Abstract
Background
Since the first documented outbreak of a novel severe acute respiratory syndrome inducing Coronavirus in China at the end of 2019 the virus has spread to all continents, leading the WHO to declare a pandemic in March 2020. While this virus primarily targets the alveoli in the lungs, multiple authors have described an increased rate of thrombo-embolic events in affected patients. We present this case of a myocardial infarction with no obstructive coronary atherosclerosis in an otherwise healthy 48-year-old patient.
Case summary
A 48-year-old female, presenting with chest pain radiating to her left shoulder with no cardiovascular risk factors other than genetic predisposition, was screened for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and tested positive. Although computed tomography angiography excluded obstructive coronary heart disease, cardiac magnetic resonance imaging showed an acute myocardial infarction with no obstructive coronary arteries of the inferior wall. The patient was treated with dual anti-platelet therapy, an angiotensin-converting-enzyme inhibitor and a statin, and assigned to a cardiac rehabilitation program.
Conclusion
We report a serious thrombo-embolic event during an oligosymptomatic SARS-CoV-2 infection in a healthy, young patient. While these two diseases may have occurred simultaneously, by chance, it is possible that the pro-thrombotic effects of the SARS-CoV-2 infection facilitated the infarction. This case further demonstrates the significant cardiovascular morbidity potentially caused by SARS-CoV-2.
Keywords: COVID-19, MINOCA, Endothelitis, Case report
Learning points
COVID-19 may cause serious thrombo-embolic complications—rarely even in patients with mild symptoms of the infection.
Computed tomography coronary angiography and cardiac magnetic resonance are particularly useful in the further evaluation of unclear acute cardiac events in Severe Acute Respiratory Syndrome Coronavirus 2 infections.
Primary Specialties involved other than cardiology
Infectious diseases, radiology.
Introduction
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) made its first appearance in the province of Wuhan, China, at the end of 2019, where it caused an outbreak of viral pneumonia. The virus then spread throughout the seven continents, causing significant morbidity and mortality and forcing the WHO to declare a pandemic in March 2020.
The virus appears to gain access to human cells through angiotensin-converting enzyme 2 (ACE2). This molecule can be identified, among others, in the type II alveolar cells of the lungs1 and in the myocardial and coronary endothelial cells of the heart.2
While the SARS-CoV-2 infection primarily affects the lungs,3 there have been multiple reports of increased risk for thrombo-embolic complications during the disease,4–6 an effect potentially mediated by immune system hyperactivity with cytokine storm as well as local and systemic inflammatory reactions.7 We present the case of a 48-year-old female who developed a myocardial infarction with no obstructive coronary arteries (MINOCA) during an oligosymptomatic SARS-CoV-2 infection.
Timeline
| Day | Event |
|---|---|
| 0 | A 48-year-old female experienced sudden pain in her chest and left shoulder |
| 1 |
First medical contact Treating physician observes pathologic electrocardiogram-findings and elevated hs-TnI values and, suspecting a non-ST-elevation myocardial infarction, refers the patient for a coronary angiography Screening reveals a Severe Acute Respiratory Syndrome Coronavirus 2 infection |
| 2 | Patient is isolated at the infectious diseases department with a suspected diagnosis of viral myocarditis; therapy started with acetylsalicylic acid, low-molecular-weight heparin and a proton-pump inhibitor |
| 3 | Coronary computed tomography angiography shows no significant coronary artery stenosis or occlusion |
| 5 | Cardiac magnetic resonance imaging shows a transmural posterior >myocardial infarction; anti-platelet therapy expanded with clopidogrel |
| 9 | Positron emission tomography confirms a transmural posterior myocardial infarction; therapy expanded with an angiotensin-converting-enzyme inhibitor |
| 10 | Transfer to other hospital |
Case presentation
We report the case of a 48-year-old female who checked into the emergency room (ER) having experienced pain in her chest and left shoulder since the previous evening. The patient was of normal weight and reported a positive family history for coronary artery disease, but denied other cardiovascular risk factors such as hypercholesterinaemia, hypertension, diabetes, or nicotine consumption. The patient had no past medical history and was taking no medication. At presentation, her vital signs were stable. The physical and cardiovascular examination revealed no abnormalities. The chest pain receded after one gram of paracetamol.
