Abstract
Chemical synapses are heterogeneous junctions formed between neurons that are specialized for the conversion of electrical impulses into the exocytotic release of neurotransmitters. Voltage-gated Ca2+ (Cav) channels play a pivotal role in this process as they are the major conduits for the Ca2+ ions that trigger the fusion of neurotransmitter-containing vesicles with the presynaptic membrane. Alterations in the intrinsic function of these channels, and their positioning within the active zone can profoundly alter the timing and strength of synaptic output. Advances in optical and electron microscopic imaging, structural biology and molecular techniques have facilitated recent breakthroughs in our understanding of the properties of Cav channels that support their presynaptic functions. Here we examine the nature of these channels, how they are trafficked to and anchored within presynaptic boutons, and the mechanisms that allow them to function optimally in shaping the flow of information through neural circuits.
Introduction
In their pioneering studies of the squid giant synapse more than 50 years ago, Bernard Katz and Ricardo Miledi established the importance of Ca2+ ions as the “essential links in the ‘electro-secretory’ coupling process of the axon terminal”1. It is now well-established that voltage-gated Ca2+ (Cav) channels transduce electrical activity into the flow of Ca2+ ions that initiate the vesicular release of neurotransmitters at synapses. Although these channels are normally comprised of a pore-forming α1 subunit and two auxiliary subunits [G] (β and α2δ), they interact directly or indirectly with a diverse array of proteins that regulate their function, modulation and localization within the presynaptic terminal.
While electrophysiological approaches have traditionally been used to characterize the biophysical properties of Cav channels and their roles in synaptic transmission(usually in rat and mouse tissue), a number of technical advances in the last decade have paved the way for new insights into how Cav channels participate in neurotransmitter release. In particular, novel imaging approaches have aided quantitative analyses of Cav channels within tiny presynaptic boutons at nanoscale resolution (BOX 1). In addition, three-dimensional views of Cav channels, unveiled by cryo-electron microscopy2,3, have provided a framework for understanding the key molecular determinants that underly the complex functioning of Cav channels at the synapse. In this Review, we will highlight recent progress towards understanding the structure and function of Cav channels, with an emphasis on those features that support the presynaptic roles of these channels in neurons. Most of the work discussed has been carried out in rodents; experiments using other species have been indicated.
Box 1| Methods to study Cav channel organization and function.
Confocal and super-resolution imaging.
The extension of confocal imaging to form the basis of multiple super-resolution imaging techniques(for review see216) has facilitated the direct visualization of Cav channels in presynaptic terminals and allowed their physical relationship to other presynaptic proteins to be determined97. This approach relies either on the existence of antibodies (or potentially nanobodies) that bind specifically to the channel itself116,129 or to inserted epitopes or fusion proteins53. It is therefore vital to verify that such epitopes do not affect the function of the channels or their interactions.
Electron microscopy:
Electron microscopic images allowed the first glimpse of vesicular release217 and revealed the exquisite detail of presynaptic structures218. Quantitative SDS-digested freeze-fracture replica labelling immuno-electron microscopy (SDS-FRL EM)206 is currently the method of choice to localize presynaptic Ca2+ channels, and involves freeze-fracture of brain tissue, followed by immunogold labelling and electron microscopy. Taking this approach, CaV2.1 antibodies have been used to label the intracellular face of presynaptic active zone membranes, allowing clusters of channels to be mapped and their relationship to other presynaptic markers determined82,88. Nevertheless, since the efficiency of antibody labeling can vary between preparations, care must be taken in interpreting quantitative estimates of Cav channel numbers82. More reliable antibodies for CaV2.2 and CaV2.3219 that are amenable to this technique are sorely needed.
Direct and indirect electrophysiology:
Presynaptic Ca2+ currents have been recorded by whole cell or cell-attached patch clamp in a number of accessible presynaptic terminals, including calyceal terminals220–222 and mossy fiber en passant terminals223. Such recordings give direct information on the composition of presynaptic calcium currents, but not their subcellular localization. The use of specific Cav blockers and knock-out animals can be used to reveal the effect of loss of particular channels224. When combined with the postsynaptic measurement of synaptic currents, the channels that are involved in release of the neurotransmitter can also be identified indirectly (for example see REF82).
Ca2+ imaging.
Intracellular Ca2+ measurements in individual presynaptic boutons can be obtained using either chemical dyes (such as Magnesium-green or Fluo-3) or genetically-encoded Ca2+ reporters225. These measurements can be performed in cultured neurons, acute or cultured slices or in vivo68,226. They allow determination of the specific Cav channels responsible for presynaptic Ca2+ entry resulting from a single action potential stimulation or from trains of action potentials.
Imaging vesicular release.
Genetically-encoded vesicle release sensors generally rely on the use of a pH-sensitive green fluorescent protein (GFP) that is targeted to the lumen of the synaptic vesicle. The change in pH when a vesicle fuses with the membrane results in an increase in fluorescence227. Styryl dyes such as FM1–43 which are loaded into synaptic vesicle membranes have also been widely used, although they have lower sensitivity and time resolution228. These measurements allow determination of the relationship between Ca2+ entry and vesicular release, as well as the specific CaV channels responsible for neurotransmitter release resulting from single and multiple action potential stimulation patterns.
Glutamate sensors.
‘Sniffer’ outside-out patches containing glutamate receptors have been used to identify the sites of glutamate release229 and, more recently, novel glutamate sensors have been developed141,230,231 that can precisely localize release sites and also allow optical quantal analysis to be performed141. The effects of Cav blockers on release from specific sites can then be determined.
Characterization of Cav channels
In the decades since the initial electrophysiological characterization of high and low voltage-activated Ca2+ currents (termed HVA and LVA currents) in invertebrates4 — and subsequently in mammalian neurons and cardiac atrial cells5,6 — the Cav channel subtypes underlying those currents have been characterized (FIG.1a). It is now clear that the division between HVA and LVA channels was rather artificial as there is a continuum of activation thresholds among the different Cav subtypes (FIG. 1b), which can be further accentuated by different auxiliary subunit combinations, as well as by alternative splicing [G] 7–9. Subsequently the use of pharmacological and biochemical tools, as well as the availability of mouse strains lacking expression of specific Cav channel subtypes, has allowed the properties of cloned Cav channel isoforms to be matched more closely with those identified in different tissues (FIG.1a).
Fig. 1|. Cav channel nomenclature and properties.
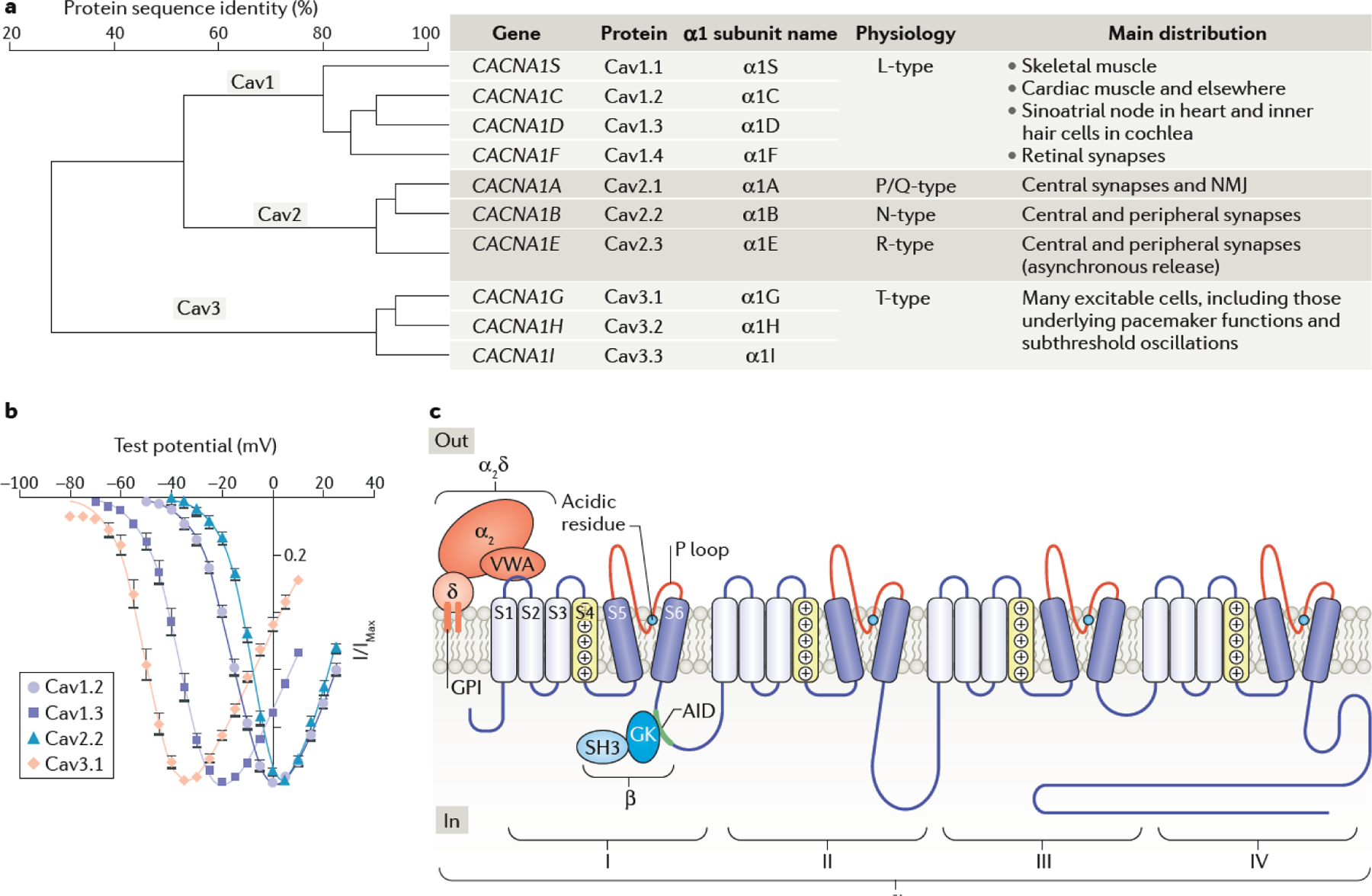
a| Schematic showing Cav channel homology (the % identity between protein sequence of the difference Cav channel isoforms), human genetic nomenclature and protein classification. The channels are divided into 3 main groups, CaV1, CaV2 and CaV3, based on homology, and then subdivided according to the individual gene products. The original name for each of the cloned α1 subunits, as well as the names derived from electrophysiological experiments are also shown. The tissue distribution and main functions of each isoform are listed. b| Normalized current-voltage relationships for Cav currents recorded from tsA201 cells. A comparison of the activation voltage ranges of Cav3.1, Cav1.3, Cav1.2 and Cav2.2 channels shows that there is a continuum of activation voltages for the different channels, rather than a clear division into low voltage activated (LVA) and high voltage activated (HVA) channels7–9,20–22. c| Schematic illustrating Cav channel subunit interactions2,29,34. The α1 subunit, contains four homologous domains (I-IV) each with 6 transmembrane segments (S1-S6), an S4 voltage sensor (yellow), containing a motif of positively charged amino acid residues, and a P-loop between S5 and S6 (red segments), which comprise the pore domain, containing key acidic residues (generally glutamate, cyan circles) involved in the selectivity filter. The α2δ subunit is shown with its GPI anchor linking it into the membrane and von Willebrand factor A (VWA) domain binding to the first extracellular loop of the α1 subunit. The two domains of the β subunit (Src homology domain (SH3) and guanylate kinase-like domain (GK)) are also shown, with the GK interacting with the α1-interaction domain (AID) motif on the I-II linker intracellular loop. Part b is reproduced, with permission from ref7. NMJ, neuromuscular junction.
Dihydropyridines aid identification of Cav1 channels.
Originally characterized in muscle and neurons10,11, Cav1 channels form a subset of HVA channels that were designated through electrophysiological experiments as ‘L-type’ channels, based on their ‘long’ openings in the presence of dihydropyridine (DHP) agonists12. Biochemical purification of DHP-binding proteins from skeletal muscle revealed that these channels (now known as Cav1.1 channels) were comprised of multiple subunits13: an α1 ion-conducting subunit (which was called α1S) and auxiliary α2, β, ϒ, and δ subunits13,14. The cloning of the genes encoding α1S (CACNA1S)14,15 and subsequently the α1 subunits of Cav1.2 channels from the heart (CACNA1C)16, Cav1.3 channels from the brain (CACNA1D)17, and Cav1.4 channels in the retina (CACNA1F)18,19 revealed key functional differences among Cav1 subtypes (FIG. 1a). For example, Cav1.3 and Cav1.4 were shown to activate at more negative voltages than Cav1.2 (FIG. 1b)20–22.
Toxins reveal the presence of distinct non-L-type channels.
The functional characterization of neuronal CaV2 channels, and the genes encoding their α1 subunits, was greatly aided by the discovery of toxins that specifically block these channels: ω-agatoxin IVA for the ‘P/Q-type’ channels (now known as Cav2.1 channels and containing α1A) 23, ω-conotoxin GVIA for the ‘N-type’ channels (now known as Cav2.2 channels and containing containing α1B)24, and SNX-482 for the ‘R-type’ channel (now known as Cav2.3 channels and containing α1E)25. The genes encoding the α1 subunits of ‘T-type’ channels (now known as Cav3.1, Cav3.2 and Cav3.3 channels) were then cloned (for review see26) (FIG. 1a). When expressed in heterologous expression systems, Cav3 channels show very hyperpolarized activation voltages [G] (FIG. 1b) and rapid inactivation as compared to Cav1 and Cav2 channels, and also do not require auxiliary subunits (for review see26).
Cav channel topology and involvement in neurotransmitter release.
Like the voltage-gated Na+ channels, Cav α1 subunits have 24 transmembrane segments organized into four homologous domains (I-IV), each with 6 transmembrane alpha-helical segments (S1-S6, FIG. 1c). The S5 and S6 segments of each domain form the channel pore [G] and selectivity filter [G], with S1-S4 contributing to a voltage-sensing domain (VSD), as further elucidated by the recent structural studies of Cav1.12 and Cav3.13. The main Cav channels involved in transmitter release in the central and peripheral nervous systems are Cav2.1 and Cav2.2, whereas Cav1.3 and Cav1.4 are key to the functioning of the specialized ribbon synapses [G] of the cochlea and retina. The current nomenclature for Cav channels is based on the identity of the ten α1 subunits identified in the mammalian genome27 (FIG. 1a). In this Review, we will adhere to the convention that ‘CavX’ will refer to the channels containing the corresponding α1 subunit.
Auxiliary Cav subunits
The properties of CaV1 and CaV2 channels are modulated by their auxiliary subunits, in ways that are key to their differing roles in neurotransmitter release.
β subunits are cytosolic modulators of Cav function.
Encoded by four genes (CACNB1–4), the β subunits of Cav channels (β1- β4) regulate various channel properties, including their levels at the cell surface and their voltage-dependent activation and inactivation (for review see28). Cav β subunits bind to an α1-interaction domain (AID) in the I-II linker of Cav1 and Cav2 channels 29. The β2 variant confers Cav channels with relatively slow voltage-dependent inactivation — an effect that can be modified further by alternative pre-mRNA splicing30,31. In the brain, β4 is the predominant ß isoform found in association with Cav2 channels32. By contrast, β2 is the major Cav β subunit that is thought to interact with Cav1 channels at cochlear and retinal ribbon synapses31,33.
α2δ subunits are post-translationally processed extracellular subunits.
The two polypeptides (α2 and δ) making up the auxiliary α2δ subunit are encoded by the same gene, of which there are four (CACNA2D1–4) (for review see 34). The gene product encodes a pre-protein, which is proteolytically processed into α2 and δ35,36. These proteins are associated with the external leaflet of the plasma membrane via a glycosyl-phosphatidylinositol (GPI) anchor37. α2δ−1–3 are all expressed in the brain, whereas α2δ−4 is most abundant in the retina38.
α2δ interacts via its von Willebrand factor A (VWA) domain primarily with the first extracellular loop of domain I of CaV1 and CaV2 α1 subunits2,39,40 and can regulate the activation and inactivation of Cav channels, although the strength of this regulation varies among Cav subtypes (for review see 34). The α2δ subunits also increase the cell surface expression of these channels, in a manner that requires the presence of Cav β subunits41. While in complex with presynaptic Cav channels (or possibly independently), α2δ subunits may engage in trans-synaptic interactions with proteins that regulate axonal wiring or other processes42–45.
ϒ subunits.
Although γ1 is a component of the skeletal Cav1.1 channel complex2, none of the γ subunits co-purify with Cav2 subtypes in the brain32 and thus are unlikely to be major components of neuronal Cav channel complexes. Although γ2 (also known as stargazin) was first interpreted to be a Cav subunit46, it and the subsequently cloned γ3–8 are now understood to be auxiliary subunits of AMPA glutamate receptors47. Thus, they will not be considered further here.
Trafficking of Cav channels
Auxiliary subunits support Cav channel assembly and trafficking.
In neuronal cell bodies, the formation of the Cav channel complex begins with the translation and folding of α1 in association with the endoplasmic reticulum, a process that may be enhanced by Ca2+ binding to the selectivity filter within the pore48 (FIG. 1c). The binding of a Cav β subunit to the cytosolic face of α1 promotes the maturation of the channel complex (FIG. 2a), protecting it from polyubiquitination and endoplasmic reticulum-associated proteasomal degradation, thus increasing its forward trafficking49–51.
Fig. 2|. Synthesis and trafficking of Cav channels.
