Abstract
Traditional medicine is gaining an increasing importance in diseases management. Besides, thyroid disease is one of the common endocrine disorders spreading at high frequency worldwide. The present work is an ethnopharmacological study aiming to identify, document and analyze aromatic and medicinal plants used in Algerian traditional medicines for thyroid disorders management. Semi-structured interviews with 120 herbalists and traditional practitioners and rural dwellers were realized in eleven locations in Algeria throughout field studies achieved from June 2017 to July 2019. Results reveal the use of 63 medicinal plants belonging to 59 genera and 34 families. The most represented botanical families were Lamiaceae, Fabaceae, Apiaceae, Amaranthaceae and Asteraceae. However, the most cited plant species were Atriplex halimus L., Bunium incrassatum (Boiss.) Amo, Nigella sativa L., Aquilaria malaccensis Lam. and Saussurea costus (Falc.) Lipsch. These species are taken alone or in mixtures of two or more ingredients from different origins such as honey, olive oil, and goat milk. Our findings revealed new therapeutic uses of 60 medicinal plants that have not been previously reported for the treatment of thyroid in Algeria. This is the first study documenting the traditional uses based on herbal medicine for thyroid management in Algeria. Our findings are relevant in the search for novel drug discovery. Obviously, it is the time to increase effective scientific studies on mechanisms of action of these medicinal plants in order to validate their popular usages.
Keywords: Traditional medicine, Ethnopharmacology, Medicinal plants, Thyroid disorders, Algeria
1. Introduction
The thyroid gland is an important butterfly-shaped organ of the human endocrine system. This soft and reddish parenchymal organ secretes thyroxine (T4) and tri-iodothyronine (T3) which play critical roles in the regulation of basal metabolism processes indispensable for normal growth and development (Soundarrajan and Kopp, 2019). Less than 1% of T4 is transported in free form while the remainder amount is transported bounded to plasma proteins. The T4 is deiodinated to T3 in the periphery. It should be noted that the thyroid produces all the circulating T4 but only 5 to 10% of circulating T3 while the rest is being derived from peripheral monodeiodination of T4 by the type I deiodinase in tissues such as heart, liver, kidney, and gut mucosa. However, the type II deiodinase provides intracellular T3 in specific sites such as central nervous system and pituitary gland (Mullur et al., 2014, Valverde-R et al., 2014). The free fraction of both T4 and T3 constitutes the active hormones. Throughout the activation of specific genes, these hormones stimulate proteo-synthesis, promote nervous system development, and regulate oxygen metabolism and calcium homeostasis (Oetting and Yen, 2007). The level of thyroid hormones production is under the control of thyroid stimulating hormone (TSH) released from the pituitary gland. On the other side, TSH level is regulated by the hypothalamus and further controlling mechanisms producing a feedback loop closed by the action of T4 and T3 on the pituitary gland (Mullur et al., 2014).
Nevertheless, thyroid disorders are among the most common endocrine diseases worldwide and are linked to numerous genetic and environmental factors although dietary iodine intake remains a key determinant of thyroid ailments. The hyperfunction of the thyroid gland suppresses the production of TSH, whereas the hypofunction stimulates the pituitary to produce further TSH (Soundarrajan and Kopp, 2019). Hyper and hypothyroidism might be due to the malfunction of thyroid gland, pituitary gland or to the hypothalamus and both forms are much more observed in women than men although the reasons are not completely elucidated. Goiter or active thyroid nodules may occur endemic in some inland regions due to dietary iodine deficiency and in many times, it may be the site of various types of tumors (Gonçalves et al., 2017).
The frequency of thyroid patients is in constant rise in Algeria affecting mainly adult female; according to WHO (2018), 2.103 new thyroid cancer cases were recorded in Algeria during 2018 among which 1.714 were women (81.5%). Clinical and epidemiological studies in Algeria have demonstrated the predominance of the papillary form (60% of cases) against vesicular form (40%). The treatment of differentiated thyroid cancer, most often revealed by a goiter (85%), consists of large thyroid surgery (91%) with lymph node dissection and ablative iratherapy (89%). The survival rate at 5, 15 and 30 years, depending on whether it is papillary or vesicular carcinoma, is respectively 95%, 94% and 79% versus 82%, 67% and 63% (Benouis et al., 2017).
Unfortunately, the health care services are usually too distant and hence inaccessible for rural populations living away from the main cities in Algeria. In addition, the health facilities are generally of poor-quality with huge inefficiencies which makes diagnosis and treatment difficult mainly for underprivileged families.
Moreover, some thyroid disorders need lifelong treatment and, usually, frequent relapses and side effects often follow drug treatments (Kim and Lee, 2019). Such hindrances led patients to search for alternative or complementary medicine to cure their ailments. Accordingly, herbal remedies have gained popularity to manage thyroid disorders as they are believed to be effective, safe and with less side effects (Bharthi et al., 2017). Several plant species are consumed worldwide to normalize thyroid hormones, to support the thyroid function as source of iodine or as thyroid suppressor (Gupta et al., 2016, Shokri et al., 2018, Verma and Jameel, 2014). Currently, Chinese herbal medicines, alone or in combination with drugs, have demonstrated significant benefits in improving thyroid function and reducing relapse rates and adverse effects compared to drugs therapy alone (Zen et al., 2007). By the same, Cunha Lima et al. (2012) reported the traditional use of numerous medicinal plants with pharmacological potential for the treatment of thyroid problems in Salvador-Bahia (Northeastern Brazil). This ethnopharmacological study revealed the presence of 31 candidates that might contain triiodothyronine (T3) and thyroxin (T4) analogs, including agonists, antagonists and many compounds capable to modulate thyroid receptor. Therefore, ethnopharmacological studies based on traditional and herbal medicines might constitute a valuable alternative strategy to discover natural hormone analogs for hormone replacement therapy.
Topographical, ecological, climatic and soil variability across Algeria has favorized the emergence of an important biodiversity represented by 3183 plant species which constitutes subsequently a substantial opportunity for screening of multiple biological compounds and active substances of interests (Boussaid et al., 2018, Taïbi et al., 2020). However, only few ethnopharmacological and ethnomedicinal studies have been realized to document the uses of this diversity by local populations in Algeria. Therefore, ethnopharmacological surveys are required to disclose medicinal plants uses for thyroid management and safeguard the local traditional knowledge (Yuan et al., 2016). In addition, it is imperative to develop a national pharmacopoeia and national guidelines of collect and uses (Taïbi et al., 2020).
The current study aims to document the ethnopharmacological relevance of medicinal plants used traditionally for the management of thyroid disorders in Algeria. The obtained data are thought to enrich national and world’s databases of traditional knowledge and safeguard the cultural heritage as recognized by the UNESCO in 2003. Up to knowledge of authors, this study represents the first ethnopharmacological investigation carried out on thyroid disorders management in Algeria and therefore, it might constitute the basis for further studies seeking for eco-friendly green drug discovery.
2. Methodology
2.1. Data collection
Ethnopharmacological study was conducted through field studies achieved during two years from June 2017 to July 2019. The study was engaged in eleven different locations in Algeria i.e. Adrar, Aflou, Chlef, Laghouat, Mila, Mostaghanem, Oran, Ouargla, Relizane, Tiaret and Tissemsilt reflecting both, the traditional and the modern regions encompassing both modern Western-style healthcare and traditional, culture-based healthcare. The regions covered in this study are characterized by their ecological and climatic diversity reflecting the most characteristic ecosystems of the country including the coasts, mountains, forests, arid high plains, steppe and Sahara. These various ecosystems shelter significant faunistic and floristic biodiversity of international importance as defined by the Ramsar Convention. This cross-validation was achieved to cover all the cultural and regional differences and to obtain representative dataset for the whole Algerian west- and center region. The interrogated local population works mainly in agriculture (animal farming, pastoralism and plant farming) and commercial sector, service industries while the other activities are of less importance. Socio-demographic characteristics of the informants are exposed in Table 1.
Table 1.
Socio-demographic characteristics of the informants.
| Socio-demographic variables | Number | Percentage (%) | |
|---|---|---|---|
| Age |
>30 | 18 | 15 |
| 30–45 | 35 | 29 | |
| 45–60 | 41 | 34 | |
| >60 | 26 | 22 | |
| Gender | Male | 76 | 63 |
| Female | 44 | 37 | |
| Education | Illiterate | 42 | 35 |
| Primary | 19 | 16 | |
| Middle | 22 | 18 | |
| Secondary | 22 | 18 | |
| University | 15 | 13 | |
In total, one hundred-twenty informants were interviewed throughout this study (n = 120). This number includes forty-three herbalists (n = 53) and sixty-seven traditional practitioners and rural dwellers (n = 67).