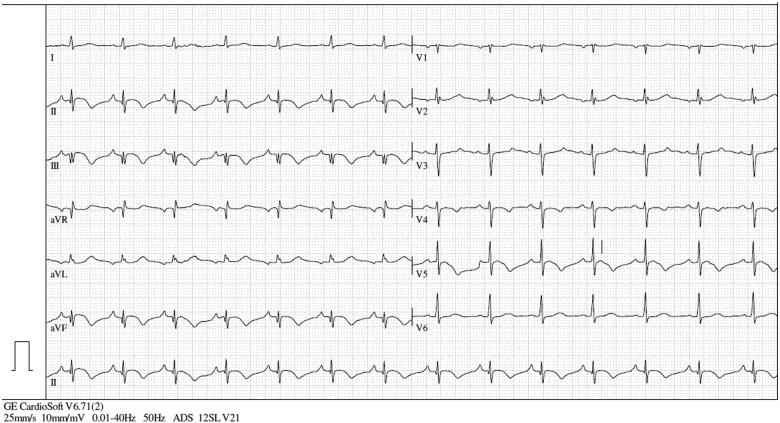
A 12-lead electrocardiogram (ECG) was performed in the ER at presentation, showing inverted T-waves in II, III, aVF, V4, V5, and V6 (Figure 1). Bedside echocardiography revealed hypokinesis in the apical inferior segment of the left ventricle. The laboratory results showed significantly increased values for high-sensitivity troponin I (upward of 25 000 pg/mL, reference range: 0.0–51.4 pg/mL) and creatine kinase (CK) (540 U/L, reference range: 0–145 U/L). Therefore, a non-ST-elevation myocardial infarction was suspected, and the local interventional cardiology centre was contacted to perform coronary angiography (CAG).
Figure 1.
ECG at presentation, showing inverted T-waves in II, III, aVF, V4, V5, and V6.
The patient was swabbed before transfer to exclude a SARS-CoV-2 infection, as per the Medical University of Innsbruck (MUI) guidelines during the SARS-CoV-2 pandemic; surprisingly she tested positive for SARS-CoV-2 on the real-time reverse transcriptase-polymerase chain reaction assay. After further questioning, she reported having experienced isolated throat discomfort since the beginning of February, but denied contact with individuals with confirmed infections or flu-like symptoms; she also denied fever, cough, malaise, dyspnoea, or anosmia.
After further discussion with the cardiology department, the ER team revised the working diagnosis to viral myocarditis and referred the patient to our infectious diseases ward, where she was promptly isolated and received cardiac and haemodynamic monitoring. We started therapy with acetylsalicylic acid, prophylactic-dose low-molecular-weight heparin, a statin, and a proton-pump inhibitor.
A computed tomography coronary angiography (CTCA) was performed to exclude a coronary origin for the complaints and for the laboratory and ECG abnormalities, which revealed no significant coronary obstruction (Coronary Artery Disease Reporting and Data System: 0; Coronary Artery Calcium – Data and Reporting System: 0) (Figure 2).
Figure 2.
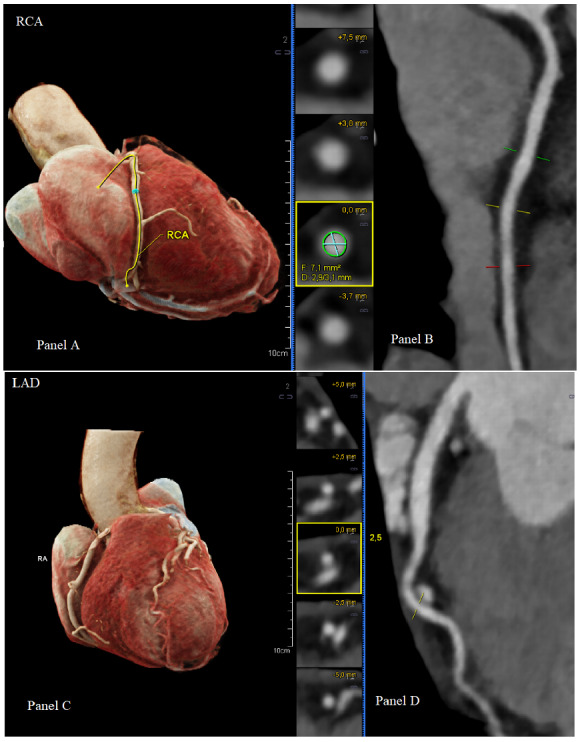
Computed tomography volume rendering technique with depiction of the right coronary artery (A) and left anterior descending artery (C). Right coronary artery showing a strong appearance with good delineation and no signs of stenosis (B). Left anterior descending artery with good delineation and no signs of stenosis (D).
