val group than in the Val/Met (p = 0.051) and Met/Met group (p = 0.022), which indicates that the main contribution was from Val/Val moderate PDM subjects (Fig. 4B). Dosage effect analysis revealed a positive dosage-dependent relationship between the Val allele and the volume of the VBM-defined right posterior hippocampal in the overall PDM group (PDM: r = 0.50, beta = 0.28, t = 3.35, p = 0.001, Cohen's f2 = 0.33; CON: r = 0.54, beta = 0.06, t = 0.70, p = 0.49, Cohen's f2 = 0.41) and the moderate PDM subgroup (moderate PDM: r = 0.53, beta = 0.40, t = 2.90, p = 0.006, Cohen's f2 = 0.39; severe PDM: r = 0.51, beta = 0.22, t = 1.90, p = 0.06, Cohen's f2 = 0.35; Fig. 4B). Note also that among Met/Met moderate PDM subjects, the volume of the VBM-defined posterior hippocampal region was negatively correlated with PCS level (the higher the PCS score, the worse the pain cognition and coping as well as hypervigilance to pain (Sullivan et al., 1995) (r = -0.70, p = 0.017; Fig. 4C).
Fig. 4.
Post-hoc analysis of PDM severity on genotype-VBM-defined hippocampal volumes. (A) Among subjects with PDM, the volume of the VBM-defined localized right posterior hippocampus was significantly greater among Val/Val subjects than among Met/Met subjects (indicated by the yellow sphere in the 3D visualization of the right hippocampal subfield segmentation). (B) This region manifested a significantly positive dosage-dependent relationship with the number of Val alleles in subjects with moderate PDM (r = 0.53, beta = 0.40, t = 2.90, p = 0.006). Genotype effects were apparent in the volume of this region among subjects with moderate PDM (p = 0.015). Post-hoc analyses revealed that the volume of this region was greater in the Val/Val group than in the Val/Met group (p = 0.051) and Met/Met group (p = 0.022). (C) In addition, the volume of this region was negatively correlated with pain catastrophizing scale level only among Met/Met subjects with moderate PDM (n = 14, r = -0.70, p = 0.017); not among Met/Met subjects with severe PDM (n = 20, r = 0.23, p = 0.37). All volumetric data were adjusted by age, concentration of estradiol, and total gray matter volume. Bar graphs show means and SEM. *p < 0.05; **p < 0.01, post-hoc Bonferroni test. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Within the context of VBM-spatial scope, the VBM-defined posterior hippocampal region confluently involved the hippocampal tail, molecular layer, CA4, CA2/3, GC-DG, CA1, and fimbria, subiculum (peak in CA1), whereas the VBM-defined anterior hippocampal region confluently involved the subiculum, molecular layer, and hippocampal fissure. VBM results provide further support for the assertion that BDNF Val66Met polymorphisms contribute to the structural plasticity of the hippocampus, as modulated by pain severity in PDM subjects. The protective effects of Val (as indexed by volumetric measurements) worked only under conditions of moderate pain and were impeached by the excessive stress associated with severe menstrual pain.
4. Discussion
Different approaches to the volumetric analysis of the hippocampus reveal different neurobiological changes at different spatial scales, and multiple approaches can be combined to shed light on subtle, diverse, transitional, and evolving facets of the hippocampal system. It has been established that the frequency of the Met allele and the proportion of Met/Met homozygotes are higher among Asians than among Caucasians (Petryshen et al., 2010). Thus, the current study (conducted in Taiwan) is well-positioned to elucidate the polyphyletic associations among all genotypes and structural representations of the hippocampus. We previously reported that the BDNF Val66Met polymorphism is associated with diverse functional expressions of the descending pain modulatory systems in cases of PDM (Wei et al., 2016b). Our data revealed potential Val allele dosage-dependent protective effects on the hippocampal structure (i.e., volume) at various spatial scales. This also means that the Met allele has a dosage-dependent deleterious effect on the hippocampal structure. Note however that at all spatial scales, the neuroprotective effects of the Val allele were observed only in the moderate PDM subgroup; i.e., not in the severe PDM subgroup. These findings suggest that BDNF Val/Val PDM subjects are resistant to stress associated with intermittent pain of moderate intensity, whereas the Met carrier PDM subjects are susceptible.
Regional differences in hippocampal volume between PDM and CON subjects in conjunction with the absence of global differences in hippocampal volume are consistent with our previous VBM findings on various sets of PDM subjects (Tu et al., 2010, Tu et al., 2013). Taken together, this is an indication that the brief nature of cyclic PDM episodes would not necessarily prompt global structural changes in the hippocampus. This assertion is corroborated by evidence in a previous study clearly demonstrating that the absence of significant changes in intrinsic functional brain architecture allow young PDM females to maintain normal psychosocial outcomes during the pain-free periovulatory phase (Lee et al., 2018). Pain disorders vary in terms of underlying nature and pain characteristics; therefore, our findings cannot be extrapolated to other chronic functional pain disorders, regardless of whether they are sustained or occur irregularly over longer periods of time. Nonetheless, it should be mentioned that patients with chronic pelvic pain due to endometriosis (secondary dysmenorrhea, cyclic pain disorder of uterine pathogenesis) presented overlapping regional changes in brain volume similar to the results from PDM studies (Tu et al., 2010, Tu et al., 2013) without regional and global changes in hippocampal volume (As-Sanie et al., 2012). Note that in the current study, we observed a positive Val allele dosage effect on the volume of the bilateral hippocampus among subjects with moderate PDM, and this effect was more pronounced in the right hippocampus.
Repeated stress due to PDM can lead to subtle yet significant alterations in the regional structure of the hippocampus, which can be detected at finer anatomical resolutions (e.g., specific subfields and VBM-defined regions of the hippocampus). Regional increases in volume under moderate pain may be partially attributed to neurogenesis induced by moderate intermittent stress. This supposition gains support from animal studies in which it has been reported that intermittent stress (e.g., 1 h/day exposure to cold water) can lead to an increase in neuronal volume, the lengthening of dendrites in pyramidal neurons, and neurogenesis in the ventral hippocampus (Pinto et al., 2015) (cf., sustained overt stress can lead to suppression of neurogenesis, neuronal injury, and neuronal death (Sapolsky et al., 1990)).