The study directed in agreement with the requirements of the declarations of Helsinki was approved by the scientific committee for ethical criterion in the department of Natural and Life Sciences, Ibn Khaldoun University of Tiaret (Algeria). Therefore, semi-structured interviews based on note-taking while interviewing the informants were conducted with the local dialect and generally took place in public spaces as described by Martin (1995). Informant consent was obtained prior to the interviews to authorize the collection, use and publication of data, then informants were asked to list aromatic and medicinal plants used for thyroid diseases management and were requested to provide detailed information about their uses. Following the International Society of Ethnobiology (ISE) code of ethics, interviews were not limited in time to allow the informants answering addressed questions spontaneously without pressure.
Interviews covered popular and vernacular names of the used plant species, used parts, mode of preparation and administration, dosage, period of treatment and toxicity among other information. Local names were provided mostly in Arabic and/or in Amazigh languages and informants were asked whether they would be willing to deliver a sample or to recognize it in photos if the material was not available. The collected specimens were pressed and dried on site then the voucher specimens were identified by specialists and conserved in the laboratory at the Faculty of Natural and Life Sciences, University of Tiaret (Algeria). The identity of plant species was verified according to the available bibliographical resources and scientific names were confirmed in accordance with the International Index of Plant Name (http://www.ipni.org) and the Plant List database (http://www.theplantlist.org).
It should be noted that diagnosis of thyroid disorders is realized by specialists’ doctors through physical exams, imaging tests and measuring the amount of thyroid hormones in blood. Patients diagnosed with thyroid disorders tend to use usually traditional medicine alongside conventional treatments.
2.2. Data analysis
The obtained ethnopharmacological data were organized in a matrix then Frequency of Citation (FC) was calculated as the sum of informants that cite a use for the medicinal plant according to Prance et al. (1987). Continuous data were represented as mean ± standard deviation while frequencies and percentages were calculated for categorical variables. All the statistical analyses were performed using the computing environment R (R Development Core Team, 2013).
3. Results
In the present study, the number of female participants was higher than that of males (63% versus 37%). Overall, the age of the informants varies from 20 to 85 years old. Around 35% of the informants were illiterate whereas 16% have just elementary instructive level. However, 13% of the informants were undergraduate or graduate from the university.
Results revealed the use of 63 medicinal plant species, belonging to 59 genera and 34 families, for thyroid disorders management in Algeria (Table 2). The Lamiaceae family provided the largest number of species (10 species) followed by Fabaceae (5 species), Apiaceae (4 species), Amaranthaceae, Asteraceae and Brassicaceae families (3 species each). The other families were represented by just two or one species each. Several reported plant species are cultivated and used either for direct consumption or vended commercially such as Allium spp., Avena sativa L., Beta vulgaris L., Citrus spp., Daucus carota L., Hordeum vulgare L., Prunus spp., Raphanus sativus L. among others. Remarkably, several species belong to the Algerian steppe region and Sahara namely Artemisia spp., Atriplex halimus L., Haloxylon scoparium Pomel, Origanum spp., Peganum harmala L. and Phoenix dactylifera L.
Table 2.
Medicinal plant species used for thyroid disorders management in Algerian traditional medicines.
| Plant family: Species | Voucher n° | Common name | Local name | FC | Plant part | Mode of preparation | Application |
|---|---|---|---|---|---|---|---|
| Amaranthaceae | |||||||
| Atriplex halimus L. | EthTK015 | Sea orache | Gtaf | 32 | Aerial parts | Decoction, infusion, powder | One glass cup of the decoction or infusion is given on empty stomach daily during one month. One glass cup of the powder is mixed with honey (jar of 350–550 mL) and a spoon is given the morning on empty stomach and at night daily during three months. |
| Beta vulgaris L. | EthTK017 | Beetroot | Barba | 3 | Leaf | Infusion, compress | One glass cup of infusion made of beetroot and marjoram is taken daily at night. Leaves are mixed with tepid olive oil and applied daily as compress on thyroid |
| Haloxylon scoparium Pomel | EthTK051 | Haloxylon | Remth | 5 | Aerial parts | Powder | One glass cup of haloxylon powder is mixed with honey (jar of 350–550 mL) and one teaspoon is given on empty stomach the morning during one month. |
| Amaryllidaceae | |||||||
| Allium cepa L. | EthTK003 | Onion | Bsal | 8 | Bulb | Juice, decoction, | One glass cup of bulb juice (100–150 mL) is mixed with 1–2 tablespoon of honey and given orally morning on empty stomach for 1–3 weeks. Half fresh bulb is applied externally with a gentle massage on thyroid areas for 5 min during one week. |
| Allium sativum L. | EthTK004 | Garlic | Thoum | 6 | Bulb | Juice, decoction, raw | 2–3 fresh cloves are eaten raw once daily on empty stomach for 1–3 weeks. |
| Annonaceae | |||||||
| Annona muricata L. | EthTK008 | Soursop | Qachta | 7 | Fruit, seed | Raw, syrup | The fresh fruit or syrup of soursop are given daily. |
| Apiaceae | |||||||
| Bunium incrassatum (Boiss.) Amo | EthTK021 | Pig Nut | Talghouda | 30 | Tuber | Powder | One tablespoon of the powder is mixed in a glass cup of tepid goat milk and taken daily on empty stomach during one month. One glass cup of the powder is mixed with honey (jar of 350–550 mL) or with ½ honey and ½ goat butter and one teaspoon is given on empty stomach the morning and at night during one month. |
| Carum carvi L. | EthTK107 | Caraway | Karwya | 1 | Seed | Infusion, powder | One glass cup of caraway seed or powder infusion is given daily. |
| Daucus carota L. | EthTK040 | Carrot | Zrodya | 1 | Root, seed | Decoction, infusion | One glass cup of carrot seed or powder infusion/decoction is given daily. |
| Petroselinum crispum (Mill.) Fuss | EthTK070 | Parsley | Maâdnous | 1 | Stem, leaf, fruit | Decoction | One glass cup of parsley decoction is given daily on empty stomach and at night |
| Arecaceae | |||||||
| Phoenix dactylifera L. | EthTK072 | Date Palm | Tmar | 6 | Fruit, nut | Raw, powder | One glass cup of fruit and nut powder is mixed with honey (jar of 350–550 mL) and one teaspoon is given on empty stomach the morning during one to two months. |
| Asteraceae | |||||||
| Anacyclus pyrethrum (L.) Lag. | EthTK108 | Spanish chamomile | Baboundj | 1 | Flowers | Infusion | One glass cup of chamomile infusion is given daily. |
| Artemisia herba-alba Asso | EthTK014 | white wormwood | Chih | 1 | Aerial parts | Infusion | One glass cup of wormwood infusion is given daily. |
| Saussurea costus (Falc.) Lipsch. | EthTK090 | Costus | Qist el Hindi | 12 | Rhizome | Infusion, decoction, powder | One to three teaspoons of costus powder is mixed in a glass cup of honey and a teaspoon is given daily on empty stomach and at night during one month. One glass cup of costus infusion/decoction is given daily on empty stomach and at night. |
| Berberidaceae | |||||||
| Berberis vulgaris L. | EthTK016 | Barberry | Oud Ghriss | 6 | Peel, bark | Powder | One tablespoon of berberis and pig nut powder are mixed with honey (jar 350–550 mL) and a spoon is given daily on empty stomach during 2–3 weeks. |
| BoraginaceaeLithodora fruticosa (L.) Griseb. | EthTK117 | Shrubby gromwell | Chingibar | 4 | Aerial parts | Infusion, decoction | One glass cup of the infusion or decoction is given daily during one month. |
| Brassicaceae | |||||||
| Eruca sativa Mill. | EthTK115 | Arugula | Jarjeer | 1 | Leaf | Raw, compress | Leaves are mixed with tepid olive oil and applied daily as compress on thyroid. |
| Lepidium sativum L. | EthTK057 | Cress | Horf, hab errchad | 8 | Seed | Raw, powder | One teaspoon of cress seeds or powder is mixed in yoghurt pot and given daily at night. 150 g of cress powder and 100 g of turmeric are mixed with honey (jar of 350–550 mL) and a tablespoon is given three times daily after meal. |
| Raphanus sativus L. | EthTK126 | Radish | Left | 6 | Root | Raw | The fresh roots are incorporated in salad or eaten uncooked. |
|
Convolvulaceae Convolvulus sabatius subsp. mauritanicus (Boiss.) Murb. |
EthTK114 | Bindweed | Shobrog | 3 | Aerial parts | Decoction, infusion | One glass cup of the decoction or infusion is given on empty stomach daily during one month |
|
Cupressaceae Juniperus phoenica L. |
EthTK054 | Phoenicean juniper | Ârâar | 3 | Leaf, fruit | Decoction, infusion | One glass cup of the decoction or infusion is given on empty stomach daily during one month. |
| Fabaceae | |||||||
| Cicer arietinum L. | EthTK112 | Chickpea | Homos | 1 | Seed | Powder | The powder of chickpea and almond one glass cup each are mixed with honey (jar 350–550 mL) and a tablespoon is given daily at night. |
