Abstract
Inflammatory organ injury and sepsis have profound impacts on the morbidity and mortality of surgical and critical care patients. MicroRNAs are small RNAs composed of 20–25 nucleotides that have a significant contribution to gene regulation. MicroRNA-147 (miR-147), in particular, has been shown to have an emerging role in different physiological functions such as cell cycle regulation and inflammatory responses. However, animal model systems to study tissue-specific functions of miR-147 during inflammatory conditions in vivo are lacking. In the present study, we characterize miR-147 expression in different organs and cell types. Next, we generated a transgenic mouse line with a floxed miR-147 gene. Subsequently, we used this mouse line to generate mice with whole-body deletion of miR-147 (miR-147 −/−) by crossing “floxed” miR-147 mice with transgenic mice expressing Cre recombinase in all tissues (CMVcre mice). Systematic analysis of miR-147 −/− mice demonstrates normal growth, development, and off-spring. In addition, deletion of the target gene in different organs was successful at baseline or during inflammation, including the heart, intestine, stomach, liver, spleen, bone marrow, lungs, kidneys, or stomach. Moreover, miR-147 −/− mice have identical baseline inflammatory gene expression compared to C57BL/6 mice, except elevated IL-6 expression in the spleen (7.5 fold, p < 0.05). Taken together, our data show the successful development of a transgenic animal model for tissue and cell-specific deletion of miR-147 that can be used to study the functional roles of miR-147 during inflammatory organ injury.
KEY WORDS: Inflammation, Organ injury, MicroRNA, Transgenic mouse model, MicroRNA-147
INTRODUCTION
Inflammation is commonly observed in organ injuries including acute respiratory distress syndrome (ARDS) [1–3], myocardial infarction [4], acute kidney injury [5–7], and in sepsis and septic shock [8, 9], which contribute significantly to the mortality and morbidity of surgical or critical care patients. Sepsis and septic shock alone affect more than 31.5 million patients globally each year [8]. Different cell populations play a distinct role in the pathogenesis of inflammatory organ injuries. For example, innate immune cells such as macrophage and neutrophils are involved in the acute phase of inflammation in response to the pathogen-associated molecular patterns (PAMP) and danger-associated molecular patterns (DAMP) and contribute to the production of early inflammatory cytokines including tissue necrosis factor (TNF) and interleukin 1 (IL-1) [10]. Endothelial cells and epithelial cells could further respond to DAMP to promote tissue injury and organ dysfunction [10]. So far, the treatment option of inflammatory organ injury involves correcting the underlining causes and supportive care [11]. Thus, understanding the pathogenesis and the search for novel therapeutic targets for inflammatory organ injury is at the center of attention. To this front, several animal models have been developed to study pathophysiology and therapeutic targets of systemic inflammation and organ dysfunction [12]. For example, cecal ligation and puncture (CLP) is one of the most commonly used models to induce systemic inflammation and organ injury mainly caused by multi-microbial infection [13].
MicroRNAs are small RNAs ranging from 20 to 25 nucleotides that are crucial for post-transcriptional gene regulation. So far, there are more than 2000 microRNAs identified in the human genome according to the most recent miRBase database (http://www.mirbase.org/). The main role of microRNAs is to regulate target gene expression through interaction with the untranslated region (UTR) [14]. It is estimated that microRNAs could target 60% of the human genes [15], indicating the critical role of microRNAs in many physiological and pathological conditions. Previous studies have suggested that microRNAs play crucial roles in development, cell cycle regulation, inflammatory responses, and many other physiological processes [14, 16–18]. For example, miR-223 functions as a regulator of macrophage and neutrophil differentiation and activation [19]. A recent study identified the shuttling of miR-223 from neutrophil to alveolar epithelial cells to provide tissue protection during acute lung injury [20], suggesting diverse mechanisms of action for microRNAs. In the clinic, microRNAs could be therapeutically targeted via several approaches [21]. Specifically, microRNA overexpression is achieved by the delivery of microRNA mimetics, while microRNA inhibition is achieved by inhibitors such as the locked nucleic acid (LNA) [21]. For example, miR-122 has been identified as an enhancer for hepatitis C virus (HCV) replication via binding to the 5′UTR of the viral genome [22]. MiR-122 LNA has been developed and studied by phase III clinical trials as HCV therapy showing promising results [23, 24]. Stemming from the functional diversity and the ease of therapeutic targeting, investigations on microRNAs have been intensive. Thus, developing mouse models to pinpoint the specific contribution of microRNAs in a particular tissue or cell type is of great importance.
MiR-147 (hsa-miR-147b or mmu-miR-147-3p) is located at chromosome 15 in humans and chromosome 2 in mouse. It has been continuously gaining attention as a key regulator of cell cycle progression and inflammatory responses by in vitro and pharmacological studies in vivo [25–32]. For example, miR-147 has been identified as the top upregulated microRNA in lung cancer cells that are tolerant to epidermal growth factor receptor inhibitor, and it orchestrates the metabolic shift of cancer cells for drug tolerance [25]. Another earlier study indicated that miR-147 is induced by toll-like receptor stimulation in macrophages and it is involved in the regulation of inflammatory responses [26]. These studies, along with many others, support the emerging role of miR-147 in the control of many biological processes both in homeostasis and in pathological conditions. Here, we developed a transgenic mouse line using the Cre-flox system for germline and conditional targeting of miR-147 in vivo. The generation of this mouse line will facilitate the study of tissue-/cell-specific contribution of miR-147 in inflammatory organ injury.
MATERIALS AND METHODS
Animals
Animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas Health Center (UTHealth) at Houston. C57BL/6J (wild-type), miR-147loxP/loxP, CMVcre mice were purchased from Jackson Laboratories (Bar Harbor, ME). All mice were housed and bred in a specific pathogen-free facility with a 12-h:12-h light:dark cycle at the Center for Laboratory Animal Medicine and Care at the UTHealth. Both gender mice with age between 8 and 12 weeks were used in this experiment. To produce whole-body deficiency of miR-147, miR-147loxP/loxP mice were crossbred with CMVcre+ mice to generate miR-147loxP/loxPCMVcre+ mice (miR-147-/-).
CLP Model
To establish endotoxin-induced organ injury models, we performed cecal ligation and puncture (CLP) procedure. Mice were anesthetized with 3–5% inhaled isoflurane for induction and 1–3% for maintenance. The cecum was ligated with 4–0 sterile suture at 1 cm from the end, and was punctured twice with a 20G needle. A small amount of feces was extracted with around 2 mm diameter. Sham operation was conducted in the same way without ligation and puncture. Buprenorphine SR was administrated via subcutaneous injection after surgery to reduce pain, and 500 μl of sterile and prewarmed saline was injected to protect from dehydration. All procedures are performed under sterile conditions. The organs were collected 24 h after the procedure.
Cell Lines
HEK293, HMEC1, T84, A539, Calu3, HK-2, Caco, HL60, and THP1 cells were purchased from ATCC and cultured according to ATCC recommended conditions. Human cardiomyocytes (HCM) and human pulmonary alveolar epithelial cells (HPAEpiC) were purchased from ScienCell (Catalog #6200 and #3200, respectively) and cultured according to the manufacturer’s instruction. Human monocyte-derived macrophages (MDM) were differentiated from monocytes as previously described [33], using monocytes isolated from peripheral blood collected from healthy volunteers.
T Cell Differentiation
Naïve CD4 T cells were isolated from spleens dissected from 8- to 12-week-old C57BL/6J mice using the STEMCell Naïve CD4 T cell isolation kit according to the manufacturer’s instruction. Isolated cells were cultured in a concentration of 2–2.5 million/ml in complete RPMI with l-glutamine supplemented with 10% heat inactivated FBS and antimicrobial reagents. T cell differentiation to Th0/Th1/Th2/Th17/Treg condition was as previously described [34]. Cells were cultured for 72 h and washed with PBS. After centrifugation, cell pellet was lysed by Trizol reagent for RNA isolation.
Isolation of Blood Neutrophils, Lymphocytes, and Monocytes
Neutrophil was isolated from blood from 8- to 12-week-old C57BL/6J mice as previously described [20]. Lymphocytes were isolated from blood from 8- to 12-week-old C57BL/6J mice as previously described [35]. Monocytes were isolated from blood from 8- to 12-week-old C57BL/6J mice using EasySep™ Mouse Monocyte Isolation Kit from STEMCELL Technologies according to the manufacturer’s recommended protocol. After centrifugation, cell pellet was lysed by Trizol reagent for RNA isolation.
