Abstract
Purpose
Emmetropization is the process of adjusting ocular growth to the focal plane in order to achieve a clear image. Chromatic light may be involved as a cue to guide this process. Achromats are color blind and lack normal cone function; they are often described as being hyperopic, indicating a failure to emmetropize. We aim to describe the refraction and refractive development in a population of genetically characterized achromats.
Methods
Refractive error data were collected retrospectively from 28 medical records of CNGB3 c.1148delC homozygous achromats. The distribution of spherical equivalent refractive error (SER) and spherical error was analyzed in adults. The refractive development in children was analyzed by documenting astigmatic refractive error and calculating median SER in 1-year age groups and by analyzing the individual development when possible.
Results
The distribution of SER and spherical error resembled a Gaussian distribution, indicating that emmetropization was disturbed in achromats, but we found indication of some decrease in SER during the first years of childhood. The prevalence of refractive errors was high and broadly distributed. Astigmatic refractive errors were frequent but did not seem to increase with age.
Conclusions
Refractive development in achromats is more complicated than a complete failure to emmetropize. The spread of refractive errors is larger than previously documented. Results presented here support the theory that chromatic cues and cone photoreceptors may play a role in emmetropization in humans but that it is not essential.
Keywords: achromatopsia, rod monochromatism, CNGB3, emmetropization, refractive error
At birth the refractive status of the human eye shows a Gaussian distribution including both myopic and hyperopic refractive errors but with the peak shifted toward hyperopia such that the majority of children are born with hyperopic eyes,1,2 moving toward a leptokurtic distribution in adulthood.3 The process of emmetropization is a vision-dependent process.4 Animal studies have shown that the eye can detect signs of defocus and adjust its axial growth to achieve a match of the axial length to the focal plane and that eye growth can be altered by visual manipulation and deprivation,5–7 indicating that the growth of the eye is regulated by feedback mechanisms that monitor signs of retinal defocus. In animals, compensation for image blur does not require an intact optic nerve, ciliary nerve, or fovea, as their eyes can compensate for optical lens-induced defocus after selective nerve severing, and foveal laser ablation does not affect refraction or the ability to develop form-deprivation myopia.8,9
Many factors may be important for providing visual cues affecting emmetropization, including the ratio between M and L cones10 and the spectral composition of light,11,12 but the experimental evidence in primates is somewhat conflicting.11,13 Cone photoreceptors seem to be important in detecting long-wave chromatic aberration (LCA) that may serve as a visual cue for emmetropization.12,14 Color vision defects may affect the refractive state of the eye in humans, as the prevalence of myopia seem to be lower in red/green color-deficient students,15 but, in other conditions caused by variants in the cone opsin genes, a high degree of myopic refractive error has been observed.16,17
Achromatopsia, or rod monochromatism, is a congenital condition characterized by complete lack of color vision, low visual acuity, photophobia, and nystagmus. Electroretinography most often shows absent or severely reduced cone responses.18 Achromatopsia is autosomal recessively inherited and has been associated with variants in six genes (CNGA3, CNGB3, GNAT2, PDE6C, PDE6H, and ATF6).19–24 CNGB3 variants are the most frequent cause in patients of European origin, and a one base pair deletion, c.1148delC, is the most frequent variant.25,26 CNGB3 encodes the β-subunit of a cyclic nucleotide-gated cation channel in cone photoreceptors that is essential for phototransduction.21 The c.1148delC variant results in a frameshift downstream of amino acid p.Thr383 and a premature stop codon, resulting in a truncated protein.27 Functional analyses have shown that the consequence of the c.1148delC variant is that no β-channel subunits are expressed on the cell surface, thereby altering channel properties essential for normal cone function.28
Refraction in achromatopsia is typically described as hyperopic,29–31 suggesting a failure to emmetropize, but the refractive development in achromatopsia has only been described in a few small studies so far.31,32 As achromatopsia is characterized by a lack of or reduced number of normally functioning cones, studying a genetically well-defined population of achromats may provide insight into the importance of color vision and cone photoreceptors in the process of human emmetropization. Studies on children under 17 years of age with mixed causes of reduced visual acuity have shown that the distribution of refractive errors does not exhibit the characteristic leptokurtosis but is more normally distributed as an indication of a failure to emmetropize.32 Emmetropization in children with low vision and nystagmus has been most extensively studied in patients with idiopathic congenital nystagmus and albinism and has shown that there is a high prevalence of spherical equivalent refractive error (SER) that does not decrease with age as an expression of failure to emmetropize.33 The most frequent refractive error in these patients seems to be hyperopia with high degrees of with-the-rule astigmatism that does seem to increase with age.33 The astigmatism in albinism has been ascribed to corneal molding effects produced by the lids during nystagmus eye movements.33,34 Hyperopic refraction is also described in Leber's congenital amaurosis.35
Recent studies have not found a correlation between visual acuity and spherical equivalent refraction33,34 (Haegerstrom-Portnoy G, et al. IOVS 2003;44:ARVO E-Abstract 4777), suggesting that the failure to emmetropize could be a result of the image blur caused by nystagmus or astigmatic refractive error and not low vision itself.34 The present study aims to retrospectively describe the refraction in an adult population of genetically characterized patients with achromatopsia and analyze the refractive development during childhood.