| Glycyrrhiza glabra L. | EthTK049 | Liquorice | Erq-Essous | 2 | Rhizome, root | Powder | One glass cup of liquorice infusion is taken daily. One glass cup of liquorice powder is mixed with honey (jar 350–550 mL) and a tablespoon is given daily on empty stomach during one month. |
| Lupinus luteus L. | EthTK118 | Yellow-lupin | Termess | 1 | Seed | Powder | One glass cup of lupin powder is mixed with honey (jar 350–550 mL) and a teaspoon is given daily on empty stomach during one month. |
| Trigonella foenum-graecum L. | EthTK101 | Fenugreek | Helba | 2 | Seed | Raw, infusion, compress | Three tablespoons of fenugreek powder are mixed with glass cup of honey and teaspoon is given three times per day. Powder is mixed with alcohol and applied daily as compress on thyroid. |
| Vicia faba L. | EthTK129 | Faba bean | Foul | 4 | Seed | Powder | One glass cup of faba bean powder is mixed with cup of honey and cup of goat butter and a teaspoon is given daily on empty stomach during one month. |
| Fagaceae | |||||||
| Quercus ilex L. | EthTK125 | Holm oak | Ballout | 2 | Fruit peel | Infusion, maceration | One glass cup of holm oak fruit peel infusion or maceration could be mixed with tablespoon of honey and given daily on empty stomach and at night. |
| Iridaceae | |||||||
| Crocus sativus L. | EthTK036 | Saffron crocus | Zaâfrane | 3 | Flower, pistil | Raw, infusion | One glass cup of saffron flower infusion could be mixed with tablespoon of honey and given daily. |
| Lamiaceae | |||||||
| Ajuga iva (L.) Schreb. | EthTK002 | Herb Ivy | Chendgoura | 12 | Stem, leaf, flower | Powder | One glass cup of the powder is mixed with honey (jar of 350–550 mL) and tablespoon is given daily on empty stomach and at night during one month. |
| Lycopus europaeus L. | EthTK119 | Gypsywort | Echbat ethib | 10 | Leaf, stem, flower | Infusion, decoction | One glass cup of gypsywort infusion/decoction is given daily. |
| Melissa officinalis L. | EthTK120 | Lemon balm | Mlissa | 11 | Aerial parts | Infusion, decoction | One glass cup of clary infusion/decoction is given daily. |
| Salvia verbenaca L. | EthTK089 | Wild clary | Kassâin raâie el hmam | 2 | Aerial parts | Infusion, decoction | One glass cup of clary infusion/decoction is given daily. |
| Ocimum basilicum L. | EthTK123 | Basil | Merdkouch | 1 | Leaf | Decoction, infusion | One glass cup of basil infusion/decoction is given daily. |
| Origanum majorana L. | EthTK124 | Marjoram | Merdkouch el kbir | 6 | Leaf | Infusion | One glass cup of marjoram infusion is given daily. |
| Origanum vulgare L. | EthTK068 | Oregano | Zaâtar berri | 2 | Leaf | Infusion | One glass cup of marjoram infusion is given daily. |
| Rosmarinus officinalis L. | EthTK085 | Rosemary | Klil, Halhal, Azir | 8 | Leaf, stem, flower | Infusion | One glass cup of Rosemary infusion is given daily. |
| Teucrium polium L. | EthTK128 | Felty germander | Joâayda | 6 | Leaf | Infusion, decoction | One glass cup of germander infusion/decoction is given daily morning on empty stomach. |
| Thymus vulgaris L. | EthTK100 | Thyme | Zaâtar | 6 | Aerial parts | Infusion | One glass cup of thyme infusion is given daily at night. |
| Lauraceae | |||||||
| Cinnamomum verum J. Presl | EthTK030 | Cinnamon | Qarfa | 4 | Peel, bark | Powder | One glass cup of the powder is mixed with honey (jar of 350–550 mL) and teaspoon is given daily on empty stomach and at night during one month. |
| Linaceae | |||||||
| Linum usitatissimum L. | EthTK058 | Flax | Zerriâat el Kettane | 5 | Seed | Raw, powder, compress | One teaspoon of flag seeds or powder is decocted in glass cup of water and given once or twice per day. Four tablespoons of flag powder are mixed with tepid water and applied daily as compress on thyroid |
| Lythraceae | |||||||
| Lawsonia inermis L. | EthTK056 | Henna | Henna | 2 | Leaf | Powder, compress | Three tablespoons of henna powder are mixed with tepid water or alcohol and applied daily as compress on thyroid |
|
Moraceae Ficus carica L. |
EthTK046 | Fig | Karmous | 10 | Fruit | Raw | Fresh Fig fruits are eaten raw daily. |
| Morus nigra L. | EthTK121 | Blackberry | Toutt aswad | 12 | Fruit | Raw | Fresh Blackberry fruits are eaten raw daily. |
|
Myrtaceae Myrtus communis L. |
EthTK122 | Myrtle | Hamblass | 1 | Leaf | Infusion | One glass cup of myrtle infusion is given daily |
| Oleaceae | |||||||
| Olea europaea L. | EthTK066 | Olive | Zitoune | 2 | Leaf | Infusion | One glass cup of olive leaves infusion is given daily |
|
Plantaginaceae Bacopa monnieri (L.) Wettst. |
EthTK110 | Waterhyssop | Brahmi | 6 | Leaf | Infusion | One glass cup of brahmi leaves infusion is given daily |
| Poaceae | |||||||
| Avena sativa L. | EthTK109 | Oat | Khortal | 8 | Seed | Powder | Avena powder is used in food preparations or can be mixed with honey, milk, butter or Yoghurt and given daily |
| Hordeum vulgare L. | EthTK052 | Barley | Zraâ, Cheêir | 1 | Seed | Raw, juice, powder | One glass cup of barley powder is mixed with ¼ glass cup of goat butter and a tablespoon is given on an empty stomach each day. |
| Punicaceae | |||||||
| Punica granatum L. | EthTK081 | Pomegranate | Rommane | 1 | Leaf, fruit, seed | Raw, juice, powder | One glass cup of pomegranate seeds and/or leaves powder is mixed with honey (jar of 350–550 mL) and one teaspoon is given on empty stomach during one month. |
| Ranunculaceae | |||||||
| Nigella sativa L. | EthTK065 | Devil in the bush | Habet el Baraka | 14 | Seed | Raw, powder | One glass cup of the powder is mixed with honey (350–450 mL) and a spoon is given daily on an empty stomach morning and at night during. |
| Rosaceae | |||||||
| Prunus amygdalus Batsch | EthTK078 | Almond | Louz el mor | 1 | Root, leaf, seed | Raw, powder, decoction | One glass cup of Almond leaf, seed and/or root powder is used as decoction or mixed with honey (350–450 mL) and a spoon is given daily on an empty stomach during one month. |
| Prunus persica (L.) Batsch | EthTK080 | Peach | Khoukh | 2 | Leaf, seed, fruit | Raw, powder | One glass cup of peach leaf and/or seeds powder is mixed with honey (350–450 mL) and a spoon is given daily on an empty stomach during one month. |
| Rutaceae | |||||||
| Citrus limon (L.) Osbeck | EthTK032 | Lemon | Limone | 1 | Fruit, peel | Juice, infusion, decoction | One glass cup of lemon juice or peel infusion/decoction is mixed with tablespoon of honey and taken daily |
| Citrus sinensis (L.) Osbeck | EthTK113 | Sweet orange | Tchina | 16 | Fruit, peel | Juice, infusion, decoction | One glass cup of orange juice or peel infusion/decoction is mixed with tablespoon of honey and taken daily. |
| Solanaceae | |||||||
| Capsicum annuum L. | EthTK024 | Pepper | Sasafinda | 1 | Fruit | Raw, decoction | One glass cup of pepper deoction is mixed with tablespoon of honey and taken daily. |
| Withania somnifera (L.) Dunal | EthTK130 | Winter cherry | Sekrane | 6 | Leaf | Infusion, decoction | One glass cup of Winter cherry infusion/decoction is taken daily. |
| Thymelaeaceae | |||||||
| Aquilaria malaccensis Lam. | EthTK009 | Agar wood | Oud | 12 | Stem, leaf, flower | Oil, decoction | One to three tablespoons of the powder is mixed with honey (350–450 mL) and a spoon is given on an empty stomach each day during 10–30 days. |
|
Verbenaceae Lippia citriodora (Palau) Kunth |
EthTK116 | Lemon verbena | Lwiza | 4 | Leaf | Infusion, Decoction | One glass cup of lemon verbena infusion or decoction is given on empty stomach daily during one month. |
| Vitaceae | |||||||
| Vitis vinifera L. | EthTK104 | Grape vine | Aneb | 6 | Leaf, fruit | Raw, powder | One glass cup of seed powder is mixed with honey (350–450 mL) and a spoon is given daily on an empty stomach morning and at night during. |
|
Xanthorrhoeaceae Aloe barbadensis Mill. |
EthTK107 | Aloe vera | Mor-w-sbar | 8 | Gel, juice | Compress | Aloe gel applied daily as compress on thyroid. |
| Zingiberaceae | |||||||
| Curcuma longa L. | EthTK039 | Turmeric | Curcum | 4 | Rhizome | Powder, decoction | One tablespoon of the turmeric powder is mixed with glass cup of goat milk and given daily. 150 g of turmeric powder, 5 g of black pepper powder and 200 g of garlic are mixed in 1 L olive oil and kept in jar for one week. The tablespoons are given three times per day during six months. |
| Zingiber officinale Roscoe | EthTK105 | Ginger | Zanjabyl | 5 | Rhizome | Powder, infusion | One teaspoon of ginger powder and one tablespoon of honey are mixed in one glass cup of tepid water and given daily. One teaspoon of ginger powder, one teaspoon of cinnamon powder and one tablespoon of honey are mixed in glass cup of tepid water and given daily. |
| Zygophyllaceae | |||||||
| Peganum harmala L. | EthTK069 | Harmel | Harmel | 1 | Seed | Raw, powder | One to three tablespoons of the powder are mixed with honey (jar of 350–450 mL) and a tablespoon is given on an empty stomach each day during 10–30 days. |
It is important to note that the majority of the inventoried plant species are native to the Mediterranean region or North Africa. Interestingly, Bunium incrassatum (Boiss.) Batt. & Trab. and Origanum floribundum Munby have been reported as Algerian endemic plant species. The use of other introduced species like Saussurea costus (Falc.) Lipsch was also reported.