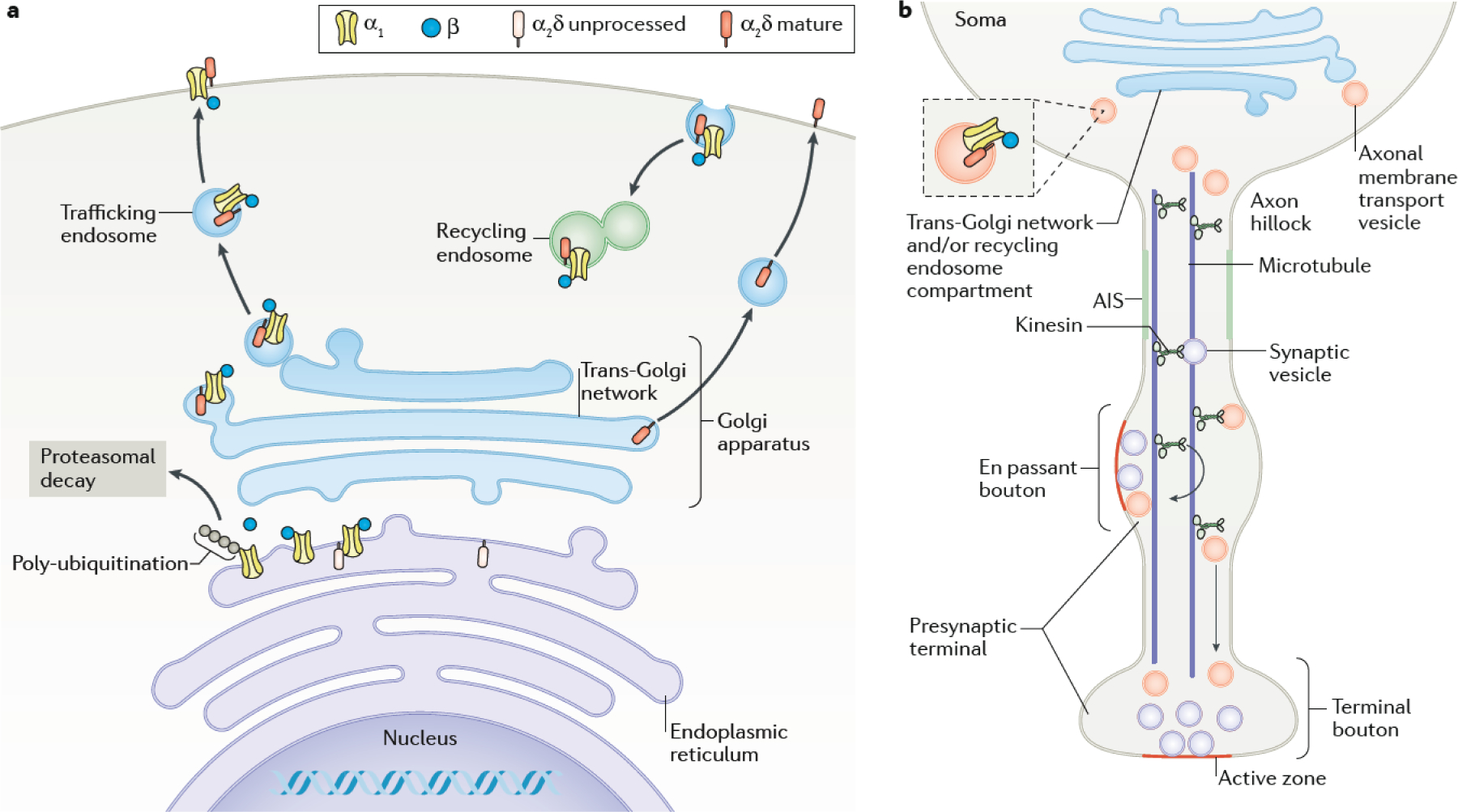
a| The synthesis of the α1 and α2δ subunits of Cav2 channels occurs on endoplasmic reticulum-associated ribosomes. The unprocessed form of α2δ is therefore synthesised entirely within the endoplasmic reticulum and attached to the endoplasmic reticulum membrane by a GPI anchor. In the endoplasmic reticulum, the α1 subunit associates with the ß subunit, which is a cytoplasmic protein. This interaction protects the α1 subunit from polyubiquitination and ER-associated degradation via the proteasome. The α2δ subunits are heavily glycosylated in the endoplasmic reticulum (there are up to 18 N-glycosylation sites to which glycans are attached) and the glycosyl moieties are further processed in the Golgi apparatus45, which is also probably the main site of its proteolytic cleavage into α2 and δ45 to form the mature protein. α2δ proteins may associate with the α1 and ß subunit complex in the endoplasmic reticulum or in the Golgi apparatus. The complex is subsequently transported in trafficking endosomes and incorporated into the plasma membrane by fusion. The calcium channel complexes are also subject to endocytosis and are recycled to the plasma membrane via recycling endosomes55,211. The α2δ proteins are also able to reach the cell surface alone. b| The transport of axonal membrane transport vesicles containing Cav channels destined for active zone membranes involves binding of axonal cargo-containing transport vesicles (axonal endosomes) from the trans-Golgi network / recycling endosome compartment to microtubules in the pre-axonal exclusion zone at the axon hillock61,212. The axon initial segment (AIS) represents a specialized region in which particular Na+ and K+ channels are concentrated, and action potentials are initiated, which may also restrict axonal trafficking. The cargo destined for en passant or terminal boutons is attached by axonal kinesins to axonal microtubules, and is released from the microtubules at presynaptic sites, as are synaptic vesicles 213.
Where in the assembly and trafficking pathway α2δ associates with the channel complex is largely unknown. Since it binds to extracellular sites on α1 (FIG. 1c), the α2δ pre-protein (that is, the uncleaved form of the protein) could co-assemble with α1 in the endoplasmic reticulum, where they are both translated (FIG. 2a). Given that the uncleaved α2δ subunit does not allow Ca2+ influx through the channel, such co-assembly with α2δ could prevent nascent Cav1 and Cav2 channels from opening and thus leaking Ca2+ out of the endoplasmic reticulum, potentially avoiding toxic elevations in cytosolic Ca2+ 52. While the α2δ pre-protein shows only ‘immature’ (that is, endoplasmic reticulum enzyme-mediated) glycosylation, the proteolytically processed α2 and δ subunits exhibit ‘mature’ (that is, Golgi enzyme-mediated) glycosylation, a process that is modulated by interaction with the multi-functional trafficking protein low density lipoprotein receptor-related protein-1 (LRP1)45. This indicates that cleavage of the α2δ pre-proteins into mature α2 and δ subunits probably begins to occur in the Golgi apparatus (FIG. 2a).
Sorting and trafficking Cav channels to presynaptic sites of action.
In the trans-Golgi network, Cav channels are packaged into trafficking endosomes (or trafficking vesicles) for sorting to their final destination (FIG. 2a). Although α2δ can be transported alone to the plasma membrane and into neurites 52 (FIG. 2a), Cav2 channels require α2δ to be present for optimal trafficking into presynaptic terminals52–54. For CaV2.2, it has been shown that this process is regulated by the adaptor protein AP-1 binding to identified motifs in region encoded by an alternatively spliced exon in the proximal C-terminus (exon 37a) and that α2δ is required for the effectiveness of AP-1 in this trafficking mechanism55. The proteolytic cleavage of α2δ in the Golgi may expose this motif, mediating exit of the channel complex from the trans-Golgi network into trafficking endosomes55. α2δ−1 has been identified at the electron microscopic level in axonal endosomes56, and biochemical analysis indicates that these α2δ−1 subunits are largely in the mature cleaved form52, in agreement with the hypothesis that α2δ proteolytic cleavage may be a prerequisite for entry into this trafficking pathway in neurons.
Within a single neuron, the Cav subtypes present in the soma, dendrites, and presynaptic terminals can vary substantially57. Like other neuronal proteins, the Cav subtypes destined for these compartments may be sorted differentially at key checkpoints58. Cav channels interact with a variety of synaptic proteins, some of which affect the presynaptic levels of these channels. However, it is not always evident whether such protein interactions are required for the initial targeting and transport of Cav channels to presynaptic terminals or whether they are involved in maintaining their clustering within presynaptic sites of action.
The segregation of trafficking endosomes containing presynaptic cargo is mediated by multiple Rab proteins59,60 and appears to occur at a pre-axonal exclusion zone within the axon hillock, prior to the axon initial segment61 (FIG. 2b). Following sorting, the anterograde transport of vesicles along microtubules employs kinesin motors, with different kinesins being involved in dendritic and axonal trafficking62,63 (FIG. 2b). Presynaptic components of the active zone may traffic as pre-formed units64, and be inserted together into the presynaptic membrane to enlarge active zones during synaptic plasticity65. α2δ may be important in this regard, since its overexpression in hippocampal neurons increases presynaptic Cav channel abundance as well as the size of the active zone cytomatrix66. Whether Cav channels are co-transported with other active zone proteins has not been established for Cav2 channels, but has been studied with respect to Cav1.4 channels in retinal photoreceptors. It has been shown that Cav1.4 is transported together with other presynaptic proteins (such as Munc13, CAST1 and RIM2) to the presynaptic terminal67.
Synapse-specific Cav functions.
In order to encode a wide range of information that can vary with development and experience, synapses are remarkably diverse in terms of their complement of proteins, and this diversity extends to Cav channels. Studies aimed at defining the contributions of specific Cav subtypes to neurotransmitter release have traditionally relied on electrophysiological recordings of postsynaptic responses to presynaptic stimulation. However, the precision of this approach is somewhat limited by the non-linearity of the relationship between Ca2+ entry and vesicular release (for recent review see 68). More recently, many novel techniques and preparations have improved our ability to directly identify presynaptic Cav channels and the Ca2+ signals they mediate at the active zone (BOX 1), and have significantly advanced our understanding of the contributions of Cav channels to vesicle release properties.
Cav2 channels regulate exocytosis at most synapses.
The fusion of synaptic vesicles with the presynaptic membrane can occur in the absence of stimulation (spontaneous release) or within milliseconds (synchronous release) or up to tens of seconds after (asynchronous release) the arrival of an action potential into the terminal (for review see 69). Most synapses utilize Cav2.1 and/or Cav2.2 channels to coordinate synchronous release, but there are few generalities that can be made regarding which Cav2 subtype(s) predominates at different synapses. Blockers of Cav2.1 and/or Cav2.2 blunt glutamatergic transmission at synapses in many brain regions70,71. While most GABA-ergic neurons in the cerebellum utilize primarily presynaptic Cav2.1 channels72,73, those in the hippocampus rely on a more variable complement of Cav channels. For example, at synapses formed with dentate granule cells, GABA release is triggered by Cav2.2 or Cav2.1 channels in the presynaptic terminals of interneurons expressing cholecystokinin (CCK) or parvalbumin (PV), respectively74.
Owing to their small size, most presynaptic terminals are not amenable to pharmacological characterization of resident Cav channels by direct patch-clamp recordings. This hurdle is overcome in analyses of large, electrotonically compact glutamatergic terminals, such those of the dentate granule cells that form synapses with CA3 pyramidal cells in the hippocampus. In patch-clamp recordings of these ‘mossy fiber’ boutons, Cav2.1 is most efficiently activated by action potentials, with contributions of Cav2.2 and Cav2.3 becoming more evident as action potential width is increased75. Consistent with its relatively negative activation voltage, Cav2.3 is the main Cav channel that is recruited by subthreshold depolarizations of mossy fiber boutons, which may account for the prominent role of this Cav2 subtype in plasticity of mossy fiber synapses76,77 and spontaneous release78. Within negative voltage ranges, such as those present in neurons at or below resting potentials, Cav3 channels have also been found to indirectly modulate neurotransmitter release at various synapses79,80.
Matching Cav2 subtypes to meet synapse demands.
Within a single terminal, the presence of multiple Cav2 subtypes with distinct properties may help to diversify synaptic responses to reflect changes in action potential firing patterns, such as changes in frequency or bursting activity. However, some synapses may require less variability in stimulus–response properties. For example, the Calyx of Held is a synapse requiring rapid and temporally precise glutamate release within the sound localization circuit of the mature auditory brainstem81. To accommodate this need, the distance between Cav channels and exocytotic release sites (that is, the coupling distance) at the mature Calyx of Held synapse is very small (<30 nm), such that the opening of only a few Cav channels to form a ‘nanodomain’ of elevated intracellular Ca2+ can trigger vesicle fusion82 (BOX 2). By contrast, before the maturation of this synapse the requirements for Cav channel coupling distance appear less stringent. It has been shown that Ca2+ chelators such as EGTA (which have a slow Ca2+ binding rate) can blunt release at the Calyx of Held before but not after hearing onset, suggesting that exocytosis at immature Calyx of Held synapses relies on the presence of a ‘microdomain’ of elevated intracellular Ca2+, resulting from the opening of multiple Cav channels that are only loosely coupled (up to ~100 nm) to readily-releasable vesicles83.
Box 2| Organization of Cav channels relative to synaptic release sites.
How many Cav channels are required to open to trigger vesicular release?
This question remains a source of controversy and the answer may vary with synapse geometry, as described below. A combinination of results from ultrastructural imaging studies and electrophysiology (in the presence of fast and slow Ca2+ chelators) is required to produce models of the arrangement between channels and release sites. At different synapses, estimates of the number of CaV channels required to open to trigger vesicle release have varied from a single channel in both chick ciliary ganglion terminals 232 and mouse hippocampal Schaffer collateral synapses233, to 3 channels or fewer at the rat basket cell-granule cell synapse of rat hippocampus234, to more than 60 in the immature rat calyx of Held synapse235.
Coupling distance between Cav channels and release sites.
Ca2+ channels have been reported to be organized in ‘nanodomains’ or ‘microdomains’ around a vesicle release site (for review see 87). These terms refer to the positioning of Cav channels involved, respectively implying tight (nanodomain) or loose (microdomain) coupling of these channels to vesicular release. In a situation in which nanodomain coupling is present, the opening of one or a few Cav channels could trigger release, whereas a larger number of Cav channels are required to contribute to the Ca2+ elevation that triggers release in a situation in which microdomain coupling is present. The quantitative definition of a nanodomain or microdomain has varied slightly across the many studies that have investigated this point; however, in general the distance between individual channels and the vesicle release site is less than ~20 nm for nanodomains and greater than ~20 nm for microdomains84 (although it seems clear that there is a continuum of distances 90 and geometries). Indeed, the coupling distance has been defined differently depending on the study. For example it has been estimated from the centre of the synaptic vesicle release site to the nearest perimeter edge of a Cav cluster82 but it has also been defined by the relative effectiveness of Ca2+ buffers with differing Ca2+ on rates to supress vesicular release 87.
The arrangement of Cav channels in relation to release sites:
Using the techniques described in Box 1 (and others), many different arrangements have been proposed for the localization of channels in relation to vesicles within active zones (see the figure). Proposals include the tethering of a single channel to each vesicle232 (not shown) or a circular arrangement of channel-preferring ‘slots’ surrounding each vesicle236 (akin to the circular arrangement of SNAREs142 (see the figure, part a), although this is not supported by electron microscopy and other findings, for example 48,82,237. A random arrangement of CaV2 channels within the active zone has also been proposed (see the figure, part b)233. Electrophysiological, electron microscopical and Ca2+-imaging data has pointed to a perimeter release model (see the figure, part c) for calyx of Held active zones, consisting of a loose cluster of ~20 – 30 CaV2 channels, with one or more vesicles located 15 – 30 nm from the perimeter of the cluster 82. Numerical simulations from this model showed a remarkable correspondence to the average number of channels in a cluster observed using electron microscopy82. According to this model, a single channel opening could result in vesicular fusion, albeit with very low probability, although action potential-mediated release normally relies on the opening of multiple channels in the cluster82. Unlike calyx of Held synapses, the high release probability of neocortical synapses does not change with maturity, despite the presence of tighter coupling and reorganization of channel topology such that CaV2.1 channels become the predominant source of Ca2+ for neurotransmitter release relative to CaV2.284, as the CaV2.1 channels cluster nearer to the vesicle (see also the figure, part d). Also seen in parallel fiber–Purkinje neuron synapses of the cerebellum, this type of organization is termed the ‘frame and centre’ model88. More ordered arrays of channels are observed in a variety of neuromuscular junctions238,239.
Box 2. Models of Cav channel arragment in the active zone.
