Key Points
Question
Is low-dose intradermal influenza vaccine a suitable alternative to regular dose intramuscular vaccine?
Findings
In this systematic review and meta-analysis including 30 studies with a total of 177 780 participants, the seroconversion rates of low doses of intradermal influenza vaccine vs the 15-µg intramuscular dose for each of the H1N1, H3N2, and B strains were not statistically significantly different. Seroprotection rates for the 9-µg and 15-µg intradermal doses were not statistically significantly different from the 15-µg intramuscular dose, except for the 15-µg intradermal dose for the H1N1 strain, which was significantly higher.
Meaning
These findings suggest that a low-dose intradermal influenza vaccine may be a suitable alternative to standard-dose intramuscular vaccine.
This systematic review and meta-analysis examines the safety and efficacy of reduced- or full-dose influenza vaccine administered intradermally vs standard-dose administered intramuscularly.
Abstract
Importance
Low-dose intradermal influenza vaccines could be a suitable alternative to full intramuscular dose during vaccine shortages.
Objective
To compare the immunogenicity and safety of the influenza vaccine at reduced or full intradermal doses with full intramuscular doses to inform policy design in the event of vaccine shortages.
Data Sources
MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials were searched for studies published from 2010 until June 5, 2020.
Study Selection
All comparative studies across all ages assessing the immunogenicity or safety of intradermal and intramuscular influenza vaccinations were included.
Data Extraction and Synthesis
Data were extracted by a single reviewer and verified by a second reviewer. Discrepancies between reviewers were resolved through consensus. Random-effects meta-analysis was conducted.
Main Outcomes and Measures
Primary outcomes included geometric mean titer, seroconversion, seroprotection, and adverse events.
Results
A total of 30 relevant studies were included; 29 studies were randomized clinical trials with 13 759 total participants, and 1 study was a cohort study of 164 021 participants. There was no statistically significant difference in seroconversion rates between the 3-µg, 6-µg, 7.5-µg, and 9-µg intradermal vaccine doses and the 15-µg intramuscular vaccine dose for each of the H1N1, H3N2, and B strains, but rates were significantly higher with the 15-µg intradermal dose compared with the 15-µg intramuscular dose for the H1N1 strain (rate ratio [RR], 1.10; 95% CI, 1.01-1.20) and B strain (RR, 1.40; 95% CI, 1.13-1.73). Seroprotection rates for the 9-µg and 15-µg intradermal doses did not vary significantly compared with the 15-µg intramuscular dose for all the 3 strains, except for the 15-µg intradermal dose for the H1N1 strain, for which rates were significantly higher (RR, 1.05; 95% CI, 1.01-1.09). Local adverse events were significantly higher with intradermal doses than with the 15-µg intramuscular dose, particularly erythema (3-µg dose: RR, 9.62; 95% CI, 1.07-86.56; 6-µg dose: RR, 23.79; 95% CI, 14.42-39.23; 9-µg dose: RR, 4.56; 95% CI, 3.05-6.82; 15-µg dose: RR, 3.68; 95% CI, 3.19-4.25) and swelling (3-µg dose: RR, 20.16; 95% CI, 4.68-86.82; 9-µg dose: RR, 5.23; 95% CI, 3.58-7.62; 15-µg dose: RR, 3.47 ; 95% CI, 2.21-5.45). Fever and chills were significantly more common with the 9-µg intradermal dose than the 15-µg intramuscular dose (fever: RR, 1.36; 95% CI, 1.03-1.80; chills: RR, 1.24; 95% CI, 1.03-1.50) while all other systemic adverse events were not statistically significant for all other doses.
Conclusions and Relevance
These findings suggest that reduced-dose intradermal influenza vaccination could be a reasonable alternative to standard dose intramuscular vaccination.
Introduction
Influenza infection causes 3 to 5 million severe illnesses and approximately half a million annual deaths globally.1 It is a highly contagious disease characterized by high fever, cough, sore throat, headache, chills, lack of appetite, and fatigue.2 Vaccinations are essential for prevention of influenza and can be administered intradermally or intramuscularly.3
Interest in intradermal influenza vaccines has arisen because of a presumed dose-sparing potential. An intradermal dose-sparing effect has been used successfully for other vaccines, such as rabies.4,5 If confirmed, this may mitigate potential vaccine shortages, which could occur from unanticipated loss of expected supplies or from excessive demand owing to high rates of infection, such as during pandemics.6 With the approval of new intradermal vaccines,2,7,8 new delivery devices have become available, including minineedles, microneedles, patches, and disposable syringe jet injectors.3,9 The recent international focus on the development of vaccines for coronavirus disease 2019 highlights the need to better understand the safety and efficacy of various vaccine delivery methods and doses.
Intradermal vaccinations are believed to have a dose-sparing effect3; therefore, smaller doses of intradermal vaccines may be sufficient to produce an antigenic response that is similar to standard intramuscular doses. This is physiologically plausible because the dermis is rich in Langerhans cells, dendritic cells that are very potent antigen-presenting cells capable of eliciting both cell-mediated and humoral immune responses via antigen presentation to CD4+ and CD8+ T cells, and eventual B cell activation to produce high levels of antigen-specific antibodies. Intramuscular injection bypasses this dermal immune system response and delivers the vaccine directly into the muscular tissue, which has relatively few resident antigen-presenting cells.10
Previous studies have compared the immunogenicity and safety of intradermal and intramuscular influenza vaccines; however, the magnitude of the effect across all populations has not been recently examined. In this study, we synthesized the published literature on the immunogenicity and safety of the influenza vaccine at reduced or regular intradermal doses compared with a regular intramuscular dose.
Methods
Literature Search
A systematic review of the literature was completed. MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials were searched for studies published from 2010 until June 5, 2020. Terms aimed at capturing the technology of interest, such as intradermal, ID injection, and Mantoux were combined using the Boolean Operator and with influenza terms. These terms were searched as keywords (title or abstract words) and as subject headings (eg, MEDLINE medical subject headings) as appropriate. The search excluded case reports, editorials, letters, and animal studies. The search strategy was developed by a research librarian and reviewed by another research librarian using the Peer Review of Electronic Search Strategies method11 (eAppendix in the Supplement). This search was supplemented by reviewing the reference lists of published systematic reviews, identified during the abstract screening, to ensure that all studies meeting the inclusion criteria were captured. This review follows the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline. This study is registered in the International Prospective Register of Systematic Reviews (PROSPERO), No. CRD42020190246.
Literature Selection
Abstracts identified through database searching were screened by a single reviewer (O.E., J.T., or L.M.); all abstracts included at this stage proceeded to full-text review. Full-text publications were screened by a single reviewer (O.E., J.T., or L.M.). Calibration with a second reviewer (O.E., J.T., or L.M.) was completed prior to abstract screening and full-text review until greater than 70% agreement was reached. Publications were included if they met all the following inclusion criteria: comparative, including randomized and nonrandomized clinical trials, studies of the immunogenicity and safety of intradermal and intramuscular influenza vaccine involving participants of any age, published between 2010 and 2020. Non–English- or French-language studies, animal studies, studies involving patients who were immunocompromised, and studies with whole-virus vaccinations were excluded.
Data Extraction
For all included studies, year of publication, country, study design, dates of recruitment, study inclusion and exclusion criteria, setting, patient characteristics, treatment protocol (eg, intention-to-treat, per-protocol), sample size, follow-up time, geometric mean titer (GMT, defined as the antilog of the arithmetic mean of the log-transformed antibody titers), seroconversion rate (percentage of participants with a 4-fold increase in hemagglutination inhibition [HAI] antibody titers) and seroprotection rates (the percentage of participants achieving an HAI titer ≥40), and all relevant outcomes were extracted by a single reviewer (O.E., J.T., or L.M.) and verified by a second reviewer (O.E. verified L.M., J.T. verified O.E., and L.M. verified J.T.) using standardized data extraction forms. Immunogenicity outcomes were extracted for only HAI assays. Discrepancies between reviewers during data extraction were resolved through consensus.