The hippocampus comprises a variety of subfields, which differ in terms of function, brain connectivity, histology, and physiology (Fanselow and Dong, 2010). Translational studies have provided evidence that the cardinal consequences of stress exposure are the suppression of neurogenesis in the dentate gyrus (DG) and dendritic remodeling in the cornu ammonis (McEwen, 2000). In humans as well as animals, early exposure to stress can affect the development of specific hippocampal subfields, and the effects on hippocampal synaptic density tend not to emerge until well after puberty (Andersen and Teicher, 2004). It appears that high density glucocorticoid binding sites render the subiculum particularly vulnerable to stress (Sarrieau et al., 1986). Given the importance of subiculum in the regulation of the HPA axis, dopaminergic responses to stress, risk for substance abuse and psychosis, and vulnerability to early trauma, our discovery of subiculum involvement is particularly intriguing (Grace, 2010, Radley and Sawchenko, 2011, Teicher et al., 2012). At the subfield scale, the significant neuroprotective effects of the Val allele (indexed by subfield volumetry) were similarly expressed in the right subiculum and hippocampal molecular layer. The CA1 and subiculum project into the medial prefrontal cortex (mPFC) and amygdala; both of which service the regulation of the hypothalamic–pituitaryadrenal (HPA) axis (Herman and Mueller, 2006, Quach et al., 2016). The HPA axis is a crucial stress regulation system enabling the organism to continually adapt to changing environmental conditions (de Kloet et al., 2005). Note that the amygdala activates the HPA axis, whereas the hippocampus/mPFC inhibits the HPA axis (Chattarji et al., 2015). The hypothalamus downregulates HPA-axis activity via a negative feedback mechanism to prevent corticosteroid-induced hippocampal atrophy (Herman et al., 2016). We were surprised to observe that Val/Val subjects exhibited hippocampal subfield enlargement only in cases of moderate PDM; i.e., not in cases of severe PDM. We speculate that the reduction in hippocampal volume among young Val/Val subjects with severe PDM and young Met carriers with moderate/severe PDM is indicative of subclinical hippocampal dysfunction under long-term and repeated clinically significant dysmenorrheal stress, which may exhaust the neuroprotective regulatory reservoir of HPA.
The neuroprotective effects of the Val allele at the microscopic scale manifest as an increase in dendritic complexity, increased neurogenesis, and a decrease in cell death through the activity-dependent secretion of BDNF (Bath and Lee, 2006). Note however that only subjects with moderate PDM demonstrated positive Val allele dosage effects on the VBM-defined left anterior hippocampus and VBM-genotype co-defined right posterior hippocampus. From a functional perspective, it has been suggested that the anterior hippocampus relates to stress, emotion, and affect, whereas the posterior hippocampus relates primarily to information processing and cognitive functions (Fanselow and Dong, 2010, Poppenk et al., 2013). From a molecular perspective, gene expression in the posterior hippocampus (e.g., Wfs1, Iyd, Itga7) correlates with cortical regions involved in information processing, whereas genes expressed (e.g., Grp, Dcn, Htr2c) in the anterior hippocampus correlate with regions involved in emotion and stress (amygdala and hypothalamus) (Fanselow and Dong, 2010). The neurotrophic model specifies that stress decreases the expression of BDNF via inhibitory GABA-ergic interneurons in limbic structures, particularly in the hippocampus (Duman and Monteggia, 2006). The Met allele of BDNF Val66Met polymorphisms is associated with poor episodic memory and hippocampal function, due to impaired intracellular trafficking and the activity-dependent secretion of BDNF (Egan et al., 2003). We observed negative Met allele dosage effects (number of Met alleles) on the volume of the VBM-defined right posterior hippocampus in the overall PDM group and a pronounced effect in the moderate PDM group. The posterior hippocampus appears to be involved in the process of fear conditioning (Kim and Fanselow, 1992). Based on Mowrer’s two-factor theory of fear conditioning (Greenberg and Burns, 2003), PDM subjects (under heightened trait anxiety (Lee et al., 2014), Supplementary Table 1) may manifest fear and/or anxiety when anticipating subsequent inevitable menstrual pain (Golub, 1976). The Met variant BDNF Val66Met polymorphism contributes to abnormalities in fear memory extinction and fear conditioning responses (Yu et al., 2009). Note that the Met/Met subjects with moderate PDM exhibited a negative correlation between the volume of the VBM-defined right posterior hippocampus and PCS, indicating pain catastrophizing as a possible corollary to pain-related disability, fear-avoidance behaviors, and/or psychological distress in that group (Severeijns et al., 2001).
Collectively, the hippocampal volume at various spatial scales was proportional to the number of Val alleles carried by moderate PDM subjects. It was surprising to observe that the neuroprotective effects were not observed in Val/Val subjects with severe PDM (i.e., local volumes were lower in cases of severe PDM than in cases of moderate PDM). These findings imply that distressful severe pain and the corresponding stress can undermine the protective effect of Val/Val homozygosity on hippocampal volume. This is similar to the observation that the PTSD symptoms of Val allele carriers were less severe than those of Met/Met homozygotes only in situations of low stress. It should be born in mind that environmental factors, such as stress, could induce epigenetic changes at the BDNF gene, thereby affecting the availability and function of the BDNF proteins (Boulle et al., 2012, Fuchikami et al., 2010).