On the basis of citation frequency, the most cited plant species by the informants to manage thyroid disorders were respectively Atriplex halimus L. (FC = 32), Bunium incrassatum (Boiss.) Amo (FC = 30), Nigella sativa L. (CF = 14), Aquilaria malaccensis Lam. (CF = 12), Saussurea costus (Falc.) Lipsch (CF = 12), Allium cepa L. and Lipidium sativum L. (CF = 8).
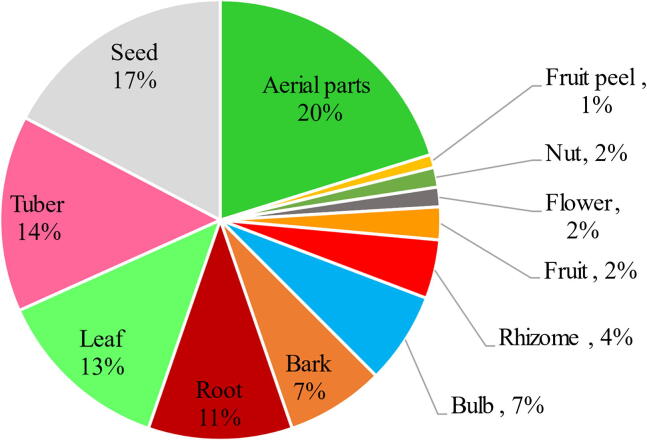
Aerial parts were the most commonly used part by the informants (around 20%) followed by seeds (17%), tubers (14%), leaves (13%) and roots (11%) as the next most likely used plant parts. Bark and bulb are used by around 7% while the use of rhizome is 4%. However, the other parts indicated in Fig. 1 are the least used by the informants.
Fig. 1.
Frequency of plant parts used for thyroid management in Algerian traditional medicines.
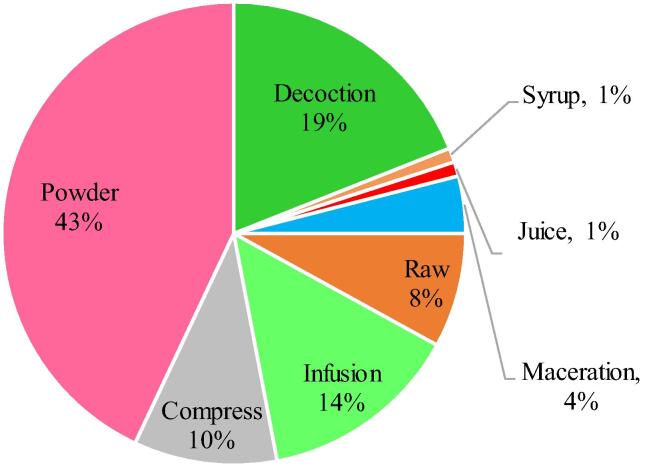
Regarding the methods of preparation, most often the indicated medicinal plants parts are crushed or powdered (43%). However, 19% are prepared as decoction through boiling the crushed tissues in water till the volume has been reduced by around the half then the filtrate is administered orally. Nevertheless, 14% of the medicinal herbs used in thyroid management were prepared by herbal infusion method and administered orally either alone or in combination with honey or goat milk. In addition, 8% of the plants are used for external application as compress. Other uses are also reported in other few cases such as maceration and transformation to juice or syrup (Fig. 2).
Fig. 2.
Frequency of mode of use of medicinal plants parts for thyroid management in Algerian traditional medicines.
Honey was widely cited by the informants in the present study (FC = 57). It was used as additive to prepare mixtures based on more than one ingredient. Informants have preconized mainly that of Ziziphus lotus flowers but most of them have stated that any pure honey could be of interest if this later is not available. Honey was administered fresh or in infusion, alone or in combination with the other natural products from different origins. In addition, informants have reported the use of milk and butter of goat (FC = 5 and 13 respectively), olive oil (FC = 5) and Yoghurt (FC = 2). Informants believe that these additives are able to enhance healing, improve the taste and reduce side effects of the herbal medicine.
It was difficult to establish standards and guidelines of uses since the dosage varies considerably among informants. Generally, the given prescriptions are undertaken by hand palm, tablespoon, teaspoon, little finger index, coffee cup or glass measures. The frequently defined doses by informants for decoction and infusion are a glass cup measure (100 to 150 mL) which could be mixed with a tablespoon of honey but patient should not add sugar. Herbs are generally crushed and ground then the powder is mixed with additives (honey, olive oil, butter, milk, water or alcohol) in order to obtain a paste which will be administered orally using spoons or applied externally as compress on thyroid. Informants usually add from few tablespoons to glass cup of the powder to a jar of honey (350 to 550 mL). Sometimes they use glass cup of goat milk or butter. For certain mixtures, informants recommend that it should be prepared one or two hours before consumption. However, other preparations are advised to be prepared and conserved for one week before use. Patients typically take one to three tablespoons or glass cups from one to three times per day (Table 2).
4. Discussion
Ethnopharmacological studies constitute a less expensive way of finding natural drug candidates for several diseases. The search for natural hormone analogs in medicinal plants to substitute synthetic compounds is extremely promising. Several molecules from medicinal plants have been described as modulators of nuclear receptors (He et al., 2012, Ong and Tan, 2007) including thyroid hormone receptors (Reis et al., 2018). The target medicinal plants molecules should not be only hormones analogs, but also further similar compounds capable to modulate existing nuclear receptors (such as ligands, agonists, antagonists, co-activators, co-repressors, and responsive elements) since transcription factors control practically the whole genetic activity and subsequently the corresponding physiology. In addition, Lin et al. (2007) have reported that the innumerable molecules contained in plant extracts might act synergistically in modulating nuclear receptors. Ong and Tan (2007) have described also that plant extracts are able to enhance the nuclear receptor activity through binding outside the hormone pocket, even when the receptor is saturated with endogenous ligands.
In the present study, most of the gathered data on traditional knowledge about thyroid management has been shared by informants having more than 50 years old. In addition, illiterate informants shared more information than those with high instructive level. Bouzid et al. (2017) and Chaachouay et al. (2019) have reported similar finding in Algeria and Morocco respectively.
