Abstract
Vonoprazan, a novel potassium-competitive acid blocker, results in greater inhibition of gastric acid secretion than proton pump inhibitors (PPI). The aim of this study was to assess the long-term outcomes of patients with PPI-resistant gastroesophageal reflux disease (GERD) treated with vonoprazan. The medical records of patients with symptomatic GERD treated with vonoprazan for 1 year were retrospectively reviewed. Changes in abdominal symptoms were assessed using the Izumo scale, a self-reported questionnaire which is useful in evaluating the symptoms of GERD, epigastric pain, postprandial distress, constipation and diarrhea, and is commonly used in routine clinical practice. A total of 30 patients were included and stratified into a non-erosive (n=22) and erosive group (n=8). At baseline, postprandial distress symptoms were significantly greater in the non-erosive group compared with the erosive group (P=0.013). Even with vonoprazan therapy, symptoms of GERD in the non-erosive group were refractory compared with the erosive group, and required additional treatment in a larger proportion of patients (45 vs. 13%). GERD symptoms in the non-erosive group significantly improved from baseline and remained better after 1 year of vonoprazan therapy, similar to the erosive group. In addition, vonoprazan improved epigastric pain and postprandial distress symptoms in the non-erosive group, and 1 year of vonoprazan therapy did not aggravate constipation or diarrhea. In conclusion, 1 year of vonoprazan therapy improves GERD symptoms in patients with PPI-resistant GERD.
Keywords: PPI, potassium-competitive acid blocker, GERD, therapeutic outcome, dyspepsia
Introduction
According to the Japanese guidelines for gastroesophageal reflux disease (GERD) 2015, a proton pump inhibitor (PPI) is the first-choice drug for the treatment of patients with GERD (1). Although PPIs have comparatively better acid inhibition properties than histamine-2 receptor antagonists, ~45% of GERD patients treated with PPI suffer from persistent GERD symptoms in an observational study (2). In patients with PPI-resistant GERD, weak acid reflux is observed even with standard-dose PPI treatment (3), and double-dose PPI cannot adequately control PPI-resistant GERD (4). Therefore, more potent acid inhibition is necessary to control symptomatic PPI-resistant GERD.
Vonoprazan, a potassium-competitive acid blocker, strongly suppresses gastric acid release by inhibiting H+-K+ exchange in the gastric parietal cells. The holding time ratios of gastric pH >4 when treated with vonoprazan is significantly higher than treatment with PPIs (5). Due to its strong and sustainable acid inhibition, vonoprazan has become the first-choice drug for GERD treatment and Helicobacter pylori (H. pylori) eradication therapy (6,7). Vonoprazan is useful in overcoming PPI-resistant GERD, and our previous study was the first to report the short-term effects of vonoprazan in patients with symptomatic PPI-resistant GERD (8). However, previous reports regarding the effect of vonoprazan on PPI-resistant GERD were limited to healing of esophageal erosions and/or short-term symptomatic improvement (9-12). To the best of our knowledge, there are no reports regarding the long-term effects of vonoprazan on symptomatic improvement in patients with PPI-resistant GERD without esophageal erosions. The aim of the present study was to evaluate the long-term outcomes of patients with PPI-resistant GERD treated with vonoprazan.
Patients and methods
Patients
Consecutive patients with PPI-resistant symptomatic GERD treated for 1 year with continuous vonoprazan therapy at the Shinozaki Medical Clinic between February 2016 and October 2020 were included in the present study, and their medical records were retrospectively reviewed. The final cohort consisted of 7 males and 23 females with a median age of 69 (age range, 35-86). Gastrointestinal symptoms were routinely evaluated using the Izumo scale, a self-reporting questionnaire (13). All patients underwent esophagogastroduodenoscopy (EGD) prior to starting vonoprazan therapy. The patients were asked to complete the Izumo scale questionnaire 0, 1, 3, 6, 9 and 12 months after starting therapy as previously reported (14). All patients visited the clinic, and vonoprazan was prescribed monthly. The treating physician confirmed the nature of abdominal symptoms at each visit. If patients reported changes in abdominal symptoms, the Izumo scale was immediately administered and the next treatment strategy considered. If GERD symptoms were not sufficiently improved, the dose was increased from 10 to 20 mg or the addition of acotiamide was considered. For patients taking acotiamide at baseline, vonoprazan was added without ceasing acotiamide. After 1 year of vonoprazan therapy, a follow-up EGD was used to evaluate the grade of erosive esophagitis. Based on the Los Angeles classification of reflux esophagitis, erosive esophagitis was defined as grade A or higher (15). The degree of gastric atrophy was evaluated based on Kimura-Takemoto classification (16). H. pylori infection status was evaluated using the serum H. pylori IgG kit (E-plate; cat. no. I-DQ77; Eiken Chemical, Co., Ltd.) according to the manufacturer's protocol, and serum IgG values of <3, 3-9, ≥10 were considered negative, undetermined and positive, respectively. In case of an indeterminate result, a 13C-urea breath test (UBIT; Otsuka Pharmaceutical, Co., Ltd.) or stool antigen test (Testmate rapid pylori antigen; Wakamoto Pharmaceutical, Co., Ltd.) were used. H. pylori eradication history was determined based on the medical record or based on the patient's recollection. The Institutional Review Board of the Shinozaki Medical Clinic approved this retrospective review. The need for informed consent was waived due to the retrospective nature of the study.
