Abstract
Objective
To examine the association between antihypertensive treatment and specific adverse events.
Design
Systematic review and meta-analysis.
Eligibility criteria
Randomised controlled trials of adults receiving antihypertensives compared with placebo or no treatment, more antihypertensive drugs compared with fewer antihypertensive drugs, or higher blood pressure targets compared with lower targets. To avoid small early phase trials, studies were required to have at least 650 patient years of follow-up.
Information sources
Searches were conducted in Embase, Medline, CENTRAL, and the Science Citation Index databases from inception until 14 April 2020.
Main outcome measures
The primary outcome was falls during trial follow-up. Secondary outcomes were acute kidney injury, fractures, gout, hyperkalaemia, hypokalaemia, hypotension, and syncope. Additional outcomes related to death and major cardiovascular events were extracted. Risk of bias was assessed using the Cochrane risk of bias tool, and random effects meta-analysis was used to pool rate ratios, odds ratios, and hazard ratios across studies, allowing for between study heterogeneity (τ2).
Results
Of 15 023 articles screened for inclusion, 58 randomised controlled trials were identified, including 280 638 participants followed up for a median of 3 (interquartile range 2-4) years. Most of the trials (n=40, 69%) had a low risk of bias. Among seven trials reporting data for falls, no evidence was found of an association with antihypertensive treatment (summary risk ratio 1.05, 95% confidence interval 0.89 to 1.24, τ2=0.009). Antihypertensives were associated with an increased risk of acute kidney injury (1.18, 95% confidence interval 1.01 to 1.39, τ2=0.037, n=15), hyperkalaemia (1.89, 1.56 to 2.30, τ2=0.122, n=26), hypotension (1.97, 1.67 to 2.32, τ2=0.132, n=35), and syncope (1.28, 1.03 to 1.59, τ2=0.050, n=16). The heterogeneity between studies assessing acute kidney injury and hyperkalaemia events was reduced when focusing on drugs that affect the renin angiotensin-aldosterone system. Results were robust to sensitivity analyses focusing on adverse events leading to withdrawal from each trial. Antihypertensive treatment was associated with a reduced risk of all cause mortality, cardiovascular death, and stroke, but not of myocardial infarction.
Conclusions
This meta-analysis found no evidence to suggest that antihypertensive treatment is associated with falls but found evidence of an association with mild (hyperkalaemia, hypotension) and severe adverse events (acute kidney injury, syncope). These data could be used to inform shared decision making between doctors and patients about initiation and continuation of antihypertensive treatment, especially in patients at high risk of harm because of previous adverse events or poor renal function.
Registration
PROSPERO CRD42018116860.
Introduction
High blood pressure (hypertension) is one of the leading modifiable risk factors for cardiovascular disease worldwide,1 and much healthcare resource is given to reducing blood pressure. In recent years, guidelines for hypertension management have recommended lower treatment targets2 3 on the basis of trials that found benefit for cardiovascular risk reduction.4 In patients with frailty and multimorbidity, however, these guidelines recommend clinical judgment because of potential risks from adverse effects of treatment.3 5
In the UK, guidelines for managing patients with multimorbidity suggest doctors weigh the risk of diseases with the benefits and risks of treatments and make personalised treatment recommendations.6 Such an approach is straightforward for the benefits of treatment when data exist from numerous meta-analyses of randomised controlled trials.7 8 9 When attempting to judge the potential harms of treatment, however, few data are available to support decision making. Existing meta-analyses focus on the overall risk of adverse events,10 11 making it difficult to distinguish between those events that might not be considered particularly serious, such as transient electrolyte abnormalities, and those resulting in severe complications and hospital admission, such as falls or acute kidney injury.
Currently few definitive data are available from meta-analyses of randomised controlled trials on the risks of specific harm outcomes that could be used to facilitate personalised decision making in patients with hypertension. We systematically reviewed evidence from trials and large observational studies to determine the association between antihypertensive treatment and specific adverse events such as falls, acute kidney injury, and electrolyte abnormalities.
Methods
We performed a systematic review and meta-analysis of randomised controlled trials and large observational studies examining the association between antihypertensive treatment and adverse events. The study is reported according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines.12 The study protocol was registered on PROSPERO (international prospective register of systematic reviews) and is available online (www.crd.york.ac.uk/prospero , CRD42018116860).
Search strategy
To capture all randomised controlled trials reporting the association between antihypertensive treatment and adverse events we searched Embase(OvidSP), Medline(OvidSP), Cochrane Central Register of Controlled Trials (CENTRAL, Cochrane Library), and the Science Citation Index (Web of Science Core Collection). Searches were undertaken from inception of the databases until 14 April 2020, and no language restrictions were applied. In this review we focused on randomised controlled trials, which are less prone to bias from confounding by indication.13 14 We also searched for large observational studies by interrogating the bibliographies of databases of electronic health records, but as few relevant data were identified and given the limitations of observational study designs we decided not to include them in the present study. Further studies were identified through searching the references of eligible full text articles and previous meta-analyses. Supplementary table 1 shows the full search strategy.
Selection of studies and inclusion and exclusion criteria
Eligible studies included participants aged 18 years or older, compared individuals receiving antihypertensive treatment (single agents) with those receiving placebo or no treatment, more antihypertensive drugs compared with fewer antihypertensive drugs, or one blood pressure target compared with another. Although these study designs examine different types of intervention, all compared more antihypertensive treatment with less antihypertensive treatment, enabling the potential association with adverse events to be determined. Trials were also required to present data describing the association between antihypertensive treatment and at least one adverse event. Randomised controlled trials were included if they reported 50 or more adverse events in each specific category or had at least 650 patient years of follow-up.
To ensure study selection and data analysis remained manageable by avoiding small, early phase mechanistic studies, we specified a priori the limit on patient years of follow-up and number of outcome events. We chose the specific criteria to ensure each included study was large enough to accrue outcome events and provide reliable effect estimates. These criteria assumed an incidence of the primary outcome (falls) of 7.8 events per 100 patient years of follow-up, which would accrue at least 50 outcome events in each study.15
We excluded studies in specialist populations (children, pregnant women), and case reports, case series, or before and after studies. At least two members of the review team (AA, MS, BP, SF, CK, AD, JPS) independently reviewed study titles, abstracts, and full text articles. At each stage, the entire review team screened a proportion of articles to ensure consistency of decision making. Disagreements were resolved by a third reviewer (JPS).
Outcome measures
Outcomes of interest were prespecified based on those reported in recent large scale trials of blood pressure lowering treatment.4 16 17 The primary outcome was falls, at any time point and by any definition given in the original study. Secondary outcomes were acute kidney injury, fractures, gout, electrolyte abnormalities (changes in potassium), hypotension, and syncope (eg, fainting) at any time point during trial follow-up. Acute kidney injury was defined as any outcome reported according to the KDIGO (kidney disease: improving global outcomes) definition.18 All other outcomes were defined according to definitions given in the original study. Additional treatment efficacy outcomes of interest included cardiovascular death, myocardial infarction, stroke, and all cause mortality.
Data extraction and quality assessment
AA, MH, LA, AD, and BL extracted data from eligible studies. Two reviewers independently entered outcome data into a Microsoft Excel spreadsheet (2016 version, Redmond, WA). A second reviewer then manually cross checked these, referring to the original source data when discrepancies were identified. After an initial consistency check involving extraction of data from 10 articles, one reviewer extracted study descriptive data.
Data were extracted on populations studied, interventions tested, length of follow-up, effect measures (estimates and confidence intervals for rate ratios, odds ratios, and hazard ratios), and numbers of patients experiencing adverse events and cardiovascular or mortality outcomes.
The methodological quality and risk of bias of individual studies was assessed using the Cochrane risk of bias tool (for randomised controlled trials).19
Data synthesis
Summary effect estimates describing the association between all antihypertensive drug classes (combined) and adverse events were derived using a random effects meta-analysis. For uncommon adverse events (approximately less than 10% of the population experience an event), rate ratios (for rate outcomes), odds ratios (for binary outcomes), and hazard ratios (for time-to-event outcomes) were considered reasonably similar and combined provided they had the same directional interpretation.20 For uncommon outcomes, we label summary effect estimates as risk ratios. For more common cardiovascular disease outcomes, we synthesised rate ratios, odds ratios, and hazard ratios separately. We used restricted maximum likelihood estimation to fit the random effects model, with 95% confidence intervals derived using the Hartung-Knapp approach to account for uncertainty in heterogeneity estimates.21 For studies with three treatment arms, we split binary and rate outcomes for the control arm into two equal groups.22 This approach is not possible for the time-to-event outcomes, and therefore we made an approximate adjustment to the standard errors.
Heterogeneity was summarised using the estimate of between study variance (τ2) and 95% prediction intervals for the treatment effect in a new study. The proportion of variability in effect estimates due to between study heterogeneity was summarised using I2.
Sensitivity analyses were undertaken focusing on adverse events reported as a reason for study withdrawal. Meta-regression was used to examine the association between observed treatment effects and study quality. Small study effects (potential publication bias) were explored using contour enhanced funnel plots for outcomes reported in 10 or more studies.23 Prespecified subgroup analyses were conducted to examine the association between treatment and adverse events by antihypertensive drug class.
No other subgroup analyses were undertaken by patient level characteristics (eg, age), owing to the risk of ecological bias.24 Aggregate data only allow relationships across studies to be examined, but these often do not reflect within study (participant level) relationships, because of aggregation bias and study level confounding.25 26 For example, those studies with a higher mean age might also have a longer mean follow-up or a higher dose of the drug; hence it is difficult to disentangle these different associations, and interpreting across study associations as if they were interactions at the individual level is potentially misleading.
All analyses were undertaken using Stata version 16 (StataCorp, College Station, TX).
Patient and public involvement
This study was developed with the help of our patient and public advisor. As a member of our study advisory group, they commented on the study protocol. We also held a focus group with seven older adults during the study to discuss broader issues related to drugs for cardiovascular disease prevention and adverse events, which informed the interpretation of this work.
Results
Study selection and characteristics
A total of 15 023 unique articles were identified from the literature searches, of which 119 records were screened from reference lists of included articles and previous meta-analyses. After screening of the title, abstract, and full text, 63 articles originating from 58 randomised controlled trials4 16 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 were eligible for inclusion (fig 1). The most common reason for exclusion at full text screening was lack of adverse event reporting (n=108) or inclusion of too few patient years of follow-up (n=104).
Fig 1.
