Abstract
Since the sudden epidemic of coronavirus disease 2019 (COVID-19), the State Administration of Traditional Chinese Medicine immediately organized experts to formulate and screen the effective prescriptions of traditional Chinese medicine according to the characteristics of the novel coronavirus infection. Qingfei Paidu decoction (QFPDD) has been proven to be effective in multi-provincial clinical trials, and has been selected as a general prescription for the treatment of COVID-19 in different stages that was later promoted to be used nationwide. This review highlights the latest advances of QFPDD, focusing on the TCM theory, mechanism analysis, clinical application of QFPDD and its future perspectives. Moreover, an in-depth discussion of some valuable issues and possible development for future research on QFPDD is also discussed, aiming to provide a novel guide to combat the global epidemic COVID-19.
Keywords: qingfei paidu decoction, novel coronavirus pneumonia, prescription principle, mechanism analysis, clinical application
1. Introduction
As of November 1, 2020, novel coronavirus pneumonia (COVID-19), has spread over 211 countries around the world including all the continents, except Antarctica with around 46.43 million cumulative confirmed cases and 1.2 million deaths due to its strong infectiousness. The prevalence of COVID-19 has surpassed that of SARS in 2003, and is recognized as a severe health menace worldwide.
Since December 1, 2019, COVID-19 was emerged in Wuhan, Hubei province, China. Subsequently, the epidemic broke out throughout the country with the floating population during the Spring Festival. The mode of transmission for COVID-19 was soon recognized to be the inhalation of droplets from sneezing and coughing or the physical contact with the mucous secretions from infected individuals. People were generally susceptible and contracting the COVID-19 infection at exponentially high rate. Due to the sudden rise in the number of COVID-19 cases, China immediately launched the nationwide strict epidemic prevention and control guidelines. According to the Law of the People’s Republic of China on the Prevention and Treatment of Infectious Diseases, the COVID-19 epidemic is listed in class-B infectious disease while it is managed in accordance with Class A infectious diseases (Xue et al., 2020). Until now, the number of cases infected by COVID-19 continues to grow around the globe, and it is predicted to be continued for longer period of time (Guan et al., 2020). Still until now, proper and effective targeted therapy, drugs or vaccines, for COVID-19 epidemic control has not been identified. The accessibility of traditional drugs based on natural origin with effective therapeutic potential and the valuable historical treatment experience provide a more prominent therapeutic approach against COVID-19. Traditional Chinese medicine (TCM) has accumulated rich experience in the long-term practice of epidemic prevention and treatment, and it is characterized by broad-spectrum immunity, universal adaptability, foresight and so on. The unique advantages of TCM have attracted more and more attention to the epidemic prevention and treatment of COVID-19 (Yang et al., 2020a). Therefore, on January 27, 2019, the National Administration of Traditional Chinese Medicine launched the “Clinical screening for effective prescriptions of TCM for the prevention and treatment of pneumonia caused by novel coronavirus (2019-nCoV) infection” under the criteria “urgent, practical and effective”; nationwide. Qingfei Paidu Decoction (QFPDD), a multi-component herbal formula, was used clinically to treat 214 confirmed cases of COVID-19 with for three consecutive days as a course of treatment in four different pilot provinces in China from January 27, 2020 to February 5, 2020. The total effective rate was more than 90%, among them more than 60% of the cases showed significant improvement of symptoms and imaging manifestations and 30% of the patients showed stability of symptoms without aggravation or worsening (Yao et al., 2020). On February 18, 2020, the National Health Commission and National Administration of TCM jointly issued document No. 145 Diagnosis and Treatment Program of Novel Coronavirus Pneumonia (Trial Sixth edition). The document proposed to officially include TCM, QFPDD, in the clinical treatment of confirmed COVID-19 cases. QFPDD has been recommended as a general treatment prescription of TCM treatment for COVID-19 and has been promoted to the whole country for its remarkable clinical effect in the clinical prescription screening (Qin et al., 2020). Throughout the country, around 28 provinces, autonomous regions, and cities have been using this prescription, which is suitable for all periods and symptoms of COVID-19. Currently its use has been extended to treat suspected cases which has also been found effective and the feedback received is good.
QFPDD is formulated by the combination of syndrome differentiation and innovation based on the four classical prescriptions in Treatise on Febrile Diseases according to the pathogenic characteristics and development laws of COVID-19 (Jin, 2020). QFPDD has manifested its potential advantages and beneficial effects for the treatment of COVID-19. In this review, after summarizing the extant literature including CNKI, PubMed, Springer, Taylor & Francis, Google Scholar, and Baidu Scholar databases and other scientific resources e.g., Chinese Pharmacopoeia, 2020 edition, postgraduate research (PhD and MSc thesis, etc.), we have systematically summarized the TCM theory, modern mechanism analysis, clinical practice and application of QFPDD, hoping that it could offer some enlightenment for the further development and propel the research forward for efficiency, safety and controllable quality of QFPDD, so as to provide strong support for the global fight against the COVID-19 (Supplementary Figure).
1.1. TCM Theory and Prescription Principle of QFPDD
According to TCM theory, the experts have reached a consensus that COVID-19 belongs to a category of phytophthora blight (Li et al., 2020a), however, different experts have different understandings of COVID-19, including damp-toxin epidemic, cold-damp epidemic, and damp-heat epidemic. Wang and Miao et al. (2020) proposed COVID-19 as a damp-toxin epidemic caused by the damp toxin that belongs to yin, with the injury of Yang as the mainline (Miao et al., 2020; Wang et al., 2020c). Some believed that COVID-19 is a cold-damp epidemic caused by noxious dampness, and the basic pathogenesis is characterized by dampness, poison, blood stasis, and closure (Tong et al., 2020; Wang et al., 2020c; Xue et al., 2020). Luo and Zeng (2020) considered that COVID-19 is a damp-heat type caused by damp-heat epidemic toxin, and the main pathogenesis is the dampness, heat block of the Qi movement, endogenesis of phlegm and its transformation into fire and toxin, cremation of toxin and the combination of heat and blood stasis (Luo et al., 2020; Zeng and Sun, 2020).
Based on comprehensive analysis of COVID-19 clinical manifestations and syndrome types issued by the National Health Commission of PRC and various provinces in response to local conditions (Table 1), it is considered that COVID-19 is a damp-heat lung plague caused by damp-heat and epidemic toxin, and the pathogenesis and evolution process can be dry, and fire, and wind. At the very beginning, the pestilence attacks from the Taiyang meridian into the Yangming meridian quickly, or straight into the three Yang meridians, which is called concurrent disease of three yang meridians. But sometimes there is cold-dampness surrounding the exterior along with intense interior pathogenic fire. Or at the beginning, the exogenous pathogenic factors invade into three Yin meridians quickly, which may conduce to the syndrome of internal blockade and external collapse or syncope and collapse syndrome. The intermingled dampness and heat block the Qi movement, turbid phlegm hence appears inside and transforms into fire and toxin, intermingled heat and stasis is its main pathogenesis. Therefore, the treatment should be focused on dispelling dampness, heat and damp toxin, clearing dampness in triple warmer as well as strengthening vital Qi to eliminate pathogenic factors (Luo and Chen, 2020; Zhou, 2020). The most typical syndrome of COVID-19 is concurrent disease of three Yang meridians, which is often common in mild, moderate and part of severe cases. Hence, QFPDD is prescribed especially for this kind of syndrome.
TABLE 1.
TCM Syndrome Types of COVID-19 in national and various provinces' prevention and control programs.