Izumo scale
The Izumo scale was developed to evaluate various abdominal symptoms simultaneously (13). The Izumo scale has been validated and is a widely used self-reporting questionnaire with five domains: GERD (Q1-3), epigastric pain syndrome (Q4-6), postprandial distress syndrome (Q7-9), constipation (Q10-12) and diarrhea (Q13-15) (17-20). Each domain has three items and each item is scored from 0-5 on a Likert scale as follows: 0, not bothered; 1, not so bothered; 2, slightly bothered; 3, bothered; 4, strongly bothered; and 5, intolerably bothered. Higher scores indicate more severe gastrointestinal symptoms. The domain-specific score is calculated as a total score of three items ranging from 0 to 15. A domain-specific score of ≥4 is considered a ‘significant symptom’ (21).
Inclusion and exclusion criteria
The inclusion criteria were: i) Continuous vonoprazan therapy for at least 1 year without cessation; ii) GERD symptoms that had persisted for >8 weeks whilst taking a standard dose of PPI; and iii) total score of GERD domain of ≥4 prior to initiation of vonoprazan therapy. The exclusion criteria were: i) discontinuation of vonoprazan therapy or lost to follow-up within 1 year; ii) lack of critical clinicopathological data; iii) H. pylori positive status; and iv) status-post distal gastrectomy. Safety analysis included all consecutively enrolled patients regardless of these criteria.
Statistical analysis
Changes in domain-specific scores of the Izumo scale during 1 year of vonoprazan therapy were compared using a Friedman test. Categorical data and continuous variables were compared between the erosive and non-erosive groups using a Fischer's exact test and a Mann-Whitney U-Test, respectively. Statistical analysis was performed using StatFlex version 7.0 (Artech). P<0.05 was considered to indicate a statistically significant difference.
Results
Characteristics and outcome of patients
Between February 2016 and October 2020, 47 patients received vonoprazan therapy for PPI-resistant symptomatic GERD, and 17 patients were excluded for the following reasons: Cessation of vonoprazan therapy due to improved GERD symptoms (n=6); lack of critical clinicopathological data (n=4); lost to follow (n=3); cessation of vonoprazan therapy due to adverse events (n=2); cessation of vonoprazan therapy due to lack of effectiveness (n=1); and H. pylori positive status (n=1). The remaining 30 patients were included in the final analysis.
Safety analysis was performed using all 47 patients, including the 30 study patients and 17 excluded patients. Adverse events occurred in two patients (4%). The first patient was a 71-year-old female who suffered from a dry mouth 3 months after initiation of vonoprazan and ceased therapy. The second patient was a 59-year-old female who suffered from constipation just after starting vonoprazan therapy, and thus ceased therapy.
Baseline characteristics and outcomes of the 30 patients are shown in Table I. First, patients were divided into two groups, a non-erosive (n=22) and erosive group (n=8), and these two groups were compared. At baseline, the Izumo scale score for postprandial distress symptoms was significantly higher in the non-erosive group compared with the erosive group (P=0.013). A total of 8 patients (8/22; 36%) in the non-erosive group had been treated with acotiamide prior to initiation of vonoprazan, and vonoprazan was prescribed in addition to acotiamide. The initial dose of vonoprazan was 20 mg once daily prior to April 2017 and subsequently lowered to 10 mg once daily. As a result, 17 patients started with a 10 mg initial dose and were maintained at that level. The remaining 13 patients received an initial dose of 20 mg and underwent dose reduction to 10 mg after 1 month.
Table I.
