Abstract
Objective
Non-alcoholic fatty liver disease (NAFLD) affects approximately one in four adults of the general population, with an important minority of cases at high risk of developing cirrhosis. We evaluated the utility of a primary care NAFLD pathway incorporating a specialist nurse-led NAFLD clinic and a two-step testing approach for advanced liver fibrosis.
Design/Method
We performed a retrospective evaluation of prospectively collected demographic and clinical data on all patients diagnosed with NAFLD and intermediate NAFLD fibrosis score seen in our nurse-led NAFLD clinic between 1 May 2014 and 30 April 2017. Patients were assessed using a specific clerking pro forma and transient elastography (TE). Discharge to primary care with lifestyle advice was considered where TE<7.9 kPa.
Results
904 patients were identified, 114 (12.6%) of whom did not meet NAFLD criteria. Among the NAFLD population (n=790 (87.4%)), TE<7.9 kPa was present in 558 patients (70.6%), 519 of whom were discharged to primary care. Selected patients were followed up in secondary care despite TE<7.9 kPa or discharged with TE≥7.9 kPa. TE was unreliable in 22 patients (2.7%). Overall, 559 (70.8%) of patients with confirmed NAFLD were discharged from the nurse-led clinic. Introduction of the new pathway was associated with increased screening for hepatitis B and C viruses in primary care, and 17 new cases of alpha-1-antitrypsin deficiency were identified.
Conclusion
An integrated primary/secondary care NAFLD pathway, including a specialist nurse-led clinic may be a useful way of managing increasing demand on secondary care hepatology services.
Keywords: fatty liver, fibrosis, chronic liver disease, primary care, non-alcoholic steatohepatitis
Significance of this study.
What is already known on this topic
Non-alcoholic fatty liver disease (NAFLD) affects around one in four adults in Western countries with an important minority at risk of developing progressive liver fibrosis, cirrhosis and its eventual complications. The challenge for primary and secondary care providers is to identify those with NAFLD at greatest risk of future liver-related complications so that they may be offered appropriate treatment and monitoring.
What this study adds
A primary–secondary care NAFLD pathway incorporating a specialist nurse-led NAFLD clinic and two-step testing approach for advanced liver fibrosis allowed 70% of patients to be discharged without the need to attend consultant clinics and identified new cases of non-NAFLD-related liver disease.
How might it impact on clinical practice in the foreseeable future
Similar pathways might be replicated elsewhere to help manage high demand for secondary care investigation of NAFLD.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the the most common cause of chronic liver disease worldwide, affecting approximately one in four adults in the UK.1 2 NAFLD represents a spectrum of disease. The vast majority of individuals with NAFLD will have simple steatosis and relatively benign liver disease requiring no specific intervention.1 However, an important minority are at higher risk of progressive liver injury, characterised by the presence of non-alcoholic steatohepatitis and hepatic fibrosis, leading to eventual cirrhosis and its complications. The prevalence of NAFLD in Western countries is increasing, and NAFLD is a leading cause of cirrhosis, hepatocellular carcinoma and indication for liver transplantation.3
The challenge for primary and secondary care providers is to identify those with NAFLD at greatest risk of future liver-related complications, so that they may be offered appropriate treatment and monitoring.4–7 The presence of advanced fibrosis is the most important determinant of clinical outcome in NAFLD,7 8 and recent UK clinical guidelines recommend that patients with suspected NAFLD are offered non-invasive assessment for liver fibrosis and cirrhosis.9–11
In 2014, we performed a service evaluation noting a 25% per annum increase in hepatology referrals from primary to secondary care from 1020 in 2012–2013 to 1288 in 2013–2014. In order to manage this increase and to identify patients most in need of secondary care, we collaborated with local clinical commissioning groups to implement a risk stratification pathway for patients with suspected NAFLD. This pathway encompassed primary care assessment to exclude major alternative causes of chronic liver disease and initial risk stratification for liver fibrosis, with a new secondary care-based nurse-led NAFLD clinic for intermediate-risk patients.
