Summary
Social hierarchy plays important roles in maintaining social structures. Despite similarity in concept, frameworks of human hierarchy have seldom been investigated in parallel with other animals. Moreover, the importance of subordination in hierarchical formation has been largely underestimated in previous research. Here we established, compared, and investigated hierarchy in children and weanling mice. Temperament assessments suggested that children who are less persistent, low emotional intensity, and withdrew easily were more likely to be subordinate in competitive scenarios independent of task characteristics and interaction experiences. The tube test further showed that conflicts between mice were not resolved by winner approach but by loser withdrawal, which was mainly determined by intrinsic subordinate status regardless of opponents. Our study presents evolutionary conserved hierarchical relationships in young and a critical role of the intrinsic subordinate characteristics in hierarchical determination. These findings provide a new perspective on social interactions with potential implications for preschool education.
Subject areas: behavioral neuroscience, biological sciences, natural sciences
Graphical abstract
Highlights
-
•
Similar social hierarchy can be established in weanling mice and young children
-
•
Social rankings in children are largely influenced by intrinsic characteristics
-
•
Conflicts in mouse tube test are not resolved by winner but by loser withdrawal
-
•
Subordinate withdrawals are determined by intrinsic status regardless of opponents
Behavioral neuroscience; biological sciences; natural sciences
Introduction
Social hierarchy is a fundamental structure for social interactions in several animal species and has a strong influence on behaviors and physiology of those species. Typically, a hierarchy is established by an agonistic interaction, such as aggression (Wang et al., 2014). Winners of such conflicts eventually dominate their group and have greater access to limited resources, such as food and mates. However, dominance comes at a cost owing to greater stress, physical danger, and metabolic demands (Huntingford and Turner, 1987). In contrast, lower-ranked individuals receive fewer resources but are subjected to less conflict and peril. Either behavioral strategy may benefit the needs of an individual, and, once established, mutual formation of stable dominance hierarchies helps all participants by limiting social conflicts and optimizing group fitness (Wang et al., 2014).
The conceptual framework of social hierarchy has been applied to human studies. Numerous observational studies have shown that dominance hierarchies form early in life and gradually become stable with age (La Freniere and Charlesworth, 1983; Pellegrini et al., 2007; Roseth et al., 2007; Strayer and Trudel, 1984). In the past decades, the stably existing social hierarchies in preschool children have promoted researchers to explore the influences of multiple factors, such as age, sex, intelligence, social skills, parenting style, moral education, and cultural background (Charafeddine et al., 2016; Hawley, 1999, 2002; Hawley and John Geldhof, 2012; Keating and Bai, 1986; McDonald et al., 2013; Neppl and Murray, 1997; Pellegrini et al., 2007; Reifen Tagar et al., 2017; Roseth et al., 2011). Children with more aggressive behaviors are usually recognized as higher-ranking status in their group (Hawley, 2007; Roseth et al., 2011; Sluckin and Smith, 1977; Strayer and Strayer, 1976). The adoption and change of resource control strategies of highly dominant children have also been the focus of child development research (Hawley, 1999, 2002; Roseth et al., 2011). Conversely, in recent years, cognitive-oriented studies have indicated that preschool children learn to make judgments of social dominance based on various cues as they gain experience interacting with other children (Brey and Shutts, 2015; Charafeddine et al., 2015; Gülgöz and Gelman, 2017; Lourenco et al., 2016; Over and Carpenter, 2015; Qu et al., 2017). Preschool children even consider social dominance from situation to situation (Charafeddine et al., 2016). This judgment ability suggests that dominance hierarchies in young children are supposed to be unstable or dynamic and would change with time and experience. However, this was not the case in past studies based on behavioral observations, raising the question of whether, in addition to direct interactions or observational learning, there is another overlooked fundamental cause impacting the formation of social hierarchies in young children. This question turns us to the influence of innate intrinsic traits in the formation of social hierarchy. An appropriate method that could possibly rule out the complexity of educational or cultural influences and directly address the issue is therefore needed.
Animal models, with a variety of associated basic or translational research assays, have been established to measure social status among these groups. For rodent species, including the mouse and rat, aggression and scent-marking assays have been used to evaluate dominance hierarchy (Wang et al., 2014). Because only adult males perform these dominance behaviors, the social hierarchies of female and young animals have rarely been explored. Compared with methods examining complicated dominance behaviors, the tube test, in which one mouse forces its opponent backward out of a tube, is relatively easy to set up and perform and has been used in recent years largely to study the molecular mechanism(s) and/or neural circuits of social dominance (Larrieu et al., 2017; Lindzey et al., 1961; Saxena et al., 2018; Tada et al., 2016; van den Berg et al., 2015; Wang et al., 2011; Yamaguchi et al., 2017; Zhou et al., 2017). One such study has indicated that social ranks of C57/B6 adult male mice are stable across multiple days, obey a linear (i.e., transitive) relationship and are consistent with the ranks defined by other methods (Wang et al., 2011). Because the only requirement for mice to complete the tube test is the ability to move forward and backward inside the tube, the assay provides a potential opportunity to investigate social ranking in young mice, who do not display dominant behaviors, such as aggression or scent-marking.
While research on social hierarchy in both humans and mice have mostly focused on dominance behaviors (Hawley, 1999, 2002; Islam, 2014; Lindzey et al., 1961; Saxena et al., 2018; Sidanius and Pratto, 2001; Sluckin and Smith, 1977; Strayer and Strayer, 1976; Wang et al., 2011; Yamaguchi et al., 2017; Zhou et al., 2017), the contribution of subordinate behavior or submissive personality to the formation of social hierarchies has largely been underestimated. Moreover, although mice have been used widely as a standard model organism for human biology, in parallel studies between human children and mice have been rare in the past, especially in behavioral research. Because few reports in recent years have found surprising similarities (Esposito et al., 2013), we believe that investigation of social hierarchy in these two species together would provide new insight into the innate intrinsic characteristics in the hierarchical formation from an evolutionary perspective. Since internal characteristics in mice cannot be clearly classified in detail (e.g. different aspects of personality or internal status) and many variables cannot be manipulated in human children (e.g. surgical or pharmacological approach), we expected that the combination and comparison of data from children and mice would be helpful for answering questions that could not be approached by only one of the species.
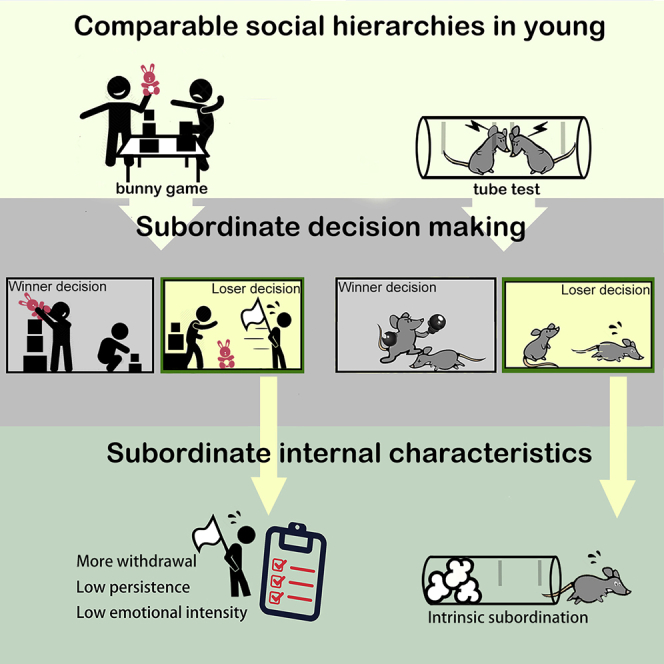
In this report, we establish and investigate hierarchical relationships in preschool-aged children and weanling mice through parallel comparable task designs. While previous studies based on observation suggested that young children’ s social hierarchy has initially formed around this time (La Freniere and Charlesworth, 1983; Strayer and Trudel, 1984), these are also the youngest stages in two species with available social tasks for evaluation of hierarchy. Our results showed that, for both species, stable and transitive social rankings develop at a young age. To explore the relationship between intrinsic characteristics and hierarchical formation, we investigated several temperament factors in children and found that while dominant children were as expected to be more likely to approach with stronger emotion expression, subordinate children showed a greater tendency toward withdrawal behavior accompanied by less persistence and less expressiveness of emotions. We next explored the causality between social hierarchy and internal status by applying the mouse tube test and showed that conflicts between two weanling mice were surprisingly not resolved by the dominant winner but by the loser with innate subordinate tendency. Our results therefore not only present a remarkable similarity in social hierarchies of humans and mice but also reveal the determining role of subordinate decision-making that has not been recognized in previous studies for hierarchical formation. These findings potentially provide new insights into the mechanisms underlying the formation of social ranking and contribute to research on social interaction in the fields of biology, psychology, and education.