Selection of studies for inclusion in review. *Hand searches of reference lists of included studies and recent meta-analyses of blood pressure lowering trials7 8 9
A total of 280 638 participants were included in the primary analyses from 58 unique randomised controlled trials. Forty eight studies compared a single drug treatment with placebo and 10 studies compared a high blood pressure target with a lower blood pressure target in the intervention and control groups (table 1). The remaining five studies either compared treatment with no treatment or compared multiple drugs with a single drug. The median duration of follow-up in the trials was 3 (interquartile range 2-4) years. Most studies were conducted in patients with at least one risk factor for cardiovascular disease in addition to hypertension.
Table 1.
Summary of included randomised controlled trials
| Trial name, year, reference | Population characteristics | Total sample | Follow-up | Mean (SD) age (years): intervention; control | Mean (SD) baseline sBP (mm Hg): intervention: control | Intervention | Comparator |
|---|---|---|---|---|---|---|---|
| AASK 200237 | African-Americans with renal disease | 1094 | 3.8 years | 54.5 (10.9); 54.7 (10.4) | 152 (25); 149 (23) | Mean atrial pressure target ≤92 mm Hg | Mean atrial pressure target 102-107 mm Hg |
| ACCORD 201016 48 | Type 2 diabetes | 4733 | 5.6 years | 62.2 (6.8); 62 (6.9) | 139.0 (16.1) 139.4 (15.5) | BP target <120 mm Hg | BP target <140 mm Hg |
| ACEi progressive renal insufficiency study group 199659 | Renal dysfunction | 583 | 3 years | 51 (13); 51 (12) | 142 (17); 144 (17) | Benazepril | Placebo |
| ADVANCE 201470 | Type 2 diabetes | 11 140 | 4.3 years | 66 (6); 66 (7) | 145 (22); 145 (21) | Perindopril+indapamide | Placebo |
| AIRE 199381 | Acute myocardial infarction+evidence of heart failure | 1986 | 15 months | 64.9 (10); 65.1 (10.8) | NS (28% hypertensive) | Ramipril | Placebo |
| ALTITUDE 201285 | Type 2 diabetes | 8561 | 2.6 years | 64.6 (9.6); 64.4 (9.9) | 137.3 (16.2); 137.3 (16.7) | Aliskiren | Placebo |
| ASPIRE 201186 | Post-myocardial infarction | 820 | 36 weeks | 61 (12); 59 (12) | 121.6 (16.1); 121.7 (16.2) | Aliskiren | Placebo |
| BEST 200187 | NYHA class III or IV heart failure | 2708 | 2 years | 60 (12.6); 60 (12.3) | 117 (18.2); 117 (17.8) | Bucindolol | Placebo |
| BHAT 198227 | Admitted to hospital with acute myocardial infarction | 3837 | 2 years | 54.7 (NS); 54.9 (NS) | 112.3 (NS); 111.7 (NS) | Propranolol | Placebo |
| Cardio-Sis 200928 | No diabetes with hypertension | 1111 | 2 years | 67 (7); 67 (7) | 163.3 (11.3); 163.3 (11.1) | BP target <120 mm Hg | BP target <130 mm Hg |
| CCS-I 199729 | Acute myocardial infarction | 14 962 | 4 weeks | 61.2 (10.7); 61 (10.6) | 127 (24); 126 (24) | Captopril | Placebo |
| CHARM -Preserved 200330 | NYHA class II-IV heart failure | 3023 | Median 36.6 months | 67.2 (11.1); 67.1 (11.1) | 136.0 (18.6); 136.3 (18.3) | Candesartan | Placebo |
| CHARM-ADDED 200331 | NYHA class II-IV heart failure | 2548 | 3.5 years | 64 (10.7); 64.1 (11.3) | 124.7 (18.6); 125.6 (18.6) | Candesartan | Placebo |
| CHARM-Alternative 200332 | Heart failure | 2028 | 2.7 years | 66.3 (11); 66.8 (10.5) | 129.9 (19.0); 130.3 (18.5) | Candesartan | Placebo |
| Collaborative Study Group 200133 | Type 2 diabetes with nephropathy | 1715 | 2.6 years | 59.3 (7.1), 59.7 (7.9); 58.3 (8.2) | 160 (20); 159 (19); 158 (20) | Irbesartan or Amlodipine | Placebo |
| CONSENSUS II 199234 | Post-myocardial infarction | 6090 | 6 months | 65.7 (NS); 65.8 (NS) | 133 (NS); 134 (NS) | Enalapril | Placebo |
| DIME 201435 | No diabetes, hypertension | 1130 | 4.4 years | 63 (10); 63 (10) | 154 (11); 154 (10) | Thiazide diuretic | No thiazide diuretic |
| Dutch TIA Trial 199336 | Previous transient ischaemic attack | 1473 | 2.6 years | 50 (NS); 54 (NS) | 157 (25) and 158 (24) | Atenolol | Placebo |
| EMPHASIS-HF 201138 | NYHA class II heart failure | 2737 | 1.75 years | 68.6 (7.7); 68.6 (7.6) | 124 (17); 124 (17) | Eplerenone | Placebo |
| EUROPA 200339 | Stable coronary heart disease without heart failure | 12 218 | 4.2 years | 60 (9); 60 (9) | 137 (16); 137 (15) | Perindopril | Placebo |
| EWPHE 199140 | >60 years with raised BP | 822 | 5 years | 72 (8); 72 (8) | 183 (16); 183 (16) | Hydrochlorothiazide+triamterene | Placebo |
| GISSI-3 199441 | Myocardial infarction within 24 hours | 9442 | 6 weeks | NS | NS | Lisinopril | No treatment |
| GISSI-AF 200942 | Atrial fibrillation and underlying CVD | 1442 | 1 year | 67.5 (9.5); 68.2 (8.9) | 138.2 (16.7); 139.0 (16.9) | Valsartan | Placebo |
| Hypertension in diabetes study IV 199643 | Type 2 diabetes | 758 | 5 years | 57 (7.9) all patients | 160 (19); 160 (20) | Atenolol or Captopril with BP target <150/<85 mm Hg | BP target <180/<105 mm Hg |
| HOPE Trial44 45 | >55 years, high CVD risk | 9297 | 5 years | 66 (7); 66 (7) | 139 (20); 139 (20) | Ramipril | Placebo |
| HOPE-3 201646 | Men >55 years and women >65 years with one CVD risk factor or more | 12 705 | 5.5 years | 65.7 (6.4); 65.8 (6.4) | 138.2 (14.7); 137.9 (14.8) | Candesartan+Hydrochlorothiazide | Placebo |
| HYVET Trial47 84 | >80 years with hypertension | 3845 | 2.1 years | 84/84 | 173 and 173 | Indapamide and/or perindopril | Placebo |
| INFINITY 201949 | >75 years, hypertension, white matter lesions | 199 | 3 years | 80.9 (4.4); 80.3 (3.8) | 149.7 (15.4); 152.0 (17.5) | sBP target ≤130 mm Hg | sBP target ≤145 mm Hg |
| Intensive Antihypertensive Treatment for Elderly 201350 | >70 years with hypertension | 724 | 4 years | 76.6 (4.6); 76.5 (4.5) | 158.8 (16.0); 160.3 (16.9) | BP target <140/90 mm Hg | BP target <150/90 mm Hg |
| I-PRESERVE 200851 | Heart failure | 4128 | 4.1 years | 72 (7); 72 (7) | 137 (15); 136 (15) | Irbesartan | Placebo |
| MACB 199552 | Referred for coronary artery bypass grafting | 967 | 2 years | Median age 64 in both groups | Median sBP 120 mm Hg in both groups | Metoprolol | Placebo |
| MERIT-HF 200053 | NYHA class II-IV heart failure | 3991 | 1 year | 63.9 (NS); 63.7 (NS) | Not stated (44% of cohort hypertensive) | Metoprolol | Placebo |
| MRC 198555 | Patients with mild hypertension | 17 354 | 5.5 years | 51 (NS); 53 (NS) | 158 (men); 165 (women) | Bendroflumethiazide or propranolol | Placebo |
| Multicentre Diltiazem Postinfarction Trial 198856 | Admitted to hospital with acute myocardial infarction | 2466 | 25 months | 58 (10); 58 (10) | NS | Diltiazem | Placebo |
| NAVIGATOR 201057 | Type 2 diabetes | 9306 | 6.3 years | 63.7 (6.8) 63.8 (6.8) | 139.4 (17.8) and 139.9 (17.1) | Valsartan | Placebo |