Isolation of Alveolar Epithelial Cells
Alveolar epithelial cells were isolated as previously described [36]. In brief, 8- to 12-week-old C57BL/6J mice were euthanized by overdose of pentobarbital. After opening up the chest cavity, lungs were perfused with 10 ml of PBS and a small incision was made at the trachea for the insertion of 20G blunt ended catheter. 1.5 ml of 5 unit/ml dispase in DMEM/F12 media was instilled intratracheally via the catheter and followed by 300 μl of 1% low melting point agarose in PBS. Lungs were removed and incubated in 0.5 ml of dispase for 45 min at room temperature. After the incubation, lung tissues were cut into small pieces and rotate for 15 min at 4°C. Digested tissues run through a 70-micron cell strainer and biotinylated antibodies for CD16/32, TER119, CD 45, and CD90 were added. Alveolar epithelial cells were negatively selected using streptavidin labeled magnetic beads. The resulting cells were incubated for 2 h to remove fibroblast. The cell pellet was lysed by Trizol reagent for RNA isolation.
Isolation of Colon Epithelial Cells
Colon epithelium was isolated from 8- to 12-week-old C57BL/6J mice as previously described [37]. Cell pellet was lysed by Trizol reagent for RNA isolation.
Isolation of Renal Tubule Cells
Kidney tubules were isolated according to a modified protocol described previously [38]. Mice were euthanized by high dose of pentobarbital sodium and the kidneys were reperfused with ice-cold PBS. The kidneys were washed with ice-cold PBS twice and were chopped into small pieces on ice. The chopped tissues were enzymatically dissociated with collagenase type II (0.25 mg/ml; Worthington) using a gentleMACS tube (Miltenyi Biotec). The tissue was incubated and dissociated in gentleMACSTM Octo Dissociator (Miltenyi Biotec) at 37°C for 30 min. After enzymatic reaction, collagenase activity was inhibited by adding one volume of Renal Epithelial Growth Medium 2 (PromoCell). To collect tubular cells, the dissociated kidney was centrifuged at 50×g for 5 min. First pellet was resuspended with Renal Epithelial Growth Medium 2 and the supernatant was centrifuged again at 50 × g for 5 min. Second pellet was resuspended in the same medium. First and second pellets were combined and used for analyses.
Quantitative Real-time Polymerase Chain Reaction
Total RNA was isolated from cultured cells and mouse tissues using a Trizol reagent (Ambion, Life Technologies) according to the manufacturer’s instructions. Reverse transcription was carried out from 50 ng of total RNA using a High-Capacity cDNA RT kit (Applied Biosystems, Thermo Fisher Scientific). TaqMan real-time PCR assay was performed to detect Il-6, Cxcl1, and 18s (internal control). TaqMan™ Gene Expression Assay (FAM): 18s (catalog number: 4351368, Assay ID: Hs99999901_s1); Il-6 (catalog number: 4351370, Assay ID: Mm00446190_m1); Cxcl1 (catalog number: 4351370, Assay ID: Mm04207460_m1). For miRNA detection, quantitative PCR was conducted in two-step PCR using TaqMan MicroRNA Assay. First, reverse transcription (RT) was performed from 10 ng total RNA using miR-147 and U6 snoRNA (internal control) primers on a Bio-Rad T100 Thermal Cycler. Second, the RT-PCR product was amplified using TaqMan MicroRNA Assay plus the TaqMan Universal PCR Master Mix on Bio-Rad CFX384 real-time system. The relative expressions of target genes were calculated using 2−∆∆Ct method after normalizing by 18s or U6 snoRNA. TaqMan™ MicroRNA Assay: miR-147 (Catalog #: 4440887, Assay ID: 002262); U6 snoRNA (Catalog #: 4440888, Assay ID: 001973).
Histopathological Analysis
Histological comparison of major organs including the brain, heart, liver, lung, stomach, intestine, spleen, and kidney was performed in 8- to 12-week-old, sex- and age-matched C57BL/6J mice and miR-147−/− mice as previously described [39]. After harvesting, tissues were fixed in 10% formaldehyde for 24 – 48 h and paraffin-embedded. Five-micrometer sections were cut and stained with hematoxylin and eosin. Pictures were taken from the slides using a Leica microscope.
Statistical Analysis
All data included were shown as mean ± standard error (SEM) of the mean. Data following normal distribution and have equal variances were compared using parametric two-sample unpaired t tests. Data that are not normally distributed were analyzed using non-parametric two-sample unpaired t tests using Mann-Whitney rank-sum tests. Comparison of three or more groups was achieved by one-way ANOVA and corrected for multiple comparisons. Detailed information of statistical analysis for each experiment shown was included in the figure legend. Statistical analyses were performed using GraphPad Prism software.
RESULTS
MiR-147 Expression in Different Cell Lines, Primary Cells, and Organs
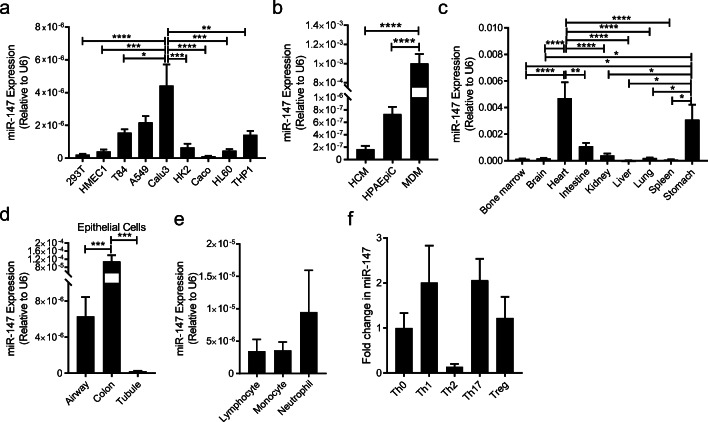
Previous studies have suggested the functional role of miR-147 in many physiological processes including cell cycle regulation and metabolism [25–32]. Here, the expression pattern of miR-147 across different cells and organs is investigated by RT-qPCR. Firstly, we measured the expression level of miR-147 by RT-qPCR in different human cell lines (Fig. 1a) and found relatively high expression levels of miR-147 in T84, A549, and Calu 3 cells. The miR-147 levels in primary cells including human cardiomyocytes (HCM), human monocyte-derived macrophages (MDM), and human pulmonary alveolar epithelial cells (HPAEpiC) are further investigated (Fig. 1b). To study the miR-147 level across different organs, we harvested different organ compartments including the bone marrow, brain, heart, intestine, kidney, liver, lung, spleen, and stomach from C57BL/6J (WT) mice, and measured the miR-147 expression by RT-qPCR. MiR-147 is mostly enriched in the intestine, stomach, and heart tissue (Fig. 1c). Subsequently, different types of epithelial cells were isolated from C57BL/6J mice and higher miR-147 levels were observed in the colon epithelial cells compared to the alveolar epithelial cells and kidney tubule epithelial cells (Fig. 1d). Furthermore, no significant differences in miR-147 expression were observed in several types of blood immune cells including neutrophils, lymphocytes, and monocytes from the peripheral blood of C57BL/6J mice (Fig. 1e). Finally, there are no significant changes in the miR-147 levels across the different T helper differentiation conditions when naïve CD4 T cells isolated from the spleen of C57BL/6J mice are differentiated into T helper 0 (Th0), T helper 1 (Th1), T helper 2 (Th2), T helper17 (Th17), and regulatory T (Treg) cells in vitro (Fig. 1f).
Fig. 1.
MiR-147 expression across different cell lines, tissue, and cells. a Transcript level of miR-147 in various human cell lines (kidney-derived cell lines including HEK293T and HK2; endothelial cell line, HMEC1; colon-derived cell lines including T84, Caco2, and HL60; lung-derived cell lines including A549 and Calu3; peripheral blood–derived cell line, THP1). Expression level was normalized to U6 snoRNA. b Transcript level of miR-147 in human primary cells including human cardiomyocytes (HCM), human pulmonary alveolar epithelial cells (HPAEpiC), and human monocyte-derived macrophages (MDM). Expression level was normalized to U6 snoRNA. c Screening of miR-147 expression in different organs collected from C57BL/6J mice. Expression level was normalized to U6 snoRNA. d Transcript level of miR-147 in murine primary epithelial cells derived from airway, colon, and renal tubules. Expression level was normalized to U6 snoRNA. e Transcript level of miR-147 in immune cells such as lymphocytes, monocytes, and neutrophils isolated from the peripheral blood of C57BL/6J mice. Expression level was normalized to U6 snoRNA. f Transcript level of miR-147 in Th0, Th1, Th2, Th17, and Treg cells differentiated from naïve CD4 T cells of C57BL/6J mice. Data was normalized to Th0. All graphs represent mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 by one-way ANOVA with Bonferroni’s multiple comparisons.