Characteristics and treatment regimens of the 30 patients.
| Characteristics | Non-erosive, n=22 | Erosive, n=8 | P-value |
|---|---|---|---|
| Age, years, median (IQR) | 69 (67-79) | 70 (65-77) | 0.605 |
| Sex, male, n (%) | 6 (27%) | 1 (13%) | 0.376 |
| Body mass index, median (IQR) | 23.5 (22.6-25.3) | 26.7 (23.8-28.8) | 0.11 |
| Current Smoker, n (%) | 3 (14%) | 0 (0%) | 0.379 |
| Alcohol use, >20 g/day, n (%) | 2 (9%) | 0 (0%) | 0.531 |
| Severity of GERD, Izumo scale score, median (IQR) | 5.5 (4.0-7.0) | 6.0 (4.8-6.3) | 0.865 |
| Severity of epigastric pain symptoms, median (IQR) | 2.5 (1.0-5.0) | 3.0 (0.0-5.0) | 0.849 |
| Severity of postprandial distress symptoms, median (IQR) | 4.0 (1.3-6.0) | 1.0 (0.0-2.3) | 0.013a |
| Severity of constipation, median (IQR) | 3.0 (0.0-5.5) | 0.5 (0.0-1.3) | 0.083 |
| Severity of diarrhea, median (IQR) | 1.0 (0.0-3.0) | 0.5 (0.0-3.0) | 0.783 |
| History of H. pylori eradication, n (%) | 7 (32%) | 4 (50%) | 0.309 |
| Previously treated with acotiamide, n (%) | 8 (36%) | 0 (0%) | 0.054 |
| Reflux esophagitis, n | |||
| Non-erosive | 22 | 0 | |
| Erosive, Los Angeles grade A/B/C/D | 0 | 2/5/1/0 | |
| Type of PPI before starting vonoprazan, n (%) | |||
| Esomeprazole | 11 (50%) | 4 (50%) | 1 |
| Lansoprazole | 3 (14%) | 3 (37%) | 0.174 |
| Omeprazole | 3 (14%) | 0 (%) | 0.379 |
| Rabeprazole | 5 (22%) | 1 (13%) | 0.480 |
| Grade of gastric atrophy, n (%) | |||
| None | 10 (45%) | 2 (25%) | 0.282 |
| Closed type | 5 (23%) | 5 (62%) | 0.056 |
| Open type | 7 (32%) | 1 (13%) | 0.287 |
| Starting dose (10/20 mg), n | 13/9 | 4/4 | 0.485 |
| Additional treatment during 1y therapy, n (%) | 10 (45%) | 1 (13%) | 0.107 |
| Dose escalation of vonoprazan | 8 | 1 | |
| Addition of acotiamide | 2 | 0 |
aP<0.05. IQR, interquartile range; GERD, gastroesophageal reflux disease; H. pylori, Helicobacter pylori; PPI, proton pump inhibitor.
During the 1 year of treatment, the proportion of patients requiring additional treatments or another sort of intervention, including dose escalation of vonoprazan or addition of acotiamide to control refractory GERD symptoms was higher in the non-erosive group compared with the erosive group (45 vs. 13%), although the difference was not significant (P=0.107; Table I). All but 1 patient in the erosive group showed mucosal healing during follow-up EGD after 1 year; the 1 patient refused follow-up EGD.
Changes in GERD symptoms
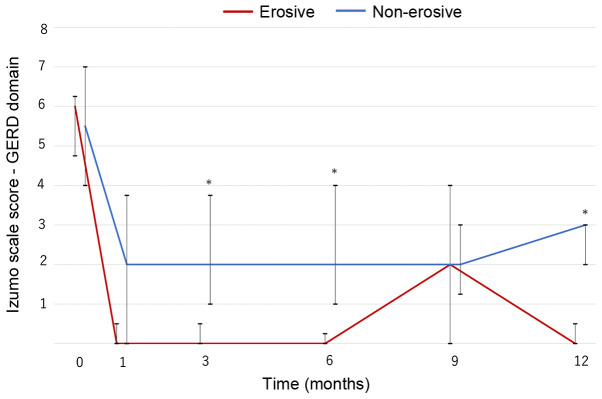
In both groups, GERD symptoms significantly improved from baseline and were maintained for 1 year (P<0.001; Fig. 1). The non-erosive group had a higher score than the erosive group except after 9 months, and significant differences between the two groups were observed at 3, 6 and 12 months (Fig. 1). Nevertheless, continuous vonoprazan therapy improved symptoms and provided good control of GERD symptoms in patients with PPI-resistant non-erosive GERD for 1 year, although the symptoms were more refractory than in patients with PPI-resistant erosive GERD.
