Abstract
Objective
Refractory ascites is an established indication for liver transplantation. While transplantation is regarded as the definitive therapy for this condition, many patients are unsuitable due to comorbidity or frailty. Alternatives such as transjugular intrahepatic portosystemic shunt (TIPSS) and large-volume paracentesis can lead to complications, including encephalopathy, circulatory and renal dysfunction, and protein–calorie deficiency that may accelerate sarcopenia. Cost and complication rates limit therapies such as alfapump. While there are data to support the use of indwelling catheters in the management of patients with malignant ascites, there is limited evidence to support their routine use in the context of end-stage liver cirrhosis. Here we describe our centres’ experience using indwelling tunnelled ascitic drains over a 6-year period.
Methods
A retrospective review of data (January 2012–May 2018) was undertaken for all patients with refractory ascites who underwent a tunnelled ascitic drain. Demographics, disease aetiology, procedure data and follow-up data were obtained through interrogation of electronic records and reports.
Results
Twenty-five drains were placed. All procedures were technically successful with no immediate complications. Six patients were readmitted following their index admission with abdominal pain and suspected infected ascites (although only two had a positive ascitic fluid culture). There were three cases of abdominal wall cellulitis and three of leakage around the tunnel site; all managed conservatively.
Conclusion
Indwelling drains appear an effective strategy for palliative management of select patients with liver cirrhosis complicated by refractory ascites who are not amenable to undergo TIPSS or transplantation. While complications can occur, these are most usually minor and can be managed on an outpatient basis.
Keywords: ascites, cirrhosis
Significance of this study.
What is already known on this topic?
The use of palliative indwelling drains for ascites complicating malignancy or even chronic heart failure is established. Large published series of the use of such drains in the setting of ascites complicating liver cirrhosis to date are largely lacking.
What this study adds?
This study adds data regarding the applicability, acceptable safety and clinical outcomes of the use of palliative tunnelled ascitic drains, in those patients deemed by a multi-disciplinary team decision not to be candidates for transplantation or other treatments for ascites.
How might it impact on clinical practice?
Use of tunnelled indwelling drains in the appropriate selected patients with liver cirrhosis and refractory ascites can be considered in a palliative setting. Careful patient selection remains key, along with centre-experience in placement of drains. Patients however if appropriate should be considered for TIPSS or transplant assessment when ascites complicates cirrhosis prior to indwelling tunnelled drains being inserted.
Summary
Tunnelled peritoneal drains are widely used in patients with malignant ascites, but there are limited data regarding their safety and effectiveness in patients with cirrhosis and refractory ascites. The data presented in our case series of 25 patients with end-stage liver disease not suitable for transjugular intrahepatic portosystemic shunt or transplantation demonstrate that these devices can be safely used in this population. However, further randomised studies are needed.
Introduction
The development of ascites is an established complication of portal hypertension occurring in up to 60% of patients within 10 years of being diagnosed with cirrhosis.1 The presence of ascites, which is refractory to diuretics, acts as a marker of poor prognosis with 50% of patients dying within 6 months of onset.2 The presence of ascites can also significantly reduce a patient’s quality of life and worsen sarcopenia. Liver transplantation (LT) should be considered in all patients as a definitive and curative intervention, although not all patients will be suitable. Radiological techniques such as insertion of a transjugular intrahepatic portosystemic shunt (TIPSS) may be considered either as a definitive (although non-curative) treatment or, in select patients, as a bridge to an LT when optimisation is needed before a patient can be listed. For patients where LT and TIPSS are inappropriate and the burden of intermittent arge volume paracentesis (LVP) is high, there are few alternative therapies available. These patients often require multiple admissions to the hospital, which can have both a cost and bed-resource implications for hospitals3 and can be associated with a significant impact on the patients with constraints on the timing of drainage and the associated burden of regular hospital attendances. The use of tunnelled peritoneal drains may represent a more durable, sustainable and compassionate solution for select patients entering the palliative phase of their disease.
When undertaken under image guidance, procedural complications related to the insertion of tunnelled peritoneal drains such as visceral injury and haemoperitoneum are almost non-existent as evidenced by the outcomes from studies of the use of these devices in patients with malignant ascites. Two recently published studies looking at patients with malignant ascites reported 100% technical success and no immediate procedural complications.4 5 However, there remains a paucity of literature describing the safety data related to the use of long-term catheters in patients with cirrhosis, although the data available suggest that these are safe within this population.6 7
To date, no prospective studies or trials in this area have been published, although interest in this area is increasing; in Denmark, a randomised controlled trial (RCT) study of LVP versus PleurX is currently recruiting patients but is not due to be completed until 2022,8 while in the UK, preliminary data from a feasibility RCT comparing LVP and drain insertion support the safety and efficacy of these devices.9
In this paper, we describe our experience using tunnelled peritoneal drains (PleurX) in patients with end-stage cirrhosis and refractory ascites who were deemed unsuitable for TIPSS or LT.