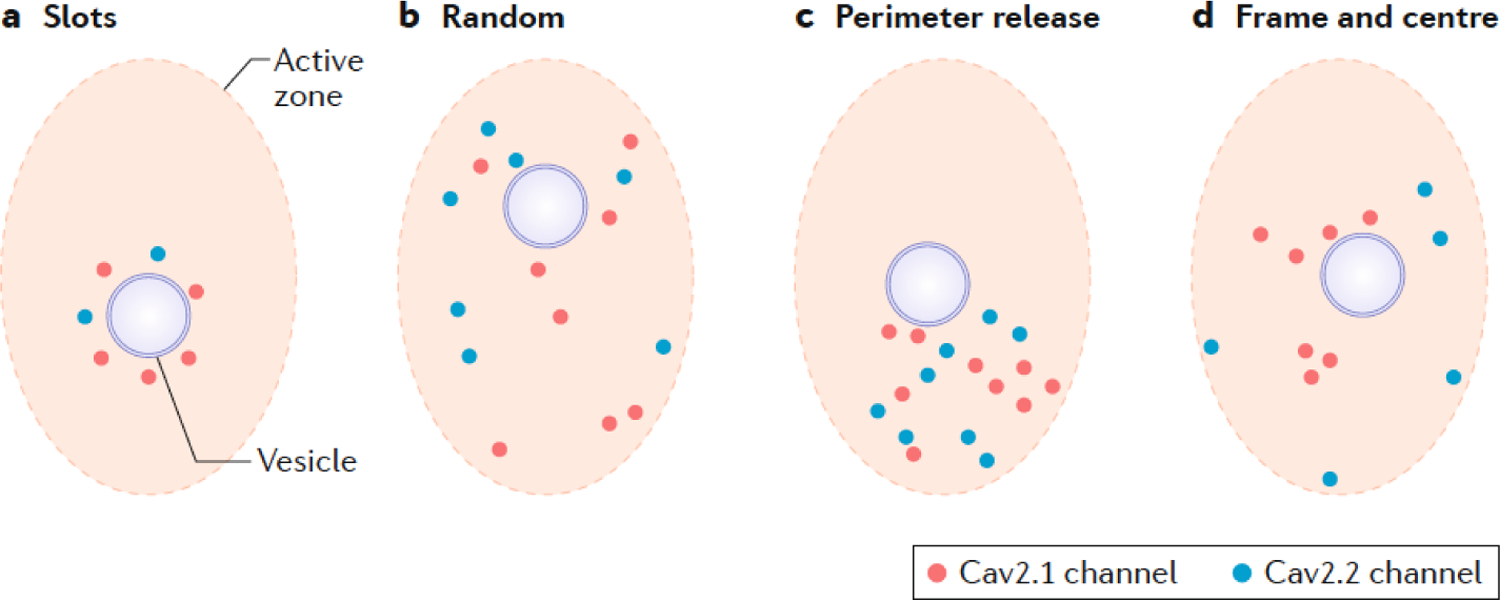
As occurs at other synapses84, the maturation of neurotransmission at the Calyx of Held is accompanied by a shift from a mixed population of Cav2 subtypes82,85 to one that is dominated by Cav2.172,83,86. Indeed, Cav2.1 is distinguished from other Cav2 subtypes at most mature central synapses in exhibiting nanodomain coupling 87. Recent evidence suggests that this property of Cav2.1 channels may relate to their unique tethering by active zone proteins. Quantitative SDS-digested freeze-fracture replica immuno-electron microscopy (SDS-FRL EM; BOX 1) of mature parallel fiber–Purkinje cell synapses in the cerebellum has revealed that Cav2.1 is selectively clustered within the active zone, where it is surrounded by CaV2.2 towards the perimeter of the active zone 88 (see part d of the figure in BOX 2). Deletion of Munc13–3, one of three members of the Munc13 family, disrupts the differential localization of Cav2.1 and CaV2.2 channels at this synapse and the developmental transition from microdomain coupling, supported by both subtypes, to nanodomain coupling supported only by Cav2.188. However, the effects of Munc13–3 in regulating coupling and localization of Cav2 subtypes may differ between synapses82 (also see BOX 2). Filament-forming septins have also been implicated in the developmental switch between microdomain and nanodomain coupling89,90.
The presence of a mixed population of Cav2 subtypes may also enable the diversification of Ca2+ sources in order to match distinct presynaptic responses to variations in activity. For example, at mossy fiber-CA3 synapses in the adult mouse hippocampus, Cav2.3 does not contribute to the Ca2+ signals that support the fast neurotransmitter release evoked by single action potentials; however, when neurons are subjected to tetanic stimulation causing brief trains of presynaptic action potentials, Cav2.3 can induce long-term and post-tetanic potentiation. This suggests that Cav2.3 is localized at a greater distance from neurotransmitter release sites than other Cav2 subtypes 76. At this synapse, Cav2.3 channels are brought into play by tetanic stimulation, which boosts presynaptic Ca2+ to levels that can support these forms of potentiation 76. At Schaffer collateral synapses in the adult mouse hippocampus, the contributions of Cav2.1, Cav2.2, and Cav2.3 also vary with different stimulus frequencies, which can affect the filtering features that are caused by GABA receptor-mediated inhibition of these synapses91. These findings suggest that the pathological up- or down-regulation of a particular Cav2 subtype (for example, as a result of a disease-causing mutation) could profoundly alter the information-encoding properties of synapses containing mixed Cav2 subtypes.
Slowly inactivating-Cav1 channels encode sensory information at ribbon synapses.
Vision, hearing and balance rely on transmission at ribbon synapses in the retina and inner ear. In contrast to the phasic, action potential-mediated neurotransmitter release that occurs at conventional synapses, ribbon synapses are specialized for analog transmission. They feature continuous glutamate release that is modulated by graded changes in the membrane potential (for review see92). One specialized feature of these synapses is the synaptic ribbon, an organelle found in cochlear and vestibular hair cells, as well as in retinal photoreceptors and bipolar cells. Among other functions, the ribbon primes vesicles for exocytosis and helps to maintain glutamate release during prolonged stimulation93 (FIG. 3).
Fig. 3|. Regulation of Cav1 channel inactivation at ribbon synapses.
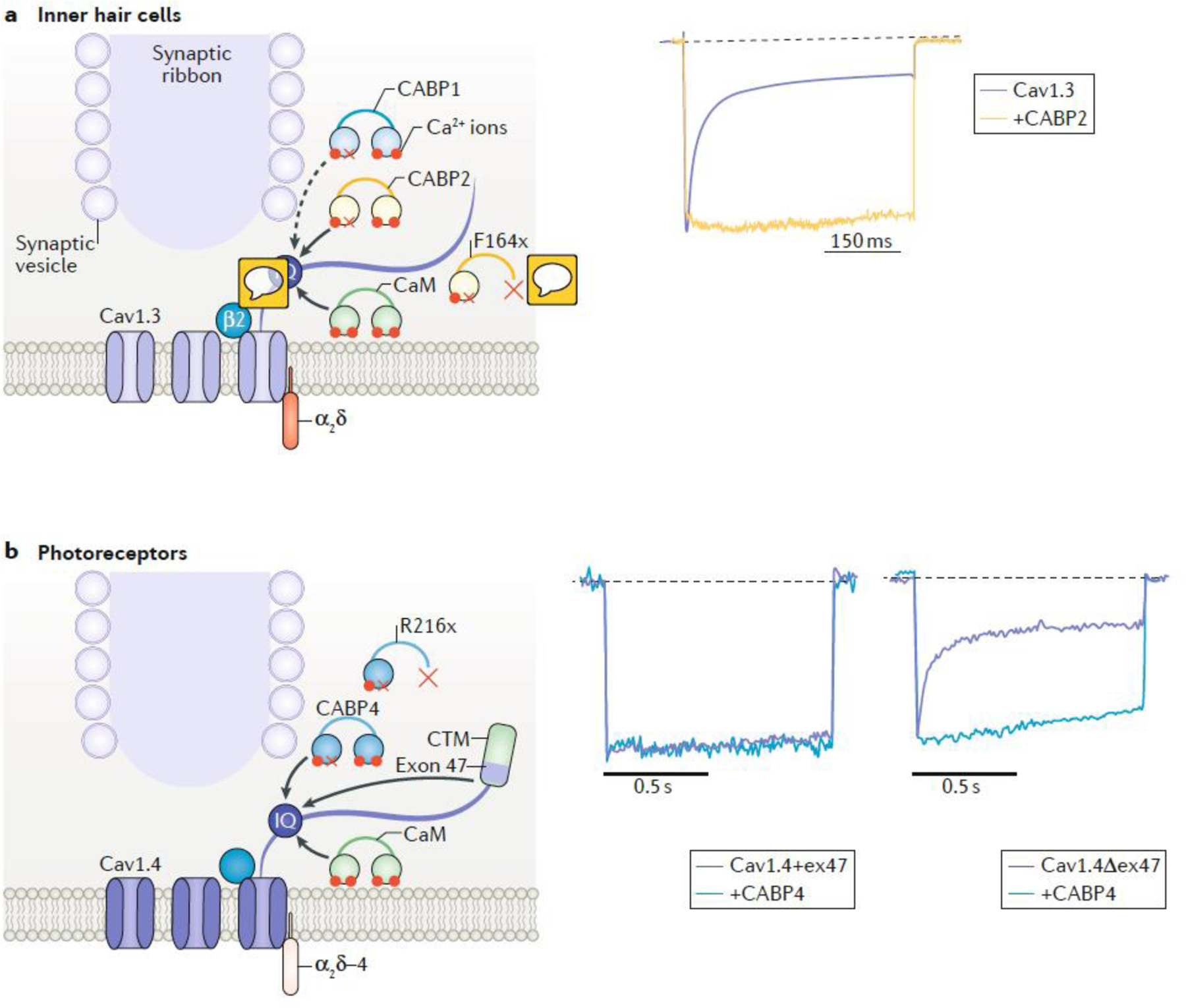
The schematics show the location of Cav1 channels at the active zones of inner hair cells (IHCs) and photoreceptors near the synaptic ribbon. The traces on the right show the normalized Ca2+ current recorded from HEK293T cells expressing Cav1 channels alone or Cav1 channels together with a calcium-binding protein (CABP). a| In many cell-types, Cav1 channels are thought to be constitutively associated with calmodulin (CaM) which is bound to an IQ-domain (IQ) in the C-terminal domain of the channel. The binding of 4 Ca2+ ions to the N- and C-terminal lobes of CaM triggers a conformational change in the channel that favors Ca2+-dependent inactivation (CDI) (reviewed in100). In IHCs, Cav1.3 channels exhibit limited inactivation in comparison to that present in other cell types21,101. This is due in part to the co-assembly of Cav1.3 with β2 subunits, which cause slow voltage-dependent inactivation33. In addition, CABP1 and/or CABP2 are Ca2+ binding proteins with a dysfunctional Ca2+ binding site in the N-terminal lobe (red x). These CABPs are thought to compete with CaM for binding to the channel thus suppressing the effects of CaM on inactivation. Right panel shows that Ca2+ currents inactivate more slowly in cells cotransfected with Cav1.3 and CABP2 than in those transfected with Cav1.3 alone105,106. CABP1 has a similar effect (not shown) 101,104. A mutation (F164X) that causes premature truncation of CABP2 inhibits its ability to suppress inactivation of Cav1.3 and causes autosomal recessive hearing loss105. b|In photoreceptor terminals, Cav1.4 channels, which are associated with β2 and extracellular α2δ−4 subunits, show little CDI. Channels containing exon 47 (Cav1.4+ex47) possess a C-terminal modulatory domain (CTM) that is thought to compete with CaM for binding to the channel (left current trace)109. Splice variants lacking exon 47 (Cav1.4Δex47) show stronger CDI, which may be due to a reduced ability of the CTM to compete with CaM for binding to the channel (right purple current trace). CABP4 slows inactivation of Cav1.4Δex47 but not of Cav1.4+ex47, possibly because the loss of exon 47 enables CaBP4 to compete effectively with CaM for binding to the channel114. A mutation (R216X) that causes premature truncation of CABP4 inhibits its ability to suppress CDI of Cav1.4 and causes vision impairment in humans205. Trace in part a is adapted, with permission from105. Traces in part b are adapted, with permission from ref 114.
A second feature of ribbon synapses is their reliance on slowly inactivating Cav1 channels: Cav1.3 in inner hair cells (IHCs) and Cav1.4 in photoreceptors94–96. In both cell types, slow voltage-dependent inactivation of Cav1 channels is thought to be conferred in part by the β2 subunit97–99. Ca2+-dependent inactivation (CDI), a process that is mediated by calmodulin [G] (CaM) binding to a consensus IQ-domain in the C-terminal domain of Cav1 channels100, is also suppressed in these channels; however, the mechanisms underlying this suppression for native Cav1.3 and Cav1.4 channels are distinct (FIG. 3).
Compared to the properties of Cav1.3 channels expressed in heterologous expression systems, these channels undergo very little CDI when expressed in IHCs101. The mechanism underlying the loss of CDI in these cells is thought to involve Ca2+-binding proteins (CABPs), a family of CaM-like proteins that suppress CDI, in part by displacing CaM from its binding site on Cav1 channels (FIG. 3a). Like CaM, CABPs possess 4 EF-hand Ca2+ binding domains, one of which is non-functional102. Due to its abundant expression in IHCs and ability to suppress CDI of Cav1.3 upon co-transfection in cell lines103–105, CABP2 initially emerged as a promising candidate for opposing CDI of Cav1.3 in IHCs. However, in IHCs of CABP2 knock-out mice, voltage-dependent inactivation of Cav1.3 was increased, but CDI was unaffected106. It is thought that this was perhaps due to the overlapping expression of another CABP family member, CABP1, in IHCs103,104 (FIG. 3a). Additional mechanisms that could suppress CDI in IHCs include alternative splicing of the mRNA encoding Cav1.3 107 and interactions of Cav1.3 with other synaptic proteins108.
Unlike Cav1.3, long splice variants of Cav1.4 show little CDI even in the absence of CABPs, due to the presence of a C-terminal modulatory domain (CTM) that competes with and/or modifies CaM binding to the channel109,110. Cav1.4 splice variants lacking some or all of the CTM, such as one lacking exon 47 (Cav1.4Δex47, Fig.3b), show strong CDI when expressed heterologously111,112. CABP4 is a CABP family member that is enriched in photoreceptor synaptic terminals113 which interacts with the CaM binding site and suppresses CDI of Cav1.4Δex47114 (FIG. 3b). Thus, it is expected that Cav1.4Δex47, and perhaps other CTM-lacking variants, would show limited CDI in the retina.
Cav organization at the synapse
Quantitative proteomic analyses suggest that the presynaptic interactome of Cav2 channels includes ~200 proteins, although not all of these are direct interactions32. The intimate association of Cav channels with proteins of the active zone could ensure that vesicular release occurs rapidly upon arrival of an action potential115, and is aligned with the relevant postsynaptic elements at the nanoscale level116 (FIG. 4a).
Fig. 4|. Organization and modulation of Cav2 channels in synapses.
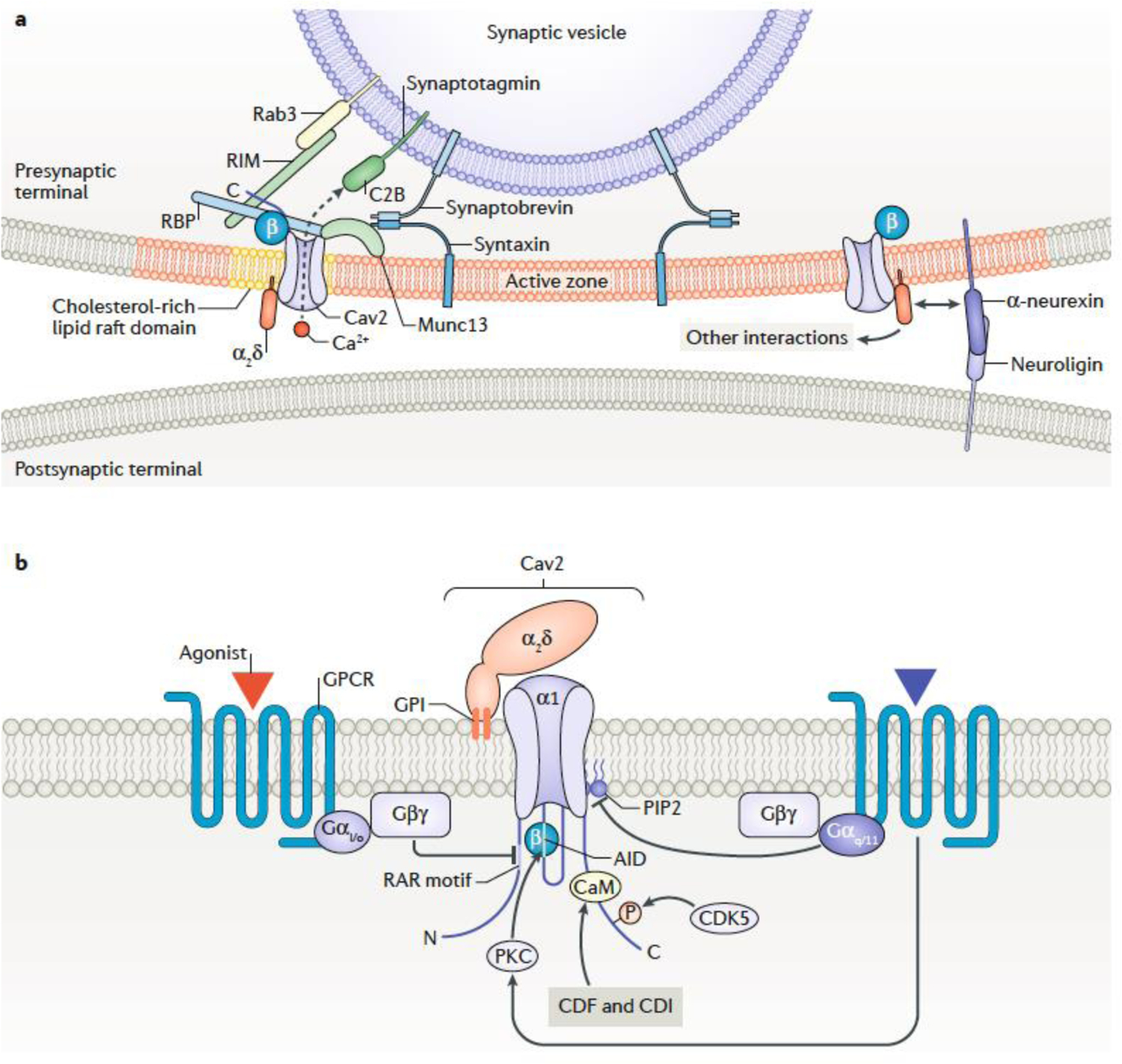
a| Schematic depicts some of the proteins known to be involved in anchoring the Cav2 channels sufficiently near to synaptic vesicles to form a nanodomain within the presynaptic active zone (for reviews see 69,87). These include Rab3, synaptotagmin (the major Ca2+ sensor, with one of its C2 domains shown) and synaptobrevin, all of which are associated with the vesicular membrane. Rab3-interacting molecules (RIM) and RIM binding proteins (RBP) are cytosolic, whereas Munc13 and syntaxin are associated with the plasma membrane. The association of the Cav ß subunit with the channel is shown. CaVß interacts with RBP and also with another scaffolding protein, CAST/ELKS (not shown). The α2δ subunit is extracellular, and may (via its GPI anchor) preferentially associate with cholesterol rich lipid raft membrane domains. It also mediates effects on Cav channels of α-neurexins, which also interact with neuroligin (postsynaptic except in Caenorhabditis elegans). Other interactions of α2δ subunits are also likely to occur at the synapse but have not been depicted for clarity. b| Schematic showing some of the pathways modulating CaV2 calcium channel function. Some G protein-coupled receptors (GPCRs) inhibit CaV2 channel activity via their Gßγ subunits. This requires the presence of a conserved RAR motif in the N-terminal sequence of the channel, and also involves Cav ß binding to the I-II linker159,162. GPCRs coupled to Gq/11 inhibit Cav2 channels by reducing levels of PIP2 (and activating PKC, which phosphorylates the AID214 and elsewhere). Modulation involving the C-terminal domain of Cav2 channels includes CDK5-mediated phosphorylation of a conserved serine of CaV2.2, which increases channel open probability178. Ca2+-dependent inactivation (CDI) (in Cav2.2 and Cav2.1) and Ca2+-dependent facilitation (CDF (in CaV2.1) are mediated by calmodulin (CaM) binding to sites in the proximal C-terminal domain182,183.