Figure 1.
Changes in GERD symptoms during the 1 year of vonoprazan therapy in the non-erosive (n=22) and erosive groups (n=8). The scores significantly decrease over the study period (P<0.001 in both groups). *P<0.05 vs. the respective erosive group in each month. Line, median; bar, interquartile range. GERD, gastroesophageal reflux disease.
Changes in dyspepsia symptoms
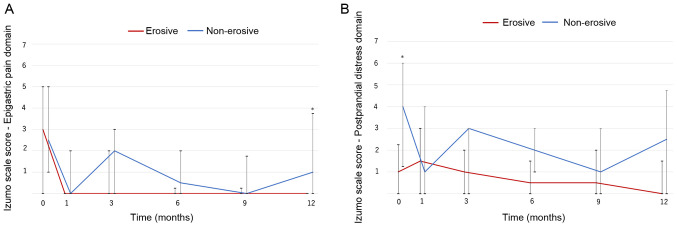
Changes in dyspepsia symptoms were also investigated (Fig. 2). The score for the epigastric pain domain significantly improved over the study period in both groups (non-erosive group, P=0.001; erosive group P=0.018; Fig. 2A). The non-erosive group had higher scores than the erosive group, and a significant difference was observed at 12 months.
Figure 2.
Changes in dyspepsia symptoms. (A) Epigastric pain domain; the scores significantly decrease over the study period in both groups (non-erosive group, P=0.001; erosive group, P=0.018). (B) Postprandial distress domain; the scores significantly decrease in the non-erosive group (non-erosive group, P=0.023; erosive group, p=0.908). *P<0.05 vs. the respective erosive group in each month. Line, median; bar, interquartile range.
Throughout the 1 year treatment period, the Izumo scale score for the postprandial distress domain significantly decreased in the non-erosive group (P=0.023). Although it was slightly higher than the erosive group, the difference was not significant (Fig. 2B). Vonoprazan therapy improved and controlled dyspepsia symptoms effectively in patients with PPI-resistant non-erosive GERD.
Changes in lower gastrointestinal symptoms
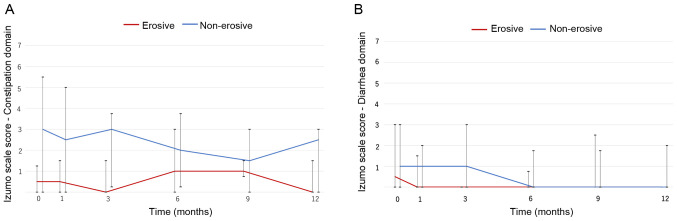
Lower gastrointestinal symptoms were evaluated using the Izumo scale. Vonoprazan did not largely affect constipation. The non-erosive group had a slightly higher score compared with the erosive group, but the difference was not significant (Fig. 3A). Similarly, vonoprazan did not notably affect diarrhea (Fig. 3B).
Figure 3.
Changes in lower gastrointestinal symptoms. (A) Constipation domain; the scores did not change significantly over the study period (non-erosive group, P=0.689; erosive group, P=0.344). (B) Diarrhea domain; the scores did not change significantly (non-erosive group, P=0.443; erosive group, P=0.451). Line, median; bar, interquartile range.
Discussion
This retrospective study of the long-term effectiveness of vonoprazan on PPI-resistant GERD showed symptomatic improvement in patients with non-erosive GERD was more refractory than in patients with erosive GERD, and that patients with non-erosive GERD were more likely to require additional treatments to control the refractory symptoms. Nevertheless, 1 year of treatment with vonoprazan resulted in significantly sustained improvement of GERD symptoms in both non-erosive and erosive GERD patients. This regimen also improved epigastric pain and postprandial distress symptoms, that frequently complicate non-erosive GERD (20). Although there are some studies showing the effect of vonoprazan on PPI-resistant GERD, they primarily report the outcomes of patients with PPI-resistant erosive GERD (10,12,22). The present study demonstrated the long-term effectiveness of vonoprazan on PPI-resistant non-erosive GERD as well as erosive GERD.
GERD remains symptomatic in ~50% of patients treated with standard doses of PPI, particularly in patients with non-erosive GERD (23). Resolution of these symptoms is important to improve the quality of life and sleep of patients regardless of the presence of esophageal erosions. In patients with PPI-resistant GERD, acid reflux is still present in 57% of patients and serves a more important determining role in nocturnal GERD symptoms than bile reflux (24). In patients with PPI-resistant non-erosive GERD, 96% of liquid reflux episodes were acidic, and acidic reflux is the major cause of persistent GERD symptoms (4). However, the number of acid reflux events is almost zero in patients treated with vonoprazan based on data from 24 h pH monitoring (25). The present study suggests that the strong acid suppression provided by vonoprazan contributes to the improvements in GERD symptoms.