| NICOLE 200358 | <75 years and previous successful angioplasty | 819 | 3 years | 60.4 (NS); 60.2 (NS) | NS (40% of cohort hypertensive) | Nisoldipine | Placebo |
| NILVAD 201860 | Alzheimer’s disease | 511 | 1.5 years | 73.1 (8.7); 72.8 (7.8) | 138 (14); 137 (14) | Nilvadipine | Placebo |
| ONTARGET 200861 | Existing vascular disease or diabetes | 25 620 | 4.5 years | 66.4 (7.2) 66.4 (7.1) 66.5 (7.3) | 141.8 (17.4); 141.7 (17.2); 141.9 (17.6) | Ramipril or telmisartan | Ramipril+telmisartan combination |
| ORIENT 201162 | Type 2 diabetes with poor renal function | 566 | 3.4 years | 59.1 (8.1)/; 59.2 (8.1) | 141.7 (17.0) 140.8 (18.0) | Olmesartan | Placebo |
| PEACE 200463 | Myocardial infarction or bypass in past 3 months | 8290 | 4.8 years | 64 (8); 64 (8) | 134 (17) and 133 (17) | Trandolapril | Placebo |
| PRoFESS 200864 | >55 years and ischaemic stroke | 20 332 | 2.5 years | 66.1 (8.6); 66.2 (8.6) | 144.1 (16.4) 144.2 (16.7) | Telmisartan | Placebo |
| PROGRESS 200165 | Previous stroke or transient ischaemic attack | 6105 | 4 years | 64 (10); 64 (10) | 147 (19); 147 (19) | Perindopril+indapamide | Placebo |
| ROADMAP Trial66 67 | Type 2 diabetes | 4447 | 3.2 years | 57.7 (8.8); 57.8 (8.6) | 137 (16); 136 (15) | Olmesartan | Placebo |
| SANDS 200968 | Native Americans with type 2 diabetes | 548 | 3 years | 55.8 (9.3); 57.4 (9.3) | 128.7 (14.7) 132.6 (16.4) | BP target <115/75 mm Hg | BP target <130/80 mm Hg |
| SENIORS 200569 | >70 years with heart failure | 2128 | 1.5 years | 76.1 (4.8); 76.1 (4.6) | 138.6 (20.1) 139.5 (21.1) | Nebivolol | Placebo |
| SHEP 199171 72 | >60 years with ISH | 4736 | 5 years | 71.6 (6.7); 71.5 (6.7) | 170.5 (9.5) 170.1 (9.2) | Chlorthalidone with or without atenolol or reserpine | Placebo |
| SOLVD 199273 | Heart failure with ejection fraction <0.35 | 2569 | 41.4 months | 60.7 (NS); 61.0 (NS) | 125.3 (NS); 124.5 (NS) | Enalapril | Placebo |
| Spironolactone and mild heart failure 201674 | NYHA class II heart failure | 139 | 10 years | 66.7 (1.3); 65.5 (1.3) | 120.6 (1.3); 121.3 (1.4) | Spironolactone+standard treatment | Standard treatment |
| SPRINT 20154 | >50 years with increased CVD risk, no diabetes | 9361 | 3.26 years | 67.9 (9.4); 67.9 (9.5) | 139.7 (15.8); 139.7 (15.4) | BP target <120 mm Hg | BP target <140 mm Hg |
| SPS3 201375 | Stroke within past 6 months | 3020 | 3.7 years | 63 (11); 63 (11) | 142 (19); 144 (19) | BP target <130 mm Hg | BP target 130-149 mm Hg |
| The Norwegian Multicenter Study 198176 | Admitted to hospital with acute myocardial infarction | 1884 | 17 months | 60.3 (NS); 61.4 (NS) | NS | Timolol | Placebo |
| TRACE 199586 | Admitted to hospital with acute myocardial infarction | 1749 | 24 to 50 months | 67.7 (NS); 67.3 (NS) | 122 (NS); 120 (NS) | Trandolapril | Placebo |
| TRANSCEND 200877 | CVD or diabetes with end organ damage | 5926 | Median 56 months | 66.9 (7.3); 66.9 (7.4) | 140.7 (16.8); 141.3 (16.4) | Telmisartan | Placebo |
| TROPHY 200678 | Prehypertensive population | 772 | 4 years | 48.6 (7.9); 48.3 (8.2) | 133.9 (4.3); 134.1 (4.2) | Candesartan | Placebo |
| VA NEPHRON-D 201379 | Type 2 diabetes+moderate to severe proteinuria | 1448 | 2.2 years | 64.5 (7.9); 64.7 (7.7) | 136.9 (16.5); 137.0 (16.0) | Losartan+lisinopril | Losartan+placebo |
| Val-HeFT 200180 | Heart failure | 5010 | 23 months | 62.4 (11.1) 63.0 (11) | 123.0 (18.4) 124.0 (18.6) | Valsartan | Placebo |
| VALIANT 200382 | Myocardial infarction with left ventricular systolic dysfunction | 11 703 | 2 years | 65.0 (11.8) 64.9 (11.8) 64.6 (11.9) | 123 (NS) overall mean | Valsartan or captopril | Valsartan+captopril (dual treatment) |
| VA-NHLBI 197883 | 21-50 years with mild hypertension | 1012 | 2 year | 37.5 (NS); 37.5 (NS) | Mean diastolic BP 93 mm Hg | Chlorthalidone and reserpine | Placebo |
CVD=cardiovascular disease; NYHA=New York Heart Association; NS=not stated; sBP=systolic blood pressure; ISH=isolated systolic hypertension.
Quality assessment
Supplementary table 2 presents the risk of bias assessment for individual trials. Most of the trials (n=40, 69%) had a low risk of bias (fig 2). Eight trials (14%) did not adequately blind outcome assessment of adverse events (or did not describe this adequately) and 12 (21%) did not adequately describe the randomisation process. Outcome reporting was complete in 52 trials (90%) trials.
Fig 2.
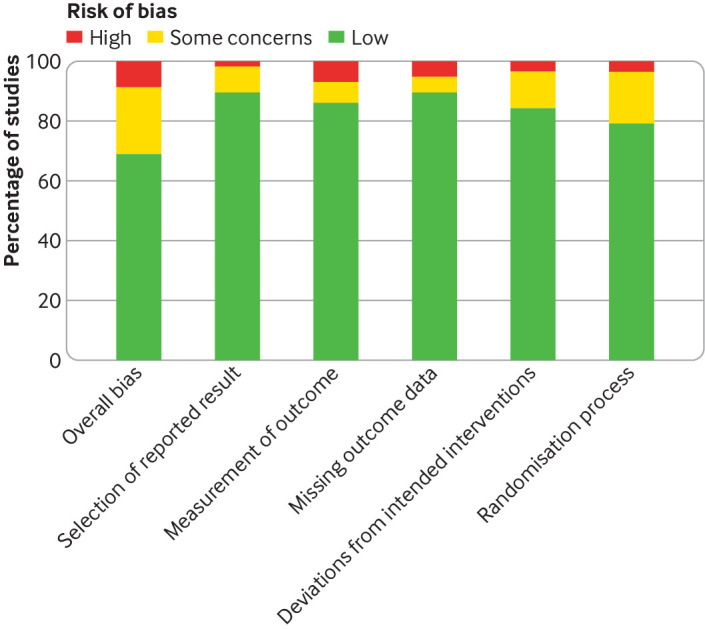
Summary of risk of bias assessment across all included randomised controlled trials
Primary outcome
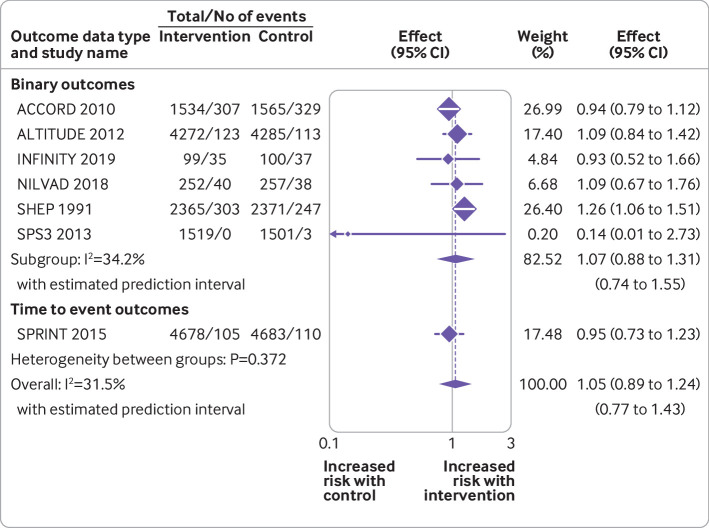
Seven randomised controlled trials reported data for the primary outcome of falls (fig 3). Data were available from 29 481 patients experiencing 1790 events. Overall, no evidence was found of an association between antihypertensive treatment and falls (summary risk ratio 1.05, 95% confidence interval 0.89 to 1.24). Little evidence was found of between study heterogeneity in this association (τ2=0.009; I2=31.5%; P=0.372). Subgroup analyses by drug type did not reveal any evidence of associations between falls and specific antihypertensive drug classes, except for thiazide diuretics, although this was based on data from just one trial (supplementary figure 1).71 More intensive treatment (ie, to lower blood pressure targets) was not associated with falls across four trials (supplementary figure 1).
Fig 3.
Random effects meta-analysis of randomised controlled trials examining the association between antihypertensive treatment and falls
Secondary outcomes
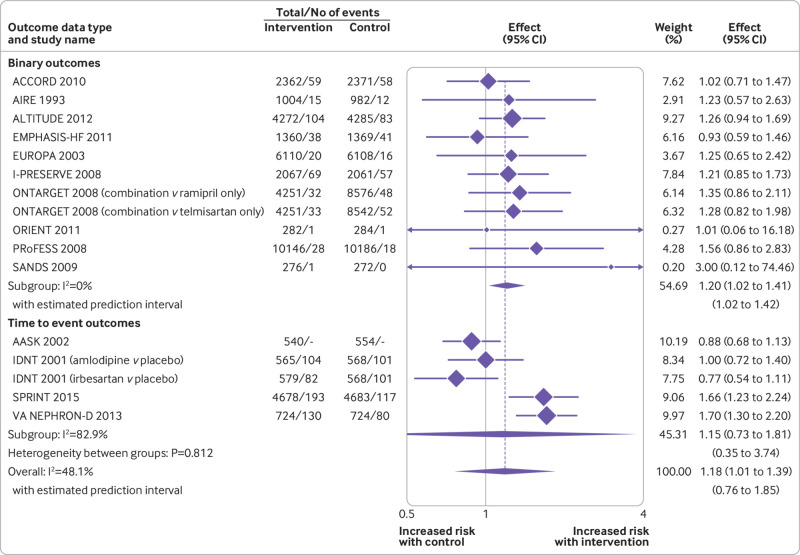
In analyses examining adverse events across all drug classes, antihypertensive treatment was associated with an increased risk of acute kidney injury (summary risk ratio 1.18, 95% confidence interval 1.01 to 1.39, n=15 studies; fig 4), hyperkalaemia (1.89, 1.56 to 2.30, n=26 studies), hypotension (1.97, 1.67 to 2.32, n=35 studies), and syncope (1.28, 1.03 to 1.59, n=16 studies) (table 2; supplementary figures 2-4), although statistical heterogeneity was significant for most outcomes (τ2=0.037 to 1.374; I2=42.9% to 85.1%). Evidence was unclear of an association between antihypertensive treatment and fractures (0.93, 0.58 to 1.48, τ2=0.062, I2=53.8%, n=5 studies; supplementary figure 5) and gout (1.54, 0.63 to 3.75, τ2=1.612, I2=94.3%, n=12 studies; supplementary figure 7), although confidence intervals were wide, partly reflecting large between study heterogeneity.
Fig 4.
Random effects meta-analysis of randomised controlled trials examining the association between antihypertensive treatment and acute kidney injury
Table 2.