A web of protein interactions regulate presynaptic Cav channel clustering.
Rab3-interacting molecules (RIMs) are key organizers of the active zone and bind via their PDZ domains to a conserved motif (DxWC) in the C-terminus of Cav2.1 and Cav2.2 channels115,117–119 (FIG. 4a). RIMs are also indirectly linked to these channels through association with RIM binding proteins (RBPs), that bind to proline-rich motifs (PxxP) in the C-terminal domain of both Cav1 and Cav2 channels120. In mice lacking RIM variants and/or RBPs, the density of Cav2.1 channels is reduced in synaptic terminals115,117,118,121. The presynaptic abundance of Cav1.3 channels is also reduced in IHCs in the absence of RIMs and RBPs122,123, indicating a conserved role for these proteins at different types of synapses.
Despite the evidence supporting a requirement for RIMs and RBPs for controlling presynaptic levels of Cav channels, ectopic expression of a Cav2.1 protein lacking the RIM and RBP binding sites at the Calyx of Held of Cav2.1 knock-out mice leads to normal presynaptic Cav current density124. A possible explanation for these discrepant results is that RIM may regulate presynaptic abundance of Cav2.1 channels via tertiary interactions with Cav β subunits (FIG. 4a). In support of this idea RBPs are known to interact with Cav β subunits118 and RIM is known to bind to CAST/ELKS125, a core active zone protein that binds to β4 126. Deletion of CAST/ELKS decreases presynaptic Ca2+ influx at inhibitory hippocampal synapses127, and reduces the clustering of Cav2.1 channels at the Calyx of Held128 and at Drosophila neuromuscular junctions129. There is likely to be redundancy in this interconnected network of proteins130, such that disrupting one point of contact may be compensated by other Cav protein interactions.
The interaction between mature α2δ and α1 subunits is also critically important for the clustering of Cav channels in presynaptic terminals52,54,131. Genetic inactivation of α2δ−1 and α2δ−4 reduces the active zone localization of Cav2.2 in primary afferent terminals in the spinal cord dorsal horn53 and Cav1.4 in photoreceptor synaptic terminals42,132. In the case of photoreceptors, Cav1.4 channels are targeted to synaptic terminals but the presynaptic level of these channels is reduced in the absence of α2δ−442,132.
It has been proposed that α2δs may engage in trans-synaptic protein interactions that ensure appropriate alignment of pre- and post-synaptic signaling complexes. In support of this idea, deletion of α2δ−2 causes misalignment of presynaptic Cav1.3 channels with postsynaptic glutamate receptors at IHC synapses43. The formation of rod to rod-bipolar cell synapses in the retina is thought to require the interaction of presynaptic α2δ−4 with the postsynaptic protein extracellular leucine-rich repeat fibronectin type III domain containing-1 (ELFN1) 42. α2δ was also found to interact with neurexins that interact trans-synaptically with neuroligins133. However in a subsequent study, although α2δ−1 mediated the effects of α-neurexin on Cav2.1 function, no specific association could be demonstrated 134, and this interaction is not required for postsynaptic recruitment of GABA receptors at inhibitory synapses44. α2δ binding to thrombospondins, a family of extracellular matrix proteins, was found to promote excitatory synaptogenesis135, although in other studies this interaction was shown to be of relatively low affinity and was only demonstrated for thrombospondin-4136,137. How such protein interactions with Cav complexes would still allow their mobility within the active zone66,138 also remains unclear.
Molecular coupling of Cav channels to release sites.
The resolution limits of confocal microscopy have previously hindered quantitative analyses of Cav coupling via imaging of fluorescently tagged channels. This hurdle has been overcome with the advent of super-resolution microscopy methods (such as stimulated emission depletion microscopy (STED) and stochastic optical reconstruction microscopy (STORM)) and SDS-FRL EM (BOX 1). STED has shown that two isoforms of the positional priming protein, unc13, form distinct scaffolding complexes that cluster at different distances from Cav channels and might underlie the developmental tightening of the coupling between Ca2+ entry and release at the Drosophila neuromuscular junction139. The mammalian ortholog of unc13, Munc13, associates with RIM and ELKS at particular synapses140, and with syntaxin at vesicular release sites141. In agreement with this, Munc13 proteins are hypothesized to form a juxta-membrane ring around each vesicular release site, together with an inner ring of oligomerized synaptotagmins, anchoring the soluble NSF attachment protein (SNAP) receptor (SNARE) proteins [G] in order to allow vesicular fusion to occur on Ca2+ entry142 (FIG. 4a).
The search for mechanisms that facilitate the coupling of Cav2 channels to vesicle release led to the identification of the ‘synprint’ site—a sequence in the cytoplasmic II-III linker of CaV2.1 and CaV2.2 that binds to SNAREs and other exocytotic proteins (for review see143). When injected into some neurons, peptides corresponding to the synprint site inhibit synaptic transmission, interpreted as competitive displacement of SNAREs from Cav2 channels, thus loosening the coupling of these channels to release sites144,145. However, mammalian synprint peptides inhibit neurotransmitter release when introduced into invertebrate neurons in which Cav2 channels lack the synprint sequence, and so it is thought that they may interfere with vesicle dynamics independent of disrupting Cav2-synaptic protein interactions146. For example, the synprint interacts with AP-2 adaptor proteins involved in clathrin-mediated endocytosis, and membrane capacitance recordings indicate that injected synprint peptides disrupt synaptic vesicle endocytosis rather than exocytosis at the Calyx of Held synapse147.
Are there proteins that interact with Cav channels that directly regulate their coupling to exocytosis? An intriguing result in this regard is the distinct coupling efficiencies of Cav2.1 channels with (Cav2.1+ex47) and without (Cav2.1Δex47) exon 47148. This exon encodes the binding sites for a number of active zone proteins including RIM and RBPs. At hippocampal synapses, Cav2.1+ex47 channels exhibited higher mobility as well as activity-dependent accumulation in synaptic nanodomains that strengthened coupling and supported greater neurotransmitter release and paired-pulse depression148. Similar results were not, however, obtained at the Calyx of Held, where a 19 amino acid sequence upstream of exon 47 was found to be necessary for rapid vesicle release124. This sequence is poorly conserved in Cav2.2 and Cav2.3 and therefore may represent a molecular determinant that affords Cav2.1 the positional advantage in controlling fast vesicular release at this synapse. Whether this site interacts with proteins that enhance Cav2.1 coupling, or rather promotes channel conformations that facilitate coupling, remains to be discovered.
Presynaptic Cav channel regulation
Modulation of presynaptic Ca2+ entry is one of the key mechanisms through which neurotransmitter release can be rapidly up- or down-regulated, and is mediated by multiple different mechanisms. In neurons, the exquisite sensitivity of such forms of neuromodulation relates to the non-linearity of the dependence of vesicular release on Ca2+ entry, such that a small change in the level of presynaptic Ca2+ can profoundly impact the amount of neurotransmitter released.
Modulation of presynaptic Cav function by active zone proteins.
In addition to regulating the presynaptic density of Cav channels and their coupling to vesiclular release, proteins within the active zone can also directly regulate Cav function. This was initially demonstrated for proteins of the synaptic vesicle release machinery —such as syntaxin, SNAP25 and synaptotagmin — which interact with Cav2 α1 subunits (reviewed in143) (FIG.4a).
Through the interaction of their C2B domains with Cav β subunits (FIG. 4a), RIM proteins can augment neurotransmitter release by markedly prolonging Cav2 and Cav1 Ca2+ currents108,118,149. However, analysis of short RIM2γ variants (which contain the C2B domain but not the PDZ and PxxP sequences that mediate direct and indirect interactions with the Cav2 C-terminal domain) suggests that the ability of RIM proteins to support neurotransmission may be due to their effects in regulating Cav2 channel abundance and/or tethering near vesicle release sites. Although RIM2γ slows inactivation of Cav2 channels in transfected HEK293T cells, it does not rescue neurotransmitter release defects in RIM1/2 double knock-out neurons150. In rod photoreceptors, RIM1 and 2 do not affect the clustering of Cav1.4 channels at the synaptic ribbon, but strongly enhance the opening of these channels that is required for evoked glutamate release151. Thus, the impact of RIM proteins on Cav channel function and the consequences for neurotransmitter release may vary with Cav channel subtype and in different types of synapse.
Regulation by G-protein coupled receptors.
A prominent route by which some G-protein coupled receptors (GPCRs) suppress neurotransmitter release is through inhibition of Cav2 channels, with CaV2.2 being more sensitive to this form of modulation than CaV2.1. Generally mediated by GPCRs that couple to Gi/Go proteins, this inhibition requires the precise positioning of GPCRs within the vicinity of the channels152 (FIG. 4b). The inhibition involves a slowing of channel activation which was first characterized in dorsal root ganglion neurons153,154, and subsequently shown to be mediated by the G-protein βγ subunits155–157. Gβγ-mediated inhibition of Cav2 channels is voltage-dependent in that it can be relieved by strong or repeated depolarizations which cause the unbinding of Gβϒ from the channel158. It was subsequently found that CaV β subunits are required for voltage-dependent inhibition of Cav2 channels by Gβγ159,160. Although Gβγ was found to interact with a QxxER motif within the AID, this site was previously identified to be partially occupied by CaVβ161. Thus other sites, including a sequence of conserved residues in the N-terminus of all the CaV2 channels, represent additional essential determinants of Gβγ modulation162 (FIG. 4b).
The modulatory role of presynaptic lipids.
The importance of the active zone lipid composition has been little explored with respect to presynaptic Cav function. Cholesterol is an important component of presynaptic membranes163, and GPI-anchored proteins, including α2δ, are concentrated in cholesterol-rich regions of the membrane, raising the possibility that cholesterol may contribute to a mechanism for clustering presynaptic channels164. Furthermore, phosphatidylinositol 4,5-bisphosphate (PIP2), a minor component of the intracellular leaflet of the plasma membrane, is important for the binding of RIM and other synaptic proteins, including synaptotagmin, to the cell membrane165,166. Indeed, rapid elevation of PIP2 in the membrane of chromaffin cells, by photo-uncaging, potentiated exocytosis167 and, on a slower time scale, lowering presynaptic PIP2 in calyx of Held terminals slowed endocytosis168.
It is also well-documented that Cav channels can be directly regulated by PIP2 (for review see169): the activation of Gq-coupled GPCRs depletes PIP2 via activation of phospholipase C, and thus inhibits CaV currents170 (FIG. 4b). This represents yet another potential mechanism for presynaptic Cav channel modulation, although it has not yet been demonstrated in synapses.
Presynaptic modulation by protein kinases.
The contribution of Cav channels to neurotransmitter release can be modulated by various protein kinases, including protein kinase C (PKC). In sympathetic neurons, the activation of PKC by phorbol-12-myristate-13-acetate (PMA) increases currents mediated by Cav2.2 channels by opposing G-protein inhibition171. The mechanism for this regulation involves PKC phosphorylation of the Gβγ binding site in the I-II linker of Cav2.2172 (FIG. 4b) and could provide a means whereby Gq-linked receptors coupled to PKC could facilitate neurotransmitter release173. As shown in Aplysia neurons, PMA can also increase neurotransmitter release by promoting the insertion of Cav2 channels in the presynaptic membrane174.
Cyclin-dependent protein kinase (CDK5) is another protein kinase implicated in the modulation of Cav channels and presynaptic function. An inhibitor of CDK5, roscovitine, was used to show that CDK5 phosphorylates the II-III loop of CaV2.1, which reduces CaV2.1 currents and inhibits the interaction of these channels with SNAP-25 and synaptotagmin175. While this mechanism could explain the effects of roscovitine in inhibiting neurotransmitter release, it is important to note that roscovitine has mixed agonist/antagonist effects on Cav channels and may regulate neurotransmission independent of CDK5176,177. In an alternative approach, a dominant-negative CDK5 construct was used to show that CDK5 phosphorylates a conserved serine residue in the C-terminal domain of Cav2.2. This was found to cause an increase in somatic Cav2.2-mediated currents (FIG. 4b), and also facilitates synaptic vesicle docking and release, possibly through enhanced Cav2.2 interactions with RIM1 and other SNAREs178 (FIG. 4a). However, a later study that used optical imaging of presynaptic boutons showed that CDK5 inhibits action potential evoked Cav2.2-mediated Ca2+ signals and vesicular release — effects that were reversed by the phosphatase calcineurin179. These disparate results could be due to distinct effects of CDK5 with respect to Cav2.2 channels in the soma compared to presynaptic terminals and indicate the importance of the balance in kinase and phosphatase activities for the synaptic functions of Cav channels.
CaV β subunits also represent a hub for phosphorylation by multiple kinases and for other post-translational modifications (for reviews see28,180). While the impact of CaV β phosphorylation on the functions of presynaptic Cav channels has yet to be explored, the interaction of Cav β with the Rem-Gem-Kir (RGK) protein, Rad, has been shown to be critical for PKA-mediated potentiation of Cav1.2 channels. However, the mechanism involves PKA phosphorylation of Rad itself, which prevents Rad-mediated inhibition of Cav1.2 181. The Cav β-Rad interaction also confers PKA-dependent regulation to Cav2.2 181, and therefore could contribute to facilitation of presynaptic Ca2+ currents by neuromodulators.
CaM and other Ca2+ binding proteins.
As has been discussed for Cav1 channels, CaM also regulates Cav2 channels; however, the mechanisms involved are distinct. In Cav2.1 channels, the association of CaM with a Ca2+ sensor (CaS) binding site in the C-terminal domain causes CDI (FIG. 4b), but also a potentiation of the Cav2.1 current (Ca2+-dependent facilitation (CDF)) which is apparent during repetitive stimuli182,183. CDI and CDF of Cav2.1 have both been reported to contribute to short-term synaptic plasticity at a variety of synapses, which can be modified by CABPs and related CaS proteins (reviewed in184). However, many of these results were obtained in electrophysiological recordings at room temperature with relatively high concentrations of extracellular Ca2+. Under more physiological conditions, CDF is relatively nominal and does not contribute significantly to short-term facilitation at synapses in the hippocampus, cerebellum, and brainstem185. CABP1, binding to the CaS site, prevents CDF and enhances CDI of Cav2.1186, and causes short-term depression of PV-expressing interneuron-CA1 pyramidal neuron synapses in the hippocampus187. Thus, CaS proteins could oppose CDF of Cav2.1 under physiological conditions in nerve terminals, helping to limit the potential for vesicle depletion at some synapses.
The modest level of Cav2.1 CDF is expected to offset the effect of CDI in promoting synaptic depression187. This could be prominent at CA3–CA1 hippocampal synapses, which rely on both Cav2.1 and Cav2.2, since Cav2.2 channels undergo CDI but not CDF188 (FIG. 4b). The lack of CDF in Cav2.2 arises from differences in regions corresponding to the IQ-like domain and upstream sequences of Cav2.2 and Cav2.1189. The inability of Cav2.2 to undergo CDF may be beneficial in nociceptive dorsal root ganglion neurons and sympathetic neurons where, if present, CDF would oppose the inhibition of Cav2.2 by neurotransmitters acting on presynaptic GPCRs in the control of pain transmission and sympathetic outflow.
Presynaptic Cav channelopathies
Considering how relatively modest changes in Cav channel function and/or positioning within the active zone can dramatically influence synaptic output, it is perhaps not surprising that genetic variations causing dysregulation of Cav channels are linked to a variety of nervous system disorders in humans.
Synapse-specific impact and effects on neuromodulation.
Missense mutations in the CACNA1A gene lead to numerous neurological disorders190. The best studied in terms of its impact on synaptic transmission is familial hemiplegic migraine type 1 (FHM-1), a rare form of migraine with aura. Two FHM-1 mutations in CACNA1A, R192Q and S218L, cause an increase in the open probability of the CaV2.1 channel and a large negative shift in the voltage-dependence of its activation190. Studies of knock-in mice bearing these mutations indicate that both mutations lead to increased action potential-evoked Ca2+ influx and a greater probability of glutamate release at cortical pyramidal cell synapses191,192. In contrast, at the calyx of Held, peak Cav2.1 current density is actually reduced, and action potential-evoked Ca2+ currents are smaller in S218L knock-in mice than in wild type mice. However, the large (~10 mV) negative shift in the half-maximal activation voltage enables the opening of some S218L mutant channels at this synapse at the resting potential, increasing basal levels of intracellular Ca2+. As a consequence, calyces from the knock-in mice still exhibit a gain-of-function, with greater spontaneous release, faster recovery from synaptic depression, and stronger synaptic strength than those of wild type mice193.