Results
Social hierarchy of preschool-aged children
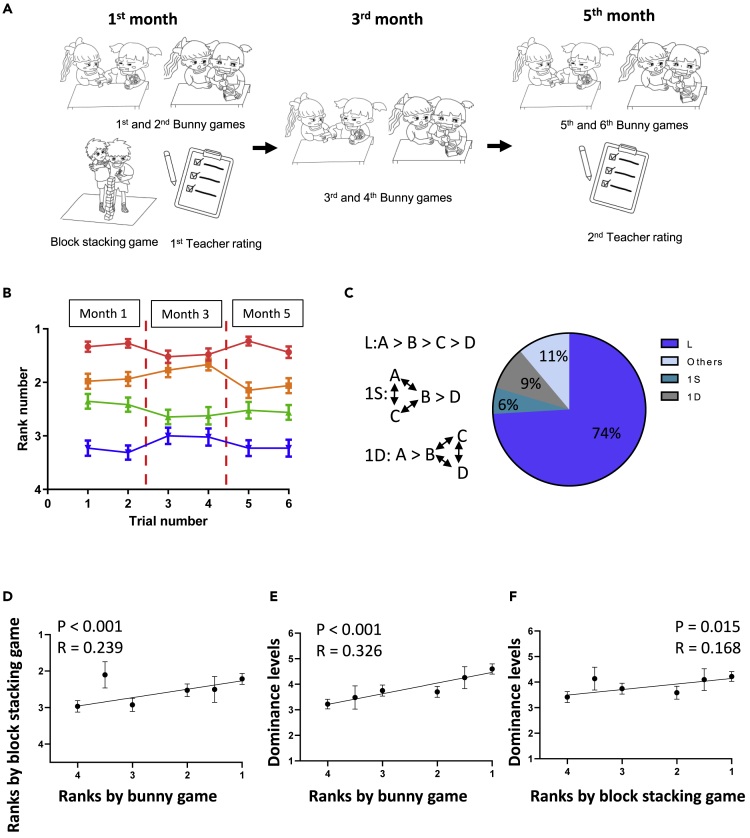
Human studies have shown that dominance hierarchies gradually become stable in preschool years (Strayer and Strayer, 1976; Strayer and Trudel, 1984). To explore the influence of innate characteristics on the formation of social hierarchy, we supposed that preschool young children would be the most appropriate participants because of their least school experience and very limited peer interaction experience. Therefore, we collected data from 216 children aged 3 to 6 years from two preschools and examined their social hierarchies from the beginning of a new semester to the end of the semester. After arranging the children as groups of four, their social rankings within the groups were examined by the bunny game that they performed twice in one day every other month during a period of 5 months (Figure 1A). The bunny game was new to the children and reflected nonconflicting competitive peer interactions. Our results showed that, as with the social patterns found for animals (Wang et al., 2011), the ranking status of young children was very stable over this 5-month period (Figure 1B). The hierarchical rankings mostly obeyed a linear relationship (i.e., they were transitive, meaning that if A is dominant over B, who is dominant over C, then A is dominant over C) (Figure 1C).
Figure 1.
Social hierarchy in preschool-aged children is stable and obeyed a linear relationship
(A) Experimental procedure for evaluating social hierarchy in children. The ranking between two children was tested by the bunny game twice consecutively in the first, third and fifth month. The blocking game was also applied to evaluate hierarchy in the first month. The teacher rating was conducted in both the first and the fifth month.
(B) Summary of social ranks defined by the bunny game for 54 groups of four preschool-aged children over a period of 5 months. Mean ± S.E.M.
(C) Possible social relationships among a group of four children and the percentage of each relationship observed (N = 54 groups).
(D) Correlation between ranks defined by bunny game and ranks by block-stacking game (Pearson Correlation, N = 216).
(E) Correlation between ranks defined by bunny game and dominance levels rated by teacher (Pearson Correlation, N = 216).
(F) Correlation between ranks defined by block-stacking game and dominance levels rated by teacher (Pearson Correlation, N = 216).
Next, we investigated the consistency of the social rankings under different conditions by comparing the rankings defined by the bunny game with rankings defined by the block-stacking game (Figure 1A). Compared with the bunny game, which requires the children to be more intellectually involved, the block-stacking game mainly demands fast physical movement. Although the task requirements for the two games were different, we found a significantly positive correlation between the rankings defined by these two independent methods (Figure 1D). More importantly, the ranks determined by either game were also consistent with evaluations of the social dominance levels made by the teachers (Figures 1A, 1E, and 1F) (Dodge and Coie, 1987). The significantly positive correlation among these different measures implied a general hierarchical relationship for both the specific conflict situations and daily social interactions.
Correlations between the individual characteristics of the children and their social hierarchy
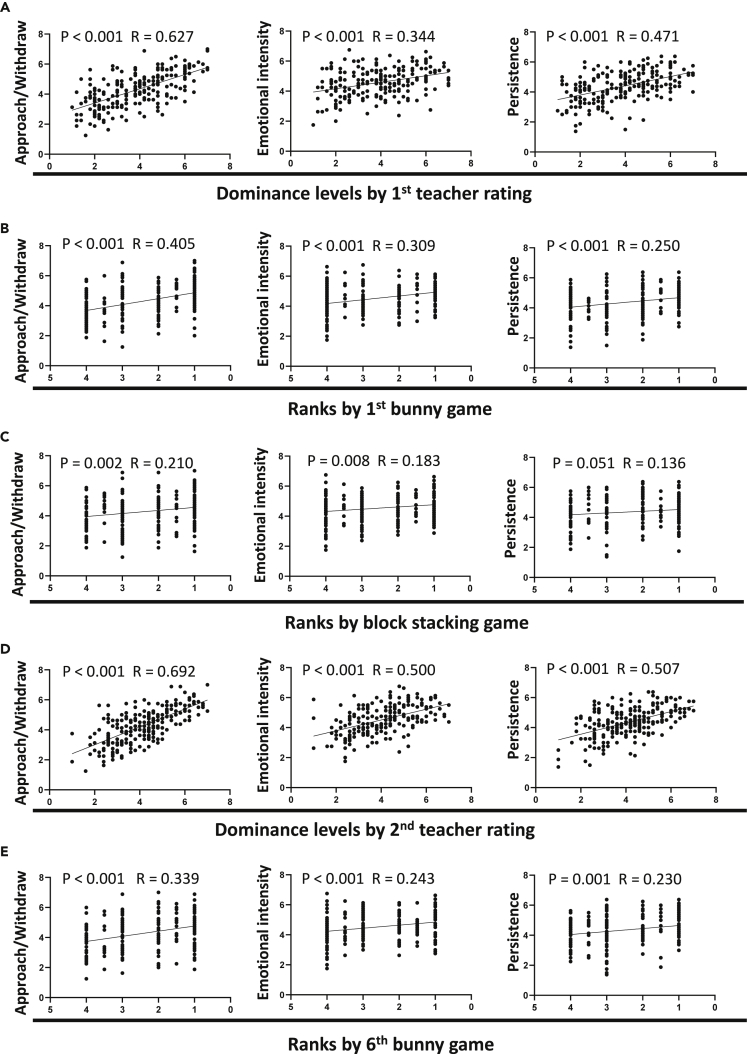
The consistency among the rankings defined by three completely different methods indicates that certain fundamental root causes, e.g., individual innate traits or personalities, might be important in forming social hierarchies of children. Consequently, since teachers' rating covered various daily social interactions of the young children, exploring the characteristics related to the questionnaire answers given by the teachers would provide insight into the hierarchies formed among those children. To examine the influences of possible individual characteristics, we tested several aspects of temperament using the Temperament Assessment Battery for Children questionnaire, which assesses the intrinsic, neurophysiological basis of an individual (Martin, 1994; Wang, 1995). We found that the rank positions defined by the ratings of the teachers in the beginning of a new semester (with limited social experience with classmates) significantly correlated with almost every aspect of temperament except for activity level (Table S1 and Figure 2A). Moreover, even though the task requirements for two competitive games were quite different, the rankings defined by either game are identically correlated with temperaments in approach/withdraw, emotion intensity and persistence (Table S1, Figures 2B and 2C). Since the preschoolers were not familiar with each other at the beginning of a new semester and thus had limited knowledge about their classmates' abilities or traits, we therefore inferred that the innate internal temperaments of children could have an important influence on the formation of a social hierarchy.