Materials and methods
Study population
A retrospective review of the hospital’s radiology department patient database was performed, identifying all patients who had a tunnelled peritoneal drain for any indication between January 2012 and April 2018. From this cohort, all those who had a tunnelled drain for ascites on a background of cirrhosis were identified. Patient demographic and preprocedural data were collected from the electronic records, including patient age, sex, the aetiology of liver disease, number of previous LVP, disease severity scores (Model for End-Stage Liver Disease (MELD) and Child-Pugh score). Procedural data were obtained from the local patient information system and radiology electronic system.
All patients considered for tunnelled drain insertion were discussed via an MDT approach involving a hepatologist, palliative care consultant and specialist liver cirrhosis nurse, where their suitability for tunnelled drain insertion was determined. Contraindications to the insertion of tunnelled drain included the presence of loculated ascites and active abdominal wall infection. Previous spontaneous bacterial peritonitis (SBP) was not an absolute contraindication but was considered when deciding on the appropriateness of tunnelled drain insertion and the need for postprocedure antibiotic use with regard to coagulopathy. While the insertion of a temporary ascitic drain can be undertaken in patients with cirrhosis and an international normalised ratio (INR) of >1.5, the insertion of a permanent ascitic drain requires tunnelling of the drain in the abdominal wall, which is associated with a higher risk of bleeding. As such, radiology department guidelines within our institution state the need for an INR of 1.5 or less. While for patients with end-stage liver disease this may not be a significant coagulopathy, for the purpose of undertaking this specific interventional radiology procedure, it is deemed to be significant and required correction before the procedure can be undertaken.
Outcomes
The primary outcome measured was event-free survival of the tunnelled peritoneal drain. This was defined as the number of days since placement of drain without intervention or removal. Event endpoints were patient death or drain removal/replacement for any reason. Secondary outcomes noted were any drain-related complication expressed according to Common Terminology Criteria for Adverse Events (CTCAE)10 and readmission rates, defined as the number of hospital admissions for complaints related to tunnelled peritoneal drain/ascites. As drains were inserted for palliative indications and managed within the community, no follow-up data on the United Kingdom Model for End stage liver disease (UKELD)/MELD scores or renal function were collected.
Statistical analysis
Data were collected and collated using Microsoft Excel 2010. Mean (±SD) and median (range) were calculated for numerical data, and frequencies were calculated for categorical data. Kaplan-Meir survival curves were calculated for event-free survival following ascitic drains.
Drain placement procedure
The procedure of placing the tunnelled peritoneal drain was as described further and as per manufacturer recommendations (PleurX, BD, USA). The use of the PleurX system in patients with cirrhosis and refractory ascites is considered an off-label indication, and this was discussed with each patient prior to insertion. The abdomen was scanned with ultrasound to identify a sufficiently large pocket of ascites. The identified area was cleaned, and a sterile field was established. Local anaesthetic (lidocaine 1%) was injected subcutaneously at the point of intended puncture and then along the proposed tunnel (approximately 10 cm long). The tunnelled portion of the drain was directed centrally towards the umbilicus, a position practical for patient comfort and drain care. An Angiocath (BD, USA) coaxial needle was then used to puncture into the peritoneal cavity under ultrasound. The sheath was then advanced, and a standard wire (0.035-inch J-tip guidewire) was placed through the sheath, in the peritoneal cavity. A small incision was made in the skin at the puncture point and a second incision 5 cm away, towards the umbilicus. The ‘peritoneal’ end of the catheter was then attached and tunnelled subcutaneously between the two incisions, towards the wire, until the Dacron cuff was palpable in the tunnel. Dilators were used over the wire before introduction of the peel-away sheath. The peel-away sheath was advanced and the catheter fed through, before removing the peel-away sheath. The incisions were then sutured, and a dressing was applied to fix the drain in place. One litre of ascites was drained in the procedure room, and the patient was then transferred back to the ward or the ambulatory care unit.