Main analyses showing meta-analysis results from trials reporting the association between antihypertensive treatment and adverse events and cardiovascular and mortality outcomes
| Outcome | No of studies | Sample size | Events | Effect size (95% CI)* | I2 (%) | τ2 | 95% prediction interval | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention group | Control group | Intervention group | Control group | |||||||
| Adverse events | ||||||||||
| Falls4 16 49 60 71 72 75 85 (primary outcome) | 7 | 14 719 | 14 762 | 913 | 877 | 1.05 (0.89 to 1.24) | 31.5 | 0.009 | 0.77 to 1.43 | |
| Acute kidney injury4 16 33 38 39 51 61 62 64 68 79 81 85 88 | 15 | 43 467 | 52 133 | 909 | 785 | 1.18 (1.01 to 1.39) | 48.1 | 0.037 | 0.76 to 1.85 | |
| Fractures16 50 60 71 72 89 | 5 | 6447 | 6466 | 230 | 267 | 0.93 (0.58 to 1.48) | 53.8 | 0.062 | 0.36 to 2.41 | |
| Gout17 35 55 83 90 | 5 | 16 524 | 16 137 | 249 | 26 | 3.84 (0.95 to 15.57) | 84.3 | 1.374 | 0.11 to 138.91 | |
| Hyperkalaemia4 16 30-34 38 41 43 45 51 57 59 62 64 68 73 74 77 79 82 85 86 91-93 | 26 | 57 604 | 61 795 | 2749 | 1880 | 1.89 (1.56 to 2.30) | 71.8 | 0.121 | 0.90 to 3.98 | |
| Hypokalaemia4 16 35 38 43 51 57 71 72 74 83 86 94 | 12 | 19 748 | 19 528 | 517 | 274 | 1.54 (0.63 to 3.75) | 94.3 | 1.612 | 0.08 to 29.98 | |
| Hypotension4 16 27 29-32 34 36 38 39 42 51-53 56 58 62 64 65 68-70 75 76 78 80-82 85-87 91 93 | 35 | 88 575 | 93 547 | 5390 | 3121 | 1.97 (1.67 to 2.32) | 85.1 | 0.132 | 0.92 to 4.18 | |
| Syncope4 16 17 27 60 61 63 64 68 75-78 81 85 87 | 16 | 51 072 | 51 189 | 644 | 543 | 1.28 (1.03 to 1.59) | 42.9 | 0.050 | 0.75 to 2.17 | |
| Cardiovascular and mortality outcomes | ||||||||||
| All cause mortality4 16 17 28 31 32 34 36 38 42 56 57 62 63 69 71 72 74 75 77 79-81 85-87 89 91 | 32 | 128 619 | 128 729 | 11 831 | 13 018 | 0.93 (0.88 to 0.98) | 50.4 | 0.008 | 0.77 to 1.12 | |
| Cardiovascular death4 16 17 30-32 36 45 51 57 61-63 69 71 72 75 77 82 85 87 91 92 | 21 | 92 676 | 92 733 | 6341 | 6890 | 0.92 (0.86 to 0.99) | 54.6 | 0.011 | 0.73 to 1.16 | |
| Myocardial infarction4 16 17 28 32 38 45 57 61-63 71 72 75 77 79 85 87 89 91 92 | 19 | 75 002 | 75 301 | 2900 | 3255 | 0.94 (0.85 to 1.03) | 40.7 | 0.013 | 0.73 to 1.21 | |
| Stroke4 16 17 28 36 38 45 57 61-64 75 77 79 85 89 92 | 17 | 104 153 | 104 366 | 3220 | 3733 | 0.84 (0.76 to 0.93) | 44.8 | 0.013 | 0.64 to 1.09 | |
Adverse events reported as risk ratios and cardiovascular and mortality outcomes reported as hazard ratios (in studies reporting outcome as time to event). Binary and rate outcomes for cardiovascular and mortality outcomes are presented in supplementary figures 15-17.
Analyses of outcomes by specific drug class showed that drugs affecting the renin angiotensin-aldosterone system were associated with acute kidney injury (1.26, 1.03 to 1.56, τ2=0.030, I2=39.0%; n=9 studies; table 3, supplementary figure 8) and hyperkalaemia (2.03, 1.67 to 2.48, τ2=0.063, I2=51.0%; n=20 studies; table 3, supplementary figure 9). These effects were larger and had less between study heterogeneity than in analyses examining the association between all antihypertensive treatments and the same outcomes (table 2 and table 3). Only a small number of studies assessed the association between diuretics and hypokalaemia (three studies) or gout (five studies), and the results of these were inconclusive (table 3; supplementary figures 10 and 11). No other drug class specific associations with adverse events were observed in the stratified analyses (supplementary figures 12-14).
Table 3.
Summary of sensitivity analyses showing important drug class specific associations between antihypertensive treatment and specific adverse events
| Outcome | Drug class | No of studies | Sample size | Events | Risk ratio (95% CI) | I2 (%) | τ2 | 95% prediction interval | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention group | Control group | Intervention group | Control group | ||||||||
| Acute kidney injury33 39 51 61 62 64 79 81 85 | RAAS | 9 | 33 686 | 42 316 | 514 | 468 | 1.26 (1.03 to1.56) | 39.0 | 0.030 | 0.80 to 1.99 | |
| Hyperkalaemia30-34 42 45 51 57 59 62 64 73 77 79 82 85 86 91-93 | RAAS | 20 | 47 122 | 51 787 | 2282 | 1541 | 2.03 (1.67 to 2.48) | 51.0 | 0.063 | 1.16 to 3.57 | |
| Hypokalaemia35 71 72 83 | Diuretics | 3 | 3154 | 3114 | 259 | 25 | 10.73 (0.32 to 354.58) | 80.9 | 1.385 | - | |
| Gout17 35 55 71 72 83 90 | Diuretics | 5 | 12 121 | 12 190 | 237 | 29 | 4.48 (0.79 to 26.54) | 85.0 | 1.547 | 0.05 to 388.68 | |
RAAS=drugs affecting the renin angiotensin-aldosterone system (eg, angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, direct renin inhibitors); diuretics=thiazide and thiazide-like diuretics.
Other generic adverse events such as falls, hypotension, syncope, and fractures were examined by drug class, but no significant drug specific effects were observed (supplementary figures 1 and 12-14).
Cardiovascular and mortality outcomes
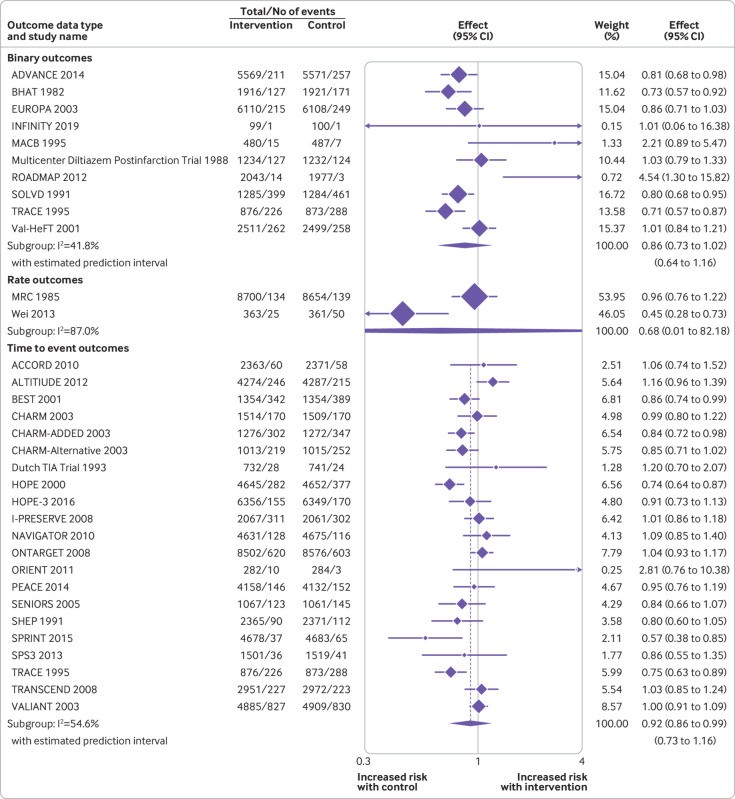
On average across studies examining outcomes using time-to-event analyses, antihypertensive treatment was associated with a reduction in cardiovascular death (hazard ratio 0.92, 95% confidence interval 0.86 to 0.99, τ2=0.011, I2=54.6%, n=21 studies; fig 5), all cause mortality (0.93, 0.88 to 0.98, τ2=0.008, I2=50.4%, n=32 studies; supplementary figure 15), and stroke (0.84, 0.76 to 0.93, τ2=0.013, I2=44.8%, n=17; supplementary figure 16) (table 2). No clear evidence was found of an association between antihypertensive treatment and myocardial infarction (supplementary figure 17).
Fig 5.
Random effects meta-analysis of randomised controlled trials examining the association between antihypertensive treatment and cardiovascular death
Sensitivity analyses
Meta-regression examining the relation between the observed treatment effects for each adverse event outcome and study quality found no clear evidence of an association (supplementary table 3). Funnel plots showed asymmetry (potential publication bias) for hyperkalaemia and hypotension events, with smaller studies missing for smaller effect estimates, but this was not evident for other adverse events examined (supplementary figures 18-22).
Supplementary figures 23-27 show the results of sensitivity analyses focusing on studies reporting adverse events that led to participant withdrawal from each trial (summarised in table 4). These analyses were limited to studies reporting acute kidney injury, gout, hyperkalaemia, hypotension, and syncope owing to availability of data. In these analyses, summary risk ratios for hyperkalaemia, hypotension, and syncope were increased compared with the primary analysis including all studies. However, there was no longer evidence that acute kidney injury was associated with antihypertensive treatment (table 4).
Table 4.