The Expression Level of MiR-147 After CLP
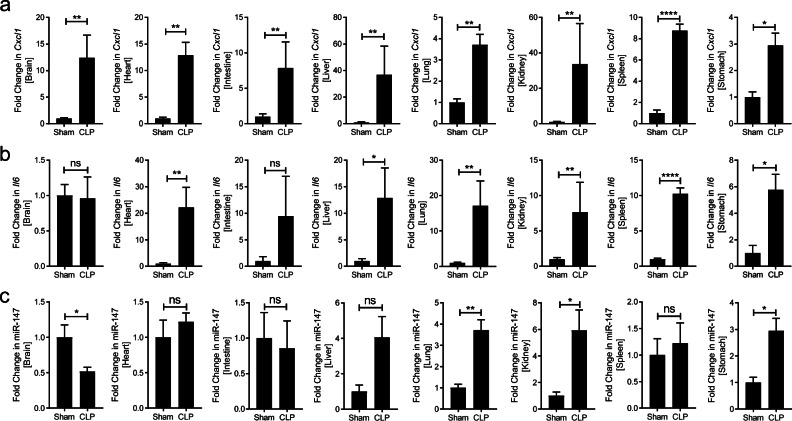
After having shown the expression level of miR-147 under baseline conditions across different organs, we next investigated the expression of miR-147 under systemic inflammatory conditions. To achieve systemic organ inflammation, CLP or sham procedure was performed in 8- to 10-week-old C57BL/6J mice and major organs including the brain, heart, intestine, kidney, liver, lung, spleen, and stomach were harvest 24 h after CLP. Firstly, to understand the inflammatory conditions in each organ, the expression level of Cxcl1 in these organs was assessed by RT-qPCR in the CLP and sham group and significant induction of Cxcl1 was found in all of the organs (Fig. 2a). In addition, previous studies have indicated an upregulation of Il6 in the CLP models. Thus, we compared the expression level of Il6 in these organs by RT-qPCR in the CLP and sham group and found significant induction of these Il6 in the majority of the organs except the brain and intestine (Fig. 2b). These results indicate profound multi-organ inflammation in the CLP group. Finally, the expression pattern of miR-147 in inflamed organs was further measured by RT-qPCR. Surprisingly, miR-147 is induced in the lung, kidney, and stomach while decreased in the brain following the CLP procedure when compared to the sham group (Fig. 2c). Taken together, these studies indicated strong systemic inflammation in all major organs following CLP, and miR-147 is selectively upregulated in the lung, kidney, and stomach while downregulated in the brain.
Fig. 2.
Organ inflammation and miR-147 levels after CLP. CLP was conducted on 8- to 12-week-old C57BL/6J mice, and the indicating organs were collected in 24 h. a, b Induction of inflammatory genes including Cxcl1 and Il6 in CLP group. c Relative transcript level of miR-147 in CLP group compared to sham group. All graphs are presented as mean ± SEM. *P < 0.05, **P < 0.01, ****P < 0.0001 relative to sham, by Mann-Whitney test.
Strategy for the Generation of MiR-147 Floxed Mice
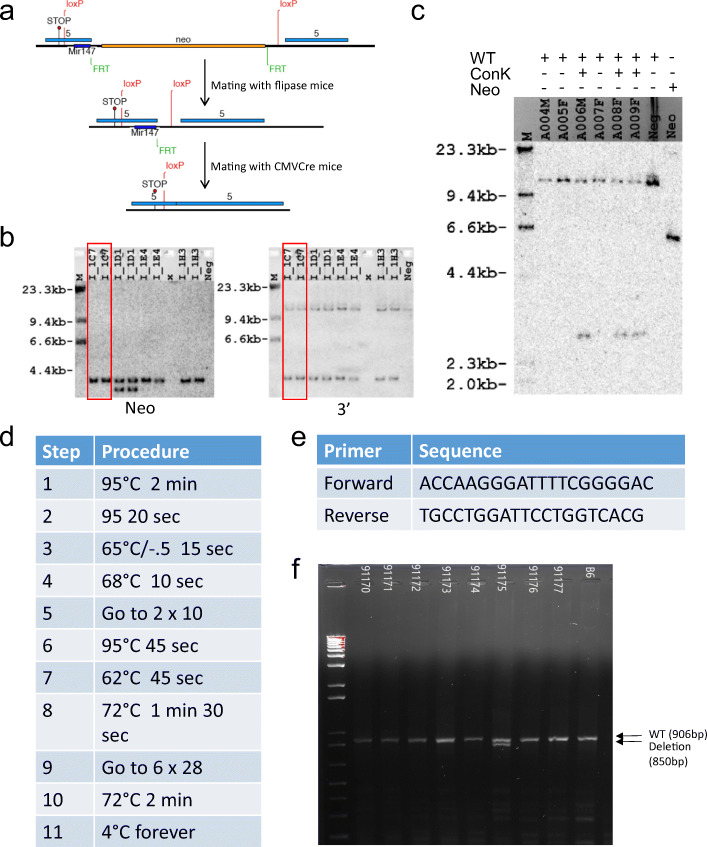
To address the function of miR-147 during organ injury, we generated a novel transgenic mouse line with a floxed miR-147 gene. Utilizing the Cre-loxP system, these mice allow us to generate mice with germline deletion of miR-147 (miR-147−/−) mice. Gene targeting of miR-147 conditional knockout was first established in Bruce4 C57BL/6 embryonic stem (ES) cells. To generate miR-147 conditional mice, a 114-bp region encompassing the miR-147 gene was floxed with loxP sites (Fig. 3a). An FRT-flanked neo cassette was also inserted within the floxed region for targeted ES cell selection by neomycin. Correctly targeted ES cells were confirmed by Southern blotting with Neo and 3′ probes (Fig. 3b). Subsequently, one of the clones with correct targeting (I-IC7) was selected for the generation of chimera mice. Breeding of chimera mice to flipase (flp) mice resulted in flp-mediated recombination at the FRT sites to delete the neo cassette. Litter from the mating of chimera and flipase mice was confirmed by Southern blot (Fig. 3c). These mice have been crossbred with CMVcre for the generation of miR-147 −/− mice. miR-147 −/− allele was confirmed by genotyping on DNA isolated from tail snip. The primer sequence and PCR protocol are shown in Fig. 3 d and e. From the representative gel picture shown in Fig. 3f, the deletion band was successfully detected in the animals. Heterozygous mice carrying miR-147 deletion allele were further bred to generate homozygous miR-147−/− mice.
Fig. 3.
Strategy for the generation of miR-147 floxed mice and miR-147−/− mice. a Schematic illustration for the generation of miR-147 conditional knockout mice. b Southern blotting with Neo and 3′ probes to confirm targeted ES cells. I-IC7 (lane 1, 2) was selected for chimera generation. c Southern blot data of DNA extracted from the litter of chimera and flipase mice. d PCR conditions for genotyping of miR-147 −/− mice. e Primer sequences are complementary to miR-147 allele. f Gel image from PCR-based genotyping to detect deletion of miR-147 allele.
Confirmation of MiR-147 Knockout and Breeding Characteristics of miR-147−/− Mice
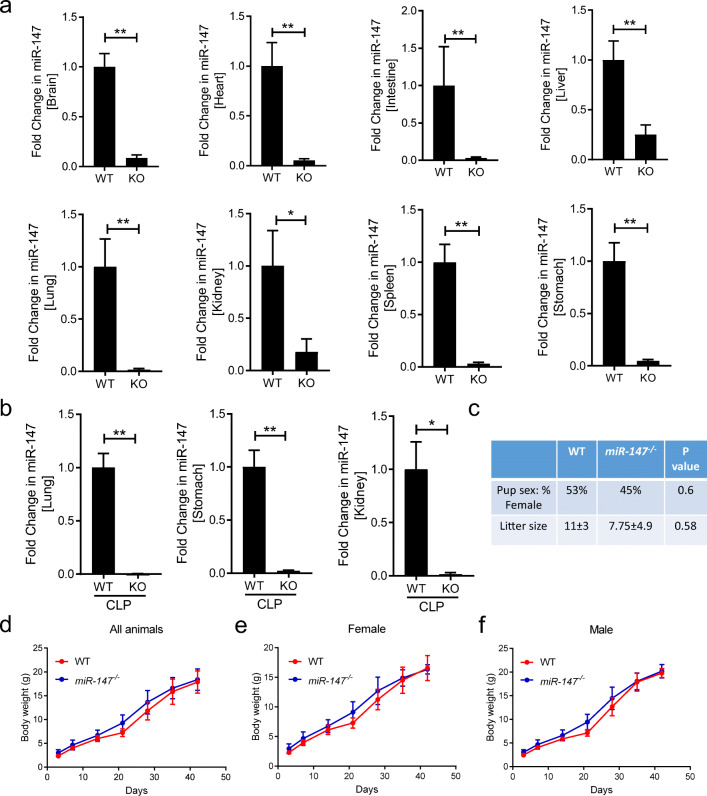
After the identification of miR-147 −/− mice, we pursue to further confirm the knockout of miR-147 under inflammatory conditions and define the breeding characteristics. Firstly, to confirm the knockout of miR-147, several major organs including the brain, heart, intestine, kidney, liver, lung, spleen, and stomach were harvested from WT or miR-147 −/− mice under baseline condition. The expression level of miR-147 was assessed by RT-qPCR and the result indicated that miR-147 is successfully deleted in all major organs (Fig. 4a). To investigate the knockout efficiency of miR-147 under inflammatory conditions, WT or miR-147 −/− mice were exposed to CLP. Tissues showing significant upregulation of miR-147 during CLP, including the lung, kidney, and stomach, were harvest 24 h later for the analysis of miR-147 expression. miR-147 −/− mice showed complete abolishment of miR-147 levels compared to WT mice (Fig. 4b), indicating successful knockout of miR-147 during inflammatory conditions. Next, the reproductive performance and growth curves were assessed in miR-147 −/− mice. A total of 30 mice in the WT group and 29 mice in the miR-147 −/− were born in the study period. There are no significant differences in litter size and gender ratio (Fig. 4c). The weight of each mouse from the WT and miR-147 −/− groups was recorded for the generation of weight curve. There is no significant difference in weight gain comparing WT and miR-147 as indicated in the growth curve (Fig. 4d). Furthermore, when separated based on gender, the weight curves are similar between WT and miR-147 −/− mice (Fig. 4e, f). Overall, the miR-147-/- mice show a successful knockout of miR-147 under inflammatory conditions and the breeding or growth of miR-147 −/− mice has similar characteristics compared to WT animals.