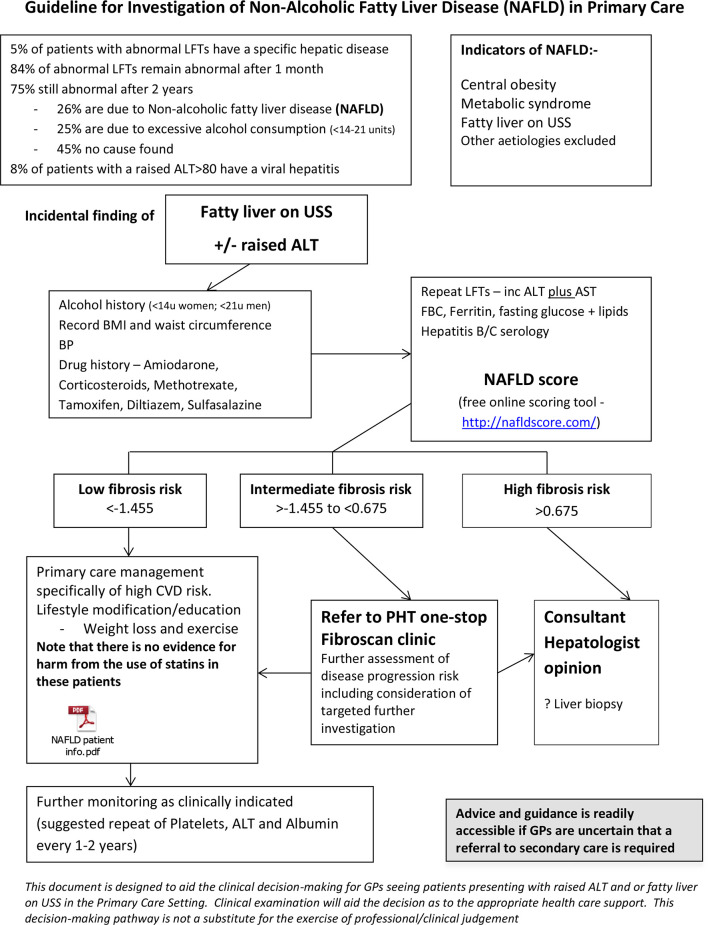
Primary care patients could enter the pathway with hepatic steatosis on ultrasound examination, identified either incidentally or on investigation for abnormal liver biochemistry (figure 1). The NAFLD-fibrosis score (NFS) was calculated in primary care for initial risk stratification.12 Those with an NFS indicating a low risk of advanced liver fibrosis were retained and managed in primary care; high-risk cases were referred to a consultant clinic; and intermediate-risk cases were referred to a twice weekly nurse-led clinic. Transient elastography (TE, Fibroscan) was performed on all patients with NAFLD referred to the nurse-led clinic and discharge to primary care with lifestyle advice considered in those with TE values of <7.9 kPa.13 General practitioners (GPs) were advised to repeat NFS at an interval of 3–5 years and again to follow the pathway in those patients with a low NFS at initial testing or who had been discharged from the nurse-led clinic. Patients who were not discharged from the nurse-led clinic were offered a follow-up appointment in a consultant clinic. Here we present an evaluation of this service after its first 3 years in operation.
Figure 1.
Portsmouth, Fareham and Gosport and Southeast Hampshire Clinical Commisioning Groups primary–secondary care NAFLD algorithm. ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; CVD, cardiovascular disease; FBC, full blood count; GP, generl practitioner; LFT, liver function test; NAFLD, non-alcoholic fatty liver disease; PHT, Portsmouth Hospitals NHS Trust; USS, ultrasound sonography.
Design/method
We conducted a retrospective evaluation of prospectively collected demographic and clinical data on all patients seen in the nurse-led NAFLD clinic at Portsmouth Hospitals NHS Trust between 1 May 2014 and 30 April 2017. Patients were seen by one of two designated hepatology clinical nurse specialists who had completed formal training in physical examination and using a specific clerking pro forma. TE was performed using a Fibroscan FS502 device (Echosens) with a standard or XL probe, according to the manufacturer’s instructions. The service evaluation was approved by and registered with the Portsmouth Hospitals NHS Trust Clinical Audit Department (ID 4241).
