Abstract
Background
The coronavirus disease 2019 pandemic has resulted in high levels of exposure of medical workers to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Hand decontamination is one of the actions recommended to reduce the risk of infection.
Aim
Two disinfectants – BIAKŌS antimicrobial skin and wound cleanser (AWC) and AWC2 (Sanara MedTech, Fort Worth, TX, USA) – were tested to determine whether they can inactivate SARS-CoV-2 upon contact or as a coating applied before contact with the virus.
Methods
The ability of AWC and AWC2 to inactivate SARS-CoV-2 was tested in liquid and dried form on plastic surfaces and porcine skin.
Findings
AWC and AWC2 were effective in reducing the infectious titre of SARS-CoV-2 in liquid form during application and in dried form 4 h after application. Virus on skin was reduced up to 2 log10-fold and 3.5 log10-fold after treatment with AWC and AWC2, respectively.
Conclusion
Application of AWC and AWC2 to skin reduces the level of SARS-CoV-2 and the risk of infection.
Keywords: COVID-19, SARS-CoV-2, Disinfection, Skin
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has affected millions of people world-wide. The aetiologic agent is severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which is a member of the family Coronaviridae, genus Betacoronavirus and subgenus Sarbecovirus. One of the most important routes of infection is direct or indirect contact between people, including a person contacting their contaminated hand with their nose or mouth, although infection can also take place through fomites, or respiratory droplets and aerosols. Therefore, handwashing is an important measure to avoid infection, but excessive handwashing can lead to problems with skin health [1,2], and handwashing four times or more per day can increase the roughness of the skin [3] which can, in turn, discourage frequent handwashing. Dry skin and dermatitis due to excessive handwashing are particularly prevalent among healthcare workers, as they work in environments with a high risk of infection. One solution to this problem could be to apply disinfectants to the skin to inactivate viruses upon contact and prevent accumulation of live viruses after the application. BIAKŌS antimicrobial skin and wound cleanser (AWC) and AWC2, an ethanol variant of AWC, are two disinfectants with components hypothesized to have antiviral activity against SARS-CoV-2 (Sanara MedTech, Fort Worth, TX, USA). These ingredients are: (i) polyhexamethylene biguanide (PHMB), a cationic antimicrobial that may attract and adhere to the negatively charged lipid layer, thereby inactivating the virus [4,5]; (ii) vicinal diols (octane-1-2-diol and ethylhexylglycerin), which are capable of disrupting lipid structures [[6], [7], [8]], such as those in the SARS-CoV-2 envelope; (iii) ethylenediamine tetracetic acid (EDTA), which is known to have antimicrobial activity that can synergize with various antimicrobials [6,9]; and (iv) poloxamer 407, a non-ionic surfactant that helps to solubilize lipids in water [10] and maintains the activity of PHMB [11] and vicinal diols [6]. PHMB, vicinal diols, EDTA and poloxamer 407 are non-volatile, and are expected to remain on the skin surface after the liquid has evaporated. Whereas AWC is water-based, AWC2 uses ethanol as a vehicle, which is likely an active component in disinfection in liquid form, but evaporates when the product dries. PHMB and vicinal diols are also hypothesized to be gentle on the skin. PHMB is commonly used for disinfection of contact lenses [12] and is proposed to make water potable [13]. Vicinal diols have dual activity as humectants and antimicrobials [8]. This study tested AWC and AWC2 in their liquid and dry forms for disinfection of skin prior to and after contact with SARS-CoV-2.
Methods
Viruses and cells
Vero E6 (CRL-1586; American Type Culture Collection, Manassas, VA, USA) cells were maintained in Dulbecco's minimal essential media (DMEM; Gibco, Thermo Fisher Scientific, Waltham, MA, USA) with 5% fetal bovine serum (FBS; Atlanta Biologicals, Flowery Branch, GA, USA), 200 mg/mL of streptomycin and 200 U/mL of penicillin (Gibco). SARS-CoV-2 virus stock (Strain USA_WA1/2020) was obtained from the World Reference Center for Emerging Viruses and Arboviruses at Vero cell passage 3, and amplified in Vero E6 cells using DMEM with 2% FBS, penicillin and streptomycin as above to generate the working stock used (Vero passage 4), which had a titre of 4 x 107 plaque-forming units (PFU)/mL.