Standard postprocedure care involved 2 hours of bed rest and half hourly observations (including heart rate, blood pressure and temperature). Patients were not routinely placed on prophylactic antibiotics following the procedure. However, those on SBP prophylaxis continued this postprocedure. Where patients had large-volume ascites at the time of drain insertion, this was drained via the PleurX prior to discharge; if a total of >5 L was removed, then 100 mL of 20% human albumin solution was given for every 2–3 L drained. Following this, albumin was not routinely given for ongoing small-volume drainage. Further care and patient training were arranged in the community in conjunction with local district nurses. The amount drained per session and the frequency of drainage were directed initially by the discharging medical team and going forward, guided by patients’ symptoms and tolerance. Clinical follow-up of patients once drainage was established was at the discretion of their hepatologist. The end of follow-up was defined as the last documented clinical contact or death.
Results
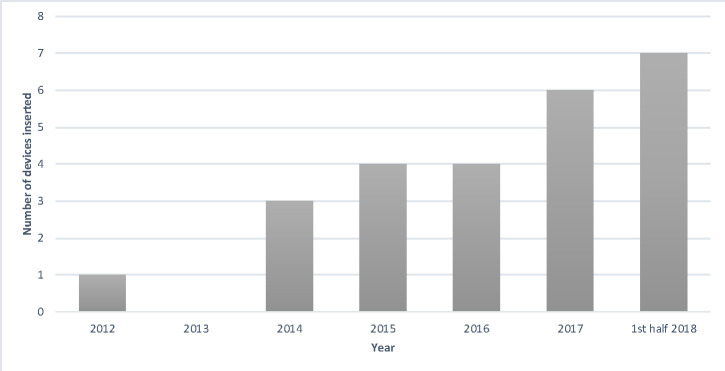
Twenty-five tunnelled peritoneal drains were placed in 25 patients during the study period with the use of the drains becoming more frequent over time (figure 1). Patient demographics are summarised in table 1. The most common aetiology for cirrhosis was alcohol-related liver disease (n=12) followed by non-alcoholic steatohepatitis (n=10), viral hepatitis (n=3), cryptogenic cirrhosis (n=2), primary sclerosing cholangitis (n=1), polycystic liver and kidney disease (n=1), and amyloid (n=1). In those patients with concurrent hepatocellular carcinoma (n=9, 36%), all diseases were confined to the liver with no extrahepatic or peritoneal disease, and the median MELD score was 15 (range 7–23), indicating the severity of their underlying liver dysfunction. Therefore, the presumed mechanism of action for the ascites was liver synthetic failure with an overall MELD score of 15 (range 7–25) for all patients.
Figure 1.
Number of PleurX drains inserted per calendar from the beginning of the use of the devices in 2012 until the end of the data collection period.
Table 1.
Patient characteristics
| Patient characteristics (n=25) | |
| Age, median (range) | 69 (48–83) |
| M:F | 17:8 |
| Concurrent HCC | 9 (36%) |
| Aetiology of cirrhosis* | |
| ARLD | 12 |
| NASH | 10 |
| Cryptogenic | 2 |
| HBV/HCV | 3 |
| Other | 3 |
| Classification of liver function | |
| MELD score, median (range) | 15 (7–25) |
| Child-Pugh classification | B, 22 (88%); C, 3 (12%) |
*n>25 as some patients had more than one aetiology to their liver disease.
ARLD, Alcohol related liver disease; F, female; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; M, male; MELD, Model for End-Stage Liver Disease; NASH, Non-alcoholic steatohepatitis.
All patients had undergone day case LVP in the 12 months prior to the procedure, with a median number of three drainage episodes required per patient (range 1–50). Seven patients (28%) had a previous episode of SBP. While LT had been considered in all 25 patients, advancing age and poor physiological reserve were deemed a contraindication in 4 cases (16%), major comorbidity in 13 cases (52%), active or past malignancy in 4 cases (16%) and ongoing alcohol use/concern regarding recidivism in 3 cases (12%). Twenty-one of the 25 patients (84%) were formally referred to palliative care services (18 via hospital-based services and 3 to local community services). Nine patients (36%) were formally assessed for TIPSS but were deemed to be inappropriate: four had previous or current hepatic encephalopathy (HE); four had a significant cardiopulmonary comorbidity; and one patient had a portal vein thrombosis, meaning that TIPSS was technically not feasible.