Methods
This retrospective study was conducted in accordance with tenets of the Declaration of Helsinki. Before data collection was initiated, approval was obtained from the Regional Danish National Committee on Health Research Ethics (jr.nr. H-19029634) and the Danish Patient Safety Authority (ref.nr. 3-3013-2911/1). To have the most uniform and genetically homogeneous population, we aimed to include all Danish patients with achromatopsia caused by homozygosity of the c.1148delC in CNGB3. Patients were identified from the Danish Family Archive for Genetic Eye Disease, the Danish Register for the Blind and Partially Sighted Children, and electronic medical charts. The Danish Family Archive for Genetic Eye Disease was started around 1985 and is a nationwide registry of heritable eye diseases with over 100 different conditions represented. The Danish Register for the Blind and Partially Sighted Children holds information on the diagnosis and other clinical data of all children 0 to 17 years of age who reside in Denmark and are known to be visually impaired or blind. Both registries are located at the National Eye Clinic for the Visually Impaired at the Kennedy Center, which has medical records dating back more than 100 years.
The diagnosis of achromatopsia was confirmed by medical record review and was based on typical clinical findings, color vision testing, typical electroretinography, and description of funduscopic findings confirmed by the results of genetic analysis. Data for refraction, visual acuity, and nystagmus were collected in the years 1949 through 2020, and follow-up ranged from 1 to 46 years. If available, we used cycloplegic refraction by an autorefraction or streak retinoscopy. If objective cycloplegic refraction was not available or specified in the medical record, prescribed correction based on subjective refraction and streak retinoscopy was used.
The right eye was chosen for analysis. Refractive error was documented in conventional form (sphere, negative cylinder, and axis) and converted into SER (sphere + 1/2 cylinder). For the adult population (≥17 years of age) the latest recorded refraction was documented. The distribution of SER and spherical refractive error was studied, as was the degree and axis of astigmatic refractive error.
To describe the development in refractive error in the pediatric group (0 to <17 years of age), the median SER, spherical refractive error, and astigmatic refractive error were recorded at available time points and documented by age in whole years. If data were available more than once within a year, data nearest the center of the age span were selected such that individual patient data were represented only once in each age group. To compare with published normative data on Danish patients median SER, spherical refractive error and astigmatic refractive error were calculated for the age group 4.5 to 7.25 years.36
Statistical Methods
As refractive data in adults and older children are not normally distributed, median values and interquartile ranges (IQRs) were chosen for analysis of refraction in different age groups. If refractive data from more than one visit were available in the period of 0 to 9 years of age, when primary emmetropization is believed to take place, refractive development in the individual was analyzed statistically accounting for the nonlinear nature of the data.
Mutti et al.37 recently analyzed the longitudinal development in all major optical components including SER in more than 250 children. They used the NLMIXED procedure in SAS Statistics 9.3 (SAS Institute, Cary, NC, USA) to fit growth models and found that the curves for most ocular variables including SER were best fit by an early exponential function in the first 1 to 2 years of age followed by a quadratic curve as age increased. Refractive error is relatively stable after 1 to 2 years of age. Not including a sex-dependent term for SER, their model followed the following equation:
The variable T equals (age – 0.25), as 3 months was the target age for their initial visit. Using the same model, we used R 3.5.2 (R Project for Statistical Computing, Vienna, Austria) to fit our refractive data using the NLMER function.
Results
We identified 33 patients who were homozygous for c.1148delC. Medical records were available for 28 of the patients. Adult refraction was available for 21 patients, who had a mean age of 46 years (range, 17–65). Refractive data in the pediatric age group was available for 23 patients, and the medical records of 12 patients provided data for more than one visit in the period of 0 to 9 years of age, thus enabling individual analysis of early refractive development.
Clinical Findings
All patients had Snellen visual acuity of 0.08 to 0.17 (1.1–0.8 LogMar) that was stable throughout the follow-up and all but three patients (n = 25) had childhood nystagmus. The most frequent fundoscopic finding was an abnormal foveal reflex (n = 16). Electroretinography was available for 20 patients and most frequently described unrecordable flicker responses or light adapted responses.