Figure 2.
Social ranks defined by teacher rating, bunny game, and block-stacking game in preschool-aged children are positively correlated with temperaments in approach/withdraw, emotion intensity, and persistence Table S1
(A) Correlation between dominance levels defined by first teacher rating and three temperaments.
(B) Correlation between ranks defined by first bunny game and three temperaments.
(C) Correlation between ranks defined by block-stacking game and three temperaments.
(D) Correlation between dominance levels defined by second teacher rating and three temperaments.
(E) Correlation between ranks defined by sixth bunny game and three temperaments (Pearson Correlation, N = 216).
See also Table S1.
Given that the social rankings can be caused by both the personal characteristics and social experiences in classroom interactions of the children, to determine whether there are personal characteristics that keep influencing the social rankings regardless of the quantity or the quality of social experience, we further investigated the relationship between temperaments and hierarchy defined by the second teacher rating (with accumulated interaction experience with classmates for five months) and the sixth bunny game (after playing six rounds in five months) at the end of the semester. We found that the correlations were extremely similar over the 5-month testing period (Table S1, Figures 2D and 2E), suggesting that the influence of intrinsic traits on rankings were not diminished or changed by learning (including the newly accumulated interactive experiences and preschool education), but in fact pre-existed and persisted over time. Taken together, by simultaneously examining the influence of temperaments on social rankings defined by different methods at the beginning and end of the semester, we found that approach/withdraw, emotion intensity and persistence were the major individual characteristics influencing the formation of social hierarchy.
Social hierarchy in weanling mice
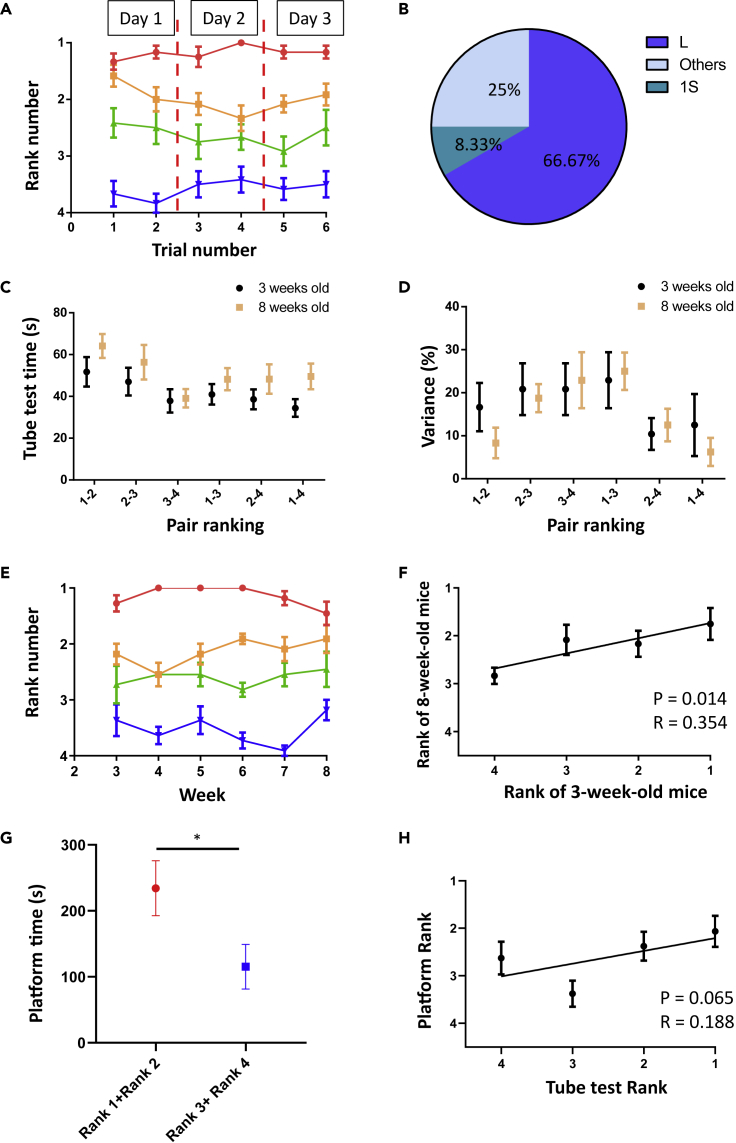
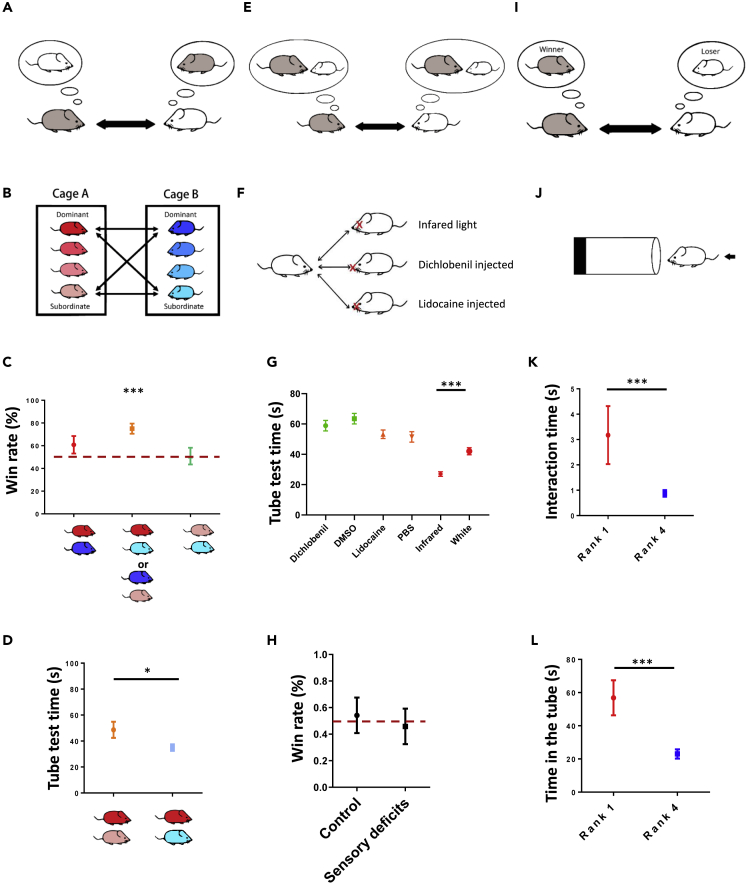
Because the behaviors or performance of children can be influenced by several human factors, to further dissect the causality between intrinsic characteristics and social hierarchy, we also investigated the natural or intrinsic hierarchical relationships in groups of C57BL/6J mice using the tube test. Similar to the bunny game and stock-stacking game in children, tube test also provides a platform to determine mice social ranks unambiguously in a short time without involving physical fighting. However, while there is no any learning procedure as well as immediate advantage/disadvantage for children to compete for bunny or stock-stacking games, the traditional tube test requires training of the mice by applying aversive air pressure to motivate individuals to enter the tube (Wang et al., 2011). In order to apply an assay comparable to the tasks for young children, we therefore modified the standard tube test to reduce confounds associated with training or stress. We first applied this untrained tube test using adult male mice and found that the mice voluntarily entered the tube and behaved naturally. For the untrained tube test, the majority of social rankings among the mice were stable across 3 days and were mostly transitive (Figures S1A and S1B). Consistent with a previous study, we also noticed that the assay time was negatively correlated with the rank numbers and rank distances between pairs of mice (Figure S1C) (Wang et al., 2011). We compared the results of the untrained tube test to the standard trained tube test and found that the assay time was generally shorter after training (Figure S1D), but the differences in the percent variance over four consecutive sessions between assays with trained and untrained mice were not significant (Figure S1E). These results indicated that the tube test can be a reliable experimental platform to study social hierarchy even without the training procedure.