In general, 63 medicinal plant species have been reported traditionally used for thyroid disorders management in Algeria. The reported plant diversity was distributed within 34 families and 59 genera. Lamiaceae, Fabaceae and Apiaceae were respectively the most represented families. The prevalence of these plant families is well known in the Algerian medicinal flora (Benarba et al., 2015, Meddour and Meddour-Sahar, 2016, Sarri et al., 2015, Sarri et al., 2014) and in the whole Mediterranean basin (González-Tejero et al., 2008). Furthermore, aerial parts including leaves and stems were the most common used plant parts cited among informants (Fig. 2). In general, the usage of leaves does not threaten the life cycle of the used plants (Bhat et al., 2013). However, the respective frequent used parts were seeds (17%), tubers (14%), roots (11%), bark and bulbs (7%) which means that the underground plant parts represent almost 40% of the total. Probably, their use is due to their richness with bioactive ingredients (Adnan et al., 2014).
Most of the used parts were ground and powdered (43%) then used as preparations with other additives and ingredients. The second mode of preparation was decoction (19%) and infusion (14%). Topical application in form of compress represents 10%. The use of dried parts as powder could be suitable for some plants however, the boiling procedure reported by the informants can cause severe degradation of the therapeutic compounds in some medicinal plants.
The previous studies undertaken in Algeria have reported only the use of Juniperus phoenicea L. (Chermat and Gharzouli, 2015), Ajuga iva (L.) Sch (Benarba et al., 2015) and Berberis vulgaris L. (Elyebdri et al., 2017) for thyroid management but no further information was provided about which type of disorders they are used for, their mode of use or their related toxicity. Actually, thyroid disorders range from harmless gland enlargement that needs no treatment to aggressive cancer. However, the most common disorders involve perturbation of thyroid hormones production (hyperthyroidism and hypothyroidism).
Basing on the frequency of citation, the most cited medicinal plant species to manage thyroid disorders were Atriplex halimus L. (FC = 32), Bunium incrassatum (Boiss.) Amo (FC = 30) and Nigella sativa L. (CF = 14). Atriplex halimus L. and Bunium incrassatum (Boiss.) Amo particularly are used for treating both hyperthyroidism and hypothyroidism, although their effects are poorly understood. To the knowledge of authors, there is no published work describing the use of Atriplex halimus L. and Bunium incrassatum (Boiss.) Batt. & Trab. for the treatment of thyroid disorders.
Practically the majority of participants have informed the use Atriplex halimus L. as anti-hyperthyroid while others reported its use as anti-hypothyroid however, almost all the informants have cited its potent use for thyroid cysts treatment. The phytochemical studies of A. halimus have demonstrated that it contains up 10% sodium chloride along with several active compounds such as phenolic acids, flavonoids, alkaloids, tannins, saponins and resins acting as potent reducing agents and singlet oxygen quenchers (Benhammou et al., 2009, Mohammedi, 2016). Kabbash and Shoeib (2012) have isolated two new flavonol glycosides from the aerial parts of A. halimus while Clauser et al. (2013) have isolated other four new glycosylated flavonoids along with other known phenolic compounds. Almost all of these molecules, mainly flavonoids glycosides, are known by their anti-hyperthyroid activity since they are able to inhibit the synthesis of thyroid hormones by acting as alternative substrates for the key enzyme in thyroid hormones biosynthesis thyroperoxidase (TPO), and therefore to a rise in TSH levels in male Sprague–Dawley rat treated by 50 mg/kg quercetin during 14 days (Giuliani et al., 2014, Gonçalves et al., 2017).
Besides, Bunium incrassatum (Boiss.) Batt. & Trab. is an economically important endemic medicinal plant in the north of Algeria (Quezel and Santa, 1963). This plant has a long history in Algeria since it contributed to save many Algerian populations from starvation during the French colonization where people resorted to dry and ground the harvested tubers of this plant to prepare bread and couscous. The dried powder of its tubers is usually used as astringent, anti-diarrheal, anti-inflammatory as well as for bronchitis and cough treatments in local traditional medicine. This species has been widely reported throughout the present study for the management of both hyper and hypothyroidism. Essential oils and extracts from some Bunium spp. have revealed a potent antioxidant activity (Shahsavari et al., 2008) but there is still insufficient data about its phytochemical composition and its use in Algeria and worldwide. At this point the study could offer valuable information for the use and valorization of such neglected genetic resources. Thereby, phytochemical studies of Bunium microcarpum (Boiss.) Freyn & Bornm., B. brachyactis (Post) H. Wolff and B. pinnatifolium Kljuykov have revealed the presence of apigenin, chlorogenic acid, isoquercitrin, rutin, pantothenic acid, esculin, quinic acid, scopoletin, coumarins, monoterppenoids and sesquiterpenes. However, the presence of angelicin, diosmin, vitexin, cosmosiin, luteolin, salcolin B, vicenin-2, naringenin, afzelin, kaempferol and orientin was species dependent (Appendino et al., 1994, Sharafati Chaleshtori et al., 2018, Talebi et al., 2018).
As well, Nigella sativa L. was the most cited plants used against hypothyroidism among several medicinal plants recommended for hypothyroid management. This species is used traditionally in different forms to treat many diseases such as asthma, bronchitis, cough, influenza, diabetes, headache, hypertension, fever, inflammation, eczema, and dizziness. N. sativa L. holds important protective immunomodulatory effects against several autoimmune diseases (Noor et al., 2015). It contains thymoquinone (2-isopropyl-5-methyl-1,4-benzoquinone) which acts as a potent antioxidant (Nagi and Mansour, 2000) and anti-inflammatory in many ailments including cancer (Woo et al., 2012, Taïbi et al., 2020).
Daily consumption of 2 g N. sativa L. powder during 8 weeks has proved to be effective against hypothyroidism in forty patients, aged between 22 and 50 years old, suffering from Hashimoto’s thyroiditis. The beneficial effects of N. sativa L. have been attributed mainly to its antioxidant potential (Farhangi et al., 2016).
Furthermore, thymoquinone decreases thyroid inflammation via the up-regulation of heme-oxygenase-1 expression and the suppression of cyclooxygenase-2 (COX-2) expression (Khader and Eckl, 2014). N. sativa L. powder administered at a dose of 1.7 mcg/kg body weight/day during eight weeks raised also T3 levels and reduced the synthesis of anti-TPO antibodies as well as the transforming growth factor (TGF)-β and interleukin (IL)–23 concentrations among forty patients with Hashimoto's thyroiditis aged between 20 and 50 years (Tajmiri et al., 2016). Wang et al. (1998) have reported that vascular endothelial growth factor (VEGF) and its receptors (Flt-1) contribute to the developmental regulation of thyroid epithelial cells in adult Wistar rats after administration of goitrogen for two weeks. Studies have revealed that thymoquinone decreases VEGF levels (Sethi et al., 2008) which are known to increase in response to the increase of TSH concentrations in patients suffering from chronic thyroiditis or thyroid cancer (Farhangi et al., 2016).
Interestingly, thymoquinone is also present in Thymus vulgaris L. (Taborsky et al., 2012) and Origanum spp. (Ahmad et al., 2019) which were also recommended by the informants as anti-hypothyroid treatment. Miler et al. (2017) and Elwan et al. (2019) have demonstrated that Citrus limon (L.) Osbeck flavanones naringenin and hesperetin, administrated orally (15 mg/kg) during four weeks as effective antioxidants, significantly inhibited lipid peroxidation and increased serum TSH in male Wistar rats. Besides, Chaturvedi et al. (1993) have reported a slight stimulation of the thyroid gland by the alcoholic extract of Saussurea lappa (Decne.) Sch.Bip (400 mg/kg body weight) administered during two weeks to Wistar rats. Nevertheless, El Mgeed et al. (2009) have demonstrated that the extract of Glycyrrhiza glabra L., prepared by boiling 2.5 g of fresh licorice in 100 mL of distilled water and administrated ad-libitum to Wistar rats during four weeks, strongly enhances hormone metabolism via pituitary and adrenal cortex regulation.
Concerning hyperthyroid management, various medicinal plants were recommended and some of them have already demonstrated anti-hyperthyroid activity in vivo. Chandra et al. (2006) have pointed out that prolonged rat’s consumption of Raphanus sativus L. induced a decrease of thyroid peroxidase activity and thyroid hormone profile. However, Parmar and Kar (2008) have demonstrated the antithyroid and antiperoxidative activities of Citrus sinensis (L.) Osbeck peel extract administered to male mice at a concentration of 25 mg/kg during ten days and they have correlated these activities to its phenolics compounds mainly to flavone glycosides, polymethoxylated flavones and hydroxycinnamate.
In addition, the anti-hyperthyroid effect of several members of the Lamiaceae family plants such as Rosmarinus officinalis L., Melissa officinalis L., Lycopus europaeus L., Ocimum basilicum L., Origanum vulgare L., Origanum majorana L., Salvia verbenaca L. and even in some members of Boraginaceae family like Lithodora fruticose (L.) Griseb. is attributed to rosmarinic acid. This molecule is supposed to inhibits TSH effects on receptor sites, constrains immunoglobulin effects on TSH receptors, and reduces peripheral conversion of thyroxine to T3 (Bharthi et al., 2017, Eric and Kathy, 2006, Miraj et al., 2017).