Sensitivity analyses showing meta-analysis results focusing on trials reporting the association between antihypertensive treatment and adverse events which led to permanent withdrawal from a trial
| Outcome | No of studies | Sample size | Events | Risk ratio (95% CI) | I2 (%) | τ2 | 95% prediction interval | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention group | Control group | Intervention group | Control group | |||||||
| Acute kidney injury38 39 61 62 64 85 | 6 | 30 672 | 39 350 | 128 | 146 | 1.34 (0.99 to 1.81) | 0.0 | 0.000 | 0.97 to 1.84 | |
| Gout17 55 | 2 | 15 998 | 15 959 | 179 | 38 | 3.41 (0.08 to 148.47) | 86.4 | 1.903 | - | |
| Hyperkalaemia30-34 38 42 45 59 62 64 82 85 92 | 13 | 34 580 | 38 953 | 398 | 189 | 2.28 (1.70 to 3.05) | 22.8 | 0.053 | 1.28 to 4.06 | |
| Hypotension27 30-32 34 38 39 42 52 53 62 64 65 70 76 80 82 85 | 18 | 51 063 | 56 030 | 1042 | 541 | 2.18 (1.84 to 2.58) | 34.0 | 0.033 | 1.43 to 3.32 | |
| Syncope17 27 61 64 77 85 | 6 | 34 146 | 34 289 | 62 | 28 | 2.17 (1.20 to 3.90) | 0.0 | 0.000 | 1.15 to 4.09 | |
Discussion
Data from random effects meta-analyses of 58 randomised controlled trials and more than 280 000 patients with hypertension confirm the known benefit of antihypertensive treatment in reducing the risk of cardiovascular disease.7 8 9 These data also confirm the association between antihypertensive treatment and adverse events10 11 and show how this association varies across some drug classes and for mild (eg, hypotension without falls) and more severe (eg, acute kidney injury, syncope) adverse events. Despite a widely held belief,95 96 no association was found between treatment and falls, but an association with syncope was observed, which is important as this can have a major impact on quality of life and health service use and could even result in death.97 98 99 100
These data will inform shared decision making around initiation and continuation of antihypertensive treatment, especially in patients with a high absolute risk of certain adverse outcomes as a result of previous events or poor renal function. Such discussions will become increasingly important as patients age and develop frailty and multimorbidity that could put them at increased risk of adverse events.101 102 103
Strengths and limitations of this study
More than 15 000 articles were screened for inclusion in this review and 58 randomised controlled trials including a large number of participants and adverse events were identified. Although power was likely to be sufficient to detect associations between antihypertensive treatment and adverse events, we observed statistically significant heterogeneity across studies, and the resulting prediction intervals were wide. Such heterogeneity might preclude pooling of some treatment effects, so caution should be exercised when interpreting the results. For acute kidney injury and hyperkalaemia events, the observed heterogeneity was partly explained by pooling of different drug classes, and heterogeneity was reduced when we focused on drugs that affect the renin angiotensin-aldosterone system. For other outcomes, the observed heterogeneity could not be explained by study quality or differences in the drug class examined in individual trials; however, populations of interest, interventions, comparators, and study designs varied widely across studies, which could have contributed to the observed variation.
As this review focused on adverse events, selective outcome reporting might also have been a problem. Evidence was found of publication bias for certain outcomes (hyperkalaemia and hypotension), confirming the findings of previous studies that showed adverse events are more likely to be reported in randomised controlled trials when they are statistically significant.104 This is understandable in the context of single trial reporting, but it would be better for the evidence base if all adverse events were reported in clinical trials to enable more complete meta-analyses in the future. It is a limitation of this review that original study authors were not contacted for these additional data.
This review focused on large randomised controlled trials with the aim of including those with at least 50 adverse events (and therefore 650 patient years of follow-up). This restriction on study size was chosen to make the review more manageable in terms of screening and analysis and avoid inclusion of numerous small early phase mechanistic studies of varying methodological quality. The cut-off for this inclusion was chosen to ensure studies provided adequately powered estimates of association between treatment and outcomes.15 It is possible that some useful trials could have been excluded, although many relevant trials were still available for inclusion.
Across all included trials, adverse events were poorly defined and probably varied across studies. For instance, many studies referred to syncope as an outcome, but did not say what type of syncopal event this might have included. A conservative approach to inclusion of outcomes was taken when possible, and only those explicitly stating the outcome of interest were included. For example, trials reporting hypotension or acute kidney injury were included, but those reporting hypotension or dizziness or renal impairment were excluded. Despite this approach, some studies were included that did not specify the thresholds used to define hypotension or acute kidney injury. This could have resulted in some relevant data for certain outcomes being missed, but this meant those that were included were likely to be sufficiently similar to enable pooling in a meta-analysis. Although the quality of adverse event ascertainment is likely to have varied between trials, it would not be expected to vary between treatment arms within trials. Thus it is unlikely that differences in the quality of adverse event ascertainment would have affected the relative treatment effects presented in this review.
We prespecified adverse events of interest based on those reported in recent large scale trials of blood pressure lowering treatment.4 16 17 Other patient focused harm outcomes, such as weight gain, sexual dysfunction, fatigue, and exercise intolerance might exist that were reported in the original trials but not captured as part of this review. However, the reporting of these events is likely to vary because many have no standardised definitions.105 106 107 Some might be captured but not reported.108 It is also important to note that randomised controlled trials often select populations with less frailty and multimorbidity who are more likely to tolerate treatment.109 Therefore, fewer adverse events might have been reported in the included trials than would be expected in the general population.
For outcomes included in meta-analyses, the time points at which they occurred varied across studies, and so the risk ratios and odds ratios provided relate to a summary across different times. We did synthesise hazard ratios when available, but these were rarely reported.
Comparison with other studies
Few previous meta-analyses have quantified the association between antihypertensive treatment and adverse events. Thomopoulos and colleagues examined the association between antihypertensive treatment and permanent discontinuation of treatment because of adverse events and found that antihypertensives were associated with a near doubling of risk (standardised relative risk 1.89, 95% confidence interval 1.51 to 2.39).10 110 This was similar to findings from our sensitivity analyses focusing on permanent withdrawal as a result of hyperkalaemia, hypotension, and syncope events. These associations were stronger than those observed in the primary analysis focusing on all adverse event reporting. It is possible that these events were more likely to be reported in the intervention group when they were considered serious enough to lead to withdrawal.104 Although the focus of this review was on adverse events, we found evidence for the beneficial effects of treatment on all cause mortality, cardiovascular mortality, and stroke, but not on myocardial infarction, as has been reported previously.4 8
Frey and colleagues11 focused on data from seven original studies investigating the harms of intensive blood pressure lowering targets (≤130 mm Hg) versus usual care (<140 mm Hg). Although this number of studies was insufficient to conduct a meta-analysis, the descriptive summary suggested that intensive blood pressure lowering might be associated with higher rates of serious adverse events. The present analysis included all trials of blood pressure lowering treatment enabling meta-analyses of the association between antihypertensive treatment and adverse events and how this association varies across mild and more severe adverse events. We identified an increased risk of acute kidney injury, hyperkalaemia, hypotension, and syncope with antihypertensive treatment.
Stratified analyses by drug class suggested that associations with acute kidney injury and hyperkalaemia were mostly driven by the use of drugs that affect the renin angiotensin-aldosterone system (eg, angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, and direct renin inhibitors). However, no evidence was found of an association with this class of drug and falls, fractures, gout, or hypokalaemia. In analyses that focused on patients prescribed diuretics, a 10-fold increase in the risk of hypokalaemia was observed, but this association was derived from only three trials, with high between study heterogeneity. The pooled effect had large confidence intervals and was not statistically significant. This null finding contrasted with previous studies that recommend routine monitoring of potassium to detect hypokalaemia in patients prescribed diuretics.111 This could be explained by the small number of included trials examining this drug class.
Much debate exists in the literature on the association between antihypertensive treatment and falls.95 96 112 113 Most data showing an association originate from observational studies,112 113 which are prone to bias from confounding by indication.14 Despite conflicting evidence, a wide held belief remains that antihypertensive treatment increases the risk of falls.95 96 This study found no evidence for an association between treatment or lower blood pressure targets and falls, but an association was found with syncope. Although syncope is a common cause of falls, not all falls are caused by syncope and therefore not all falls will be related to blood pressure lowering treatment.114 In addition, reporting of falls might vary among participants (ie, not all participants will be admitted to hospital or see their primary care doctor after a fall) and participants might be more likely to be withdrawn from a trial when experiencing events that could be considered precursors to falls and fractures (eg, hypotension). If this were the case and hypotension events are not dealt with by treating doctors, the incidence of serious falls and fractures associated with antihypertensive treatment could be greater in routine clinical practice.
Policy implications
The present data clearly show the benefits and harms of antihypertensive treatment for specific cardiovascular outcomes and adverse events. The data also highlight that certain adverse events might be specific to certain drug classes (eg, renin angiotensin-aldosterone system drugs and acute kidney injury or hyperkalaemia). This detail is important because some adverse events reported in randomised controlled trials might be considered relatively mild and worth the risk when weighed against the substantial benefits of treatment. These new data will allow patients and clinicians to take into consideration these benefits and risks, as has been recommended in clinical guidelines.6 This is particularly important now that guidelines for the management of hypertension across the world increasingly recommend more intensive treatment,2 3 5 115 but with conflicting blood pressure targets, meaning a personalised approach is required for each patient.
The present data should ideally be combined with information about an individual’s absolute risk of each harm outcome to make informed, personalised treatment decisions. This process is complex and requires real time data, which suggests that tools embedded in electronic health records will be the way forward. Further work is needed to understand better the results of this meta-analysis (which summarises average risk ratios across all participants and studies) in the context of individualised absolute risks so that treatment initiation and discontinuation can be targeted at those with the most to gain.116 In the absence of such information, doctors should focus on patients who have experienced previous adverse events or have poor renal function.17 110 117
Conclusions
This review found no evidence of an association between antihypertensive treatment and falls (primary outcome) or fractures but did show a variation in the association between antihypertensive treatment and mild (eg, hypotension without falls) and more severe (eg, acute kidney injury, syncope) adverse events. Some effects were found to be specific to the drug class used. In patients at high risk of drug harms because of previous adverse events or poor renal function, these data should be used to inform shared decision making between doctors and patients around initiation and continuation of antihypertensive treatment.
What is already known on this topic
Many meta-analyses exist of randomised controlled trials that examine the efficacy of antihypertensive treatment, but few have studied potential harms
Existing meta-analyses have focused on the association between antihypertensive treatment and all adverse events, grouping mild and more serious outcomes
The association between antihypertensive treatment and specific adverse events is unclear
What this study adds
In a meta-analysis of 58 randomised controlled trials, including 280 638 participants, no evidence was found of an association between antihypertensive treatment and falls (primary outcome) or fractures
Evidence was, however, found of an association between antihypertensive treatment and potentially both mild (hypotension) and more severe (acute kidney injury, syncope) adverse events
These data might be used to inform shared decision making between doctors and patients about the benefits and harms of initiation and continuation of antihypertensives, especially in those at high risk of harm because of previous adverse events or poor renal function
Acknowledgments
We thank Margaret Ogden for her advice as a patient and public contributor to this project, and Lucy Curtin for administrative support throughout the project.
The STRAtifying Treatments In the multi-morbid Frail elderlY (STRATIFY) investigators include the authors and: Amitava Banerjee, associate professor in clinical data science and honorary consultant cardiologist, Institute of Health Informatics, University College London; Andrew Clegg, professor of geriatric medicine, University of Leeds and Bradford Teaching Hospitals NHS Foundation Trust; John Gladman, professor of medicine of older people, School of Medicine, University of Nottingham; Simon Griffin, professor of primary care, Department of Public Health and Primary Care, Primary Care Unit, University of Cambridge; and Margaret Ogden, patient and public involvement advisor.