We next used the untrained tube test to examine social hierarchy in young mice. When we tested 2-week-old mouse pups, right before they were weaned, we failed to establish a robust social ranking because the pups had difficulty finishing the assay. For the 3-week-old mice who had just been weaned, most were able to complete the assay successfully, and we observed a stable and mostly transitive social hierarchy (Figures 3A and 3B). Comparison between adult and weanling mice showed no difference in either the assay time or variance across four consecutive trials (Figures 3C and 3D). Notably, we found that the ranks of the weanling mice were well maintained as the mice matured to 8 weeks of age, i.e., became adults (Figure 3E). The correlation of rank positions between 3- and 8-week-old mice were weak but statistical significance (Figure 3F). Lastly, since young mice do not perform aggression, platform competition assay, in which mice in water compete for a platform to stand, was developed to validate the social ranks by tube test (Figure 3G). We found dominant mice (first and second rank mice) generally have more time on the platform than subordinate mice (third and fourth mice). The positive correlation between ranks defined by tube test and ranks by platform competition were also close to significant (Figure 3H), implying a certain degree of consistency between two assays. Taken together, our results suggested that a stable social hierarchy can be established in 3-week-old weanling mice using the untrained tube test and that these social rankings could potentially reflect the hierarchical relationship in adults.
Figure 3.
Social hierarchy in weanling mice can be established using the untrained tube test Figure S1
(A) Summary of the social ranks of the weanling mice as determined by the untrained tube test performed two times per day over three days (N = 12 cages).
(B) Percentage of each hierarchy relationship observed in weanling mice (N = 12 cages).
(C) Comparison of assay time across four trials for the 3- and 8-week-old mice (pair 1-2: p = 0.186, pair 2-3: p = 0.370, pair 3-4: p = 0.561, pair 1-3: p = 0.259, pair 2-4: p = 0.267, pair 1-4: p = 0.057, unpaired t test or Mann-Whitney test, N = 12 pairs).
(D) Comparison of assay variation across four trials for the 3- and 8-week-old mice (pair 1-2: p = 0.369, pair 2-3: p > 0.999, pair 3-4: p = 0.883, pair 1-3: p = 0.626, pair 2-4: p > 0.999, pair 1-4: p = 0.784, Mann-Whitney test, N = 12 pairs).
(E) Summary of social ranks tested every week from 3-week-old to 8-week-old (N = 12 cages). (F) Correlation of social ranks for the 3- and 8-week-old mice (Pearson Correlation, N = 12 cages).
(G) Total time of dominant and subordinate mice on the platform in platform competition assay (p = 0.011, Mann-Whitney test, N = 12 cages).
(H) Correlation of social ranks defined by tube test and platform competition assay (Pearson Correlation, N = 12 cages). Mean ± S.E.M.
See also Figure S1.
The loser in the untrained tube test determined the social hierarchy of the group
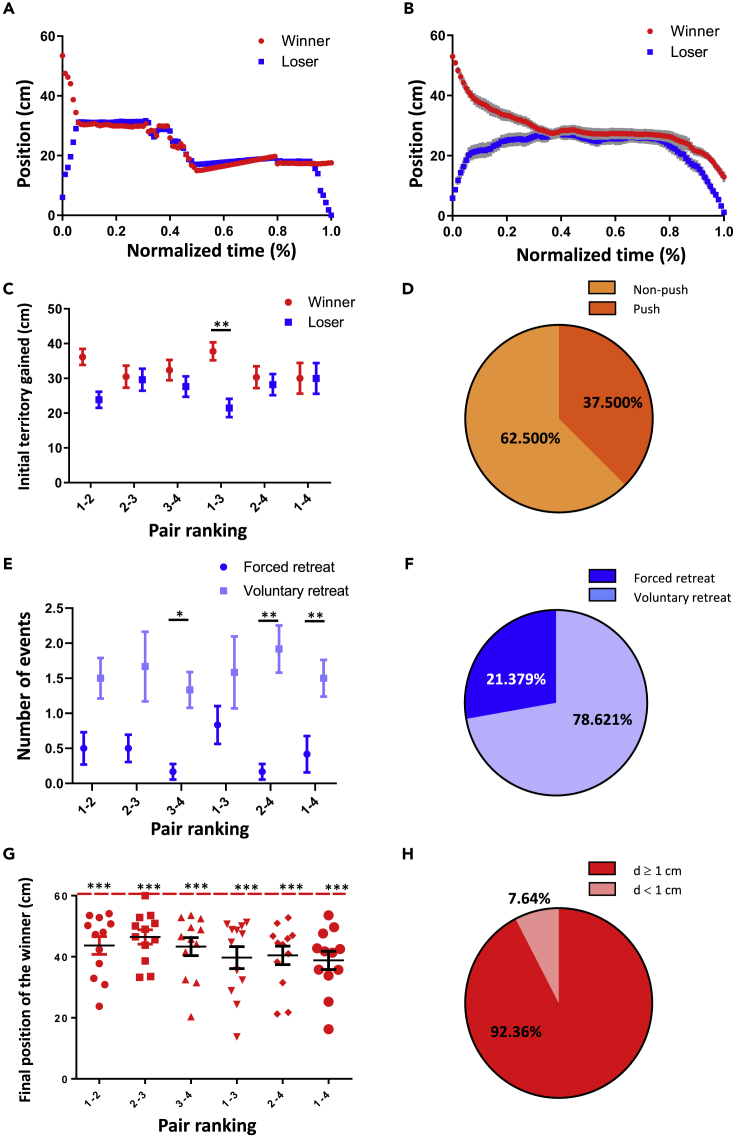
What roles do winners and losers play to determine the outcome of the tube test? Controlling and aggressive actions of a dominant male are thought to limit and influence the response of a subordinate individual (Benton and Brain, 1979; Darwin, 1871; Long, 1972; Wang et al., 2014); however, our study in children also implied that those of lower rank were more likely to withdraw and be both less expressive and less persistent, which may contribute to the formation of their social hierarchy. Although previous studies using the tube test mainly quantified winners and described the trial as ending when one mouse ‘forced’ another to retreat out of the tube (Lindzey et al., 1961), the behaviors of the losers have never been considered carefully. By examining the behaviors and positions of the mice in detail during the tube test with weanling mice (Figures 4A and 4B and Video S1), we first found that the behaviors of the two competing individuals during the early phases of the assay were hardly distinguishable. Although rank-1 mice tended to gain slightly more territory, in most cases both winners and losers acquired similar territory in the tube and stood holding their positions (Figure 4C). Unexpectedly, less than half of the winners physically pushed the losers during the assay (Figure 4D). Resolution of the social encounters within the tube was therefore not determined by one-sided assertive and dominance behavior of the ultimate winner. Instead, detail examination of 145 loser retreat events in 72 tests showed that the assay was mostly ended by a shift by the ultimate loser from being socially engaged to unilaterally retreating voluntarily (Figures 4E and 4F). Most importantly, although some winners moved forward as the losers retreated, the assays almost always ended with a certain distance between the winner and its forward exit, which indicated that losers left the tube without direct contact with the winners (Figure 4G). Among the encounters, only <8% concluded with the winner forcibly pushing the loser out, which was defined as a distance between the two animals of <1 cm once the loser had fully exited the tube (Figure 4H). Similar phenomenon was also observed in the adult mice (Figures S2A−S2C) and indicated that the social rank based on the untrained tube test was mainly determined by the loss of motivation and persistence by the subordinate mice and was followed by their withdrawal rather than by an aggressive advance of the dominant mice.