Refraction in the Adult Population
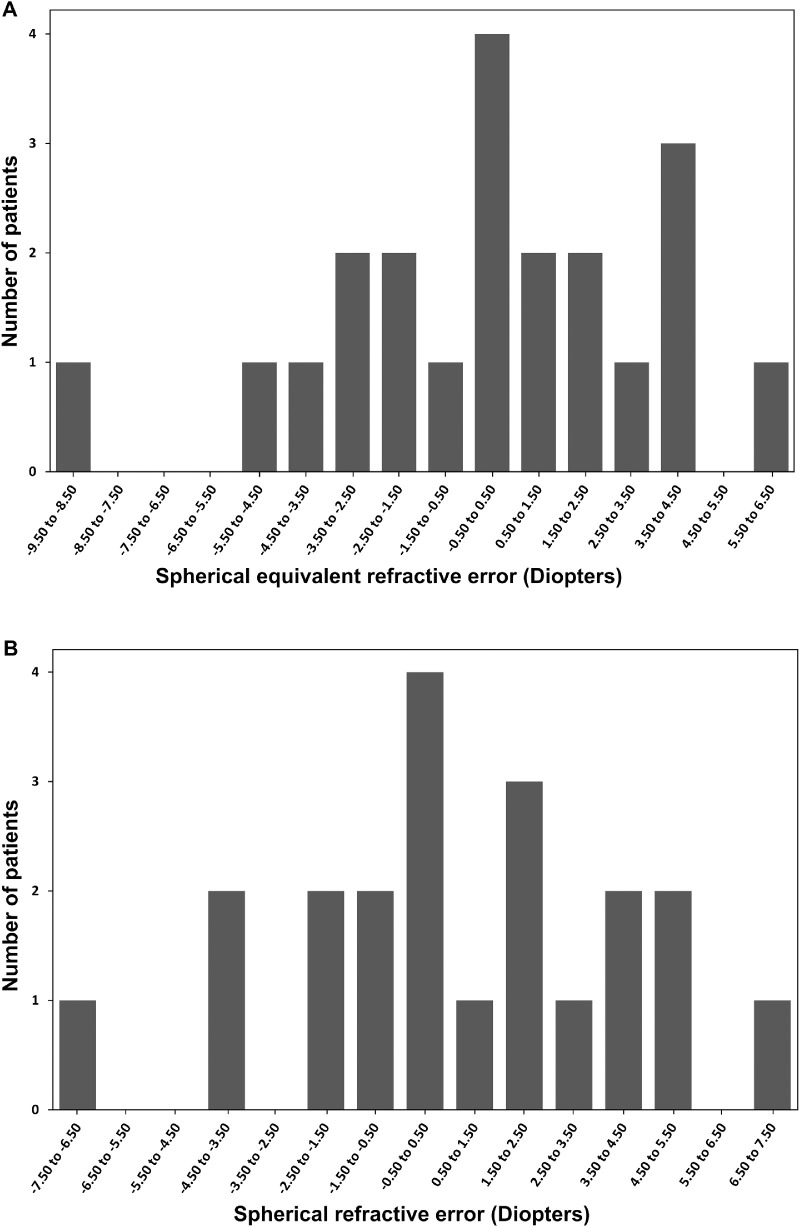
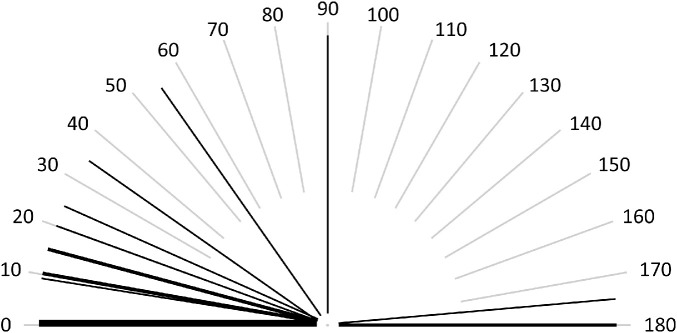
Spherical equivalent refraction ranged from –8.63 to +4.50 diopters (D) with a median of 0.00 D (IQR, 4.1). Neither the distribution of SER nor spherical refractive error followed a leptokurtic distribution (Fig. 1). Both SER and spherical error showed a broader distribution resembling a more Gaussian distribution. The majority of patients (43%, n = 9) were hyperopic with a SER ≥ +0.50; 38% (n = 8) were myopic with a SER ≤ –0.50; and 19% (n = 4) were emmetropic. Astigmatic refractive error greater than –0.50 was found in 76% of patients (n = 16) and ranged from –5.25 to –1.00 with a median of –1.50 (IQR, 1.25); the majority (81%, n = 13) had with-the-rule astigmatism (Fig. 2). Median values, prevalence, and IQRs for refractive errors are summarized in Table 1.
Figure 1.
Distribution of SER (A) and spherical refractive errors (B) in the adult population.
Figure 2.
The axis of astigmatic refractive error (negative cylinder) in the adult population; each thin line represents an individual patient, and bolder lines represent more than one patient. The majority (81%, n = 13) had with-the-rule astigmatism (between 0°–30° and 150°–180°).
Table 1.