Quality Assessment
The quality of randomized clinical trials was assessed using the Cochrane Handbook Risk of Bias Assessment Tool version 5.1.0.12 Each study was assessed using 5 criteria broadly covering the areas of randomization, deviation from intended intervention, missing outcome data, measurement of outcome, and selection of reporting result. Each criterion was assigned a rating of low, some, or high concerns.
The quality of observational studies was assessed using the Newcastle Ottawa Scale. Each study was assessed across 3 categories: selection, comparability, and outcome. Items within selection and comparability were assigned up to 1 star for high quality, while items within comparability were assigned a maximum of 2 stars, with a maximum total possible score of 9 stars.
Quality assessment was completed by a single reviewer and verified by a second reviewer (quality assessment by single reviewers and verified in pairs: O.E. verified L.M., J.T. verified O.E., and L.M. verified J.T.). Discrepancies were resolved through discussion. Studies were not excluded based on quality assessment.
Statistical Analysis
Random-effects meta-analysis was conducted using the DerSimonian and Laird estimator13 for tau. Statistical heterogeneity was assessed using the I2 measure, with values greater or less than 50% considered high and low heterogeneity, respectively. A continuity correction of 0.5 was used, where appropriate, allowing the inclusion of zero-total event trials.14 Stratified analyses by dose were completed for the GMT, seroconversion, seroprotection, influenza or influenza-like illness, and adverse events. For dose stratification, different intradermal vaccine doses (3, 6, 7.5, 9 and 15 µg) were separately compared with a 15-µg intramuscular dose for each outcome. Only immunogenicity outcomes for days 21 through 30 after vaccination were analyzed. Subgroup analyses of immunogenicity outcomes were conducted for studies involving participants aged 60 years or older. Risk ratios (RRs) were calculated for categorical outcomes, and the ratio of geometric means calculated for GMT, as described by Friedrich el al.15 Publication bias for small studies with missing small effect sizes was assessed using an Egger test16 when the number of studies was greater than 4. When the Egger test was statistically significant (P < .05), the Duval-Tweedie trim-and-fill method17 was used to adjust for funnel plot asymmetry. All analyses were completed in R statistical software version 3.6.1 (R Project for Statistical Computing). P values were 2-sided, and statistical significance was set at P < .05. For RR comparisons, statistical significance was inferred from the 95% CIs, and actual P values were not generated. Data were analyzed from July 2 through 16, 2020.
Results
Study Characteristics
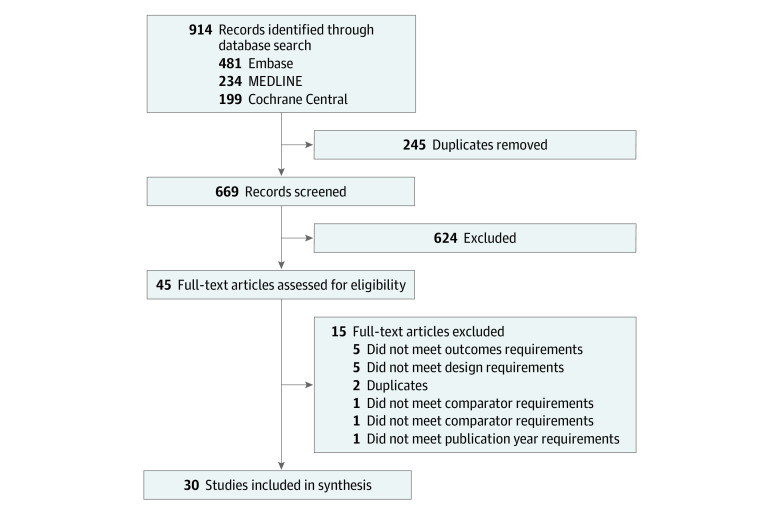
The search strategy yielded 914 unique citations, 245 of which were excluded after deduplication, and 624 were excluded after abstract review. A total of 45 studies proceeded to full-text review (Figure); of these, 15 studies were excluded for inappropriate study design for the aims of this review (5 studies), incorrect outcome (5 studies), duplicate publication (2 studies), incorrect study population (1 study), and publication year not of interest (1 study). A total of 30 relevant studies were included in the final data set (Figure).
Figure. Flowchart of Included Studies.
Of 30 included studies, 29 studies were randomized clinical trials with a total of 13 759 participants,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46 and 1 study was a cohort study of 164 021 participants (Table 1).47 Sixteen studies were multi-center18,19,20,22,23,29,31,33,34,37,38,41,43,44,47; 12 studies were single-center24,25,26,27,28,30,32,35,36,39,40,45; and 2 studies did not report the setting.21,42 Approximately half of the studies (14 studies)18,19,21,22,24,25,26,27,29,34,41,43,44,46 involved only participants aged 60 years or older or reported data for participants aged 60 years or older.
Table 1. Characteristics of Included Studies.
| Source | Country | Participants | Dose, μg | Virus strains | Outcomes | Maximum follow-up, d | ||
|---|---|---|---|---|---|---|---|---|
| No. | Age, range, y | ID | IM | |||||
| Ansaldi et al,19 2013 | Italy | 47 | ≥60 | 15 | 15 | A/H1N1 (A/California/7/2009, A/Genoa/1/11, A/Genoa/6/11, A/Genoa/24/11), A/H3N2 (A/Perth/16/2009), B Strain (B/Brisbane/60/2008) | AEs, GMT, MFI, Sc, Sp | 90 |
| Ansaldi et al,18 2012 | France, Belgium, Lithuania, Italy | 50 | ≥60 | 15 | 15 | A/H3N2 (Wisconsin/67/05, Genoa62/05, Genoa03/07, Brisbane/10/07, Genoa02/07,Genoa03/06) | GMT, MFI, Sc, Sp | 21 |
| Arnou et al,20 2010 | France, Italy, Belgium, Lithuania | 1676 | 18-60 | 9 | 15 | A/H1N1 (A/New Caledonia/20/99),A/H3N2(A/Wisconsin/67/2005), B Strain (B/Malaysia/2506/2004) | AEs, GMT, MFI, Sc, Sp | 21 |
| Boonnak et al,21 2017 | Thailand | 221 | 60-88 | 15 | 15 | A/H1N1(A/California/07/09), A/H3N2 (A/Songhka/308/13), B Strain (B/Phuket/287/13) | AEs, GMT, Sc, Sp | 60 |
| Camiloni et al,22 2014 | Italy | 80 | 64−100 | 15 | 15 | A/H1N1 (A/California/7/09,A/Perugia/06/12, A/Perugia/20/12, A/Perugia/44/12, A/Perugia/50/12), A/H3N2 (A/Perth/16/09), B Strain (B/Brisbane/60/08) | GMT, MFI, Sc, Sp | 180 |
| Carter et al,23 2019 | US | 106 | 18-69 | 9 | 15 | A/H1N1 (A/California/04/2009), A/H3N2 (A/Victoria/361/2011, A/Texas/50/2012), B Strain (B/Texas/6/2011,B/Wisconsin/1/2010, B/Massachusetts/2/2012) | AEs, GMT, Sc | 21 |
| Chan et al,24 2014 | Hong Kong | 100 | ≥65 | 15 | 15 | A/H1N1 (A/Victoria/361/2011), A/H3N2 (A/California/7/2009), B Strain (B/Massachusetts/2/2012) | AEs, GMT, MFI, Sc, Sp | 180 |
| Chi et al,25 2010 | US | 130 | ≥65 | 9 | 15 | A/H1N1 (A/Solomon Islands/3/2006), A/H3N2 (A/Wisconsin/67/2005), B Strain (B/Malaysia/2506/2004) | AEs, GMT, Sp | 28 |