Controlling PPI-resistant non-erosive GERD is more difficult than erosive GERD. In patients with naïve symptomatic GERD, erosive GERD is more easily treatable than non-erosive GERD (23). Unlike symptomatic erosive GERD, symptoms in patients with non-erosive GERD are largely influenced by visceral hypersensitivity, impaired intestinal motility coordination, gastric accommodation disorders and psychiatric disorders (1). In the present study, postprandial distress symptoms and constipation were more common in the non-erosive group compared with the erosive group at baseline. This suggests that acid reflux serves a lesser role in causing these symptoms in patients with non-erosive GERD than in patients with erosive GERD. Additional therapies such as prokinetics, dietary manipulation and psychiatric medications may be necessary to treat patients with PPI-resistant non-erosive GERD (1).
The effectiveness of PPI on dyspepsia symptoms is well demonstrated based on a meta-analysis, and is slightly superior to that of prokinetics (26). Direct introduction of hydrochloric acid into the stomach induces dysmotility-like predominant dyspeptic symptoms, including a heavy feeling, bloating and belching (27). Introducing hydrochloric acid directly into the duodenum also induces dyspeptic symptoms (28). Therefore, gastric acid surely influences dyspeptic symptoms, such as epigastric pain and postprandial distress. Suppression of gastric acid by vonoprazan partially improved dyspepsia symptoms in the present study.
It is well-established that the acid suppressing effect of vonoprazan is greater than that of PPIs, and this more potent suppression contributes to its effectiveness in the treatment of patients with PPI-resistant GERD (5). Patients with extensive metabolism of the CYP2C19 genotype have a higher risk of PPI-resistant reflux esophagitis than those with poor metabolism (29); the acid suppression effect of vonoprazan is not influenced by the CYP2C19 genotype (5). An open-label cross-over study showed significantly superior acid suppression by vonoprazan compared with rabeprazole, and the pH4 holding time ratios were 88.4 and 53.8% in patients treated with 20 mg vonoprazan and 20 mg rabeprazole, respectively (30). A Japanese randomized controlled trial showed improved pH4 holding times following 8 weeks of 20 mg and 40 mg vonoprazan therapy for PPI-resistant erosive GERD (9). Another Japanese study reported that acid clearance time and the number of reflux events were significantly reduced following treatment with 20 mg/day vonoprazan (10). The nocturnal acid suppression effect of vonoprazan is superior to that of PPI (31), and in another study it was shown that changing therapy to vonoprazan from PPI resulted in resolution of GERD symptoms within a few days (12). Vonoprazan exhibits a rapid onset, long duration of action and strong acid suppression effects, and thus successfully improved GERD symptoms in patients with PPI-resistant GERD in the present study.
A phase III trial in naïve non-erosive GERD patients did not show a significant difference between vonoprazan and placebo groups regarding the proportion of days without heartburn in the full-analysis-set. Additionally, the per-protocol-set analysis showed a significant difference, and complete heartburn resolution in the fourth week of treatment was higher in vonoprazan group compared with the placebo group (P=0.002) (32). Unlike the phase III trial, all patients included in the present study did not have naïve symptomatic GERD, but instead PPI-resistant symptomatic GERD. Therapeutic susceptibility to PPI-resistant GERD is different from naïve GERD (1). In the present study, the effectiveness of vonoprazan on long-term outcomes in patients with PPI-resistant non-erosive GERD were determined. Randomized controlled studies are necessary to confirm these preliminary results.
A recent Japanese prospective study showed that long-term maintenance therapy with vonoprazan for PPI-resistant erosive GERD was effective (22). All patients included in the present study had suffered from persistent, recurrent and/or refractory GERD symptoms over a long period of time. Therefore, vonoprazan therapy was not ceased just after improvement of the GERD symptoms. Based on these results, it is suggested that long-term maintenance of vonoprazan therapy should be considered as a standard treatment for patients with PPI-resistant symptomatic GERD.
The present study has some limitations. First, this study was a retrospective observational study. Second, CYP3A4 or CYP2C19 polymorphisms were not examined; vonoprazan and PPIs are primarily metabolized by CYP3A4 and CYP2C19, respectively (1,33). Third, the types of PPI used before starting vonoprazan differed between patients. However, the observation period in the present study is longer than previous studies, and patients were continuously treated with vonoprazan. Additionally, patients with active H. pylori infections that can influence gastric acid secretion and motility were excluded.