| Countries and regions | Mild | Moderate/Ordinary | Severe | Critical | Convalescence |
| National health commission | Cold-dampness retention lung syndrome; damp-heat retention lung syndrome | Pathogenic dampness retention lung syndrome; cold-dampness stagnating the lung | Syndrome of epidemic toxin obstructing lung; syndrome of flaring heat in qifen and yingfen | Syndrome of internal blockade and external collapse | Lung and spleen qi deficiency, deficiency of both qi and yin |
| Hubei province | Syndrome of heat-toxin invading lung | Pathogenic dampness retention lung syndrome | Syndrome of accumulated dampness-toxicity | Syndrome of blazing heat-toxin | NA |
| Heilongjiang province | Damp warm retention lung syndrome | Phlegm-heat retention lung syndrome | Syndrome of pathogenic toxin obstructing lung | Syndrome of pathogenic toxin clouding orifices | Pathogenic factors residue, deficiency of both qi and yin |
| Beijing province | Syndrome of epidemic toxin invading lungs | NA | Epidemic toxin retention lung syndrome | Syndrome of epidemic toxin obstructing lung | Deficiency of both qi and yin |
| Shanghai province | Noxious dampness retention lung syndrome | NA | Syndrome of heat-toxin obstructing lung | Syndrome of internal blockade and external collapse | Lung and spleen qi deficiency, deficiency of both qi and yin |
| Guangdong province | Pathogenic dampness stagnating the lung, cardinal disadvantageous; syndrome of pathogenic heat congesting lung, impairment of the ascending and descending function of the lung | NA | Pathogenic heat obstructing lung syndrome, obstruction of fu-qi; warm-heat obstructing lung syndrome | Syndrome of internal blockade and external collapse | Pathogenic factors residue, deficiency of both qi and yin, deficiency of both lung and spleen |
| Jiangxi province | Noxious dampness retention lung syndrome, cardinal disadvantageous | Heat-toxin with dampness syndrome, impairment of the ascending and descending function of the lung | Syndrome of heat-toxin obstructing lung, obstruction of fu-qi | Syndrome of internal blockade and external collapse | NA |
| Shanxi province | Syndrome of exterior tightened by cold-dampness, impairment of fluid due to heat retention; syndrome of heat-toxin invading lung; external-cold and internal-heat | NA | Heat-toxin retention lung syndrome | Syndrome of internal blockade and external collapse | Syndrome of lingering heat, deficiency of both qi and yin |
| Tianjin province | Syndrome of heat-toxin invading lung | Pathogenic dampness retention lung syndrome | Syndrome of accumulated dampness-toxicity | Syndrome of blazing heat-toxin | NA |
| Yunnan province | Dampness-heat retention lung syndrome | Pathogenic heat retention lung syndrome | Syndrome of pathogenic toxin obstructing lung | Syndrome of internal blockade and external collapse | NA |
| Sichuan province | Wind heat with dampness syndrome; wind chill with dampness syndrome | Pathogenic dampness retention lung syndrome; dampness-heat retention lung syndrome | Pathogenic heat retention lung syndrome; epidemic toxin obstructing lung syndrome | Syndrome of internal blockade and external collapse | Pathogenic factors residue, deficiency of both qi and yin |
| Gansu province | Syndrome of warm pathogen attacking lung | Warm-heat retention lung syndrome | Syndrome of warm toxin obstructing lung | Syndrome of internal blockade and external collapse | NA |
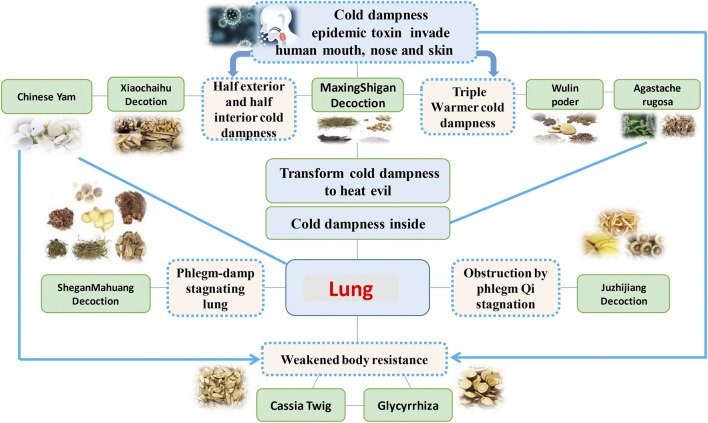
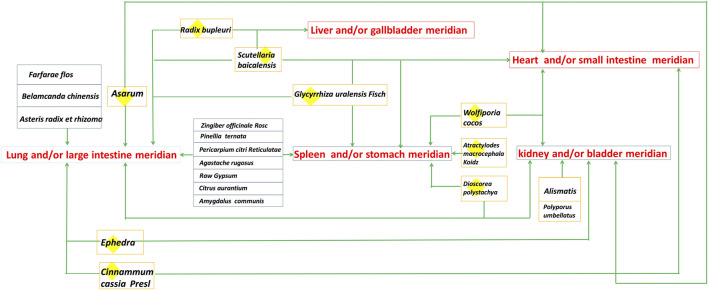
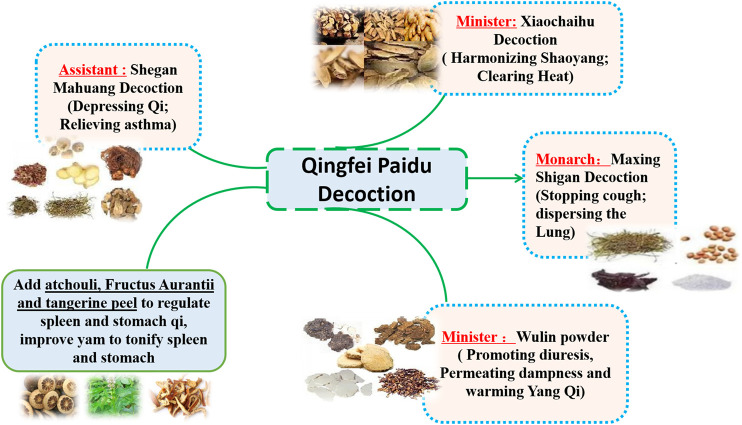
QFPDD is composed of 21 TCMs, including Herba Ephedrae (Ephedra sinica Stapf; 9 g), Radix Glycyrrhizae (Glycyrrhiza uralensis Fisch.; 6 g; baked), Semen Armeniacae Amarum (Prunus armeniaca L.; 9 g), Raw Gypsum (15–30 g; first decocted), Ramulus Cinnamomi (Cinnamomum cassia (L.) J.Presl; 9 g), Rhizoma Alismatis (Alisma plantago-aquatica Linn.; 9 g), Polyporus Umbellatus (Polyporus umbellaru (Pers.) Fr.; 9 g), Rhizoma Atractylodis Macrocephalae (Atractylodes macrocephala Koidz.; 9 g), Poria (Poria cocos (Schw.) Wolf.; 15 g), Radix Bupleuri (Bupleurum chinensis DC.; 16 g), Radix Scutellariae (Scutellaria baicalensis Georgi; 6 g), Rhizome Pinelliae Preparata (Pinellia ternata (Thunb.) Breit.; 9 g; processed with ginger), Rhizoma Zingiberis Recens (Zingiber officinale Roscoe; 9 g), Radix Asteris (Aster tataricus Linn. f.; 9 g), Flos Farfarae (Tussilago farfara Linn.; 9 g), Rhizoma Belamcandae (Iris domestica (L.) Goldblatt & Mabb.; 9 g), Herba Asari (Asarum sieboldii Miq.; 6 g), Rhizoma Dioscoreae (Dioscorea oppositifolia L.; 12 g), Fructus Aurantii Immaturus (Citrus sinensis Osbeck; 6 g), Pericarpium Citri Reticulatae (Citrus aurantium L.; 6 g), and Herba Pogostemonis (Pogostemon cablin (Blanco) Benth.; 9 g). This prescription is mainly composed of Maxing Shigan decoction, Shegan Mahuang decoction, Xiaochaihu decoction and Wuling powder. In addition, it also incorporates Daqinglong decoction, Juzhijiang decoction, Fuling Xingren Gancao decoction, etc. QFPDD is a syncretic innovation of classical prescriptions from Treatise on Febrile Diseases, which act on different stages and viscera of water, dampness, phlegm, and fluid (Fan et al., 2020). This formula is suitable for the pathogenesis of COVID-19, affecting cold, dryness, damp toxin and dampness, and can effectively improve the symptoms. TCM theory and composition mechanism of QFPDD are summarized in Figure 1. The meridian tropisms of drugs in QFPDD are shown in Figure 2, where the top meridian tropism in QFPDD is lung meridian, indicating that drugs in QFPDD are mainly specific for lung diseases. The prescriptions of QFPDD are synergistic and complementary and the prescription principle of QFPDD is shown in Figure 3. Maxing Shigan decoction is to relieve exterior Taiyang syndrome, relieve superficies and ventilate lung Qi, clear heat and relieve panting; Shegan Mahuang decoction (Fructus Jujubae and Fructus Schisandra chinensis were taken out) is for lowering the adverse Qi and resolving fluid ventilate lung Qi, dispelling phlegm and relieving cough; Xiaochaihu decoction is for harmonizing half-superficies and half-interior Shaoyang syndrome, and large dose of raw gypsum is used to clear interior heat of the Yangming meridian, and Wuling powder is to warm the triple energizer and transform Qi and remove dampness by promoting diuresis; Juzhijiang decoction can activate Qi and dispel phlegm; Herba Pogostemonis can exorcise toxins and eliminate dampness; and Rhizoma Dioscoreae can strengthen the spleen and supplement the lung (Shen et al., 2020; Wang and Jin, 2020). The combination of Ramulus Cinnamomi and Radix Glycyrrhizae can nourish Yang and support healthy energy. QFPDD is not made up of drugs but multiple concordant prescriptions contributing to get twice the result with half the effort, so that the damp-heat and epidemic toxin can be quickly discharged (Wang et al., 2020a).
FIGURE 1.
TCM theory and composition mechanism of durgs in QFPDD.
FIGURE 2.
Drug‐meridian network of QFPDD.
FIGURE 3.
Compatibility of QFPDD in prescription.
1.2. Mechanism Analysis of QFPDD
As described earlier, QFPDD contains a total of 21 TCMs, therefore, it is difficult to clearly explain the complex mechanisms of QFPDD in the treatment of COVID-19. Modern research on TCM holds that Chinese herbal compound formula plays an omnidirectional and overall regulatory role in the body due to the characteristics of multi-components, multi-targets and multi-path of the formula.
Recently, based on the reported components of QFPDD, several research groups have adopted the method of network pharmacology, molecular docking, and computer-aided drug design to provide data and clues for the multi-directional exploration of the material basis and pharmacodynamic mechanism of QFPDD in the treatment of COVID-19. Xu et al. (2020b) used the network pharmacology to screen significant effective compounds and key targets. Using TCMSP database, 148 related targets of 302 bioactive components in QFPDD were screened. Another database, GeneCards, using “COVID-19”, “2019-nCoV” and “Novel Coronavirus Pneumonia” as keywords, was used to screen 362 COVID-19 related targets where a total of 23 intersection targets were obtained by Venn analysis. By using the CentiScaPe plug-in of Cytoscape software, the network topology diagram of the 10 significant effective compounds, i.e., quercetin, luteolin, naringenin, kaempferol, beta-sitosterol, stigmasterol, baicalein, isorhamnetin, nobiletin, and wogonin (Table 2); and five pivotal targets, i.e., PTGS2, NOS2, PPARG, MAPK14, and PTGS1 were analyzed (Table 3). The results of molecular docking of the above most significant compound, quercetin, and target, PTGS2, with the highest degree value showed that the binding and interaction ability between these molecules was strong. The Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of the key targets were done using the Cluster Profiler package of R software, which showed that significant compounds such as quercetin, luteolin, naringin, kaempferol, and baicalein have expectorant, antitussive, antiviral and anti-inflammatory effects in various degrees. The key targets were mainly concentrated in 144 related signaling pathways including IL-17, tuberculosis, human cytomegalovirus infection, TNF, MAPK, Hepatitis B, etc. (Table 4). It contained 28 biological effects including cytokine receptor binding, MAP kinase activity and phosphatase binding to regulate and control metabolism, immune regulation, lung function, inflammation, and other physiological processes (Xu et al., 2020b). Xu et al. (2020a) showed that 217 related targets of 186 active components and 200 COVID-19 related targets were screened, and 51 common drug-disease targets were obtained by Venn analysis. Then, five significantly effective compounds i.e., quercetin, luteolin, kaempferol, naringin, and isorhamnetin were obtained by using the CentiScaPe plug-in of Cytoscape software to further construct the network topology diagram. The GO and KEGG pathway enrichment analysis indicated that the key targets were mainly concentrated in 30 related signal pathways such as IL-17, NF-κB, TNF, MAPK, Th17, etc. It involved several biological functions such as inflammation, immune regulation, neuroprotection, reduction of lung injury, and other physiological processes (Xu et al., 2020a).