Procedure outcomes
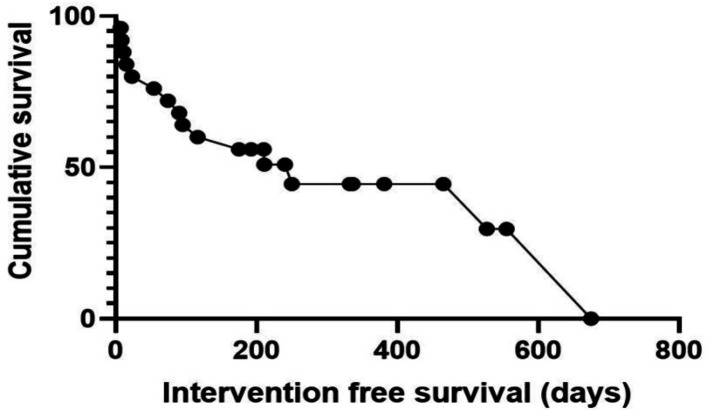
Drain placement was technically successful in all 25 cases. There were no immediate complications after drain placement and no complications that developed during the index admission of the patient. Fourteen drains (56%) were carried out as day case procedures through the hospital ambulatory care unit with the remainder carried out during an inpatient stay. The median length of stay post drain was 3 days (range 0–33). Delayed complications are summarised in table 2 and are graded according to the CTCAE. The median time to the last follow-up or death was 90 days (range 8–675). Ten of the patients (40%) had no recorded drain-related complications or readmissions. Of the remaining 15 patients, 6 (40%) were readmitted following their index admission. Two patients had a positive ascitic fluid culture (one grew acinobacter and the other a vancomycin-sensitive enterococcus) and were treated with appropriate antibiotics. Two patients had negative ascitic fluid cultures (one had an ascitic white cell count of >250) but were treated with antibiotics. The other two patients (one of which was admitted to an external hospital) were treated for presumed SBP without ascitic fluid sampling. Symptoms settled in all patients and none required drain removal. None of these patients developed event recurrence. Minor complications (not requiring readmission) included three cases of superficial cellulitis, managed with oral antibiotics and three cases of leakage around the tunnel site, which were treated definitively with a purse-string suture again. One drain was removed as it became blocked and a muliti-disciplnary meeting (MDT) decision was made not to replace it. The Kaplan-Meier curve (figure 2) demonstrates the survival of patients with tunnelled peritoneal drains. More than 50% of patients survived >200 days with drains in situ. This is in keeping with published mortality rates for patients with refractory ascites.
Table 2.
Adverse events
| Complication | Number (%) | Intervention | |
| Minor (CTCAE grade 2) |
Leakage | 3 (12) | Suture |
| Cellulitis | 3 (12) | Oral antibiotics | |
| Abdominal pain | 4 (16) | Empirical antibiotics | |
| Major (CTCAE grade 3) |
Drain malfunction | 1 (4) | Blocked, removed |
| Positive ascitic fluid culture | 2 (8) | Antibiotics |
CTCAE, Common Terminology Criteria for Adverse Events.
Figure 2.
Kaplan-Meier survival curve demonstrating intervention-free drain survival.
Discussion
This study, to our knowledge, represents one of the largest cohort of patients with cirrhosis undergoing insertion of a tunnelled peritoneal drain for the management of refractory ascites. While insertion of this device is regarded as standard practice in the palliation of patients with recurrent malignant ascites, its use in patients with underlying cirrhosis is not yet widespread and its utility, safety and cost effectiveness in this context remain to be established. Currently, LVP is the standard of care for patients with refractory ascites not suitable for TIPSS or LT, and is known to be a safe procedure with a relatively low complication rate.2 This approach, however, can be burdensome for patients entering a palliative phase of their illness. The convenience, durability and ease of use of these indwelling drains facilitate community management and can prevent frequent hospital admissions for paracentesis. In terms of cost, a review by National Institute for Health and Clinical Evidence suggested a cost saving of £1051 per patient when compared with inpatient LVP with malignant ascites.11 However, savings are not seen in patients where LVP can be offered on an outpatient basis. Further data are needed to compare ambulatory LVP and peritoneal drain insertion in patients with cirrhosis.
Our experience confirms that the indwelling catheters can be used over long periods (with 50% surviving over 200 days) with an acceptable rate of complications which are similar to those reported in other studies. Where complications did occur in our series, all patients who experienced a drain leak were managed as outpatients with insertion of a purse-string suture, and those who developed superficial cellulitis were managed with oral antibiotics at home. The most significant complication was that of culture-proven bacterial peritonitis which was diagnosed in two patients (8%). This complication has also been reported in other studies of tunnelled ascitic drains for both non-malignant and malignant ascites and in our series was successfully managed with intravenous antibiotics with the drain left in situ. Neither of the two patients in our cohort was systemically unwell or had signs of sepsis.