The presubiculum receives subcortical inputs from the anterior thalamic nuclei and relays projections of hippocampal formation through the entorhinal cortex to the amygdala (Brother and Finch, 1985, Bubb et al., 2017). This pathway is involved in the processing of emotional memory (Yaniv et al., 2003). The volume reduction in the bilateral presubiculum in PDM subjects (in contrast to overall PDM vs. CON subjects) could not be attributed to focal neurogenesis or synaptogenesis. It can be better explained as subclinical aberrant neuronal networking under the effects of repeated pain-related stress (Ezzati et al., 2014, Vaculik et al., 2019), which might be further effectuated by gene expression, such as BDNF Val66Met polymorphisms (Wei et al., 2016b).
Under stringent statistical criteria, only the regional volume of the left anterior hippocampus was significantly larger in the PDM group than in the CON group. Under a more liberal threshold, the regional volume of the right hippocampus demonstrated a similar trend (uncorrected p < 0.001). We observed that BDNF Val66Met allele dosage effects were more prominent in the right hippocampus, which may be associated with the hemispheric functional asymmetry of the hippocampus. It has been shown in human studies and animal models (Chen et al., 2006, Faris et al., 2020, Montag et al., 2010) that the BDNF Met allele exhibits increased anxiety-related behaviors under stress, which in turn may increase the risk of psychiatric mood disorders, suggestive of gene (BDNF Val66Met polymorphism) - environment (stress) interactions. Negative stimuli with aversive emotions tend to engage the right hippocampus more (Holt et al., 2005) for emotional processing (Ahern and Schwartz, 1985). Furthermore, the right ventral hippocampus tends to be more adaptive than the left hippocampus in regulating anxiety levels (Sakaguchi and Sakurai, 2017). We posit that the pronounced adaptive response in the right hippocampus might be mediated through genetic modulation (e.g., Val variant).
There are three possible explanations for the dosage-dependent negative effects of the Met allele on anxiety-related behavior in PDM subjects: 1) Neuroimaging is more sensitive than the observation of behavioral manifestations in a clinical setting (Rose and Donohoe, 2013); 2) The complex neurodynamics of pain-laden behavior cannot be attributed only to the hippocampus (Liotti et al., 2000, Zidda et al., 2018) as alluded to in our previous PDM fMRI and imaging genetics studies (Wei et al., 2016a, Wu et al., 2016); 3) Behavioral heterogeneity among three polymorphic genotypes. Our findings indicated that insufficient BDNF expression and defective fear-memory extinction specifically in Met carrier PDM subjects may underpin vulnerability to intermittent stress, which in turn could lead to hippocampal derangement and dysfunction in response to cyclic menstrual pain of significant magnitude over an extended period of time. We argued that in Met/Met PDM subjects, the observed lack of a compensatory increase in volume as a reactive response to moderate pain could be explained by an absence of protection contribution from the Val allele; however, the compensatory effects might be inconspicuous/ineffective in cases where the magnitude of the increase does not clearly indicate a main effect of genotype or a between-genotype difference when confronting severe PDM-related stress.
In the current study, we opted not to perform quantitative analysis of menstrual cycle status (e.g., menstrual bleeding status and biochemical content of menstrual effluent), due to the fact that we were more concerned with obtaining a confirmed diagnosis of PDM by a gynecological clinic. Furthermore, the pain-free state was our primary concern in seeking to elucidate genotype-laden trait changes in the hippocampal structure under the effects of long-term PDM (Tu et al., 2010). Nonetheless, it would be interesting to elucidate the aforementioned aspects of menstrual cycle status in a future study in order to gain a more complete understanding of the association between menstrual pain and brain plasticity.
Our findings indicate that the BDNF Met/Met polymorphism can render an individual susceptible to deleterious effects (e.g., hippocampal volume) resulting from adverse early life events (PDM in the current study), whereas the BDNF Val/Val polymorphism appears to provide protective effects at the level of the hippocampus. It has been shown that adverse early life events (e.g., maltreatment during childhood) could differentially predict hippocampal volumes (Perez et al., 2017, Rabl et al., 2014). One recent retrospective, population-based cohort study in the UK (Chandan et al., 2020) reported a link between exposure to maltreatment during early childhood and an elevated risk of developing fibromyalgia, chronic fatigue syndrome, chronic lower back pain, restless leg syndrome, and irritable bowel syndrome. Note that they did not observe a statistical correlation between maltreatment during early childhood and the development of temporomandibular joint disorder, chronic headache, interstitial cystitis, vulvodynia, chronic prostatitis, or myofascial pain syndrome. To the best of our knowledge, no previous study has reported a link between maltreatment during childhood and PDM. In the current study, efforts were made to exclude subjects with any reported neurological or psychiatric disorder.
5. Limitations
A number of points deserves further consideration. First, this study focused on a narrow age range (20–26 y/o) to reduce between-subject variance attributable to maturational or aging effects on the hippocampus. Note also that all of the subjects enrolled in this study were highly educated. Second, we did not collect cortisol samples due to the difficulties in arranging appointments to comply with the menstrual cycle. Saliva or blood cortisol levels can be used as indicators of HPA-axis activation; however, difficulties in controlling diurnal variations (Edwards et al., 2001) would call into question the verity of the assessments. Thus, only behavioral and psychological measurements (e.g., STAI, BAI) were used as indirect indicators of anxiety levels associated with the stress response and HPA-axis function. High stress is generally viewed as a risk factor of dysmenorrhea in various groups of susceptible women, such as students and workers (Nohara et al., 2011, Wang et al., 2004). The sensitivity of hippocampal neurons to stress and glucocorticoids has been confirmed in a host of species (Conrad, 2008); however, the relationship between cortisol concentrations (reflecting stress-induced HPA-axis activity) and dysmenorrhea are inconsistent. Compared to controls in the menstrual phase, PDM has been associated with elevated cortisol concentrations in some studies (Yang et al., 2019) and lower concentrations in other studies (Vincent et al., 2011). Despite the fact that most studies have reported a positive correlation between stress levels and cortisol secretion (Van Eck et al., 1996), exposure to chronic intermittent stressors may lead to desensitization/dysfunction of the HPA axis and a corresponding decrease in cortisol concentrations (Rosal et al., 2004), particularly in female patients (Meewisse et al., 2007, Van Cauter et al., 1996). Third, we did not analyze BDNF expression in blood samples because peripheral BDNF concentrations (e.g., plasma) do not accurately represent BDNF concentrations in the brain (Lanz et al., 2012). Fourth, the current study suffered from a relatively small and unbalanced sample, a common difficulty in genetic neuroimaging research. Note that compared to many neuroimaging studies, the sample size in the current study was by no means deficient (in total, 115 PDM and 117 CON subjects). Nonetheless, our findings should be considered preliminary and subject to further verification using samples of greater scope. Finally, the cross-sectional design used in the current study precludes establishing a direct causal link between variations in hippocampus volume and the subsequent development of chronic pain disorders. Nonetheless, the current study underscores the potential benefits of identifying the neurobiological consequences of PDM, which may prefigure and augment neuroimaging abnormalities associated with a variety of chronic functional pain disorders.