TABLE 2.
The key active compounds of QFPDD.
| Compounds | References | Compounds | References |
| Quercetin | Xu et al. (2020a), Zhou et al. (2020), Duan et al. (2020), Xu et al. (2020b), Wu et al., 2020b | Beta-sitosterol | Xu et al. (2020b), Wu et al., 2020b |
| Luteolin | Xu et al. (2020a), Zhou et al. (2020), Duan et al. (2020), Xu et al. (2020b), Wu et al., 2020b | Wogonin | Zhou et al. (2020), Duan et al. (2020), Xu et al. (2020b) |
| Kaempferol | Xu et al. (2020a), Zhou et al. (2020), Duan et al. (2020), Wu et al., 2020b, Xu et al. (2020b) | Baicalein | Xu et al. (2020b) |
| Naringenin | Xu et al. (2020a), Zhou et al. (2020), Duan et al. (2020), Xu et al. (2020a), Xu et al. (2020b) | Nobiletin | Xu et al. (2020b) |
| Isorhamnetin | Xu et al. (2020a), Xu et al. (2020b) | Stigmasterol | Xu et al. (2020b) |
TABLE 3.
The main key targets of QFPDD in the treatment of COVID-19.
| Key targets | References | Key targets | References |
| Cell tumor antigen p53 (TP53) | Peng et al. (2020), Duan et al. (2020), Zhou et al. (2020) | Caspase 3 (CASP3) | Peng et al. (2020), Xu et al. (2020a), Yan et al. (2020), Duan et al. (2020), Zhou et al. (2020) |
| Protein kinase B1(Akt1) | Peng et al. (2020), Wu et al., 2020b | Janus kinase 2 (JAK2) | Peng et al. (2020) |
| Nuclear factor nuclear transcription factor-κB p105 subunit (NFKB1) | Peng et al. (2020) | Nuclear factor transcription factor-κB p100 subunit (NFKB2) | Peng et al. (2020) |
| Nuclear factor p65 subunit (RELA) | Peng et al. (2020) | Calmodulin 1 (CALM1) | Peng et al. (2020), Xu et al. (2020a) |
| Adenylate cyclase type 1 (ADCY1) | Peng et al. (2020) | Eukaryotic translation initiation factor 2, subunit 3 (EIF2S3) | Peng et al. (2020) |
| Adenylate cyclase type 2 (ADCY2) | Peng et al. (2020) | B-cell CLL/lymphoma 2 (BCL2) | Peng et al. (2020), Xu et al. (2020a), Zhou et al. (2020) |
| Heat shock protein α A1 (HSP90AA1) | Peng et al. (2020) | Protein kinase C-delta (PRKCD) | Peng et al. (2020) |
| Adenylate cyclase type 5 (ADCY5) | Peng et al. (2020) | Jun proto-oncogene (JUN) | Peng et al. (2020), Wu et al., 2020b |
| Recombinant human glucocorticoid receptor (NR3C1) | Peng et al. (2020) | Prostaglandin-endoperoxide synthase 2 (PTGS2) | Xu et al. (2020a), Xu et al. (2020b), Duan et al. (2020), Zhou et al. (2020) |
| Mitogen-activated protein kinase 8 (MAPK8) | Xu et al. (2020a), Duan et al. (2020), Zhou et al. (2020), Wu et al., 2020b | Prostaglandin-endoperoxide synthase 1 (PTGS1) | Xu et al. (2020a), Xu et al. (2020b), Zhou et al. (2020) |
| Mitogen-activated protein kinase 3 (MAPK3) | Peng et al. (2020), Xu et al. (2020a), Zhou et al. (2020) | Dipeptidyl peptidase-4 (DPP4) | Xu et al. (2020a), Yan et al. (2020) |
| Human NK-κB inhibited protein α (NFKBIA) | Peng et al. (2020) | V-rel reticuloendotheliosis viral oncogene homolog A (RELA) | Xu et al. (2020a), Zhou et al. (2020) |
| Bcl2-associated X protein (BAX) | Xu et al. (2020a), Zhou et al. (2020) | V-fos FBJ murine osteosarcoma viral oncogene homolog (FOS) | Xu et al. (2020a), Zhou et al. (2020) |
| Apolipoprotein D (APOD) | Xu et al. (2020a) | Lymphocyte specific tyrosine kinase (LCK) | Peng et al. (2020) |
| Peroxisome proliferative activated receptor, gamma (PPARG) | Xu et al. (2020a), Xu et al. (2020b), Zhou et al. (2020) | Signal transducerand activator of transcription 1(STAT1) | Xu et al. (2020a), Zhou et al. (2020) |
| Nitric oxide synthase (NOS2) | Xu et al. (2020a), Xu et al. (2020b), Zhou et al. (2020) | Retinoblastoma 1 (RB1) | Xu et al. (2020a), Duan et al. (2020), Zhou et al. (2020) |
| Mitogen-activated protein kinase 14 (MAPK14) | Xu et al. (2020a), Xu et al. (2020b), Duan et al. (2020), Zhou et al. (2020) | Interleukin-6 (IL-6) | Xu et al. (2020a), Zhou et al. (2020), Wu et al., 2020b |
| Heme oxygenase (decycling) 1 (HMOX1)) | Xu et al. (2020a), Duan et al. (2020), Zhou et al. (2020) | Apoptosis-related cysteine peptidase (CASP8) | Xu et al. (2020a), Zhou et al. (2020) |
| Intercellular adhesion molecule 1 (ICAM1) | Xu et al. (2020a), Duan et al. (2020), Zhou et al. (2020)Z | Superoxide dismutase 1 (SOD1) | Xu et al. (2020a), Zhou et al. (2020) |
| Epidermal growth factor receptor (EGFR) | Xu et al. (2020a), Duan et al. (2020), Zhou et al. (2020) | Protein kinase C alpha type (PRKCA) | Xu et al. (2020a), Zhou et al. (2020) |
| Bcl-2-like protein 1 (BCL2L1) | Xu et al. (2020a), Zhou et al. (2020) | Heat shock 70 kDa protein 5 (HSPA5) | Xu et al. (2020a), Zhou et al. (2020) |
| Mitogen-activated protein kinase 1 (MAPK1) | Xu et al. (2020a), Duan et al. (2020), Wu et al., 2020b | Interleukin-1β (IL-1β) | Xu et al. (2020a), Duan et al. (2020) |
| Chemokine (C-C motif) ligand 2 (CCL2) | Xu et al. (2020a), Duan et al. (2020), Zhou et al. (2020) | Protein kinase C beta type (PRKCB) | Xu et al. (2020a), Zhou et al. (2020) |
| Serine protease inhibitor protein E1 (SERPINE1) | Xu et al. (2020a), Zhou et al. (2020) | Nitric oxide synthase 3 (NOS3) | Xu et al. (2020a), Zhou et al. (2020) |
| Interleukin-2 (IL-2) | Xu et al. (2020a), Zhou et al. (2020) | Heat shock 27 kDa protein 1 (HSPB1) | Xu et al. (2020a), Zhou et al. (2020) |
| Interleukin-1α (IL-lα) | Xu et al. (2020a), Zhou et al. (2020) | Poly ADP-ribose polymerase 1 (PARP1) | Xu et al. (2020a), Zhou et al. (2020) |
| Chemokine CXC motif ligand 2 (CXCL2) | Xu et al. (2020a), Zhou et al. (2020) | Chemokine CXC motif ligand 11 (CXCL11) | Xu et al. (2020a), Zhou et al. (2020) |
| C-reactive protein (CRP) | Xu et al. (2020a), Zhou et al. (2020) | Chemokine CXC motif ligand 10 (CXCL10) | Xu et al. (2020a), Zhou et al. (2020) |
| CD40 ligand (CD40LG) | Xu et al. (2020a), Zhou et al. (2020) | BCL2-antagonist of cell death (BAD) | Xu et al. (2020a), Zhou et al. (2020) |
| Interferon regulatory factor 1 (IRF1) | Xu et al. (2020a), Zhou et al. (2020) | Catalase (CAT) | Xu et al. (2020a), Duan et al. (2020), Zhou et al. (2020) |
| Phospholipase A2 (PLA2G4A) | Xu et al. (2020a), Zhou et al. (2020) | cAMP responsive element binding protein 1 (CREB1) | Xu et al. (2020a), Zhou et al. (2020) |
| Cyclin D3 (CCND3) | Xu et al. (2020a) | Myeloid cell leukemia sequence 1 (MCLl) | Xu et al. (2020a) |
| Interleukin-4 (IL-4) | Xu et al. (2020a), Duan et al. (2020), Zhou et al. (2020) | Cyclin-dependent kinase 4 (CDK4) | Xu et al. (2020a), Zhou et al. (2020) |
| Angiotensin I-converting enzyme (ACE) | Yan et al. (2020) | Glucose-6-phosphate dehydrogenase (G6PD) | Xu et al. (2020a), Zhou et al. (2020) |
| Angiotensin I-converting enzyme 2 (ACE2) | Yan et al. (2020) | Furin (FURIN) | Yan et al. (2020) |
| Angiotensin II type 1 receptor (AT1R/AGTR1) | Yan et al. (2020) | Caspase 6 (CASP6) | Yan et al. (2020) |
| Myeloid cell Leukemia sequence 1 (MCL1) | Yan et al. (2020), Zhou et al. (2020) | Polymerase (DNA directed), delta 1, catalytic subunit 125 kDa (POLD1) | Yan et al. (2020) |
| Tumor necrosis factor (TNF) | Yan et al. (2020), Zhou et al. (2020) | Interleukin- 10 (IL-10) | Duan et al. (2020), Zhou et al. (2020) |
| Interferon, gamma (IFNG) | Duan et al. (2020), Zhou et al. (2020) | Interleukin-8 (IL-8) | Duan et al. (2020), Zhou et al. (2020) |
| Transforming growth factor, beta 1 (TGFB1) | Zhou et al. (2020) |
TABLE 4.