| Chuaychoo et al,26 2019 | Thailand | 80 | ≥60 | 9 | 15 | A/H1N1 (A/California/7/2009), A/H3N2 (A/Perth/16/2009), B Strain (B/Brisbane/60/2008) | AEs, GMT, Sc, Sp | 365 |
| Chuaychoo et al,27 2016 | Thailand | 149 | 60-94 | 9 | 15 | A/H1N1 (A/California/7/2009), A/H3N2 (A/Perth/16/2009), B Strain (B/Brisbane/60/2008) | AEs, GMT, Sc, Sp, Influenza infection | 28 |
| Chuaychoo et al,28 2010 | Thailand | 156 | 36-91 | 6 | 15 | A/H1N1 (A/New Caledonia/20/99), A/H3N2 (A/California/7/2004), B Strain (B/Malaysia/2506/2004) | AEs, GMT, Sc, Sp | 365 |
| Della Cioppa et al,29 2014 | Germany, Poland, Belgium | 257 | ≥65 | 6 or 12 | 15 or 30 | A/H3N2 (A/Uruguay/716/2007) | AEs, GMT, MFI, Sc, Sp | 22 |
| Esposito et al,30 2011 | Italy | 112 | ≥3 | 9 or 15 | 15 | A/H1N1 (A/California/7/2009), A/H3N2 (A/Perth/16/2009), B Strain (B/Brisbane/60/2008) | AEs, GMT, MFI, Sc, Sp | 28 |
| Frenck et al,31 2011 | US | 1571 | 18-64 | 3, 6, or 9 | 15 | A/H1N1 (A/New Caledonia/20/99 IVR-116), A/H3N2 (A/Wyoming/03/2003 (an A/Fujian/411/2002-like strain), B Strain (B/Jiangsu/10/2003 (a B/Jiangsu/361/2002-like strain)) | AEs, GMT, MFI, Sc, Sp | 21 |
| Garg et al,32 2016 | Thailand | 80 | 18-60 | 15 | 15 | A/H1N1, A/H3N2, B Strain | AEs, GMT, MFI, Sc, Sp | 30 |
| Gorse et al,33 2013 | US | 3868 | 18-64 | 9 | 15 | A/H1N1 (A/Brisbane/59/07), A/H3N2 (A/Uruguay/716/2007 X-175CA), B Strain (B/Florida/04/2006 Yamagata-like) | AEs, GMT, Sc, Sp | 28 |
| Han et al,34 2013 | South Korea | 120 | ≥18 | 9 or 15 | 15 | A/H1N1 (A/California/7/2009), A/H3N2 (A/Perth/16/2009), B Strain (B/Brisbane/60/2008) | AEs, GMT, Sc, Sp | 21 |
| Hung et al,35 2016 | China | 160 | 18-30 | 15 | 15 | A/H1N1 (A/California/07/2009, Prototype A/WSN/1933, A/HK/408027/09, A/H3N2 (A/Victoria/361/2011, A/HK/485197/14), B Strain (B/Massachusetts/2/2012), Others (B/HK/418078/11) | AEs, GMT, MFI, Sc, Sp | 21 |
| Hung et al,37 2014 | China | 93 | ≥21 | 15 | 15 | A/H1N1 (A/California/07/2009), A/H3N2 (A/Perth/16/2009), B Strain (B/Brisbane/60/2008) | AEs, GMT, MFI, Sc, Sp, Influenza | 365 |
| Hung et al,36 2012 | China | 262 | ≥21 | 3 or 9 | 15 | A/H1N1 (A/California/07/2009), A/H3N2 (A/Perth/16/2009), B Strain (B/Brisbane/60/2008) | AEs, GMT, MFI, Sc, Sp | 21 |
| Leung et al,38 2017 | US | 336 | 18-64 | NR | NR | A/H1N1 (A/California/07/2009), A/H3N2 (A/Perth/16/2009), B Strain (B/Brisbane/60/2008) | AEs, GMT, MFI, Sc, Sp | 28 |
| Levin et al,46 2016 | NR | 370 | ≥65 | 7.5 or 15 | 15 | A/H1N1 (A/California/07/2009), A/H3N2 (A/Victoria/361/2011), B Strain (B/Wisconsin/1/2010) | AE, Sc, Sp | 90 |
| Levin et al,45 2014 | Switzerland | 280 | 18-60 | 3, 4.5, or 6 | 15 | A/H1N1 (A/SolomonIslands/3/2006), A/H3N2 (A/Wisconsin/67/2005), B Strain (B/Malaysia/2506/2004) | AEs, GMT, MFI, Sc, Sp | 21 |
| Nougarede et al,39 2014 | France | 80 | 18-40 | 9 | 15 | A/H1N1 (A/SolomonIslands/3/2006), A/H3N2 (A/Wisconsin/67/2005), B Strain (B/Malaysia/2506/2004) | AEs, GMT, MFI, Sc, Sp, influenza-like illness | 180 |
| Patel et al,40 2010 | US | 100 | 18-40 | 3 or 9 | 15 | H5N1 (A/Vietnam/1203/2004) | AEs, GMT,Sc, Sp | 28 |
| Seo et al,41 2014 | South Korea | 364 | ≥65 | 15 | 15 | A/H1N1 (A/California/7/2009), A/H3N2 (A/Perth/16/2009), B Strain (B/Brisbane/60/2008) | AEs, GMT, MFI, Sc, Sp | 180 |
| Song et al,42 2013 | South Korea | 96 | 18-30 | 3 or 7.5 | 15 | A/H1N1 (A/New Caledonia/20/99), A/H3N2 (A/Wisconsin/67/2005), B Strain (B/Malaysia/2506/2004) | GMT, Sc, Sp | 180 |
| Tsang et al,43 2014 | US | 1912 | ≥65 | 15 or 21 | 15 | A/H1N1 (A/Solomon Islands/3/2006), A/H3N2 (A/Wisconsin/67/2005), B Strain (B/Malaysia/2506/2004) | AEs, GMT, MFI, Sc, Sp | 28 |
| Van Damme et al,44 2010 | France, Belgium | 795 | ≥65 | 15 | 15 | A/H1N1 (A/Solomon Islands/3/2006), A/H3N2 (/Wisconsin/67/2005), B Strain (B/Malaysia/2506/2004) | AEs, GMT, MFI, Sc, Sp | 21 |
| Puig Barbera et al,47 2014a | Spain | 164021 | ≥65 | 15 | 15 | A/H1N1 (A/California/7/2009), A/H3N2 (A/Perth/16/2009), B Strain (B/Brisbane/60/2008) | Influenza | |
Abbreviations: AE, adverse events; GMT, geometric mean titer; ID, intradermal; IM, intramuscular; NR, not reported; MFI, mean fold increase; Sc, seroconversion rate; Sp, seroprotection rate.
All other included studies were randomized clinical trials, but Puig Barbera et al47 was a cohort study.
Quality Assessment
Most studies had bias stemming from the randomization process; 6 studies were at low risk of bias20,23,24,30,32,35; and 2 studies were at high risk.28,34 All but 2 low-risk studies23,35 had some risk of bias due to deviations from intended interventions. All included studies had low risk of bias due to missing outcome data. All but 1 high-risk study34 were of low risk of bias stemming from the measurement of outcomes. Lastly, all studies were of concern of bias regarding selection of the reported results. Overall, all studies except 2 high-risk studies,28,34 were of some concern for bias (eFigure 1 in the Supplement).
The only included cohort study was allocated 9 out of a possible 9 stars.47 It was judged to be representative of the exposed population. Exposure were ascertained from secure records, and outcomes were ascertained from record linkage. The cohorts were comparable, and follow-up was long and adequate.
Meta-analyses
Seroconversion
Although there was high heterogeneity, no statistically significant difference in seroconversion rates was found between the 3-µg, 6-µg, 7.5-µg, and 9-µg intradermal vaccine doses vs the 15-µg intramuscular vaccine dose for each of the H1N1, H3N2, and B strains. The doses represent the amount of hemagglutinin present in each vaccine. Furthermore, the difference in the seroconversion rate for the H3N2 strain was also not statistically significant between the 15-µg intradermal dose and 15-µg intramuscular doses, but the seroconversion rate was significantly higher with the 15-µg intradermal dose compared with 15-µg intramuscular doses for the H1N1 strain (RR, 1.10; 95% CI, 1.01-1.20) and B strain (RR, 1.40; 95% CI, 1.13-1.73) (Table 2; eFigures 2-8 in the Supplement).