The local pathology results database (APEX) was interrogated for all hepatitis B surface antigen (HBsAg) and hepatitis C antibody (HCV Ab) tests from primary care between 1 November 2011 and 31 October 2016, thus including 30 months before and after the NAFLD pathway was implemented. Community HIV serology requests were measured as ‘controls’ for overall bloodborne virus testing and associated initiatives. APEX was interrogated for primary care AST:ALT ratio requests as a proxy measurement for use of the NAFLD pathway. Data were scrutinised for the number of tests per month pre- and post-implementation, the number of new diagnoses and the number of those diagnoses attributable to the NAFLD pathway based on previous laboratory results and clinical details on the request form.
Results
A total of 904 patients were seen in the nurse-led NAFLD clinic during the 3-year evaluation period. One hundred fourteen patients (12.6%) were identified as having liver disease due to a recognised cause other than NAFLD. One hundred five patients (11.6%) were identified as consuming alcohol at levels above those consistent with a diagnosis of NAFLD (women >14 units/week, men >21 units/week). Two patients (0.2%) were diagnosed with autoimmune hepatitis, one (0.1%) with primary biliary cholangitis, two (0.2%) with chronic hepatitis C, one (0.1%) with chronic hepatitis B and three (0.3%) with haemochromatosis. Seven hundred ninety patients (87.4%) were diagnosed with NAFLD (male=418 female=372, mean age 59 years (range 17–91), mean weight 98.7 kg (range 53–189) and mean body mass index (BMI) 34.4 (range 19–64)).
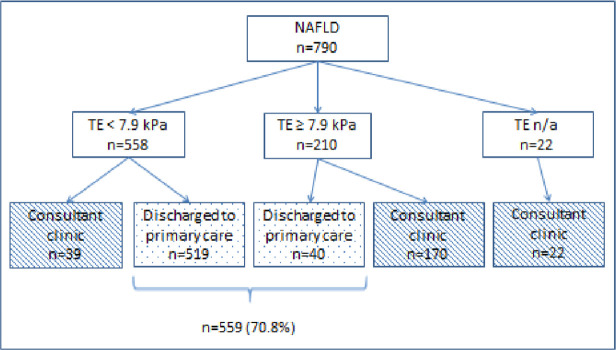
Among the NAFLD population (n=790), TE values of <7.9 kPa were present in 558 patients (70.6%), 519 of whom were discharged to primary care (65.7% of the referred NAFLD population). Thirty-nine patients with NAFLD (4.9%) with TE values of <7.9 kPa were followed up in consultant clinics for other reasons such as mild alpha-1 antitrypsin (A1AT) deficiency, features of potentially more advanced liver disease (eg, thrombocytopenia/splenomegaly) or abdominal symptoms requiring further assessment. 210 patients (26.6%) had TE readings≥7.9 kPa, 170 of whom were subsequently seen in a consultant clinic, as well as 22 patients (2.7%) in whom TE was unreliable. 40 patients with Fibroscan readings≥7.9 kPa were discharged to primary care, 27 of whom had readings of 7.9–8.4 kPa and 13 of 8.5–8.9 kPa. These patients had been discussed with a Consultant at or following the nurse-led visit and deemed not requiring formal Consultant-clinic review on the basis of age, co-morbidity, low risk of advanced liver disease and/or patient preference. Overall, of 790 referred patients with NAFLD, 559 (70.8%) were discharged from the nurse-led clinic (figure 2). No patients were re-referred via the pathway during the evaluation period.
Figure 2.
Discharge destination of patients with NAFLD according to TE result. n/a, not applicable; NAFLD, non-alcoholic fatty liver disease; TE, transient elastography.
Among the NAFLD cohort, 367 patients (46.5%) had type 2 diabetes mellitus, 371 (47.0%) had hypertension, 359 (45.4%) dyslipidaemia and 102 (12.9%) had a history of cardiac disease. 251 (32%) of the NAFLD cohort were aged 65 years or greater.