Although GABA release at synapses between fast-spiking interneurons and cortical pyramidal cells relies primarily on Cav2.1, transmission at these synapses is surprisingly unaltered in FHM-1 mutant mice191. Moreover, the enhanced activation of Cav2.1 in pyramidal cells is not found for these channels in GABA-ergic interneurons of FHM-1 mutant mice194. GABA-ergic neurons may express long Cav2.1 splice variants containing exon 47, in which the effects of FHM-1 mutations are nominal195. Alternatively, the complement of Cav β subunits, which can affect the impact of some FHM1 mutations196, may differ in excitatory and inhibitory interneurons. Regardless of the underlying mechanism, heightened glutamate release in the absence of corresponding alterations in inhibitory synaptic inputs to cortical pyramidal cells is thought to fuel cortical spreading depression, a neurophysiological hallmark of migraine aura in humans, that is also observed in the FHM-1 mutant mice190.
Presynaptic Cav channels and neuropsychiatric disease.
Genes encoding Cav channel subunits have emerged as prominent risk alleles for a variety of neuropsychiatric disorders. In the largest genome-wide analysis of schizophrenia to date, genes encoding Cav channel subunits (CACNA1C, CACNB2, and CACNA1I) and Cav-interacting proteins (RIMs and neuroligins) were identified among the 108 disease-associated loci197. Moreover, autism-associated mutations in the RIM3 gene were found to disrupt the effects of RIM3 in suppressing Cav2.1 inactivation, resulting in impaired neurotransmitter release198. Mice lacking the Cav β-anchoring and regulatory protein (BARP), which suppresses Cav2 and Cav1.2 channel function199, show an enhancement in phenotypes that are characteristic of schizophrenia and autism such as working memory, flexibility, and sociability 200. Thus, our understanding of how dysregulation of Cav channels gives rise to neuropsychiatric disease could benefit from studies of mouse strains lacking expression of such Cav-modulatory proteins.
Fragile X syndrome is a genetic form of intellectual disability, often associated with autism, which is characterized by a loss of expression of Fragile X mental retardation protein (FMRP). One of the pleiotropic effects of FMRP knockout is disruption of synaptic function 201. Cav2 channels have been shown to interact with FMRP 202 and FMRP overexpression was found to decrease the forward trafficking of CaV2.2 203. Furthermore loss of FMRP causes an increase in presynaptic Ca2+ entry and vesicular release in both primary afferent and hippocampal presynaptic terminals 202,203. Thus, interference with normal CaV2 trafficking and function may be one of the many ways in which of loss of FMRP has a disruptive effect on synaptic function in Fragile X syndrome.
Altered interactions with synaptic proteins at ribbon synapses.
The impact of disease-causing mutations in Cav channel-interacting partners is well-illustrated at photoreceptor synapses, where a mutation causing premature truncation of CABP4 (R216X) results in congenital stationary night blindness type 2 (CSNB2)-related visual impairment204. The truncation removes the C-terminal EF-hand domains of CABP4 and prevents the capacity of CABP4 to enhance the voltage-dependent activation of long Cav1.4 variants113,205 (FIG. 3b). Loss of such regulation by CABP4 is expected to decrease the availability of Cav1.4 channels at the membrane potential of photoreceptors in darkness, thus reducing the sensitivity of neurotransmitter release modulation to light stimuli. A remarkably similar mutation in CABP2 (F164X) causes an autosomal recessive hearing impairment. Like R216X in CABP4, F164X deletes the C-terminal EF-hands of CABP2, which greatly impairs its ability to bind Ca2+ and to regulate Cav1.3105 (FIG. 3a). Such defects in Cav1.3 function could underlie the abnormalities in glutamate release at IHC synapses and of hearing that have been observed in CABP2 knock-out mice106. However, CABP2 and CABP4 are likely to interact with a variety of other effectors presynaptically, and so disease-causing mutations in the encoding genes could have complex effects on synaptic function.
Conclusions and future directions
Advances in multiple techniques, including electron microscopy and super-resolution imaging, electrophysiology, time-resolved optical techniques and simulations have greatly enhanced our current knowledge of the principles governing the organization and function of Cav channels in various types of synaptic boutons82,84,116,206. The picture that emerges is that the exquisite molecular diversity of mature synapses and the changes in synaptic function that occur during development in many cases depend on alterations in presynaptic Cav channel properties. The up- or down-regulation of Cav channels within the active zone can be achieved via a myriad of mechanisms, allowing for input-specific forms of neuromodulation onto the dendritic arbour of individual neurons (FIG. 5a, b). In cell types with multiple active zones (such as IHCs) the distinct recruitment of different Cav channel-regulatory mechanisms at different release sites offers a convenient mechanism for generating the heterogeneous forms of afferent activity that may be needed to cover a wide dynamic range of sensory stimuli207 (FIG. 5c).
Fig. 5|. Differential synaptic recruitment of Cav channel regulatory mechanisms.
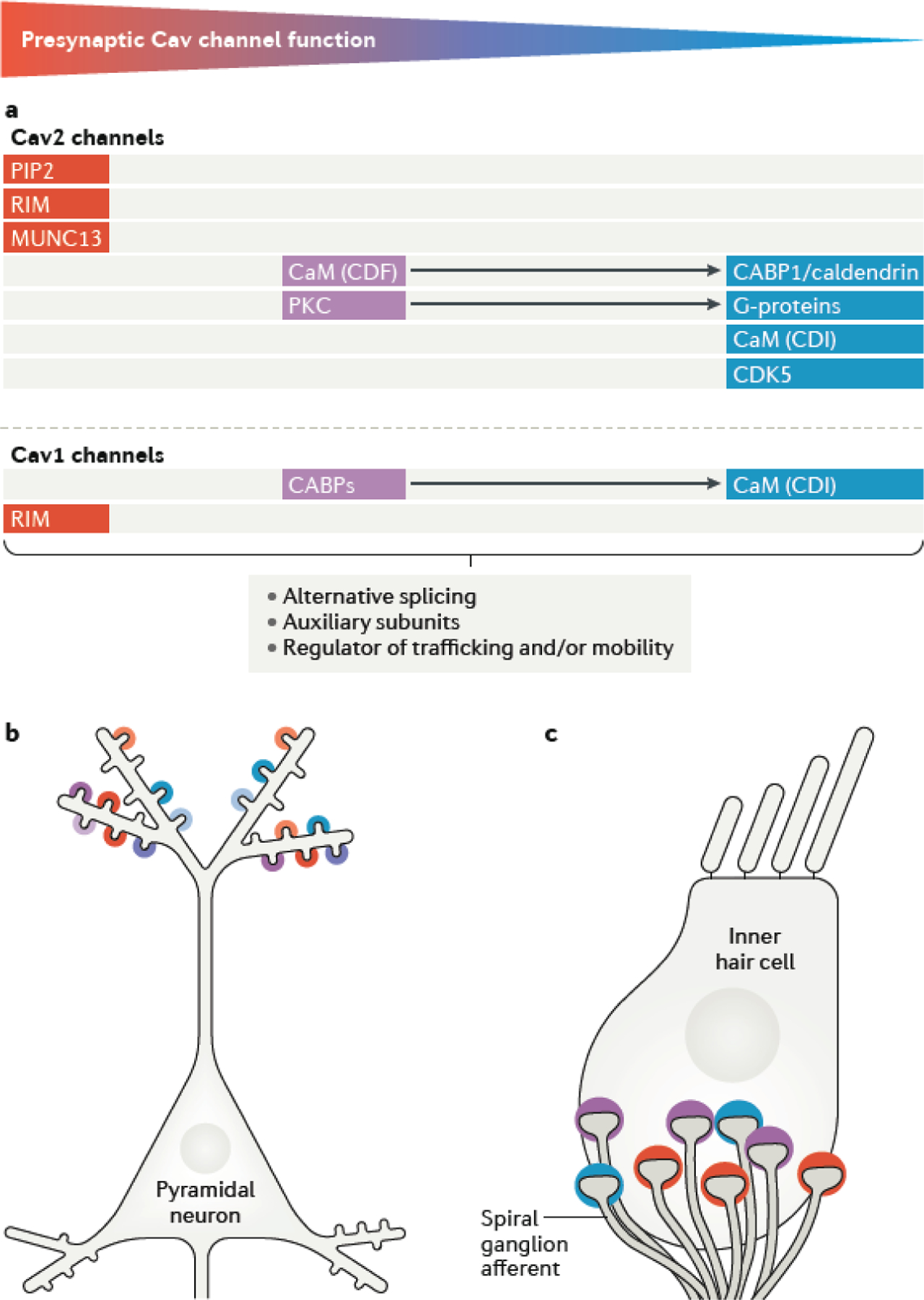
a| Cav2 channel–protein interactions that promote or inhibit presynaptic Cav channel function (depicted along the color-coded gradient to indicate strength of stimulatory (red) and inhibitory (blue) modulation) are major determinants of the amount of neurotransmitter release at the synapse88,100,105,106,108,113,115,117,118,121–123,153,154,170–172,175,179,182,183,186. Arrows between proteins indicate that they exhibit opposing forms of regulation. Distinct forms of alternative splicing, different auxiliary subunits, and bidirectional regulators of trafficking/mobility are also expected to have either stimulatory or inhibitory effects on presynaptic Cav channel function.b| These diverse modulatory mechanisms may add to observed variations in the nanoscale topographies of synapses, resulting in heterogeneity in synapse strength and plasticity that is needed for the computational robustness of neural circuits215. c| In cell-types that utilize multiple active zones such as inner hair cells (IHCs), inter-synaptic differences in Cav channel modulation and therefore neurotransmission could increase the complexity of information that can be encoded and integrated postynaptically. The individual active zones of a single IHC provide input to functionally distinct spiral ganglion afferent neurons. Such presynaptic diversity of Cav channel properties likely contributes to the ability of spiral ganglion neurons to represent the richness of sound information through their variable firing rates207. Colored arcs represent presynaptic inputs to postsynaptic sites of a neuron (B) and IHC (C) and are coded to represent intensity of presynaptic Cav Ca2+ signals based on modulatory mechanisms described in part a.
Yet, there are still many gaps in our understanding. We still do not know the routes by which Cav channels are trafficked to the active zone, and whether they are conserved for the different Cav channels found at conventional and ribbon synapses. With improved methods to label Cav channels for ultrastructural analyses, we will be able to identify the organelles that carry Cav channels to and from the plasma membrane and determine whether the insertion and retention of Cav channels in the active zone are dynamically regulated by the pattern of electrical activity. These approaches will be particularly informative for studies of how differential sorting and trafficking of Cav2.1 and Cav2.2 might underlie the distinct coupling of these Cav2 subtypes to neurotransmitter release57,82,85.
As members of the presynaptic interactome of Cav channels continue to be identified, advances in imaging technologies208 should help resolve the temporal and spatial dynamics of key interactions of Cav channels with synaptic proteins in relation to vesicle release, not only for synapses in culture but also in intact neural circuits. In vivo proximity labeling techniques that can identify components of Cav microenvironment 181, combined with synapse-specific proteomic strategies209 are now in place to quantify how activity-dependent modifications in Cav complexes contribute to synaptic plasticity in both healthy and diseased states of the nervous system.
The differences in the intrinsic properties and modulation of Cav subtypes in heterologous expression systems has led to the assumption that such differences contribute to the distinct roles of Cav channels at various synapses. An important challenge for future studies will be to validate this assumption in intact neural circuits. Viral methodologies that enable the expression of the entire Cav α1 subunit124 in neurons should facilitate future analyses both ex vivo and in vivo of structure–function relationships of Cav channels and the ways in which they contribute to presynaptic function in mature and developing circuits. Furthermore, the importance of G-protein modulation of presynaptic Cav channels in a variety of neurological contexts can now be resolved at the level of individual synapses using cell type-specific targeting of GPCR ligands210.
As the combination of techniques that are available to researchers continues to evolve, it is clear that new results will continue to surprise in this field for many years to come.
Acknowledgements
This work was supported by a Wellcome Trust Investigator award 206279/Z/17/Z (ACD) and NIH grants NS084190, DC009433, EY026817 (AL).
Glossary
- Auxiliary subunits
Subunits that modify the activity and/or trafficking of the pore-forming subunit
- Alternative splicing
The process in which certain exons are included or excluded to produce multiple different mRNAs from a single gene
- Activation voltages
The membrane potentials at which a channel open probability increases, which can be quantified in terms of the voltage for half-maximal activation
- Channel pore
The ion conduction pathway of a channel
- Selectivity filter
The part of the channel pore that restricts passage to particular ions, for example for Ca2+ ions within a Cav channel
- Ribbon synapses
Synapses characterized by a synaptic ribbon that is specialized for the transmission of sensory information
- Calmodulin
A multi-functional Ca2+ sensing protein found in all eukaryotic cells
- Soluble N-ethylmaleimide-Sensitive Factor Attachment Protein (SNAP) receptor (SNARE) proteins
Members of a protein complex that mediates the fusion of vesicles with another membrane, for example the plasma membrane
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Katz B & Miledi R Ionic requirements of synaptic transmitter release. Nature 215, 651, (1967). [DOI] [PubMed] [Google Scholar]
- 2.Wu J, Yan Z, Li Z, Qian X, Lu S, Dong M, Zhou Q & Yan N Structure of the voltage-gated calcium channel Cav1.1 at 3.6 A resolution. Nature 537, 191–196, (2016). [DOI] [PubMed] [Google Scholar]
- 3.Zhao Y, Huang G, Wu Q, Wu K, Li R, Lei J, Pan X & Yan N Cryo-EM structures of apo and antagonist-bound human Cav3.1. Nature 576, 492–497, (2019). [DOI] [PubMed] [Google Scholar]
- 4.Hagiwara S, Ozawa S & Sand O Voltage clamp analysis of two inward current mechanisms in the egg cell membrane of a starfish. J. Gen. Physiol 65, 617–644, (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carbone E & Lux HD A low voltage-activated fully inactivating Ca channel in vertebrate sensory neurones. Nature 310, 501–502, (1984). [DOI] [PubMed] [Google Scholar]
- 6.Bean BP Two kinds of calcium channels in canine atrial cells. Journal of General Physiology 86, 1–30, (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helton TD, Xu W & Lipscombe D Neuronal L-type calcium channels open quickly and are inhibited slowly. J. Neurosci 25, 10247–10251, (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray AC, Raingo J & Lipscombe D Neuronal calcium channels: splicing for optimal performance. Cell Calcium 42, 409–417, (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao P, Zhang HY & Soong TW Alternative splicing of voltage-gated calcium channels: from molecular biology to disease. Pflugers Arch 458, 481–487, (2009). [DOI] [PubMed] [Google Scholar]
- 10.Reuter H Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature 301, 569–574, (1983). [DOI] [PubMed] [Google Scholar]
- 11.Nowycky MC, Fox AP & Tsien RW Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature 316, 440–446, (1985). [DOI] [PubMed] [Google Scholar]
- 12.Hess P, Lansman JB & Tsien RW Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature 311, 538–544, (1984). [DOI] [PubMed] [Google Scholar]
- 13.Takahashi M, Seager MJ, Jones JF, Reber BFX & Catterall WA Subunit structure of dihydropyridine-sensitive calcium channels from skeletal muscle. Proceedings of the National Academy of Sciences of the United States of America 84, 5478–5482, (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanabe T, Takeshima H, Mikami A, Flockerzi V, Takahashi H, Kangawa K, Kojima M, Matsuo H, Hirose T & Numa S Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature 328, 313–318, (1987). [DOI] [PubMed] [Google Scholar]
- 15.Ellis SB, Williams ME, Ways NR, Brenner R, Sharp AH, Leung AT, Campbell KP, McKenna E, Koch WJ, Hui A, Schwartz A & Harpold MM Sequence and expression of mRNAs encoding the α1 and α2 subunits of a DHP-sensitive calcium channel. Science 241, 1661–1664, (1988). [DOI] [PubMed] [Google Scholar]
- 16.Mikami A, Imoto K, Tanabe T, Niidome T, Mori Y, Takeshima H, Narumiya S & Numa S Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature 340, 230–233, (1989). [DOI] [PubMed] [Google Scholar]
- 17.Williams ME, Feldman DH, McCue AF, Brenner R, Velicelebi G, Ellis SB & Harpold MM Structure and functional expression of α1, α2, and β subunits of a novel human neuronal calcium channel subtype. Neuron 8, 71–84, (1992). [DOI] [PubMed] [Google Scholar]
- 18.Bech-Hansen NT, Naylor MJ, Maybaum TA, Pearce WG, Koop B, Fishman GA, Mets M, Musarella MA & Boycott KM Loss-of-function mutations in a calcium channel α1 subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nature Genetics 19, 264–267, (1998). [DOI] [PubMed] [Google Scholar]
- 19.Strom TM et al. An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nature Genetics 19, 260–263, (1998). [DOI] [PubMed] [Google Scholar]
- 20.Xu WF & Lipscombe D Neuronal Cav1.3α1 L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. Journal of Neuroscience 21, 5944–5951, (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koschak A, Reimer D, Huber I, Grabner M, Glossmann H, Engel J & Striessnig J alpha 1D (Cav1.3) subunits can form L-type Ca2+ channels activating at negative voltages. Journal of Biological Chemistry 276, 22100–22106, (2001). [DOI] [PubMed] [Google Scholar]
- 22.Koschak A, Reimer D, Walter D, Hoda JC, Heinzle T, Grabner M & Striessnig J Cav1.4alpha1 subunits can form slowly inactivating dihydropyridine-sensitive L-type Ca2+ channels lacking Ca2+-dependent inactivation. J. Neurosci 23, 6041–6049, (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Starr TVB, Prystay W & Snutch TP Primary structure of a calcium channel that is highly expressed in the rat cerebellum. Proceedings of the National Academy of Sciences of the United States of America 88, 5621–5625, (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mori Y, Friedrich T, Kim MS, Mikami A, Nakai J, Ruth P, Bosse E, Hofmann F, Flockerzi V, Furuichi T &. Primary structure and functional expression from complementary DNA of a brain calcium channel. Nature 350, 398–402, (1991). [DOI] [PubMed] [Google Scholar]
- 25.Soong TW, Stea A, Hodson CD, Dubel SJ, Vincent SR & Snutch TP Structure and functional expression of a member of the low voltage-activated calcium channel family. Science 260, 1133–1136, (1993). [DOI] [PubMed] [Google Scholar]
- 26.Perez-Reyes E Molecular physiology of low-voltage-activated T-type calcium channels. Physiological Reviews 83, 117–161, (2003). [DOI] [PubMed] [Google Scholar]
- 27.Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez-Reyes E, Schwartz A, Snutch TP, Tanabe T, Birnbaumer L, Tsien RW & Catterall WA Nomenclature of voltage-gated calcium channels. Neuron 25, 533–535, (2000). [DOI] [PubMed] [Google Scholar]
- 28.Buraei Z & Yang J The beta subunit of voltage-gated Ca2+ channels. Physiol Rev 90, 1461–1506, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pragnell M, De Waard M, Mori Y, Tanabe T, Snutch TP & Campbell KP Calcium channel β-subunit binds to a conserved motif in the I-II cytoplasmic linker of the α1-subunit. Nature 368, 67–70, (1994). [DOI] [PubMed] [Google Scholar]
