Abstract
Tofacitinib is an oral, Janus kinase (JAK) molecule, which selectively inhibits Janus-associated tyrosine kinases JAK1 and JAK3. It has already shown efficacy in the treatment of rheumatoid arthritis and the prevention of organ allograft rejection in kidney transplantation. Two separate phase III placebo-controlled trials, assessing 8-week efficacy of tofacitinib induction for ulcerative colitis (UC), demonstrated superiority when compared with placebo. Tofacitinib also demonstrated robust efficacy versus placebo in the 52-week maintenance component of the same study. Tofacitinib has been recommended by the National Institute for Health and Care Excellence as an effective treatment option for adult patients with moderate to severe UC when conventional therapy or a biological agent cannot be tolerated or the disease has responded inadequately or lost response to treatment. We review the guidelines and provide brief commentary on the post hoc analysis related to lipid increases and thromboembolism risk, which have lead to changes in current therapeutic guidance.
Keywords: ulcerative colitis, inflammatory bowel disease
Key points.
Tofacitinib is an oral, Janus-associated tyrosine kinase inhibitor, which has been recommended by the National Institute for Health and Care Excellence for the management of moderate to severe ulcerative colitis (UC).
As an oral, small molecule, tofacitinib may render reduced immunogenicity compared with biological treatment.
The recommended regimen is an induction dose of 10 mg two times per day for 8 weeks followed by a maintenance dose of 5 mg two times per day.
However, a higher dosing regimen can be used in patients who have used anti-tumour necrosis factor inhibitors previously, but the risks of venous thromboembolism should be considered in decision-making.
There is a higher risk of herpes zoster infection in patients on tofacitinib, especially those on higher doses.
There is evidence of an increased dose-related risk of venous thromboembolism on higher dosing tofacitinib and so it should be used very cautiously in certain patient groups.
Commentary
Background
Tofacitinib has recently been recommended by the National Institute of Health and Care Excellence (NICE) (NICE Technology appraisal guidance (TA547)) as a cost-effective option for treating moderately to severely active ulcerative colitis (UC) in adults when conventional therapy or a biological agent cannot be tolerated or the disease has responded inadequately or lost response to treatment.1
Tofacitinib is a small, oral, Janus kinase (JAK) molecule. It selectively inhibits the Janus-associated tyrosine kinases JAK1 and JAK3. These molecules are integral for the propagation of intracellular signalling pathways through autophosphorylation of signal transducers and activators of transcription (STATs), which drive cytokine production (figure 1). Tofacitinib is thought to inhibit signalling of gamma-chain containing cytokines interleukin (IL)-2, 4, 7, 9, 15 and 21. These cytokines are integral to lymphocyte activation, function and proliferation, and have been implicated in the pathogenesis of UC.2 Equally, tofacitinib has shown efficacy for prevention of organ allograft rejection3 and is already approved for the treatment of patients with moderately to severely active rheumatoid arthritis (RA).4 5 The safety in the RA population has been reported based on clinical trial data from >7000 patients and >22 000 patient-years of exposure accumulated through 9 years of treatment.4 5 Tofacitinib as an oral agent offers potential solution to some of the burden associated with non-oral alternative, for example, intravenous and subcutaneous. Such burdens include the need to visit infusion units, issues relating to needle phobias and dependence on homecare companies.
Figure 1.