A1AT deficiency among the NAFLD population
A1AT deficiency is a chronic, under-recognised metabolic disease that may cause significant liver and lung injury.14 15 The responsible genetic defect affects 1 in 3000–5000 individuals. Seventeen patients with NAFLD (2.2%) had A1AT levels below the lower limit of normal (1.1 g/L). All patients were given lifestyle advice within the nurse-led clinic and offered A1AT phenotyping. Hepatology consultant follow-up was initiated regardless of liver Fibroscan evaluation score to discuss phenotyping results. A1AT phenotypes are shown in figure 3.
Figure 3.
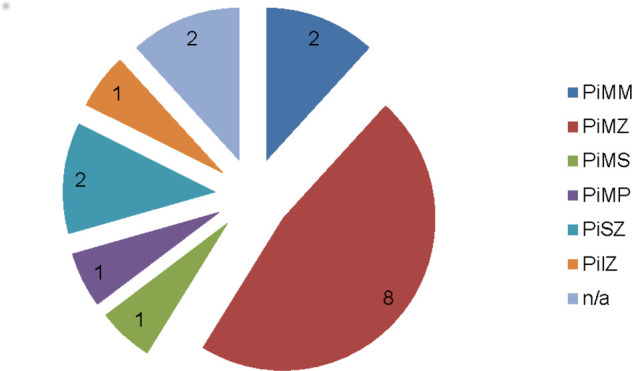
A1AT phenotype of patients with non-alcoholic fatty liver disease with incidental A1AT deficiency (n=17). A1AT, alpha-1 antitrypsin; n/a, not applicable.
Increased uptake of bloodborne virus and AST:ALT ratio testing in primary care
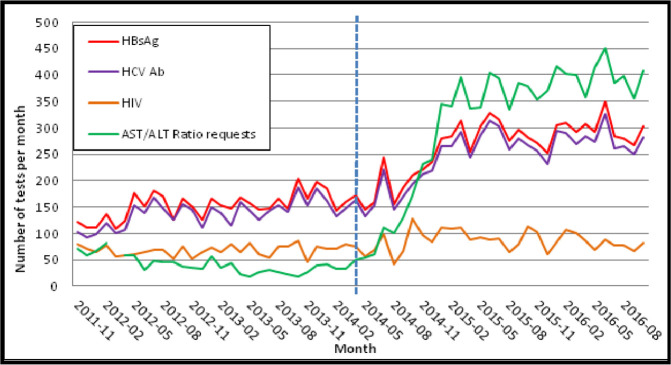
During the baseline period (November 2011–April 2014), there were a mean of 173 HBsAg and 139 anti-HCV tests per month requested from primary care. Following the introduction of the NAFLD pathway, mean monthly testing (May 2014–October 2016) increased to 295 HBsAg and 250 anti-HCV tests per month (p<0.001 for both). A proportionately higher increase in primary care testing for AST/ALT ratio was observed following the introduction of the NAFLD pathway from a mean of 42–315 per month (p<0.0001). Across the same period, HIV testing remained stable with a mean of 68 tests per month prior to and 86 per month following introduction of the pathway (p=0.20, NS) (figure 4).
Figure 4.
Local primary care bloodborne virus and AST:ALT ratio testing (2011–2016). The dashed line represents introduction of the local non-alcoholic fatty liver disease algorithm in April 2014. ALT, alanine transaminase; AST, aspartate transaminase; HBsAg, hepatitis B surface antigen; HCV Ab, hepatitis C antibody.
Implementation of the NAFLD pathway was associated with an increase in new HBsAg and anti-HCV Ab-positive diagnoses, although the proportion of positive tests decreased (table 1). Four new HBsAg and HCV Ab-positive diagnoses were directly attributable to NAFLD pathway assessment on clinical review.
Table 1.
Impact of the NAFLD pathway on hepatitis B and C virus testing in primary care
| HBV | Pre-NAFLD pathway | Post-NAFLD pathway | P value | HCV | Pre-NAFLD pathway | Post-NAFLD pathway | P value |
| Total number of HBsAg tests performed | 4586 | 8005 | – | Total number of anti-HCV tests performed | 4169 | 7504 | – |
| Mean number of HBsAg tests per month | 173 | 295 | <0.001 | Mean number of anti-HCV tests per month | 139 | 250 | <0.001 |
| Total number of HBsAg positives | 75 | 68 | – | Total number of anti-HCV positives | 164 | 189 | – |
| Positivity rate (%) | 1.64 | 0.85 | – | Positivity rate (%) | 3.93 | 2.52 | – |
| New HBsAg positives | 47 | 57 | – | New anti-HCV positives | 64 | 76 | – |
| Directly attributable to the NAFLD pathway | n/a | 4 | – | Directly attributable to the NAFLD pathway | n/a | 4 | – |
HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; n/a, not applicable; NAFLD, non-alcoholic fatty liver disease.