6. Conclusions
In conclusion, the BDNF Val66Met polymorphism is implicated in the exquisite structural vulnerability of the hippocampus to PDM. The neuroprotective effect of the Val variant on the hippocampus is modulated and constrained by the severity of menstrual pain. This current study, combining structural brain imaging and genetic analysis, corroborates the results obtained in our previous research based on functional imaging and genetic analysis (Wei et al., 2016b). Our current findings indicate that Met/Met homozygotic females with severe PDM are particularly susceptible to pain chronification (Baliki et al., 2012, Hashmi et al., 2013). Years of repeated PDM stress can induce structural and functional changes in the hippocampus in accordance with the BDNF Val66Met genotype and pain severity. This triad study on PDM combined genotype with endophenotype imaging results and clinical phenotypes to explain the high prevalence of chronic functional pain disorders among females later in life (compared to males) (Wei et al., 2016b, Wei et al., 2017). Pain and stress early in life are recognized as harbingers of reduced quality-of-life (see also Supplementary Table 1); however, they may also be predictive of more-severe or chronic pain later in life (Berkley, 2013, Victoria and Murphy, 2016). The interaction between genetic attributes and the effects of severe pain on the resilience of the brain helps to explain individual differences in the way that PDM is experienced. It is also possible that this interaction influences the coping mechanisms adopted by PDM subjects, which may in turn affect their vulnerability to other chronic pain disorders. Thus, tackling cases of moderate to severe PDM vigorously and as early as possible could help to prevent the chronification of pain in the brain (Berkley, 2013). Note that there is no evidence indicating whether the observed sexual dimorphism in the BDNF-genotypic predilection to hippocampal structural dynamics is generalizable to other pain disorders. This issue should be considered in future genetic neuroimaging studies as well as in the clinical treatment of PDM and chronic functional pain disorders.
CRediT authorship contribution statement
Wei-Chi Li: Conceptualization, Methodology, Investigation, Formal analysis, Data curation, Writing - original draft, Visualization. Hsiang-Tai Chao: Investigation, Resources. Ming-Wei Lin: Resources, Data curation. Horng-Der Shen: Resources, Data curation. Li-Fen Chen: Conceptualization, Methodology, Resources, Writing - original draft, Writing - review & editing, Supervision, Funding acquisition. Jen-Chuen Hsieh: Conceptualization, Methodology, Resources, Writing - review & editing, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank all participants for their support and contribution to this study. We appreciate the assistance of Intan Low, Chou-Ming Cheng, Tzu-Ling Tzeng, Ching-Ju Yang, Chih-Che Chou, Dr. Lin-Chien Lee, Dr. Tzu-Chen Yeh, Li-Kai Cheng, Tzu-Yi Hong, Ian-Ting Chu, Yi-Ling Yeh, Man-Shan Hung, Pin-Hsuan Lin, Yu-Hsiang Liu, and Wen-Chi Lu with participant recruitment and experiments, and Dr. Cheng-Hao Tu for precious inputs to MRI data analyses. The study was supported by the Taiwan Ministry of Science and Technology (NSC 100-2314-B-010-006-MY3, NSC 100-2629-B-010-001, NSC 101-2629-B-010-001, NSC 102-2629-B-010-001, MOST-106-2629-B-010-001-MY3, MOST 108-2314-B-010-026, MOST 109-2314-B-010-037-MY2), Taipei Veterans General Hospital (V100D-001), Taipei Veterans General Hospital – National Taiwan University Hospital Joint Research Program (VN103-05, VN104-03, VN105-03), The Aim for the Top University Plan of the Ministry of Education, and The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education for National Yang-Ming University.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102576.
Contributor Information
Li-Fen Chen, Email: lfchen@nycu.edu.tw.