Besides, the use of Prunus spp. and Linum usitatissimum L. as anti-hyperthyroid remedy could be due to the presence of cyanogenic glycosides compounds i.e. amygdalin, dhurrin, and linamarin. These compounds are known to generate thiocyanate and thiocyanate-like which are monovalent anions with a molecular size similar to that of iodide and therefore, they compete with iodide at the thyroid peroxidase level which inhibit its assimilation into thyroglobulin (Chandra, 2010).
The use of multiple medicinal therapies based on mixing two or more natural products even combined with chemical drugs may increase the effectiveness of such medicines (Ait Abderrahim et al., 2019a, Ait Abderrahim et al., 2019b). The medicinal plants reported in this study were used in preparations mixed with other ingredients like honey, milk and butter. Honey of Ziziphus lotus L. which is the most cited in this study was recently characterized by Zerrouk et al. (2018). Honey holds several bioactive compounds such as polyphenols and flavonoids (Waheed et al., 2019). It was reported to have antioxidant (Almasaudi et al., 2016, Ait Abderrahim et al., 2017), anti-inflammatory and estrogenic effects (Porcza et al., 2016), apoptotic, immunomodulatory, antiproliferative (Jaganathan et al., 2015) and anticarcinogenic action (Subramanian et al., 2016). Besides, consumption of milk or dairy products (butter and Yoghurt) is correlated with a reduced risk of numerous types of cancer (Jeyaraman et al., 2019). These properties are attributed to the variety of components such as fat compounds, conjugated linoleic acid, and proteins such as casein and vitamins (A, B, C, D) which have been proved to have protective and/or anticarcinogenic properties (Davoodi et al., 2013).
It is important to know that traditional medicines are not free of side effects and many substances might induce health problems if dosage and method of administration are inappropriate (Izzo et al., 2016). Despite their therapeutical properties, some of the reported plant species are toxic at higher doses and thus are not recommended for patients i.e. Peganum harmala L., Artemisia herba-alba Asso, Berberis vulgaris L., Crocus sativus L., Haloxylon scoparium Pomel, Juniperus phoenicea L., Lawsonia inermis L., Lepidium sativum L., Rosmarinus officinalis L. (Hammiche et al., 2013). Besides, cyanogenic plants like Prunus spp. rich with glycosides, cyanogen and amygdaline might cause weakness, nausea, vomiting, diarrhea and spasms followed by terminal coma and death due to cyanide poisoning at higher doses (Chaouali et al., 2013). Therefore, patients must have sufficient information about their preparation, dosage and toxicity. In fact, ethnopharmacological studies are not only appreciated for conservation of traditional knowledge and resources but also valuable for the population’s healthcare and drug discovery (Orhan, 2014).
5. Conclusion
Thyroid disorders are predominant worldwide and its management remains controversial. Ethnopharmacological studies provide significant therapeutic evidences on medicinal plants since their uses are linked directly to the presence of active compounds and healing properties.
This is the first study documenting the traditional uses based on herbal medicine for thyroid management in Algeria. The obtained results reveal an important local knowledge along with a variety and a large number of aromatic and medicinal plants used in Algerian traditional medicine to treat thyroid disorders. Overall, informants have described 63 medicinal plants belonging to 59 genera and 34 families. The most represented botanical families were Lamiaceae, Fabaceae, Apiaceae, Amaranthaceae and Asteraceae respectively. However, the most cited plant species were Atriplex halimus L., Bunium incrassatum (Boiss.) Amo, Nigella sativa L., Aquilaria malaccensis Lam. and Saussurea costus (Falc.) Lipsch. Evidently, it is the time to increase effective scientific studies on mechanisms of action of these medicinal plants in order to validate their popular usages.
Author contributions
TK designed study, analyzed the data, interpreted the results and wrote manuscript; All authors carried out field studies, identified medicinal plants, collected, prepared and revised data, read and approved the manuscript.
Declaration of Competing Interest
The authors declare that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Acknowledgements
Authors would like to acknowledge the local community in general and informants in particular for their valuable information and support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adnan M., Ullah I., Tariq A., Murad W., Azizullah A., Khan A.L., Ali N. Ethnomedicine use in the war affected region of northwest Pakistan. J. Ethnobiol. Ethnomed. 2014;10 doi: 10.1186/1746-4269-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad A., Mishra R.K., Vyawahare A., Kumar A., Rehman M.U., Qamar W., Khan A.Q., Khan R. Thymoquinone (2-Isopropyl-5-methyl-1, 4-benzoquinone) as a chemopreventive/anticancer agent: Chemistry and biological effects. Saudi Pharmaceut. J. 2019;27(8):1113–1126. doi: 10.1016/j.jsps.2019.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait Abderrahim L., Taïbi K., Ait Abderrahim C. Assessment of the Antimicrobial and Antioxidant Activities of Ziziphus lotus and Peganum harmala. Iranian J. Sci. Technol. Trans. A: Sci. 2017;43(2):409–414. [Google Scholar]
- Ait Abderrahim L., Taïbi K., Ait Abderrahim N., Alomery A.M., Abdellah F., Alhazmi A.S., Aljassabi S. Protective effects of melatonin and N-acetyl cysteine against oxidative stress induced by microcystin-LR on cardiac muscle tissue. Toxicon. 2019;169:38–44. doi: 10.1016/j.toxicon.2019.08.005. [DOI] [PubMed] [Google Scholar]
- Ait Abderrahim L., Taïbi K., Ait Abderrahim N., Boussaid M., Rios-Navarro C., Ruiz-Saurí A. Euphorbia honey and garlic: Biological activity and burn wound recovery. Burns. 2019;45(7):1695–1706. doi: 10.1016/j.burns.2019.05.002. [DOI] [PubMed] [Google Scholar]
- Almasaudi, S.B., El-Shitany, N.A., Abbas, A.T., Abdel-dayem, U.A., Ali, S.S., Al Jaouni, S.K., Harakeh, S., 2016. Antioxidant, anti-inflammatory, and antiulcer potential of manuka honey against gastric ulcer in rats. Oxidative medicine and cellular longevity 2016. [DOI] [PMC free article] [PubMed]
- Appendino G., Çetin Özen H., Jakupovic J. Prenylated isocoumarins from Bunium paucifolium. Phytochemistry. 1994;36(2):531–532. [Google Scholar]
- Benarba, B., Belabid, L., Righi, K., Bekkar, A.a., Elouissi, M., Khaldi, A., Hamimed, A., 2015. Ethnobotanical study of medicinal plants used by traditional healers in Mascara (North West of Algeria). Journal of Ethnopharmacology 175, 626–637. [DOI] [PubMed]
- Benhammou N., Bekkara F.A., Kadifkova Panovska T. Antioxidant activity of methanolic extracts and some bioactive compounds of Atriplex halimus. C. R. Chim. 2009;12(12):1259–1266. [Google Scholar]
- Benouis A., Bekkouche Z., Merad M.S., Loudjedi L., Khelil H., Berber N. Thyroid cancer in western Algeria: histopathological and epidemiological study. Journal of Cancer Therapy. 2017;08(7):672–682. [Google Scholar]
- Bharthi V., Kavya N.P., Shubhashree M.N., Bhat S. Herbal approach to management of thyroid disease-a review. J. Ayurvedic Herb. Med. 2017;3(1):48–52. [Google Scholar]
- Bhat J.A., Kumar M., Bussmann R.W. Ecological status and traditional knowledge of medicinal plants in Kedarnath Wildlife Sanctuary of Garhwal Himalaya, India. J. Ethnobiol. Ethnomed. 2013;9(1):1. doi: 10.1186/1746-4269-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussaid M., Taïbi K., Ait Abderrahim L., Ennajah A. Genetic diversity of Ziziphus lotus natural populations from Algeria based on fruit morphological markers. Arid Land Res. Manage. 2018;32(2):184–197. [Google Scholar]