Discussion
We have described the utility of a specialist nurse-led NAFLD clinic as part of a new integrated primary–secondary care pathway for NAFLD, including two-step testing for advanced liver fibrosis. Over two-thirds of 790 patients with NAFLD identified in primary care as being at intermediate risk of advanced liver fibrosis could be discharged to primary care after a single nurse-led clinic appointment and transient elastographic staging of liver fibrosis. This has meant that referral to consultant clinics of patients at low risk of significant liver disease is avoided, thus freeing up appointments for those patients with the greatest clinical need.
Nurses undertaking the NAFLD clinic have significant experience of working with patients with liver disease and have completed training in physical assessment and history taking. In addition to assessing liver fibrosis using TE, they can identify alternative causes of liver disease. Over one in eight patients initially referred with a primary care diagnosis of NAFLD had an alternative cause of chronic liver disease at the nurse-led clinic appointment, in particular, alcohol-related liver disease. This emphasises a need for improved education of primary care practitioners in the identification of excess alcohol use. At the request of primary care commissioners, routine investigation for rarer causes of chronic liver disease, such as autoimmune liver disease and A1AT deficiency, was not part of the initial primary care assessment of patients with fatty liver. However, further refinement of the pathway to include such testing could help ensure patients were directed to the most appropriate clinic. The nurse-led clinic is also an important opportunity to offer patients signposting to support services such as the complex obesity service and well-being service, and refer onwards for consultant assessment those with more advanced disease or other relevant comorbidities.
TE is a useful tool in this setting and provides a liver stiffness measurement to evaluate the degree of liver fibrosis.16 However, there are limitations to its use. Studies demonstrated that even with the XL probe, 10% of patients with a BMI of >28 kg/m2 had a difference of two fibrosis stages when compared with evaluation by liver biopsy.13 The expertise of the nurses delivering the clinic is again important, as they can recognise the limitations of TE and its need to be interpreted with validated clinical screening tools such as the NAFLD fibrosis score and the clinical context. Patients with NAFLD who are assessed as having a low risk of advanced fibrosis not only avoid unnecessary further appointments but also receive a comprehensive evaluation and valuable lifestyle advice, with guidance provided to primary care physicians for ongoing monitoring. This approach is in keeping with recent recommendations on management of NAFLD at the primary–secondary care interface.9 11
Another potential benefit of our primary–secondary NAFLD care pathway is that it promotes a structured approach to identifying other causes of chronic liver disease in the community. For example, following implementation of the integrated primary–secondary care pathway, we observed increased community testing for viral hepatitis, including the identification of new cases of hepatitis B and C directly attributable to the NAFLD pathway. Although the number of new cases identified was modest, we suggest implementation of a similar approach to NAFLD could have an even greater impact on viral hepatitis diagnosis in areas of higher hepatitis B or C virus prevalence.
In addition to new viral hepatitis diagnoses, the NAFLD algorithm identified a small cohort of patients with A1AT deficiency. The significance of A1AT deficiency in NAFLD is not well described. However, the PiMZ phenotype, which was the most prevalent phenotype identified in our cohort, may have a role in worsening liver disease due to NAFLD,15 17 and we have offered such patients long-term secondary care follow-up.
One limitation of our evaluation is that we have not directly quantified the proportion of patients identified with NAFLD in primary care that were not referred, for example, because they had a low-risk NFS or the pathway was not followed. However, others have shown that approximately 57% of primary care diagnosed patients with NAFLD in the UK have a low risk NFS1 and that a two-step primary care-based algorithm using alternative non-invasive fibrosis markers to NFS reduced unnecessary primary care NAFLD referrals by 81%.18 We observed a significant and sustained increase in primary care AST:ALT ratio testing following implementation of the NAFLD pathway. AST:ALT ratio is a component part of the NFS,12 and this increase serves as a useful indicator of uptake of the new pathway by primary care physicians. Adoption was encouraged by several education sessions held for referring GPs prior to and following launch of the new pathway, raising awareness of NAFLD and associated risk factors in primary care, where most patients with NAFLD will first present.