Fig. 4.
Confirmation of miR-147 KO in various tissues and breeding characteristics and growth curve of miR-147−/− (KO) mice. a Basal transcript level of miR-147 in the brain, heart, intestine, liver, lung, kidney, spleen, and stomach from C57BL/6J (WT) and miR-147−/− mice (KO) (n = 6/group). b Transcript level of miR-147 in the lung, stomach, and kidney from WT and miR-147−/− mice following CLP procedure (n = 6 for WT; n = 5 for KO mice). c Comparison of gender ratio and average litter size from WT and KO mice that were used for the present study. P value from Fisher’s exact test and unpaired t test, respectively. d Growth curve based on body weight of all animals from WT and KO mice over 42 days. e Growth curve based on body weight of females from WT and KO mice. f Growth curve based on body weight of males from WT and KO mice. Graphs for mRNA expression represent mean ± SEM and growth curves represent mean ± SD. *P < 0.05, **P < 0.01 relative to WT, by Mann-Whitney test.
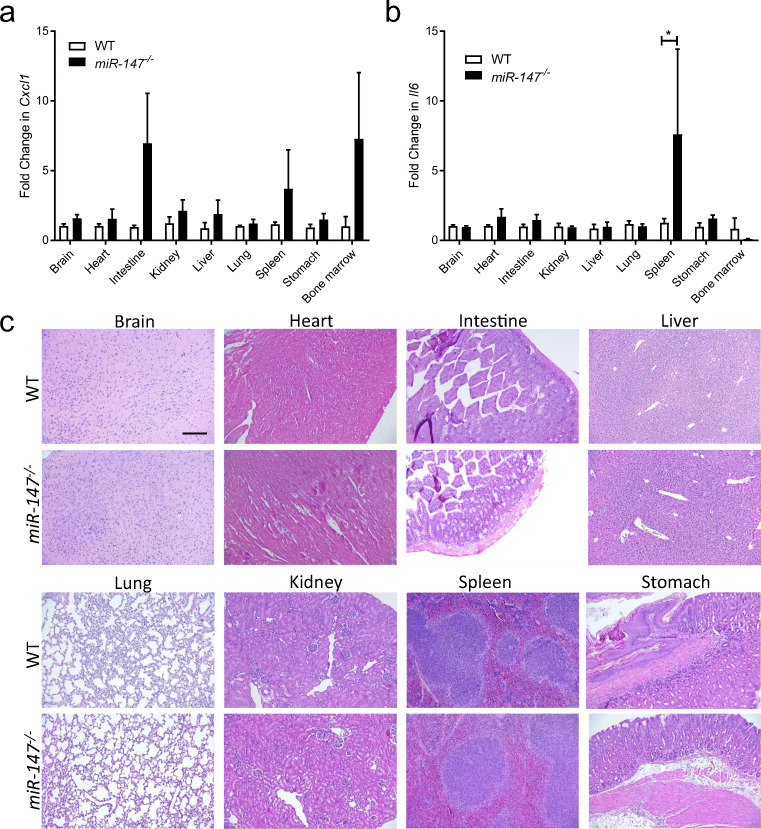
Baseline Inflammation in miR-147 −/− Mice
After confirming the successful knockout of miR-147 in several organs following CLP, we next investigated the baseline inflammation in major organs and tissues to further characterize miR-147 −/− mice. For this purpose, we harvested organs and tissues including the bone marrow, brain, heart, intestine, kidney, liver, lung, spleen, and stomach, from age- and gender-matched WT or miR-147 −/− mice and measured the expression level of Cxcl1 and Il6 by RT-qPCR. Statistically significant differences were not detected in the expression level of Cxcl1 in different organs or tissues when comparing WT and miR-147 −/− mice (Fig. 5a). However, the Il6 level is upregulated in the spleen from miR-147 −/− mice compared to that from WT mice, suggesting potential increases in the baseline inflammation in the spleen resulted from miR-147 deletion (Fig. 5b). Furthermore, we performed histological analysis on major organs including the brain, heart, intestine, kidney, liver, lung, spleen, and stomach, from age- and gender-matched WT or miR-147 −/− mice by H&E staining. From the histological analysis, we did not observe significant abnormality in miR-147 −/− mice when compared to WT animals (Fig. 5c). Taken together, these studies suggest that miR-147 −/− mice have comparable baseline inflammation and histological characteristics in major organs with WT animals, except for an elevated Il6 expression in the spleen.
Fig. 5.
Characterization of miR-147−/− mice. a, b Baseline mRNA expression level of inflammatory genes including Cxcl1 and Il6 in various organs isolated from WT and miR-147−/− mice (KO). c Representative images of H&E staining from mouse tissues including the brain, heart, intestine, liver, lung, kidney, spleen, and stomach to compare histology of WT and miR-147−/− mice (scale bar: 200 μm). All graphs represent mean ± SEM. *P < 0.05 (n = 6 mice/group) relative to WT, by two-way ANOVA with Bonferroni’s multiple comparisons.
DISCUSSION
The present study aimed at establishing transgenic animal models to study miR-147 in organ injury. Previous studies had indicated a crucial role of miR-147 in key biological processes such as cell cycle regulation and inflammatory response. Thus, we investigated miR-147 expression levels in different cell lines, different organs, and different immune cell populations under baseline conditions. Following organ injury achieved by CLP, we observed systemic inflammation across all examined organs and miR-147 upregulation in the lung, stomach, and kidney. Furthermore, to address the function of miR-147 during organ injury, we generated a novel transgenic mouse line with a floxed miR-147 gene and crossed the mice with CMVcre for germline deletion of miR-147. The successful deletion of miR-147 was confirmed as miR-147−/− mice showed completely abolished miR-147 expression under inflammatory conditions. Next, we assessed the reproductive performance and growth curves of the miR-147−/− mice and observed no significant difference in weight gain between WT and miR-147−/− mice. After confirming the successful knockout of miR-147 in several organs following CLP, we next investigated the baseline inflammation in major organs and tissues using RT-qPCR and histological analysis. Results from these analyses suggest that miR-147−/− mice have baseline inflammation and histological characteristics in major organs comparable with WT animals, except an elevated Il6 expression in the spleen. Taken together, these studies confirmed the successful generation of transgenic animals to study the role of miR-147 in organ injury.
MicroRNAs are studied in vivo by several different strategies. First and foremost, pharmacological overexpression and inhibition of microRNAs are essential tools for the therapeutic targeting of microRNAs [21]. For instance, delivery of microRNA mimic results in the overexpression of target microRNA and downregulation of the target genes. On the other hand, microRNA inhibition could be achieved by treatment of LNA or antagomirs. Expression of microRNAs in vivo could be achieved by microRNAScope, which will localize and visualize mature microRNAs on tissue slides. Transgenic animal models are instrumental for studying the functional role of microRNAs in vivo, especially models with conditional potential for cell- or tissue-specific manipulation of microRNAs [40]. Transgenic mice could be generated with the injection of a transgene into the fertilized eggs and including an exogenous promoter would facilitate the constitutive or tissue-specific overexpression [41]. Constitutive or conditional knockout of microRNAs could be achieved by gene-targeting vectors using several systems including Cre-loxP system [42] and flp-FRT system [43]. Moreover, several systems have been established for inducible modification of microRNAs that could also have the potential for inducible knockout or overexpression, including the tetracycline (Tet)-inducible system [44] and the Cre-ER(T) system [45]. Combining the inducible system with the conditional system results in powerful tools to study the temporal and tissue-specific functional role of microRNAs.