In conclusion, vonoprazan therapy for 1 year improved GERD symptoms in patients with PPI-resistant GERD. Long-term vonoprazan therapy does not adversely affect lower gastrointestinal symptoms. The present study is the first study of long-term outcomes of patients with symptomatic PPI-resistant non-erosive GERD treated with vonoprazan.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
SS conceived and designed the study, collected the data, performed the data analysis and interpretation and drafted the manuscript. HO and AKL drafted the manuscript, and performed the data analysis and interpretation. YH, YM and HY performed the data analysis and interpretation. All authors read and approved the final manuscript. HO and YH confirm the authenticity of all the raw data.
Ethics approval and consent to participate
The present study was approved by the Institutional Review Board (approval no. ID#30-R001). The need for written informed consent was waived due to the retrospective design of the study.
Patient consent for publication
Not applicable.
Competing interests
SS, HO and YM have received honoraria from Takeda and Otsuka Pharmaceuticals. HY has received honoraria from Takeda Pharmaceutical. All the other authors declare that they have no conflicts of interest.
References
- 1.Iwakiri K, Kinoshita Y, Habu Y, Oshima T, Manabe N, Fujiwara Y, Nagahara A, Kawamura O, Iwakiri R, Ozawa S, et al. Evidence-based clinical practice guidelines for gastroesophageal reflux disease 2015. J Gastroenterol. 2016;51:751–767. doi: 10.1007/s00535-016-1227-8. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag H, Becher A, Jones R. Systematic review: Persistent reflux symptoms on proton pump inhibitor therapy in primary care and community studies. Aliment Pharmacol Ther. 2010;32:720–737. doi: 10.1111/j.1365-2036.2010.04406.x. [DOI] [PubMed] [Google Scholar]
- 3.Frazzoni M, Conigliaro R, Melotti G. Weakly acidic refluxes have a major role in the pathogenesis of proton pump inhibitor-resistant reflux oesophagitis. Aliment Pharmacol Ther. 2011;33:601–606. doi: 10.1111/j.1365-2036.2010.04550.x. [DOI] [PubMed] [Google Scholar]
- 4.Iwakiri K, Sano H, Tanaka Y, Kawami N, Umezawa M, Futagami S, Hoshihara Y, Nomura T, Miyashita M, Sakamoto C. Characteristics of symptomatic reflux episodes in patients with non-erosive reflux disease who have a positive symptom index on proton pump inhibitor therapy. Digestion. 2010;82:156–161. doi: 10.1159/000309483. [DOI] [PubMed] [Google Scholar]
- 5.Kagami T, Sahara S, Ichikawa H, Uotani T, Yamade M, Sugimoto M, Hamaya Y, Iwaizumi M, Osawa S, Sugimoto K, et al. Potent acid inhibition by vonoprazan in comparison with esomeprazole, with reference to CYP2C19 genotype. Aliment Pharmacol Ther. 2016;43:1048–1059. doi: 10.1111/apt.13588. [DOI] [PubMed] [Google Scholar]
- 6.Mori H, Suzuki H. Role of acid suppression in acid-related diseases: proton pump inhibitor and potassium-competitive acid blocker. J Neurogastroenterol Motil. 2019;25:6–14. doi: 10.5056/jnm18139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shinozaki S, Kobayashi Y, Osawa H, Sakamoto H, Hayashi Y, Lefor AK, Yamamoto H. Effectiveness and safety of vonoprazan versus proton pump inhibitors for second-line Helicobacter pylori eradication therapy: systematic review and meta-analysis. Digestion. 2020:1–7. doi: 10.1159/000504939. [DOI] [PubMed] [Google Scholar]