Liquid disinfectant tests
Working in biosafety level 3 (BSL-3) conditions, 900 μL of AWC, AWC2 or phosphate-buffered saline (PBS) was tested in triplicate. The disinfectant or PBS (negative control) was added to 100 μL of SARS-CoV-2 stock [containing 1 x 106 plaque-forming units (PFU)] in a microcentrifuge tube, mixed by gentle pulse-vortexing, and incubated at room temperature (RT) for 30 s, 1 min or 10 min. Samples were titrated by plaque assay with a limit of detection of 4 x 102 PFU/mL. To test whether serial dilution neutralizes the activity of AWC or AWC, these disinfectants were diluted serially using DMEM containing 2% FBS, streptomycin and penicillin as described above. Dilutions of 1:10, 1:100, 1:1,000, 1:10,000 and 1:100,000 were mixed with 100 μL of virus containing 4 x 106 PFU. These virus–disinfectant mixtures were incubated at 37˚C at 5% CO2, and titrated by plaque assay.
Disinfectant coating tests on plastic
Fifty microlitres of PBS, AWC or AWC2 was added to each well of a 96-well plate and allowed to dry for 16 h under ambient conditions in a biosafety cabinet. Next, 50 μL of SARS-CoV-2 stock containing 1 x 104 PFU of virus was added to each well and incubated for 10 min or 1 h at RT in triplicate wells. The contents of each well were retrieved by washing with 250 μL of DMEM containing 2% FBS supplemented with penicillin and streptomycin as described above. Titrations by plaque assay were performed to determine the amount of virus retrieved for each condition.
Disinfection of porcine skin
For experiments involving skin, a piece of porcine skin approximately 1.27 cm in diameter was purchased from iFyber (Ithaca, NY, USA), and shipped frozen after chlorine gas sterilization [14]. Skin was washed in 50 mL of PBS prior to the experiments to remove excess chlorine, and transported to a BSL-3 laboratory for viral experiments. To test disinfection of virus on skin, pieces of porcine skin were transferred to a 12-well plate, and 25 μL of virus containing 1 x 106 PFU was added to each piece of porcine skin on the epidermal side. After 5 min of incubation, 25 μL of disinfectant (AWC, AWC2 or PBS) was added to the epidermal surface and incubated at RT for 1 min, 5 min or 10 min in triplicate. The pieces of porcine skin were subsequently washed with a micropipette using 1 mL of DMEM medium (with 5% FBS, penicillin and streptomycin), and then the medium and skin were placed in a microcentrifuge tube and vortexed for 30 s to retrieve the virus. Finally, 250 μL of this medium was used for plaque assays, with the limit of detection (LOD) being 4 PFU per replicate.
To test whether the disinfectants could form a coating on the skin, providing protection against subsequent viral exposure, pieces of skin were dipped into a 50-mL conical tube containing 30 mL of PBS, AWC or AWC2 for 30 s in a BSL-3 laboratory. Skin pieces were transferred to 12-well plates and incubated for 4 h at RT with 60% relative humidity (RH). Subsequently, 25 μL of SARS-CoV-2 containing 2 x 105 PFU was added to the outer epidermal surface and allowed to incubate at RT for 10 min, 30 min or 1 h. Following incubation, the skin and any liquid left on the epidermis were transferred to a microcentrifuge tube containing 1 mL of DMEM media with 2% FBS, penicillin and streptomycin, and 250 μL was used for plaque assays.
Plaque assays
Plaque assays were performed in a BSL-3 laboratory using Vero E6 cells, as described previously [15].