Another limitation is that we have not examined the outcome of the 192 patients who were referred for consultant clinic assessment. This is an important area for future analysis in order to determine the performance of the pathway in identifying cases of advanced fibrosis and cirrhosis.
A potential drawback of our NAFLD pathway that became apparent following its introduction is the significant impact of age on the NFS. We and others have identified that this can result in a high number of false-positive results in elderly patients. We have found that a sizeable proportion (32%) of patients referred with intermediate-risk NFS are aged 65 or older. McPherson et al 19 examined the role of age as a confounding factor when using non-invasive scoring systems for the accurate diagnosis of advanced NAFLD fibrosis. They identified that NFS, which was principally developed and validated in patients aged 35–65 years, had an unacceptably low specificity for advanced fibrosis of 20% in patients aged ≥65 years, resulting in a high false-positive rate. To address this, they proposed new thresholds for use in patients aged ≥65 years, which improve specificity and sensitivity. Analysis of the first 739 patients seen in our nurse-led NAFLD clinic suggested that using the new cut-offs would have prevented referral of 144/236 (61%) over 65 years and 144/739 (20%) of total referrals. We have since incorporated these thresholds into our primary–secondary care NAFLD pathway.
While we have not conducted a formal health economic evaluation, we believe our pathway may prove cost effective when compared with alternative approaches. In a recent modelling study, based on 10 000 simulated patients, investigators compared multiple strategies for evaluating NAFLD wholly in primary or secondary care, or using hybrid models (similar to our own). The authors concluded that major costs are saved if primary care physicians can recognise and manage early disease, referring those with advanced fibrosis to secondary care.20 Furthermore, the cost to our service commissioners of a nurse-led NAFLD clinic appointment is lower than a new-patient consultant clinic appointment, where unselected patients would otherwise be seen.
In conclusion, our model appears to be a successful means of managing NAFLD at the primary–secondary care interface. A recent survey of UK specialists in gastroenterology and hepatology by the UK NAFLD group identified high demand for secondary care investigation of abnormal liver biochemistry (largely related to NAFLD) and high variability in use of primary and secondary care non-invasive NAFLD fibrosis assessment, with only 22% of respondents reporting the presence of local NAFLD guidelines.21 The authors of the survey recommended greater implementation of non-invasive disease staging in NAFLD, and we believe ours is an approach that could be introduced elsewhere. Our data suggest nurse-led clinics can help deliver more appropriately stratified care for people with NAFLD, particularly when coupled with an integrated pathway between primary and secondary care.
Acknowledgments
The authors thank the staff of the Queen Alexandra Hospital Hepatology Department for their contribution to the NAFLD pathway and the general practitioners of Portsmouth, Fareham & Gosport and Southeast Hampshire Clinical Commissioning Groups for providing the patients for this study.
Footnotes
Contributors: AJF, KF, KG, JKD, PH and RJA contributed to the design and implementation of the care pathway. AJF, KF, KG, KB, JKD and RJA contributed to the design of the service evaluation, the analysis of the results and the writing of the manuscript. PH reviewed and commented on the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This study was approved as a quality improvement project by the Portsmouth Hospitals NHS Trust Clincial Audit Department (approval ID 4241).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No data are available. Due to institutional restrictions, there are no data that can be shared. However, further information can be obtained from the corresponding author.