- 8.Shinozaki S, Osawa H, Hayashi Y, Sakamoto H, Kobayashi Y, Lefor AK, Yamamoto H. Vonoprazan 10 mg daily is effective for the treatment of patients with proton pump inhibitor-resistant gastroesophageal reflux disease. Biomed Rep. 2017;7:231–235. doi: 10.3892/br.2017.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwakiri K, Sakurai Y, Shiino M, Okamoto H, Kudou K, Nishimura A, Hiramatsu N, Umegaki E, Ashida K. A randomized, double-blind study to evaluate the acid-inhibitory effect of vonoprazan (20 mg and 40 mg) in patients with proton-pump inhibitor-resistant erosive esophagitis. Therap Adv Gastroenterol. 2017;10:439–451. doi: 10.1177/1756283X17705329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamashita H, Kanamori A, Kano C, Hashimura H, Matsumoto K, Tsujimae M, Yoshizaki T, Momose K, Obata D, Eguchi T, et al. The Effects of switching to vonoprazan, a novel potassium-competitive acid blocker, on gastric acidity and reflux patterns in patients with erosive esophagitis refractory to proton pump inhibitors. Digestion. 2017;96:52–59. doi: 10.1159/000478255. [DOI] [PubMed] [Google Scholar]
- 11.Okuyama M, Nakahara K, Iwakura N, Hasegawa T, Oyama M, Inoue A, Ishizu H, Satoh H, Fujiwara Y. Factors associated with potassium-competitive acid blocker non-response in patients with proton pump inhibitor-refractory gastroesophageal reflux disease. Digestion. 2017;95:281–287. doi: 10.1159/000475658. [DOI] [PubMed] [Google Scholar]
- 12.Hoshino S, Kawami N, Takenouchi N, Umezawa M, Hanada Y, Hoshikawa Y, Kawagoe T, Sano H, Hoshihara Y, Nomura T, et al. Efficacy of vonoprazan for proton pump inhibitor-resistant reflux esophagitis. Digestion. 2017;95:156–161. doi: 10.1159/000456072. [DOI] [PubMed] [Google Scholar]
- 13.Kakuta E, Yamashita N, Katsube T, Kushiyama Y, Suetsugu H, Furuta K, Kinoshita Y. Abdominal symptom-related QOL in individuals visiting an outpatient clinic and those attending an annual health check. Intern Med. 2011;50:1517–1522. doi: 10.2169/internalmedicine.50.5390. [DOI] [PubMed] [Google Scholar]
- 14.Shinozaki S, Osawa H, Kobayashi Y, Sakamoto H, Hayashi Y, Miura Y, Kawarai Lefor A, Yamamoto H. Long-term outcomes of patients with symptomatic gastroesophageal reflux disease treated with vonoprazan. Scand J Gastroenterol. 2018;53:897–904. doi: 10.1080/00365521.2018.1486883. [DOI] [PubMed] [Google Scholar]
- 15.Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler SJ, et al. Endoscopic assessment of oesophagitis: Clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172–180. doi: 10.1136/gut.45.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;1:87–97. [Google Scholar]
- 17.Furuta K, Ishihara S, Sato S, Miyake T, Ishimura N, Koshino K, Tobita H, Moriyama I, Amano Y, Adachi K, et al. Development and verification of the Izumo Scale, new questionnaire for quality of life assessment of patients with gastrointestinal symptoms. Nihon Shokakibyo Gakkai Zasshi. 2009;106:1478–1487. (In Japanese) [PubMed] [Google Scholar]
- 18.Fujishiro M, Kushiyama A, Yamazaki H, Kaneko S, Koketsu Y, Yamamotoya T, Kikuchi T, Sakoda H, Suzuki R, Kadowaki T. Gastrointestinal symptom prevalence depends on disease duration and gastrointestinal region in type 2 diabetes mellitus. World J Gastroenterol. 2017;23:6694–6704. doi: 10.3748/wjg.v23.i36.6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinoshita Y, Chiba T. Characteristics of Japanese patients with chronic gastritis and comparison with functional dyspepsia defined by ROME III criteria: Based on the large-scale survey, FUTURE study. Intern Med. 2011;50:2269–2276. doi: 10.2169/internalmedicine.50.5678. FUTURE Study Group. [DOI] [PubMed] [Google Scholar]
- 20.Okimoto E, Ishimura N, Morito Y, Mikami H, Shimura S, Uno G, Tamagawa Y, Aimi M, Oshima N, Kawashima K, et al. Prevalence of gastroesophageal reflux disease in children, adults, and elderly in the same community. J Gastroenterol Hepatol. 2015;30:1140–1146. doi: 10.1111/jgh.12899. [DOI] [PubMed] [Google Scholar]
- 21.Shinozaki S, Osawa H, Sakamoto H, Hayashi Y, Kawarai Lefor A, Yamamoto H. The effect of acotiamide on epigastric pain syndrome and postprandial distress syndrome in patients with functional dyspepsia. J Med Invest. 2016;63:230–235. doi: 10.2152/jmi.63.230. [DOI] [PubMed] [Google Scholar]