Jen-Chuen Hsieh, Email: jchsiehibru@nycu.edu.tw, jchsieh@vghtpe.gov.tw.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Ahern G.L., Schwartz G.E. Differential lateralization for positive and negative emotion in the human brain: EEG spectral analysis. Neuropsychologia. 1985;23(6):745–755. doi: 10.1016/0028-3932(85)90081-8. [DOI] [PubMed] [Google Scholar]
- Ameade E., Mohammed B. Menstrual Pain Assessment: Comparing Verbal Rating Scale (VRS) with Numerical Rating Scales (NRS) as Pain Measurement Tools. Int J Womens Health Wellness. 2016;2:017. [Google Scholar]
- Andersen S.L., Teicher M.H. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29(11):1988–1993. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- As-Sanie S., Harris R.E., Napadow V., Kim J., Neshewat G., Kairys A., Williams D., Clauw D.J., Schmidt-Wilcke T. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain. 2012;153:1006–1014. doi: 10.1016/j.pain.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki M.N., Petre B., Torbey S., Herrmann K.M., Huang L., Schnitzer T.J., Fields H.L., Apkarian A.V. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat. Neurosci. 2012;15(8):1117–1119. doi: 10.1038/nn.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath K.G., Lee F.S. Variant BDNF (Val66Met) impact on brain structure and function. Cogn. Affect Behav. Neurosci. 2006;6(1):79–85. doi: 10.3758/cabn.6.1.79. [DOI] [PubMed] [Google Scholar]
- Berkley K.J. Primary dysmenorrhea: an urgent mandate. Pain: Clin Update. 2013;21:1–8. [Google Scholar]
- Boulle F., van den Hove D.L.A., Jakob S.B., Rutten B.P., Hamon M., van Os J., Lesch K.-P., Lanfumey L., Steinbusch H.W., Kenis G. Epigenetic regulation of the BDNF gene: implications for psychiatric disorders. Mol. Psychiatry. 2012;17(6):584–596. doi: 10.1038/mp.2011.107. [DOI] [PubMed] [Google Scholar]
- Breivik H., Borchgrevink P.C., Allen S.M., Rosseland L.A., Romundstad L., Breivik Hals E.K., Kvarstein G., Stubhaug A. Assessment of pain. Br. J. Anaesth. 2008;101(1):17–24. doi: 10.1093/bja/aen103. [DOI] [PubMed] [Google Scholar]
- Brooks G.P., Johanson G.A. Sample size considerations for multiple comparison procedures in ANOVA. J. Mod. Appl. Stat. Methods. 2011;10(1):97–109. [Google Scholar]
- Brother L.A., Finch D.M. Physiological evidence for an excitatory pathway from entorhinal cortex to amygdala in the rat. Brain Res. 1985;359(1-2):10–20. doi: 10.1016/0006-8993(85)91407-6. [DOI] [PubMed] [Google Scholar]
- Bubb E.J., Kinnavane L., Aggleton J.P. Hippocampal - diencephalic - cingulate networks for memory and emotion: An anatomical guide. Brain Neurosci. Adv. 2017;1:1–20. doi: 10.1177/2398212817723443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandan J.S., Keerthy D., Zemedikun D.T., Okoth K., Gokhale K.M., Raza K., Bandyopadhyay S., Taylor J., Nirantharakumar K. The association between exposure to childhood maltreatment and the subsequent development of functional somatic and visceral pain syndromes. EClinicalMedicine. 2020;23:100392. doi: 10.1016/j.eclinm.2020.100392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattarji S., Tomar A., Suvrathan A., Ghosh S., Rahman M.M. Neighborhood matters: divergent patterns of stress-induced plasticity across the brain. Nat. Neurosci. 2015;18(10):1364–1375. doi: 10.1038/nn.4115. [DOI] [PubMed] [Google Scholar]
- Chen Z.-Y., Jing D., Bath K.G., Ieraci A., Khan T., Siao C.-J., Herrera D.G., Toth M., Yang C., McEwen B.S., Hempstead B.L., Lee F.S. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314(5796):140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coco A.S. Primary dysmenorrhea. Am. Fam. Physician. 1999;60:489–496. [PubMed] [Google Scholar]
- Conrad C.D. Chronic stress-induced hippocampal vulnerability: the glucocorticoid vulnerability hypothesis. Rev. Neurosci. 2008;19:395–411. doi: 10.1515/revneuro.2008.19.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet E.R., Joëls M., Holsboer F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 2005;6(6):463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Duman R.S., Monteggia L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry. 2006;59(12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Edwards S., Clow A., Evans P., Hucklebridge F. Exploration of the awakening cortisol response in relation to diurnal cortisol secretory activity. Life Sci. 2001;68(18):2093–2103. doi: 10.1016/s0024-3205(01)00996-1. [DOI] [PubMed] [Google Scholar]
- Egan M.F., Kojima M., Callicott J.H., Goldberg T.E., Kolachana B.S., Bertolino A., Zaitsev E., Gold B., Goldman D., Dean M., Lu B., Weinberger D.R. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Ezzati A., Zimmerman M.E., Katz M.J., Sundermann E.E., Smith J.L., Lipton M.L., Lipton R.B. Hippocampal subfields differentially correlate with chronic pain in older adults. Brain Res. 2014;1573:54–62. doi: 10.1016/j.brainres.2014.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow M.S., Dong H.W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faris A., Chech P.S., Ling K.H. Single nucleotide polymorphism of BDNF Val66Met (rs6265) and its association to neuropsychiatric disorders. Neurosci res notes. 2020;3:9–26. [Google Scholar]
- Fortin N.J., Agster K.L., Eichenbaum H.B. Critical role of the hippocampus in memory for sequences of events. Nat. Neurosci. 2002;5:458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchikami M., Yamamoto S., Morinobu S., Takei S., Yamawaki S. Epigenetic regulation of BDNF gene in response to stress. Psychiatry Investig. 2010;7:251–256. doi: 10.4306/pi.2010.7.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze E., Vermetten E., Bremner J.D. MR-based in vivo hippocampal volumetrics: 2. Findings in neuropsychiatric disorders. Mol. Psychiatry. 2005;10:160–184. doi: 10.1038/sj.mp.4001579. [DOI] [PubMed] [Google Scholar]