Statistical analyses
Statistical analyses of plaque assays were performed by log10-transforming viral titres and performing one-way analysis of variance with Sidak's multiple comparison test. The LOD was defined as the lowest amount of virus that could be detected in one replicate for each assay. In all analyses, P≤0.05 was considered to indicate significance.
Results
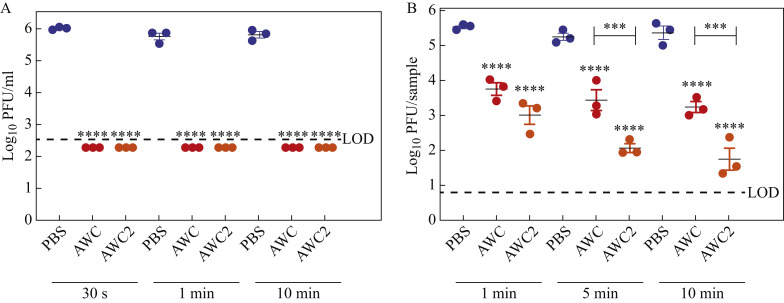
Hand disinfectants are expected to inactivate virus either during or after application, depending when exposure to the pathogen takes place. To test whether liquid AWC and AWC2 have rapid disinfectant activity against SARS-CoV-2, 100 μL of viral stock (containing 1 x 106 PFU) was mixed with 900 μL of PBS (as a non-inactivating control), AWC or AWC2, and incubated for 30 s, 1 min or 10 min prior to performing plaque assays. The PBS control had a viral titre of approximately 1 x 106 PFU/mL, whereas in the presence of AWC and AWC2, viral titres were reduced to below the LOD (<400 PFU/mL) at all timepoints (Figure 1 A); this showed that both disinfectants have potent antiviral activity with as little as 30 s of contact. To determine whether these disinfectants can act on skin under conditions that mimic hand disinfection, 25 μL of SARS-CoV-2 stock containing 1 x 106 PFU was added to a piece of porcine skin on the epidermal side. Five minutes later (without drying), 25 μL of PBS, AWC or AWC2 was added to the epidermis, and allowed to contact the virus at RT for 1 min, 5 min or 10 min. These volumes were chosen to avoid liquid spreading beyond the epidermal surface. The authors were able to retrieve 5 x 105 PFU/sample for PBS in all three incubation times (Figure 1B). In comparison, viral titres were reduced by approximately 2 log10-fold after 1 min, 5 min or 10 min of incubation (Figure 1B) with AWC. AWC2, which is ethanol based, decreased viral titres further than AWC, by 2.5 log10-fold at 1 min, 3 log10-fold at 5 min, and 3.5 log10-fold at 10 min of incubation (Figure 1B). These data suggest that AWC and AWC2 could both function as effective hand disinfectants against SARS-CoV-2.
Figure 1.
Liquid BIAKŌS antimicrobial skin and wound cleanser (AWC) and AWC2 have disinfectant effects against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). (A) SARS-CoV-2 titres were reduced after contact with liquid AWC or AWC2. The virus was added to liquid AWC or AWC2 in a 1:10 ratio and the mixture was titrated. (B) AWC and AWC2 disinfected SARS-CoV-2 from skin. SARS-CoV-2 was added to porcine skin and an equal volume of disinfectant was added. Error bars indicate standard deviations. One-way analysis of variance with Sidak's multiple comparison test was used to assess significance. ∗∗∗∗P<0.001. LOD, limit of detection; PBS, phosphate-buffered saline; PFU, plaque-forming units.