Figure 4.
The outcome of the tube test for weanling mice is determined by the withdrawal behavior of the losers Figure S2
(A) A representative kymograph of mouse positions during the tube test assay (see also Video S1).
(B) Kymograph of mouse positions in an average of 12 pairs.
(C) Initial territory gained by the winners and losers in the tube test (pair 1-2: p = 0.052, pair 2-3: p = 0.8936, pair 3-4: p = 0.4362, pair 1-3: p = 0.009, pair 2-4: p = 0.732, pair 1-4: p = 0.996, paired t test or Wilcoxon signed-rank test, N = 12 pairs, four trials for each pair).
(D) Percentage of winners physically pushing losers during the assay (N = 72 trials).
(E) Number of forced retreat or voluntary retreat of losers during the assay (pair 1-2: p = 0.056, pair 2-3: p = 0.120, pair 3-4: p = 0.009, pair 1-3: p = 0.240, pair 2-4: p = 0.005, pair 1-4: p = 0.039, Wilcoxon signed-rank test, N = 12 pairs).
(F) Percentage of forced retreat and voluntary retreat of losers during the assay (N = 145 retreats in 72 trials).
(G) Final positions of the winners compared with their exit end of the tube at the end of the assay (pair 1-2: p < 0.001, pair 2-3: p < 0.001, pair 3-4: p < 0.001, pair 1-3: p < 0.001, pair 2-4: p < 0.001, pair 1-4: p < 0.001, one-sample t test or one-sample Wilcoxon signed-rank test, N = 12 pairs, four trials for each pair).
(H) Percentage of winners found at a distance (D) from the exit of ≥1 cm and <1 cm at the end of the assay (N = 288 trials). ∗ = p < 0.05, ∗∗ = p < 0.01, ∗∗∗ = p < 0.001. Mean ± S.E.M.
See also Figure S2.
Subordination is driven by internal status
The social rankings in weanling mice were not simply determined by weight or general activity (Figures S3A and S3B). There were also no detectable differences in anxiety level and exploration activity (Figures S3C–S3E). How does a mouse decide to switch from persistence to retreat? Since group-housing prior to the test may provide an opportunity for mice to learn and associate the individual social signals of each cagemate with the previous outcomes of their social experiences (Brennan and Kendrick, 2006; Camats Perna and Engelmann, 2017; Hurst et al., 2001), one possibility is that the low-ranking individuals could recognize the learned social signature of their opponent to determine whether they should persist or withdraw in a given encounter (Figure 5A). To evaluate the role of social memory in promoting social withdrawal, we compared each individual's social outcome against its cagemates with whom they had prior social interactions and against strangers with whom no prior social experience existed (Figure 5B). We reasoned that if individual recognition was guiding the withdrawal behavior of the losers in the tube test, we would expect that (1) the number of times a mouse wins against an unknown individual would be unbiased (50%) and that (2) on average the assay time between two strangers would be longer than that between two cagemates because there would be no recognizable information that could be used to resolve the conflict. Instead, we found that dominant males mostly dominated against unknown subordinate opponents (Figure 5C). The assay time across four consecutive trials, surprisingly, was shorter between two strangers than between two cagemates (Figure 5D). These results, therefore, did not support a role for associative social memories or individual recognition driving the hierarchy in the untrained tube test.
Figure 5.
The withdrawal behavior of the subordinate mice is determined by their internal status Figure S3
(A) Associative memory model. An individual's unique social cues evoke a memory of previous social experiences.
(B) Stranger assay. The tube test was applied to compare the behavior of two cagemates (individuals housed together) vs. two strangers (individuals housed in different cages).
(C) The win rate for paired strangers compared with the expected rate of 50% (dashed line) (rank-1 vs. rank-1: p = 0.188, N = 15 pairs; rank-1 vs. rank-4: p < 0.001, N = 30 pairs; rank-4 vs. rank-4: p = 0.991, N = 15 pairs, one-sample Wilcoxon signed-rank test).
(D) Assay time across four trials for two cagemates and two strangers (p = 0.0179, unpaired t test, N = 12 pairs of cagemates, N = 30 pairs of strangers).
(E) Specialized social cue(s) model. Dominant winners emit an instructive social signal(s) that promotes retreat by the losers.
(F) Sensory removal. Social hierarchy was evaluated using mice without normal sensory sensations. Olfactory cues were removed by intraperitoneal injection of dichlobenil. Tactile cues were blocked by injection of lidocaine into the whisker pads of the mice. Visual signals were eliminated by performing the experiments under infrared light.
(G) Assay time of all paired conditions across four trials for evaluation of the social hierarchy in mice that lacked a sensory input (dichlobenil vs. DMSO mice: p = 0.57, lidocaine vs. PBS mice: p = 0.581, infrared-light condition vs. white-light condition: p < 0.001, Mann-Whitney test, N = 60–72 pairs) (see also Figure S4).
(H) Win rate for control mice (DMSO + PBS) and mice without olfaction and somatosensation (dichlobenil + lidocaine) compared with the expected rate of 50% (dashed line) under infrared light (Intact: p = 0.877, Sensory deficits: p = 0.877, one-sample Wilcoxon signed-rank test, N = 10 pairs).
(I) Internal status model. Losers are less persistent and more likely to withdraw when obstructed.
(J) Blocker assay. Further forward motion of winners or losers was blocked by an immovable object placed midway in the tube.
(K) Assay time across four trials that higher-ranked mice and lower-ranked mice interacted with the blocking material (p < 0.001, Mann-Whitney test, N = 10 pairs).
(L) Assay time across four trials that higher-ranked mice and lower-ranked mice spent in the tube containing the blocking material (p < 0.001, Mann-Whitney test, N = 10 pairs). ∗ = p < 0.05, ∗∗∗ = p < 0.001. Mean ± S.E.M.
See also Figure S3.
Instead of social memory, most social cues function as instructive signals to induce a receiver's response (Figure 5E). For example, specialized olfactory cues or pheromones have been suggested to trigger specific dominance behaviors or to promote aggressive motor patterns in mice (Chamero et al., 2007; Stowers et al., 2002). In the tube test, if subordinate-specific behaviors or social withdrawal are induced by signals released from dominant opponents, mice without sensory perceptions should not retreat and have enhanced persistence in the tube (Figure 5F). We would then expect that (1) there is an increase in the assay time for mice without sensory inputs and that (2) such mice would be more dominant than mice with intact sensation. However, when we compared mice injected with dichlobenil, which eliminated olfaction (Yoon et al., 2005), to dimethyl sulfoxide (DMSO) control mice, we did not find longer assay time in anosmic mice (Figures 5G and S4A). There was also no difference in assay time between mice injected with lidocaine at the whisker pad, which blocked somatosensation, and phosphate buffered saline (PBS)-injected control mice (Mizuno et al., 2018) (Figures 5G and S4B). In addition, when the tube test with untrained mice was conducted under infrared light to minimize visual cues (Jacobs et al., 1999), the assay times were even shorter than when the assay was performed under white-light conditions (Figures 5G and S4C). This shorter assay time may be due to reduced anxiogenic effect caused by white light. The win rates for mice without both olfactory and tactile sensations and for control mice injected with DMSO and PBS were ∼50% under infrared light, suggesting that the outcomes of the competitive interactions between intact mice and mice with sensory deficits were random (Figure 5H). Therefore, we obtained no evidence suggesting that the subordinate withdrawal of the mice was triggered by instructive social signals.
Our experiments did not support roles for social memory or instructive odors underlying the differences in social persistence, as shown by the tube test. Alternatively, a third possible model suggests that the behavioral switch from persistence to withdrawal can be simply determined by an animal's intrinsic status rather than via social signals (Figure 5I). If this was true, we would expect that losers would retreat more readily from everything regardless of the identity of their opponents. For the blocker assay, the material in the tube prevented further forward movement to the exit of the tube (Figure 5J). Notably, the higher-ranked animals spent significantly more time interacting with the blocking material (Figure 5K). The losers withdrew and retreated out of the tube much faster than the winners (Figure 5L), which indicated that the losers inherently were less persistent and preferred to withdraw regardless of whether the obstruction was a mouse or a blockage of the tube. In summary, while our experiments cannot support the first and second models, the blocker assay evidently indicated that the withdrawal decision of subordinate animals can be solely made by intrinsic status without involvement of the opponents' identity.