Web extra.
Extra material supplied by authors
Supplementary information: additional tables 1-3 and figures 1-27
Contributors: JPS conceived the study and wrote the protocol with FDRH, RJM, RS, and RR. NR did the literature searches. AA, MS, BP, SF, CK, AD, and JPS screened articles for inclusion. MH, AA, LA, AD, and BL extracted data for analysis. MH undertook the meta-analysis and produced forest plots and summary results, under supervision of RR. AA and JPS wrote the first draft of the manuscript. All authors revised the manuscript and approved the final version. JPS is the guarantor for this work and accepts full responsibility for the conduct of the study, had access to the data, and controlled the decision to publish. The corresponding author (JPS) attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study was funded by the Wellcome Trust and Royal Society through a Sir Henry Dale fellowship held by JPS (ref 211182/Z/18/Z) and the National Institute for Health Research (NIHR) School for Primary Care (project 430). JPS also receives funding through an NIHR Oxford Biomedical Research Centre (BRC) senior fellowship. RJMcM is supported by an NIHR senior investigator award. FDRH acknowledges part support from the NIHR SPCR, the NIHR CLAHRC Oxford, and the NIHR Oxford BRC. BL is supported by a Fonds de recherche du Québec – Santé Postdoctoral Training Fellowship. KIES is funded by an NIHR School for Primary Care Research launching fellowship. MS is supported by the NIHR Oxford BRC. SLF is part funded by the NIHR Oxford BRC and NIHR Applied Research Collaborations Oxford and Thames Valley. JUS was funded by a Cancer Research UK Prevention fellowship (C55650/A21464). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. The sponsor and funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: authors had financial support from the Wellcome Trust, Royal Society, Cancer Research UK, Fonds de recherche du Québec–Santé and National Institute for Health Research for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required.
The manuscript’s guarantor (JPS) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned (and, if relevant, registered) have been explained.
Data sharing: Requests for data sharing should be sent to the corresponding author at james.sheppard@phc.ox.ac.uk.
Dissemination to participants and related patient and public communities: No participants were included in this work. The findings of this work, including a lay summary of the results, will be made available on the study website (www.phc.ox.ac.uk/research/stratified-treatments/studies/stratifying-treatments-in-the-multi-morbid-frail-elderly-stratify-antihypertensives).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903-13. . 10.1016/S0140-6736(02)11911-8 [DOI] [PubMed] [Google Scholar]
- 2. Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. Hypertension 2018;71:e13-115. 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 3. Williams B, Mancia G, Spiering W, et al. ESC Scientific Document Group 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021-104. . 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 4. Wright JT, Jr, Williamson JD, Whelton PK, et al. SPRINT Research Group A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med 2015;373:2103-16. . 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Institute for Health and Care Excellence. Hypertension in adults: diagnosis and management. NICE guideline [NG136]. https://www.nice.org.uk/guidance/ng136. Published 2019.
- 6.NICE. Overview | Multimorbidity: clinical assessment and management | Guidance | NICE. 2016. https://www.nice.org.uk/guidance/ng56. Accessed May 29, 2020.
- 7. Weiss J, Freeman M, Low A, et al. Benefits and harms of intensive blood pressure treatment in adults aged 60 years or older: A systematic review and meta-analysis. Ann Intern Med 2017;166:419-29. . 10.7326/M16-1754 [DOI] [PubMed] [Google Scholar]
- 8. Brunström M, Carlberg B. Association of Blood Pressure Lowering With Mortality and Cardiovascular Disease Across Blood Pressure Levels: A Systematic Review and Meta-analysis. JAMA Intern Med 2018;178:28-36. . 10.1001/jamainternmed.2017.6015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016;387:957-67. . 10.1016/S0140-6736(15)01225-8 [DOI] [PubMed] [Google Scholar]
- 10. Thomopoulos C, Parati G, Zanchetti A. Effects of blood-pressure-lowering treatment in hypertension: 9. Discontinuations for adverse events attributed to different classes of antihypertensive drugs: meta-analyses of randomized trials. J Hypertens 2016;34:1921-32. . 10.1097/HJH.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 11. Frey L, Gravestock I, Pichierri G, Steurer J, Burgstaller JM. Serious adverse events in patients with target-oriented blood pressure management: a systematic review. J Hypertens 2019;37:2135-44. . 10.1097/HJH.0000000000002176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006-12. . 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 13. Caparrotta TM, Dear JW, Colhoun HM, Webb DJ. Pharmacoepidemiology: Using randomised control trials and observational studies in clinical decision-making. Br J Clin Pharmacol 2019;85:1907-24. . 10.1111/bcp.14024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Freemantle N, Marston L, Walters K, Wood J, Reynolds MR, Petersen I. Making inferences on treatment effects from real world data: propensity scores, confounding by indication, and other perils for the unwary in observational research. BMJ 2013;347:f6409. . 10.1136/bmj.f6409 [DOI] [PubMed] [Google Scholar]
- 15. Stalenhoef PA, Diederiks JP, de Witte LP, Schiricke KH, Crebolder HFJ. Impact of gait problems and falls on functioning in independent living persons of 55 years and over: a community survey. Patient Educ Couns 1999;36:23-31. . 10.1016/S0738-3991(98)00071-8 [DOI] [PubMed] [Google Scholar]
- 16. Cushman WC, Evans GW, Byington RP, et al. ACCORD Study Group Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575-85. . 10.1056/NEJMoa1001286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lonn EM, Bosch J, López-Jaramillo P, et al. HOPE-3 Investigators Blood-Pressure Lowering in Intermediate-Risk Persons without Cardiovascular Disease. N Engl J Med 2016;374:2009-20. . 10.1056/NEJMoa1600175 [DOI] [PubMed] [Google Scholar]
- 18. Section 2: AKI Definition. Kidney Int Suppl (2011) 2012;2:19-36. 10.1038/kisup.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higgins JPT, Altman DG, Gøtzsche PC, et al. Cochrane Bias Methods Group. Cochrane Statistical Methods Group The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. . 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Perneger TV. Estimating the relative hazard by the ratio of logarithms of event-free proportions. Contemp Clin Trials 2008;29:762-6. . 10.1016/j.cct.2008.06.002 [DOI] [PubMed] [Google Scholar]
- 21. Hartung J, Knapp G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat Med 2001;20:3875-89. . 10.1002/sim.1009 [DOI] [PubMed] [Google Scholar]
- 22. Rücker G, Cates CJ, Schwarzer G. Methods for including information from multi-arm trials in pairwise meta-analysis. Res Synth Methods 2017;8:392-403. . 10.1002/jrsm.1259 [DOI] [PubMed] [Google Scholar]
- 23. Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. . 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 24. Sedgwick P. Understanding the ecological fallacy. BMJ 2015;351:h4773. . 10.1136/bmj.h4773 [DOI] [PubMed] [Google Scholar]
- 25. Fisher DJ, Carpenter JR, Morris TP, Freeman SC, Tierney JF. Meta-analytical methods to identify who benefits most from treatments: daft, deluded, or deft approach? BMJ 2017;356:j573. . 10.1136/bmj.j573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Riley RD, Debray TPA, Fisher D, et al. Individual participant data meta-analysis to examine interactions between treatment effect and participant-level covariates: Statistical recommendations for conduct and planning. Stat Med 2020;39:2115-37. . 10.1002/sim.8516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Friedman LM. A randomized trial of propranolol in patients with acute myocardial infarction. I. Mortality results. JAMA 1982;247:1707-14. . 10.1001/jama.1982.03320370021023 [DOI] [PubMed] [Google Scholar]
- 28. Verdecchia P, Staessen JA, Angeli F, et al. Cardio-Sis investigators Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet 2009;374:525-33. . 10.1016/S0140-6736(09)61340-4 [DOI] [PubMed] [Google Scholar]
- 29. Chinese Cardiac Study (CCS-1) Collaborative Group Oral captopril versus placebo among 14,962 patients with suspected acute myocardial infarction: a multicenter, randomized, double-blind, placebo controlled clinical trial. Chinese Cardiac Study (CCS-1) Collaborative Group. Chin Med J (Engl) 1997;110:834-8. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=med4&AN=9772413. [PubMed] [Google Scholar]
- 30. Yusuf S, Pfeffer MA, Swedberg K, et al. CHARM Investigators and Committees Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003;362:777-81. . 10.1016/S0140-6736(03)14285-7 [DOI] [PubMed] [Google Scholar]
- 31. McMurray JJV, Östergren J, Swedberg K, et al. CHARM Investigators and Committees Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet 2003;362:767-71. . 10.1016/S0140-6736(03)14283-3 [DOI] [PubMed] [Google Scholar]
- 32. Granger CB, McMurray JJV, Yusuf S, et al. CHARM Investigators and Committees Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet 2003;362:772-6. . 10.1016/S0140-6736(03)14284-5 [DOI] [PubMed] [Google Scholar]
- 33. Lewis EJ, Hunsicker LG, Clarke WR, et al. Collaborative Study Group Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001;345:851-60. . 10.1056/NEJMoa011303 [DOI] [PubMed] [Google Scholar]
- 34. Swedberg K, Held P, Kjekshus J, Rasmussen K, Rydén L, Wedel H. Effects of the early administration of enalapril on mortality in patients with acute myocardial infarction. Results of the Cooperative New Scandinavian Enalapril Survival Study II (CONSENSUS II). N Engl J Med 1992;327:678-84. . 10.1056/NEJM199209033271002 [DOI] [PubMed] [Google Scholar]