Main enriched signaling pathways of QFPDD in the treatment of COVID-19.
| Pathway name | References | Pathway name | References |
|---|---|---|---|
| Adherens junction | Zhao et al. (2020) | AGE-RAGE signaling pathway in diabetic complications | Xu et al. (2020b), Xu et al. (2020a) |
| Focal adhesion | Zhao et al. (2020) | C-type lectin receptor signaling pathway | Xu et al. (2020b), Xu et al. (2020a) |
| Osteoclast differentiation | Zhao et al. (2020), Xu et al. (2020b), Wu et al., 2020b | HIF-1 signaling pathway | Xu et al. (2020b), Xu et al. (2020a), Wu et al., 2020b |
| Estrogen signaling pathway | Zhao et al. (2020) | Toxoplasmosis | Xu et al. (2020b), Wu et al., 2020b, Xu et al. (2020a), Duan et al. (2020) |
| Thyroid hormone signaling pathway | Zhao et al. (2020), Wu et al., 2020b | Yersinia infection | Xu et al. (2020b) |
| Relaxin signaling pathway | Zhao et al. (2020) | Hepatitis B | Xu et al. (2020b), Xu et al. (2020a), Wu et al., 2020b |
| Prolactin signaling pathway | Zhao et al. (2020), Wu et al., 2020b | NOD-like receptor signaling pathway | Xu et al. (2020b), Wu et al., 2020b, Duan et al. (2020), Zhou et al. (2020) |
| Oxytocin signaling pathway | Zhao et al. (2020) | Kaposi sarcoma-associated herpesvirus infection | Xu et al. (2020b), Xu et al. (2020a) |
| Glucagon signaling pathway | Zhao et al. (2020) | Pertussis | Xu et al. (2020b), Wu et al., 2020b, Xu et al. (2020a), Duan et al. (2020) |
| Th17 cell differentiation | Zhao et al. (2020), Xu et al. (2020b) | Leishmaniasis | Xu et al. (2020b), Wu et al., 2020b, Xu et al. (2020a), Duan et al. (2020) |
| B cell receptor signaling pathway | Zhao et al. (2020), Wu et al., 2020b | Endocrine resistance | Xu et al. (2020b) |
| T cell receptor signaling pathway | Zhao et al. (2020), Wu et al., 2020b | FoxO signaling pathway | Xu et al. (2020b), Wu et al., 2020b, Duan et al. (2020) |
| Neurotrophin signaling pathway | Zhao et al. (2020) | Prion diseases | Xu et al. (2020b) |
| Dopaminergic synapse | Zhao et al. (2020) | Pancreatic cancer | Wu et al., 2020b, Xu et al. (2020b) |
| ErbB signaling pathway | Zhao et al. (2020), Wu et al., 2020b | Hepatitis C | Wu et al., 2020b, Duan et al. (2020) |
| MAPK signaling pathway | Zhao et al. (2020), Duan et al. (2020), Zhou et al. (2020) | Ras signaling pathway | Wu et al., 2020b |
| PI3K-Akt signaling pathway | Zhao et al. (2020), Xu et al. (2020b), Wu et al., 2020b | Bladder cancer | Wu et al., 2020b |
| TNF signaling pathway | Zhao et al. (2020), Wu et al., 2020b, Xu et al. (2020b), Duan et al. (2020), Zhou et al. (2020) | Prostate cancer | Wu et al., 2020b |
| Wnt signaling pathway | Zhao et al. (2020) | Melanama | Wu et al., 2020b |
| VEGF signaling pathway | Zhao et al. (2020), Xu et al. (2020b), Wu et al., 2020b | Thyroid hormone signaling pathway | Wu et al., 2020b |
| Ribosome | Zhao et al. (2020) | Chronic myeloid leukemia | Wu et al., 2020b |
| IL-17 signaling pathway | Xu et al. (2020b), Xu et al. (2020a) | Glioma | Wu et al., 2020b |
| Chagas disease (American trypanosomiasis) | Xu et al. (2020b), Wu et al., 2020b, Xu et al. (2020a), Duan et al. (2020) | Endometrial cancer | Wu et al., 2020b |
| Tuberculosis | Xu et al. (2020b), Wu et al., 2020b, Xu et al. (2020a), Duan et al. (2020) | Influenza A | Wu et al., 2020b, Xu et al. (2020b), Duan et al. (2020) |
| Human cytomegalovius infection | Xu et al. (2020b), Xu et al. (2020a) | Toll-like receptor signaling pathway | Wu et al., 2020b, Xu et al. (2020a), Duan et al. (2020), Zhou et al. (2020), Yang et al. (2020a) |
| Epithelial cell signaling in helicobacter pylori infection | Wu et al., 2020b | Salmonella infection | Wu et al., 2020b, Duan et al. (2020) |
| Melanoma | Wu et al., 2020b | Colorectal cancer | Wu et al., 2020b |
| RIG-I-like receptor signaling pathway | Wu et al., 2020b | Small cell lung cancer | Wu et al., 2020b |
| Herpes simplex infection | Wu et al., 2020b, Duan et al. (2020) | Non-alcoholic fatty liver disease | Wu et al., 2020b |
| Shigellosis | Wu et al., 2020b | HTLV-I infection | Wu et al., 2020b |
| Cytosolic DNA-sensing pathway | Wu et al., 2020b | Apoptosis | Xu et al. (2020a) |
| Acute myeloid leukemia | Wu et al., 2020b | Human immunodeficiency virus 1 infection | Xu et al. (2020a) |
| Measlea | Xu et al. (2020a) | Proteoglycans in cancer | Wu et al., 2020b |
| Non-small cell lung cancer | Wu et al., 2020b | Glutamatergic synapse | Jin et al. (2020) |
| Amphetamine addiction | Jin et al. (2020) | Long-term potentiation | Jin et al. (2020) |
| Long-term depressio | Jin et al. (2020) | Retrograde endocannabinoid signaling | Jin et al. (2020) |
| Cocaine addiction | Jin et al. (2020) | Nitrogen metabolism | Jin et al. (2020) |
| Nicotine addiction | Jin et al. (2020) | Neuroactive ligand-receptor interaction | Jin et al. (2020), Chen et al. (2020a) |
| Interleukin-4 and interleukin-13 signaling | Peng et al. (2020) | Interleukin-1 processing | Peng et al. (2020) |
| Adrenoceptors | Peng et al. (2020) | IκBα variant leads to EDA-ID | Peng et al. (2020) |
| CLEC7A/inflammasome pathway | Peng et al. (2020) | DEx/H-box helicases activate type I IFN and inflammatory cytokines production | Peng et al. (2020) |
| G alpha (s) signaling events | Peng et al. (2020) | G alpha(z) signaling events | Peng et al. (2020) |
| Tp53 regulates transcription of DNA repair | Peng et al. (2020) | RIP-mediated NF-κB activation via ZBP1 | Peng et al. (2020) |
| Interleukin-21 signaling | Peng et al. (2020) | PI5P, PP2A and IER3 regulate PI3K/Akt signaling | Peng et al. (2020) |
| Interleukin-2 signaling | Peng et al. (2020) | Signaling by SCF-KIT | Peng et al. (2020) |
| Erythropoietin activatesPphosphoinositide-3-kinase (PI3K) | Peng et al. (2020) | Activation of the AP-1 family of transcription factors | Peng et al. (2020) |
| Interleukin-10 signaling | Peng et al. (2020) | Interleukin receptor SHC signaling | Peng et al. (2020) |
| Adenylate cyclase inhibitory pathway | Peng et al. (2020) | Calmodulin induced events | Peng et al. (2020) |
| Inflammatory bowel disease (IBD) | Duan et al. (2020) | Cytokine-cytokine receptor interaction | Duan et al. (2020), Zhou et al. (2020) |
| Rheumatoid arthritis | Duan et al. (2020) | Amebiasis | Duan et al. (2020) |
| African trypanosomiasis | Duan et al. (2020) | Malaria | Duan et al. (2020) |
| Dteroid biosynthesis | Chen et al. (2020a) | PPAR signaling pathway | Chen et al. (2020a) |
| Adipocytokine signaling pathway | Chen et al. (2020a) | Steroid hormone biosynthesis | Chen et al. (2020a) |
Zhao et al. revealed that 464 compounds of QFPDD corresponded to 790 different putative targets, of which 232 targets were co-expressed with angiotensin-converting enzyme 2 (ACE2), the receptor of 2019-nCoV. Main signaling pathways regulated by key targets of QFPDD are shown in Table 3, where the main targets are concentrated on two types of disease pathways i.e., virus infection and lung injury. In addition, 48 important targets interacted densely with six proteins of HIV, indicating its potential antiviral effect. Key targets regulated a series of signaling pathways in biological processes such as endocrine system, immune system, translation, nervous system, and signal transduction (Zhao et al., 2020).