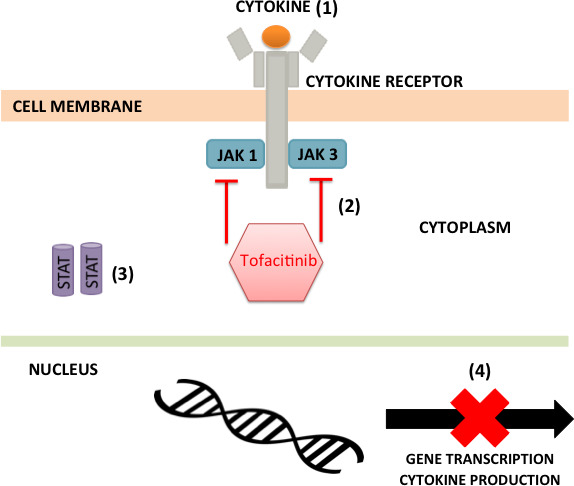
The mechanism of action of tofacitinib: (1) a cytokine binding to its cell surface receptor leads to receptor polymerisation; (2) tofacitinib inhibits the phosphorylation and activation of the Janus-associated tyrosine kinases (JAKs), JAK1 and JAK3; (3) JAKs cannot phosphorylate the cytokine receptors, therefore the receptors cannot dock signal transducers and activators of transcription (STATs); (4) as STATs are not phosphorylated or activated, gene transcription/cytokine production is inhibited.
There are approximately 150 000 people with UC in England, of whom around half have moderate to severe disease (Mayo score 6–12), with peak incidences between 15–25 years and 55–65 years.1 For this reason, an oral therapeutic agent for the treatment of patients is a welcomed addition for inflammatory bowel disease specialists.
Guidance overview
The NICE guidelines,1 released first in November 2018, focus on the outcomes of the three OCTAVE phase III, randomised, double-blind, placebo-controlled trials: I, II and OCTAVE Sustain.6 In OCTAVE I and II induction studies, patients with moderately to severely active UC despite previous conventional therapy or therapy with a tumour necrosis factor antagonist (anti-TNF) were randomly assigned to receive induction therapy with tofacitinib (10 mg two times per day, bd) or placebo for 8 weeks, with the primary endpoint being clinical remission at week 8. In the OCTAVE Sustain trial, patients who had a clinical response to induction therapy were randomly assigned to receive maintenance therapy with tofacitinib (either 5 or 10 mg bd) or placebo for 52 weeks with the primary end point being in remission at 52 weeks.
In both the OCTAVE I and II trials, tofacitinib 10 mg bd demonstrated efficacy and superiority when compared with placebo at 8 weeks in inducing remission (p<0.01). Specifically, for OCTAVE Induction 1 trial, remission at 8 weeks occurred in 18.5% of the patients in the tofacitinib group versus 8.2% in the placebo group (p=0.007); while for OCTAVE Induction 2 trial, remission occurred in 16.6% versus 3.6% (p<0.001). In the OCTAVE Sustain trial, remission at 52 weeks occurred in 34.3% of the patients in the 5 mg bd tofacitinib group and 40.6% in the 10 mg bd tofacitinib group versus 11.1% in the placebo group (p<0.001 for both comparisons with placebo).6 In both trials, outcomes were the same regardless of whether the patient had received prior anti-TNF therapy or not. A separate study demonstrated how this clinical improvement could equate to improved quality of life with better Inflammatory Bowel Disease Questionnaire (IBDQ) scores.7 In contrast, studies in patients suffering from Crohn’s disease (CD) were disappointing and the primary endpoint of clinical remission could not be met in the respective phase II induction and maintenance trials. Subsequently, the clinical development of tofacitinib was discontinued in CD.8 Filgotinib, another JAK inhibitor, shows promise for the treatment of moderate-severe CD, with more studies required.
In the OCTAVE studies,2 infections occurred at higher rates with tofacitinib than with placebo. The rate of serious infection was higher with tofacitinib in the induction trials but similar across treatment groups in the maintenance trial (OCTAVE sustain 5 mg=1% and 10 mg=0.5%). A numerically higher rate of herpes zoster infection was observed in the 10 mg tofacitinib group (5.1%) than in the placebo group (0.5%) or the 5 mg tofacitinib group (1.5%). Across all three trials, adjudicated non-melanoma skin cancer occurred in five patients who received tofacitinib and in one who received placebo. Malignancy risk was difficult to assess as many of the patients developing malignancy during the studies had had prior exposure to thiopurines. Adjudicated cardiovascular events occurred in five patients who received tofacitinib and in none who received placebo. Compared with placebo, tofacitinib was associated with increased lipid levels.