- 35. Ueda S, Morimoto T, Ando S, et al. DIME Investigators A randomised controlled trial for the evaluation of risk for type 2 diabetes in hypertensive patients receiving thiazide diuretics: Diuretics In the Management of Essential hypertension (DIME) study. BMJ Open 2014;4:e004576. . 10.1136/bmjopen-2013-004576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Algra A, van Gijn J, Jaap Kappelle L, van Latum CJ, Koudstaal PJ, The Dutch TIA Trial Study Group Trial of secondary prevention with atenolol after transient ischemic attack or nondisabling ischemic stroke. Stroke 1993;24:543-8. . 10.1161/01.STR.24.4.543 [DOI] [PubMed] [Google Scholar]
- 37. Wright JT, Jr, Bakris G, Greene T, et al. African American Study of Kidney Disease and Hypertension Study Group Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 2002;288:2421-31. . 10.1001/jama.288.19.2421 [DOI] [PubMed] [Google Scholar]
- 38. Zannad F, McMurray JJV, Krum H, et al. EMPHASIS-HF Study Group Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011;364:11-21. . 10.1056/NEJMoa1009492 [DOI] [PubMed] [Google Scholar]
- 39. Fox KM, Bertrand M, Ferrari R, et al. EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet 2003;362:782-8. . 10.1016/S0140-6736(03)14286-9 [DOI] [PubMed] [Google Scholar]
- 40. Staessen J. The determinants and prognostic significance of serum uric acid in elderly patients of the European Working Party on High Blood Pressure in the Elderly trial. Am J Med 1991;90(SUPPL. 1):50S-4S. . 10.1016/0002-9343(91)90439-5 [DOI] [PubMed] [Google Scholar]
- 41. GISSI-3: effects of lisinopril and transdermal glyceryl trinitrate singly and together on 6-week mortality and ventricular function after acute myocardial infarction. Gruppo Italiano per lo Studio della Sopravvivenza nell’infarto Miocardico. Lancet 1994;343:1115-22. https://www.ncbi.nlm.nih.gov/pubmed/7910229. [PubMed] [Google Scholar]
- 42. Disertori M, Latini R, Barlera S, et al. GISSI-AF Investigators Valsartan for prevention of recurrent atrial fibrillation. N Engl J Med 2009;360:1606-17. . 10.1056/NEJMoa0805710 [DOI] [PubMed] [Google Scholar]
- 43. Stratton I, Manley S, Holman R, Turner R. Hypertension in Diabetes Study IV. Therapeutic requirements to maintain tight blood pressure control. Diabetologia 1996;39:1554-61. . 10.1007/s001250050614 [DOI] [PubMed] [Google Scholar]
- 44. Gerstein HC, Yusuf S, Mann JFE, et al. Heart Outcomes Prevention Evaluation Study Investigators Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet 2000;355:253-9. . 10.1016/S0140-6736(99)12323-7 [DOI] [PubMed] [Google Scholar]
- 45. Gianni M, Bosch J, Pogue J, et al. Effect of long-term ACE-inhibitor therapy in elderly vascular disease patients. Eur Heart J 2007;28:1382-8. . 10.1093/eurheartj/ehm017 [DOI] [PubMed] [Google Scholar]
- 46. Yusuf S, Lonn E, Pais P, et al. HOPE-3 Investigators Blood-Pressure and Cholesterol Lowering in Persons without Cardiovascular Disease. N Engl J Med 2016;374:2032-43. . 10.1056/NEJMoa1600177 [DOI] [PubMed] [Google Scholar]
- 47. Peters R, Beckett N, Burch L, et al. The effect of treatment based on a diuretic (indapamide) +/- ACE inhibitor (perindopril) on fractures in the Hypertension in the Very Elderly Trial (HYVET). Age Ageing 2010;39:609-16. . 10.1093/ageing/afq071 [DOI] [PubMed] [Google Scholar]
- 48. Margolis KL, Palermo L, Vittinghoff E, et al. Intensive blood pressure control, falls, and fractures in patients with type 2 diabetes: the ACCORD trial. J Gen Intern Med 2014;29:1599-606. . 10.1007/s11606-014-2961-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. White WB, Wakefield DB, Moscufo N, et al. Effects of intensive versus standard ambulatory blood pressure control on cerebrovascular outcomes in older people (INFINITY). Circulation 2019;140:1626-35. . 10.1161/CIRCULATIONAHA.119.041603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wei Y, Jin Z, Shen G, et al. Effects of intensive antihypertensive treatment on Chinese hypertensive patients older than 70 years. J Clin Hypertens (Greenwich) 2013;15:420-7. . 10.1111/jch.12094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Massie BM, Carson PE, McMurray JJ, et al. I-PRESERVE Investigators Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008;359:2456-67. . 10.1056/NEJMoa0805450 [DOI] [PubMed] [Google Scholar]
- 52. The MACB Study Group Effect of metoprolol on death and cardiac events during a 2-year period after coronary artery bypass grafting. Eur Heart J 1995;16:1825-32. https://www.ncbi.nlm.nih.gov/pubmed/8682014. 10.1093/oxfordjournals.eurheartj.a060835 [DOI] [PubMed] [Google Scholar]
- 53. Hjalmarson A, Goldstein S, Fagerberg B, et al. MERIT-HF Study Group Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). JAMA 2000;283:1295-302. . 10.1001/jama.283.10.1295 [DOI] [PubMed] [Google Scholar]
- 54. Miall WE, Greenberg G, Brennan PJ. The Medical Research Council’s trial of treatment for mild hypertension. Curr Med Res Opin 1982;8:47-56. 10.1185/03007998209110130. [DOI] [Google Scholar]
- 55. Medical Research Council Working Party MRC trial of treatment of mild hypertension. Br Med J (Clin Res Ed). 1985;29:97-104. 10.2307/29519852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Abrams J, Bigger JT, Multicenter Diltiazem Postinfarction Trial Research Group The effect of diltiazem on mortality and reinfarction after myocardial infarction. N Engl J Med 1988;319:385-92. . 10.1056/NEJM198808183190701 [DOI] [PubMed] [Google Scholar]
- 57. McMurray JJ, Holman RR, Haffner SM, et al. NAVIGATOR Study Group Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med 2010;362:1477-90. . 10.1056/NEJMoa1001121 [DOI] [PubMed] [Google Scholar]
- 58. Dens JA, Desmet WJ, Coussement P, et al. Long term effects of nisoldipine on the progression of coronary atherosclerosis and the occurrence of clinical events: the NICOLE study. Heart 2003;89:887-92. . 10.1136/heart.89.8.887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Maschio G, Alberti D, Janin G, et al. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. N Engl J Med 1996;334:939-45. . 10.1056/NEJM199604113341502 [DOI] [PubMed] [Google Scholar]
- 60. Lawlor B, Segurado R, Kennelly S, et al. NILVAD Study Group Nilvadipine in mild to moderate Alzheimer disease: A randomised controlled trial. PLoS Med 2018;15:e1002660. 10.1371/journal.pmed.1002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yusuf S, Teo KK, Pogue J, et al. ONTARGET Investigators Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008;358:1547-59. . 10.1056/NEJMoa0801317 [DOI] [PubMed] [Google Scholar]
- 62. Imai E, Chan JCN, Ito S, et al. ORIENT study investigators Effects of olmesartan on renal and cardiovascular outcomes in type 2 diabetes with overt nephropathy: a multicentre, randomised, placebo-controlled study. Diabetologia 2011;54:2978-86. . 10.1007/s00125-011-2325-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Braunwald E, Domanski MJ, Fowler SE, et al. PEACE Trial Investigators Angiotensin-converting-enzyme inhibition in stable coronary artery disease. N Engl J Med 2004;351:2058-68. . 10.1056/NEJMoa042739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yusuf S, Diener HC, Sacco RL, et al. PRoFESS Study Group Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med 2008;359:1225-37. . 10.1056/NEJMoa0804593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. MacMahon S, Neal B, Tzourio C, et al. PROGRESS Collaborative Group Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001;358:1033-41. . 10.1016/S0140-6736(01)06178-5 [DOI] [PubMed] [Google Scholar]
- 66. Haller H, Ito S, Izzo JL, Jr, et al. ROADMAP Trial Investigators Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med 2011;364:907-17. . 10.1056/NEJMoa1007994 [DOI] [PubMed] [Google Scholar]
- 67. Menne J, Izzo JL, Jr, Ito S, et al. ROADMAP investigators Prevention of microalbuminuria in patients with type 2 diabetes and hypertension. J Hypertens 2012;30:811-8, discussion 818. . 10.1097/HJH.0b013e328351856d [DOI] [PubMed] [Google Scholar]
- 68. Weir MR, Yeh F, Silverman A, et al. Safety and feasibility of achieving lower systolic blood pressure goals in persons with type 2 diabetes: the SANDS trial. J Clin Hypertens (Greenwich) 2009;11:540-8. . 10.1111/j.1751-7176.2009.00121.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Flather MD, Shibata MC, Coats AJS, et al. SENIORS Investigators Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J 2005;26:215-25. https://academic.oup.com/eurheartj/article/26/3/215/2888055. 10.1093/eurheartj/ehi115 [DOI] [PubMed] [Google Scholar]
- 70. Chalmers J, Arima H, Woodward M, et al. Effects of combination of perindopril, indapamide, and calcium channel blockers in patients with type 2 diabetes mellitus: results from the Action In Diabetes and Vascular Disease: Preterax and Diamicron Controlled Evaluation (ADVANCE) trial. Hypertension 2014;63:259-64. . 10.1161/HYPERTENSIONAHA.113.02252 [DOI] [PubMed] [Google Scholar]
- 71. SHEP Cooperative Research Group Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991;265:3255-64. . 10.1001/jama.1991.03460240051027 [DOI] [PubMed] [Google Scholar]
- 72. Franse LV, Pahor M, Di Bari M, Somes GW, Cushman WC, Applegate WB. Hypokalemia associated with diuretic use and cardiovascular events in the Systolic Hypertension in the Elderly Program. Hypertension 2000;35:1025-30. 10.1161/01.HYP.35.5.1025. [DOI] [PubMed] [Google Scholar]
- 73. Yusuf S, Pitt B, Davis CE, Hood WB, Jr, Cohn JN, SOLVD Investigators Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med 1992;327:685-91. . 10.1056/NEJM199209033271003 [DOI] [PubMed] [Google Scholar]
- 74. Wu J-L, Hou D-Y, Ma G-L, et al. Effects of long-term low-dose spironolactone treatment in patients with New York Heart Association functional class II heart failure: a 10-year prospective study. Int J Clin Exp Med 2016;9:15689-98. [Google Scholar]
- 75. Benavente OR, Coffey CS, Conwit R, et al. SPS3 Study Group Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013;382:507-15. . 10.1016/S0140-6736(13)60852-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Norwegian Multicenter Study Group Timolol-induced reduction in mortality and reinfarction in patients surviving acute myocardial infarction. N Engl J Med 1981;304:801-7. . 10.1056/NEJM198104023041401 [DOI] [PubMed] [Google Scholar]