Wu et al. (2020b) showed that the QFPDD compound-pneumonia target network contained 292 compounds and 214 corresponding potential targets and the top five pivotal targets were AKT serine/threonine kinase 1 (AKT1), interleukin-6 (IL-6), mitogen-activated protein kinase 8 (MAPK8), mitogen-activated protein kinase 1 (MAPK1), and jun proto-oncogene (JUN). The GO and KEGG enrichment analysis and screening yielded 122 related signaling pathways, including non-small cell lung cancer, small cell lung cancer, hypoxia inducible factor-1, toll-like receptor signaling pathway, T cell receptor signaling pathway and other pathways related to pneumonia. Moreover, the same enrichment analysis also included TNF signaling pathway, P13k-Akt signaling pathway, MAPK signaling pathway, B cell receptor signaling pathway, apoptosis, and other pathways related to the reduction of lung injury (Table 4). The molecular docking results indicated that some core compounds such as ergosterol, shionone, tussilagone, etc. of the TCMs present in QFPDD had a certain degree of binding activity for 2019-nCoV 3C-like protease (3CLpro) and ACE2. It was worthwhile pointing out that ergosterol is the only one that can form a hydrogen bond with 3CLpro of 2019-nCoV (Wu et al., 2020b). In another study by Yan et al. (2020) QFPDD compound-2019-nCoV and COVID-19 target-biological function network was screened, it contained 163 active ingredients, 10 protein targets, and 42 biological functions such as renin-angiotensin regulation of blood volume and systemic arterial blood pressure to treat COVID-19. The results of preliminary molecular docking showed that the core ingredients had a good affinity with SARS-CoV-2 3CL hydrolase to form complexes with stable conformations and high binding energy, indicating that QFPDD might treat COVID-19 through RAS signaling pathway (Yan et al., 2020). Cytokine storm is considered one of the central causes of clinical sudden deterioration of COVID-19. It has been reported that QFPDD had an inhibitory effect on cytokine storm in the treatment of COVID-19 by acting on multiple targets and pathways with multiple components (Zhou et al., 2020). Duan et al. (2020) revealed that QFPDD had a potential common action mechanism in the treatment of SARS, MERS, and COVID-19. 337 corresponding targets of 246 components in QFPDD and 148 common disease-related targets for SARS, MERS, and COVID-19 were screened, and 44 common drug-disease targets were obtained by Venn analysis. The GO and KEGG pathway enrichment analysis of the key targets indicated that the key targets were mainly concentrated in 77 related signal pathways such as pertussis, tuberculosis, MAPK, FoxO, TNF, NOD-like receptor signaling pathways, and other pathways related to viral pneumonia. biological angiogenesis, immune response, nitric oxide synthesis and cell apoptosis might be the potential common mechanisms of QFPDD in the treatment of SARS, MERS, and COVID-19 (Duan et al., 2020).
In addition, Peng et al. (2020) constructed the interaction network of Formula-Herb-Disease-Targets-Pathways based on the three main clinical symptoms of COVID-19: pneumonia, fever, and cough. The research results indicated that key-targets such as cell tumor antigen p53 (tp53), protein kinase B1 (Akt1), nuclear factor nuclear transcription factor-κB (NK-κB) p105 subunit (NFKB1), nuclear factor p65 subunit (RELA), human NK-κB inhibited protein α (NFKBIA), etc. were mainly related to the regulation of apoptosis and immune response, inflammatory response, improving lung function, etc. The GO and KEGG pathway enrichment analysis indicated that the 103 key targets were mainly concentrated in the signal pathways such as interleukin signaling, adrenoceptors, seven members of the family of c-type lectin domains A (CLEC7A)/inflammasome pathway, phosphoinositide-3-kinase (PI3K)/protein kinase B (Akt) inflammatory signaling pathway, tp53 regulates transcription of DNA repair, etc. which might be the main pathways related to QFPDD’s effect on the treatment of COVID-19 accompany with lung injury, fever, cough, and other symptoms (Peng et al., 2020).
Based on computer-aided drug design, Jin et al. (2020) systematically explored and analyzed the material basis and molecular mechanism of QFPDD in the three aspects of detoxification, anti-inflammatory storm, and diuresis-removing dampness. Molecular docking virtual screening was performed based on the 2,740 compounds in QFPDD and the targets including ACE2, interleukin-6 receptor (IL-6R), and aquaporins (APQ). The mechanism of action was predicted by reverse target prediction, GO and KEGG pathway enrichment analysis for Atractylodes macrocephala, Polyporus umbellatus, Poria cocos, and Alisma plantago-aquatica. Research showed that Xiaochaihu decoction ranked the first in the number of potentially active compounds to block the virus and suppress inflammatory storm among the five classic prescriptions of QFPDD. The top three most prominent drugs to block the key binding sites of the virus were Radix Glycyrrhizae, Herba Ephedrae, and Citrus aurantium, while the top three to suppress inflammatory storm were Radix Glycyrrhizae, Radix Asteris, and Radix Bupleuri. Quercetin and its derivatives, the potential dual-target active compounds, had a high binding ability to ACE2 and IL-6R targets. Atractylodes macrocephala, Cinnamomum cassia, Poria cocos, Polyporus umbellatus, and Alisma plantago-aquatica lacked compounds that blocked viruses and suppressed inflammatory storms, but dehydroeburicoic acid, scopoletin, alismoxide and alpha-D-galactose contained in the above drugs had the potential binding ability with AQP4. Each component of the sub-medical prescription is reasonably compatible and plays a role in prevention and treatment through multi-point cooperation and complementary advantages. The interaction between these targets can form a molecular network, and it is found that many active components of QFPDD play a role in virus invasion, virus replication, and multiple organ damage (Jin et al., 2020). Furthermore, Chen et al. (2020a) divided QFPDD into five functional units (four sub-medical prescriptions and the rest) in the light of the compatibility theory of TCM. Results showed that all the five functional units had a positive effect on COVID-19 independently, and it involved physiological processes such as inflammation, bacterial and viral responses, immune system, signaling transduction, etc (Chen et al., 2020a). Yang et al. (2020c) also reported the chemical composition and pharmacological mechanism of QFPDD which indicated the thrombin and Toll-like receptor (TLR) signaling pathway were suggested to be main pathways for Maxing Shigan decoction mediated anti-inflammatory effects (Yang et al., 2020c).
1.3. In Vivo Distribution and Metabolomics of QFPDD
Liu et al. (2020b) investigated the main chemical constituents in QFPDD and the tissues distribution of the main absorbed constituents in mice following oral administration of QFPDD. As shown in Table 5, a total of 39 compounds were identified from QFPDD using UHPLC-Q-Orbitrap HRMS. After administered QFPDD in mice (2.6 g/100 g, ig), 12, 9, 10, 8, 9, and 10 constituents were identified in serum, heart, lung, spleen, liver, and kidney, respectively. The results showed that these nine constituents (ephedrine, pseudoephedrine, amygdalin, prunasin, liquiritin, hyperoside, hesperidin, baicalin, and risflorentin) could be quickly absorbed into the circulation system and then widely distributed in various tissues. At 0.5 h, except baicalin, the exposure of the other eight target components reached a peak in serum and tissues. The exposure of baicalin was peaked at 2 or 4 h. At 0.5 h, the exposure of target components to lung tissue was ranked as follows: ephedrine (2,759.11 ± 784.39 ng/g), prunasin (1819.7 ± 427.28 ng/g), pseudoephedrine (880.6 ± 287.97 ng/g), amygdalin (304.43 ± 234.7 ng/g), hesperidin (78.33 ± 38.38 ng/g), risflorentin (8.62 ± 4.66 ng/g), baicalin (8.53 ± 1.91 ng/g), hyperoside (7.72 ± 1.63 ng/g), liquiritin (7.68 ± 5.19 ng/g). At 2 h, ephedrine (776.61 ± 148.4 ng/g), prunasin (173.77 ± 58.21 ng/g), pseudoephedrine (84.68 ± 59.04 ng/g), baicalin (49.33 ± 17.06 ng/g), amygdalin (1.26 ± 0.26 ng/g) (Liu et al., 2020b). Furthermore, Wu et al. (2020a) indicated that treatment with QFPDD (1.5, 6 g/kg/day, p.o.) for continued 5 days, could significantly regulate the host metabolism and gut microbiota composition in rats such as enriched romboutsia, turicibacter, and clostridium_sensu_stricto_1, and decreased norank_f_Lachnospiraceae. The results from GC-MS and LC-MS/MS identified a total of 23 and 43 differential metabolites respectively that were altered by QFPDD. The metabolic pathways of these differential metabolites included glycerophospholipid metabolism, linoleic acid metabolism, TCA cycle, and pyruvate metabolism (Wu et al., 2020a).
TABLE 5.
Components of QFPDD distribution in the organs.