- 30.Takahashi SX, Mittman S & Colecraft HM Distinctive modulatory effects of five human auxiliary β2 subunit splice variants on L type calcium channel gating. Biophysical Journal 84, 3007–3021, (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee A, Wang S, Williams B, Hagen J, Scheetz TE & Haeseleer F Characterization of Cav1.4 Complexes (alpha11.4, beta2, and alpha2delta4) in HEK293T Cells and in the Retina. J. Biol. Chem 290, 1505–1521, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller CS, Haupt A, Bildl W, Schindler J, Knaus HG, Meissner M, Rammner B, Striessnig J, Flockerzi V, Fakler B & Schulte U Quantitative proteomics of the Cav2 channel nano-environments in the mammalian brain. Proc Natl Acad Sci U S A 107, 14950–14957, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neef J, Gehrt A, Bulankina AV, Meyer AC, Riedel D, Gregg RG, Strenzke N & Moser T The Ca2+ channel subunit beta2 regulates Ca2+ channel abundance and function in inner hair cells and is required for hearing. J. Neurosci 29, 10730–10740, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dolphin AC Calcium channel auxiliary alpha(2)delta and beta subunits: trafficking and one step beyond. Nat. Rev. Neurosci 13, 542–555, (2012). [DOI] [PubMed] [Google Scholar]
- 35.Jay SD, Sharp AH, Kahl SD, Vedvick TS, Harpold MM & Campbell KP Structural characterization of the dihydropyridine-sensitive calcium channel α2-subunit and the associated δ peptides. Journal of Biological Chemistry 266, 3287–3293, (1991). [PubMed] [Google Scholar]
- 36.De Jongh KS, Warner C & Catterall WA Subunits of purified calcium channels. α2 and δ are encoded by the same gene. Journal of Biological Chemistry 265, 14738–14741, (1990). [PubMed] [Google Scholar]
- 37.Davies A, Kadurin I, Alvarez-Laviada A, Douglas L, Nieto-Rostro M, Bauer CS, Pratt WS & Dolphin AC The α2δ subunits of voltage-gated calcium channels form GPI-anchored proteins, a post-translational modification essential for function. Proc. Natl. Acad. Sci. U. S. A 107, 1654–1659, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qin N, Yagel S, Momplaisir ML, Codd EE & D’Andrea MR Molecular cloning and characterization of the human voltage-gated calcium channel α2δ−4 subunit. Molecular Pharmacology 62, 485–496, (2002). [DOI] [PubMed] [Google Scholar]
- 39.Dahimene S, Page KM, Kadurin I, Ferron L, Ho DY, Powell GT, Pratt WS, Wilson SW & Dolphin AC The α2δ-like Protein Cachd1 Increases N-type Calcium Currents and Cell Surface Expression and Competes with α2δ−1. Cell Reports 25, 1610–1621, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Canti C, Nieto-Rostro M, Foucault I, Heblich F, Wratten J, Richards MW, Hendrich J, Douglas L, Page KM, Davies A & Dolphin AC The metal-ion-dependent adhesion site in the Von Willebrand factor-A domain of alpha2delta subunits is key to trafficking voltage-gated Ca2+ channels. Proc. Natl. Acad. Sci. USA 102, 11230–11235, (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cassidy JS, Ferron L, Kadurin I, Pratt WS & Dolphin AC Functional exofacially tagged N-type calcium channels elucidate the interaction with auxiliary alpha2delta-1 subunits. Proc. Natl. Acad. Sci. U. S. A 111, 8979–8984, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Fehlhaber KE, Sarria I, Cao Y, Ingram NT, Guerrero-Given D, Throesch B, Baldwin K, Kamasawa N, Ohtsuka T, Sampath AP & Martemyanov KA The Auxiliary Calcium Channel Subunit alpha2delta4 Is Required for Axonal Elaboration, Synaptic Transmission, and Wiring of Rod Photoreceptors. Neuron 93, 1359–1374 e1356, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fell B, Eckrich S, Blum K, Eckrich T, Hecker D, Obermair GJ, Munkner S, Flockerzi V, Schick B & Engel J alpha2delta2 Controls the Function and Trans-Synaptic Coupling of Cav1.3 Channels in Mouse Inner Hair Cells and Is Essential for Normal Hearing. J Neurosci 36, 11024–11036, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geisler S, Schopf CL, Stanika R, Kalb M, Campiglio M, Repetto D, Traxler L, Missler M & Obermair GJ Presynaptic alpha2delta-2 Calcium Channel Subunits Regulate Postsynaptic GABAA Receptor Abundance and Axonal Wiring. J Neurosci 39, 2581–2605, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kadurin I, Rothwell SW, Lana B, Nieto-Rostro M & Dolphin AC LRP1 influences trafficking of N-type calcium channels via interaction with the auxiliary alpha2delta-1 subunit. Sci Rep 7, 43802, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Letts VA, Felix R, Biddlecome GH, Arikkath J, Mahaffey CL, Valenzuela A, Bartlett FS, Mori Y, Campbell KP & Frankel WN The mouse stargazer gene encodes a neuronal Ca2+ channel gamma subunit. Nature Genetics 19, 340–347, (1998). [DOI] [PubMed] [Google Scholar]
- 47.Tomita S, Chen L, Kawasaki Y, Petralia RS, Wenthold RJ, Nicoll RA & Bredt DS Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J. Cell Biol 161, 805–816, (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meyer JO, Dahimene S, Page KM, Ferron L, Kadurin I, Ellaway JIJ, Zhao P, Patel T, Rothwell SW, Lin P, Pratt WS & Dolphin AC Disruption of the key Ca2+ binding site in the selectivity filter of neuronal voltage-gated calcium channels inhibits channel trafficking. Cell Reports 29, 22–33, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waithe D, Ferron L, Page KM, Chaggar K & Dolphin AC β-Subunits Promote the Expression Of Cav2.2 channels by reducing their proteasomal degradation. J Biol. Chem 286, 9598–9611, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Altier C, Garcia-Caballero A, Simms B, You H, Chen L, Walcher J, Tedford HW, Hermosilla T & Zamponi GW The Cavβ subunit prevents RFP2-mediated ubiquitination and proteasomal degradation of L-type channels. Nature Neuroscience 14, 173–180, (2010). [DOI] [PubMed] [Google Scholar]
- 51.Page KM, Rothwell SW & Dolphin AC The CaVbeta subunit protects the I-II loop of the voltage-gated calcium channel, CaV2.2, from proteasomal degradation but not oligo-ubiquitination. J Biol Chem 291, 20402–20416, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kadurin I, Ferron L, Rothwell SW, Meyer JO, Douglas LR, Bauer CS, Lana B, Margas W, Alexopoulos O, Nieto-Rostro M, Pratt WS & Dolphin AC Proteolytic maturation of α2δ represents a checkpoint for activation and neuronal trafficking of latent calcium channels ELife 5, e21143, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nieto-Rostro M, Ramgoolam K, Pratt WS, Kulik A & Dolphin AC Ablation of alpha2delta-1 inhibits cell-surface trafficking of endogenous N-type calcium channels in the pain pathway in vivo. Proc Natl Acad Sci U S A 115, E12043–E12052, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferron L, Kadurin I & Dolphin AC Proteolytic maturation of alpha2delta controls the probability of synaptic vesicular release. Elife 7, e37507, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Macabuag N & Dolphin AC Alternative splicing in CaV2.2 regulates neuronal trafficking via adaptor protein complex-1 adaptor protein binding motifs. Journal of Neuroscience 35, 14636–14652, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bauer CS, Nieto-Rostro M, Rahman W, Tran-Van-Minh A, Ferron L, Douglas L, Kadurin I, Ranjan YS, Fernandez-Alacid L, Millar NS, Dickenson AH, Lujan R & Dolphin AC The Increased Trafficking of the Calcium Channel Subunit alpha(2)delta-1 to Presynaptic Terminals in Neuropathic Pain Is Inhibited by the alpha(2)delta Ligand Pregabalin. Journal of Neuroscience 29, 4076–4088, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doughty JM, Barnes-Davies M, Rusznak Z, Harasztosi C & Forsythe ID Contrasting Ca2+ channel subtypes at cell bodies and synaptic terminals of rat anterioventral cochlear bushy neurones. J Physiol 512, 365–376, (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bonifacino JS Adaptor proteins involved in polarized sorting. The Journal of Cell Biology 204, 7–17, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koike S & Jahn R SNAREs define targeting specificity of trafficking vesicles by combinatorial interaction with tethering factors. Nat Commun 10, 1608, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Binotti B, Jahn R & Chua JJ Functions of Rab Proteins at Presynaptic Sites. Cells 5, E6, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Britt DJ, Farias GG, Guardia CM & Bonifacino JS Mechanisms of Polarized Organelle Distribution in Neurons. Front Cell Neurosci 10, 88, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jacobson C, Schnapp B & Banker GA A Change in the Selective Translocation of the Kinesin-1 Motor Domain Marks the Initial Specification of the Axon. Neuron 49, 797–804, (2006). [DOI] [PubMed] [Google Scholar]
- 63.Jenkins B, Decker H, Bentley M, Luisi J & Banker G A novel split kinesin assay identifies motor proteins that interact with distinct vesicle populations. J Cell Biol 198, 749–761, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhai RG, Vardinon-Friedman H, Cases-Langhoff C, Becker B, Gundelfinger ED, Ziv NE & Garner CC Assembling the presynaptic active zone: a characterization of an active zone precursor vesicle. Neuron 29, 131–143, (2001). [DOI] [PubMed] [Google Scholar]
- 65.Bell ME, Bourne JN, Chirillo MA, Mendenhall JM, Kuwajima M & Harris KM Dynamics of nascent and active zone ultrastructure as synapses enlarge during long-term potentiation in mature hippocampus. J Comp Neurol 522, 3861–3884, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schneider R, Hosy E, Kohl J, Klueva J, Choquet D, Thomas U, Voigt A & Heine M Mobility of calcium channels in the presynaptic membrane. Neuron 86, 672–679, (2015). [DOI] [PubMed] [Google Scholar]
- 67.Regus-Leidig H, Tom DS, Specht D, Meyer L & Brandstatter JH Early steps in the assembly of photoreceptor ribbon synapses in the mouse retina: the involvement of precursor spheres. J. Comp Neurol 512, 814–824, (2009). [DOI] [PubMed] [Google Scholar]
- 68.Dittman JS & Ryan TA The control of release probability at nerve terminals. Nat Rev Neurosci 20, 177–186, (2019). [DOI] [PubMed] [Google Scholar]
- 69.Kaeser PS & Regehr WG Molecular mechanisms for synchronous, asynchronous, and spontaneous neurotransmitter release. Annu Rev Physiol 76, 333–363, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wheeler DB, Randall A & Tsien RW Changes in action potential duration alter reliance of excitatory synaptic transmission on multiple types of Ca2+ channels in rat hippocampus. Journal of Neuroscience 16, 2226–2237, (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takahashi T & Momiyama A Different types of calcium channels mediate central synaptic transmission. Nature 366, 156–158, (1993). [DOI] [PubMed] [Google Scholar]
- 72.Iwasaki S, Momiyama A, Uchitel OD & Takahashi T Developmental changes in calcium channel types mediating central synaptic transmission. Journal of Neuroscience 20, 59–65, (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stephens GJ, Morris NP, Fyffe REW & Robertson B The Ca(v)2.1/alpha 1A (P/Q-type) voltage-dependent calcium channel mediates inhibitory neurotransmission onto mouse cerebellar Purkinje cells. European Journal of Neuroscience 13, 1902–1912, (2001). [DOI] [PubMed] [Google Scholar]
- 74.Hefft S & Jonas P Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat Neurosci 8, 1319–1328, (2005). [DOI] [PubMed] [Google Scholar]
- 75.Li L, Bischofberger J & Jonas P Differential gating and recruitment of P/Q-, N-, and R-type Ca2+ channels in hippocampal mossy fiber boutons. J Neurosci 27, 13420–13429, (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dietrich D, Kirschstein T, Kukley M, Pereverzev A, von der BC, Schneider T & Beck H Functional specialization of presynaptic Cav2.3 Ca2+ channels. Neuron 39, 483–496, (2003). [DOI] [PubMed] [Google Scholar]
- 77.Breustedt J, Vogt KE, Miller RJ, Nicoll RA & Schmitz D Alpha1E-containing Ca2+ channels are involved in synaptic plasticity. Proc Natl Acad Sci U S A 100, 12450–12455, (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ermolyuk YS, Alder FG, Surges R, Pavlov IY, Timofeeva Y, Kullmann DM & Volynski KE Differential triggering of spontaneous glutamate release by P/Q-, N- and R-type Ca2+ channels. Nat. Neurosci 16, 1754–1763, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carbone E, Calorio C & Vandael DH T-type channel-mediated neurotransmitter release. Pflugers Arch 466, 677–687, (2014). [DOI] [PubMed] [Google Scholar]
- 80.Huang Z, Lujan R, Kadurin I, Uebele VN, Renger JJ, Dolphin AC & Shah MM Presynaptic HCN1 channels regulate Ca(V)3.2 activity and neurotransmission at select cortical synapses. Nat. Neurosci 14, 478–486, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kopp-Scheinpflug C, Steinert JR & Forsythe ID Modulation and control of synaptic transmission across the MNTB. Hear Res 279, 22–31, (2011). [DOI] [PubMed] [Google Scholar]
- 82.Nakamura Y, Harada H, Kamasawa N, Matsui K, Rothman JS, Shigemoto R, Silver RA, DiGregorio DA & Takahashi T Nanoscale distribution of presynaptic Ca(2+) channels and its impact on vesicular release during development. Neuron 85, 145–158, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fedchyshyn MJ & Wang LY Developmental transformation of the release modality at the calyx of Held synapse. J Neurosci 25, 4131–4140, (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bornschein G, Eilers J & Schmidt H Neocortical High Probability Release Sites Are Formed by Distinct Ca(2+) Channel-to-Release Sensor Topographies during Development. Cell Rep 28, 1410–1418, (2019). [DOI] [PubMed] [Google Scholar]
- 85.Ishikawa T, Kaneko M, Shin HS & Takahashi T Presynaptic N-type and P/Q-type Ca2+ channels mediating synaptic transmission at the calyx of Held of mice. J. Physiol 568, 199–209, (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Iwasaki S & Takahashi T Developmental changes in calcium channel types mediating synaptic transmission in rat auditory brainstem. Journal of Physiology (Lond. ) 509, 419–423, (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eggermann E, Bucurenciu I, Goswami SP & Jonas P Nanodomain coupling between Ca(2)(+) channels and sensors of exocytosis at fast mammalian synapses. Nat Rev Neurosci 13, 7–21, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kusch V, Bornschein G, Loreth D, Bank J, Jordan J, Baur D, Watanabe M, Kulik A, Heckmann M, Eilers J & Schmidt H Munc13–3 Is Required for the Developmental Localization of Ca(2+) Channels to Active Zones and the Nanopositioning of Cav2.1 Near Release Sensors. Cell Rep 22, 1965–1973, (2018). [DOI] [PubMed] [Google Scholar]
- 89.Yang YM, Fedchyshyn MJ, Grande G, Aitoubah J, Tsang CW, Xie H, Ackerley CA, Trimble WS & Wang LY Septins regulate developmental switching from microdomain to nanodomain coupling of Ca(2+) influx to neurotransmitter release at a central synapse. Neuron 67, 100–115, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fekete A, Nakamura Y, Yang YM, Herlitze S, Mark MD, DiGregorio DA & Wang LY Underpinning heterogeneity in synaptic transmission by presynaptic ensembles of distinct morphological modules. Nat Commun 10, 826, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ricoy UM & Frerking ME Distinct roles for Cav2.1–2.3 in activity-dependent synaptic dynamics. J Neurophysiol 111, 2404–2413, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matthews G & Fuchs P The diverse roles of ribbon synapses in sensory neurotransmission. Nat Rev Neurosci 11, 812–822, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Snellman J, Mehta B, Babai N, Bartoletti TM, Akmentin W, Francis A, Matthews G, Thoreson W & Zenisek D Acute destruction of the synaptic ribbon reveals a role for the ribbon in vesicle priming. Nat Neurosci 14, 1135–1141, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H & Striessnig J Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell 102, 89–97, (2000). [DOI] [PubMed] [Google Scholar]
- 95.Mansergh F, Orton NC, Vessey JP, Lalonde MR, Stell WK, Tremblay F, Barnes S, Rancourt DE & Bech-Hansen NT Mutation of the calcium channel gene Cacna1f disrupts calcium signaling, synaptic transmission and cellular organization in mouse retina. Hum. Mol. Genet 14, 3035–3046, (2005). [DOI] [PubMed] [Google Scholar]