Inflammation is commonly observed in many pathogenic conditions including infection, acute organ injury, and chronic organ dysfunctions [46–54]. Initially intended as a response to help resolve tissue injury, inflammation could become excessive and uncontrolled, which leads to further tissue injury and, occasionally, systemic inflammation [55–59]. For example, uncontrolled alveolar epithelial inflammation results in ARDS [1, 16, 60, 61], which is the common and main cause of death in COVID-19 [62, 63]. Several previous studies have suggested the importance of endogenous anti-inflammatory pathways for the control of organ inflammation. These pathways include the hypoxia-inducible factor signaling pathway [61, 64, 65], purinergic signaling pathway [66–75], microRNAs [19, 20, 76–78], resolvins [79–81], and many others [82–90]. Along these lines, those pathways not only facilitate tissue protection during pulmonary injuries but also contribute to other inflammatory organ conditions such as myocardial infarction [91–98], kidney injury [99–101], and inflammatory bowel diseases [102–108]. Thus far, the majority of the studies on miR-147 focuses on the regulation of different biological processes such as cell proliferation and drug tolerance in cancer cells [29, 31, 109–112]. Additional studies indicated that miR-147 is crucial for the mechanical stretch-induced apoptosis in myoblast [30]. Moreover, a recent study suggested that miR-147 is reduced during rat myocardial infarction models and overexpression of miR-147 provides cardiac protection [113]. Taken together, miR-147loxP/loxP mice could facility the study of miR-147 in different organs and cell types during inflammatory organ injury by breeding with different Cre recombinase transgenic mice.
The functional role of miR-147 in inflammation has also been indicated by several studies. For instance, a study from Liu et al. demonstrated that miR-147 overexpression in macrophages could dampen TLR activation–induced cytokine production in vitro [26]. Furthermore, a recent study demonstrated that virulent factor from the Mycobacterium marinum downregulated miR-147 levels and overexpression of miR-147 dampened Mycobacterium marinum–induced cytokine production in murine macrophage cell lines in vitro [28]. These studies imply the functional role of miR-147 during infection and inflammatory stimulations in macrophages, and potentially other myeloid cell populations. Our successful generation of floxed miR-147 transgenic mice will facilitate the study of cell-specific function in vivo. Several Cre recombinase mouse lines have been developed to target different populations of macrophages and other myeloid cells [114, 115]. However, most of these Cre recombinase transgenic mice, including LysM-Cre, Csf1r-Cre, CD11b-Cre, F4/80-Cre, and CX3CR1-Cre, have limited ability in the specific targeting of a certain myeloid cell population. Recent studies suggested that hCD68-rtTA transgenic system could facilitate the selective and inducible targeting of CD11b+ macrophages, including pulmonary recruited and interstitial macrophages [116–118]. Crossbreeding of miR-147loxP/loxP mice with hCD68-rtTA and Teto-Cre mice will facilitate the functional study of miR-147 in macrophages in vivo.
CONCLUSION
In this study, we have generated a transgenic mouse line with a floxed miR-147 gene (miR-147loxP/loxP) and crossing the miR-147loxP/loxP mice with CMVcre mice successfully generated mice with germline deletion of miR-147 (miR-147−/−). Firstly, we demonstrate the successful deletion of the target gene in different organs under baseline or inflammatory conditions in organs that showed induction of miR-147 during inflammation. Furthermore, we show that miR-147 −/− mice experience normal growth, development, and off-spring. Moreover, miR-147 −/− mice have identical baseline inflammatory gene expression compared to C57BL/6 mice, except elevated IL-6 expression in the spleen (7.5 fold, p < 0.05). Taken together, our data show that we have successfully developed a transgenic animal model for tissue- and cell-specific deletion of miR-147 that can be used to study the functional role of miR-147 during inflammation.
Acknowledgements
We would like to acknowledge Yanyu Wang, Ph.D., for critically reviewing the data presented in this manuscript.
Author Contribution
B. Kim designed and performed the experiments, analyzed the data, and drafted the manuscript. V. Guaregua, X. Chen, C. Zhao, W. Yeow, and N. K. Berg performed the experiments and analyzed the data. H. K. Eltzschig edited the manuscript and provided critical advice on the study. X. Yuan designed and performed the experiments, analyzed the data, and drafted and finalized the manuscript.
Funding
This work is supported by the American Thoracic Society Unrestricted Grant, American Heart Association Career Development Award (19CDA34660279), American Lung Association Catalyst Award (CA-622265), the Center for Clinical and Translational Sciences, McGovern Medical School Pilot Award (1UL1TR003167–01), and Parker B. Francis Fellowship (to X. Yuan); and National Institutes of Health (Bethesda, Maryland) Grant Nos. R01HL154720, R01DK122796, R01DK109574, R01HL133900 and Department of Defense Grant No: W81XWH2110032 (to H. K. Eltzschig).
Data Availability
The data that support this study are available upon request.
Declarations
Ethics Approval
All included studies have approval from the IACUC or CPHS committee at the University of Texas Health Science Center at Houston.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Williams GW, Berg NK, Reskallah A, Yuan X, Eltzschig HK. Acute respiratory distress syndrome. Anesthesiology. 2020;134(2):270–282. doi: 10.1097/ALN.0000000000003571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaramillo-Rocha V. Acute respiratory distress syndrome. The New England Journal of Medicine. 2017;377(19):1903–1904. doi: 10.1056/NEJMc1711824. [DOI] [PubMed] [Google Scholar]
- 3.Blanch L, Fernandez R, Mancebo J. Acute respiratory distress syndrome. The New England Journal of Medicine. 1995;332(24):1649. [PubMed] [Google Scholar]
- 4.Eckle T, Eltzschig HK. Toll-like receptor signaling during myocardial ischemia. Anesthesiology. 2011;114(3):490–492. doi: 10.1097/ALN.0b013e31820a4d78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia S, Lin H, Liu H, Lu Z, Wang H, Fan S, Li N. Honokiol attenuates sepsis-associated acute kidney injury via the inhibition of oxidative stress and inflammation. Inflammation. 2019;42(3):826–834. doi: 10.1007/s10753-018-0937-x. [DOI] [PubMed] [Google Scholar]
- 6.Carney EF. Acute kidney injury: proximal tubule cells modulate inflammation after renal injury. Nature Reviews. Nephrology. 2015;11(5):254. doi: 10.1038/nrneph.2015.40. [DOI] [PubMed] [Google Scholar]
- 7.Dirkes S. Sepsis and inflammation: impact on acute kidney injury. Nature Reviews Nephrology. 2013;40(2):125–132. [PubMed] [Google Scholar]
- 8.Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nature Reviews. Disease Primers. 2016;2:16045. doi: 10.1038/nrdp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420(6917):885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 10.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 11.Lelubre C, Vincent JL. Mechanisms and treatment of organ failure in sepsis. Nature Reviews Nephrology. 2018;14(7):417–427. doi: 10.1038/s41581-018-0005-7. [DOI] [PubMed] [Google Scholar]
- 12.Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nature Reviews Drug Discovery. 2005;4(10):854–865. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- 13.Toscano MG, Ganea D, Gamero AM. Cecal ligation puncture procedure. Journal of Visualized Experiments. 2011;51:2860. doi: 10.3791/2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neudecker V, Yuan X, Bowser JL, Eltzschig HK. MicroRNAs in mucosal inflammation. Journal of Molecular Medicine (Berlin, Germany) 2017;95(9):935–949. doi: 10.1007/s00109-017-1568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee LK, Medzikovic L, Eghbali M, Eltzschig HK, Yuan X. The role of microRNAs in acute respiratory distress syndrome and sepsis, from targets to therapies: a narrative review. Anesthesia and Analgesia. 2020;131:1471–1484. doi: 10.1213/ANE.0000000000005146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu W, You R, Yuan X, Yang T, Samuel EL, Marcano DC, Sikkema WK, Tour JM, Rodriguez A, Kheradmand F, et al. The microRNA miR-22 inhibits the histone deacetylase HDAC4 to promote T(H)17 cell-dependent emphysema. Nature Immunology. 2015;16(11):1185–1194. doi: 10.1038/ni.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cata JP, Gorur A, Yuan X, Berg NK, Sood AK, Eltzschig HK. Role of micro-RNA for pain after surgery: narrative review of animal and human studies. Anesthesia and Analgesia. 