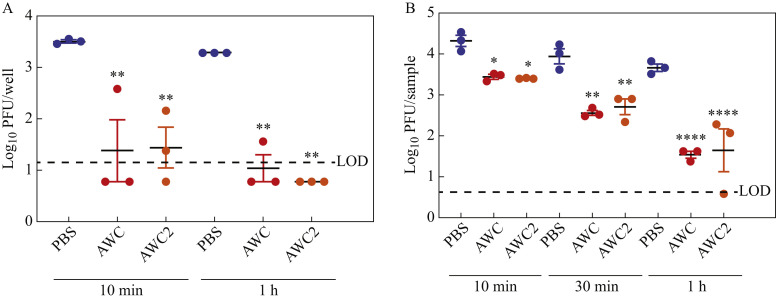
To determine whether dried AWC and AWC2 coatings retained activity against SARS-CoV-2, 50 μL of PBS, AWC or AWC2 was added to each well of a 96-well plate and allowed to dry overnight. Next, 50 μL of virus containing 1 x 104 PFU was added to each well and incubated at RT for 10 min or 1 h. For the PBS control condition, 3.5 and 3.3 log10 PFU of virus was recovered after 10 min and 1 h of incubation, respectively. Both AWC and AWC2 reduced the average viral titre by ≥2 log10-fold after 10 min of treatment, with AWC reducing the viral titre of two of three samples to below the LOD, and AWC2 reducing one of three samples to below the LOD (Figure 2 A). After 1 h of incubation, AWC reduced the viral titre by ≥2.2 log10-fold, with the measurement of two of three samples falling below the LOD, and AWC2 reduced the viral titre by 2.5 log10-fold or more, with all samples falling below the LOD (Figure 2A).
Figure 2.
Dried BIAKŌS antimicrobial skin and wound cleanser (AWC) and AWC2 have disinfectant effects against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). (A) Dried AWC and AWC2 disinfected SARS-CoV-2 on a polystyrene surface. AWC and AWC2 were allowed to dry on the bottom of a 96-well plate and then put in contact with virus. (B) Dried AWC and AWC2 on porcine skin disinfected against SARS-CoV-2. Porcine skins were dipped in AWC or AWC2, allowed to dry and then virus was added. Error bars indicate standard deviations. One-way analysis of variance with Sidak's multiple comparison test was used to assess significance. ∗P≤0.05; ∗∗P≤0.01; ∗∗∗∗P≤0.0001. LOD, limit of detection; PBS, phosphate-buffered saline; PFU, plaque-forming units.
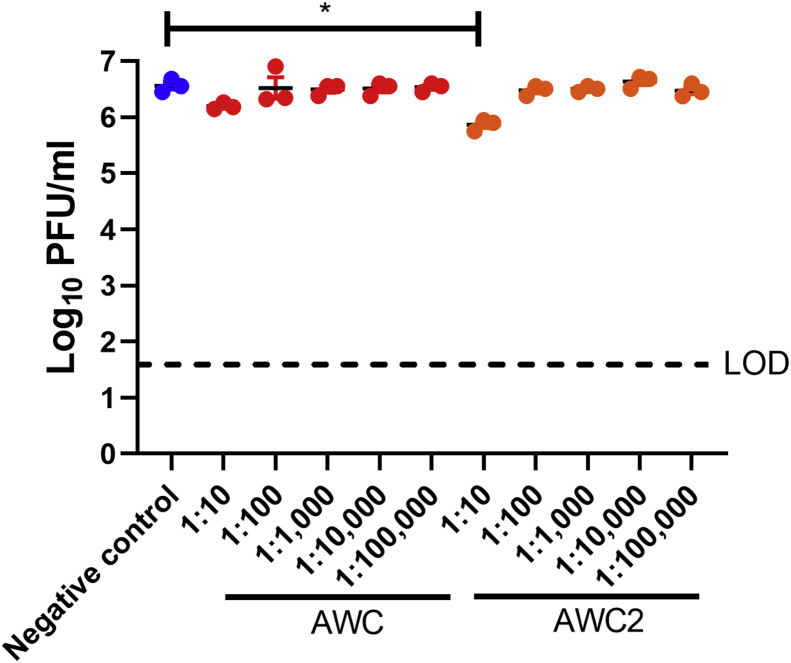
To mimic conditions in which a person applied disinfectant to their hand and allowed it to dry, porcine skin was immersed in PBS, AWC or AWC2 for 30 s, transferred to a plate and incubated for 4 h at RT and 60% RH. After 4 h, 25 μL of SARS-CoV-2 containing 2 x 105 PFU was added to the epidermal surface and incubated at RT for 10 min, 30 min or 1 h. After 10 min of incubation, both AWC and AWC2 were able to reduce the viral titre approximately 10-fold in comparison with the PBS control (Figure 2B). After 30 min of incubation, there was a reduction in viral titre of >10-fold, and after 1 h of incubation, the reduction was 100-fold in comparison with PBS, suggesting that application of AWC or AWC2 to skin prior to viral contact can reduce viral levels and the risk of infection and spread (Figure 2B). To ensure that AWC and AWC2 were neutralized by the serial dilution of the plaque assays, AWC and AWC2 were diluted serially 1:10 through 1:100,000 and mixed with 100 μL of virus stock containing 4 x 106 PFU. The antiviral activity of AWC was neutralized completely by dilution at 1:10. AWC2 had some activity remaining at 1:10 dilution but not at the subsequent dilutions (Figure S1, see online supplementary material).