- 22.Tanabe T, Hoshino S, Kawami N, Hoshikawa Y, Hanada Y, Takenouchi N, Goto O, Kaise M, Iwakiri K. Efficacy of long-term maintenance therapy with 10-mg vonoprazan for proton pump inhibitor-resistant reflux esophagitis. Esophagus. 2019;16:377–381. doi: 10.1007/s10388-019-00676-x. [DOI] [PubMed] [Google Scholar]
- 23.Fass R, Sifrim D. Management of heartburn not responding to proton pump inhibitors. Gut. 2009;58:295–309. doi: 10.1136/gut.2007.145581. [DOI] [PubMed] [Google Scholar]
- 24.Hershcovici T, Jha LK, Cui H, Powers J, Fass R. Night-time intra-oesophageal bile and acid: A comparison between gastro-oesophageal reflux disease patients who failed and those who were treated successfully with a proton pump inhibitor. Aliment Pharmacol Ther. 2011;33:837–844. doi: 10.1111/j.1365-2036.2011.04583.x. [DOI] [PubMed] [Google Scholar]
- 25.Masaoka T, Kameyama H, Yamane T, Yamamoto Y, Takeuchi H, Suzuki H, Kitagawa Y, Kanai T. Pathophysiology of potassium-competitive acid blocker-refractory gastroesophageal reflux and the potential of potassium-competitive acid blocker test. J Neurogastroenterol Motil. 2018;24:577–583. doi: 10.5056/jnm18036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinto-Sanchez MI, Yuan Y, Hassan A, Bercik P, Moayyedi P. Proton pump inhibitors for functional dyspepsia. Cochrane Database Syst Rev. 2017;11(CD011194) doi: 10.1002/14651858.CD011194.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miwa H, Nakajima K, Yamaguchi K, Fujimoto K, Veldhuyzen VAN, Zanten SJ, Kinoshita Y, Adachi K, Kusunoki H, Haruma K. Generation of dyspeptic symptoms by direct acid infusion into the stomach of healthy Japanese subjects. Aliment Pharmacol Ther. 2007;26:257–264. doi: 10.1111/j.1365-2036.2007.03367.x. [DOI] [PubMed] [Google Scholar]
- 28.Ishii M, Manabe N, Kusunoki H, Kamada T, Sato M, Imamura H, Shiotani A, Hata J, Haruma K. Real-time evaluation of dyspeptic symptoms and gastric motility induced by duodenal acidification using noninvasive transnasal endoscopy. J Gastroenterol. 2008;43:935–941. doi: 10.1007/s00535-008-2303-5. [DOI] [PubMed] [Google Scholar]
- 29.Ichikawa H, Sugimoto M, Sugimoto K, Andoh A, Furuta T. Rapid metabolizer genotype of CYP2C19 is a risk factor of being refractory to proton pump inhibitor therapy for reflux esophagitis. J Gastroenterol Hepatol. 2016;31:716–726. doi: 10.1111/jgh.13233. [DOI] [PubMed] [Google Scholar]
- 30.Takeuchi T, Furuta T, Fujiwara Y, Sugimoto M, Kasugai K, Kusano M, Okada H, Suzuki T, Higuchi T, Kagami T, et al. Randomised trial of acid inhibition by vonoprazan 10/20 mg once daily vs rabeprazole 10/20 mg twice daily in healthy Japanese volunteers (SAMURAI pH study) Aliment Pharmacol Ther. 2020;51:534–543. doi: 10.1111/apt.15641. [DOI] [PubMed] [Google Scholar]
- 31.Sakurai Y, Mori Y, Okamoto H, Nishimura A, Komura E, Araki T, Shiramoto M. Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects - a randomised open-label cross-over study. Aliment Pharmacol Ther. 2015;42:719–730. doi: 10.1111/apt.13325. [DOI] [PubMed] [Google Scholar]
- 32.Kinoshita Y, Sakurai Y, Takabayashi N, Kudou K, Araki T, Miyagi T, Iwakiri K, Ashida K. Efficacy and safety of vonoprazan in patients with nonerosive gastroesophageal reflux disease: a randomized, placebo-controlled, phase 3 study. Clin Transl Gastroenterol. 2019;10(e00101) doi: 10.14309/ctg.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugimoto M, Ban H, Hira D, Kamiya T, Otsuka T, Inatomi O, Bamba S, Terada T, Andoh A. Letter: CYP3A4/5 genotype status and outcome of vonoprazan-containing Helicobacter pylori eradication therapy in Japan. Aliment Pharmacol Ther. 2017;45:1009–1010. doi: 10.1111/apt.13959. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.