To further confirm the diagnosis of viral myocarditis, a cardiac magnetic resonance imaging (CMR) was arranged. Surprisingly, CMR showed features of myocardial oedema restricted to the mid-ventricular to apical territory of the right coronary artery (RCA), as evidenced on T2 short tau inversion recovery sequence (signal intensity ratio of myocardium over skeletal muscle 2.3, Figure 3A) and on T1 maps (increased native T1 of 1313 ms at inferior wall; reference value 980 ms, Figure 3B). Based on subendocardial to partially transmural late gadolinium enhancement in the mid-ventricular to apical inferior wall, an acute myocardial infarction was diagnosed (Figure 3C,D).
Figure 3.
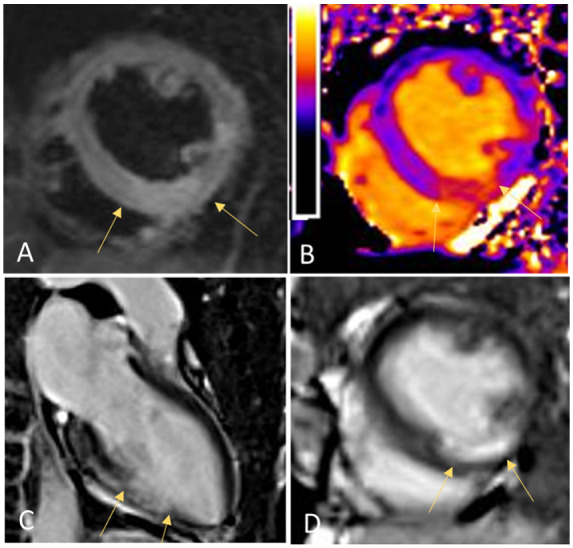
Cardiac magnetic resonance imaging showing myocardial oedema restricted to mid-ventricular to apical territory of the right coronary artery as evidenced on T2 short tau inversion recovery sequence (signal intensity ratio of myocardium over skeletal muscle 2.3, A) and on T1 maps (increased native T1 of 1313 ms at inferior wall; reference value 980 ms, B). Signs of acute myocardial infarction, based on subendocardial to partially transmural late gadolinium enhancement in the mid-ventricular to apical inferior wall (C and D).
Meanwhile, troponin T and CK values decreased (Figure 4), and episodes of mild chest pain were sufficiently controlled with paracetamol.
Figure 4.
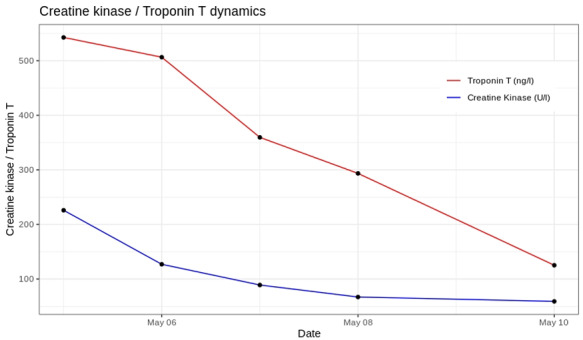
Trend of troponin T and CK values.
After further communication with our interventional cardiology department the diagnosis was revised to a posterior MINOCA; the cardiologists advised against a CAG, due to the absence of coronary obstruction on the CTCA scan.
Further diagnostics with cardiac positron emission tomography–computed tomography showed evidence of reduced metabolic activity in the area affected by the infarction (Figure 5).
Figure 5.