- Bouzid A., Chadli R., Bouzid K. Étude ethnobotanique de la plante médicinale Arbutus unedo L. dans la région de Sidi Bel Abbés en Algérie occidentale. Phytothérapie. 2017;15(6):373–378. [Google Scholar]
- Chaachouay N., Benkhnigue O., Fadli M., El Ayadi R., Zidane L. Ethnobotanical study of medicinal plants used to treat osteoarticular diseases in the Moroccan Rif, Morocco. J. Pharm. Pharm. Res. 2019;7(6):454–470. [Google Scholar]
- Chandra A.K. Chapter 42 - Goitrogen in food: cyanogenic and flavonoids containing plant foods in the development of goiter. In: Watson R.R., Preedy V.R., editors. Bioactive Foods in Promoting Health. Academic Press; San Diego: 2010. pp. 691–716. [Google Scholar]
- Chandra A.K., Mukhopadhyay S., Ghosh D., Tripathy S. Effect of radish (Raphanus sativus Linn.) on thyroid status under conditions of varying iodine intake in rats. Indian J. Exp. Biol. 2006;44(8):653–661. [PubMed] [Google Scholar]
- Chaouali, N., Gana, I., Dorra, A., Khelifi, F., Nouioui, A., Masri, W., Belwaer, I., Ghorbel, H., Hedhili, A., 2013. Potential Toxic Levels of Cyanide in Almonds (Prunus amygdalus), Apricot Kernels (Prunus armeniaca), and Almond Syrup. ISRN Toxicol 2013, 610648-610648. [DOI] [PMC free article] [PubMed]
- Chaturvedi P., Tripathi P., Pandey S., Singh U., Tripathi Y.B. Effect of Saussurea lappa alcoholic extract on different endocrine glands in relation to glucose metabolism in the rat. Phytother. Res. 1993;7(2):205–207. [Google Scholar]
- Chermat S., Gharzouli R. Ethnobotanical study of medicinal flora in the north east of Algeria – an empirical knowledge in Djebel Zdimm (Setif) J. Mater. Sci. Eng. A. 2015;1–2:50–59. [Google Scholar]
- Clauser M., Dall'Acqua S., Loi M.C., Innocenti G. Phytochemical investigation on Atriplex halimus L. from Sardinia. Nat. Prod. Res. 2013;27(20):1940–1944. doi: 10.1080/14786419.2013.793684. [DOI] [PubMed] [Google Scholar]
- Cunha Lima S.T., Merrigan T.L., Rodrigues E.D. Laura Sterian Ward I., editor. new insights into some old and some new issues. IntechOpen. 2012 [Google Scholar]
- Davoodi H., Esmaeili S., Mortazavian A.M. Effects of milk and milk products consumption on cancer: a review. Compr. Rev. Food Sci. Food Saf. 2013;12(3):249–264. [Google Scholar]
- El Mgeed A.A., Bstawi M., Mohamed U., Gabbar M.A. Histopathological and biochemical effects of green tea and/or licorice aqueous extracts on thyroid functions in male albino rats intoxicated with dimethylnitrosamine. Nutr Metab (Lond) 2009;6 doi: 10.1186/1743-7075-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwan H.A.M., Dawood D.H., Abd El-Aziz El-Shafei S.M., Abd El-Mohsen Abd El-Rahman A., Abdel-Latif S.A., Mohany M., Alqahtani F., Alqahtani S., Al-Rejaie S.S. The Potential Role of Citrus limon Powder as a Natural Feed Supplement to Boost the Productive Performance, Antioxidant Status, and Blood Biochemistry of Growing Rabbits. Animals (Basel) 2019;9(7):426. doi: 10.3390/ani9070426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elyebdri N., Boumediou A., Addoun S. Ethnobotanical study on the usage of toxic plants in traditional medicine in the city center of Tlemcen, Algeria. Int. J. Pharm. Pharm. Sci. 2017;131 [Google Scholar]
- Eric Y., Kathy A. Botanical medicine for thyroid regulation. Alternat. Complement. Therap. 2006;12(3):107–112. [Google Scholar]
- Farhangi M.A., Dehghan P., Tajmiri S., Abbasi M.M. The effects of Nigella sativa on thyroid function, serum Vascular Endothelial Growth Factor (VEGF) – 1, Nesfatin-1 and anthropometric features in patients with Hashimoto’s thyroiditis: a randomized controlled trial. BMC Complement Altern. Med. 2016;16(1):471. doi: 10.1186/s12906-016-1432-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani C., Bucci I., Di Santo S., Rossi C., Grassadonia A., Piantelli M., Monaco F., Napolitano G. The flavonoid quercetin inhibits thyroid-restricted genes expression and thyroid function. Food Chem. Toxicol. 2014;66:23–29. doi: 10.1016/j.fct.2014.01.016. [DOI] [PubMed] [Google Scholar]
- Gonçalves C.F.L., de Freitas M.L., Ferreira A.C.F. Flavonoids, thyroid iodide uptake and thyroid cancer – a review. Int. J. Mol. Sci. 2017;18(6):1247. doi: 10.3390/ijms18061247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Tejero M.R., Casares-Porcel M., Sánchez-Rojas C.P., Ramiro-Gutiérrez J.M., Molero-Mesa J., Pieroni A., Giusti M.E., Censorii E., de Pasquale C., Della A., Paraskeva-Hadijchambi D., Hadjichambis A., Houmani Z., El-Demerdash M., El-Zayat M., Hmamouchi M., ElJohrig S. Medicinal plants in the Mediterranean area: synthesis of the results of the project Rubia. J. Ethnopharmacol. 2008;116(2):341–357. doi: 10.1016/j.jep.2007.11.045. [DOI] [PubMed] [Google Scholar]
- Gupta A., Wamankar S., Gidwani B., Deep Kaur C. Herbal drugs for thyroid treatment. Int. J. Pharm. Biol. Sci. 2016;6(1):62–70. [Google Scholar]
- Hammiche V., Merad R., Azzouz M. Plantes toxiques à usage médicinal du pourtour méditerranéen. In: Spiegler V., editor. Springer. Paris; 2013. [Google Scholar]
- He Y.-Q., Ma G.-Y., Peng J.-N., Ma Z.-Y., Hamann M.T. Liver X receptor and peroxisome proliferator-activated receptor agonist from Cornus alternifolia. Biochim Biophys Acta. 2012;1820(7):1021–1026. doi: 10.1016/j.bbagen.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo A.A., Hoon-Kim S., Radhakrishnan R., Williamson E.M. A critical approach to evaluating clinical efficacy, adverse events and drug interactions of herbal remedies. Phytother. Res. 2016;30(5):691–700. doi: 10.1002/ptr.5591. [DOI] [PubMed] [Google Scholar]
- Jaganathan S.K., Balaji A., Vellayappan M.V., Asokan M.K., Subramanian A.P., John A.A., Supriyanto E., Razak S.I., Marvibaigi M. A review on antiproliferative and apoptotic activities of natural honey. Anti-Cancer Agents Med. Chem. 2015;15(1):48–56. doi: 10.2174/1871520614666140722084747. [DOI] [PubMed] [Google Scholar]
- Jeyaraman M.M., Abou-Setta A.M., Grant L., Farshidfar F., Copstein L., Lys J., Gottschalk T., Desautels D., Czaykowski P., Pitz M., Zarychanski R. Dairy product consumption and development of cancer: an overview of reviews. BMJ Open. 2019;9(1) doi: 10.1136/bmjopen-2018-023625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbash A., Shoeib N. Chemical and biological investigation of some secondary metabolites in Atriplex halimus growing in Egypt. Nat. Prod. Commun. 2012;7(11):1465–1468. [PubMed] [Google Scholar]
- Khader M., Eckl P.M. Thymoquinone: an emerging natural drug with a wide range of medical applications. Iran J Basic Med Sci. 2014;17(12):950–957. [PMC free article] [PubMed] [Google Scholar]
- Kim, M., Lee, B.-C., 2019. Therapeutic Effect of Scutellaria baicalensis on L-Thyroxine-Induced Hyperthyroidism Rats. Evidence-Based Complementary and Alternative Medicine 2019, Article ID 3239649.
- Lin F.M., Chen L.R., Lin E.H., Ke F.C., Chen H.Y., Tsai M.J., Hsiao P.W. Compounds from Wedelia chinensis synergistically suppress androgen activity and growth in prostate cancer cells. Carcinogenesis. 2007;28(12):2521–2529. doi: 10.1093/carcin/bgm137. [DOI] [PubMed] [Google Scholar]
- Martin G. Chapman et Hall; London: 1995. Ethnobotany—A manual of methods. [Google Scholar]
- Meddour, R., Meddour-Sahar, O., 2016. Medicinal plants and their traditional uses in Kabylia (Tizi Ouzou, Algeria). Arabian Journal of Medicinal and Aromatic Plants; Vol 1, No 2 (2015) 1, 137–151.