Discussion
By investigating social hierarchy in both children and weanling mice together, our study aimed to uncover the importance of individuals' intrinsic characteristics in hierarchical formation. Through simply behavioral observation and manipulation, we provide evidences related to three aspects of social hierarchy in the young. First, a stable social hierarchy can be established similarly in young children and mice. Second, social ranking in children is largely influenced by intrinsic characteristics independent of the social experience, not only the active approach in dominants but also withdrawal or retreating in subordinates. Third, in the tube test, individuals with subordinate tendency play the determining role in the formation of a social hierarchy. We carefully ruled out the effects of past interaction experiences and other confounding dominance cues from competitors. Our study not only shows a remarkable similarity in social hierarchy between children and weanling mice but also reveals the critical role of subordinate characteristics in hierarchical formation. We believe that this simple but novel concept has not been well recognized in previous studies.
The concept of dominance hierarchy in preschool-aged children has been suggested either by observing a small sample size or questionnaire rating by teachers in previous studies (Dodge and Coie, 1987; Hawley, 1999, 2002; Pellegrini et al., 2007; Roseth et al., 2007; Sluckin and Smith, 1977; Strayer and Strayer, 1976; Vaughn and Waters, 1981). To our knowledge, this is the first study to adopt multiple methods to evaluate young children's dominance rank in such a large sample of over two hundred preschool children in a five-month longitudinal design. Through the carefully designed tasks that depend on substantially different cues, types of interactions, behavioral requirements, and outcome measures, the consistent results we got therefore strongly support the possibility that intrinsic traits fundamentally influence the formation of social hierarchy. More importantly, we present our findings in parallel with mouse hierarchy, reflecting the similarity between these two species. Given that dominance hierarchy has been studied extensively in children, it is surprising that the hierarchy in young mice has not been reported previously. Most studies concerning social hierarchy in mice have focused on dominance behaviors, e.g., aggression and scent-marking, and were, therefore, performed using adult males. One study recently applied the tube test to investigate the influence of social isolation on ranking in the juvenile rat (Tada et al., 2016), but it did not provide general information concerning the hierarchical relationships in a group. Our study shows a detectable ranking among weanling mice, providing a fundamental basis for exploring social interactions in young animals and having potential implications for human children.
While previous studies have mostly focused on the dynamics of peer dominant relationship or on the changes in children's controlling strategies with interactive experiences or education (Hawley, 1999, 2002; La Freniere and Charlesworth, 1983; Roseth et al., 2011), our findings suggest stable and persisting influences of innate intrinsic traits in the formation of social hierarchy. Although the relationship between temperament and social dominance may have been examined in previous studies of adults, it was defined either as one dimension of personality or just one aspect of emotion (Burgoon et al., 1998; Hall et al., 2005; Keltner et al., 2003; Mehrabian, 1996; Russell and Mehrabian, 1977). The influence of temperament on social dominance of young children has rarely been explored. Even if there was, the focus was just on one single aspect of temperament (persistence) (Hawley and Little, 1999). Our work therefore provides a more comprehensive examination of the relationship between social rankings and multiple temperaments and suggests that certain fundamental individual traits are especially crucial in determining social hierarchies. Children who can adopt a more accepting attitude toward a novel social situation (approach/withdraw), tend to express emotions more strongly (emotional intensity), and to continue with difficult learning tasks (persistence) would gain a higher rank in their social group. In contrast, regardless of the task characteristics and past social experience, children who withdraw more rapidly from a new situation, are less daring in expressing emotions and exhibit less persistence are more likely become subordinates. Our findings suggest that social hierarchies are strongly determined by certain intrinsic traits of the individual during the very beginning of social interactions. The formation of social hierarchy might be not caused solely by someone dominating or controlling others but also by someone with submissive characteristics allowing the other to successfully become the winner. This phenomenon prompted us to reconsider the possible even more important role of subordinates in the formation of social hierarchies.
Our study in mice further confirms this concept concerning the determining role of the loser in conflict situations. The untrained tube test shared some important features with the bunny game or stock-stacking game for us to investigate these two species comparably. First, there is no any form of physical fighting. Second, the conflicts are resolved in a short time with no ambiguous condition between winner and loser. Third, active motivation is important since there is no any punishment or disadvantage for losers. Using this untrained tube test, we found that hierarchy surprisingly was not determined by displays of antagonism from winners or by a lack of such dominance displays by losers. Instead, the outcomes were determined by the behavior of the losers as they disengaged from the conflict. Since winners needed only to hold their competitive intention, it was the losers who changed their action to resolve a conflict. A hierarchy basically cannot be formed without a loser or subordinate decision. From an evolutionary perspective, the decision to withdraw or be subordinate is an important survival strategy, because losing a conflict without avoiding the winner might be lethal for the loser. Consequently, an intrinsic, neurophysiological basis for the hierarchical position of an individual helps the social hierarchy of a group to form easily and remain stable without the need for fighting or other interactions. It is, therefore, important to uncover the mechanism(s) underlying the decision(s) of losers to understand the fundamental structure of a social hierarchy. Glucocorticoid hormones, for example, have been reported to play a crucial role in social rank attainment in rodents (Papilloud et al., 2020; Timmer and Sandi, 2010). While the effect of glucocorticoid on aggression have been investigated extensively (Mikics et al., 2004, 2007), it would be interesting to explore the influence of the hormones on subordinate behaviors. The untrained tube test, thus, presents as a novel platform to evaluate tendency toward persistence or withdrawal, and may help to explore subordinate decisions in the future.
We believe that both dominance and subordinate individuals play important roles in hierarchy determination. Unfortunately, most assays for social behaviors in mice have focused only on dominance displays, whereas the behavior of subordinate animals was simply not assessed (Wang et al., 2014). Our study provides evidence showing that, in certain circumstance, subordinate individuals can play the major role to resolve the conflict. Like studies involving nonhumans, human studies concerning social hierarchy have focused on primarily dominance behaviors, whether the target behavior was aggression or resource control (Sluckin and Smith, 1977; Strayer and Strayer, 1976). In a preschool classroom, aggressive and coercive-controlling behaviors are not preferred and thus attract the attention of and intervention by the teacher. However, our study shows that young children with retreating, less-emotionally expressive, and less persistent temperaments are most likely to become subordinate. Although this may have evolutionary roots or significance, this silent formation of a social hierarchy with an absence of noticeable conflicts may mean that some children lose their opportunities to play toys, use classroom resources, or partake in important learning experiences without being noticed by the teacher. The findings of this study, therefore, provide an important reminder to educators that more attention should be paid to subordinates in the classroom rather than to only the dominant students.
Limitations of the study
In this study, we applied surgical and pharmacological manipulations in mice to study the influence of internal status on conflict solving, which cannot be conducted in human children. We also study children temperaments to figure out crucial internal characteristics on the formation of social hierarchy, which cannot be classified clearly in mice. Our study therefore made a breakthrough to use the two species to answer different questions and reveal information that could not be approached solely by one of them. However, compared to the study of a single species, cross-species behavioral research is more difficult in data comparison and interpretation. Although each of our research tasks has been carefully designed and the consistent results solidified our discovery, this research still has many unresolved limitations.