Table 2. Seroconversion, Seroprotection, and GMT of Intradermal Doses vs Standard 15-μg Intramuscular Dose of Influenza Vaccine.
| Intradermal dose | Studies, No. | Risk ratio (95% CI) | I2 |
|---|---|---|---|
| Seroconversion | |||
| H1N1 | |||
| 3 µg | 2 | 1.77 (0.43-7.28) | 82.6 |
| 6 µg | 3 | 1.00 (0.78-1.28) | 87.7 |
| 7.5 µg | 3 | 1.01 (0.80-1.28) | 0 |
| 9 µg | 10 | 1.02 (0.93-1.12) | 59 |
| 15 µg | 16 | 1.10 (1.01-1.20)a | 50.5 |
| H3N2 | |||
| 3 µg | 2 | 1.14 (0.56-2.31) | 81.3 |
| 6 µg | 3 | 0.98 (0.97-1.00) | 0 |
| 7.5 µg | 3 | 0.92 (0.63-1.33) | 63.8 |
| 9 µg | 11 | 1.01 (0.95-1.06) | 38 |
| 15 µg | 17 | 1.07 (0.99-1.17) | 43.2 |
| B Strain | |||
| 3 µg | 2 | 1.46 (0.67-1.99) | 53.5 |
| 6 µg | 3 | 0.95 (0.68-1.32) | 88.3 |
| 7.5 µg | 3 | 1.21 (0.79-1.85) | 43.9 |
| 9 µg | 11 | 0.95 (0.84-1.08) | 57.1 |
| 15 µg | 16 | 1.40 (1.13-1.73)a | 59.1 |
| Seroprotection | |||
| H1N1 | |||
| 3 µg | 3 | 1.00 (0.78-1.28) | 87.7 |
| 6 µg | 3 | 0.93 (0.88-0.99)a | 37.5 |
| 7.5 µg | 3 | 1.07 (1.01-1.12)a | 0 |
| 9 µg | 12 | 1.00 (0.98-1.03) | 33 |
| 15 µg | 17 | 1.05 (1.01-1.09)a | 43.6 |
| H3N2 | |||
| 3 µg | 3 | 0.98 (0.97-1.00) | 0 |
| 6 µg | 3 | 1.00 (0.99-1.01) | 0 |
| 7.5 µg | 3 | 1.01 (0.96-1.06) | 36.6 |
| 9 µg | 12 | 1.00 (0.99-1.00) | 0 |
| 15 µg | 18 | 1.01 (0.99-1.02) | 25.9 |
| B Strain | |||
| 3 µg | 3 | 0.95 (0.68-1.32) | 88.3 |
| 6 µg | 3 | 0.92 (0.86-0.98)a | 0 |
| 7.5 µg | 3 | 1.13 (0.78-1.66) | 58.2 |
| 9 µg | 12 | 0.99 (0.95-1.03) | 50 |
| 15 µg | 16 | 1.03 (0.97-1.09) | 48.5 |
| GMTb | |||
| H1N1 | |||
| 3 µg | 3 | 1.00 (0.54-1.84) | 99.9 |
| 6 µg | 2 | 0.88 (0.85-0.90)a | 65.1 |
| 9 µg | 11 | 1.04 (0.99-1.10) | 99.8 |
| 15 µg | 11 | 1.17 (0.95-1.42) | 99.9 |
| H3N2 | |||
| 3 µg | 3 | 0.90 (0.68-1.18) | 99.4 |
| 6 µg | 3 | 1.09 (0.90-1.32) | 99 |
| 9 µg | 11 | 1.08 (1.05-1.12)a | 99.4 |
| 15 µg | 11 | 1.16 (0.96-1.41) | 100 |
| B strain | |||
| 3 µg | 3 | 0.80 (0.46-1.38) | 99.9 |
| 6 µg | 2 | 0.82 (0.67-1.01) | 98.9 |
| 9 µg | 11 | 0.93 (0.86-1.01) | 99.9 |
| 15 µg | 11 | 1.21 (1.11-1.32)a | 99.8 |
Abbreviation: GMT, geometric mean titer.
P < 05.
Data are expressed as ratio of means for GMT.
Seroprotection
Seroprotection rates were significantly lower with the 6-µg intradermal dose vs the 15-μg intramuscular dose for the H1N1 strain (RR, 0.93; 95% CI, 0.88-0.99) and B strain (RR, 0.92; 95% CI, 0.86-0.98). For the 9-µg intradermal doses, seroprotection rates were not statistically significant compared with the 15-µg intramuscular dose for all 3 strains. The seroprotection rates for 15-µg intradermal and 15-µg intramuscular doses were also not statistically significantly different for H3N2 and B strains; however, the seroprotection rate for intradermal doses was significantly higher for the H1N1 strain compared with the 15-μg intramuscular dose (RR, 1.05; 95% CI,1.01-1.09) (Table 2; eFigures 9-15 in the Supplement).
GMT
Although there was high heterogeneity, the GMTs were not statistically significantly different between the 3-µg and 6-µg intradermal doses and the 15-µg intramuscular dose for the 3 strains, except for a significant decrease for H1N1 observed with the 6-µg intradermal dose (RR, 0.88; 95% CI, 0.85-0.90). Similarly, GMTs were not statistically significant for the H1N1 and B strains when the 9-µg intradermal doses were compared with the 15-µg intramuscular dose, but GMT was significantly higher for the 9-µg intradermal dose of the H3N2 strain (RR, 1.08; 95% CI, 1.05-1.12). The 15-µg intradermal dose showed no statistically significant difference with the 15-µg intramuscular dose for the H1N1 and the H3N2 strains. However, the 15-µg intradermal dose was associated with significantly higher GMT for the B strain (RR, 1.21; 95% CI, 1.11-1.32) (Table 2; eFigures 16-21 in the Supplement).
Immunogenicity in Older Adults
Subgroup analyses for immunogenicity in adults aged 60 years and older did not show statistically significant difference between the 9-µg intradermal dose and the 15-µg intramuscular doses, with respect to seroconversion, seroprotection, or GMT for each of the 3 strains. There was high heterogeneity among the studies. Seroprotection rates did not differ significantly between the 15-µg intradermal dose vs 15-µg intramuscular dose for the 3 strains, while seroconversion rate was significantly higher with the 15-µg intradermal dose compared with the 15-µg intramuscular dose for the B strain (RR, 1.41; 95% CI, 1.13- 1.75), as was GMT (RR, 1.19; 95% CI, 1.09-1.30) (Table 3).
Table 3. Immunogenicity of Intradermal Doses vs Standard 15-µg Intramuscular Dose of Influenza Vaccine Among Participants ≥60 Years.
| Intradermal dose | Pooled studies, No. | Risk ratio (95% CI) | I2 |
|---|---|---|---|
| Seroconversion | |||
| H1N1 | |||
| 9 µg | 2 | 1.01 (0.58-1.77) | 87 |
| 15 µg | 13 | 1.11 (1.00-1.24) | 57.3 |
| H3N2 | |||
| 9 µg | 2 | 1.02 (0.83-1.25) | 0 |
| 15 µg | 14 | 1.12 (1.00-1.25) | 52.1 |
| B strain | |||
| 9 µg | 2 | 1.00 (0.60-1.67) | 0 |
| 15 µg | 13 | 1.41 (1.13-1.75)a | 59 |
| Seroprotection | |||
| H1N1 | |||
| 9 µg | 4 | 0.98 (0.88-1.09) | 24.1 |
| 15 µg | 14 | 1.04 (1.00-1.09) | 55.1 |
| H3N2 | |||
| 9 µg | 4 | 1.03 (0.94-1.12) | 0 |
| 15 µg | 11 | 1.01 (0.99-1.03) | 38.6 |
| B strain | |||
| 9 µg | 4 | 0.95 (0.71-1.27) | 0 |
| 15 µg | 12 | 1.03 (0.97-1.09) | 45.3 |
| GMT | |||
| H1N1 | |||
| 9 µg | 4 | 0.96 (0.75-1.23)b | 99.3 |
| 15 µg | 11 | 1.11 (0.89-1.39)b | 100 |
| H3N2 | |||
| 9 µg | 4 | 1.07 (0.80-1.44)b | 99.5 |
| 15 µg | 7 | 1.13 (0.92-1.40)b | 100 |
| B strain | |||
| 9 µg | 4 | 0.93 (0.72-1.20)b | 99.5 |
| 15 µg | 9 | 1.19 (1.09-1.30)a,b | 99.8 |
Abbreviation: GMT, geometric mean titer.