Median Refractive Error and Prevalence for Children Ages 4.5 to 7.25 Years and Adults
| Percent (n) | |||||||
|---|---|---|---|---|---|---|---|
| Age Group (n) | Median SER (IQR) | Median Spherical Error (IQR) | Median Astigmatic (IQR) | Astigmatic Error ≤ –0.50 | With-the-Rule Astigmatism | Myopes, SER ≤ –0.50 | Hyperopes, SER ≥ +0.50 |
| 4.5–7.25 y (11) | +2.37 (3) | +3.0 (2.3) | –1.50 (1.5) | 72 (8) | 100 (8) | 0 (0) | 81.8 (9) |
| 17–65 y (21) | 0.00 (4,3) | +0.50 (4) | –1.50 (1.3) | 76.2 (16) | 81.3 (13) | 38.1 (9) | 42.9 (9) |
Refraction in the Pediatric Population
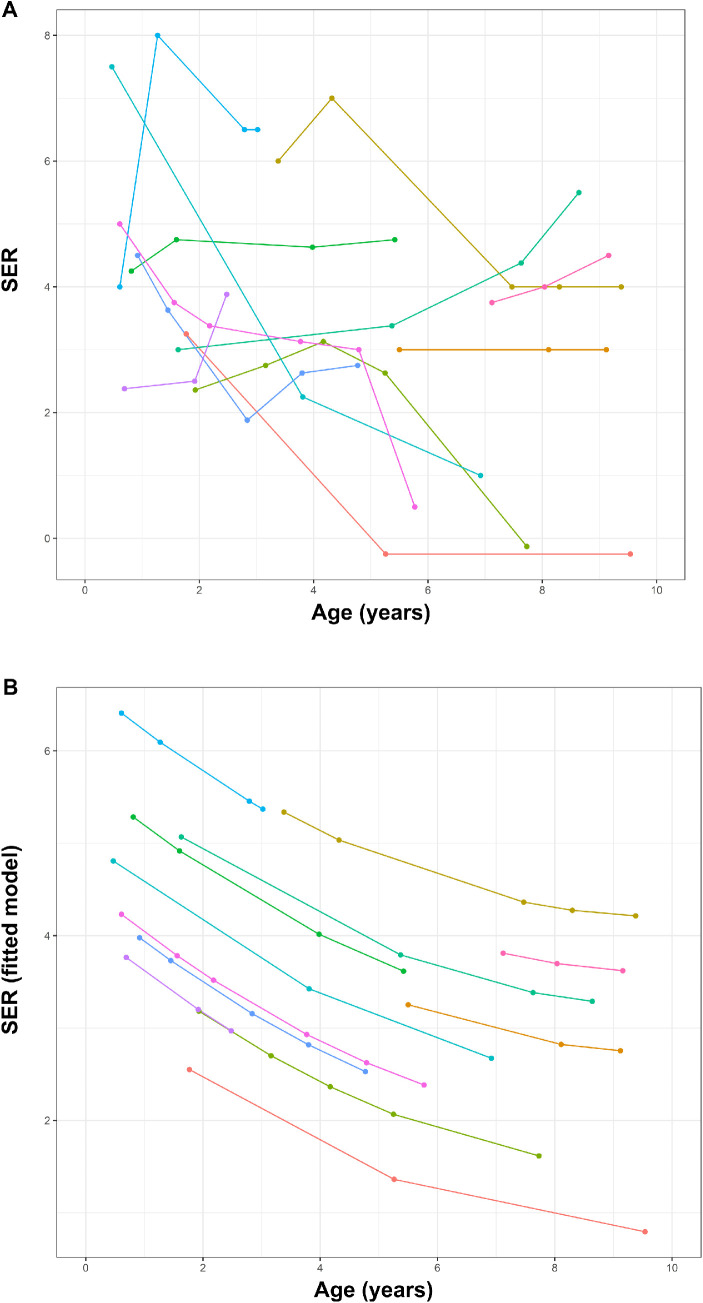
The median spherical equivalent, spherical refractive error, and astigmatic refractive error at different ages throughout childhood are shown in Table 2. From 0 to 7 years, there was a tendency toward a decrease in both median SER and spherical error. This decrease stopped after 7 years of age. The development in SER for individual patients is shown in Figure 3, where we, when accounting for the nature of our data using a nonlinear random regression model, also found a decrease in SER during the first years of life. Median SER, spherical refractive error, and astigmatic refractive error in the age group 4.5 to 7.25 years are summarized in Table 1.
Table 2.