- Gilbertson M.W., Shenton M.E., Ciszewski A., Kasai K., Lasko N.B., Orr S.P., Pitman R.K. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat. Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub S. The magnitude of premenstrual anxiety and depression. Psychosom. Med. 1976;38:4–12. doi: 10.1097/00006842-197601000-00002. [DOI] [PubMed] [Google Scholar]
- Grace A.A. Dopamine system dysregulation by the ventral subiculum as the common pathophysiological basis for schizophrenia psychosis, psychostimulant abuse, and stress. Neurotox. Res. 2010;18:367–376. doi: 10.1007/s12640-010-9154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J., Burns J.W. Pain anxiety among chronic pain patients: specific phobia or manifestation of anxiety sensitivity? Behav. Res. Ther. 2003;41:223–240. doi: 10.1016/s0005-7967(02)00009-8. [DOI] [PubMed] [Google Scholar]
- Hashmi J.A., Baliki M.N., Huang L., Baria A.T., Torbey S., Hermann K.M., Schnitzer T.J., Apkarian A.V. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain. 2013;136:2751–2768. doi: 10.1093/brain/awt211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J.P., Hayes S., Miller D.R., Lafleche G., Logue M.W., Verfaellie M. Automated measurement of hippocampal subfields in PTSD: Evidence for smaller dentate gyrus volume. J. Psychiatr. Res. 2017;95:247–252. doi: 10.1016/j.jpsychires.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidari F., Afshari M., Moosazadeh M. Prevalence of fibromyalgia in general population and patients, a systematic review and meta-analysis. Rheumatol. Int. 2017;37:1527–1539. doi: 10.1007/s00296-017-3725-2. [DOI] [PubMed] [Google Scholar]
- Herman J.P., McKlveen J.M., Ghosal S., Kopp B., Wulsin A., Makinson R., Scheimann J., Myers B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr Physiol. 2016;6:603–621. doi: 10.1002/cphy.c150015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J.P., Mueller N.K. Role of the ventral subiculum in stress integration. Behav. Brain Res. 2006;174:215–224. doi: 10.1016/j.bbr.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Hofer M., Pagliusi S.R., Hohn A., Leibrock J., Barde Y.A. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990;9:2459–2464. doi: 10.1002/j.1460-2075.1990.tb07423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E.J., Reichardt L.F. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Coupland N.J., Lebel R.M., Carter R., Seres P., Wilman A.H., Malykhin N.V. Structural changes in hippocampal subfields in major depressive disorder: a high-field magnetic resonance imaging study. Biol. Psychiatry. 2013;74:62–68. doi: 10.1016/j.biopsych.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Iacovides S., Avidon I., Baker F.C. What we know about primary dysmenorrhea today: a critical review. Hum Reprod Update. 2015;21:762–778. doi: 10.1093/humupd/dmv039. [DOI] [PubMed] [Google Scholar]
- Kim E.J., Pellman B., Kim J.J. Stress effects on the hippocampus: a critical review. Learn Mem. 2015;22:411–416. doi: 10.1101/lm.037291.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.J., Fanselow M.S. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Lanz T.A., Bove S.E., Pilsmaker C.D., Mariga A., Drummond E.M., Cadelina G.W., Adamowicz W.O., Swetter B.J., Carmel S., Dumin J.A. Robust changes in expression of brain-derived neurotrophic factor (BDNF) mRNA and protein across the brain do not translate to detectable changes in BDNF levels in CSF or plasma. Biomarkers. 2012;17:524–531. doi: 10.3109/1354750X.2012.694476. [DOI] [PubMed] [Google Scholar]
- Lee L.C., Chen Y.H., Lin C.S., Li W.C., Low I., Tu C.H., Chou C.C., Cheng C.M., Yeh T.C., Chen L.F., Chao H.T., Hsieh J.C. Unaltered intrinsic functional brain architecture in young women with primary dysmenorrhea. Sci. Rep. 2018;8:12971. doi: 10.1038/s41598-018-30827-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L.C., Tu C.H., Chen L.F., Shen H.D., Chao H.T., Lin M.W., Hsieh J.C. Association of brain-derived neurotrophic factor gene Val66Met polymorphism with primary dysmenorrhea. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.C., Tu C.H., Chao H.T., Yeh T.C., Chen L.F., Hsieh J.C. High prevalence of incidental brain findings in primary dysmenorrhoea. Eur. J. Pain. 2015;19:1071–1074. doi: 10.1002/ejp.639. [DOI] [PubMed] [Google Scholar]
- Liotti M., Mayberg H.S., Brannan S.K., McGinnis S., Jerabek P., Fox P.T. Differential limbic–cortical correlates of sadness and anxiety in healthy subjects: implications for affective disorders. Biol. Psychiatry. 2000;48:30–42. doi: 10.1016/s0006-3223(00)00874-x. [DOI] [PubMed] [Google Scholar]
- Lisofsky N., Martensson J., Eckert A., Lindenberger U., Gallinat J., Kuhn S. Hippocampal volume and functional connectivity changes during the female menstrual cycle. Neuroimage. 2015;118:154–162. doi: 10.1016/j.neuroimage.2015.06.012. [DOI] [PubMed] [Google Scholar]
- Liu M.G., Chen J. Roles of the hippocampal formation in pain information processing. Neurosci. Bull. 2009;25:237–266. doi: 10.1007/s12264-009-0905-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell R.M., Ford A.C. Effect of gender on prevalence of irritable bowel syndrome in the community: systematic review and meta-analysis. Am. J. Gastroenterol. 2012;107:991–1000. doi: 10.1038/ajg.2012.131. [DOI] [PubMed] [Google Scholar]
- Maleki N., Becerra L., Brawn J., McEwen B., Burstein R., Borsook D. Common hippocampal structural and functional changes in migraine. Brain Struct. Funct. 2013;218:903–912. doi: 10.1007/s00429-012-0437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae C.S., O'Shea A.M., Boissoneault J., Vatthauer K.E., Robinson M.E., Staud R., Perlstein W.M., Craggs J.G. Fibromyalgia patients have reduced hippocampal volume compared with healthy controls. J. Pain Res. 2015;8:47–52. doi: 10.2147/JPR.S71959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Weiss J.M., Schwartz L.S. Selective retention of corticosterone by limbic structures in rat brain. Nature. 1968;220:911–912. doi: 10.1038/220911a0. [DOI] [PubMed] [Google Scholar]