2020;130(6):1638–1652. doi: 10.1213/ANE.0000000000004767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan X, Berg N, Lee JW, Le TT, Neudecker V, Jing N, Eltzschig H. MicroRNA miR-223 as regulator of innate immunity. Journal of Leukocyte Biology. 2018;104(3):515–524. doi: 10.1002/JLB.3MR0218-079R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neudecker V, Brodsky KS, Clambey ET, Schmidt EP, Packard TA, Davenport B, Standiford TJ, Weng T, Fletcher AA, Barthel L, et al. Neutrophil transfer of miR-223 to lung epithelial cells dampens acute lung injury in mice. Science Translational Medicine. 2017;9(408):eaah5360. doi: 10.1126/scitranslmed.aah5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee TJ, Yuan X, Kerr K, Yoo JY, Kim DH, Kaur B, Eltzschig HK. Strategies to modulate microRNA functions for the treatment of cancer or organ injury. Pharmacological Reviews. 2020;72(3):639–667. doi: 10.1124/pr.119.019026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309(5740):1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 23.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y, et al. Treatment of HCV infection by targeting microRNA. The New England Journal of Medicine. 2013;368(18):1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 24.Bandiera S, Pfeffer S, Baumert TF. Zeisel MB: miR-122--a key factor and therapeutic target in liver disease. Journal of Hepatology. 2015;62(2):448–457. doi: 10.1016/j.jhep.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Zhang WC, Wells JM, Chow KH, Huang H, Yuan M, Saxena T, Melnick MA, Politi K, Asara JM, Costa DB, Bult CJ, Slack FJ. miR-147b-mediated TCA cycle dysfunction and pseudohypoxia initiate drug tolerance to EGFR inhibitors in lung adenocarcinoma. Nature Metabolism. 2019;1(4):460–474. doi: 10.1038/s42255-019-0052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu G, Friggeri A, Yang Y, Park YJ, Tsuruta Y. Abraham E: miR-147, a microRNA that is induced upon Toll-like receptor stimulation, regulates murine macrophage inflammatory responses. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(37):15819–15824. doi: 10.1073/pnas.0901216106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee CG, McCarthy S, Gruidl M, Timme C, Yeatman TJ. MicroRNA-147 induces a mesenchymal-to-epithelial transition (MET) and reverses EGFR inhibitor resistance. PLoS One. 2014;9(1):e84597. doi: 10.1371/journal.pone.0084597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuo X, Wang L, Bao Y, Sun J. The ESX-1 virulence factors downregulate mir-147-3p in Mycobacterium marinum-infected macrophages. Infection and Immunity. 2020;88(6):e00088–e00020. doi: 10.1128/IAI.00088-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Zhang HE, Liu Z. MicroRNA-147 suppresses proliferation, invasion and migration through the AKT/mTOR signaling pathway in breast cancer. Oncology Letters. 2016;11(1):405–410. doi: 10.3892/ol.2015.3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du Y, Yang F, Lv D, Zhang Q, Yuan X. MiR-147 inhibits cyclic mechanical stretch-induced apoptosis in L6 myoblasts via ameliorating endoplasmic reticulum stress by targeting BRMS1. Cell Stress & Chaperones. 2019;24(6):1151–1161. doi: 10.1007/s12192-019-01037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang E, Liu Q, Wang Y, Wang H, He L, Jin X, Li N. MicroRNA miR-147b promotes tumor growth via targeting UBE2N in hepatocellular carcinoma. Oncotarget. 2017;8(69):114072–114080. doi: 10.18632/oncotarget.23120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chu G, Zhang J, Chen X. Serum level of microRNA-147 as diagnostic biomarker in human non-small cell lung cancer. Journal of Drug Targeting. 2016;24(7):613–617. doi: 10.3109/1061186X.2015.1116539. [DOI] [PubMed] [Google Scholar]
- 33.Kelly A, Grabiec AM, Travis MA. Culture of human monocyte-derived macrophages. Methods in Molecular Biology. 2018;1784:1–11. doi: 10.1007/978-1-4939-7837-3_1. [DOI] [PubMed] [Google Scholar]
- 34.Flaherty S, Reynolds JM. Mouse naive CD4+ T cell isolation and in vitro differentiation into T cell subsets. Journal of Visualized Experiments. 2015;98:52739. doi: 10.3791/52739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim JF, Berger H, Su IH. Isolation and activation of murine lymphocytes. Journal of Visualized Experiments. 2016;116:54596. doi: 10.3791/54596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mercer PF, Johns RH, Scotton CJ, Krupiczojc MA, Konigshoff M, Howell DC, McAnulty RJ, Das A, Thorley AJ, Tetley TD, et al. Pulmonary epithelium is a prominent source of proteinase-activated receptor-1-inducible CCL2 in pulmonary fibrosis. American Journal of Respiratory and Critical Care Medicine. 2009;179(5):414–425. doi: 10.1164/rccm.200712-1827OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frey MR, Edelblum KL, Mullane MT, Liang D, Polk DB. The ErbB4 growth factor receptor is required for colon epithelial cell survival in the presence of TNF. Gastroenterology. 2009;136(1):217–226. doi: 10.1053/j.gastro.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding W, Yousefi K, Shehadeh LA. Isolation, characterization, and high throughput extracellular flux analysis of mouse primary renal tubular epithelial cells. Journal of Visualized Experiments. 2018;136:57718. doi: 10.3791/57718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang D, Cui Y, Li B, Luo X, Li B, Tang Y. A comparative study of the characterization of miR-155 in knockout mice. PLoS One. 2017;12(3):e0173487. doi: 10.1371/journal.pone.0173487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pal AS, Kasinski AL. Animal models to study microRNA function. Advances in Cancer Research. 2017;135:53–118. doi: 10.1016/bs.acr.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanahan D, Wagner EF, Palmiter RD. The origins of oncomice: a history of the first transgenic mice genetically engineered to develop cancer. Genes & Development. 2007;21(18):2258–2270. doi: 10.1101/gad.1583307. [DOI] [PubMed] [Google Scholar]
- 42.Xiong F, Wei ZQ, Zhu ZY, Sun YH. Targeted expression in zebrafish primordial germ cells by Cre/loxP and Gal4/UAS systems. Marine Biotechnology. 2013;15(5):526–539. doi: 10.1007/s10126-013-9505-4. [DOI] [PubMed] [Google Scholar]
- 43.Deng CX. Conditional knockout mouse models of cancer. Cold Spring Harbor Protocols. 2014;2014(12):1217–1233. doi: 10.1101/pdb.top074393. [DOI] [PubMed] [Google Scholar]
- 44.Das AT, Tenenbaum L, Berkhout B. Tet-on systems for doxycycline-inducible gene expression. Current Gene Therapy. 2016;16(3):156–167. doi: 10.2174/1566523216666160524144041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim H, Kim M, Im SK, Fang S. Mouse Cre-LoxP system: general principles to determine tissue-specific roles of target genes. Laboratory Animal Research. 2018;34(4):147–159. doi: 10.5625/lar.2018.34.4.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan X, Chang CY, You R, Shan M, Gu BH, Madison MC, Diehl G, Perusich S, Song LZ, Cornwell L, et al. Cigarette smoke-induced reduction of C1q promotes emphysema. JCI Insight. 2019;5(13):e124317. doi: 10.1172/jci.insight.124317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poth JM, Brodsky K, Ehrentraut H, Grenz A, Eltzschig HK. Transcriptional control of adenosine signaling by hypoxia-inducible transcription factors during ischemic or inflammatory disease. Journal of Molecular Medicine (Berlin, Germany) 2013;91(2):183–193. doi: 10.1007/s00109-012-0988-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Millien VO, Lu W, Mak G, Yuan X, Knight JM, Porter P, Kheradmand F, Corry DB. Airway fibrinogenolysis and the initiation of allergic inflammation. Annals of the American Thoracic Society. 2014;11(Suppl 5):S277–S283. doi: 10.1513/AnnalsATS.201403-105AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le TT, Berg NK, Harting MT, Li X, Eltzschig HK, Yuan X. Purinergic signaling in pulmonary inflammation. Frontiers in Immunology. 2019;10:1633. doi: 10.3389/fimmu.2019.01633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hong MJ, Gu BH, Madison MC, Landers C, Tung HY, Kim M, Yuan X, You R, Machado AA, Gilbert BE, Soroosh P, Elloso M, Song L, Chen M, Corry DB, Diehl G, Kheradmand F. Protective role of gammadelta T cells in cigarette smoke and influenza infection. Mucosal Immunology. 2018;11(3):894–908. doi: 10.1038/mi.2017.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu F, Huang JH, Yuan XY, Huang WS, Chen YH. Characterization of immunity induced by M2e of influenza virus. Vaccine. 2007;25(52):8868–8873. doi: 10.1016/j.vaccine.2007.09.056. [DOI] [PubMed] [Google Scholar]