| Name | CAS No | Distribution | |||||
| Serum | Liver | Heart | Spleen | Lung | Kidney | ||
| Synephrine | 94-07-5 | - | - | - | - | - | - |
| Dihydroxyacetone | 96-26-4 | - | - | - | - | - | - |
| Gallic acid monohydrate | 5,995-86-8 | - | - | - | - | - | - |
| Neochlorogenic acid | 906-33-2 | - | - | - | - | - | - |
| (1R,2S)-2-(Methylamino)-1-phenylpropan-1-ol | 299-42-3 | + | + | + | + | + | + |
| Pseudoephedrine | 90-82-4 | + | + | + | + | + | + |
| Caffeic acid | 331-39-5 | - | - | - | - | - | - |
| Chlorogenic acid | 327-97-9 | - | - | - | - | - | - |
| Cryptochlorogenic acid | 905-99-7 | - | - | - | - | - | - |
| (R)-amygdalin | 29,883-15-6 | + | + | + | + | + | + |
| Benzeneacetonitrile, a-(b-D-glucopyranosyloxy)-, (aR)- | 99-18-3 | + | + | + | + | + | + |
| (-)-3,5-Dicaffeoyl quinic acid | 89,919-62-0 | - | - | - | - | - | - |
| Ferulic acid | 1,135-24-6 | - | - | - | - | - | - |
| Liquiritin | 551-15-5 | + | + | + | + | + | + |
| Isochlorogenic acid B | 14,534-61-3 | - | - | - | - | - | - |
| 3,5-Dicaffeoylquinic acid | 2,450-53-5 | + | - | - | - | + | + |
| Hyperoside | 482-36-0 | + | + | + | - | + | + |
| Rutin | 153-18-4 | - | - | - | - | - | - |
| Resveratrol | 501-36-0 | - | - | - | - | - | - |
| Naringen | 4,493-40-7 | - | - | - | - | - | - |
| Hesperiden | 520-26-3 | + | + | + | + | + | + |
| Isochlorogenic acid C | 57,378-72-0 | - | - | - | - | - | - |
| Cinnamaldehyde | 14,371-10-9 | - | - | - | |||
| Baicalin | 21,967-41-9 | + | + | + | + | + | + |
| Quercetin | 117-39-5 | - | - | - | - | - | - |
| Luteolin | 491-70-3 | - | - | - | - | - | - |
| Kaempferol | 520-18-3 | - | - | - | - | - | - |
| Irisflorentin | 41,743-73-1 | + | + | + | + | + | + |
| Gingerol | 23,513-14-6 | - | - | - | - | - | - |
| 2,5,7-trimethoxyphenanthren-3-ol | 51,415-00-0 | - | - | - | - | - | - |
| Asarinin | 133-04-0 | - | - | - | - | - | - |
| Glycyrrhizic acid | 1,405-86-3 | + | - | - | - | - | - |
| (8)-Gingerol | 23,513-08-8 | - | - | - | - | - | - |
| Atractylenolide I | 73,069-13-3 | - | - | - | - | - | - |
| Saikosaponin A | 20,736-09-8 | - | - | - | - | - | - |
| Tussilagone | 104012-37-5 | - | - | - | - | - | - |
| (10)-Gingerol | 23,513-15-7 | - | - | - | - | - | - |
| Alisol B,23-acetate | 19,865-76-0 | + | - | - | - | - | - |
| Pachymic acid | 29,070-92-6 | - | - | - | - | - | - |
1.4. Clinical Application and Practice of QFPDD
QFPDD is taken as water decoction, once a day, administrated in the morning and at night separately, 40 min after meals and total of three doses as a course of treatment (Jiang and Chen, 2020). If possible, half a bowl of rice water can be taken after taking the decoction every time, and those suffering from body fluid deficiency can take one bowl of rice water (Tian et al., 2020). Diagnosis and Treatment Program of COVID-19 (Seventh edition) issued by National Health Commission of the PCR has clearly stated that TCM treatment requires syndrome differentiation and treatment based on the local climate characteristics and different physical constitution. QFPDD, as a general prescription, could not take into account individual differences and may bring some related adverse reactions. Common adverse reactions of QFPDD include nausea and vomiting, dizziness, dermatitis, etc. Wang et al. (2020b) collected information about the entire diagnosis and treatment of 98 confirmed cases of COVID-9 treated with QFPDD in Sichuan province, and found that during the course of QFPDD treatment, four patients had nausea and vomiting, two patients had dizziness, one patient had a rash, and the incidence of adverse reactions was 7.14% (Wang et al., 2020b). In addition, Hu et al. revealed the observation on clinical effect of Qingfei Paidu granules in the treatment of 76 confirmed cases of COVID-9 in Hubei province, and found that during the course of Qingfei Paidu granules treatment, two patients had mild diarrhea, one patient had nausea and vomiting, one patient suffered from pruritus, and the incidence of adverse reactions was 5.26%, but above adverse reaction symptoms were mild and disappeared without special treatment (Hu et al., 2020). As shown in Table 6, some clinical observation of Qingfei Paidu prescription with different dosage forms in the treatment of COVID-19 indicated that QFPDD could effectively improve the symptoms and the effective rate is above 80%. The specific clinical indicators of TCM syndromes and main laboratory indices and safety observation which reflect the efficacy of QFPDD are shown in Table 7 and Table 8, respectively. For each course of treatment, clinicians should objectively evaluate the efficacy and actual adverse reactions of QFPDD to adjust the prescription appropriately.
TABLE 6.
Observation on clinical effect of Qingfei Paidu prescription with different dosage forms in the treatment of COVID-19.
| No | The number of cases | Pharmaceutical dosage form | Course of treatment | Cure rate (%) | Total effective rate (%) | Province | References |
| 1 | 76 cases | Granules | 5 days as a course of treatment, three courses of treatment | 65.79% | 88.16% | Hubei province | Hu et al. (2020) |
| 2 | 98 cases | Decoction | 3 days as a course of treatment, three courses of treatment | 41.13% | 92.09% | Sichuan province | Wang et al., 2020b |
| 3 | 30 cases | Decoction | 3 days as a course of treatment, three courses of treatment | NA | 83.335 | Hubei province | Li et al., 2020b |
| 4 | 151 cases | Mixture | 3 days as a course of treatment, three courses of treatment | 43.70% | 90.07% | Sichuan province | Lai et al. (2020) |
| 5 | 108 cases | Decoction | 3 days as a course of treatment, three courses of treatment | NA | 91.67% | Hubei province | Meng et al. (2020) |
| 6 | 214 cases | Decoction | 3 days as a course of treatment, three courses of treatment | NA | 90% | Shanxi, Hebei, Shaanxi, Heilongjiang province |
General Office of National Health Commission, State Administration of Traditional Chinese Medicine (2020) |
TABLE 7.
Clinical symptom rating scale of TCM syndromes of COVID-19.
| Primary symptoms | Normal (0 point) | Slight (2 points) | Medium (4 points) | Severe (6 points) |
| Fever | ≤37.2°C | 37.2°C–38.2°C | 38.3°C–39.0°C | >39.0°C |
| Cough | None | Occasionally, with a single cough | Often, but does not affect work and rest | Cough frequently with more than one cough, cause vomiting, affects work and rest |
| Asthma | The respiration is stable and the frequency is within the normal range of the corresponding age | Exceeding the upper limit of the normal value of the corresponding age (≤10 times/min), there is no flaring of nares and three concave sign | Exceeding the upper limit of the normal value of the corresponding age (11–20times/min), and/or intermittent wheezing, flapping of nasal wings, three concave sign | Exceeding the upper limit of the normal value of the corresponding age (≥21times/min), and/or continuous wheezing, flaring of nares, three concave sign |
| Expectoration | None | There is an occasional sound of phlegm in the throat and a small amount of sputum | The phlegm sound in the throat is hissing and the phlegm is yellow | There is a roar of phlegm sound in the throat and a large amount of yellow-phlegm |
| Nasal obstruction | None | Occasionally. It doesn’t affect breathing through the nose | Patients often have the nasal obstruction during the day | Obvious nasal obstruction patients have to breathe through the mouths |
| Nasal discharge | None | Occasionally | Patients have runny nose in the morning and at night | Continuously |
| Dry mouth | None | Occasionally | Sometimes | Continuously |
| Pharyngalgia | None | Slightly | Dry pain, pain when swallowing | Burning pain, sharp pain when swallowing |
| Hypodynamia | Normal | Slightly | Obvious | General weakness |
| Anorexia | Normal | Poor appetite | Loss of appetite | The appetite is extremely poor, or the patients refuse to eat |
| Diarrhea | None | Less than 3 times a day Loose stool | Three to six times a day Loose stool | More than 7 times a day The stool is watery |
| Secondary symptoms | Normal (0 point) | Slight (1 point) | Moderate(2 points) | Severe (4 points) |
| Complexion | Normal | Flushing of face and lusterless complexion | Flushing of face and dim complexion | Pallor and dim complexion |
| Palpitation | None | Mildly | Sometimes | Continuously |
| Abdominal distension | None | Occasional abdominal distension or postprandial abdominal distension | Abdominal distension is severe, up to 6 hours a day | Abdominal distension all day long |
| Aversion to cold | None | Slightly | Moderately | Shivering |
| Cyanosis | None | Slight cyanosis [P(O2)50 mmHg–80 mmHg,SaO2 80%–90%] | Moderate cyanosis [P(O2)30 mmHg–50 mmHg,SaO2 60%–80%] | Severe cyanosis [P(O2)<30 mmHg,SaO2 <60%] |
| Hyperhidrosis | None | Usually the skin is slightly moist or occasionally hot and sweating | Usually the skin is moist, sweating if you move a little; hectic fever on the chest and back, sweating repeatedly | Sweat usually and sweat like washing with moving |
| Short breath | None | Slightly | Shortness of breath increases after exercise | Obviously affecting work and daily life |
| Insomnia | Normal | Difficulty falling asleep | Difficulty falling asleep, sleep lightly | Hard to sleep |
| Urination | Normal | Slightly yellow | Dark yellow | Dark urine |
| Tongue manifestation | Normal (0 point) | Abnormal (2 points) | ||
| Tongue property | Light red tongue | Red or dark-red tongue, or with ecchymosis, or prickly tongue | ||
| Coated tongue | The tongue coating is thin and white | Tongue coating is yellow, thick, greasy, etc. | ||
| Pulse | Normal (0 point) | Abnormal (2 points) | ||
| Pulse | Normal pulse | Irregular-rapid pulse, irregularly intermittent pulse, regularly intermittent pulse, etc. | ||
TABLE 8.
The main laboratory indices and safety observation in the treatment of COVID-19.
If the patient does not have a fever, the dosage of raw gypsum should be reduced, otherwise, the dosage of raw gypsum should be increased. If the symptoms are improved but not cured, the second course should be added. If the patient has other basic diseases, the second course of the prescription shall be modified according to the actual situation. (You et al., 2020). If the symptoms disappear, the patients can stop taking the medicine in the second course of treatment. For patients with obvious deficiency of spleen Yang, 15 g of raw gypsum can be used in the prescription; for patients with the deficiency of stomach Yin, the method of nourishing Yin and eliminating dampness can be followed for empirical treatment and for those with excessive sweating, high blood pressure, palpitation, and insomnia, the dosage of the prescription can be appropriately reduced, or the dosage of yam can be increased. In the case of hepatic insufficiency, clinicians should analyze the causes of hepatic insufficiency, stop taking drugs if necessary, or add liver protection therapy (Dong et al., 2020; Lai et al., 2020). As for the dosage of Herba Asari, QFPDD is used up to 6 g, although it does not follow “the dosage of Herba Asari is not more than 5 g”, it is still in the range of commonly used clinical dosage and its fluctuation, which is more suitable for the patients with cold-dampness-yang injury and severe deficient cold. For those with severe heat and dampness, the dosage of Herba Asari should be reduced as appropriate (Liu et al., 2020a). Some scholars also have recommended the modified QFPDD combined with western medicine such as alpha-interferon, oseltamivir, chloroquine phosphate, arbidol, ribavirin in the treatment of COVID-19, and found that it was more effective than the treatment of western medicine alone, which could significantly shorten the patient’s hospitalization time, the time of clinical symptom improvement and the time of lung CT improvement (Fang et al., 2020; Li et al., 2020b; Yang et al., 2020b).
2. Conclusion and Perspectives
COVID-19 is a new type of infectious disease. Western medicine mainly focuses on symptomatic relief. TCM has been applied for treating epidemics for thousands of years, and many clinicians have conducted in-depth research on COVID-19 etiology, pathogenesis, and syndrome differentiation. Since TCM played a huge role in the treatment of SARS in China in 2003, the National Health Commission and the National Administration of TCM jointly issued the “New Coronavirus Infection Pneumonia Diagnosis and Treatment Program (Fourth, Fifth, Sixth, Seventh and Trial Eighth edition)”, which advocated the integration of Chinese and Western medicine, strived to shorten the course of the disease, improve clinical efficacy and reduce the incidence and mortality of critically ill patients (Lu and Lu, 2020; Xie, 2020).