First, from behavioral display to outcome measurements, there are still substantial differences among bunny game, block-stacking and tube tests. Although consistent results based on these different tasks solidified our discovery, interpretation of the data need to be very careful. For example, while data from tube test provided proof of concept that social conflict can be mainly determined by subordinate decision, the same conclusion cannot be inferred from our children study. Second, in spite of that our study emphasized the determining role of the subordination on resolving the conflict, we had no intention to decline the contribution of dominate individuals involved in the subordinate decision. The development of new and comparable tasks in both species will be therefore important to answer whether conflict can be resolved solely by subordinate decision without any influence from dominant others. Third, although our data examining three different potential models suggested that the withdrawal of subordinate mice are mainly caused by their intrinsic status, i.e., being less persistent, we, however, cannot completely exclude the influence of opponents or associated memory involved in subordinate retreat in the tube test. Further studies will be needed to clarify the involvements of the other two models in tube test assay. Given that social stress is known to affect social hierarchy and enhance social memory (Cordero and Sandi, 2007), the roles of stress hormones should be focused in particular. Finally, we found that the formations of social hierarchy in children and mice are influenced by temperament or intrinsic status, the underlying neurocognitive or physiological mechanisms, however, were not investigated in this study and remain to be further explored. Future research in the examination of neural activity during conflicts should put more focus on the decision-making of subordinate behaviors. And once again, we believe that comparative studies between human and model organisms would provide us more information about the neural basis as well as evolutionary mechanisms behind hierarchical formation.
Resource availability
Lead contact
Tsung-Han Kuo Email: thkuo@life.nthu.edu.tw.
Materials availability
This study did not generate any new unique reagent.
Data and code availability
This study did not use any custom code, software, or algorithm.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank the children who participated in this research and their parents for allowing them to do so. We also thank the members of the Chou and Kuo labs for experimental help. The work was supported by the Ministry of Science and Technology (MOST 107-2410-H-007-070-MY2 to Y-J. C.) (MOST 108-2636-B-007-002 Young Scholar Fellowship to T-H. K.), by the Higher Education Sprout Project funded by Ministry of Education and Ministry of Science and Technology (Grants 107Q2721E1 and 108Q2721E1 of the Interdisciplinary Research Project to Y-J. C. and T-H. K.) (the Bioresource Conservation Research Center to T-H. K.) (the Brain Research Center to T-H. K.).
Author contributions
YJ Chou, YH Lu, YS Su, and TH Kuo designed the experiments; YJ Chou, YH Lu, YK Ma, and YS Su performed the experiments; YJ Chou, YH Lu, YS Su, and TH Kuo analyzed the data, and YJ Chou and TH Kuo wrote the manuscript.
Declaration of interests
The authors declare no competing financial interests.
Published: February 19, 2021
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2021.102073.
Contributor Information
Yu-Ju Chou, Email: chouyuju@mail.nd.nthu.edu.tw.
Tsung-Han Kuo, Email: thkuo@life.nthu.edu.tw.
Supplemental information
References
- Benton D., Brain P.F. Behavioural comparisons of isolated, dominant and subordinate mice. Behav. Process. 1979;4:211–219. doi: 10.1016/0376-6357(79)90002-0. [DOI] [PubMed] [Google Scholar]
- Brennan P.A., Kendrick K.M. Mammalian social odours: attraction and individual recognition. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006;361:2061–2078. doi: 10.1098/rstb.2006.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brey E., Shutts K. Children use nonverbal cues to make inferences about social power. Child Dev. 2015;86:276–286. doi: 10.1111/cdev.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoon J.K., Johnson M.L., Koch P.T. The nature and measurement of interpersonal dominance. Commun. Monogr. 1998;65:308–335. [Google Scholar]
- Camats Perna J., Engelmann M. Recognizing others: rodent's social memories. Curr. Top. Behav. Neurosci. 2017;30:25–45. doi: 10.1007/7854_2015_413. [DOI] [PubMed] [Google Scholar]
- Chamero P., Marton T.F., Logan D.W., Flanagan K., Cruz J.R., Saghatelian A., Cravatt B.F., Stowers L. Identification of protein pheromones that promote aggressive behaviour. Nature. 2007;450:899–902. doi: 10.1038/nature05997. [DOI] [PubMed] [Google Scholar]
- Charafeddine R., Mercier H., Clément F., Kaufmann L., Berchtold A., Reboul A., Van der Henst J.-B. How preschoolers use cues of dominance to make sense of their social environment. J. Cogn. Dev. 2015;16:587–607. [Google Scholar]
- Charafeddine R., Mercier H., Clément F., Kaufmann L., Reboul A., der Henst V. Children’s allocation of resources in social dominance situations. Dev. Psychol. 2016;52:1843. doi: 10.1037/dev0000164. [DOI] [PubMed] [Google Scholar]
- Cordero M.I., Sandi C. Stress amplifies memory for social hierarchy. Front. Neurosci. 2007;1:175–184. doi: 10.3389/neuro.01.1.1.013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C. John Murray; 1871. The Descent of Man, and Selection in Relation to Sex. [Google Scholar]
- Dodge K.A., Coie J.D. Social-information-processing factors in reactive and proactive aggression in children's peer groups. J. Pers. Soc. Psychol. 1987;53:1146–1158. doi: 10.1037//0022-3514.53.6.1146. [DOI] [PubMed] [Google Scholar]
- Esposito G., Yoshida S., Ohnishi R., Tsuneoka Y., Rostagno Mdel C., Yokota S., Okabe S., Kamiya K., Hoshino M., Shimizu M. Infant calming responses during maternal carrying in humans and mice. Curr. Biol. 2013;23:739–745. doi: 10.1016/j.cub.2013.03.041. [DOI] [PubMed] [Google Scholar]
- Gülgöz S., Gelman S.A. Who's the boss? Concepts of social power across development. Child Dev. 2017;88:946–963. doi: 10.1111/cdev.12643. [DOI] [PubMed] [Google Scholar]
- Hall J.A., Coats E.J., LeBeau L.S. Nonverbal behavior and the vertical dimension of social relations: a meta-analysis. Psychol. Bull. 2005;131:898–924. doi: 10.1037/0033-2909.131.6.898. [DOI] [PubMed] [Google Scholar]
- Hawley P.H. The ontogenesis of social dominance: a strategy-based evolutionary perspective. Dev. Rev. 1999;19:97–132. [Google Scholar]
- Hawley P.H. Social dominance and prosocial and coercive strategies of resource control in preschoolers. Int. J. Behav. Dev. 2002;26:167–176. [Google Scholar]
- Hawley P.H. Social dominance in childhood and adolescence: why social competence and aggression may go hand in hand. In: Hawley P.H., Little T.D., Rodkin P.C., editors. Aggression Adaptation: The Bright Side to Bad Behavior. Routledge; 2007. pp. 1–29. [Google Scholar]
- Hawley P.H., John Geldhof G. Preschoolers’ social dominance, moral cognition, and moral behavior: an evolutionary perspective. J. Exp. Child Psychol. 2012;112:18–35. doi: 10.1016/j.jecp.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Hawley P.H., Little T.D. On winning some and losing some: a social relations approach to social dominance in toddlers. Merrill-Palmer Q. 1999;45:185–214. [Google Scholar]
- Huntingford F., Turner A.K. Springer; 1987. Animal Conflict. [Google Scholar]
- Hurst J.L., Payne C.E., Nevison C.M., Marie A.D., Humphries R.E., Robertson D.H., Cavaggioni A., Beynon R.J. Individual recognition in mice mediated by major urinary proteins. Nature. 2001;414:631–634. doi: 10.1038/414631a. [DOI] [PubMed] [Google Scholar]
- Islam G. Social dominance theory. In: Teo T., editor. Encyclopedia of Critical Psychology. Springer; 2014. pp. 1779–1781. [Google Scholar]