- 96.Liu X, Kerov V, Haeseleer F, Majumder A, Artemyev N, Baker SA & Lee A Dysregulation of Ca(v)1.4 channels disrupts the maturation of photoreceptor synaptic ribbons in congenital stationary night blindness type 2. Channels (Austin) 7, 514–523, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Neef J, Urban NT, Ohn T-L, Frank T, Jean P, Hell SW, Willig KI & Moser T Quantitative optical nanophysiology of Ca2+ signaling at inner hair cell active zones. Nature Communications 9, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Katiyar R, Weissgerber P, Roth E, Dorr J, Sothilingam V, Garcia Garrido M, Beck SC, Seeliger MW, Beck A, Schmitz F & Flockerzi V Influence of the beta2-Subunit of L-Type Voltage-Gated Cav Channels on the Structural and Functional Development of Photoreceptor Ribbon Synapses. Invest Ophthalmol Vis Sci 56, 2312–2324, (2015). [DOI] [PubMed] [Google Scholar]
- 99.Ball SL, Powers PA, Shin HS, Morgans CW, Peachey NS & Gregg RG Role of the beta(2) subunit of voltage-dependent calcium channels in the retinal outer plexiform layer. Invest Ophthalmol. Vis. Sci 43, 1595–1603, (2002). [PubMed] [Google Scholar]
- 100.Ben-Johny M & Yue DT Calmodulin regulation (calmodulation) of voltage-gated calcium channels. J Gen Physiol 143, 679–692, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang PS, Alseikhan BA, Hiel H, Grant L, Mori MX, Yang W, Fuchs PA & Yue DT Switching of Ca2+-dependent inactivation of Ca(v)1.3 channels by calcium binding proteins of auditory hair cells. J. Neurosci 26, 10677–10689, (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Haeseleer F, Sokal I, Verlinde CL, Erdjument-Bromage H, Tempst P, Pronin AN, Benovic JL, Fariss RN & Palczewski K Five members of a novel Ca(2+)-binding protein (CABP) subfamily with similarity to calmodulin. J Biol Chem 275, 1247–1260, (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang T, Scholl ES, Pan N, Fritzsch B, Haeseleer F & Lee A Expression and Localization of CaBP Ca2+ Binding Proteins in the Mouse Cochlea. PLoS One 11, e0147495, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cui G, Meyer AC, Calin-Jageman I, Neef J, Haeseleer F, Moser T & Lee A Ca2+-binding proteins tune Ca2+-feedback to Cav1.3 channels in mouse auditory hair cells. J. Physiol 585, 791–803, (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schrauwen I et al. A mutation in CABP2, expressed in cochlear hair cells, causes autosomal-recessive hearing impairment. Am J Hum Genet 91, 636–645, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Picher MM, Gehrt A, Meese S, Ivanovic A, Predoehl F, Jung S, Schrauwen I, Dragonetti AG, Colombo R, Van Camp G, Strenzke N & Moser T Ca(2+)-binding protein 2 inhibits Ca(2+)-channel inactivation in mouse inner hair cells. Proc Natl Acad Sci U S A 114, E1717–E1726, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shen Y, Yu D, Hiel H, Liao P, Yue DT, Fuchs PA & Soong TW Alternative splicing of the Ca(v)1.3 channel IQ domain, a molecular switch for Ca2+-dependent inactivation within auditory hair cells. J Neurosci 26, 10690–10699, (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gebhart M, Juhasz-Vedres G, Zuccotti A, Brandt N, Engel J, Trockenbacher A, Kaur G, Obermair GJ, Knipper M, Koschak A & Striessnig J Modulation of Cav1.3 Ca2+ channel gating by Rab3 interacting molecule. Mol Cell Neurosci 44, 246–259, (2010). [DOI] [PubMed] [Google Scholar]
- 109.Singh A, Hamedinger D, Hoda JC, Gebhart M, Koschak A, Romanin C & Striessnig J C-terminal modulator controls Ca2+-dependent gating of Ca(v)1.4 L-type Ca2+ channels. Nat. Neurosci 9, 1108–1116, (2006). [DOI] [PubMed] [Google Scholar]
- 110.Wahl-Schott C, Baumann L, Cuny H, Eckert C, Griessmeier K & Biel M Switching off calcium-dependent inactivation in L-type calcium channels by an autoinhibitory domain. Proc Natl Acad Sci U S A 103, 15657–15662, (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Williams B, Haeseleer F & Lee A Splicing of an automodulatory domain in Cav1.4 Ca(2+) channels confers distinct regulation by calmodulin. J Gen Physiol 150, 1676–1687, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tan GM, Yu D, Wang J & Soong TW Alternative splicing at C terminus of Ca(V)1.4 calcium channel modulates calcium-dependent inactivation, activation potential, and current density. J. Biol. Chem 287, 832–847, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Haeseleer F, Imanishi Y, Maeda T, Possin DE, Maeda A, Lee A, Rieke F & Palczewski K Essential role of Ca2+-binding protein 4, a Cav1.4 channel regulator, in photoreceptor synaptic function. Nat Neurosci 7, 1079–1087, (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Haeseleer F, Williams B & Lee A Characterization of C-terminal Splice Variants of Cav1.4 Ca2+ Channels in Human Retina. J Biol Chem 291, 15663–15673, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Han Y, Kaeser PS, Sudhof TC & Schneggenburger R RIM Determines Ca(2+) Channel Density and Vesicle Docking at the Presynaptic Active Zone. Neuron 69, 304–316, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Biederer T, Kaeser PS & Blanpied TA Transcellular Nanoalignment of Synaptic Function. Neuron 96, 680–696, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kaeser PS, Deng L, Wang Y, Dulubova I, Liu X, Rizo J & Sudhof TC RIM Proteins Tether Ca(2+) Channels to Presynaptic Active Zones via a Direct PDZ-Domain Interaction. Cell 144, 282–295, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kiyonaka S et al. RIM1 confers sustained activity and neurotransmitter vesicle anchoring to presynaptic Ca2+ channels. Nat. Neurosci 10, 691–701, (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hirano M, Takada Y, Wong CF, Yamaguchi K, Kotani H, Kurokawa T, Mori MX, Snutch TP, Ronjat M, De Waard M & Mori Y C-terminal splice variants of P/Q-type Ca2+ channel CaV2.1 alpha1 subunits are differentially regulated by Rab3-interacting molecule proteins. J Biol Chem 292, 9365–9381, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hibino H, Pironkova R, Onwumere O, Vologodskaia M, Hudspeth AJ & Lesage F RIM binding proteins (RBPs) couple Rab3-interacting molecules (RIMs) to voltage-gated Ca(2+) channels. Neuron 34, 411–423, (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Acuna C, Liu X & Sudhof TC How to Make an Active Zone: Unexpected Universal Functional Redundancy between RIMs and RIM-BPs. Neuron 91, 792–807, (2016). [DOI] [PubMed] [Google Scholar]
- 122.Jung S et al. Rab3-interacting molecules 2alpha and 2beta promote the abundance of voltage-gated CaV1.3 Ca2+ channels at hair cell active zones. Proc Natl Acad Sci U S A 112, E3141–3149, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Krinner S, Butola T, Jung S, Wichmann C & Moser T RIM-Binding Protein 2 Promotes a Large Number of CaV1.3 Ca(2+)-Channels and Contributes to Fast Synaptic Vesicle Replenishment at Hair Cell Active Zones. Front Cell Neurosci 11, 334, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lubbert M, Goral RO, Satterfield R, Putzke T, van den Maagdenberg AM, Kamasawa N & Young SM Jr. A novel region in the CaV2.1 alpha1 subunit C-terminus regulates fast synaptic vesicle fusion and vesicle docking at the mammalian presynaptic active zone. Elife 6, e28412, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lu J, Li H, Wang Y, Sudhof TC & Rizo J Solution structure of the RIM1alpha PDZ domain in complex with an ELKS1b C-terminal peptide. J Mol Biol 352, 455–466, (2005). [DOI] [PubMed] [Google Scholar]
- 126.Kiyonaka S, Nakajima H, Takada Y, Hida Y, Yoshioka T, Hagiwara A, Kitajima I, Mori Y & Ohtsuka T Physical and functional interaction of the active zone protein CAST/ERC2 and the beta-subunit of the voltage-dependent Ca(2+) channel. J Biochem 152, 149–159, (2012). [DOI] [PubMed] [Google Scholar]
- 127.Liu C, Bickford LS, Held RG, Nyitrai H, Sudhof TC & Kaeser PS The active zone protein family ELKS supports Ca2+ influx at nerve terminals of inhibitory hippocampal neurons. J Neurosci 34, 12289–12303, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dong W, Radulovic T, Goral RO, Thomas C, Suarez Montesinos M, Guerrero-Given D, Hagiwara A, Putzke T, Hida Y, Abe M, Sakimura K, Kamasawa N, Ohtsuka T & Young SM Jr. CAST/ELKS Proteins Control Voltage-Gated Ca(2+) Channel Density and Synaptic Release Probability at a Mammalian Central Synapse. Cell Rep 24, 284–293, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kittel RJ, Wichmann C, Rasse TM, Fouquet W, Schmidt M, Schmid A, Wagh DA, Pawlu C, Kellner RR, Willig KI, Hell SW, Buchner E, Heckmann M & Sigrist SJ Bruchpilot promotes active zone assembly, Ca2+ channel clustering, and vesicle release. Science 312, 1051–1054, (2006). [DOI] [PubMed] [Google Scholar]
- 130.Held RG & Kaeser PS ELKS active zone proteins as multitasking scaffolds for secretion. Open Biol 8, 170258, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hoppa MB, Lana B, Margas W, Dolphin AC & Ryan TA alpha2delta expression sets presynaptic calcium channel abundance and release probability. Nature 486, 122–125, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kerov V et al. alpha2delta-4 is required for the molecular and structural organization of rod and cone photoreceptor synapses. J Neurosci 38, 6145–6160, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tong X-J, López-Soto EJ, Li L, Liu H, Nedelcu D, Lipscombe D, Hu Z & Kaplan JM Retrograde Synaptic Inhibition Is Mediated by α-Neurexin Binding to the α2δ Subunits of N-Type Calcium Channels. Neuron 95, 1–15, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brockhaus J, Schreitmuller M, Repetto D, Klatt O, Reissner C, Elmslie K, Heine M & Missler M alpha-Neurexins Together with alpha2delta-1 Auxiliary Subunits Regulate Ca(2+) Influx through Cav2.1 Channels. J Neurosci 38, 8277–8294, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eroglu C et al. Gabapentin Receptor alpha2delta-1 Is a Neuronal Thrombospondin Receptor Responsible for Excitatory CNS Synaptogenesis. Cell 139, 380–392, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.El-Awaad E, Pryymachuk G, Fried C, Matthes J, Isensee J, Hucho T, Neiss WF, Paulsson M, Herzig S, Zaucke F & Pietsch M Direct, gabapentin-insensitive interaction of a soluble form of the calcium channel subunit alpha2delta-1 with thrombospondin-4. Sci Rep 9, 16272, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lana B, Page KM, Kadurin I, Ho S, Nieto-Rostro M & Dolphin AC Thrombospondin-4 reduces binding affinity of [3H]-gabapentin to calcium-channel α2δ−1-subunit but does not interact with α2δ−1 on the cell-surface when co-expressed. Scientific Reports 6, 24531, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mercer AJ, Chen M & Thoreson WB Lateral mobility of presynaptic L-type calcium channels at photoreceptor ribbon synapses. J. Neurosci 31, 4397–4406, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bohme MA et al. Active zone scaffolds differentially accumulate Unc13 isoforms to tune Ca(2+) channel-vesicle coupling. Nat Neurosci 19, 1311–1320, (2016). [DOI] [PubMed] [Google Scholar]
- 140.Kawabe H et al. ELKS1 localizes the synaptic vesicle priming protein bMunc13–2 to a specific subset of active zones. J Cell Biol 216, 1143–1161, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sakamoto H, Ariyoshi T, Kimpara N, Sugao K, Taiko I, Takikawa K, Asanuma D, Namiki S & Hirose K Synaptic weight set by Munc13–1 supramolecular assemblies. Nat Neurosci 21, 41–49, (2018). [DOI] [PubMed] [Google Scholar]
- 142.Bello OD, Jouannot O, Chaudhuri A, Stroeva E, Coleman J, Volynski KE, Rothman JE & Krishnakumar SS Synaptotagmin oligomerization is essential for calcium control of regulated exocytosis. Proc Natl Acad Sci U S A 115, E7624–E7631, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zamponi GW Regulation of presynaptic calcium channels by synaptic proteins. J Pharmacol. Sci 92, 79–83, (2003). [DOI] [PubMed] [Google Scholar]
- 144.Mochida S, Sheng ZH, Baker C, Kobayashi H & Catterall WA Inhibition of neurotransmission by peptides containing the synaptic protein interaction site of N-type Ca2+ channels. Neuron 17, 781–788, (1996). [DOI] [PubMed] [Google Scholar]
- 145.Keith RK, Poage RE, Yokoyama CT, Catterall WA & Meriney SD Bidirectional modulation of transmitter release by calcium channel/syntaxin interactions in vivo. J Neurosci 27, 265–269, (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Spafford JD, Munno DW, Van NP, Feng ZP, Jarvis SE, Gallin WJ, Smit AB, Zamponi GW & Syed NI Calcium channel structural determinants of synaptic transmission between identified invertebrate neurons. J. Biol. Chem 278, 4258–4267, (2003). [DOI] [PubMed] [Google Scholar]
- 147.Watanabe H, Yamashita T, Saitoh N, Kiyonaka S, Iwamatsu A, Campbell KP, Mori Y & Takahashi T Involvement of Ca2+ channel synprint site in synaptic vesicle endocytosis. J Neurosci 30, 655–660, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Heck J, Parutto P, Ciuraszkiewicz A, Bikbaev A, Freund R, Mitlohner J, Andres-Alonso M, Fejtova A, Holcman D & Heine M Transient Confinement of CaV2.1 Ca(2+)-Channel Splice Variants Shapes Synaptic Short-Term Plasticity. Neuron 103, 66–79, (2019). [DOI] [PubMed] [Google Scholar]
- 149.Uriu Y, Kiyonaka S, Miki T, Yagi M, Akiyama S, Mori E, Nakao A, Beedle AM, Campbell KP, Wakamori M & Mori Y Rab3-interacting molecule gamma isoforms lacking the Rab3-binding domain induce long lasting currents but block neurotransmitter vesicle anchoring in voltage-dependent P/Q-type Ca2+ channels. J Biol Chem 285, 21750–21767, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kaeser PS, Deng L, Fan M & Sudhof TC RIM genes differentially contribute to organizing presynaptic release sites. Proc. Natl. Acad. Sci. U. S. A 109, 11830–11835, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Grabner CP, Gandini MA, Rehak R, Le Y, Zamponi GW & Schmitz F RIM1/2-Mediated Facilitation of Cav1.4 Channel Opening Is Required for Ca2+-Stimulated Release in Mouse Rod Photoreceptors. J Neurosci 35, 13133–13147, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dolphin AC G protein modulation of voltage-gated calcium channels. Pharmacol Rev 55, 607–627, (2003). [DOI] [PubMed] [Google Scholar]
- 153.Holz GGI, Rane SG & Dunlap K GTP-binding proteins mediate transmitter inhibition of voltage- dependent calcium channels. Nature 319, 670–672, (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dolphin AC, Forda SR & Scott RH Calcium-dependent currents in cultured rat dorsal root ganglion neurones are inhibited by an adenosine analogue. Journal of Physiology (Lond. ) 373, 47–61, (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T & Catterall WA Modulation of Ca2+ channels by G-protein βgamma subunits. Nature 380, 258–262, (1996). [DOI] [PubMed] [Google Scholar]
- 156.Ikeda SR Voltage-dependent modulation of N-type calcium channels by G protein βgamma subunits. Nature 380, 255–258, (1996). [DOI] [PubMed] [Google Scholar]
- 157.Kajikawa Y, Saitoh N & Takahashi T GTP-binding protein βgamma subunits mediate presynaptic calcium current inhibition by GABAB receptor. Proceedings of the National Academy of Sciences of the United States of America 98, 8054–8058, (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zamponi GW & Snutch TP Decay of prepulse facilitation of N type calcium channels during G protein inhibition is consistent with binding of a single Gβgamma subunit. Proceedings of the National Academy of Sciences of the United States of America 95, 4035–4039, (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Meir A, Bell DC, Stephens GJ, Page KM & Dolphin AC Calcium channel β subunit promotes voltage-dependent modulation of α1B by Gβγ. Biophysical Journal 79, 731–746, (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang Y, Chen YH, Bangaru SD, He L, Abele K, Tanabe S, Kozasa T & Yang J Origin of the voltage dependence of G-protein regulation of P/Q-type Ca2+ channels. J. Neurosci 28, 14176–14188, (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Van Petegem F, Clark KA, Chatelain FC & Minor DL Jr. Structure of a complex between a voltage-gated calcium channel beta-subunit and an alpha-subunit domain. Nature 429, 671–675, (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Page KM, Canti C, Stephens GJ, Berrow NS & Dolphin AC Identification of the amino terminus of neuronal Ca2+ channel α1 subunits α1B and α1E as an essential determinant of G protein modulation. Journal of Neuroscience 18, 4815–4824, (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jia JY, Lamer S, Schumann M, Schmidt MR, Krause E & Haucke V Quantitative proteomics analysis of detergent-resistant membranes from chemical synapses: evidence for cholesterol as spatial organizer of synaptic vesicle cycling. Mol Cell Proteomics 5, 2060–2071, (2006). [DOI] [PubMed] [Google Scholar]