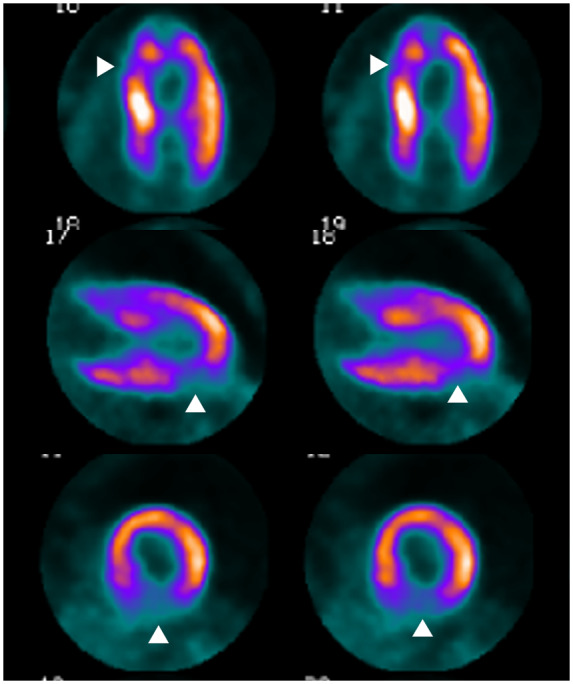
Transmural myocardial infarction with reduced metabolism in the posterior wall.
Videos 1.
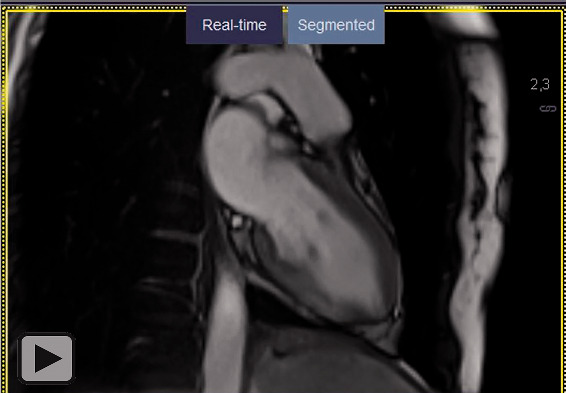
Two-chamber long-axis view/short axis view of a CINE steady-state-free-precession- (SSFP) sequence after application of gadolinium-based contrast media revealing hypokinesia of the mid-ventricular to apical portion of the inferior wall.
Videos 2.
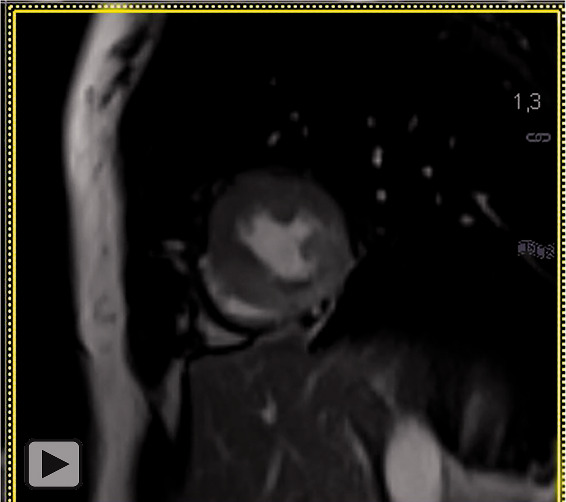
Two-chamber long-axis view/short axis view of a CINE steady-state-free-precession- (SSFP) sequence after application of gadolinium-based contrast media revealing hypokinesia of the mid-ventricular to apical portion of the inferior wall.
We prescribed dual anti-platelet therapy and an angiotensin-converting-enzyme inhibitor. A beta-blocker was not started due to a resting heart rate of 60 beats per minute. After two negative SARS-CoV-2 swab results in over 24 h and absence of COVID-19 typical symptoms, the patient was no longer considered infectious and could be assigned to a cardiac rehabilitation program. Follow-up echocardiography 2 days after discharge revealed a normal ejection fraction (58%) despite persistent inferior apical akinesia.