- Meewisse M.L., Reitsma J.B., de Vries G.J., Gersons B.P., Olff M. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br. J. Psychiatry. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- Montag C., Basten U., Stelzel C., Fiebach C.J., Reuter M. The BDNF Val66Met polymorphism and anxiety: support for animal knock-in studies from a genetic association study in humans. Psychiatry Res. 2010;179:86–90. doi: 10.1016/j.psychres.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Murer M.G., Boissiere F., Yan Q., Hunot S., Villares J., Faucheux B., Agid Y., Hirsch E., Raisman-Vozari R. An immunohistochemical study of the distribution of brain-derived neurotrophic factor in the adult human brain, with particular reference to Alzheimer's disease. Neuroscience. 1999;88:1015–1032. doi: 10.1016/s0306-4522(98)00219-x. [DOI] [PubMed] [Google Scholar]
- Mutso A.A., Radzicki D., Baliki M.N., Huang L., Banisadr G., Centeno M.V., Radulovic J., Martina M., Miller R.J., Apkarian A.V. Abnormalities in hippocampal functioning with persistent pain. J. Neurosci. 2012;32:5747–5756. doi: 10.1523/JNEUROSCI.0587-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohara M., Momoeda M., Kubota T., Nakabayashi M. Menstrual cycle and menstrual pain problems and related risk factors among Japanese female workers. Ind. Health. 2011;49:228–234. doi: 10.2486/indhealth.ms1047. [DOI] [PubMed] [Google Scholar]
- O'Mara S. The subiculum: what it does, what it might do, and what neuroanatomy has yet to tell us. J. Anat. 2005;207:271–282. doi: 10.1111/j.1469-7580.2005.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazmany E., Ly H.G., Aerts L., Kano M., Bergeron S., Verhaeghe J., Peeters R., Tack J., Dupont P., Enzlin P., Van Oudenhove L. Brain responses to vestibular pain and its anticipation in women with Genito-Pelvic Pain/Penetration Disorder. Neuroimage Clin. 2017;16:477–490. doi: 10.1016/j.nicl.2017.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez D.L., Matin N., Barsky A., Costumero-Ramos V., Makaretz S.J., Young S.S., Sepulcre J., LaFrance W.C., Jr., Keshavan M.S., Dickerson B.C. Cingulo-insular structural alterations associated with psychogenic symptoms, childhood abuse and PTSD in functional neurological disorders. J. Neurol. Neurosurg. Psychiatry. 2017;88:491–497. doi: 10.1136/jnnp-2016-314998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryshen T.L., Sabeti P.C., Aldinger K.A., Fry B., Fan J.B., Schaffner S.F., Waggoner S.G., Tahl A.R., Sklar P. Population genetic study of the brain-derived neurotrophic factor (BDNF) gene. Mol. Psychiatry. 2010;15:810–815. doi: 10.1038/mp.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E.A. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr. Opin. Neurobiol. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Pinto V., Costa J.C., Morgado P., Mota C., Miranda A., Bravo F.V., Oliveira T.G., Cerqueira J.J., Sousa N. Differential impact of chronic stress along the hippocampal dorsal-ventral axis. Brain Struct. Funct. 2015;220:1205–1212. doi: 10.1007/s00429-014-0713-0. [DOI] [PubMed] [Google Scholar]
- Poppenk J., Evensmoen H.R., Moscovitch M., Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn Sci. 2013;17:230–240. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Quach T.T., Lerch J.K., Honnorat J., Khanna R., Duchemin A.M. Neuronal networks in mental diseases and neuropathic pain: Beyond brain derived neurotrophic factor and collapsin response mediator proteins. World J Psychiatry. 2016;6:18–30. doi: 10.5498/wjp.v6.i1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabl U., Meyer B.M., Diers K., Bartova L., Berger A., Mandorfer D., Popovic A., Scharinger C., Huemer J., Kalcher K., Pail G., Haslacher H., Perkmann T., Windischberger C., Brocke B., Sitte H.H., Pollak D.D., Dreher J.C., Kasper S., Praschak-Rieder N., Moser E., Esterbauer H., Pezawas L. Additive gene-environment effects on hippocampal structure in healthy humans. J. Neurosci. 2014;34:9917–9926. doi: 10.1523/JNEUROSCI.3113-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley J.J., Sawchenko P.E. A common substrate for prefrontal and hippocampal inhibition of the neuroendocrine stress response. J. Neurosci. 2011;31:9683–9695. doi: 10.1523/JNEUROSCI.6040-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosal M.C., King J., Ma Y., Reed G.W. Stress, social support, and cortisol: inverse associations? Behav. Med. 2004;30:11–21. doi: 10.3200/BMED.30.1.11-22. [DOI] [PubMed] [Google Scholar]
- Rose E.J., Donohoe G. Brain vs behavior: an effect size comparison of neuroimaging and cognitive studies of genetic risk for schizophrenia. Schizophr. Bull. 2013;39:518–526. doi: 10.1093/schbul/sbs056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi Y., Sakurai Y. Left-right functional asymmetry of ventral hippocampus depends on aversiveness of situations. Behav. Brain Res. 2017;325:25–33. doi: 10.1016/j.bbr.2017.02.028. [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M., Uno H., Rebert C.S., Finch C.E. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J. Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrieau A., Dussaillant M., Agid F., Philibert D., Agid Y., Rostene W. Autoradiographic localization of glucocorticosteroid and progesterone binding sites in the human post-mortem brain. J. Steroid Biochem. 1986;25:717–721. doi: 10.1016/0022-4731(86)90300-6. [DOI] [PubMed] [Google Scholar]