P < .05.
Data are expressed as ratio of means for GMT.
Influenza Infection or Influenza-Like Illness
A meta-analysis of 4 studies reporting clinical outcomes showed that the risk of influenza or influenza-like illness was significantly lower with intradermal vaccines compared with intramuscular vaccines (RR, 0.62; 95% CI, 0.49-0.77).27,37,39,47 However, there was no significant difference between the 2 routes of administration at intradermal dosages of 9 µg (RR, 0.61; 95% CI, 0.19-1.91)27,39 or 15 µg (RR, 0.68; 95% CI, 0.43-1.08).37,47
Adverse Events
Local adverse events, including erythema, swelling, induration, pruritus, and ecchymosis, were significantly higher across the dose spectrum of intradermal vaccines compared with the standard intramuscular dose, particularly erythema (3-µg dose: RR, 9.62; 95% CI, 1.07-86.56; 6-µg dose: RR, 23.79; 95% CI, 14.42-39.23; 9-µg dose: RR, 4.56; 95% CI, 3.05-6.82; 15-µg dose: RR, 3.68; 95% CI, 3.19-4.25) and swelling (3-µg dose: RR, 20.16; 95% CI, 4.68-86.82; 9-µg dose: RR, 5.23; 95% CI, 3.58-7.62; 15-µg dose: RR, 3.47 ; 95% CI, 2.21-5.45). There was high heterogeneity among the pooled studies. Pain did not differ significantly between the 6-µg, 9-µg, or 15-µg intradermal doses vs the 15-µg intramuscular dose but was significantly lower with the 3-µg intradermal dose (Table 4; eFigures 22-25 in the Supplement). Differences in systemic adverse events, including headache, fever, malaise, arthralgia, myalgia, and nausea, were not statistically significant between the low intradermal doses and the standard intramuscular dose, and fever (RR, 1.36; 95% CI, 1.03-1.80) and chills (RR, 1.24; 95% CI, 1.03-1.50) were more common with the 9-µg intradermal dose than 15-µg intramuscular dose (Table 4; eFigures 26-29 in the Supplement).
Table 4. Local and Systemic Adverse Events Risks With Intradermal Doses vs 15 µg Intramuscular Dose of Influenza Vaccine.
| Intradermal dose | Pooled studies, No. | Risk ratio (95% CI) | I2 |
|---|---|---|---|
| Local adverse events | |||
| Ecchymosis | |||
| 9 µg | 7 | 1.67 (1.12-2.48)a | 55 |
| 15 µg | 9 | 1.06 (0.73-1.57) | 0 |
| Erythema | |||
| 3 µg | 3 | 9.62 (1.07-86.56)a | 97.2 |
| 6 µg | 2 | 23.79 (14.42-39.23)a | 0 |
| 9 µg | 14 | 4.56 (3.05-6.82)a | 93.9 |
| 15 µg | 16 | 3.68 (3.19-4.25)a | 8.8 |
| Induration | |||
| 9 µg | 5 | 3.27 (1.65-6.46)a | 95.4 |
| 15 µg | 9 | 2.98 (2.32-3.84)a | 42.6 |
| Pain | |||
| 3 µg | 4 | 0.34 (0.20-0.56)a | 21.9 |
| 6 µg | 2 | 0.98 (0.38-2.49) | 68.3 |
| 9 µg | 12 | 0.95 (0.86-1.05) | 34.4 |
| 15 µg | 16 | 0.94 (0.72-1.21) | 61.3 |
| Pruritus | |||
| 6 µg | 2 | 15.22 (4.77-48.54)a | 0 |
| 9 µg | 9 | 4.24 (3.16-5.70)a | 56.2 |
| 15 µg | 6 | 4.01 (3.13-5.15)a | 0 |
| Swelling | |||
| 3 µg | 2 | 20.16 (4.68-86.82)a | 51.3 |
| 9 µg | 13 | 5.23 (3.58-7.62)a | 84.4 |
| 15 µg | 12 | 3.47 (2.21-5.45)a | 71.9 |
| Systemic adverse events | |||
| Arthralgia | |||
| 15 µg | 3 | 1.17 (0.39-3.53) | 22.7 |
| Chills and shivering | |||
| 9 µg | 7 | 1.24 (1.03-1.50)a | 0 |
| 15 µg | 10 | 1.08 (0.78-1.51) | 0 |
| Fever | |||
| 6 µg | 2 | 0.54 (0.17-1.71) | 34.5 |
| 9 µg | 11 | 1.36 (1.03-1.80)a | 0 |
| 15 µg | 13 | 0.89 (0.59-1.34) | 0 |
| Headache | |||
| 3 µg | 2 | 1.09 (0.86-1.37) | 0 |
| 6 µg | 2 | 0.83 (0.39-1.78) | 68 |
| 9 µg | 13 | 1.03 (0.96-1.11) | 0 |
| 15 µg | 9 | 1.16 (0.94-1.45) | 0 |
| Malaise | |||
| 9 µg | 7 | 1.05 (0.94-1.20) | 7.1 |
| 15 µg | 14 | 0.97 (0.78-1.22) | 0 |
| Myalgia | |||
| 9 µg | 12 | 1.24 (0.93-1.65) | 74.8 |
| 15 µg | 9 | 0.84 (0.63-1.12) | 29.4 |
| Nausea | |||
| 9 µg | 3 | 0.93 (0.37-2.31) | 0 |
| 15 µg | 2 | 1.05 (0.33-3.33) | 0 |
P < .05.
Publication Bias
The Egger test for publication bias was statistically significant for the 15-µg intradermal and intramuscular doses comparison for the B strain seroconversion rate (intercept: 0.97; 95% CI, 0.21-1.73, P = .02) and the H3N2 strain seroprotection rate (intercept: 1.80; 95% CI, 0.43-3.17, P = .02). Bias correction using the trim-and-fill method did not change the statistical significance of the unadjusted results (eFigures 30-35 in the Supplement).
Discussion
This systematic review and meta-analysis found that immunogenicity resulting from 3-µg, 6-µg, 7.5-µg and 9-µg influenza intradermal vaccination doses was not significantly different from full-dose 15-µg intramuscular vaccination for most viral strains, irrespective of patient age. However, the 15-µg intradermal vaccine showed significantly better immunogenicity for some of the outcomes and strains, suggesting that the immunological response may be dose-related. The risk of local adverse events, such as erythema, induration, swelling, and ecchymosis, was reduced with intramuscular vaccination; however, the risk of pain did not differ significantly between the 2 administration methods, with the exception of the 3-µg intradermal dose, which significantly lowered the risk of pain. The risks of systemic adverse events, such as headache, malaise, myalgia, and arthralgia, were similar with both administration methods.