Median Refractive Error in 1-Year Age Intervals for the Pediatric Population
| Median (IQR) | |||
|---|---|---|---|
| Age,* y (n) | SER | Sphere | Astigmatic Error |
| 0–1 (7) | +4.25 (1.6) | +4.50 (1.75) | –0.75 (1.5) |
| 1–2 (8) | +3.43 (1.1) | +4.25 (2.13) | –0.38 (0.9) |
| 2–3 (4) | +3.63 (1.5) | +4.50 (1.4) | –1.25 (1.2) |
| 3–4 (7) | +3.12 (2.6) | +4.0 (2.5) | –0.75 (1.8) |
| 4–5 (5) | +3.00 (0.4) | +3.25 (0.75) | –0.50 (1.3) |
| 5–6 (6) | +2.82 (2.3) | +2.88 (2.3) | –1.50 (1.1) |
| 6–7 (2) | +2.50 (1.5) | +2.0 (1) | –1.0 (1.0) |
| 7-8 (4) | +3.86 (1.3) | +4.25 (1.7) | –0.75 (0.8) |
| 8–9 (7) | +4.00 (1.5) | +4.50 (1.5) | –1.0 (0.5) |
| 9–10 (5) | +3.00 (4.0) | +3.00 (3.75) | –1.0 (1.0) |
| 10–11 (4) | +1.63 (5.2) | +2.50 (3.75) | –1.25 (1.4) |
| 11–12 (5) | 0.00 (4.5) | 0.00 (5) | –0.50 (1.0) |
| 12–13 (1) | –0.25 (0) | +0.75 (0) | –2.00 (0) |
| 13–14 (4) | +1.75 (2.1) | +2.75 (1.1) | –1.50 (1.5) |
| 14–15 (6) | +3.25 (2.9) | +0.75 (2.1) | –1.00 (1.3) |
| 15–16 (2) | +0.13 (1.2) | +0.75 (1.8) | –1.25 (1.3) |
| 16–17 (8) | –0.13 (1.4) | +0.50 (2.3) | –1.25 (1.1) |
Children are represented only once in each age group but may be represented in more than one group.
Figure 3.
Development of SER in individual patients is shown as (A) a conventional spaghetti plot and (B) after fitting our data to the nonlinear random regression model as proposed by Mutti et al.37 Different colors represent the individual patients.
Discussion
Achromatopsia is characterized by color blindness and a lack or reduced number of normally functioning cones; it provides a perfect sample to study the importance of color perception and cone photoreceptors in the process of emmetropization in humans. The present study collected retrospective refractive error data from 28 patients with achromatopsia caused by homozygosity for c.1148delC in CNGB3. The advantage of selecting these patients is that the genetic cause is known, confirming the clinical diagnosis, and there is no genetic variance.
We found that emmetropization was abnormal in this population. The prevalence of refractive error was high, and the distribution of SER and spherical refractive error in adults did not exhibit the characteristic leptokurtic distribution3,38 but resembled a more Gaussian distribution (Fig. 1). We found a wider range of refractive error than previously described for achromats.31,32 Although emmetropization in our population of achromats seemed to follow a different pattern than the background population, we did observe a reduction of median SER and sphere during the first 7 years of life, suggesting that some form of emmetropization takes place.
Comparing the distribution of refractive errors in the age group 4 to 7.5 years to published Danish normative data,36 we found that our population of achromats was more hyperopic and had a larger with-the-rule astigmatic refractive error. Applying the model for childhood development in ocular variables including SER presented by Mutti et al.,37 we found that for our population of achromats there was no exponential decrease in SER in the first 2 years of life; however, our data can be fitted to the quadratic phase with a decrease in SER continuing past the first 2 years of life.
The prevalence of refractive error was higher in our adult population than published normative data for Danish adults in the age group 30 to 60 years,38 for which the prevalence of emmetropia was 42% and the prevalence of myopia was 31%. In our population, 19% were emmetropic, and the remainder were either myopic (38%) or hyperopic (43%). We found a similar median astigmatic refractive error of –1.50 D in the pediatric and adult age groups, contrasting with the age-related increase in astigmatic refractive error observed in idiopathic congenital nystagmus and albinism.33 In albinism, the increase in astigmatism with age has been ascribed to corneal remodeling as a consequence of nystagmus. The majority of our patients had childhood nystagmus, but the direction and amplitude were unfortunately not described in the medical records; hence, it is unknown how it would affect corneal remolding, especially in cases with multidirectional nystagmus. Others have found that CNGB3 achromatopsia is predominantly associated with a pendular nystagmus with vertical elements.39 Future larger studies on different forms of congenital nystagmus with well-documented characterization of nystagmus are necessary to further elucidate the role of nystagmus on astigmatism.