- 77. Yusuf S, Teo K, Anderson C, et al. Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) Investigators Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet 2008;372:1174-83. 10.1016/S0140-6736(08)61242-8. [DOI] [PubMed] [Google Scholar]
- 78. Julius S, Nesbitt SD, Egan BM, et al. Trial of Preventing Hypertension (TROPHY) Study Investigators Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med 2006;354:1685-97. . 10.1056/NEJMoa060838 [DOI] [PubMed] [Google Scholar]
- 79. Fried LF, Emanuele N, Zhang JH, et al. VA NEPHRON-D Investigators Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013;369:1892-903. . 10.1056/NEJMoa1303154 [DOI] [PubMed] [Google Scholar]
- 80. Cohn JN, Tognoni G, Valsartan Heart Failure Trial Investigators A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001;345:1667-75. . 10.1056/NEJMoa010713 [DOI] [PubMed] [Google Scholar]
- 81. The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. Lancet 1993;342:821-8. [PubMed] [Google Scholar]
- 82. Pfeffer MA, McMurray JJV, Velazquez EJ, et al. Valsartan in Acute Myocardial Infarction Trial Investigators Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003;349:1893-906. . 10.1056/NEJMoa032292 [DOI] [PubMed] [Google Scholar]
- 83. Neurath HM, Goldman AI, Lavin MA, et al. Evaluation of drug treatment in mild hypertension: VA-NHLBI feasibility trial. Plan and preliminary results of a two-year feasibility trial for a multicenter intervention study to evaluate the benefits versus the disadvantages of treating mild hypertension. Prepared for the Veterans Administration-National Heart, Lung, and Blood Institute Study Group for Evaluating Treatment in Mild Hypertension. Ann N Y Acad Sci 1978;304:267-92. 10.1111/j.1749-6632.1978.tb25604.x. [DOI] [PubMed] [Google Scholar]
- 84. Beckett NS, Peters R, Fletcher AE, et al. HYVET Study Group Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008;358:1887-98. . 10.1056/NEJMoa0801369 [DOI] [PubMed] [Google Scholar]
- 85. Parving HH, Brenner BM, McMurray JJV, et al. ALTITUDE Investigators Baseline characteristics in the Aliskiren Trial in Type 2 Diabetes Using Cardio-Renal Endpoints (ALTITUDE). J Renin Angiotensin Aldosterone Syst 2012;13:387-93. . 10.1177/1470320311434818 [DOI] [PubMed] [Google Scholar]
- 86. Solomon SD, Shin SH, Shah A, et al. Aliskiren Study in Post-MI Patients to Reduce Remodeling (ASPIRE) Investigators Effect of the direct renin inhibitor aliskiren on left ventricular remodelling following myocardial infarction with systolic dysfunction. Eur Heart J 2011;32:1227-34. . 10.1093/eurheartj/ehq522 [DOI] [PubMed] [Google Scholar]
- 87. Eichhorn EJ, Domanski MJ, Krause-Steinrauf H, Bristow MRLP, Lavori PW, Beta-Blocker Evaluation of Survival Trial Investigators A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med 2001;344:1659-67. . 10.1056/NEJM200105313442202 [DOI] [PubMed] [Google Scholar]
- 88. Wright JT, Jr, Agodoa L, Contreras G, et al. African American Study of Kidney Disease and Hypertension Study Group Successful blood pressure control in the African American Study of Kidney Disease and Hypertension. Arch Intern Med 2002;162:1636-43. . 10.1001/archinte.162.14.1636 [DOI] [PubMed] [Google Scholar]
- 89. Peters R, Beckett N, Burch L, et al. The effect of treatment based on a diuretic (indapamide) +/- ACE inhibitor (perindopril) on fractures in the Hypertension in the Very Elderly Trial (HYVET). Age Ageing 2010;39:609-16. . 10.1093/ageing/afq071 [DOI] [PubMed] [Google Scholar]
- 90. Amery A, Birkenhäger W, Brixko P, et al. Influence of antihypertensive drug treatment on morbidity and mortality in patients over the age of 60 years. European Working Party on High blood pressure in the Elderly (EWPHE) results: sub-group analysis on entry stratification. J Hypertens Suppl 1986;4:S642-7. https://www.ncbi.nlm.nih.gov/pubmed/3475430. [PubMed] [Google Scholar]
- 91. Køber L, Torp-Pedersen C, Carlsen JE, et al. Trandolapril Cardiac Evaluation (TRACE) Study Group A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 1995;333:1670-6. . 10.1056/NEJM199512213332503 [DOI] [PubMed] [Google Scholar]
- 92. Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G, Heart Outcomes Prevention Evaluation Study Investigators Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 2000;342:145-53. . 10.1056/NEJM200001203420301 [DOI] [PubMed] [Google Scholar]
- 93. Menne J, Izzo JL, Jr, Ito S, et al. ROADMAP investigators Prevention of microalbuminuria in patients with type 2 diabetes and hypertension. J Hypertens 2012;30:811-8, discussion 818. . 10.1097/HJH.0b013e328351856d [DOI] [PubMed] [Google Scholar]
- 94. Verdecchia P, Staessen JA, Angeli F, et al. Cardio-Sis investigators Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet 2009;374:525-33. 10.1016/S0140-6736(09)61340-4. [DOI] [PubMed] [Google Scholar]
- 95. Benetos A, Petrovic M, Strandberg T. Hypertension Management in Older and Frail Older Patients. Circ Res 2019;124:1045-60. . 10.1161/CIRCRESAHA.118.313236 [DOI] [PubMed] [Google Scholar]
- 96. Cai A, Calhoun DA. Antihypertensive Medications and Falls in the Elderly. Am J Hypertens 2018;31:281-3. . 10.1093/ajh/hpx203 [DOI] [PubMed] [Google Scholar]
- 97. O’Brien H, Anne Kenny R. Syncope in the Elderly. Eur Cardiol 2014;9:28-36. . 10.15420/ecr.2014.9.1.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Marrison VK, Fletcher A, Parry SW. The older patient with syncope: practicalities and controversies. Int J Cardiol 2012;155:9-13. . 10.1016/j.ijcard.2010.10.055 [DOI] [PubMed] [Google Scholar]
- 99. da Silva RM. Syncope: epidemiology, etiology, and prognosis. Front Physiol 2014;5:471. . 10.3389/fphys.2014.00471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sun BC. Quality-of-life, health service use, and costs associated with syncope. Prog Cardiovasc Dis 2013;55:370-5. . 10.1016/j.pcad.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 101. Tinetti ME, Han L, Lee DSH, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med 2014;174:588-95. . 10.1001/jamainternmed.2013.14764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Benetos A, Labat C, Rossignol P, et al. Treatment with multiple blood pressure medications, achieved blood pressure, and mortality in older nursing home residents: The PARTAGE study. JAMA Intern Med 2015;175:989-95. . 10.1001/jamainternmed.2014.8012 [DOI] [PubMed] [Google Scholar]
- 103. Mansfield KE, Nitsch D, Smeeth L, Bhaskaran K, Tomlinson LA. Prescription of renin-angiotensin system blockers and risk of acute kidney injury: a population-based cohort study. BMJ Open 2016;6:e012690. . 10.1136/bmjopen-2016-012690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chan AW, Hróbjartsson A, Haahr MT, Gøtzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291:2457-65. . 10.1001/jama.291.20.2457 [DOI] [PubMed] [Google Scholar]
- 105. Phillips R, Hazell L, Sauzet O, Cornelius V. Analysis and reporting of adverse events in randomised controlled trials: a review. BMJ Open 2019;9:e024537. . 10.1136/bmjopen-2018-024537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Pitrou I, Boutron I, Ahmad N, Ravaud P. Reporting of safety results in published reports of randomized controlled trials. Arch Intern Med 2009;169:1756-61. . 10.1001/archinternmed.2009.306 [DOI] [PubMed] [Google Scholar]
- 107. Finsterer J, Mahjoub SZ. Fatigue in healthy and diseased individuals. Am J Hosp Palliat Care 2014;31:562-75. . 10.1177/1049909113494748 [DOI] [PubMed] [Google Scholar]
- 108. Golder S, Loke YK, Wright K, Norman G. Reporting of Adverse Events in Published and Unpublished Studies of Health Care Interventions: A Systematic Review. PLoS Med 2016;13:e1002127. 10.1371/journal.pmed.1002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sheppard JP, Lown M, Burt J, et al. Generalizability of Blood Pressure Lowering Trials to Older Patients: Cross-Sectional Analysis. J Am Geriatr Soc 2020;68:2508-15. . 10.1111/jgs.16749 [DOI] [PubMed] [Google Scholar]
- 110. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering treatment in hypertension: 8. Outcome reductions vs. discontinuations because of adverse drug events - meta-analyses of randomized trials. J Hypertens 2016;34:1451-63. . 10.1097/HJH.0000000000000972 [DOI] [PubMed] [Google Scholar]
- 111. Clayton JA, Rodgers S, Blakey J, Avery A, Hall IP. Thiazide diuretic prescription and electrolyte abnormalities in primary care. Br J Clin Pharmacol 2006;61:87-95. . 10.1111/j.1365-2125.2005.02531.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kahlaee HR, Latt MD, Schneider CR. Association Between Chronic or Acute Use of Antihypertensive Class of Medications and Falls in Older Adults. A Systematic Review and Meta-Analysis. Am J Hypertens 2018;31:467-79. . 10.1093/ajh/hpx189 [DOI] [PubMed] [Google Scholar]
- 113. Lipsitz LA, Habtemariam D, Gagnon M, et al. Reexamining the Effect of Antihypertensive Medications on Falls in Old Age. Hypertension 2015;66:183-9. 10.1161/HYPERTENSIONAHA.115.05513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Brignole M. Distinguishing syncopal from non-syncopal causes of fall in older people. Age Ageing 2006;35(Suppl 2):ii46-50. 10.1093/ageing/afl086 [DOI] [PubMed] [Google Scholar]
- 115. Qaseem A, Wilt TJ, Rich R, Humphrey LL, Frost J, Forciea MA, Clinical Guidelines Committee of the American College of Physicians and the Commission on Health of the Public and Science of the American Academy of Family Physicians Pharmacologic treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: A clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2017;166:430-7. . 10.7326/M16-1785 [DOI] [PubMed] [Google Scholar]
- 116. Sheppard JP, Burt J, Lown M, et al. OPTIMISE Investigators Effect of Antihypertensive Medication Reduction vs Usual Care on Short-term Blood Pressure Control in Patients With Hypertension Aged 80 Years and Older: The OPTIMISE Randomized Clinical Trial. JAMA 2020;323:2039-51. . 10.1001/jama.2020.4871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Souverein PC, Van Staa TP, Egberts AC, De la Rosette JJ, Cooper C, Leufkens HG. Use of alpha-blockers and the risk of hip/femur fractures. J Intern Med 2003;254:548-54. https://onlinelibrary.wiley.com/doi/pdf/10.1111/j.1365-2796.2003.01227.x. 10.1111/j.1365-2796.2003.01227.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: additional tables 1-3 and figures 1-27