In the process of the treatment of COVID-19, under the guidance of TCM theory, based on clinical practice and patient-oriented principle combined with data mining and basic research of modern biology and pharmacology, China established treatment methods for different stages and syndromes in different regions by systematically sorting out several classic and effective prescriptions and quickly put them into the clinical application (Zhang et al., 2020). Given the current epidemic situation of COVID-19, early intervention of TCM has played an important role in this epidemic control. Chinese and western advantages complement each other, which has a definite curative effect in reducing fever and other symptoms, controlling disease progression and reducing complications. QFPDD was selected and recommended by the National Administration of TCM as a general prescription for treating different stages of COVID-19. QFPDD is combined with multiple prescriptions and has the properties and flavors of pungent-warm and pungent-cool, aiming at the pathogenesis of COVID-19, including cold, dampness, heat, toxin, and deficiency (Chen et al., 2020b). QFPDD has the functions of dispelling cold and dampness, eliminating heat and turbidity, promoting and nourishing lung and spleen, detoxifying and removing pathogenic factors, etc. Modern pharmacologic studies have also confirmed the anti-inflammatory, antiviral and immunological functions of QFPDD which is attributed to the multi-component, multi-target, and multi-pathway characteristics of TCM. QFPDD is also a widely accepted prescription for treating COVID-19 based on its successful and effective clinical observations. The successful use of QFPDD in this novel viral pneumonia epidemic has confirmed the advantages of TCM in treating emergencies. However, at present, the mechanism of QFPDD is still unclear. It is necessary to further comprehensively evaluate the efficacy and safety of QFPDD and clearly explain the complex mechanisms of QFPDD in the treatment of COVID-19 through systematic reviews and meta-analysis (Gao et al., 2020). Currently, there is a lack of extended research with sufficient breadth and depth and the current research has just focused on QFPDD TCM theory, clinical experience, network pharmacology, etc., with only a small number of clinical research samples. In the follow-up research, it is not only essential to carry out more comprehensive chemical composition characterization, pharmacokinetic and pharmacodynamic studies in vitro and in vivo, but also extended clinical data should be evaluated to elucidate the material basis and systematically explain the effectiveness of QFPDD against COVID-19, and further provide a theoretical basis for the clinical scientific and rational application of QFPDD in the prevention and clinical treatment of COVID-19.
Author Contributions
RW, YM, RQW, LP, GL, and SJY conceived and designed the review; QS, QGP, LD and MM reviewed the literature; RW and YM wrote the manuscript.
Funding
This work was supported by National Administration of Traditional Chinese Medicine (Grant NO. 2020ZYLCYJ02?3); Sichuan Administration of traditional Chinese Medicine [(Grant NO. 2020yj018) and (Grant NO. 2020yj024)]; the fellowship of China Postdoctoral Science Foundation (Grant NO. 2020M683365); the National Traditional Chinese Medicine Clinical Research Base (Grant NO. (2020)33). The authors are sincerely thankful to all individuals who were involved in this study.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are thankful to Jiali Liu for assistance with the literature search. We also thank Ting Wang for valuable advice.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.589714/full#supplementary-material.
References
- Chen J., Wang Y. K., Gao Y., Hu L. S., Yang J. W., Wang J. R. (2020a). Protection against COVID-19 injury by Qingfei paidu decoction via anti-viral, anti-inflammatory activity and metabolic programming. Biomed. Pharmacother. 129, 110281 10.1016/j.biopha.2020.110281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. L., Ge G. B., Rong Y., Fu W., Zheng M. Y., Zhao Y. F. (2020b). Application and research progress of traditional Chinese medicine in prevention and treatment of corona virus disease 2019. Shanghai J. Tradit. Chin. Med. 3, 1–8. 10.16306/j.1008-861x.2020.03.001 [DOI] [Google Scholar]
- Dong L., Yang X., Zhang L. S., Li Y. Q., Wang R. Q., Yang S. J. (2020). Treatment of 2 cases of COVID-19 with Qingfei Paidu decoction based on syndrome differentiation of traditional Chinese and Western medicine. Chin. Med. Pharmaco Clin. 36 (2), 55–58. 10.13412/j.cnki.zyyl.20200323.002 [DOI] [Google Scholar]
- Duan H. J., Long X. Z., Du L. D., Ning Y. M., Cao R. B., Ren Y. (2020). Potential common action and mechanism of Qingfei paidu decoction in the treatment of SARS, MERS and COVID-19. Chin. Med. Pharmaco Clin. 36 (04), 29–35. 10.13412/j.cnki.zyyl.20200706.001 [DOI] [Google Scholar]
- Fan J. X., Qin X. M., Li Z. Y. (2020). Study on mechanism of Farfarae Flos in Qingfei Paidu decoction against COVID-19 based on network pharmacology and molecular docking. Chin. Tradit. Herbal Drugs 51 (09), 2317–2325. 10.7501/j.issn.0253-2670.2020.09.005 [DOI] [Google Scholar]
- Fang L., Zhu Q. G., Cheng W., Zhan C., Fang X. M., Guo C. Y. (2020). Retrospective analysis on 308 cases of COVID-19 and clinical application protocol of Kangyi Qiangshen Gong exercise prescription. Shanghai J. Tradit. Chin. Med. 54 (05), 40–45. 10.16305/j1007-1334.2020.05.095 [DOI] [Google Scholar]
- Gao K., Song Y. P., Chen H., Zhao L. Tao., Ma Li. (2020). Therapeutic efficacy of Qingfei Paidu decoction combined with antiviral drugs in the treatment of corona virus disease 2019: a protocol for systematic review and meta-analysis. Medicine 99 (99), e20489 10.1097/MD.0000000000020489 [DOI] [PubMed] [Google Scholar]
- General Office of National Health Commission, State Administration of Traditional Chinese Medicine (2020). Recommending the treatment novel coronavirus infected lung with integrated traditional Chinese and Western Medicine Notice on using “Qingfei Paidu Decoction” in inflammation. https://mp.weixin.qq.com/s/PeeHDFnRf8_T5g0JnPF6A (Accessed February 20, 2020).
- Guan W. J., Ni Z. Y., Hu Y., Liang W. H., Ou C. Q., He J. X. (2020). Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 382 (18), 1708–1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G. M., He C. X., Sun Q. L., Wan B. B., Li Y. B., Gao J. Y. (2020). Preliminary study on the clinical efficacy of “Qingfei Detox Granule” in the treatment of COVID-19. Tianjin J. Tradit. Chin. Med. 37 (09), 999–1004. 10.11656/j.issn.1672-1519.2020.09.09 [DOI] [Google Scholar]
- Jiang Q. Q.,, Chen X. Y. (2020). Thoughts on the quick treatment of COVID-19 with Qingfei Paidu decoction based on prescription and syndrome differentiation. J. Tradit. Chin. Med. 61 (14), 1204–1206. 10.13288/j.11-2166/r.2020.14.003 [DOI] [Google Scholar]
- Jin S. Y. (2020). Since ancient times epidemic prevention prescriptions, Qingfei detoxification soup and different. J. Tradit. Chin. Med. 61 (10), 835–836. 10.13288/j.11-2166/r.2020.10.003 [DOI] [Google Scholar]
- Jin X. J., Guan R. N., Mao J. J., Wang Y. R., Wang F., Li C. X. (2020). Exploration of the material basis of Qing-Fei-Pai-Du-Tang with multi-target system treating COVID-19 based on CADD. Chin. Tradit. Herbal Drugs 51 (08), 1984–1995. 10.7501/j.issn.0253-2670.2020.08.002 [DOI] [Google Scholar]
- Lai H., Yin W. X., Zhao F. L., Chen L., Yang S. J., Mi X. Q. (2020). Effect of self - developed fuzheng bixie formula and qingfei paidu mixture on the prevention and treatment of the coronavirus disease 2019. China Pharm. 29 (7), 9–11. 10.3969/j.issn.1006-4931.2020.07.002 [DOI] [Google Scholar]
- Li C. B., Su Y., Liu Y. Q., Xue X., Gong H. X., Li T. T. (2020a). Traditional Chinese medicine theory and modern pharmacology mechanism of qingfei paidu decoction in treating coronavirus disease 2019. J. Tradit. Chin. Med. 61 (15), 1299–1302. 10.13288/j.11-2166/r.2020.15.003 [DOI] [Google Scholar]
- Li K. Y., An W., Xia F., Chen M., Yang P., Liao Y. L. (2020b). Observation on clinical effect of modified qingfei paidu decoction in treatment of COVID-19. Chin. Tradit. Herbal Drugs 51 (8), 2046–2049. 10.7501/j.issn.0253-2670.2020.08.008 [DOI] [Google Scholar]
- Liu H. L., Hu X. H., He L., Liu S., Xu J. Q. (2020a). The principle, dosage and method of decoction for QFPDD are overviewed. Shanxi J. Tradit. Chin. Med. 41 (5), 560–562. 10.3969/j.issn.1000-7369.2020.05.002 [DOI] [Google Scholar]
- Liu W., Ge G. B., Wang Y. L., Huang K., Chen J. M., Wang C. H. (2020b). Chemical profiling and tissue distribution study of Qingfei Paidu Decoction in mice using UHPLC-Q-Orbitrap HRMS. Chin. Tradit. Herbal Drugs 51 (08), 2035–2045. 10.7501/j.issn.0253-2670.2020.08.007 [DOI] [Google Scholar]
- Lu Z. Z.,, Lu X. S. (2020). “Qingfei paidu decoction” shows the efficacy and confidence of Chinese medicine in anti-epidemic. J. Tradit. Chin. Med. 61 (10),833–834. 10.13288/j.11-2166/r.2020.10.002 [DOI] [Google Scholar]
- Luo Y. F.,, Chen J. T. (2020). “Three-in-one system” treatment of novel coronavirus pneumonia. Fujian J. Tradit. Chin. Med. 51 (02), 12–14. 10.13260/j.cnki.jfjtcm.011987 [DOI] [Google Scholar]
- Luo H., Tang Q. L., Shang Y. X., Liang S. B., Yang M., Robinson N. (2020). Can Chinese medicine be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chin. J. Integr. Med. 26 (4), 243–250. 10.1007/s11655-020-3192-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J. H., He Y., Chen Q., Gao Q., Chen Y. G., An J. (2020). A retrospective study on the treatment of COVID-19 type ordinary/type severe with qingfei paidu decoction.1-7. http://kns.cnki.net/kcms/detail/42.1204.R.20200831.1613.012.html.