The effect that color perception has on emmetropization is not fully understood. Animal studies on non-primates have shown a myopic shift after prolonged exposure to monochromatic long-wavelength (red) light and a more hyperopic shift in short-wavelength (blue) light due to LCA.40 Rearing animals under quasimonochromatic conditions does not prevent recovery from deprivation-induced myopia or refractive compensation for optically imposed defocus, implying that chromatic cues are not the only parameter guiding emmetropization. In primates, the role of LCA is less convincing. Smith et al.11 found that reducing chromatic cues with filters interfered with emmetropization, but the effect was opposite of what was expected from LCA. Liu et al.13 found that two out of nine monkeys kept under red light conditions developed myopia, but no significant difference in refraction was observed in monkeys kept in blue light, suggesting that long-wavelength light may be a risk factor in myopia development and that LCA can interfere with refraction. Humans with red/green color vision deficiency are less myopic that individuals with normal color vision.15 In other conditions caused by variants in the cone opsin genes, a high degree of myopic refractive error has been documented.16,17 For humans to perceive colors, functioning cone photoreceptors and an intact optic nerve are required. But, because an intact optic nerve is not required for emmetropization to occur, the detection of LCA probably relies more on the composition of the cone photoreceptor mosaic. A recent study indicated that the presence of non-functional cones may also impact refractive error.41 Chromatic properties of light play a role in accommodation in humans,40 but the role of accommodation on emmetropization is not clear. The eye can still compensate for optical lens-induced defocus in the absence of accommodation as a result of ciliary nerve section, at least in chickens.8
Our results seem to support the notion that color perception and cone photoreceptors play a role in emmetropization but that it is not essential. Refractive development in our color-blind population was abnormal, but some degree of emmetropization did take place. The inability to detect colors may not be the only reason for retinal blur in our population. The blur caused by nystagmus and astigmatism may also play a role.
Adjusting eye growth to compensate for optically induced defocus and form deprivation does not depend on the fovea in primates9 and may rely on a local peripheral retinal mechanism,42 but which part of the retina/sclera drives this is not known. Studies on mice seem to indicate that rod photoreceptors might be important, as rod knockout mice do not develop form-deprivation myopia.43
The retrospective nature of the study limits the rigor of the data, as the preferred clinical information was not always documented in the medical records (specifically, the use of cycloplegia), especially in records of older date. The lack of consistent use of cycloplegia is a limitation in our study design, as accommodation in determination of the refractive error, especially in the pediatric population, may affect spherical refraction in a less hyperopic direction and challenge comparison. In the patients for whom refractive data before and after cycloplegic drops were available, we did not find an indication of impaired accommodation. Where cycloplegic refraction was not specified, documented refractive data were the prescribed correction based on subjective refraction and, most often, on repeated streak retinoscopy. Streak retinoscopy was performed by examiners experienced in determining the refractive error in visually impaired children. Despite this limitation, we chose this retrospective design to be able to accumulate as large a dataset with as long a follow-up as possible, considering the rarity of achromatopsia. The cohort size is small, however, thus placing limitations on our interpretation of the data, especially in the pediatric group.
A further limitation of our study is that there was no analysis of functional parameters. Achromats could be completely without functional cones or they may have some residual cone function. The selection of a genetically homogeneous population was an attempt to minimize this, but in achromatopsia and other hereditary retinal disorders the relationship between genotype and phenotype is not always predictable. However, methods currently available (e.g., color vision testing, electroretinography, adaptive optics) are not sensitive enough to detect small variance in morphology or function of the cone mosaic, and the rarity of achromatopsia makes it difficult to compare a large number of patients.
In conclusion, the refractive development in our population of c.1148delC homozygous CNGB3 achromats was not normal, but the refractive development in achromats is more complicated than a complete failure to emmetropize, as there were signs of some degree of emmetropization, which seems to support the notion that cone photoreceptors are important in providing cues to guide emmetropization but they are not essential. Although we did find that the majority of our population (43%) were hyperopic, the spread in refractive errors was large, and the prevalence of myopia contradicts the previously suggested notion that achromats are predominantly hyperopic.29,30 The astigmatic refractive error was higher than for the normal population, but there was no indication of an increase with age as has been seen in other types of congenital nystagmus. Our knowledge of emmetropization is based mainly on animal studies, but the retinal structure varies greatly among species. Studying emmetropization in selected patient groups may provide future insights into the normal emmetropization process in humans.
Acknowledgments
The authors thank Lene Theil Skovgaard, Section of Biostatistics, Department of Public Health, University of Copenhagen, for her help with statistical analysis.
Supported by grants from Fight for Sight Denmark, Dag Lenards Foundation, Danish Eye Research Foundation, and Synoptikfonden.