- Seltman H.J. Experimental design and analysis. Carnegie Mellon University; Pennsylvania: 2018. Contrasts and Custom Hypotheses; pp. 319–338. [Google Scholar]
- Severeijns R., Vlaeyen J.W., van den Hout M.A., Weber W.E. Pain catastrophizing predicts pain intensity, disability, and psychological distress independent of the level of physical impairment. Clin. J. Pain. 2001;17:165–172. doi: 10.1097/00002508-200106000-00009. [DOI] [PubMed] [Google Scholar]
- Sullivan M.J., Bishop S.R., Pivik J. The pain catastrophizing scale: development and validation. Psychol. Assess. 1995;7:524–532. [Google Scholar]
- Teicher M.H., Anderson C.M., Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc. Natl. Acad. Sci. U S A. 2012;109:E563–572. doi: 10.1073/pnas.1115396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C.H., Niddam D.M., Chao H.T., Chen L.F., Chen Y.S., Wu Y.T., Yeh T.C., Lirng J.F., Hsieh J.C. Brain morphological changes associated with cyclic menstrual pain. Pain. 2010;150:462–468. doi: 10.1016/j.pain.2010.05.026. [DOI] [PubMed] [Google Scholar]
- Tu C.H., Niddam D.M., Yeh T.C., Lirng J.F., Cheng C.M., Chou C.C., Chao H.T., Hsieh J.C. Menstrual pain is associated with rapid structural alterations in the brain. Pain. 2013;154:1718–1724. doi: 10.1016/j.pain.2013.05.022. [DOI] [PubMed] [Google Scholar]
- Vachon-Presseau E., Roy M., Martel M.O., Caron E., Marin M.F., Chen J., Albouy G., Plante I., Sullivan M.J., Lupien S.J., Rainville P. The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans. Brain. 2013;136:815–827. doi: 10.1093/brain/aws371. [DOI] [PubMed] [Google Scholar]
- Vaculik M.F., Noorani A., Hung P.S., Hodaie M. Selective hippocampal subfield volume reductions in classic trigeminal neuralgia. Neuroimage Clin. 2019;23 doi: 10.1016/j.nicl.2019.101911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauter E., Leproult R., Kupfer D.J. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J. Clin. Endocrinol. Metab. 1996;81:2468–2473. doi: 10.1210/jcem.81.7.8675562. [DOI] [PubMed] [Google Scholar]
- Van Eck M., Berkhof H., Nicolson N., Sulon J. The effects of perceived stress, traits, mood states, and stressful daily events on salivary cortisol. Psychosom. Med. 1996;58:447–458. doi: 10.1097/00006842-199609000-00007. [DOI] [PubMed] [Google Scholar]
- Victoria N.C., Murphy A.Z. Exposure to Early Life Pain: Long Term Consequences and Contributing Mechanisms. Curr. Opin Behav. Sci. 2016;7:61–68. doi: 10.1016/j.cobeha.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent K., Warnaby C., Stagg C.J., Moore J., Kennedy S., Tracey I. Dysmenorrhoea is associated with central changes in otherwise healthy women. Pain. 2011;152:1966–1975. doi: 10.1016/j.pain.2011.03.029. [DOI] [PubMed] [Google Scholar]
- Wang L., Wang X., Wang W., Chen C., Ronnennberg A.G., Guang W., Huang A., Fang Z., Zang T., Wang L., Xu X. Stress and dysmenorrhoea: a population based prospective study. Occup. Environ. Med. 2004;61:1021–1026. doi: 10.1136/oem.2003.012302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S.Y., Chao H.T., Tu C.H., Li W.C., Low I., Chuang C.Y., Chen L.F., Hsieh J.C. Changes in functional connectivity of pain modulatory systems in women with primary dysmenorrhea. Pain. 2016;157:92–102. doi: 10.1097/j.pain.0000000000000340. [DOI] [PubMed] [Google Scholar]
- Wei S.Y., Chao H.T., Tu C.H., Lin M.W., Li W.C., Low I., Shen H.D., Chen L.F., Hsieh J.C. The BDNF Val66Met polymorphism is associated with the functional connectivity dynamics of pain modulatory systems in primary dysmenorrhea. Sci. Rep. 2016;6:23639. doi: 10.1038/srep23639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S.Y., Chen L.F., Lin M.W., Li W.C., Low I., Yang C.J., Chao H.T., Hsieh J.C. The OPRM1 A118G polymorphism modulates the descending pain modulatory system for individual pain experience in young women with primary dysmenorrhea. Sci. Rep. 2017;7:39906. doi: 10.1038/srep39906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woon F.L., Sood S., Hedges D.W. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2010;34:1181–1188. doi: 10.1016/j.pnpbp.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Wu T.H., Tu C.H., Chao H.T., Li W.C., Low I., Chuang C.Y., Yeh T.C., Cheng C.M., Chou C.C., Chen L.F., Hsieh J.C. Dynamic Changes of Functional Pain Connectome in Women with Primary Dysmenorrhea. Sci. Rep. 2016;6:24543. doi: 10.1038/srep24543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.W., Slakter M.J. Increasing the precision of data analysis: planned comparisons versus omnibus tests. Nurs. Res. 1990;39:251–253. [PubMed] [Google Scholar]
- Yang L., Dun W., Li K., Yang J., Wang K., Liu H., Liu J., Zhang M. Altered amygdalar volume and functional connectivity in primary dysmenorrhoea during the menstrual cycle. Eur. J. Pain. 2019;23:994–1005. doi: 10.1002/ejp.1368. [DOI] [PubMed] [Google Scholar]
- Yaniv D., Vouimba R.M., Diamond D.M., Richter-Levin G. Simultaneous induction of long-term potentiation in the hippocampus and the amygdala by entorhinal cortex activation: mechanistic and temporal profiles. Neuroscience. 2003;120:1125–1135. doi: 10.1016/s0306-4522(03)00386-5. [DOI] [PubMed] [Google Scholar]
- Yu H., Wang Y., Pattwell S., Jing D., Liu T., Zhang Y., Bath K.G., Lee F.S., Chen Z.Y. Variant BDNF Val66Met polymorphism affects extinction of conditioned aversive memory. J. Neurosci. 2009;29:4056–4064. doi: 10.1523/JNEUROSCI.5539-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidda F., Andoh J., Pohlack S., Winkelmann T., Dinu-Biringer R., Cavalli J., Ruttorf M., Nees F., Flor H. Default mode network connectivity of fear- and anxiety-related cue and context conditioning. Neuroimage. 2018;165:190–199. doi: 10.1016/j.neuroimage.2017.10.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.