- 164.Davies A, Douglas L, Hendrich J, Wratten J, Tran-Van-Minh A, Foucault I, Koch D, Pratt WS, Saibil H & Dolphin AC The calcium channel α2δ−2 subunit partitions with CaV2.1 in lipid rafts in cerebellum: implications for localization and function. J. Neurosci 26, 8748–8757, (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.de Jong APH, Roggero CM, Ho MR, Wong MY, Brautigam CA, Rizo J & Kaeser PS RIM C2B Domains Target Presynaptic Active Zone Functions to PIP2-Containing Membranes. Neuron 98, 335–349, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lauwers E, Goodchild R & Verstreken P Membrane Lipids in Presynaptic Function and Disease. Neuron 90, 11–25, (2016). [DOI] [PubMed] [Google Scholar]
- 167.Walter AM et al. Phosphatidylinositol 4,5-bisphosphate optical uncaging potentiates exocytosis. Elife 6, e30203, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Taoufiq Z, Eguchi K & Takahashi T Rho-kinase accelerates synaptic vesicle endocytosis by linking cyclic GMP-dependent protein kinase activity to phosphatidylinositol-4,5-bisphosphate synthesis. J Neurosci 33, 12099–12104, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rodriguez-Menchaca AA, Adney SK, Zhou L & Logothetis DE Dual Regulation of Voltage-Sensitive Ion Channels by PIP(2). Front Pharmacol 3, 170, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hille B, Dickson EJ, Kruse M, Vivas O & Suh BC Phosphoinositides regulate ion channels. Biochim. Biophys. Acta 1851, 844–856, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhu Y & Ikeda SR Modulation of Ca2+-channel currents by protein kinase C in adult rat sympathetic neurons. Journal of Neurophysiology 72, 1549–1560, (1994). [DOI] [PubMed] [Google Scholar]
- 172.Hamid J, Nelson D, Spaetgens R, Dubel S, Snutch TP & Zamponi G Identification of an integration center for cross-talk between protein kinase C and G protein modulation of N type calcium channels. Journal of Biological Chemistry 274, 6195–6202, (1999). [DOI] [PubMed] [Google Scholar]
- 173.Martin R, Bartolome-Martin D, Torres M & Sanchez-Prieto J Non-additive potentiation of glutamate release by phorbol esters and metabotropic mGlu7 receptor in cerebrocortical nerve terminals. J Neurochem 116, 476–485, (2011). [DOI] [PubMed] [Google Scholar]
- 174.Groten CJ & Magoski NS PKC enhances the capacity for secretion by rapidly recruiting covert voltage-gated Ca2+ channels to the membrane. J Neurosci 35, 2747–2765, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tomizawa K, Ohta J, Matsushita M, Moriwaki A, Li ST, Takei K & Matsui H Cdk5/p35 regulates neurotransmitter release through phosphorylation and downregulation of P/Q-type voltage-dependent calcium channel activity. J Neurosci 22, 2590–2597, (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yan Z, Chi P, Bibb JA, Ryan TA & Greengard P Roscovitine: a novel regulator of P/Q-type calcium channels and transmitter release in central neurons. J Physiol 540, 761–770, (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Buraei Z & Elmslie KS The separation of antagonist from agonist effects of trisubstituted purines on CaV2.2 (N-type) channels. J Neurochem 105, 1450–1461, (2008). [DOI] [PubMed] [Google Scholar]
- 178.Su SC, Seo J, Pan JQ, Samuels BA, Rudenko A, Ericsson M, Neve RL, Yue DT & Tsai LH Regulation of N-type voltage-gated calcium channels and presynaptic function by cyclin-dependent kinase 5. Neuron 75, 675–687, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kim SH & Ryan TA Balance of calcineurin Aalpha and CDK5 activities sets release probability at nerve terminals. J Neurosci 33, 8937–8950, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dolphin AC β subunits of voltage-gated calcium channels. J. Bioeng. Biomemb 35, 599–620, (2003). [DOI] [PubMed] [Google Scholar]
- 181.Liu G et al. Mechanism of adrenergic CaV1.2 stimulation revealed by proximity proteomics. Nature 577, 695–700, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lee A, Wong ST, Gallagher D, Li B, Storm DR, Scheuer T & Catterall WA Ca2+/calmodulin binds to and modulates P/Q-type calcium channels. Nature 399, 155–159, (1999). [DOI] [PubMed] [Google Scholar]
- 183.DeMaria CD, Soong TW, Alseikhan BA, Alvania RS & Yue DT Calmodulin bifurcates the local Ca2+ signal that modulates P/Q- type Ca2+ channels. Nature 411, 484–489, (2001). [DOI] [PubMed] [Google Scholar]
- 184.Nanou E & Catterall WA Calcium Channels, Synaptic Plasticity, and Neuropsychiatric Disease. Neuron 98, 466–481, (2018). [DOI] [PubMed] [Google Scholar]
- 185.Weyrer C, Turecek J, Niday Z, Liu PW, Nanou E, Catterall WA, Bean BP & Regehr WG The Role of CaV2.1 Channel Facilitation in Synaptic Facilitation. Cell Rep 26, 2289–2297, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lee A, Westenbroek RE, Haeseleer F, Palczewski K, Scheuer T & Catterall WA Differential modulation of Cav2.1 channels by calmodulin and Ca2+-binding protein 1. Nature Neuroscience 5, 210–217, (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Nanou E, Lee A & Catterall WA Control of Excitation/Inhibition Balance in a Hippocampal Circuit by Calcium Sensor Protein Regulation of Presynaptic Calcium Channels. J Neurosci 38, 4430–4440, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Liang H, DeMaria CD, Erickson MG, Mori MX, Alseikhan BA & Yue DT Unified mechanisms of Ca2+ regulation across the Ca2+ channel family. Neuron 39, 951–960, (2003). [DOI] [PubMed] [Google Scholar]
- 189.Thomas JR, Hagen J, Soh D & Lee A Molecular moieties masking Ca(2+)-dependent facilitation of voltage-gated Cav2.2 Ca(2+) channels. J Gen Physiol 150, 83–94, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pietrobon D CaV2.1 channelopathies. Pflugers Arch 460, 375–393, (2010). [DOI] [PubMed] [Google Scholar]
- 191.Tottene A, Conti R, Fabbro A, Vecchia D, Shapovalova M, Santello M, Van den Maagdenberg AM, Ferrari MD & Pietrobon D Enhanced excitatory transmission at cortical synapses as the basis for facilitated spreading depression in Ca(v)2.1 knockin migraine mice. Neuron 61, 762–773, (2009). [DOI] [PubMed] [Google Scholar]
- 192.Vecchia D, Tottene A, van den Maagdenberg AM & Pietrobon D Abnormal cortical synaptic transmission in CaV2.1 knockin mice with the S218L missense mutation which causes a severe familial hemiplegic migraine syndrome in humans. Front Cell Neurosci 9, 8, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Di Guilmi MN, Wang T, Inchauspe CG, Forsythe ID, Ferrari MD, Van den Maagdenberg AM, Borst JG & Uchitel OD Synaptic gain-of-function effects of mutant Cav2.1 channels in a mouse model of familial hemiplegic migraine are due to increased basal [Ca2+]i. J. Neurosci 34, 7047–7058, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Vecchia D, Tottene A, van den Maagdenberg AM & Pietrobon D Mechanism underlying unaltered cortical inhibitory synaptic transmission in contrast with enhanced excitatory transmission in CaV2.1 knockin migraine mice. Neurobiol Dis 69, 225–234, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Adams PJ, Garcia E, David LS, Mulatz KJ, Spacey SD & Snutch TP Ca(V)2.1 P/Q-type calcium channel alternative splicing affects the functional impact of familial hemiplegic migraine mutations: implications for calcium channelopathies. Channels (Austin) 3, 110–121, (2009). [DOI] [PubMed] [Google Scholar]
- 196.Mullner C, Broos LA, Van den Maagdenberg AM & Striessnig J Familial hemiplegic migraine type 1 mutations K1336E, W1684R, and V1696I alter Cav2.1 Ca2+ channel gating: evidence for beta-subunit isoform-specific effects. J Biol. Chem 279, 51844–51850, (2004). [DOI] [PubMed] [Google Scholar]
- 197.Consortium, S. W. G. o. t. P. G. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Takada Y, Hirano M, Kiyonaka S, Ueda Y, Yamaguchi K, Nakahara K, Mori MX & Mori Y Rab3 interacting molecule 3 mutations associated with autism alter regulation of voltage-dependent Ca(2)(+) channels. Cell Calcium 58, 296–306, (2015). [DOI] [PubMed] [Google Scholar]
- 199.Beguin P et al. BARP suppresses voltage-gated calcium channel activity and Ca2+-evoked exocytosis. J Cell Biol 205, 233–249, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nakao A, Miki T, Shoji H, Nishi M, Takeshima H, Miyakawa T & Mori Y Comprehensive behavioral analysis of voltage-gated calcium channel beta-anchoring and -regulatory protein knockout mice. Front Behav Neurosci 9, 141, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bassell GJ & Warren ST Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron 60, 201–214, (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ferron L, Nieto-Rostro M, Cassidy JS & Dolphin AC Fragile X mental retardation protein controls synaptic vesicle exocytosis by modulating N-type calcium channel density. Nat. Commun 5, 3628, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ferron L, Novazzi CG, Moreno C, Pilch KS, Ramgoolam K & Dolphin AC FMRP regulates presynaptic localization of neuronal voltage gated calcium channels Neurobiology of Disease (2020). January 25:104779. doi: 10.1016/j.nbd.2020.104779. [Epub ahead of print]] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zeitz C, Kloeckener-Gruissem B, Forster U, Kohl S, Magyar I, Wissinger B, Matyas G, Borruat FX, Schorderet DF, Zrenner E, Munier FL & Berger W Mutations in CABP4, the gene encoding the Ca2+-binding protein 4, cause autosomal recessive night blindness. Am J Hum Genet 79, 657–667, (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Shaltiel L, Paparizos C, Fenske S, Hassan S, Gruner C, Rotzer K, Biel M & Wahl-Schott CA Complex regulation of voltage-dependent activation and inactivation properties of retinal voltage-gated Cav1.4 L-type Ca2+ channels by Ca2+-binding protein 4 (CaBP4). J Biol Chem 287, 36312–36321, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Miki T, Kaufmann WA, Malagon G, Gomez L, Tabuchi K, Watanabe M, Shigemoto R & Marty A Numbers of presynaptic Ca(2+) channel clusters match those of functionally defined vesicular docking sites in single central synapses. Proc Natl Acad Sci U S A 114, E5246–E5255, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ohn TL, Rutherford MA, Jing Z, Jung S, Duque-Afonso CJ, Hoch G, Picher MM, Scharinger A, Strenzke N & Moser T Hair cells use active zones with different voltage dependence of Ca2+ influx to decompose sounds into complementary neural codes. Proc Natl Acad Sci U S A 113, E4716–4725, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Saka SK et al. Immuno-SABER enables highly multiplexed and amplified protein imaging in tissues. Nat Biotechnol 37, 1080–1090, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wang YZ & Savas JN Uncovering Discrete Synaptic Proteomes to Understand Neurological Disorders. Proteomes 6, E30, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Shields BC, Kahuno E, Kim C, Apostolides PF, Brown J, Lindo S, Mensh BD, Dudman JT, Lavis LD & Tadross MR Deconstructing behavioral neuropharmacology with cellular specificity. Science 356, eaaj2161, (2017). [DOI] [PubMed] [Google Scholar]
- 211.Tran-Van-Minh A & Dolphin AC The alpha2delta ligand gabapentin inhibits the Rab11-dependent recycling of the calcium channel subunit alpha2delta-2. J Neurosci 30, 12856–12867, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Farias GG, Guardia CM, Britt DJ, Guo X & Bonifacino JS Sorting of Dendritic and Axonal Vesicles at the Pre-axonal Exclusion Zone. Cell Rep 13, 1221–1232, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Guedes-Dias P, Nirschl JJ, Abreu N, Tokito MK, Janke C, Magiera MM & Holzbaur ELF Kinesin-3 Responds to Local Microtubule Dynamics to Target Synaptic Cargo Delivery to the Presynapse. Curr Biol 29, 268–282, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cooper CB, Arnot MI, Feng ZP, Jarvis SE, Hamid J & Zamponi GW Cross-talk between G-protein and protein kinase C modulation of N-type calcium channels is dependent on the G-protein beta subunit isoform. J. Biol. Chem 275, 40777–40781, (2000). [DOI] [PubMed] [Google Scholar]
- 215.Rebola N, Reva M, Kirizs T, Szoboszlay M, Lorincz A, Moneron G, Nusser Z & DiGregorio DA Distinct Nanoscale Calcium Channel and Synaptic Vesicle Topographies Contribute to the Diversity of Synaptic Function. Neuron 104, 693–710 e699, (2019). [DOI] [PubMed] [Google Scholar]
- 216.Sigal YM, Zhou R & Zhuang X Visualizing and discovering cellular structures with super-resolution microscopy. Science 361, 880–887, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Heuser JE & Reese TS Structural changes after transmitter release at the frog neuromuscular junction. J Cell Biol 88, 564–580, (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Harlow ML, Ress D, Stoschek A, Marshall RM & McMahan UJ The architecture of active zone material at the frog’s neuromuscular junction. Nature 409, 479–484, (2001). [DOI] [PubMed] [Google Scholar]
- 219.Parajuli LK, Nakajima C, Kulik A, Matsui K, Schneider T, Shigemoto R & Fukazawa Y Quantitative regional and ultrastructural localization of the Ca(v)2.3 subunit of R-type calcium channel in mouse brain. J. Neurosci 32, 13555–13567, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Borst JGG & Sakmann B Calcium current during a single action potential in a large presynaptic terminal of the rat brainstem. Journal of Physiology (Lond.) 506, 143–157, (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Stanley EF Calcium currents in a vertebrate presynaptic nerve terminal: the chick ciliary ganglion calyx. Brain Research 505, 341–345, (1989). [DOI] [PubMed] [Google Scholar]
- 222.Takahashi T, Forsythe ID, Tsujimoto T, Barnes-Davies M & Onodera K Presynaptic calcium current modulation by a metabotropic glutamate receptor. Science 274, 594–597, (1996). [DOI] [PubMed] [Google Scholar]
- 223.Bischofberger J, Geiger JR & Jonas P Timing and efficacy of Ca2+ channel activation in hippocampal mossy fiber boutons. J Neurosci 22, 10593–10602, (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Inchauspe CG, Martini FJ, Forsythe ID & Uchitel OD Functional compensation of P/Q by N-type channels blocks short-term plasticity at the calyx of held presynaptic terminal. J. Neurosci 24, 10379–10383, (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Akerboom J et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J Neurosci 32, 13819–13840, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Sabatini BL & Regehr WG Optical measurement of presynaptic calcium currents. Biophysical Journal 74, 1549–1563, (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Miesenbock G, De Angelis DA & Rothman JE Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394, 192–195, (1998). [DOI] [PubMed] [Google Scholar]
- 228.Wang C & Zucker RS Regulation of synaptic vesicle recycling by calcium and serotonin. Neuron 21, 155–167, (1998). [DOI] [PubMed] [Google Scholar]
- 229.Allen TG The ‘sniffer-patch’ technique for detection of neurotransmitter release. Trends Neurosci 20, 192–197, (1997). [DOI] [PubMed] [Google Scholar]
- 230.Hires SA, Zhu Y & Tsien RY Optical measurement of synaptic glutamate spillover and reuptake by linker optimized glutamate-sensitive fluorescent reporters. Proc Natl Acad Sci U S A 105, 4411–4416, (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Marvin JS et al. An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat Methods 10, 162–170, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Stanley EF Single calcium channels and acetylcholine release at a presynaptic nerve terminal. Neuron 11, 1007–1011, (1993). [DOI] [PubMed] [Google Scholar]
- 233.Scimemi A & Diamond JS The number and organization of Ca2+ channels in the active zone shapes neurotransmitter release from Schaffer collateral synapses. J Neurosci 32, 18157–18176, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Bucurenciu I, Bischofberger J & Jonas P A small number of open Ca2+ channels trigger transmitter release at a central GABAergic synapse. Nat Neurosci 13, 19–21, (2010). [DOI] [PubMed] [Google Scholar]
- 235.Borst JG & Sakmann B Calcium influx and transmitter release in a fast CNS synapse. Nature 383, 431–434, (1996). [DOI] [PubMed] [Google Scholar]
- 236.Cao YQ, Piedras-Renteria ES, Smith GB, Chen G, Harata NC & Tsien RW Presynaptic Ca2+ channels compete for channel type-preferring slots in altered neurotransmission arising from Ca2+ channelopathy. Neuron 43, 387–400, (2004). [DOI] [PubMed] [Google Scholar]
- 237.Lubbert M, Goral RO, Keine C, Thomas C, Guerrero-Given D, Putzke T, Satterfield R, Kamasawa N & Young SM Jr. CaV2.1 alpha1 Subunit Expression Regulates Presynaptic CaV2.1 Abundance and Synaptic Strength at a Central Synapse. Neuron 101, 260–273 e266, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Liu KS et al. RIM-binding protein, a central part of the active zone, is essential for neurotransmitter release. Science 334, 1565–1569, (2011). [DOI] [PubMed] [Google Scholar]
- 239.Engel AG Review of evidence for loss of motor nerve terminal calcium channels in Lambert-Eaton myasthenic syndrome. Annals of the New York Academy of Sciences 635, 246–258, (1991). [DOI] [PubMed] [Google Scholar]