- 52.Wu F, Yuan XY, Huang WS, Chen YH. Heterosubtypic protection conferred by combined vaccination with M2e peptide and split influenza vaccine. Vaccine. 2009;27(43):6095–6101. doi: 10.1016/j.vaccine.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 53.Wu F, Yuan XY, Li J, Chen YH. The co-administration of CpG-ODN influenced protective activity of influenza M2e vaccine. Vaccine. 2009;27(32):4320–4324. doi: 10.1016/j.vaccine.2009.04.075. [DOI] [PubMed] [Google Scholar]
- 54.Shan M, Yuan X, Song LZ, Roberts L, Zarinkamar N, Seryshev A, Zhang Y, Hilsenbeck S, Chang SH, Dong C, et al. Cigarette smoke induction of osteopontin (SPP1) mediates T(H)17 inflammation in human and experimental emphysema. Science Translational Medicine. 2012;4(117):117ra119. doi: 10.1126/scitranslmed.3003041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhavani S, Yuan X, You R, Shan M, Corry D, Kheradmand F. Loss of peripheral tolerance in emphysema. phenotypes, exacerbations, and disease progression. Annals of the American Thoracic Society. 2015;12(Suppl 2):S164–S168. doi: 10.1513/AnnalsATS.201503-115AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, Herridge M, Randolph AG, Calfee CS. Acute respiratory distress syndrome. Nature Reviews. Disease Primers. 2019;5(1):18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 58.Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. The New England Journal of Medicine. 2012;367(24):2322–2333. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vuerich M, Mukherjee S, Robson SC, Longhi MS. Control of gut inflammation by modulation of purinergic signaling. Frontiers in Immunology. 2020;11:1882. doi: 10.3389/fimmu.2020.01882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ben Salem C. Acute respiratory distress syndrome. The New England Journal of Medicine. 2017;377(19):1904. doi: 10.1056/NEJMc1711824. [DOI] [PubMed] [Google Scholar]
- 61.Yuan X, Lee JW, Bowser JL, Neudecker V, Sridhar S, Eltzschig HK. Targeting hypoxia signaling for perioperative organ injury. Anesthesia and Analgesia. 2018;126(1):308–321. doi: 10.1213/ANE.0000000000002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Briggs FBS, Gianfrancesco MA, George MF. More on Covid-19 in immune-mediated inflammatory diseases. The New England Journal of Medicine. 2020;383(8):796–797. doi: 10.1056/NEJMc2018011. [DOI] [PubMed] [Google Scholar]
- 63.Cao X. COVID-19: immunopathology and its implications for therapy. Nature Reviews. Immunology. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vohwinkel CU, Hoegl S, Eltzschig HK. Hypoxia signaling during acute lung injury. Journal of Applied Physiology (Bethesda, MD: 1985) 2015;119(10):1157–1163. doi: 10.1152/japplphysiol.00226.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eckle T, Brodsky K, Bonney M, Packard T, Han J, Borchers CH, Mariani TJ, Kominsky DJ, Mittelbronn M, Eltzschig HK. HIF1A reduces acute lung injury by optimizing carbohydrate metabolism in the alveolar epithelium. PLoS Biology. 2013;11(9):e1001665. doi: 10.1371/journal.pbio.1001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ehrentraut H, Clambey ET, McNamee EN, Brodsky KS, Ehrentraut SF, Poth JM, Riegel AK, Westrich JA, Colgan SP, Eltzschig HK. CD73+ regulatory T cells contribute to adenosine-mediated resolution of acute lung injury. The FASEB Journal. 2013;27(6):2207–2219. doi: 10.1096/fj.12-225201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eckle T, Kewley EM, Brodsky KS, Tak E, Bonney S, Gobel M, Anderson D, Glover LE, Riegel AK, Colgan SP, Eltzschig HK. Identification of hypoxia-inducible factor HIF-1A as transcriptional regulator of the A2B adenosine receptor during acute lung injury. Journal of Immunology. 2014;192(3):1249–1256. doi: 10.4049/jimmunol.1100593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. The Journal of Clinical Investigation. 2008;118(10):3301–3315. doi: 10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schingnitz U, Hartmann K, Macmanus CF, Eckle T, Zug S, Colgan SP, Eltzschig HK. Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. Journal of Immunology. 2010;184(9):5271–5279. doi: 10.4049/jimmunol.0903035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Belikoff BG, Vaickus LJ, Sitkovsky M, Remick DG. A2B adenosine receptor expression by myeloid cells is proinflammatory in murine allergic-airway inflammation. Journal of Immunology. 2012;189(7):3707–3713. doi: 10.4049/jimmunol.1201207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Antonioli L, Blandizzi C, Pacher P, Hasko G. The purinergic system as a pharmacological target for the treatment of immune-mediated inflammatory diseases. Pharmacological Reviews. 2019;71(3):345–382. doi: 10.1124/pr.117.014878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Antonioli L, Pacher P, Vizi ES, Hasko G. CD39 and CD73 in immunity and inflammation. Trends in Molecular Medicine. 2013;19(6):355–367. doi: 10.1016/j.molmed.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hasko G, Cronstein B. Regulation of inflammation by adenosine. Frontiers in Immunology. 2013;4:85. doi: 10.3389/fimmu.2013.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends in Immunology. 2004;25(1):33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 75.He W, Cronstein BN. Adenosine A1 receptor regulates osteoclast formation by altering TRAF6/TAK1 signaling. Purinergic Signal. 2012;8(2):327–337. doi: 10.1007/s11302-012-9292-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu XM, Ji KQ, Wang HY, Zhao Y, Jia J, Gao XP, Zang B. MicroRNA-339-3p alleviates inflammation and edema and suppresses pulmonary microvascular endothelial cell apoptosis in mice with severe acute pancreatitis-associated acute lung injury by regulating Anxa3 via the Akt/mTOR signaling pathway. Journal of Cellular Biochemistry. 2018;119(8):6704–6714. doi: 10.1002/jcb.26859. [DOI] [PubMed] [Google Scholar]
- 77.Xie T, Liang J, Liu N, Wang Q, Li Y, Noble PW, Jiang D. MicroRNA-127 inhibits lung inflammation by targeting IgG Fcgamma receptor I. Journal of Immunology. 2012;188(5):2437–2444. doi: 10.4049/jimmunol.1101070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xue H, Li MX. MicroRNA-150 protects against cigarette smoke-induced lung inflammation and airway epithelial cell apoptosis through repressing p53: MicroRNA-150 in CS-induced lung inflammation. Human & Experimental Toxicology. 2018;37(9):920–928. doi: 10.1177/0960327117741749. [DOI] [PubMed] [Google Scholar]
- 79.Croasdell A, Thatcher TH, Kottmann RM, Colas RA, Dalli J, Serhan CN, Sime PJ, Phipps RP. Resolvins attenuate inflammation and promote resolution in cigarette smoke-exposed human macrophages. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2015;309(8):L888–L901. doi: 10.1152/ajplung.00125.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abdulnour RE, Sham HP, Douda DN, Colas RA, Dalli J, Bai Y, Ai X, Serhan CN, Levy BD. Aspirin-triggered resolvin D1 is produced during self-resolving gram-negative bacterial pneumonia and regulates host immune responses for the resolution of lung inflammation. Mucosal Immunology. 2016;9(5):1278–1287. doi: 10.1038/mi.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hsiao HM, Sapinoro RE, Thatcher TH, Croasdell A, Levy EP, Fulton RA, Olsen KC, Pollock SJ, Serhan CN, Phipps RP, Sime PJ. A novel anti-inflammatory and pro-resolving role for resolvin D1 in acute cigarette smoke-induced lung inflammation. PLoS One. 2013;8(3):e58258. doi: 10.1371/journal.pone.0058258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Robb CT, Regan KH, Dorward DA, Rossi AG. Key mechanisms governing resolution of lung inflammation. Seminars in Immunopathology. 2016;38(4):425–448. doi: 10.1007/s00281-016-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Basil MC, Levy BD. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nature Reviews. Immunology. 2016;16(1):51–67. doi: 10.1038/nri.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annual Review of Immunology. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 85.Liu J, Du J, Cheng X, Zhang X, Li Y, Fu X, Chen X. Effect of netrin-1 anti-inflammatory factor on acute lung injury in sepsis rats. Medical Science Monitor. 2019;25:7928–7935. doi: 10.12659/MSM.917279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ko CL, Lin JA, Chen KY, Hsu AC, Wu SY, Tai YT, Lin KH, Chung WC, Li MH. Netrin-1 dampens hypobaric hypoxia-induced lung injury in mice. High Altitude Medicine & Biology. 2019;20(3):293–302. doi: 10.1089/ham.2018.0116. [DOI] [PubMed] [Google Scholar]
- 87.Mirakaj V, Thix CA, Laucher S, Mielke C, Morote-Garcia JC, Schmit MA, Henes J, Unertl KE, Kohler D, Rosenberger P. Netrin-1 dampens pulmonary inflammation during acute lung injury. American Journal of Respiratory and Critical Care Medicine. 2010;181(8):815–824. doi: 10.1164/rccm.200905-0717OC. [DOI] [PubMed] [Google Scholar]