Discussion
A previous study found that vicinal diols, EDTA and PHMB, which are components of AWC and AWC2, act synergistically as antimicrobials against meticillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa biofilms [6]. The present results demonstrate that AWC and AWC2 have disinfectant activity against SARS-CoV-2, both in liquid and dry forms, and suggest that the application of AWC and AWC2 to skin before or after contact with a virus can reduce the risk of human infection and spread. Although the present results of skin disinfection with SARS-CoV-2 are limited, the disinfectant activity of AWC and AWC2 in suspension are similar to the disinfectant activity of ethanol and isopropanol formulations [16]. In their dried forms, AWC and AWC2 were similarly effective at inactivating SARS-CoV-2. In contrast, in liquid form, AWC2 was more effective for decontamination of porcine skin than AWC, possibly because it is based on ethanol whereas AWC is based on water. As AWC does not contain ethanol, its formulation may be advantageous for application in hospital settings where handwashing is frequent, as ethanol has been shown to dry the skin [17].
Handwashing with soap and water is recommended to prevent SARS-CoV-2 infection, and AWC and AWC2 should not replace handwashing. However, these products may be suitable for use after, or between, hand washes. Handwashing four times or more per day can be associated with drier skin and dermatitis, and medical workers have a higher prevalence of dermatitis due to frequent handwashing [1,3]. Therefore, AWC and AWC2 could be used to help keep medical workers safe, whilst avoiding skin conditions arising from other hand hygiene products.
Acknowledgements
The authors wish to thank their colleagues in Weaver Laboratory for their suggestions, and Dr Sasha Azar for critical reading of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhin.2021.02.004.
Conflict of interest statement
This work was supported by Rochal Industries, LLC, which developed BIAKŌS AWC and AWC2. RM is employed by Rochal Industries, LLC.
Funding source
Rochal Industries, LLC.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Figure S1.
Serial dilutions of BIAKŌS antimicrobial skin and wound cleanser (AWC) and AWC2 neutralize the antiviral activity of these disinfectants prior to infection of cell monolayers. AWC and AWC2 were diluted in Dulbecco's minimal essential media to the indicated concentration and incubated with severe acute respiratory syndrome coronavirus-2. One-way analysis of variance with Sidak's multiple comparison test was used to assess significance. ∗P≤0.05. LOD, limit of detection; PBS, phosphate-buffered serum; PFU, plaque-forming units.