NICE recommendations
Based on these results, NICE approved the use of tofacitinib for the management of moderately to severe UC in adults whose disease has responded inadequately to, or who cannot tolerate conventional or biological therapy.1 The benefits cited for tofacitinib were as follows:
Tofacitinib being orally administered, therefore offering convenience to patients and the healthcare service.
Tofacitinib offered a reduced chance of immunogenicity—with subsequent loss of response over time compared with biologics—given its small molecule nature.
NICE therefore recommended the following dosing regimen in patients not exposed to anti-TNF drugs:
Induction: 10 mg orally bd for 8 weeks*.
Maintenance: 5 mg orally bd.
*If adequate therapeutic benefit is not achieved by week 8, the induction dose could be continued for a further 8 weeks (16 weeks in total). If by week 16 there is still no therapeutic benefit, induction should be discontinued.
However, in patients exposed to anti-TNFs previously:
In patients who responded inadequately to anti-TNFs, NICE recommended tofacitinib be continued at 10 mg bd as maintenance dose.
If clinical response decreases when on tofacitinib 5 mg bd maintenance therapy, NICE recommended increasing the dose to 10 mg bd.
These recommendations are summarised in table 1.
Table 1.
Nice recommended tofacitinib dosing regimen for patients with moderately to severely active UC1
| Anti-TNF naïve dosing | Previous anti-TNF exposure dosing | |
| Induction (8 weeks) | 10 mg bd | 10 mg bd |
| Maintenance | 5 mg bd | 10 mg bd |
| Loss of response | Increase to 10 mg bd | – |
bd, two times per day; TNF, tumour necrosis factor.
Post guideline publication recommendations regarding thromboembolic risk and lipids
In May 2019, following the publication of the NICE recommendations,1 the US Food and Drug Administration (FDA)9 and then the European Medicine Agency10 expressed concerns over the results of an ongoing study in RA patients (study A3921133) which showed an increase risk of venous thromboembolism (deep venous thrombosis/pulmonary embolism, DVT/PE) and death when the higher dose of 10 mg bd was used. After this, the Medicines and Healthcare products Regulatory Agency (MHRA) in March 2020 recommended11 the following:
For any dose and in any indication, exercise caution when considering tofacitinib in patients who have known risk factors for venous thromboembolism, in addition to their underlying disease.
Maintenance treatment for UC at the 10 mg bd dose is not recommended in patients with known risk factors for venous thromboembolism, unless there is no suitable alternative treatment.
Listed risk factors for venous thromboembolism include:
Previous venous thromboembolism.
Patients undergoing major surgery.
Immobilisation.
Myocardial infarction (within previous 3 months).
Heart failure.
Use of combined hormonal contraceptives or hormone replacement therapy.
Inherited coagulation disorder.
Malignancy.
Other venous thromboembolism risk factors that should be considered include age, obesity (body mass index ≥30 kg/m2), diabetes, hypertension and smoking status.
In the open-label, long-term extension arm (OLE) of OCTAVE,12 post hoc analysis of patients with UC, during tofacitinib exposure, showed one patient had a DVT and four had PEs. These occurred during the OLE study with a total of 1157 patients, most had received the 10 mg bd dosage and had venous thromboembolism risk factors, for example, active UC, supporting the MHRA cautious recommendations. Similarly, studies have shown that increases in lipids such as cholesterol occur in patients on treatment with tofacitinib, demonstrating that lipids should be monitored actively in patients on treatment.13
Therefore, the risks of higher dosing must be balanced against the potential benefits of dose escalation. The results of OCTAVE Open14 have recently been published showing that following tofacitinib de‐escalation to 5 mg bd (in patients already in remission on 10 mg bd), most patients maintained remission, although 25.4% lost remission at 12 months. For those patients who responded to induction treatment, who dose‐escalated following treatment failure on 5 mg twice daily maintenance therapy, 49.1% achieved remission by 12 months.14
Footnotes
Twitter: @DrPhilipJSmith
Contributors: AN, AB and PJS prepared the manuscript. AN and PJS prepared the tables and figures. All authors reviewed and approved the manuscript before submission.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: PJS is an Associate Editor of Frontline Gastroenterology and the Digital and Education Editor of Gut.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. National Institute for Health and Care Excellence (NICE) Tofacitinib for moderately to severely active ulcerative colitis. Technology appraisal guidance [TA547] 2018.