- 88.Li J, Ruffenach G, Kararigas G, Cunningham CM, Motayagheni N, Barakai N, Umar S, Regitz-Zagrosek V, Eghbali M. Intralipid protects the heart in late pregnancy against ischemia/reperfusion injury via Caveolin2/STAT3/GSK-3beta pathway. Journal of Molecular and Cellular Cardiology. 2017;102:108–116. doi: 10.1016/j.yjmcc.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Korner A, Schlegel M, Theurer J, Frohnmeyer H, Adolph M, Heijink M, Giera M, Rosenberger P, Mirakaj V. Resolution of inflammation and sepsis survival are improved by dietary Omega-3 fatty acids. Cell Death and Differentiation. 2018;25(2):421–431. doi: 10.1038/cdd.2017.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mirakaj V, Dalli J, Granja T, Rosenberger P, Serhan CN. Vagus nerve controls resolution and pro-resolving mediators of inflammation. The Journal of Experimental Medicine. 2014;211(6):1037–1048. doi: 10.1084/jem.20132103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, Walker LA, Lowes BD, Han J, Borchers CH, Buttrick PM, Kominsky DJ, Colgan SP, Eltzschig HK. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nature Medicine. 2012;18(5):774–782. doi: 10.1038/nm.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bonney S, Kominsky D, Brodsky K, Eltzschig H, Walker L, Eckle T. Cardiac Per2 functions as novel link between fatty acid metabolism and myocardial inflammation during ischemia and reperfusion injury of the heart. PLoS One. 2013;8(8):e71493. doi: 10.1371/journal.pone.0071493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Halade GV, Kain V, Serhan CN. Immune responsive resolvin D1 programs myocardial infarction-induced cardiorenal syndrome in heart failure. The FASEB Journal. 2018;32(7):3717–3729. doi: 10.1096/fj.201701173RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cai ZP, Parajuli N, Zheng X, Becker L. Remote ischemic preconditioning confers late protection against myocardial ischemia-reperfusion injury in mice by upregulating interleukin-10. Basic Research in Cardiology. 2012;107(4):277. doi: 10.1007/s00395-012-0277-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eltzschig HK, Bonney SK, Eckle T. Attenuating myocardial ischemia by targeting A2B adenosine receptors. Trends in Molecular Medicine. 2013;19(6):345–354. doi: 10.1016/j.molmed.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kain V, Ingle KA, Colas RA, Dalli J, Prabhu SD, Serhan CN, Joshi M, Halade GV. Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. Journal of Molecular and Cellular Cardiology. 2015;84:24–35. doi: 10.1016/j.yjmcc.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koeppen M, Lee JW, Seo SW, Brodsky KS, Kreth S, Yang IV, Buttrick PM, Eckle T, Eltzschig HK. Hypoxia-inducible factor 2-alpha-dependent induction of amphiregulin dampens myocardial ischemia-reperfusion injury. Nature Communications. 2018;9(1):816. doi: 10.1038/s41467-018-03105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kohler D, Eckle T, Faigle M, Grenz A, Mittelbronn M, Laucher S, Hart ML, Robson SC, Muller CE, Eltzschig HK. CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation. 2007;116(16):1784–1794. doi: 10.1161/CIRCULATIONAHA.107.690180. [DOI] [PubMed] [Google Scholar]
- 99.Duffield JS, Hong S, Vaidya VS, Lu Y, Fredman G, Serhan CN, Bonventre JV. Resolvin D series and protectin D1 mitigate acute kidney injury. Journal of Immunology. 2006;177(9):5902–5911. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- 100.Zarbock A, Schmidt C, Van Aken H, Wempe C, Martens S, Zahn PK, Wolf B, Goebel U, Schwer CI, Rosenberger P, et al. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA. 2015;313(21):2133–2141. doi: 10.1001/jama.2015.4189. [DOI] [PubMed] [Google Scholar]
- 101.Carney EF. Diabetic nephropathy: Netrin-1 expression in proximal tubular epithelial cells protects against kidney inflammation and injury. Nature Reviews. Nephrology. 2012;8(12):681. doi: 10.1038/nrneph.2012.228. [DOI] [PubMed] [Google Scholar]
- 102.Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, Blumberg RS, Serhan CN. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(21):7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ishida T, Yoshida M, Arita M, Nishitani Y, Nishiumi S, Masuda A, Mizuno S, Takagawa T, Morita Y, Kutsumi H, Inokuchi H, Serhan CN, Blumberg RS, Azuma T. Resolvin E1, an endogenous lipid mediator derived from eicosapentaenoic acid, prevents dextran sulfate sodium-induced colitis. Inflammatory Bowel Diseases. 2010;16(1):87–95. doi: 10.1002/ibd.21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bowser JL, Phan LH, Eltzschig HK. The hypoxia-adenosine link during intestinal inflammation. Journal of Immunology. 2018;200(3):897–907. doi: 10.4049/jimmunol.1701414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aherne CM, Collins CB, Eltzschig HK. Netrin-1 guides inflammatory cell migration to control mucosal immune responses during intestinal inflammation. Tissue Barriers. 2013;1(2):e24957. doi: 10.4161/tisb.24957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eltzschig HK, Rivera-Nieves J, Colgan SP. Targeting the A2B adenosine receptor during gastrointestinal ischemia and inflammation. Expert Opinion on Therapeutic Targets. 2009;13(11):1267–1277. doi: 10.1517/14728220903241666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grenz A, Clambey E, Eltzschig HK. Hypoxia signaling during intestinal ischemia and inflammation. Current Opinion in Critical Care. 2012;18(2):178–185. doi: 10.1097/MCC.0b013e3283514bd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Morote-Garcia JC, Rosenberger P, Nivillac NM, Coe IR, Eltzschig HK. Hypoxia-inducible factor-dependent repression of equilibrative nucleoside transporter 2 attenuates mucosal inflammation during intestinal hypoxia. Gastroenterology. 2009;136(2):607–618. doi: 10.1053/j.gastro.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 109.Li ZY, Yang L, Liu XJ, Wang XZ, Pan YX, Luo JM. The long noncoding RNA MEG3 and its target miR-147 regulate JAK/STAT pathway in advanced chronic myeloid leukemia. EBioMedicine. 2018;34:61–75. doi: 10.1016/j.ebiom.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ning X, Wang C, Zhang M, Wang K. Ectopic expression of miR-147 inhibits stem cell marker and epithelial-mesenchymal transition (EMT)-related protein expression in colon cancer cells. Oncology Research. 2019;27(4):399–406. doi: 10.3727/096504018X15179675206495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shen J, Niu W, Zhang H, Jun M, Zhang H. Downregulation of microRNA-147 inhibits cell proliferation and increases the chemosensitivity of gastric cancer cells to 5-fluorouracil by directly targeting PTEN. Oncology Research. 2018;26(6):901–911. doi: 10.3727/096504017X15061902533715. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 112.Li F, Wang X, Yang L. MicroRNA-147 targets BDNF to inhibit cell proliferation, migration and invasion in non-small cell lung cancer. Oncology Letters. 2020;20(2):1931–1937. doi: 10.3892/ol.2020.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu CG, Huang C. MicroRNA-147 inhibits myocardial inflammation and apoptosis following myocardial infarction via targeting HIPK2. European Review for Medical and Pharmacological Sciences. 2020;24(11):6279–6287. doi: 10.26355/eurrev_202006_21526. [DOI] [PubMed] [Google Scholar]
- 114.Abram CL, Roberge GL, Hu Y, Lowell CA. Comparative analysis of the efficiency and specificity of myeloid-Cre deleting strains using ROSA-EYFP reporter mice. Journal of Immunological Methods. 2014;408:89–100. doi: 10.1016/j.jim.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shi J, Hua L, Harmer D, Li P, Ren G. Cre driver mice targeting macrophages. Methods in Molecular Biology. 2018;1784:263–275. doi: 10.1007/978-1-4939-7837-3_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McCubbrey AL, Barthel L, Mould KJ, Mohning MP, Redente EF, Janssen WJ. Selective and inducible targeting of CD11b+ mononuclear phagocytes in the murine lung with hCD68-rtTA transgenic systems. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2016;311(1):L87–L100. doi: 10.1152/ajplung.00141.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Iqbal AJ, McNeill E, Kapellos TS, Regan-Komito D, Norman S, Burd S, Smart N, Machemer DE, Stylianou E, McShane H, et al. Human CD68 promoter GFP transgenic mice allow analysis of monocyte to macrophage differentiation in vivo. Blood. 2014;124(15):e33–e44. doi: 10.1182/blood-2014-04-568691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pillai MM, Hayes B, Torok-Storb B. Inducible transgenes under the control of the hCD68 promoter identifies mouse macrophages with a distribution that differs from the F4/80 - and CSF-1R-expressing populations. Experimental Hematology. 2009;37(12):1387–1392. doi: 10.1016/j.exphem.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support this study are available upon request.