References
- 1. Armstrong MJ, Houlihan DD, Bentham L, et al. Presence and severity of non-alcoholic fatty liver disease in a large prospective primary care cohort. J Hepatol 2012;56:234–40. 10.1016/j.jhep.2011.03.020 [DOI] [PubMed] [Google Scholar]
- 2. Younossi Z, Henry L. Contribution of Alcoholic and Nonalcoholic Fatty Liver Disease to the Burden of Liver-Related Morbidity and Mortality. Gastroenterology 2016;150:1778–85. 10.1053/j.gastro.2016.03.005 [DOI] [PubMed] [Google Scholar]
- 3. Goldberg D, Ditah IC, Saeian K, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the Waitlist for liver transplantation. Gastroenterology 2017;152:1090–9. 10.1053/j.gastro.2017.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dyson JK, Anstee QM, McPherson S. Non-Alcoholic fatty liver disease: a practical approach to diagnosis and staging. Frontline Gastroenterol 2014;5:211–8. 10.1136/flgastro-2013-100403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Teli MR, James OF, Burt AD, et al. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology 1995;22:1714–9. 10.1002/hep.1840220616 [DOI] [PubMed] [Google Scholar]
- 6. Yilmaz Y, Dolar E, Ulukaya E, et al. Soluble forms of extracellular cytokeratin 18 may differentiate simple steatosis from nonalcoholic steatohepatitis. World J Gastroenterol 2007;13:837 10.3748/wjg.v13.i6.837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389–97. 10.1053/j.gastro.2015.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ekstedt M, Hagström H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015;61:1547–54. 10.1002/hep.27368 [DOI] [PubMed] [Google Scholar]
- 9. National Institute for Health and Care Excellence Non-Alcoholic fatty liver disease (NAFLD): assessment and management (NICE guideline 49), 2016. [PubMed] [Google Scholar]
- 10. National Institute for Health and Care Excellence Cirrhosis in over 16S: assessment and management (NICE guideline 50), 2016. [PubMed] [Google Scholar]
- 11. Newsome PN, Cramb R, Davison SM, et al. Guidelines on the management of abnormal liver blood tests. Gut 2018;67:6–19. 10.1136/gutjnl-2017-314924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846–54. 10.1002/hep.21496 [DOI] [PubMed] [Google Scholar]
- 13. Wong VW-S, Vergniol J, Wong GL-H, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010;51:454–62. 10.1002/hep.23312 [DOI] [PubMed] [Google Scholar]
- 14. Stoller JK, Aboussouan LS. Alpha1-antitrypsin deficiency. Lancet 2005;365:2225–36. 10.1016/S0140-6736(05)66781-5 [DOI] [PubMed] [Google Scholar]
- 15. Townsend SA, Edgar RG, Ellis PR, et al. Systematic review: the natural history of alpha-1 antitrypsin deficiency, and associated liver disease. Aliment Pharmacol Ther 2018;47:877–85. 10.1111/apt.14537 [DOI] [PubMed] [Google Scholar]
- 16. Sandrin L, Fourquet B, Hasquenoph J-M, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol 2003;29:1705–13. 10.1016/j.ultrasmedbio.2003.07.001 [DOI] [PubMed] [Google Scholar]
- 17. Regev A, Guaqueta C, Molina EG, et al. Does the heterozygous state of alpha-1 antitrypsin deficiency have a role in chronic liver diseases? interim results of a large case-control study. J Pediatr Gastroenterol Nutr 2006;43 Suppl 1:S30–5. 10.1097/01.mpg.0000226387.56612.1e [DOI] [PubMed] [Google Scholar]
- 18. Srivastava A, Gailer R, Tanwar S, et al. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J Hepatol 2019;71:371–8. 10.1016/j.jhep.2019.03.033 [DOI] [PubMed] [Google Scholar]
- 19. McPherson S, Hardy T, Dufour J-F, et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol 2017;112:740–51. 10.1038/ajg.2016.453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tapper EB, Hunink MGM, Afdhal NH, et al. Cost-Effectiveness analysis: risk stratification of nonalcoholic fatty liver disease (NAFLD) by the primary care physician using the NAFLD fibrosis score. PLoS One 2016;11:e0147237 10.1371/journal.pone.0147237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sheridan DA, Aithal G, Alazawi W, et al. Care standards for non-alcoholic fatty liver disease in the United Kingdom 2016: a cross-sectional survey. Frontline Gastroenterol 2017;8:252–9. 10.1136/flgastro-2017-100806 [DOI] [PMC free article] [PubMed] [Google Scholar]