- 2. Schreiber S, Rosenstiel P, Hampe J, et al. Activation of signal transducer and activator of transcription (STAT) 1 in human chronic inflammatory bowel disease. Gut 2002;51:379–85. 10.1136/gut.51.3.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Busque S, Vincenti FG, Tedesco Silva H, et al. Efficacy and safety of a Tofacitinib-based immunosuppressive regimen after kidney transplantation: results from a long-term extension trial. Transplant Direct 2018;4:e380. 10.1097/TXD.0000000000000819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wollenhaupt J, Silverfield J, Lee EB, et al. Safety and efficacy of tofacitinib, an oral Janus kinase inhibitor, for the treatment of rheumatoid arthritis in open-label, longterm extension studies. J Rheumatol 2014;41:837–52. 10.3899/jrheum.130683 [DOI] [PubMed] [Google Scholar]
- 5. Sandborn WJ, Panés J, D'Haens GR, et al. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin Gastroenterol Hepatol 2019;17:1541–50. 10.1016/j.cgh.2018.11.035 [DOI] [PubMed] [Google Scholar]
- 6. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;376:1723–36. 10.1056/NEJMoa1606910 [DOI] [PubMed] [Google Scholar]
- 7. Paschos P, Katsoula A, Giouleme O, et al. Tofacitinib for induction of remission in ulcerative colitis: systematic review and meta-analysis. Ann Gastroenterol 2018;31:572–82. 10.20524/aog.2018.0276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rogler G JAK efficacy in Crohn’s disease. J Crohns Colitis 2019:pii: jjz186 10.1093/ecco-jcc/jjz186 [DOI] [Google Scholar]
- 9. US Food and Drug Administration Safety trial finds risk of blood clots in the lungs and death with higher dose of tofacitinib (Xeljanz) in rheumatoid arthritis patients; FDA to investigate, 2019. Available: https://www.fda.gov/media/120485/download [Accessed 12 Apr 2020].
- 10. European Medicines Agency Restrictions in use of Xeljanz while EMA reviews risk of blood clots in lungs, 2019. Available: https://www.ema.europa.eu/en/documents/referral/xeljanz-article-20-procedure-restrictions-use-xeljanz-while-ema-reviews-risk-blood-clots-lungs_en.pdf [Accessed 12 Apr 2020].
- 11. Medicines and Healthcare products Regulatory Agency Tofacitinib (Xeljanz): new measures to minimise risk of venous thromboembolism and of serious and fatal infections, 2020. Available: https://www.gov.uk/drug-safety-update/tofacitinib-xeljanz-new-measures-to-minimise-risk-of-venous-thromboembolism-and-of-serious-and-fatal-infections [Accessed 12 Apr 2020].
- 12. Sandborn WJ, Panés J, Sands BE, et al. Venous thromboembolic events in the tofacitinib ulcerative colitis clinical development programme. Aliment Pharmacol Ther 2019;50:1068–76. 10.1111/apt.15514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sands BE, Taub PR, Armuzzi A, et al. Tofacitinib treatment is associated with modest and reversible increases in serum lipids in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2020;18:123–32. 10.1016/j.cgh.2019.04.059 [DOI] [PubMed] [Google Scholar]
- 14. Sands BE, Armuzzi A, Marshall JK, et al. Efficacy and safety of tofacitinib dose de-escalation and dose escalation for patients with ulcerative colitis: results from OCTAVE open. Aliment Pharmacol Ther 2020;51:271–80. 10.1111/apt.15555 [DOI] [PMC free article] [PubMed] [Google Scholar]