Discussion
Multiple authors have reported an increased rate of thrombo-embolic events in patients infected by SARS-CoV-2.4–6 Abnormal coagulation parameters also correlate with disease severity.8 The pro-thrombotic effects of SARS-CoV-2 are currently under investigation and could be enhanced by immune system hyperactivity with cytokine storm and pronounced local and systemic inflammatory reactions.7 The presence of angiotensin converting enzyme 2 on the endothelium of coronary arteries may also lead to endothelitis with increased local pro-thrombotic effect.9
While pre-existing cardiovascular disease is a documented risk factor for severe SARS-CoV-2 infection,10 the literature of cases and studies analysing the effects of the virus on the heart and cardiovascular diseases is currently in the preliminary phase. Reported cardiac complications include arrhythmia, myocarditis and cardiac shock,11 with increased myocardial injury biomarkers often being associated with severe disease and higher rate of ICU admissions.3
Venous and arterial thrombo-embolic complications have been reported in 31% of patients with severe disease,6 but rare cases have also been observed in mild infections.12
We believe that the pro-thrombotic or endothelitis-inducing effects of the SARS-CoV-2 infection may have caused either local coronary thrombosis or formation of a distal embolus and facilitated the MINOCA in our patient, and that the blood clot dissolved in the period leading up to the CTCA. Alternatively, MINOCA and the oligosymptomatic SARS-CoV-2 infection may have coincidentally occurred simultaneously, as independent pathological processes.
Conclusion
With this case report, we present a serious acute cardiac event in the context of an otherwise paucisymptomatic SARS-CoV-2 infection. Further analysis and explanation of the possible pathogenic mechanisms leading to MINOCA and other cardiovascular complications during this pandemic are urgently warranted.
Lead author biography

Dr Burkert is a third-year resident physician currently specializing in internal medicine at the Medical University of Innsbruck. He completed his medical school at the University of Perugia. His research focuses on infectious diseases under the guidance of Prof. Günter Weiss and Prof. Rosa Bellmann-Weiler.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidelines.
Conflict of interest: none declared.
Funding: none declared.
Supplementary Material
Contributor Information
Francesco Robert Burkert, Internal Medicine Department II, Medical University Innsbruck, Landeskrankenhaus Innsbruck, Anichstraße 35, 6020 Innsbruck, Austria.
Lukas Niederreiter, Internal Medicine Department II, Medical University Innsbruck, Landeskrankenhaus Innsbruck, Anichstraße 35, 6020 Innsbruck, Austria.
Wolfgang Dichtl, Internal Medicine Department II, Medical University Innsbruck, Landeskrankenhaus Innsbruck, Anichstraße 35, 6020 Innsbruck, Austria.
Agnes Mayr, Radiology Department, Medical University Innsbruck, Innsbruck, Austria.
Irene Virgolini, Nuclear Medicine Department, Medical University Innsbruck, Innsbruck, Austria.
Andrea Klauser, Radiology Department, Medical University Innsbruck, Innsbruck, Austria.
Günter Weiss, Internal Medicine Department II, Medical University Innsbruck, Landeskrankenhaus Innsbruck, Anichstraße 35, 6020 Innsbruck, Austria.
Rosa Bellmann-Weiler, Internal Medicine Department II, Medical University Innsbruck, Landeskrankenhaus Innsbruck, Anichstraße 35, 6020 Innsbruck, Austria.
References
- 1. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H.. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203:631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen L, Li X, Chen M, Feng Y, Xiong C.. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res 2020;116:1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J. et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T. et al. Humanitas COVID-19 Task Force. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 2020;191:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boscolo A, Spiezia L, Correale C, Sella N, Pesenti E, Beghetto L. et al. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost 2020;120:1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers D, Kant KM. et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;191:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Joly BS, Siguret V, Veyradier A.. Understanding pathophysiology of hemostasis disorders in critically ill patients with COVID-19. Intensive Care Med 2020;46:1603–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tang N, Li D, Wang X, Sun Z.. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lazaridis C, Vlachogiannis NI, Bakogiannis C, Spyridopoulos I, Stamatelopoulos K, Kanakakis I. et al. Involvement of cardiovascular system as the critical point in coronavirus disease 2019 (COVID-19) prognosis and recovery. Hellenic J Cardiol 2020;S1109–9666(20):30093–30102. doi: 10.1016/j.hjc.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu Z, McGoogan JM.. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239. [DOI] [PubMed] [Google Scholar]
- 11. Kochi AN, Tagliari AP, Forleo GB, Fassini GM, Tondo C.. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol 2020;31:1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levolger S, Bokkers RPH, Wille J, Kropman RHJ, de Vries JPM.. Arterial thrombotic complications in COVID-19 patients. J Vasc Surg Cases Innov Tech 2020;6:454–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.