While the outcomes of this study are positive, it is not without its limitations. Compared with the data in malignant ascites, it is a small case series with data having been collected retrospectively, which prevented any detailed comparative subanalyses of data.
In addition, data on quality of life scores were not collected within our case series. Given the nature of the patient cohort, it would be beneficial for any prospective studies to incorporate quality of life data collection predrain and postdrain insertion. The role of prophylactic antibiotics also requires further study, especially in view of risks of antibiotic resistance and Clostridium difficile.
Conclusion
Tunnelled peritoneal drains appear an effective strategy for community management and palliation of refractory ascites in patients with end-stage cirrhosis. The regular use of indwelling peritoneal catheters has the potential to prevent repeated hospital admission, save on inpatient costs and allow patients to be managed in their own homes. The use of the catheters is associated with an acceptable rate of manageable complications in patients entering a palliative phase of their illness. Appropriate patient selection, multidisciplinary discussion and shared protocols for the use of the drains are vital.
Footnotes
MC and RT contributed equally.
Contributors: MC, JMcD, JS, FT, AH, SK and NR were involved in the conception and planning of this paper. MC, RT, JMcD, NA, SB and SK were involved in the data acquisition and analysis. MC, RT, SK and NR were involved in creating the first draft of this paper. MC, RT, JS, FT, AH, RJ, AW, SK and NR were involved in redrafting the paper. MC, SK and NR revised the paper prior to final submission.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: AH has spoken at meetings sponsored by PleurX.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Ginés P, Quintero E, Arroyo V, et al. Compensated cirrhosis: natural history and prognostic factors. Hepatology 1987;7:122–8. 10.1002/hep.1840070124 [DOI] [PubMed] [Google Scholar]
- 2. European Association for the Study of the Liver EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol 2010;53:397–417. 10.1016/j.jhep.2010.05.004 [DOI] [PubMed] [Google Scholar]
- 3. Hudson B, Round J, Georgeson B, et al. Cirrhosis with ascites in the last year of life: a nationwide analysis of factors shaping costs, health-care use, and place of death in England. Lancet Gastroenterol Hepatol 2018;3:95–103. 10.1016/S2468-1253(17)30362-X [DOI] [PubMed] [Google Scholar]
- 4. Knight JA, Thompson SM, Fleming CJ, et al. Safety and effectiveness of palliative tunneled peritoneal drainage catheters in the management of refractory malignant and non-malignant ascites. Cardiovasc Intervent Radiol 2018;41:753–61. 10.1007/s00270-017-1872-1 [DOI] [PubMed] [Google Scholar]
- 5. Maleux G, Indesteege I, Laenen A, et al. Tenckhoff tunneled peritoneal catheter placement in the palliative treatment of malignant ascites: technical results and overall clinical outcome. Radiol Oncol 2016;50:197–203. 10.1515/raon-2016-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Macken L, Joshi D, Messenger J, et al. Palliative long-term abdominal drains in refractory ascites due to end-stage liver disease: a case series. Palliat Med 2017;31:671–5. 10.1177/0269216316671281 [DOI] [PubMed] [Google Scholar]
- 7. Solbach P, Höner Zu Siederdissen C, Taubert R, et al. Home-Based drainage of refractory ascites by a permanent-tunneled peritoneal catheter can safely replace large-volume paracentesis. Eur J Gastroenterol Hepatol 2017;29:539–46. 10.1097/MEG.0000000000000837 [DOI] [PubMed] [Google Scholar]
- 8. Peritoneal catheter versus repeated paracentesis for ascites in cirrhosis. Available: https://ClinicalTrials.gov/show/NCT03027635
- 9. Macken L, Mason L, Gage H, et al. PTU-021 Long-term palliative abdominal drains vs large-volume paracentesis in cirrhosis-related refractory ascites: multi-centre feasibility RCT (REDUCe). Gut 2019;68:A122. [Google Scholar]
- 10. Services UDoHaH Common terminology criteria for adverse events (CTCAE) version 5.0 2017, 2017. Available: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf
- 11. Excellence NIfHaC PleurX peritoneal catheter drainage system for vacuum-assisted drainage of treatment-resistant, recurrent malignant ascites, 2012. [DOI] [PubMed] [Google Scholar]