Disclosure: M.K.G. Andersen, N; L. Kessel, N
References
- 1. Goldschmidt E Refraction in the newborn. Acta Ophthalmol. 1969; 47(3): 570–578. [DOI] [PubMed] [Google Scholar]
- 2. Mayer DL Cycloplegic refractions in healthy children aged 1 through 48 months. Arch Ophthalmol. 2001; 119(11): 1625–1628. [DOI] [PubMed] [Google Scholar]
- 3. Sorsby A, Sheridan M, Leary GA, Benjamin B. Vision, visual acuity, and ocular refraction of young men: findings in a sample of 1,033 subjects. Br Med J. 1960; 1(5183): 1394–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rabin J, Van Sluyters RC, Malach R. Emmetropization: a vision-dependent phenomenon. Invest Ophthalmol Vis Sci. 1981; 20(4): 561–564. [PubMed] [Google Scholar]
- 5. Wildsoet C Active emmetropization–evidence for its existence and ramifications for clinical practice. Ophthalmic Physiol Opt. 1977; 17(4): 279–290. [PubMed] [Google Scholar]
- 6. Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004; 43(4): 447–468. [DOI] [PubMed] [Google Scholar]
- 7. Zhu X, Park TW, Winawer J, Wallman J. In a matter of minutes, the eye can know which way to grow. Invest Ophthalmol Vis Sci. 2005; 46(7): 2238–2241. [DOI] [PubMed] [Google Scholar]
- 8. Wildsoet CF Neural pathways subserving negative lens-induced emmetropization in chicks–insights from selective lesions of the optic nerve and ciliary nerve. Curr Eye Res. 2003; 27(6): 371–385. [DOI] [PubMed] [Google Scholar]
- 9. Huang J, Hung L-F, Smith EL. Effects of foveal ablation on the pattern of peripheral refractive errors in normal and form-deprived infant rhesus monkeys (Macaca mulatta). Invest Ophthalmol Vis Sci. 2011; 52(9): 6428–6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gisbert S, Schaeffel F. M to L cone ratios determine eye sizes and baseline refractions in chickens. Exp Eye Res. 2018; 172: 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smith EL, Hung L-F, Arumugam B, Holden BA, Neitz M, Neitz J. Effects of long-wavelength lighting on refractive development in infant rhesus monkeys. Invest Ophthalmol Vis Sci. 2015; 56(11): 6490–6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gawne TJ, Siegwart JT, Ward AH, Norton TT. The wavelength composition and temporal modulation of ambient lighting strongly affect refractive development in young tree shrews. Exp Eye Res. 2017; 155: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu R, Hu M, He JC, et al.. The Effects of Monochromatic Illumination on Early Eye Development in Rhesus Monkeys. Invest Ophthalmol Vis Sci. 2014; 55(3): 1901–1909. [DOI] [PubMed] [Google Scholar]
- 14. Rucker FJ, Wallman J. Cone signals for spectacle-lens compensation: differential responses to short and long wavelengths. Vision Res. 2008; 48(19): 1980–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qian Y-S, Chu R-Y, He JC, et al.. Incidence of myopia in high school students with and without red-green color vision deficiency. Invest Ophthalmol Vis Sci. 2009; 50(4): 1598–15605. [DOI] [PubMed] [Google Scholar]
- 16. Orosz O, Rajta I, Vajas A, et al.. Myopia and late-onset progressive cone dystrophy associate to LVAVA/MVAVA exon 3 interchange haplotypes of opsin genes on chromosome X. Invest Ophthalmol Vis Sci. 2017; 58(3): 1834–1842. [DOI] [PubMed] [Google Scholar]
- 17. Gardner JC, Liew G, Quan Y-H, et al.. Three different cone opsin gene array mutational mechanisms; genotype-phenotype correlation and functional investigation of cone opsin variants. Hum Mutat. 2014; 35(11): 1354–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Andréasson S, Tornqvist K. Electroretinograms in patients with achromatopsia. Acta Ophthalmol (Copenh). 2009; 69(6): 711–716. [DOI] [PubMed] [Google Scholar]
- 19. Ansar M, Santos-Cortez RLP, Saqib MAN, et al.. Mutation of ATF6 causes autosomal recessive achromatopsia. Hum Genet. 2015; 134(9): 941–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kohl S, Baumann B, Rosenberg T, et al.. Mutations in the cone photoreceptor G-protein α-subunit gene GNAT2 in patients with achromatopsia. Am J Hum Genet. 2002; 71(2): 422–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kohl S Mutations in the CNGB3 gene encoding the beta-subunit of the cone photoreceptor cGMP-gated channel are responsible for achromatopsia (ACHM3) linked to chromosome 8q21. Hum Mol Genet. 2000; 9(14): 2107–2116. [DOI] [PubMed] [Google Scholar]