- Miao Q., Cong X. D., Wang B., Wang Y. G., Zhang Z. D. (2020). Understanding and thinking of novel coronavirus pneumonia in traditional Chinese medicine. J. Tradit. Chin. Med. 61 (4), 286–288. 10.13288/j.11-2166/2020.04.003 [DOI] [Google Scholar]
- Peng X. J., Yang X. J., Xu G., Chen Y. B., Yang C. H., Gong W. L., et al. (2020). Investigating clinical efficacy and mechanism of qingfei paidu decoction for treatment of COVID-19 based on integrative pharmacology. Chin. J. Exp. Tradit. Med. Form 26 (16), 6–13. 10.13422/j.cnki.syfjx.20201638 [DOI] [Google Scholar]
- Qin Y. H., Hu F. L., Ge J. W. (2020). Discussion on the use of series TCM prescriptions for preventing novel coronavirus infection. Hunan J. Tradit. Chin. Med . 40 (2), 129–133. 10.3969/j.issn.1674-070X.2020.02.002 [DOI] [Google Scholar]
- Shen A. M., Zhang W., Wu Z., Wang W. L., Hua J. J. (2020). TCM Theory analysis of Qing-Fei-Pai-Du-Tang in treating COVID-19. Liaoning J. Tradit. Chin. Med. 47, 106–108. 10.13192/j.issn.1000-1719.2020.03.033 [DOI] [Google Scholar]
- Tian Z. H., Xiang J. J., Ge J., Qin K. L., Li Y. Y., Wang K. (2020). Novel coronavirus pneumonia treated by qingfei paidu decoction: theoretical analysis and clinical practice. World J. Tradit. Chin. Med. 15 (04), 497–501. 10.3969/jissn.1673-7202.2020.04.005 [DOI] [Google Scholar]
- Tong X. L., Li X. Y., Zhao L. H., Li Q. W., Yang Y. Y., Lin Y. Q., et al. (2020). Discussion on traditional Chinese medicine prevention and treatment strategies of coronavirus disease 2019 (COVID-19) from the perspective of “Cold-dampness pestilence”. J. Tradit. Chin. Med. 61 (6), 465–470+553. 10.13288j.11-2166/r.2020.06.003 [DOI] [Google Scholar]
- Wang G.,, Jin J. S. (2020). A preliminary study on traditional Chinese medicine understanding of novel coronavirus pneumonia. Tianjin J. Tradit. Chin. Med. 37 (3), 247–250. 10.11656/j.issn.1672-1519.2020.03.03 [DOI] [Google Scholar]
- Wang H., Wang D. F., Song H. X., Ma X. R., Zou D. X., Miao J. X. (2020a). Discussion on the composing principle of “Qingfei Paidu Decoction” in the treatment of anti-COVID-19 from the theory of syndrome-differentiation of the six meridians in treatise on febrile diseases. J. Hainan Med. Univ. 26 (19), 1441–1445. 10.13210/j.cnki.jhmu.20200805.002 [DOI] [Google Scholar]
- Wang R. Q., Yang S. J., Xie C. G., Shen Q. L., Li M. Q., Lei X. (2020b). Clinical efficacy of qingfei detox decoction in the treatment of COVID-19. Chin. Med. Pharmaco Clin. 36 (1), 13–18. 10.13412/j.cnki.zyyl.20200303.002 [DOI] [Google Scholar]
- Wang Y. G., Qi W. S., Ma J. J., Ruan L. G., Lu Y. R., Li X. C. (2020c). Clinical features of novel coronavirus (2019-ncov) pneumonia and its treatment based on syndrome differentiation. J. Tradit. Chin. Med. 61 (04), 281–285. 10.13288/j.11-2166/r.2020.04.002 [DOI] [Google Scholar]
- Wu G. S., Zhong J., Zheng N. N., Wang C. R., Jin H. L., Ge G. B. (2020a). Investigation of modulating effect of qingfei paidu decoction on host metabolism and gut microbiomein rats. Chin. J. Chin. Mater. Med. 45 (15), 3726–3739. 10.19540/j.cnki.cjcmm.20200609.201 [DOI] [PubMed] [Google Scholar]
- Wu H., Wang J. Q., Yang Y. W., Li T. Y., Cao Y. J., Qu Y. X. (2020b). Preliminary exploration of the mechanism of Qingfei Paidu decoction against novel coronavirus pneumonia based on network pharmacology and molecular docking technology. Acta Pharm. Sin. 55 (3), 374–383. 10.16438/j.0513-4870.2020-0136 [DOI] [Google Scholar]
- Xie M. (2020). Thoughts on the prescription and application of traditional Chinese medicine Qingfei Paidu decoction for prevention and treatment of COVID-19. J. Tradit. Chin. Med. 61 (13), 1105–1109. 10.13288/j.11-2166/r.2020.13.001 [DOI] [Google Scholar]
- Xu D. Y., Xu Y. L., Wang Z. W., Lv Y. L., Zhu H. L., Song T. (2020a). Study on the novel coronavirus pneumonia mechanism based on network pharmacology. Chin. Med. Pharmaco Clin. 36 (1), 26–32. 10.13412/j.cnki.zyyl.20200305.001 [DOI] [Google Scholar]
- Xu T. F., He C. G., Yang K. (2020b). Network pharmacology-based study on material basis and mechanism of Qingfei Paidu Decoction against novel. Nat. Prod. Res. Dev. 32 (6), 901–908. 10.16333/j.1001-6880.2020.6.001 [DOI] [Google Scholar]
- Xue B. S., Yao K. W., Xue Y. X. (2020). Theoretical analysis on the rapid and effective treatment of COVID-19 with “lung clearing and detoxification decoction”. J. Tradit. Chin. Med. 61 (6), 461–462. 10.13288/j.11-2166/r.2020.06.001 [DOI] [Google Scholar]
- Yan H. Y., Zhou Y., Zhou C. C. (2020). Mechanism of Qingfei Paidu decoction for treatment of COVID-19: analysis based on network pharmacology and molecular docking technology. J. South. Med. Univ. 40 (5), 616–623. 10.12122/j.issn.1673-4254.2020.05.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Lv X. D., Pang L. J., Wang L. L., Cong G. Q., Zhang H. Y. (2020a). Analysis of novel coronavirus pneumonia treatment with Chinese herbal compound. J. Hainan Med. Univ. 26 (13), 961–966. 10.13210/j.cnki.jhmu.20200515.002 [DOI] [Google Scholar]
- Yang P. Y., Huang X. Z., Yang M. B., Zhang X. (2020b). Advantages of novel coronavirus pneumonia treated by Qingfei detox soup combined with Lopinavir and Ritonavir Tablets from the perpective of pathology. J. Shanxi Univ. Tradit. Chin. Med. 43 (3), 1–4. 10.13424/j.cnki.jsctcm.2020.03.001 [DOI] [Google Scholar]
- Yang R. C., Yang R. C., Ruocong Yang, Hao Liu, Chen Bai, Yingchao Wang, Xiaohui Zhang, Rui Guo, et al. (2020c). Chemical composition and pharmacological mechanism of Qingfei Paidu Decoction and Ma Xing Shi Gan Decoction against Coronavirus Disease 2019 (COVID-19): in silico and experimental study. Pharmacol Res. 157, 104820 10.1016/j.phrs.2020.104820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J., Shi X. Y., Chen Q., Fan S. M., Yang R. X., Peng B. (2020). Theoretical study on corona virus disease 2019 treated by Qingfei Paidu decoction. Liaoning J. Tradit. Chin. Med. 47 (5), 94–98. 10.13192/j.issn.1000-1719.2020.05.029 [DOI] [Google Scholar]
- You Y. N., Yan H., Wang S. C., Lou X. H., Zhao X. (2020). Therapeutic strategy of traditional Chinese medicine for COVID-19. Drug Eva. Res. 43 (4), 613–619. 10.7501/j.issn.1674-6376.2020.04.005 [DOI] [Google Scholar]
- Zeng N. W.,, Sun G. (2020). The analysis and treatment to damp heat damaging yin type of COVID-19. Jiangsu J. Tradit. Chin. Med. 52 (5), 24–26. 10.19844/j.cnki.1672-397X.2020.00.009 [DOI] [Google Scholar]
- Zhang R. Z., Yang Y. Y., Hu J. P. (2020). Interpret the meaning of qingfei detoxication decoction from the importance of protecting Yang from disease. Clin J. Tradit. Chin. Med. 32 (05), 825–828. 10.16448/j.cjtcm.2020.0505 [DOI] [Google Scholar]
- Zhao J., Tian S. S., Yang J., Liu J. F., Zhang W. D. (2020). Investigating mechanism of Qing-Fei-Pai-Du-Tang for treatment of COVID-19 by network pharmacology. Chin. Tradit. Herbal Drugs 51 (4), 829–835. 10.7501/j.issn.0253-2670.2020.04.001 [DOI] [Google Scholar]
- Zhou Y. X. (2020). Discussion on TCM Etiology, pathogenesis and treatment of COVD-19. J. Shaanxi Univ. Tradit. Chin. Med. 43 (05), 28–32. 10.13424/j.cnki.jsctcm.2020.05.007 [DOI] [Google Scholar]
- Zhou M. Q., Yang L. P., Ma H. J., Cheng C. C., Zhang Y. X., Zhang J. K. (2020). Network pharmacological study of Qingfei Paidu decoction intervening on cytokine storm mechanism of COVID-19. J. Hainan Med. Univ. 26 (10), 721–729. 10.13210/j.cnki.jhmu.20200507.003 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.