References
- 1.Smit H.A., Burdorf A., Coenraads P.J. Prevalence of hand dermatitis in different occupations. Int J Epidemiol. 1993;22:288–293. doi: 10.1093/ije/22.2.288. [DOI] [PubMed] [Google Scholar]
- 2.O'Connell K.A., Enos C.W., Prodanovic E. Handwashing-induced dermatitis during the COVID-19 pandemic. Am Fam Physician. 2020;102:327–328. [PubMed] [Google Scholar]
- 3.Kampf G., Ennen J. Regular use of a hand cream can attenuate skin dryness and roughness caused by frequent hand washing. BMC Dermatol. 2006;6:1. doi: 10.1186/1471-5945-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbert P., Moore L.E. Cationic antiseptics: diversity of action under a common epithet. J Appl Microbiol. 2005;99:703–715. doi: 10.1111/j.1365-2672.2005.02664.x. [DOI] [PubMed] [Google Scholar]
- 5.Romanowski E.G., Yates K.A., O'Connor K.E., Mah F.S., Shanks R.M., Kowalski R.P. Evaluation of polyhexamethylene biguanide (PHMB) as a disinfectant for adenovirus. JAMA Ophthalmol. 2013;131:495–498. doi: 10.1001/jamaophthalmol.2013.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salamone A.B., Salamone J.C., McMahon R.E., Poleon S., Bionda N., D'Arpa P. Synergistic effect and antibiofilm activity of a skin and wound cleanser. Wounds. 2020;32:208–216. [PubMed] [Google Scholar]
- 7.Murphy O., O'Dwyer V., Lloyd-McKernan A. The efficacy of tea tree face wash, 1, 2-octanediol and microblepharoexfoliation in treating Demodex folliculorum blepharitis. Cont Lens Anterior Eye. 2018;41:77–82. doi: 10.1016/j.clae.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Suchomel M., Weinlich M., Kundi M. Influence of glycerol and an alternative humectant on the immediate and 3-hours bactericidal efficacies of two isopropanol-based antiseptics in laboratory experiments in vivo according to EN 12791. Antimicrob Resist Infect Control. 2017;6:72. doi: 10.1186/s13756-017-0229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finnegan S., Percival S.L. EDTA: an antimicrobial and antibiofilm agent for use in wound care. Adv Wound Care. 2015;4:415–421. doi: 10.1089/wound.2014.0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Percival S.L., Chen R., Mayer D., Salisbury A.M. Mode of action of poloxamer-based surfactants in wound care and efficacy on biofilms. Int Wound J. 2018;15:749–755. doi: 10.1111/iwj.12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yanai R., Ueda K., Nishida T., Toyohara M., Mori O. Effects of ionic and surfactant agents on the antimicrobial activity of polyhexamethylene biguanide. Eye Contact Lens. 2011;37:85–89. doi: 10.1097/ICL.0b013e31820cebc3. [DOI] [PubMed] [Google Scholar]
- 12.Lucas A.D., Gordon E.A., Stratmeyer M.E. Analysis of polyhexamethylene biguanide in multipurpose contact lens solutions. Talanta. 2009;80:1016–1019. doi: 10.1016/j.talanta.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 13.Asiedu-Gyekye I.J., Mahmood A.S., Awortwe C., Nyarko A.K. Toxicological assessment of polyhexamethylene biguanide for water treatment. Interdiscip Toxicol. 2015;8:193–202. doi: 10.1515/intox-2015-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Q., Phillips P.L., Sampson E.M., Progulske-Fox A., Jin S., Antonelli P. Development of a novel ex vivo porcine skin explant model for the assessment of mature bacterial biofilms. Wound Repair Regen. 2013;21:704–714. doi: 10.1111/wrr.12074. [DOI] [PubMed] [Google Scholar]
- 15.Campos R.K., Jin J., Rafael G.H., Zhao M., Liao L., Simmons G. Decontamination of SARS-CoV-2 and other RNA viruses from N95 level meltblown polypropylene fabric using heat under different humidities. ACS Nano. 2020;14:14017–14025. doi: 10.1021/acsnano.0c06565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kratzel A., Todt D., V'Kovski P., Steiner S., Gultom M., Thao T.T.N. Inactivation of severe acute respiratory syndrome coronavirus 2 by WHO-recommended hand rub formulations and alcohols. Emerg Infect Dis. 2020;26:1592–1595. doi: 10.3201/eid2607.200915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steere A.C., Mallison G.F. Handwashing practices for the prevention of nosocomial infections. Ann Intern Med. 1975;83:683–690. doi: 10.7326/0003-4819-83-5-683. [DOI] [PubMed] [Google Scholar]