The findings of this study are similar to those by Marra et al48 and 2 studies by Pileggi et al,49,50 which found no statistically significant difference between the different intradermal influenza vaccine doses and the 15-µg intramuscular influenza vaccine dose. It should be noted that Pileggi et al included studies involving only participants who were immunocompromised in one of their studies49 and only older adults in another.50 However, our systematic review excluded participants who were immunocompromised and carried out sensitivity analysis of studies involving older adults, given that old age51 and immunocompromise52 are known to attenuate immunological response. Although local skin reactions were more common with intradermal vaccinations, these reactions are generally well-accepted by vaccinees,53,54 who also find the microinjection systems to be more tolerable than the regular needles.54 These reactions are generally transient, with comparable rates of pain as intramuscular vaccination.55 Furthermore, the development of novel intradermal vaccine delivery systems, such as self-administrable patches with coated microprojections or biodegradable needles, could potentially improve vaccine acceptance and uptake.56 None of the studies in our review reported the use of nonneedle delivery systems. Intradermal administration requires advanced technical skill and special needles that present feasibility barriers to implementation. In Canada, an intradermal influenza vaccine is available off-label; however, most pharmacists are not licensed to administer intradermally despite administering approximately 30% of influenza doses every year.57
The results for immunogenicity and safety outcomes for this systematic review and meta-analysis were derived from only randomized clinical trials. This suggests a high level of evidence for these outcomes. The cohort study data were only included in the meta-analysis for influenza or influenza-like illness.
Limitations
This study has some limitations. One limitation was the heterogeneity among the included studies, particularly with respect to the GMT outcome. This may be associated with the variation in the characteristics of the study participants, including age and comorbidities. However, heterogeneity persisted after stratifying the meta-analyses by age group. Other possible causes of heterogeneity include variations in vaccine factors, such as the use of adjuvants and differences in vaccine brands and delivery systems. Additionally, although the DerSimonian and Laird estimator of the between-study variance used in this study is the most commonly used method,58 it tends to produce narrower CIs, which may be less conservative in the representation of uncertainty in the estimation of between-study heterogeneity, especially when the number of studies included in the meta-analysis is small.58,59
Conclusions
The findings of this systematic review and meta-analysis suggest that given the similarity in immunogenicity between the reduced dose intradermal and full dose intramuscular influenza vaccine, low-dose intradermal vaccine could be a reasonable alternative to standard-dose intramuscular vaccination. It will be important to determine if this dose-sparing finding holds true across age groups and for newer vaccines, particularly when recent high-dose formulations have demonstrated improved immunogenicity in older adults in whom immune responses have historically struggled.
eAppendix. Search Strategy
eFigure 1. Quality Assessment of Randomized Clinical Trials
eFigure 2. Seroconversion: 3 μg ID vs 15 μg IM
eFigure 3. Seroconversion: 6 μg ID vs 15 μg IM
eFigure 4. Seroconversion: 7.5 μg ID vs 15 μg IM
eFigure 5. Seroconversion: 9 μg ID vs 15 μg IM in All Population
eFigure 6. Seroconversion: in Older Adults 9 μg ID vs 15 μg IM
eFigure 7. Seroconversion: 15 μg ID vs 15 μg IM
eFigure 8. Seroconversion: in Older Adults 15 μg ID vs 15 μg IM
eFigure 9. Seroprotection: 3 μg ID vs 15 μg IM
eFigure 10. Seroprotection: 6 μg ID vs 15 μg IM
eFigure 11. Seroprotection: 7.5 μg ID vs 15 μg IM
eFigure 12. Seroprotection: 9 μg ID vs 15 μg IM
eFigure 13. Seroprotection Older Adults: 9 μg ID vs 15 μg IM
eFigure 14. Seroprotection: 15 μg ID vs 15 μg IM
eFigure 15. Seroprotection in Older Adults: 15 μg ID vs 15 μg IM
eFigure 16. GMT: 3 μg ID vs 15 μg IM
eFigure 17. GMT: 6 μg ID vs 15 μg IM
eFigure 18. GMT: 9 μg ID vs 15 μg IM
eFigure 19. GMT: in Older Adults 9 μg ID vs 15 μg IM
eFigure 20. GMT: 15 μg ID vs 15 μg IM
eFigure 21. GMT in Older Adults: 15 μg ID vs 15 μg IM
eFigure 22. Local Adverse Events: 3 μg ID vs 15 μg IM
eFigure 23. Local Adverse Events: 6 μg ID vs 15 μg IM
eFigure 24. Local Adverse Events: 9 μg ID vs 15 μg IM
eFigure 25. Local Adverse Events: 15 μg ID vs 15 μg IM
eFigure 26. Systemic Adverse Events: 3 μg ID vs 15 μg IM
eFigure 27. Systemic Adverse Events: 6 μg ID vs 15 μg IM
eFigure 28. Systemic Adverse Events: 9 μg ID vs 15 μg IM
eFigure 29. Systemic Adverse Events: 15 μg ID vs 15 μg IM
eFigure 30. Funnel Plot of Seroconversion: 9 μg ID vs 15 μg IM
eFigure 31. Funnel Plot of Seroprotection: 9 μg vs 15 μg
eFigure 32. Funnel Plot of GMT: 9 μg ID vs 15 μg IM
eFigure 33. Funnel Plot of Seroconversion: 15 μg ID vs 15 μg IM
eFigure 34. Funnel Plot of Seroprotection: 15 μg ID vs 15 μg IM
eFigure 35. Funnel Plot of GMT: 15 μg ID vs 15 μg IM
References
- 1.World Health Organization Influenza (seasonal). Revised November 2018. Accessed July 12, 2020. https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal)
- 2.Bragazzi NL, Orsi A, Ansaldi F, Gasparini R, Icardi G. Fluzone intra-dermal (Intanza/Istivac intra-dermal): an updated overview. Hum Vaccin Immunother. 2016;12(10):2616-2627. doi: 10.1080/21645515.2016.1187343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hickling JK, Jones KR, Friede M, Zehrung D, Chen D, Kristensen D. Intradermal delivery of vaccines: potential benefits and current challenges. Bull World Health Organ. 2011;89(3):221-226. doi: 10.2471/BLT.10.079426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization WHO recommends the intradermal route for rabies post-exposure prophylaxis. Accessed September 9, 2020. https://www.who.int/rabies/rabies_post_immunization/en/
- 5.Gongal G, Sampath G. Introduction of intradermal rabies vaccination—a paradigm shift in improving post-exposure prophylaxis in Asia. Vaccine. 2019;37(suppl 1):A94-A98. doi: 10.1016/j.vaccine.2018.08.034 [DOI] [PubMed] [Google Scholar]
- 6.La Montagne JR, Fauci AS. Intradermal influenza vaccination--can less be more? N Engl J Med. 2004;351(22):2330-2332. doi: 10.1056/NEJMe048314 [DOI] [PubMed] [Google Scholar]
- 7.US Food and Drug Administration Approval letter—Fluzone quadrivalent and Fluzone high-dose quadrivalent. Accessed July 12, 2020. https://www.fda.gov/media/139728/download
- 8.Roos R; Center for Infectious Disease Research and Policy Europe approves Sanofi's intradermal flu vaccine. News release. February 26, 2009. Accessed January 5, 2021. https://www.cidrap.umn.edu/news-perspective/2009/02/europe-approves-sanofis-intradermal-flu-vaccine
- 9.Levin Y, Kochba E, Hung I, Kenney R. Intradermal vaccination using the novel microneedle device MicronJet600: past, present, and future. Hum Vaccin Immunother. 2015;11(4):991-997. doi: 10.1080/21645515.2015.1010871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romani N, Flacher V, Tripp C, Sparber F, Ebner S, Stoitzner P. Targeting skin dendritic cells to improve intradermal vaccination. In: Teunissen MBM, ed. Intradermal Immunization. Springer; 2011:113-138. doi: 10.1007/82_2010_118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40-46. doi: 10.1016/j.jclinepi.2016.01.021 [DOI] [PubMed] [Google Scholar]