- Jacobs G.H., Fenwick J.C., Calderone J.B., Deeb S.S. Human cone pigment expressed in transgenic mice yields altered vision. J. Neurosci. 1999;19:3258–3265. doi: 10.1523/JNEUROSCI.19-08-03258.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating C., Bai D. Children's attributions of social dominance from facial cues. Child Dev. 1986;57:1269–1276. [Google Scholar]
- Keltner D., Gruenfeld D.H., Anderson C. Power, approach, and inhibition. Psychol. Rev. 2003;110:265–284. doi: 10.1037/0033-295x.110.2.265. [DOI] [PubMed] [Google Scholar]
- La Freniere P., Charlesworth W.R. Dominance, attention, and affiliation in a preschool group: a nine-month longitudinal study. Ethol. Sociobiol. 1983;4:55–67. [Google Scholar]
- Larrieu T., Cherix A., Duque A., Rodrigues J., Lei H., Gruetter R., Sandi C. Hierarchical status predicts behavioral vulnerability and nucleus accumbens metabolic profile following chronic social defeat stress. Curr. Biol. 2017;27:2202–2210 e2204. doi: 10.1016/j.cub.2017.06.027. [DOI] [PubMed] [Google Scholar]
- Lindzey G., Winston H., Manosevitz M. Social dominance in inbred mouse strains. Nature. 1961;191:474–476. doi: 10.1038/191474a0. [DOI] [PubMed] [Google Scholar]
- Long S.Y. Hair-nibbling and whisker-trimming as indicators of social hierarchy in mice. Anim. Behav. 1972;20:10–12. doi: 10.1016/s0003-3472(72)80167-2. [DOI] [PubMed] [Google Scholar]
- Lourenco S.F., Bonny J.W., Schwartz B.L. Children and adults use physical size and numerical alliances in third-party judgments of dominance. Front. Psychol. 2016;6:2050. doi: 10.3389/fpsyg.2015.02050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R.P. Temperament assessment battery for children (TABC) Infant Ment. Health J. 1994;15:238. [Google Scholar]
- McDonald K.L., Baden R.E., Lochman J.E. Parenting influences on the social goals of aggressive children. Appl. Dev. Sci. 2013;17:29–38. [Google Scholar]
- Mehrabian A. Pleasure-arousal-dominance: a general framework for describing and measuring individual differences in temperament. Curr. Psychol. 1996;14:261–292. [Google Scholar]
- Mikics E., Barsy B., Haller J. The effect glucocorticoids on aggressiveness in established colonies of rats. Psychoneuroendocrino. 2007;32:160–170. doi: 10.1016/j.psyneuen.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Mikics E., Kruk M.R., Haller J. Genomic and non-genomic effects of glucocorticoids on aggressive behavior in male rats. Psychoneuroendocrino. 2004;29:618–635. doi: 10.1016/S0306-4530(03)00090-8. [DOI] [PubMed] [Google Scholar]
- Mizuno H., Ikezoe K., Nakazawa S., Sato T., Kitamura K., Iwasato T. Patchwork-type spontaneous activity in neonatal barrel cortex layer 4 transmitted via thalamocortical projections. Cell Rep. 2018;22:123–135. doi: 10.1016/j.celrep.2017.12.012. [DOI] [PubMed] [Google Scholar]
- Neppl T.K., Murray A.D. Social dominance and play patterns among preschoolers: gender comparisons. Sex Roles. 1997;36:381–393. [Google Scholar]
- Over H., Carpenter M. Children infer affiliative and status relations from watching others imitate. Dev. Sci. 2015;18:917–925. doi: 10.1111/desc.12275. [DOI] [PubMed] [Google Scholar]
- Papilloud A., Weger M., Bacq A., Zalachoras I., Hollis F., Larrieu T., Battivelli D., Grosse J., Zanoletti O., Parnaudeau S. The glucocorticoid receptor in the nucleus accumbens plays a crucial role in social rank attainment in rodents. Psychoneuroendocrino. 2020;112:104538. doi: 10.1016/j.psyneuen.2019.104538. [DOI] [PubMed] [Google Scholar]
- Pellegrini A.D., Roseth C.J., Mliner S., Bohn C.M., Van Ryzin M., Vance N., Cheatham C.L., Tarullo A. Social dominance in preschool classrooms. J. Comp. Psychol. 2007;121:54–64. doi: 10.1037/0735-7036.121.1.54. [DOI] [PubMed] [Google Scholar]
- Qu C., Ligneul R., Van der Henst J.-B., Dreher J.-C. An integrative interdisciplinary perspective on social dominance hierarchies. Trends Cogn. Sci. 2017;21:893–908. doi: 10.1016/j.tics.2017.08.004. [DOI] [PubMed] [Google Scholar]
- Reifen Tagar M., Hetherington C., Shulman D., Koenig M. On the path to social dominance? Individual differences in sensitivity to intergroup fairness violations in early childhood. Pers. Individ. Differ. 2017;113:246–250. [Google Scholar]
- Roseth C.J., Pellegrini A.D., Bohn C.M., Van Ryzin M., Vance N. An observational, longitudinal study of preschool dominance and rates of social behavior. J. Sch. Psychol. 2007;45:479–497. [Google Scholar]
- Roseth C.J., Pellegrini A.D., Dupuis D.N., Bohn C.M., Hickey M.C., Hilk C.L., Peshkam A. Preschoolers' bistrategic resource control, reconciliation, and peer regard. Soc. Dev. 2011;20:185–211. [Google Scholar]
- Russell J.A., Mehrabian A. Evidence for a three-factor theory of emotions. J. Res. Pers. 1977;11:273–294. [Google Scholar]
- Saxena K., Webster J., Hallas-Potts A., Mackenzie R., Spooner P.A., Thomson D., Kind P., Chattarji S., Morris R.G.M. Experiential contributions to social dominance in a rat model of fragile-X syndrome. Proc. Biol. Sci. 2018;285:20180294. doi: 10.1098/rspb.2018.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidanius J., Pratto F. Cambridge University Press; 2001. Social Dominance: An Intergroup Theory of Social Hierarchy and Oppression. [Google Scholar]
- Sluckin A.M., Smith P.K. Two approaches to the concept of dominance in preschool children. Child Dev. 1977;48:917–923. [Google Scholar]
- Stowers L., Holy T.E., Meister M., Dulac C., Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Strayer F.F., Strayer J. An ethological analysis of social agonism and dominance relations among preschool children. Child Dev. 1976;47:980–989. [Google Scholar]
- Strayer F.F., Trudel M. Developmental changes in the nature and function of social dominance among young children. Ethol. Sociobiol. 1984;5:279–295. [Google Scholar]
- Tada H., Miyazaki T., Takemoto K., Takase K., Jitsuki S., Nakajima W., Koide M., Yamamoto N., Komiya K., Suyama K. Neonatal isolation augments social dominance by altering actin dynamics in the medial prefrontal cortex. Proc. Natl. Acad. Sci. U S A. 2016;113:E7097–E7105. doi: 10.1073/pnas.1606351113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmer M., Sandi C. A role for glucocorticoids in the long-term establishment of a social hierarchy. Psychoneuroendocrino. 2010;35:1543–1552. doi: 10.1016/j.psyneuen.2010.05.011. [DOI] [PubMed] [Google Scholar]
- van den Berg W.E., Lamballais S., Kushner S.A. Sex-specific mechanism of social hierarchy in mice. Neuropsychopharmacology. 2015;40:1364–1372. doi: 10.1038/npp.2014.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn B.E., Waters E. Attention structure, sociometric status, and dominance: interrelations, behavioral correlates, and relationships to social competence. Dev. Psychol. 1981;17:275. [Google Scholar]
- Wang F., Kessels H.W., Hu H. The mouse that roared: neural mechanisms of social hierarchy. Trends Neurosci. 2014;37:674–682. doi: 10.1016/j.tins.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Wang F., Zhu J., Zhu H., Zhang Q., Lin Z., Hu H. Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science. 2011;334:693–697. doi: 10.1126/science.1209951. [DOI] [PubMed] [Google Scholar]
- Wang P.L. Psychological Publishing Co., Ltd.; 1995. Early Childhood Development Assessment and Guidance. [Google Scholar]
- Yamaguchi Y., Lee Y.A., Kato A., Jas E., Goto Y. The roles of dopamine D2 receptor in the social hierarchy of rodents and primates. Sci. Rep. 2017;7:43348. doi: 10.1038/srep43348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H., Enquist L.W., Dulac C. Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell. 2005;123:669–682. doi: 10.1016/j.cell.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Zhou T., Zhu H., Fan Z., Wang F., Chen Y., Liang H., Yang Z., Zhang L., Lin L., Zhan Y. History of winning remodels thalamo-PFC circuit to reinforce social dominance. Science. 2017;357:162–168. doi: 10.1126/science.aak9726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not use any custom code, software, or algorithm.