- 22. Chang B, Grau T, Dangel S, et al.. A homologous genetic basis of the murine cpfl1 mutant and human achromatopsia linked to mutations in the PDE6C gene. Proc Natl Acad Sci USA. 2009; 106(46): 19581–19586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kohl S, Marx T, Giddings I, et al.. Total colourblindness is caused by mutations in the gene encoding the α-subunit of the cone photoreceptor cGMP-gated cation channel. Nat Genet. 1998; 19(3): 257–259. [DOI] [PubMed] [Google Scholar]
- 24. Kohl S, Coppieters F, Meire F, et al.. A nonsense mutation in PDE6H causes autosomal-recessive incomplete achromatopsia. Am J Hum Genet. 2012; 91(3): 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kohl S, Varsanyi B, Antunes GA, et al.. CNGB3 mutations account for 50% of all cases with autosomal recessive achromatopsia. Eur J Hum Genet. 2005; 13(3): 302–308. [DOI] [PubMed] [Google Scholar]
- 26. Wiszniewski W, Lewis RA, Lupski JR.. Achromatopsia: the CNGB3 p.T383fsX mutation results from a founder effect and is responsible for the visual phenotype in the original report of uniparental disomy 14. Hum Genet. 2007; 121(3-4): 433–439. [DOI] [PubMed] [Google Scholar]
- 27. Khan NW, Wissinger B, Kohl S, Sieving PA. CNGB3 achromatopsia with progressive loss of residual cone function and impaired rod-mediated function. Invest Ophthalmol Vis Sci. 2007; 48(8): 3864–3871. [DOI] [PubMed] [Google Scholar]
- 28. Peng C, Rich ED, Varnum MD. Achromatopsia-associated mutation in the human cone photoreceptor cyclic nucleotide-gated channel CNGB3 subunit alters the ligand sensitivity and pore properties of heteromeric channels. J Biol Chem. 2003; 278(36): 34533–34540. [DOI] [PubMed] [Google Scholar]
- 29. Aboshiha J, Dubis AM, Carroll J, Hardcastle AJ, Michaelides M. The cone dysfunction syndromes. Br J Ophthalmol. 2016; 100(1): 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Simunovic MP, Moore AT. The cone dystrophies. Eye (Lond). 1998; 12(pt 3b): 553–565. [DOI] [PubMed] [Google Scholar]
- 31. Evans NM, Fielder AR, Major DL. Ametropia in congenital cone deficiency—achromatopsia: a defect of emmetropization? Clin Vis Sci. 1989; 4: 129–136. [Google Scholar]
- 32. Nathan J, Kiely PM, Crewther SG, Crewther DP. Disease-associated visual image degradation and spherical refractive errors in children. Am J Optom Physiol Opt. 1985; 62(10): 680–688. [DOI] [PubMed] [Google Scholar]
- 33. Healey N, McClelland JF, Saunders KJ, Jackson AJ. Longitudinal study of spherical refractive error in infantile nystagmus syndrome. Ophthalmic Physiol Opt. 2014; 34(3): 369–375. [DOI] [PubMed] [Google Scholar]
- 34. Sampath V, Bedell HE. Distribution of refractive errors in albinos and persons with idiopathic congenital nystagmus. Optom Vis Sci. 2002; 79(5): 292–299. [DOI] [PubMed] [Google Scholar]
- 35. Wagner RS, Caputo AR, Nelson LB, Zanoni D. High hyperopia in Leber's congenital amaurosis. Arch Ophthalmol. 1985; 103(10): 1507–1509. [DOI] [PubMed] [Google Scholar]
- 36. Sandfeld L, Weihrauch H, Tubaek G, Mortzos P. Ophthalmological data on 4.5- to 7-year-old Danish children. Acta Ophthalmol. 2018; 96(4): 379–383. [DOI] [PubMed] [Google Scholar]
- 37. Mutti DO, Sinnott LT, Lynn Mitchell G, et al.. Ocular component development during infancy and early childhood. Optom Vis Sci. 2018; 95(11): 976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kessel L, Hougaard JL, Mortensen C, Jorgensen T, Lund-Andersen H, Larsen M. Visual acuity and refractive errors in a suburban Danish population: Inter99 Eye Study. Acta Ophthalmol Scand. 2004; 82(1): 19–24. [DOI] [PubMed] [Google Scholar]
- 39. Hirji N, Theodorou M, Bainbridge JW, Venturi N, Michaelides M. Nystagmus and optical coherence tomography findings in CNGB3-associated achromatopsia. J AAPOS. 2020; 24(2): 82.e1–82.e7. [DOI] [PubMed] [Google Scholar]
- 40. Seidemann A, Schaeffel F. Effects of longitudinal chromatic aberration on accommodation and emmetropization. Vision Res. 2002; 42(21): 2409–2417. [DOI] [PubMed] [Google Scholar]
- 41. Patterson EJ, Kalitzeos A, Kasilian M, et al.. Residual cone structure in patients with X-linked cone opsin mutations. Invest Ophthalmol Vis Sci. 2018; 59(10): 4238–4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smith EL, Hung L-F, Huang J, Blasdel TL, Humbird TL, Bockhorst KH. Effects of optical defocus on refractive development in monkeys: evidence for local, regionally selective mechanisms. Invest Ophthalmol Vis Sci. 2010; 51(8): 3864–3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. na Park H, Jabbar SB, Tan CC, et al.. Visually-driven ocular growth in mice requires functional rod photoreceptors. Invest Ophthalmol Vis Sci. 2014; 55(10): 6272–6279. [DOI] [PMC free article] [PubMed] [Google Scholar]