- 12.The Cochrane Collaboration's Tool for Assessing Risk of Bias In: Higgins JPT, Altman DG, Sterne JAC, eds. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Collaboration; 2017. [Google Scholar]
- 13.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 14.Friedrich JO, Adhikari NK, Beyene J. Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC Med Res Methodol. 2007;7(1):5. doi: 10.1186/1471-2288-7-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedrich JO, Adhikari NK, Beyene J. Ratio of geometric means to analyze continuous outcomes in meta-analysis: comparison to mean differences and ratio of arithmetic means using empiric data and simulation. Stat Med. 2012;31(17):1857-1886. doi: 10.1002/sim.4501 [DOI] [PubMed] [Google Scholar]
- 16.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455-463. doi: 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 18.Ansaldi F, Canepa P, Ceravolo A, et al. Intanza 15 mcg intradermal influenza vaccine elicits cross-reactive antibody responses against heterologous A(H3N2) influenza viruses. Vaccine. 2012;30(18):2908-2913. doi: 10.1016/j.vaccine.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 19.Ansaldi F, Orsi A, de Florentiis D, et al. Head-to-head comparison of an intradermal and a virosome influenza vaccine in patients over the age of 60: evaluation of immunogenicity, cross-protection, safety and tolerability. Hum Vaccin Immunother. 2013;9(3):591-598. doi: 10.4161/hv.23240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnou R, Eavis P, Pardo JR, Ambrozaitis A, Kazek MP, Weber F. Immunogenicity, large scale safety and lot consistency of an intradermal influenza vaccine in adults aged 18-60 years: randomized, controlled, phase III trial. Hum Vaccin. 2010;6(4):346-354. doi: 10.4161/hv.6.4.10961 [DOI] [PubMed] [Google Scholar]
- 21.Boonnak K, Dhitavat J, Thantamnu N, et al. Immune responses to intradermal and intramuscular inactivated influenza vaccine among older age group. Vaccine. 2017;35(52):7339-7346. doi: 10.1016/j.vaccine.2017.10.106 [DOI] [PubMed] [Google Scholar]
- 22.Camilloni B, Basileo M, Di Martino A, Donatelli I, Iorio AM. Antibody responses to intradermal or intramuscular MF59-adjuvanted influenza vaccines as evaluated in elderly institutionalized volunteers during a season of partial mismatching between vaccine and circulating A(H3N2) strains. Immun Ageing. 2014;11:10. doi: 10.1186/1742-4933-11-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carter C, Houser KV, Yamshchikov GV, et al. ; VRC 703 study team . Safety and immunogenicity of investigational seasonal influenza hemagglutinin DNA vaccine followed by trivalent inactivated vaccine administered intradermally or intramuscularly in healthy adults: an open-label randomized phase 1 clinical trial. PLoS One. 2019;14(9):e0222178. doi: 10.1371/journal.pone.0222178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan TC, Hung IF, Chan KH, et al. Immunogenicity and safety of intradermal trivalent influenza vaccination in nursing home older adults: a randomized controlled trial. J Am Med Dir Assoc. 2014;15(8):607.e5-607.e12. doi: 10.1016/j.jamda.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 25.Chi RC, Rock MT, Neuzil KM. Immunogenicity and safety of intradermal influenza vaccination in healthy older adults. Clin Infect Dis. 2010;50(10):1331-1338. doi: 10.1086/652144 [DOI] [PubMed] [Google Scholar]
- 26.Chuaychoo B, Kositanont U, Niyomthong P, et al. Comparison of immunogenicity between intradermal and intramuscular injections of repeated annual identical influenza virus strains post-pandemic (2011-2012) in COPD patients. Hum Vaccin Immunother. 2019;16(6):1371-1379. doi: 10.1080/21645515.2019.1692559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chuaychoo B, Kositanont U, Rittayamai N, et al. The immunogenicity of the intradermal injection of seasonal trivalent influenza vaccine containing influenza A(H1N1)pdm09 in COPD patients soon after a pandemic. Hum Vaccin Immunother. 2016;12(7):1728-1737. doi: 10.1080/21645515.2016.1149276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chuaychoo B, Wongsurakiat P, Nana A, Kositanont U, Maranetra KN. The immunogenicity of intradermal influenza vaccination in COPD patients. Vaccine. 2010;28(24):4045-4051. doi: 10.1016/j.vaccine.2010.04.006 [DOI] [PubMed] [Google Scholar]
- 29.Della Cioppa G, Nicolay U, Lindert K, et al. A dose-ranging study in older adults to compare the safety and immunogenicity profiles of MF59-adjuvanted and non-adjuvanted seasonal influenza vaccines following intradermal and intramuscular administration. Hum Vaccin Immunother. 2014;10(6):1701-1710. doi: 10.4161/hv.28618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esposito S, Daleno C, Picciolli I, et al. Immunogenicity and safety of intradermal influenza vaccine in children. Vaccine. 2011;29(44):7606-7610. doi: 10.1016/j.vaccine.2011.08.021 [DOI] [PubMed] [Google Scholar]
- 31.Frenck RW Jr, Belshe R, Brady RC, et al. Comparison of the immunogenicity and safety of a split-virion, inactivated, trivalent influenza vaccine (Fluzone) administered by intradermal and intramuscular route in healthy adults. Vaccine. 2011;29(34):5666-5674. doi: 10.1016/j.vaccine.2011.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garg S, Thongcharoen P, Praphasiri P, et al. Randomized controlled trial to compare immunogenicity of standard-dose intramuscular versus intradermal trivalent inactivated influenza vaccine in HIV-infected men who have sex with men in Bangkok, Thailand. Clin Infect Dis. 2016;62(3):383-391. doi: 10.1093/cid/civ884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gorse GJ, Falsey AR, Johnson CM, et al. Safety and immunogenicity of revaccination with reduced dose intradermal and standard dose intramuscular influenza vaccines in adults 18-64 years of age. Vaccine. 2013;31(50):6034-6040. doi: 10.1016/j.vaccine.2013.09.012 [DOI] [PubMed] [Google Scholar]
- 34.Hoon Han S, Hee Woo J, Weber F, et al. Immunogenicity and safety of Intanza/IDflu intradermal influenza vaccine in South Korean adults: a multicenter, randomized trial. Hum Vaccin Immunother. 2013;9(9):1971-1977. doi: 10.4161/hv.25295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hung IFN, Zhang AJ, To KKW, et al. Topical imiquimod before intradermal trivalent influenza vaccine for protection against heterologous non-vaccine and antigenically drifted viruses: a single-centre, double-blind, randomised, controlled phase 2b/3 trial. Lancet Infect Dis. 2016;16(2):209-218. doi: 10.1016/S1473-3099(15)00354-0 [DOI] [PubMed] [Google Scholar]
- 36.Hung IF, Levin Y, To KK, et al. Dose sparing intradermal trivalent influenza (2010/2011) vaccination overcomes reduced immunogenicity of the 2009 H1N1 strain. Vaccine. 2012;30(45):6427-6435. doi: 10.1016/j.vaccine.2012.08.014 [DOI] [PubMed] [Google Scholar]
- 37.Hung IF, Zhang AJ, To KK, et al. Immunogenicity of intradermal trivalent influenza vaccine with topical imiquimod: a double blind randomized controlled trial. Clin Infect Dis. 2014;59(9):1246-1255. doi: 10.1093/cid/ciu582 [DOI] [PubMed] [Google Scholar]