- Miler M., Jarić I., Živanović J., Ajdžanović V., Tanić N., Milošević V., Šošić-Jurjević B. Citrus flavanones mildly interfere with pituitary-thyroid axis in old-aged male rats. Acta Histochem. 2017;119(3):292–301. doi: 10.1016/j.acthis.2017.02.005. [DOI] [PubMed] [Google Scholar]
- Miraj S., Rafieian K., Kiani S. Melissa officinalis L: a review study with an antioxidant prospective. J. Evid. Based Complement. Altern. Med. 2017;22(3):385–394. doi: 10.1177/2156587216663433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammedi Z. Resistance, pharmacology properties and nutritional value of a shrub from arid environments Atriplex halimus. Res. J. .Med. Plants. 2016;10:10–18. [Google Scholar]
- Mullur R., Liu Y.-Y., Brent G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014;94(2):355–382. doi: 10.1152/physrev.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagi M.N., Mansour M.A. Protective effect of thymoquinone against doxorubicin-induced cardiotoxicity in rats: a possible mechanism of protection. Pharmacol. Res. 2000;41(3):283–289. doi: 10.1006/phrs.1999.0585. [DOI] [PubMed] [Google Scholar]
- Noor N.A., Fahmy H.M., Mohammed F.F., Elsayed A.A., Radwan N.M. Nigella sativa amliorates inflammation and demyelination in the experimental autoimmune encephalomyelitis-induced Wistar rats. Int. J. Clin. Exp. Pathol. 2015;8(6):6269–6286. [PMC free article] [PubMed] [Google Scholar]
- Oetting A., Yen P.M. New insights into thyroid hormone action. Best Practice Res. Clin. Endocrinol. Metabol. 2007;21(2):193–208. doi: 10.1016/j.beem.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Ong V.Y.C., Tan B.K.H. Novel phytoandrogens and lipidic augmenters from Eucommia ulmoides. BMC Complement Altern. Med. 2007;7:3. doi: 10.1186/1472-6882-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orhan I.E. Pharmacognosy: science of natural products in drug discovery. Bioimpacts. 2014;4(3):109–110. doi: 10.15171/bi.2014.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcza L.M., Simms C., Chopra M. Honey and cancer: current status and future directions. Diseases. 2016;4(4):30. doi: 10.3390/diseases4040030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prance G.T., Balée W., Boom B.M., Carneiro R.L. Quantitative ethnobotany and the case for conservation in ammonia. Conserv. Biol. 1987;1(4):296–310. [Google Scholar]
- Parmar H.S., Kar A. Antiperoxidative, antithyroidal, antihyperglycemic and cardioprotective role of Citrus sinensis peel extract in male mice. Phytother Res. 2008;22(6):791–795. doi: 10.1002/ptr.2367. [DOI] [PubMed] [Google Scholar]
- Quezel, P., Santa, S., 1963. Nouvelle flore de l'Algérie et des régions désertiques méridionales. p. No. 581.965 Q588.
- R Development Core Team, R.T., 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria http://www.r-project.org.
- Estrogen and thyroid hormone receptor activation by medicinal plants from Bahia, Brazil. Reis L.T.C., da Silva M.R.D., Costa S.L., Velozo E.d.S., Batista R., da Cunha Lima S.T., editors. Medicines (Basel) 2018;5(1):8. doi: 10.3390/medicines5010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarri M., Boudjelal A., Hendel N., Sarri D., Benkhaled A. Flora and ethnobotany of medicinal plants in the southeast of the capital of Hodna (Algeria) Arab. J. Med. Aromat. Plant. 2015;1(1):2015. [Google Scholar]
- Sarri M., Mouyet F.Z., Benziane M., Cheriet A. Traditional use of medicinal plants in a city at steppic character (M’sila, Algeria) J. Pharm. Pharm. Res. 2014;2(2):31–35. [Google Scholar]
- Sethi G., Ahn K.S., Aggarwal B.B. Targeting nuclear factor-κB activation pathway by Thymoquinone: role in suppression of antiapoptotic gene products and enhancement of apoptosis. 2008;6(6):1059–1070. doi: 10.1158/1541-7786.MCR-07-2088. [DOI] [PubMed] [Google Scholar]
- Shahsavari N., Barzegar M., Sahari M.A., Naghdibadi H. Antioxidant activity and chemical characterization of essential oil of Bunium persicum. Plant Foods Hum. Nutr. 2008;63(4):183–188. doi: 10.1007/s11130-008-0091-y. [DOI] [PubMed] [Google Scholar]
- Sharafati Chaleshtori F., Saholi M., Sharafati Chaleshtori R. Chemical Composition, Antioxidant and Antibacterial Activity of Bunium persicum, Eucalyptus globulus, and Rose Water on Multidrug-Resistant Listeria Species. J Evid Based Integr Med. 2018;23 doi: 10.1177/2515690X17751314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokri Z., Khoshbin M., Koohpayeh A., Abbasi N., Bahmani F., Rafieian-Kopaei M., Beyranvand F. Thyroid diseases: Pathophysiology and new hopes in treatment with medicinal plants and natural antioxidants. Int. J. Green Pharm. 2018;12(3):S473–S482. [Google Scholar]
- Soundarrajan M., Kopp P.A. Thyroid hormone biosynthesis and physiology. In: Eaton J.L., editor. Thyroid disease and reproduction: a clinical guide to diagnosis and management. Springer International Publishing; Cham: 2019. pp. 1–17. [Google Scholar]
- Subramanian A.P., John A.A., Vellayappan M.V., Balaji A., Jaganathan S.K., Mandal M., Supriyanto E. Honey and its phytochemicals: plausible agents in combating colon cancer through its diversified actions. J. Food Biochem. 2016;40(4):613–629. [Google Scholar]
- Taborsky J., Kunt M., Kloucek P., Lachman J., Zeleny V., Kokoska L. Identification of potential sources of thymoquinone and related compounds in Asteraceae, Cupressaceae, Lamiaceae, and Ranunculaceae families. Cent. Eur. J. Chem. 2012;10(6):1899–1906. [Google Scholar]
- Taïbi K., Ait Abderrahim L., Ferhat K., Betta S., Taïbi F., Bouraada F., Boussaid M. Ethnopharmacological study of natural products used for traditional cancer therapy in Algeria. Saudi Pharm. J. 2020;28(11):1451–1465. doi: 10.1016/j.jsps.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajmiri S., Farhangi M.A., Dehghan P. Nigella Sativa treatment and serum concentrations of thyroid hormones, transforming growth factor β (TGF-β) and interleukin 23 (IL-23) in patients with Hashimoto’s Thyroiditis. Eur. J. Integrative Med. 2016;8(4):576–580. [Google Scholar]
- Talebi M., Moghaddam M., Ghasemi Pirbalouti A. Variability in essential oil content and composition of Bunium persicum Boiss. populations growing wild in northeast of Iran. J. Essent. Oil Res. 2018;30(4):258–264. [Google Scholar]
- Valverde-R C., Orozco A., Carlos Solís-S J., Robles-Osorio L. Chapter 22 - Iodothyronine Deiodinases: Emerging Clinical Crossroads. In: Ulloa-Aguirre A., Conn P.M., editors. Cellular Endocrinology in Health and Disease. Academic Press; Boston: 2014. pp. 365–377. [Google Scholar]
- Verma P., Jameel K. Studies on traditional treatment of thyroid by the tribals of Chitrakoot District. Uttar Pradesh Int. J. Sci. Res. 2014;3(10):1370–1373. [Google Scholar]
- Waheed M., Hussain M.B., Javed A., Mushtaq Z., Hassan S., Shariati M.A., Khan M.U., Majeed M., Nigam M., Mishra A.P., Heydari M. Honey and cancer: a mechanistic review. Clin. Nutrit. 2019;38(6):2499–2503. doi: 10.1016/j.clnu.2018.12.019. [DOI] [PubMed] [Google Scholar]
- Wang, J., Milosveski, V., Schramek, C., Fong, G., Becks, G., Hill, D., 1998. Presence and possible role of vascular endothelial growth factor in thyroid cell growth and function. 157(1), 5. [DOI] [PubMed]
- WHO, 2018. Global Health Observatory. who.int/gho/database/en/, in: Geneva, S.W.H.O. (Ed.).
- Woo C.C., Kumar A.P., Sethi G., Tan K.H.B. Thymoquinone: potential cure for inflammatory disorders and cancer. Biochem. Pharmacol. 2012;83(4):443–451. doi: 10.1016/j.bcp.2011.09.029. [DOI] [PubMed] [Google Scholar]
- Yuan H., Ma Q., Ye L., Piao G. The traditional medicine and modern medicine from natural products. Molecules. 2016;21(5):559. doi: 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zen, X.X., Yuan, Y., Liu, Y., Wu, T.X., Han, S., 2007. Chinese herbal medicines for hyperthyroidism. Cochrane Database Syst Rev 2007(2), CD005450-CD005450. [DOI] [PMC free article] [PubMed]
- Zerrouk S., Seijo M.C., Escuredo O., Rodríguez-Flores M.S. Characterization of Ziziphus lotus (jujube) honey produced in Algeria. J. Apic. Res. 2018;57(1):166–174. [Google Scholar]