- 38.Leung DYM, Jepson B, Beck LA, et al. A clinical trial of intradermal and intramuscular seasonal influenza vaccination in patients with atopic dermatitis. J Allergy Clin Immunol. 2017;139(5):1575-1582.e8. doi: 10.1016/j.jaci.2016.12.952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nougarede N, Bisceglia H, Rozières A, et al. Nine μg intradermal influenza vaccine and 15 μg intramuscular influenza vaccine induce similar cellular and humoral immune responses in adults. Hum Vaccin Immunother. 2014;10(9):2713-2720. doi: 10.4161/hv.29695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel SM, Atmar RL, El Sahly HM, Cate TR, Keitel WA. A phase I evaluation of inactivated influenza A/H5N1 vaccine administered by the intradermal or the intramuscular route. Vaccine. 2010;28(17):3025-3029. doi: 10.1016/j.vaccine.2009.10.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seo YB, Choi WS, Lee J, Song JY, Cheong HJ, Kim WJ. Comparison of the immunogenicity and safety of the conventional subunit, MF59-adjuvanted, and intradermal influenza vaccines in the elderly. Clin Vaccine Immunol. 2014;21(7):989-996. doi: 10.1128/CVI.00615-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song JY, Cheong HJ, Noh JY, et al. Long-term immunogenicity of the influenza vaccine at reduced intradermal and full intramuscular doses among healthy young adults. Clin Exp Vaccine Res. 2013;2(2):115-119. doi: 10.7774/cevr.2013.2.2.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsang P, Gorse GJ, Strout CB, et al. Immunogenicity and safety of Fluzone intradermal and high-dose influenza vaccines in older adults ≥65 years of age: a randomized, controlled, phase II trial. Vaccine. 2014;32(21):2507-2517. doi: 10.1016/j.vaccine.2013.09.074 [DOI] [PubMed] [Google Scholar]
- 44.Van Damme P, Arnou R, Kafeja F, et al. Evaluation of non-inferiority of intradermal versus adjuvanted seasonal influenza vaccine using two serological techniques: a randomised comparative study. BMC Infect Dis. 2010;10:134. doi: 10.1186/1471-2334-10-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levin Y, Kochba E, Kenney R. Clinical evaluation of a novel microneedle device for intradermal delivery of an influenza vaccine: are all delivery methods the same? Vaccine. 2014;32(34):4249-4252. doi: 10.1016/j.vaccine.2014.03.024 [DOI] [PubMed] [Google Scholar]
- 46.Levin Y, Kochba E, Shukarev G, Rusch S, Herrera-Taracena G, van Damme P. A phase 1, open-label, randomized study to compare the immunogenicity and safety of different administration routes and doses of virosomal influenza vaccine in elderly. Vaccine. 2016;34(44):5262-5272. doi: 10.1016/j.vaccine.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 47.Puig-Barberà J, Natividad-Sancho A, Calabuig-Pérez J, et al. Intradermal and virosomal influenza vaccines for preventing influenza hospitalization in the elderly during the 2011-2012 influenza season: a comparative effectiveness study using the Valencia health care information system. Vaccine. 2014;32(42):5447-5454. doi: 10.1016/j.vaccine.2014.07.095 [DOI] [PubMed] [Google Scholar]
- 48.Marra F, Young F, Richardson K, Marra CA. A meta-analysis of intradermal versus intramuscular influenza vaccines: immunogenicity and adverse events. Influenza Other Respir Viruses. 2013;7(4):584-603. doi: 10.1111/irv.12000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pileggi C, Lotito F, Bianco A, Nobile CG, Pavia M. Immunogenicity and safety of intradermal influenza vaccine in immunocompromized patients: a meta-analysis of randomized controlled trials. BMC Infect Dis. 2015;15:427. doi: 10.1186/s12879-015-1161-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pileggi C, Mascaro V, Bianco A, Nobile CG, Pavia M. Immunogenicity and safety of intradermal influenza vaccine in the elderly: a meta-analysis of randomized controlled trials. Drugs Aging. 2015;32(10):857-869. doi: 10.1007/s40266-015-0303-8 [DOI] [PubMed] [Google Scholar]
- 51.Chen WH, Kozlovsky BF, Effros RB, Grubeck-Loebenstein B, Edelman R, Sztein MB. Vaccination in the elderly: an immunological perspective. Trends Immunol. 2009;30(7):351-359. doi: 10.1016/j.it.2009.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kunisaki KM, Janoff EN. Influenza in immunosuppressed populations: a review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect Dis. 2009;9(8):493-504. doi: 10.1016/S1473-3099(09)70175-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eizenberg P, Booy R, Naser N, Mason G, Stamboulian D, Weber F. Acceptance of Intanza 9 μg intradermal influenza vaccine in routine clinical practice in Australia and Argentina. Adv Ther. 2011;28(8):640-649. doi: 10.1007/s12325-011-0042-0 [DOI] [PubMed] [Google Scholar]
- 54.Reygrobellet C, Viala-Danten M, Meunier J, Weber F, Nguyen VH. Perception and acceptance of intradermal influenza vaccination: patient reported outcomes from phase 3 clinical trials. Hum Vaccin. 2010;6(4):336-345. doi: 10.4161/hv.6.4.10753 [DOI] [PubMed] [Google Scholar]
- 55.Ansaldi F, Durando P, Icardi G. Intradermal influenza vaccine and new devices: a promising chance for vaccine improvement. Expert Opin Biol Ther. 2011;11(3):415-427. doi: 10.1517/14712598.2011.557658 [DOI] [PubMed] [Google Scholar]
- 56.Hung IFN, Yuen K-Y. Immunogenicity, safety and tolerability of intradermal influenza vaccines. Hum Vaccin Immunother. 2018;14(3):565-570. doi: 10.1080/21645515.2017.1328332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Canadian Pharmacists Association Pharmacists' expanded scope of practice. Accessed September 9, 2020. https://www.pharmacists.ca/pharmacy-in-canada/scope-of-practice-canada/
- 58.Deeks JJ, Higgins JP, Altman DG, eds. Analysing data and undertaking meta-analyses Higgins JPT, Thomas J, Chandler J, et al. , eds. Cochrane Handbook for Systematic Reviews of Interventions. 2020:241-284. [Google Scholar]
- 59.Veroniki AA, Jackson D, Viechtbauer W, et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods. 2016;7(1):55-79. doi: 10.1002/jrsm.1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Search Strategy
eFigure 1. Quality Assessment of Randomized Clinical Trials
eFigure 2. Seroconversion: 3 μg ID vs 15 μg IM
eFigure 3. Seroconversion: 6 μg ID vs 15 μg IM
eFigure 4. Seroconversion: 7.5 μg ID vs 15 μg IM
eFigure 5. Seroconversion: 9 μg ID vs 15 μg IM in All Population
eFigure 6. Seroconversion: in Older Adults 9 μg ID vs 15 μg IM
eFigure 7. Seroconversion: 15 μg ID vs 15 μg IM
eFigure 8. Seroconversion: in Older Adults 15 μg ID vs 15 μg IM
eFigure 9. Seroprotection: 3 μg ID vs 15 μg IM
eFigure 10. Seroprotection: 6 μg ID vs 15 μg IM
eFigure 11. Seroprotection: 7.5 μg ID vs 15 μg IM
eFigure 12. Seroprotection: 9 μg ID vs 15 μg IM
eFigure 13. Seroprotection Older Adults: 9 μg ID vs 15 μg IM
eFigure 14. Seroprotection: 15 μg ID vs 15 μg IM
eFigure 15. Seroprotection in Older Adults: 15 μg ID vs 15 μg IM
eFigure 16. GMT: 3 μg ID vs 15 μg IM
eFigure 17. GMT: 6 μg ID vs 15 μg IM
eFigure 18. GMT: 9 μg ID vs 15 μg IM
eFigure 19. GMT: in Older Adults 9 μg ID vs 15 μg IM
eFigure 20. GMT: 15 μg ID vs 15 μg IM
eFigure 21. GMT in Older Adults: 15 μg ID vs 15 μg IM
eFigure 22. Local Adverse Events: 3 μg ID vs 15 μg IM
eFigure 23. Local Adverse Events: 6 μg ID vs 15 μg IM
eFigure 24. Local Adverse Events: 9 μg ID vs 15 μg IM
eFigure 25. Local Adverse Events: 15 μg ID vs 15 μg IM
eFigure 26. Systemic Adverse Events: 3 μg ID vs 15 μg IM
eFigure 27. Systemic Adverse Events: 6 μg ID vs 15 μg IM
eFigure 28. Systemic Adverse Events: 9 μg ID vs 15 μg IM
eFigure 29. Systemic Adverse Events: 15 μg ID vs 15 μg IM
eFigure 30. Funnel Plot of Seroconversion: 9 μg ID vs 15 μg IM
eFigure 31. Funnel Plot of Seroprotection: 9 μg vs 15 μg
eFigure 32. Funnel Plot of GMT: 9 μg ID vs 15 μg IM
eFigure 33. Funnel Plot of Seroconversion: 15 μg ID vs 15 μg IM
eFigure 34. Funnel Plot of Seroprotection: 15 μg ID vs 15 μg IM
eFigure 35. Funnel Plot of GMT: 15 μg ID vs 15 μg IM